Abstract
Background
Early intraoperative hypotension is associated with acute kidney and myocardial injury in patients undergoing noncardiac surgery. Precise arterial blood pressure measurement before and during the induction of general anaesthesia may avert early intraoperative hypotension. However, rapid arterial cannulation in anxious, conscious patients can be challenging. We describe the protocol for a randomised controlled trial designed to test the hypothesis that readily available, handheld ultrasound-guided arterial cannulation is the optimal method in conscious patients undergoing noncardiac surgery.
Methods
Participants >45 yr undergoing noncardiac surgery expected to last >120 min and requiring an overnight hospital stay will be eligible. We will randomly allocate participants to undergo cannulation of the radial artery in the non-dominant arm before the induction of general or regional anaesthesia using either handheld ultrasound-guided dynamic needle position technique or palpation. The primary outcome is first-pass successful arterial cannulation, analysed by intention-to-treat. Secondary outcomes include adequacy/characteristics of the arterial waveform and complications within 24 h of cannulation. We will require 118 patients to demonstrate a doubling of successful first-pass arterial cannulation, from ∼30% using the palpation approach (α=0.05; 1–β=0.1).
Results
This study has been approved by the NHS Health Research Authority and Health Care Research Wales (21/WA/0403) and commenced recruitment in May 2022.
Conclusions
This study will establish whether handheld ultrasound-guided arterial cannulation before the induction of anaesthesia should be the standard of care in patients at risk of developing perioperative organ injury after noncardiac surgery.
Clinical trial registration
Keywords: arterial cannulation, blood pressure, perioperative medicine, ultrasound, ultrasound guided
Perioperative hypotension is associated with organ injury and increased mortality in patients undergoing noncardiac surgery.1,2 Even brief episodes of relative hypotension after the induction of general anaesthesia are associated with an increased risk of organ injury.1 Intraoperative BP monitoring with arterial catheters detects hypotension twice as frequently as oscillometric measurements.3 However, large limits of agreement have been observed between noninvasive brachial cuff oscillometry vs invasive BP measurements in critically ill patients.4 Provided that the arterial BP waveform is of sufficient quality,5,6 invasive measurement before the induction of anaesthesia may enable the prompt recognition and treatment of hypotension, which may impact on clinical outcomes.7,8 In conscious patients undergoing coronary revascularisation for ST-segment elevation myocardial infarction, invasive arterial BP monitoring reduced complications and the time to achieve vascular reperfusion.9 Notably, comparable prospective studies are sparse in noncardiac surgery.8
In contrast to central venous cannulation,10,11 the evidence base for the use of ultrasound in arterial cannulation remains unclear.5 The most recent European Society of Anaesthesiology and Intensive Care (ESAIC) (perioperative use of ultrasound [PERSEUS] vascular access) guidelines on ultrasound-guided cannulation of an artery during elective procedures concur that the quality of evidence on which to base recommendations is generally weak, with relatively few RCTs that have a high degree of heterogeneity.12 The advent of high-resolution low-cost handheld ultrasound probes suggests that image-guided intra-arterial access could be widely adopted. However, establishing first-pass invasive arterial BP monitoring before induction of anaesthesia with ultrasound has not been demonstrated (Table 1) in conscious, non-sedated patients at risk of perioperative organ injury,13 in whom sedation is very likely to alter arterial BP.14 Highly experienced cardiac anaesthetists failed to find any advantage of ultrasound-guided arterial cannulation in sedated patients before cardiac surgery.15 This may reflect the lack of systematic examination of the dynamic needle-tip positioning approach, which appears to be superior,16 although a recent systematic review suggests higher-quality studies are needed.17
Table 1.
Summary of studies detailing radial artery catheterisation in conscious participants. EMLA, eutectic mixture of local anaesthetic; N, no; SA, short axis; Y, yes.
| Study | No. of patients (control/ultrasound) | Location | Outcomes | Approach (short/long axis) | Anaesthetised | Sedation | Operator choice of side | Definition of first-attempt success (defines ‘attempt’ and ‘success’ | Mode of analgesia | EMLA | Waveform analysis | First-attempt success rate |
Notes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Palpation |
Ultrasound |
|||||||||||||||
| Events | Total | Events | Total | |||||||||||||
| Shiver and colleagues20 (2006) | 60 (30/30) | Emergency department | (i) First-attempt success (ii) Number of attempts (iii) Time (iv) Number of cannulas used |
SA | N | Not mentioned | Y | Y; catheter insertion | Not described | N | N | 15 (50%) | 30 | 26 (87%) | 30 | |
| Seto and colleagues21 (2015) | 698 (351/347) | Cardiac catheterisation laboratory | (i) First-attempt success (ii) Number of attempts (iii) Time |
SA | N | Y; as per local practice | Y | Y; catheter insertion | Intra-arterial or s.c. lidocaine as per local practice | N | N | 44% | 65% | Intra-arterial verapamil/nitroglycerine for spasm prophylaxis | ||
| Hansen and colleagues22 (2014) | 40 (40/40) | Operating theatre (cardiac) | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Number of catheters |
SA | N | Not mentioned | N | Y; catheter insertion | Lidocaine s.c. for palpation group only | N | N | 23 (58%) | 40 | 38 (95%) | 40 | Crossover study |
| Levin and colleagues23 (2003) | 69 (35/34) | Operating theatre | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Number of catheters |
SA | N | Not mentioned | Y | Y; catheter insertion | Lidocaine s.c. | N | N | 12 (34%) | 35 | 21 (62%) | 34 | |
| Peters and colleagues15 (2015) | 125 (62/63) | Operating theatre (cardiac) | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Number of redirects (v) Complications |
SA | N | Y, as per local practice | Y | Y; catheter insertion | Lidocaine s.c. | N | N | 56% | 71% | No significant difference between palpation/ultrasound | ||
| Ueda and colleagues24 (2015) | 749 (256/249/Doppler 254) | Operating theatre | (i) First-attempt success (ii) Time (iii) Complications |
SA | Y and N; at discretion of anaesthetist | Not mentioned | Y | Y; transduced arterial waveform | Lidocaine s.c. | N | N | 101 (39%) | 256 | 132 (53%) | 249 | |
| Zaremski and colleagues25 (2013) | 183 (91/92) | Cardiac catheterisation laboratory | (i) ‘Primary’ success rate (ii) Time |
Operator discretion | N | Not mentioned | Y | Number of attempts not clearly defined; catheter insertion | Not described | N | N | 87% | 87% | No difference between palpation/ultrasound | ||
| Tangwiwat and colleagues26 (2016) | 100 (50/50) | Operating theatre (neurosurgery) | (i) First-attempt success (ii) Time (iii) Complications |
Both | N | Not mentioned | Y | Y; catheter insertion | Not described | N | N | 65% | 69% | No significant difference between palpation/ultrasound | ||
| Kiberenge and colleagues27 (2018) | 260 (128/132) | Operating theatre | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Number of catheters |
SA | Y and N; at discretion of anaesthetist | Not mentioned | Y | Y; transduced arterial waveform | Not described | N | N | 48% | 83% | |||
| Gopalasingam and colleagues28 (2017) | 40 (40/40) | Operating theatre (cardiac) | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Number of catheter (v) Pain |
SA | N | Not mentioned | N | Y; catheter insertion | Lidocaine injection | N | N | 70% | 90% | Crossover study | ||
| Gibbons and colleagues29 (2020) | 40 (20/20) | Emergency department | (i) First-attempt success (ii) Time (iii) Number of attempts (iv) Complications |
SA | N | Not mentioned | Y | Y; catheter insertion | Lidocaine injection | N | N | 0% | 75% | |||
| Yu and colleagues30 (2019) | 60 (30/30) | Operating theatre | (i) First-attempt success (ii) Time (iii) Complications |
SA | N | Not mentioned | Y | Y; catheter insertion | Lidocaine injection | N | N | 73% | 97% | |||
| Men and colleagues31 (2022) | 92 (46/46) | Operating theatre (obstetrics) | (i) First-attempt success (ii) Time (iii) Number of attempts |
SA | N | Not mentioned | Y | Y; transduced arterial waveform | Median nerve block with lidocaine | N | N | Ultrasound-guided cannulation in both groups: control 78% vs median nerve block 96% | ||||
EMLA, eutectic mixture of local anaesthetic; N, no; SA, short axis; Y, yes.
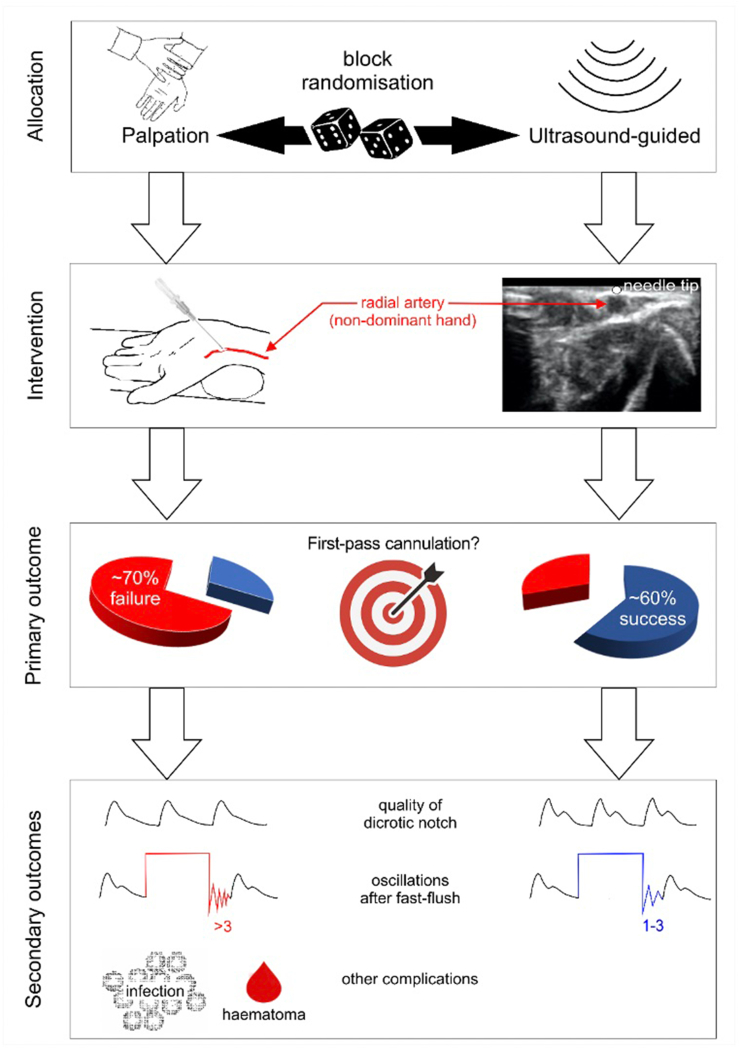
Herein, we describe the protocol for an RCT designed to examine the hypothesis that arterial cannulation guided by readily available handheld ultrasound technology is superior to the more frequently used palpation technique (standard-of-care) in conscious patients before the induction of anaesthesia at increased risk of organ injury and increased mortality after noncardiac surgery (Fig 1). For the first time, this trial specifically addresses cannulation in non-sedated patients and examines whether ultrasound-guided placement reduces underdamping/resonance phenomena, which are key factors in determining the utility of physiological information derived from the radial arterial waveform.18,19
Fig 1.
Study hypothesis and outcomes.
Methods
Study design
This multicentre, randomised, single-blind controlled trial has been approved by the NHS Health Research Authority and Health Care Research Wales (21/WA/0403) and registered on ClinicalTrials.gov (NCT05249036). Patient recruitment began in May 2022 at an NHS Trust in England, UK.
Inclusion criteria
-
(i)
Patients scheduled to undergo major elective or urgent (not requiring intervention in <24 h) noncardiac surgery under general or neuraxial anaesthesia
-
(ii)
Age ≥45 yr
-
(iii)
Requiring overnight hospital stay
Exclusion criteria
-
(i)
Anatomical deformity local to the radial artery on the non-dominant upper limb, detected either clinically or by pre-scanning with ultrasound (by an independent investigator)
-
(ii)
Cannulation, or attempted cannulation, of the radial artery on the non-dominant upper limb within the preceding 24 h
-
(iii)
Overlying infection on the non-dominant upper limb
-
(iv)
Surgical site on the non-dominant upper limb
Recruitment and screening
Eligible patients will be identified by a member of the research team either at preoperative assessment clinics or on the surgical ward if the surgical procedure is scheduled at least 24 h later. They will be given a patient information leaflet detailing the trial with an explanation of the aims, methods, anticipated benefits, and potential hazards of the trial. A member of the research team will approach potential participants and screen them against the eligibility criteria before asking for consent. All eligible patients who undergo screening will be recorded on a screening log on the investigator site file even if they decline the study. Written informed consent will be obtained on the day of surgery.
Informed consent
The principal investigator or a suitably qualified nominee will obtain written informed consent from each participant. All potential participants are free to refuse to enter the trial or to withdraw at any time during the trial for any reason. If new safety information results in significant changes in the risk/benefit assessment, the patient information sheet and consent form will be reviewed or amended accordingly. The participants will be informed that, as defined by the UK Policy Framework for Health and Social Care Research, all documentation will be stored for a minimum of 20 yr at the end of the study.
Investigator training
The intervention will be carried out by a senior Fellowship of the Royal College of Anaesthetists (FRCA)-qualified anaesthetist who has completed a programme of training in handheld ultrasound-guided cannulation of the radial artery. Each named investigator is required to successfully complete a programme of training run by the lead investigators. The anaesthetist must have completed the study training package (Supplementary data 1).
Randomisation
Participants are randomly assigned (1:1) by block randomisation (by centre) to either handheld ultrasound or palpation-guided cannulation on the day of surgery to ensure a balance in sample size across groups over time (Simple + randomisation service; Sealed Envelope Ltd, London, UK). Investigators will log on to a secure web-based randomisation file to obtain a unique patient identification number and allocation to a treatment group.
Procedures
Topical local anaesthetic cream will be applied 1–4 cm above the crease of the wrist on the ventral forearm of the non-dominant upper limb approximately 45 min before cannulation. Several RCTs have reported that eutectic mixture of local anaesthetic (EMLA) is superior to local infiltration by reducing pain associated with radial artery cannulation and improving the success rate of the procedure compared with subcutaneous local lidocaine infiltration.32, 33, 34 EMLA may also avoid the potential confounding of injected lidocaine influencing the development of local vasospasm,35 bleeding, and bruising. The non-dominant hand will be draped to avoid the participant viewing the procedure and placed at the optimal extension angle of 45° for radial artery cannulation.36 Because multiple studies have demonstrated the presence of compensatory recruitable collateral vessels that appear to be functionally recruited with ischaemia, we do not routinely undertake a modified Allen's test. This is consistent with current American Heart Association guidelines for radial arterial canulation, which observes that, ‘Routine application of the Allen or Barbeau test is not a useful triage strategy, and an abnormal test should not preclude transradial access’.37 Pre-scanning Vscan™ (GE Healthcare, Chalfont St Giles, UK) will be conducted by an independent operator to quantify radial artery diameter and any anatomical abnormalities. The Vscan probe enables cloud storage of the images.
After randomisation, under sterile conditions (chlorhexidine), the radial artery will be cannulated using a 20G catheter (Leadercath Arterial; Vygon, Swindon, UK). Cannulation will take place before the induction of general anaesthesia. Sedation will not be used to facilitate cannulation. If the participant is allocated to the ultrasound guidance group, the radial artery will be cannulated using the out-of-plane dynamic needle tip positioning technique (Fig 1) using a handheld ultrasound probe.38 Visualisation of the needle tip using the central axis guide and intra-arterial localisation will be required to ensure consistency of technique. For participants allocated to the palpation technique group, the investigator will use their usual palpation technique using the same positioning approach. A maximum of one attempt will be allowed, defined by a single needle puncture of the skin. Further attempts to cannulate in the conscious patient will be left to the discretion of the attending clinical team. In this case, further cannulation attempts may take place for clinical indications but are not stipulated by the study protocol. An example of such a situation may be a patient with critical aortic stenosis who requires invasive haemodynamic monitoring before induction of anaesthesia.
Data collection
On the morning of surgery, clinical data and operation type will be recorded (Supplementary data 2). The electronic patient record will be reviewed at 24 h after surgery for defined complications related to the cannulation. Data will be entered electronically on a secure database (Research Electronic Data Capture [REDCAP]).39
Bias and blinding
Participants will be masked to the intervention allocation by draping of the non-dominant hand. Researchers performing arterial cannulation cannot be blinded to the allocation. However, the characteristics of the arterial waveform will be assessed by an independent investigator who is masked to the treatment allocation.
Primary outcome
The primary outcome is the successful transduction of an arterial waveform after cannulation of the radial artery at the first attempt.
Secondary outcomes
-
(i)
Arterial waveform characteristics: the presence of dicrotic notch or two to three oscillations after fast flush of the arterial line indicates a satisfactory quality of waveform.5
-
(ii)
Adequacy of arterial cannulation in the first 24 h after cannulation, including the need for replacement within 24 h of the procedure
-
(iii)
Central–radial BP gradient, using noninvasive BP obtained immediately before the cannulation
-
(iv)
Complications related to arterial cannulation at 24 h (including bleeding, thrombosis, and infection)
Sample size
The most similar ultrasound-specific study to ours reported a first-pass success rate for ultrasound-guided radial artery catheterisation using the short-axis approach of 42% in patients undergoing cardiac angiography, although in this study liberal use of sedation was reported (Table 1). Because our study is not performed under general anaesthesia, we estimated a conservative patient withdrawal rate of 10%, a technical failure rate of 5%, and hence an approximately 35% success rate for patients randomly allocated to the palpation group. Therefore, 118 consecutive patients will be assigned to either ultrasound-guided or palpation technique to examine whether handheld ultrasound-guided cannulation improves first-pass cannulation from 35% using the palpation technique to 70% (α=0.01; 1–β=0.9; StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX).
Statistical analysis
An independent trial statistician will analyse the primary outcome by intention to treat. Analyses will follow the intention-to-treat principle, whereby all randomised patients will be included in the analysis and analysed according to the treatment to which they were randomised. Patients will be included in the analysis, regardless of whether the treatment they received was compliant with the protocol. Patients with missing outcome data will not be excluded from the analysis. For the analysis of the primary outcome, each secondary outcome, and all process measures, we will present the following information:
-
(i)
The number of patients included in each analysis, by treatment group
-
(ii)
A summary statistic (e.g. mean [standard deviation] and number [%]), by treatment group
-
(iii)
The estimated treatment effect
-
(iv)
A 95% confidence interval for the estimated treatment effect
-
(v)
A two-sided P-value
Sensitivity analysis
A multilevel analysis will cater for the number of operators that perform procedures. There will be no more than 10 highly experienced FRCA-qualified operators with experience of >200 radial arterial cannulations plus the ultrasound study-specific training package.
Trial management and data monitoring
The sponsor organisation is Queen Mary University of London (Joint Research Management Office, London, UK). Daily trial management will be coordinated by a trial management group consisting of the chief investigator and their support staff. An independent study committee will oversee the trial, including assessing the safety of the intervention, reviewing relevant new external evidence, and monitoring the overall conduct of the trial. This committee consists of an independent clinical triallist, lay representative, and an independent chair. Any protocol modifications will be reported by the chief investigator to the trial sponsor.
Adverse events
Any adverse events (other than pre-specified complications) directly related to the trial intervention will be recorded.
Confidentiality
Information related to participants will be kept confidential and managed in accordance with the Data Protection Act (UK), NHS Caldicott Principles (UK), Research Governance Framework for Health and Social Care (UK), and the conditions of the Research Ethics Committee approval. The principal investigator will maintain in strict confidence trial documents (e.g. patients' written consent forms) and patients' confidentiality. Representatives of the trial management team will require access to patient notes for quality assurance purposes and source data verification.
Auditing
The sponsor will have oversight of the trial conduct. The trial team will ensure compliance with the requirements of Good Clinical Practice, including data quality control and safety reporting.
Dissemination plans
Data arising from the research will be made available to the scientific community in a timely and responsible manner. A detailed scientific report will be submitted to a widely accessible scientific journal. Authorship of the final article(s), interim publications, or abstracts will be decided according to active participation in the design, accrual of eligible patients, and statistical analysis. Contributing/participating investigators will be acknowledged in the final article. We have included Kate Rivett as a lay study member, who contributed to the design of the patient information sheet. Findings from the study will be made available at www.qmul.ac.uk/ccpmg/projects/.
Authors' contributions
Study protocol design: VL-P-K, GLA, TCE.
Drafting of article: all authors.
Subsequent revisions: all authors
Declarations of interest
GLA is editor of the British Journal of Anaesthesia and performs consultancy work for GlaxoSmithKline unrelated to this work. The other authors declare no competing financial interests.
Funding
The London Clinic (clinical research fellowship) to VL-P-K; National Institute for Health and Care Research Advanced Fellowship (NIHR 300097) to GLA; British Heart Foundation programme grant (RG/19/5/34463) to GLA.
Acknowledgements
The London Clinic has had/will have no role in study design, collection, management, analysis, interpretation of data, writing of the report; and the decision to submit the report for publication.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2022.100111.
Contributor Information
Gareth L. Ackland, Email: g.ackland@qmul.ac.uk.
the Arterial Cannulation with Ultrasound Investigators:
Stephen Barrett, Mevan Gooneratne, Shaun Montagu May, Matthew Mitchard, Timothy O'Neill, Jennifer Overend, Tom EF. Abbott, Ching-Ling Pang, Archchana Radhakrishnan, Toby Reynolds, Mehul Vadher, Parvesh Verma, Matthew Wikner, and Andrew Wood
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sessler D.I., Bloomstone J.A., Aronson S., et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122:563–574. doi: 10.1016/j.bja.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Wesselink E.M., Kappen T.H., Torn H.M., Slooter A.J.C., van Klei W.A. Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. 2018;121:706–721. doi: 10.1016/j.bja.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 3.Naylor A.J., Sessler D.I., Maheshwari K., et al. Arterial catheters for early detection and treatment of hypotension during major noncardiac surgery: a randomized trial. Anesth Analg. 2020;131:1540–1550. doi: 10.1213/ANE.0000000000004370. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann T., Cox E.G.M., Wiersema R., et al. Non-invasive oscillometric versus invasive arterial blood pressure measurements in critically ill patients: a post hoc analysis of a prospective observational study. J Crit Care. 2020;57:118–123. doi: 10.1016/j.jcrc.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Saugel B., Kouz K., Meidert A.S., Schulte-Uentrop L., Romagnoli S. How to measure blood pressure using an arterial catheter: a systematic 5-step approach. Crit Care. 2020;24:172. doi: 10.1186/s13054-020-02859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackland G.L., Brudney C.S., Cecconi M., et al. Perioperative Quality Initiative consensus statement on the physiology of arterial blood pressure control in perioperative medicine. Br J Anaesth. 2019;122:542–551. doi: 10.1016/j.bja.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Abbott T.E.F., Howell S., Pearse R.M., Ackland G.L., Vision-UK Investigators Mode of blood pressure monitoring and morbidity after noncardiac surgery: a prospective multicentre observational cohort study. Eur J Anaesthesiol. 2021;38:468–476. doi: 10.1097/EJA.0000000000001443. [DOI] [PubMed] [Google Scholar]
- 8.Ackland G.L., Abbott T.E.F. Hypotension as a marker or mediator of perioperative organ injury: a review. Br J Anaesth. 2022;128:915–930. doi: 10.1016/j.bja.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y., Liu J., Peng W., Wang A., Guo L., Xu Z. Comparison of invasive blood pressure monitoring versus normal non-invasive blood pressure monitoring in ST-elevation myocardial infarction patients with percutaneous coronary intervention. Injury. 2022;53:1108–1113. doi: 10.1016/j.injury.2021.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Brass P., Hellmich M., Kolodziej L., Schick G., Smith A.F. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. doi: 10.1002/14651858.CD011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brass P., Hellmich M., Kolodziej L., Schick G., Smith A.F. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. doi: 10.1002/14651858.CD006962.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamperti M., Biasucci D.G., Disma N., et al. European Society of Anaesthesiology guidelines on peri-operative use of ultrasound-guided for vascular access (PERSEUS vascular access) Eur J Anaesthesiol. 2020;37:344–376. doi: 10.1097/EJA.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 13.White L., Halpin A., Turner M., Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116:610–617. doi: 10.1093/bja/aew097. [DOI] [PubMed] [Google Scholar]
- 14.Sneyd J.R., Absalom A.R., Barends C.R.M., Jones J.B. Hypotension during propofol sedation for colonoscopy: a retrospective exploratory analysis and meta-analysis. Br J Anaesth. 2022;128:610–622. doi: 10.1016/j.bja.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters C., Schwarz S.K., Yarnold C.H., Kojic K., Kojic S., Head S.J. Ultrasound guidance versus direct palpation for radial artery catheterization by expert operators: a randomized trial among Canadian cardiac anesthesiologists. Can J Anaesth. 2015;62:1161–1168. doi: 10.1007/s12630-015-0426-8. [DOI] [PubMed] [Google Scholar]
- 16.Bai B., Tian Y., Zhang Y., Yu C., Huang Y. Dynamic needle tip positioning versus the angle-distance technique for ultrasound-guided radial artery cannulation in adults: a randomized controlled trial. BMC Anesthesiol. 2020;20:231. doi: 10.1186/s12871-020-01152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi J., Shen J., Xiang Z., Liu X., Lu T., Tao X. Dynamic needle tip positioning versus palpation and ultrasound for arteriovenous puncture: a meta-analysis. Ultrasound Med Biol. 2021;47:2233–2242. doi: 10.1016/j.ultrasmedbio.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Foti L., Michard F., Villa G., Ricci Z., Romagnoli S. The impact of arterial pressure waveform underdamping and resonance filters on cardiac output measurements with pulse wave analysis. Br J Anaesth. 2022;129 doi: 10.1016/j.bja.2022.03.024. e6–8. [DOI] [PubMed] [Google Scholar]
- 19.Romagnoli S., Ricci Z., Quattrone D., et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18:644. doi: 10.1186/s13054-014-0644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiver S., Blaivas M., Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13:1275–1279. doi: 10.1197/j.aem.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Seto A.H., Roberts J.S., Abu-Fadel M.S., et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial) JACC Cardiovasc Interv. 2015;8:283–291. doi: 10.1016/j.jcin.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Hansen M.A., Juhl-Olsen P., Thorn S., Frederiksen C.A., Sloth E. Ultrasonography-guided radial artery catheterization is superior compared with the traditional palpation technique: a prospective, randomized, blinded, crossover study. Acta Anaesthesiol Scand. 2014;58:446–452. doi: 10.1111/aas.12299. [DOI] [PubMed] [Google Scholar]
- 23.Levin P.D., Sheinin O., Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31:481–484. doi: 10.1097/01.CCM.0000050452.17304.2F. [DOI] [PubMed] [Google Scholar]
- 24.Ueda K., Bayman E.O., Johnson C., Odum N.J., Lee J.J. A randomised controlled trial of radial artery cannulation guided by Doppler vs. palpation vs. ultrasound. Anaesthesia. 2015;70:1039–1044. doi: 10.1111/anae.13062. [DOI] [PubMed] [Google Scholar]
- 25.Zaremski L., Quesada R., Kovacs M., Schernthaner M., Uthoff H. Prospective comparison of palpation versus ultrasound-guided radial access for cardiac catheterization. J Invasive Cardiol. 2013;25:538–542. [PubMed] [Google Scholar]
- 26.Tangwiwat S., Pankla W., Rushatamukayanunt P., Waitayawinyu P., Soontrakom T., Jirakulsawat A. Comparing the success rate of radial artery cannulation under ultrasound guidance and palpation technique in adults. J Med Assoc Thai. 2016;99:505–510. [PubMed] [Google Scholar]
- 27.Kiberenge R.K., Ueda K., Rosauer B. Ultrasound-guided dynamic needle tip positioning technique versus palpation technique for radial arterial cannulation in adult surgical patients: a randomized controlled trial. Anesth Analg. 2018;126:120–126. doi: 10.1213/ANE.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 28.Gopalasingam N., Hansen M.A., Thorn S., Sloth E., Juhl-Olsen P. Ultrasound-guided radial artery catheterisation increases the success rate among anaesthesiology residents: a randomised study. J Vasc Access. 2017;18:546–551. doi: 10.5301/jva.5000702. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons R.C., Zanaboni A., Saravitz S.M., Costantino T.G. Ultrasound guidance versus landmark-guided palpation for radial arterial line placement by novice emergency medicine interns: a randomized controlled trial. J Emerg Med. 2020;59:911–917. doi: 10.1016/j.jemermed.2020.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y., Lu X., Fang W., Liu X., Lu Y. Ultrasound-guided artery cannulation technique versus palpation technique in adult patients in pre-anesthesia room: a randomized controlled trial. Med Sci Monit. 2019;25:7306–7311. doi: 10.12659/MSM.916252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Men X., Wang Q., Hu W.S., et al. Median nerve block increases the success rate of radial artery cannulation in women with gestational hypertension undergoing cesarean section. BMC Anesthesiol. 2022;22:248. doi: 10.1186/s12871-022-01793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joly L.M., Spaulding C., Monchi M., Ali O.S., Weber S., Benhamou D. Topical lidocaine-prilocaine cream (EMLA) versus local infiltration anesthesia for radial artery cannulation. Anesth Analg. 1998;87:403–406. doi: 10.1097/00000539-199808000-00032. [DOI] [PubMed] [Google Scholar]
- 33.Russell G.N., Desmond M.J., Fox M.A. Local anesthesia for radial artery cannulation: a comparison of a lidocaine-prilocaine emulsion and lidocaine infiltration. J Cardiothorac Anesth. 1988;2:309–312. doi: 10.1016/0888-6296(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 34.Smith M., Gray B.M., Ingram S., Jewkes D.A. Double-blind comparison of topical lignocaine-prilocaine cream (EMLA) and lignocaine infiltration for arterial cannulation in adults. Br J Anaesth. 1990;65:240–242. doi: 10.1093/bja/65.2.240. [DOI] [PubMed] [Google Scholar]
- 35.Ricci J.A., Koolen P.G., Shah J., Tobias A.M., Lee B.T., Lin S.J. Comparing the outcomes of different agents to treat vasospasm at microsurgical anastomosis during the papaverine shortage. Plast Reconstr Surg. 2016;138:401. doi: 10.1097/PRS.0000000000002430. –8e. [DOI] [PubMed] [Google Scholar]
- 36.Kucuk A., Yuce H.H., Yalcin F., Boyaci F.N., Yildiz S., Yalcin S. Forty-five degree wrist angulation is optimal for ultrasound guided long axis radial artery cannulation in patients over 60 years old: a randomized study. J Clin Monit Comput. 2014;28:567–572. doi: 10.1007/s10877-014-9552-z. [DOI] [PubMed] [Google Scholar]
- 37.Mason P.J., Shah B., Tamis-Holland J.E., et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/HCV.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.Y., Kim K.N., Jeong M.A., Lee B.S., Lim H.J. Ultrasound-guided dynamic needle tip positioning technique for radial artery cannulation in elderly patients: a prospective randomized controlled study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.