We read with great interest articles by Wang et al 1 and Hegyi et al,2 in which the authors reported that alcohol increased the risk of hereditary susceptibility to chronic pancreatitis. These results indicated an interaction effect between environmental and genetic risk factors on the development of pancreatitis. Epidemiological data suggested that alcohol abuse increased the risk of acute pancreatitis (AP) in people with type 2 diabetes mellitus (adjusted HR 86.3 (65.3–111.0)).3 However, no study investigated the synergistic effect between obesity and alcohol excess on AP development. Here we report the combination of acute alcohol intake and obesity causes AP with multiorgan injury (MOI) in mice, mediated by visceral adipocyte tissue (VAT) lipolysis.
The schedule of high-fat feeding and ethanol administration is shown in figure 1A. Body weight was significantly higher in the high-fat (obese) than the chow (lean) group after 12 weeks (figure 1B). Acute ethanol administration in obese mice induced significant increases in pancreatic histopathology scores (oedema, inflammation and necrosis; figure 1C), elevated circulating pancreatic enzymes (figure 1D), pancreatic and lung myeloperoxidase, and serum interleukin-6 levels (figure 1E). Time-course changes in this obese alcoholic acute pancreatitis (OA-AP) model showed pancreatic injury parameters were significantly elevated from 3 to 6 hours after the first ethanol injection, with rises in MOI indices (online supplemental figure 1A–F); almost all parameters peaked at 12 hours. In contrast, acute ethanol administration in lean mice caused only mild pancreatic oedema without discernable pancreatic necrosis or elevations of MOI indices.
gutjnl-2022-326958supp001.pdf (83.5KB, pdf)
Figure 1.
Alcohol predisposes obese mice to AP with systemic organ injury. (A) Experimental protocol of establishing OA-AP. C57BL/6 J mice were fed a CD (lean) or HFD (obese) for 12 weeks, then were injected intraperitoneally with 2 g/kg EtOH two times at 1 hour apart: (B) Body weight. (C) Representative images of pancreatic histopathology and histopathological scores (oedema, inflammation and necrosis; magnification ×200), (D) Serum amylase and lipase and (E) pancreatic MPO, lung MPO, and serum IL-6 levels of the OA-AP mice. (F) Serum FFA levels and (G) FFA and glycerol release in collected adipose tissues. (H) Amylase and lipase levels in epididymal adipose tissue. (I) Immunoblot analysis of ATGL proteins in epididymal adipose tissue. In all experiments, mice were sacrificed at 12 hours after the first injection of EtOH and assessed for disease severity and/or lipolytic parameters. *P<0.05, **P<0.01, ***P<0.001. AP, acute pancreatitis; ATGL, adipose triglyceride lipase; CD, control diet; Ctrl, control; EtOH, ethanol; FFA, free fatty acid; HFD, high-fat diet; IL, interleukin; MPO, myeloperoxidase; ns, not significant; OA-AP, obese alcoholic acute pancreatitis.
We speculated that lipolysis from excess abdominal fat is critical to OA-AP, releasing free fatty acids (FFAs) from ethanol-induced VAT lipolysis. Indeed, fat saponification was seen in the peritoneal cavity and around the pancreas of ethanol-treated obese mice (online supplemental figure 2A, B). Circulating baseline FFA levels were higher in obese mice than in lean mice, which were further increased after acute ethanol administration (figure 1F). FFA and glycerol release over 3 hours from freshly isolated epididymal VAT of ethanol-treated obese mice was higher than that of lean mice (figure 1G). While pancreatic amylase or pancreatic triglyceride lipase (PNLIP) were comparable (figure 1H), adipose triglyceride lipase (ATGL) of ethanol-treated epididymal VAT taken from obese mice was, however, significantly higher than from lean mice (figure 1I).
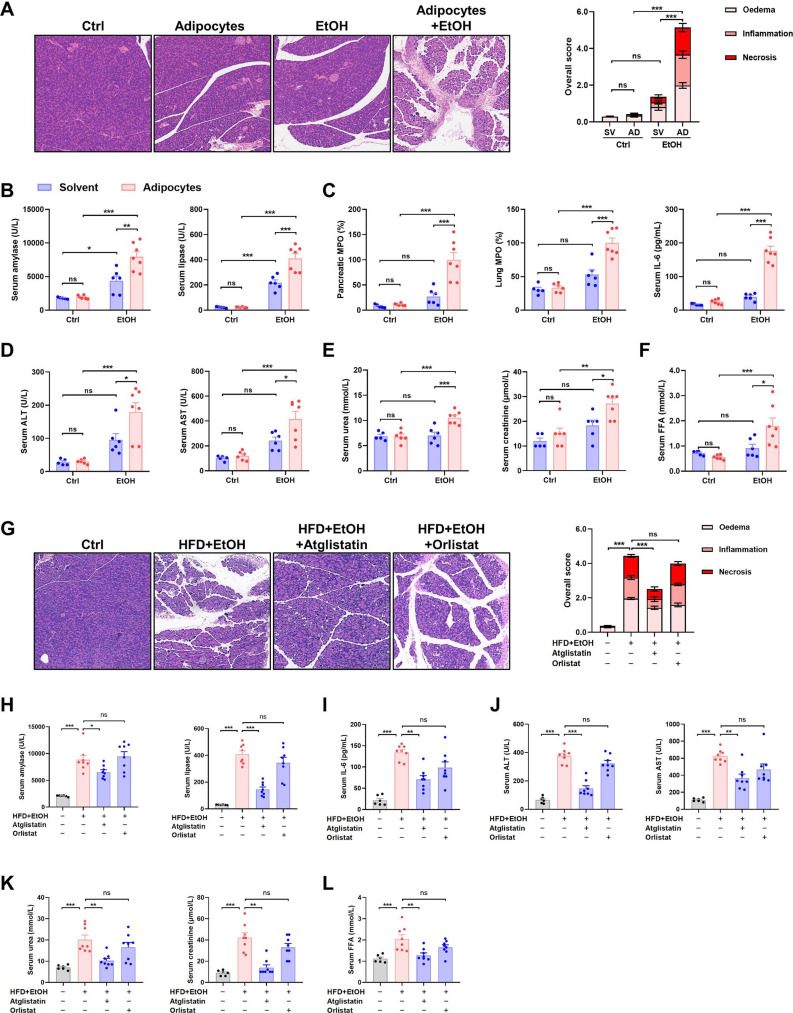
To confirm our hypothesis, we injected ethanol and adipocytes simultaneously into the abdominal cavity of lean mice, which recapitulated all features of OA-AP (figure 2A–F). Inhibition of lipolysis using specific ATGL inhibitor atglistatin significantly reduced pancreas histopathology scores, serum pancreatic enzymes, serum MOI indices and serum FFA levels, while PNLIP inhibitor orlistat had a minimal effect (figure 2G–L). These findings indicate ethanol-induced VAT lipolysis via ATGL activation is central to the pathogenesis of OA-AP. This mechanism parallels the protective systemic effects of ATGL inhibition in burn injury,4 which is distinct from systemic lipotoxicity consequent on leakage of PNLIP from the injured pancreas.5 Interestingly, we found both atglistatin and orlistat were protective against caerulein-induced AP in obese mice (online supplemental figure 2C–G), mirroring patients with COVID-19 where PNLIP-mediated and ATGL-mediated lipotoxicity may both take place after disease onset.6
Figure 2.
Obesity and acute alcohol intake cause AP through visceral adipocyte lipolysis mediated by ATGL. Lean mice received intraperitoneal injection adipocytes 1 hour prior to two injection of 2 g/kg EtOH at a 1-hour interval: (A) Representative images of pancreatic histopathology (magnification ×200) and histopathological scores. (B) Serum amylase and lipase, and (C) pancreatic MPO, lung MPO and serum IL-6 levels. (D) Serum ALT and AST levels, (E) serum urea and creatinine and urea levels, and (F) serum FFA levels. In another experiment, the effect of an ATGL inhibitor, atglistatin, and a pancreatic lipase inhibitor, orlistat, in the OA-AP mice was tested. (G) Representative images of pancreatic histopathology (magnification ×200) and histopathological scores. (H) Serum amylase and lipase activity, (I) serum IL-6 levels, (J) serum ALT and AST levels, (K) serum urea and creatinine levels, and (L) serum FFA levels. In all experiments, mice were sacrificed at 12 hours after the first injection of EtOH and were assessed for disease severity and/or lipolytic parameters. *P<0.05, **P<0.01, ***P<0.001. ALT, alanine aminotransferase; AP, acute pancreatitis; AST; aspartate aminotransferase; ATGL, adipose triglyceride lipase; Ctrl, control; EtOH, ethanol; FFA, free fatty acid; HFD, high-fat diet; IL, interleukin; MPO, myeloperoxidase; ns, not significant; OA-AP, obese alcoholic acute pancreatitis.
In summary, our study reports that obesity and alcohol act synergistically in the pathogenesis of onset and development of MOI in OA-AP through induction of ATGL-mediated VAT lipolysis. High amounts of ethanol alone may be insufficient to induce clinical AP and is not sufficient to induce murine experimental AP.7 A genetic predisposition or a susceptible precondition may be required,1 2 8 9 if not the presence of a cofactor, as in murine fatty acid ethyl ester AP induced by ethanol with palmitoleic or palmitic acid.7 10 Obesity is an alternative, which our model suggests may be targeted by ATGL inhibition.
Footnotes
Contributors: XY and LY are joint first authors. QX and WHu are joint senior authors. WHu, QX and XY designed the study. QX and WHu obtained funding and supervised students. XY and WHu drafted the manuscript. XY, LY, MY, LD and TL planned and conducted the experiments and analysed the data. WHe, XF and RS had important intellectual input. JX and RS critically revised the manuscript. All authors read and approved the final version of the article.
Funding: National Nature Science Foundation of China (number 81973632, to WHu; number 81860122, to WHe; number 81774120, to QX; number 81800575 to TL; numbers 92157205 and 81 970561 to XF); Programme of Science and Technology Department of Sichuan Province (number 22ZDYF1206, to XY); UK NIHR Senior Investigator Award (to RS).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Wang Y-C, Mao X-T, Yu D, et al. Alcohol amplifies the association between common variants at PRSS1-PRSS2 locus and chronic pancreatitis in a dose-dependent manner. Gut 2022;71:2369–71. 10.1136/gutjnl-2021-326670 [DOI] [PubMed] [Google Scholar]
- 2. Hegyi E, Tóth AZ, Vincze Áron, et al. Alcohol-dependent effect of PRSS1-PRSS2 haplotype in chronic pancreatitis. Gut 2020;69:1713–5. 10.1136/gutjnl-2019-319729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai S-W, Muo C-H, Liao K-F, et al. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol 2011;106:1697–704. 10.1038/ajg.2011.155 [DOI] [PubMed] [Google Scholar]
- 4. Kaur S, Auger C, Barayan D, et al. Adipose-specific ATGL ablation reduces burn injury-induced metabolic derangements in mice. Clin Transl Med 2021;11:e417. 10.1002/ctm2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Oliveira C, Khatua B, Noel P, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest 2020;130:1931–47. 10.1172/JCI132767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hegyi P, Szakács Z, Sahin-Tóth M. Lipotoxicity and cytokine storm in severe acute pancreatitis and COVID-19. Gastroenterology 2020;159:824–7. 10.1053/j.gastro.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang W, Booth DM, Cane MC, et al. Fatty acid ethyl ester synthase inhibition ameliorates ethanol-induced Ca2+-dependent mitochondrial dysfunction and acute pancreatitis. Gut 2014;63:1313–24. 10.1136/gutjnl-2012-304058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–54. 10.1038/ng.2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farooq A, Richman CM, Swain SM, et al. The role of phosphate in alcohol-induced experimental pancreatitis. Gastroenterology 2021;161:982–95. 10.1053/j.gastro.2021.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maléth J, Balázs A, Pallagi P, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology 2015;148:427–39. 10.1053/j.gastro.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2022-326958supp001.pdf (83.5KB, pdf)