Abstract
Introduction
The effect of disease-modifying therapies (DMT) on vaccine responses is largely unknown. Understanding the development of protective immunity is of paramount importance to fight the COVID-19 pandemic.
Objective
To characterise humoral immunity after mRNA-COVID-19 vaccination of people with multiple sclerosis (pwMS).
Methods
All pwMS in Norway fully vaccinated against SARS-CoV-2 were invited to a national screening study. Humoral immunity was assessed by measuring anti-SARS-CoV-2 SPIKE RBD IgG response 3–12 weeks after full vaccination, and compared with healthy subjects.
Results
528 pwMS and 627 healthy subjects were included. Reduced humoral immunity (anti-SARS-CoV-2 IgG <70 arbitrary units) was present in 82% and 80% of all pwMS treated with fingolimod and rituximab, respectively, while patients treated with other DMT showed similar rates as healthy subjects and untreated pwMS. We found a significant correlation between time since the last rituximab dose and the development of humoral immunity. Revaccination in two seronegative patients induced a weak antibody response.
Conclusions
Patients treated with fingolimod or rituximab should be informed about the risk of reduced humoral immunity and vaccinations should be timed carefully in rituximab patients. Our results identify the need for studies regarding the durability of vaccine responses, the role of cellular immunity and revaccinations.
Keywords: multiple sclerosis, immunology, COVID-19
Introduction
While people with multiple sclerosis (pwMS) do not have an increased risk of SARS-CoV-2 infection or severe COVID-19 disease per se, the risk is elevated in the presence of comorbidities, higher age, greater MS-associated disability, progressive disease course and ongoing treatment with certain disease-modifying therapies (DMT).1–6 Early initiation of treatment with high-efficacy DMT seems to be the single most important factor in reducing long-term disability in pwMS.7 8 Specific DMT are, however, associated with an increased risk of infections.9 Expert organisations worldwide recommend that all pwMS should be vaccinated against COVID-19.10 There is some evidence of reduced humoral immunity after mRNA-COVID-19 vaccines among patients treated with fingolimod and rituximab11 12 and there is a need for better understanding of vaccine responses among patients treated with DMT. The aim of this article is to report the first results of a nationwide study designed to assess the development of immunity after COVID-19 vaccination in pwMS. We also report on two incidents of revaccination in pwMS treated with fingolimod and rituximab who showed no antibody response after the first two doses of mRNA vaccine.
Methods
Study population
All pwMS in Norway were invited to participate in this study via the National MS Registry and Biobank, social media and web page advertising. Invitation letters were disseminated digitally containing an electronic link/QR-code leading to a digital consent form, a questionnaire and a blood test form. Inclusion criteria were MS diagnosis, signed consent and completed COVID-19 vaccination (ie, either two vaccine doses or past COVID-19 and one vaccine dose). Healthy subjects were recruited among fully vaccinated health workers and blood donors. We report on all patients who donated a blood sample by 30 June 2021.
Antibody measurement
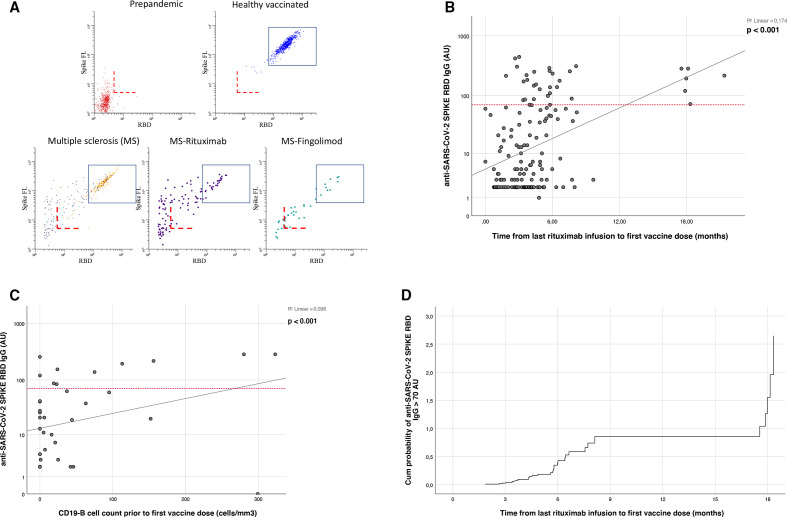
Antibodies to full length Spike (HexaPro) from SARS-CoV-2 and the receptor-binding domain (RBD) were measured using a bead-based flow cytometric assay13 in all included patients 3–12 weeks after full vaccination. Post-immunisation IgG titres were used as a correlate of protection,14 and reduced immunity was assumed in cases of IgG <70 arbitrary units (AU) corresponding to a level which was lower than found in 99% of all healthy vaccinated subjects. IgG levels <5 AU were defined as no antibody response, while IgG levels between 5 and 70 AU were defined as weak antibody response (figure 1). Calibration to the WHO international standard showed that 70 AU corresponds to approximately 40 binding antibody units per millilitre (BAU/mL).
Figure 1.
(A) COVID-19 vaccine response in healthy individuals and people with MS. The dot plots show levels of antibodies to SARS-CoV-2 Spike Protein (y-axis) and the receptor-binding domain (RBD) for indicated cohort. The dashed red lines correspond to assay cut-off determined for sero-prevalence screening (5 anti-SARS-CoV-2 SPIKE RBD IgG arbitrary units), while the rectangle shows the range of signals measured for more than >99% of vaccinated healthy individuals (<70 anti-SARS-CoV-2 SPIKE RBD IgG arbitrary units). The lower end corresponds to approximately 40 WHO binding antibody units (BAUs). (B) Scatter plot showing the correlation between time since last rituximab infusion and anti-SARS-CoV-2 SPIKE RBD IgG levels (AU). The red reference line on the Y-axis is positioned at 70 AU. (C) Scatter plot showing the correlation between CD19+ B-lymphocyte count (cells/mm3) prior to first vaccine dose and anti-SARS-CoV-2 SPIKE RBD IgG levels (AU, n=47, median of 2 months between CD19+ B-lymphocyte count and first vaccine dose). The red reference line on the Y axis is positioned at 70 AU. (D) Cox proportional-hazard model showing the cumulative probability of mounting a normal immune response (anti-SARS-CoV-2 SPIKE RBD IgG >70 AU) in relation to time since last rituximab infusion.
Data collection
Demographic, disease-specific and treatment-specific variables were acquired through a digital questionnaire and from the Norwegian MS registry and Biobank if needed. Information regarding COVID-19 vaccines was extracted from the Norwegian Immunization Registry, while relevant information regarding COVID-19 disease was extracted from the Norwegian Surveillance System for Communicable Diseases.
Statistics
Continuous and categorical variables were compared using Mann-Whitney and Fisher exact tests, respectively. A p value less than 0.05 was considered statistically significant. Correlations were assessed by Spearman p. Hazard ratios were assessed using Cox proportional-hazard models. Statistical analysis was conducted using SPSS V.26.
Results
Serum from 627 healthy subjects and 528 pwMS were available for analyses by 30 June 2021. Clinical and demographic variables are presented in table 1.
Table 1.
Clinical and demographic variables of patients with multiple sclerosis and healthy subjects that received COVID-19 vaccination
| Study population | Patients with MS, n=528 | Healthy subjects, n=627 | ||||||
| Cladribine, n=75 | Alemtuzumab, n=34 | Natalizumab, n=37 | S1P-R mod., n=61 | Anti-CD20, n=183 | Other DMT, n=95 | Untreated, n=55 | ||
| Follow-up after second vaccine, days | ||||||||
| Median | 28 | 24 | 33 | 28 | 32 | 36 | 38 | 18 |
| 25–75 IQR | 19 | 28 | 20 | 20 | 21 | 18 | 23 | 14 |
| Gender, n (%) | ||||||||
| Females | 67 (89) | 27 (79) | 31 (84) | 44 (72) | 144 (79) | 72 (76) | 38 (69) | 435 (69) |
| Males | 8 (11) | 7 (21) | 6 (16) | 17 (28) | 39 (21) | 23 (24) | 17 (31) | 192 (31) |
| Age, years | ||||||||
| Median | 46 | 40 | 46 | 50 | 48 | 56 | 58 | 50 |
| 25–75 IQR | 15 | 15 | 18 | 14 | 15 | 15 | 18 | 21 |
| Disease duration, years | ||||||||
| Median | 4 | 11 | 9 | 12 | 6 | 14 | 16 | |
| 25–75 IQR | 10 | 8 | 12 | 7 | 11 | 13 | 17 | |
| Time from last Tx dose to vaccination, months | ||||||||
| Median | 8 | 51 | 2 | 4 | ||||
| 25–75 IQR | 9 | 30 | 3 | 3 | ||||
| Patients with SARS-CoV-2 SPIKE RBD IgG >70 AU | ||||||||
| n, (%) | 69 (92) | 33 (97) | 33 (97) | 11 (18) | 37 (20) | 91 (96) | 53 (96) | 618 (99) |
| SARS-CoV-2 RBD IgG titre | ||||||||
| Median | 217 | 261 | 183 | 5 | 6 | 209 | 187 | 283 |
| 25–75 IQR | 156 | 95 | 119 | 17 | 55 | 102 | 133 | 145 |
DMT, disease-modifying therapies; S1P-R mod., sphingosine-1-phosphate receptor modulator.
The majority of all patients received BNT162b2 (81% as the first, and 86% as the second dose), followed by mRNA-1273 (14% and 14%) and ChAdOx1-S (5% and 0%) of all cases. In the 10 (2%) post-COVID-19 disease patients only one dose was given. The mean time between two inoculations was 36 days (95% CI 35 to 38 days) and did not differ between the different DMT. The most frequent DMT was rituximab (38%) followed by cladribine (16%), fingolimod (13%), natalizumab (8%) and alemtuzumab (7%). Other DMT included dimethyl fumarate (6%), teriflunomide (5%), interferons (3%), glatiramer acetate (3%) and ocrelizumab (1%).
Reduced humoral immunity was present in pwMS treated with fingolimod (82% of all 61 patients, 54% without antibody response) and rituximab (80% of all 183 patients, 48% without antibody response), while patients treated with other DMT showed similar rates as healthy subjects and untreated pwMS (figure 1A). Longer time since last rituximab infusion and higher CD19-B cell counts were associated with higher levels of protective antibodies (r2=0.174, p<0.001 and r2=0.098, p<0.001) (figure 1B, C). The cumulative probability of mounting a normal immune response in relation to time since last rituximab infusion is illustrated in figure 1D.
Two patients treated with fingolimod and rituximab were identified in our cohort without antibody response (despite completed vaccination) who underwent additional immunisations (1 and 3 months after full vaccination, respectively). Increasing antibody levels were observed in both cases after additional vaccine doses (from <5 AU to 19 and 21 AU, 14 days after 1 and 2 extra doses, respectively).
Additionally, we identified three patients (two on rituximab, one on fingolimod) with no antibody response post-COVID-19. Antibody levels >70 AUs were observed in these three patients 4, 5 and 6 weeks after a single vaccine dose, respectively.
Discussion
We present the first results of a nationwide study of COVID-19 vaccine response in pwMS. Our results demonstrate a normal humoral immune response in most patients, including those receiving cladribine, alemtuzumab and natalizumab, as well as untreated patients with MS. Treatment with anti-CD20 monoclonal antibodies (rituximab and ocrelizumab) and sphingosine-1-phosphate receptor (S1PR) modulators (fingolimod) are associated with attenuated humoral responses.
Our results are in line with previous reports of a decreased humoral immune response in pwMS treated with S1PR modulators and anti-CD20 therapies.12 15 However, a larger proportion of patients on fingolimod in our study showed a normal antibody response despite similar absolute lymphocyte count.12 15 Importantly, we demonstrated that almost one-third of patients in these treatment groups produced an attenuated, but present antibody response. We also found that three patients with no antibody response post-COVID-19 disease developed protective antibody levels after one dose of vaccination, and that two patients with no antibody response despite two immunisations acquired a weak antibody response after further vaccinations, suggesting that patients who fail to respond to initial immunisation may have a potential to respond to further vaccination. We found a positive association between time since last rituximab infusion and antibody response, as has been suggested, but not shown previously.12
The main strength of this study is the national cohort design. Although we report on the largest number of patients using high-efficacy treatments to date, our results are based on observational data with limited follow-up and the number of serological samples is not yet sufficient to give a full description of vaccine responses in the entire MS population. Selection bias might be present among early repliers. Another weakness of this study is the lack of clinical details (eg, disease courses, the grade of disability), and data regarding patients recently treated with alemtuzumab, while the number of patients in some treatment groups are low. Furthermore, we only report data regarding IgG responses as a correlate of humoral immunity while the adaptive immune response to SARS-CoV-2 seems to depend not only on virus-specific antibodies but also on cellular responses.16
Although absent humoral immunity after full vaccination is frequent in pwMS treated with rituximab and fingolimod, many also have normal or low antibody responses. Our data indicate that all pwMS should be encouraged to follow immunisation programmes. Vaccinations should preferably be given outside the time interval of one to 1–4 months past rituximab treatment, as the chance of robust IgG response is small until around 5 months after treatment (and then increases markedly), but we underline that vaccination in this time window may induce some humoral response and should be considered individually. Patients treated with S1P-modulators and anti-CD20 therapies should be informed about the risk of attenuated vaccine responses and tested for antibody responses after completed vaccination.
A study of the effect of revaccination in patients with low or no antibody response after two immunisations is initiated following these results.
Acknowledgments
We express our gratitude to Haakon Haugaa and Andreas Barratt-Due for their help regarding the recruitment of healthy subjects.
Footnotes
Correction notice: This article has been corrected since it first published. Author name Kjell-Morten Myhr has been corrected.
Contributors: MK is the submitting and corresponding author. He contributed to the conception and design of the work, collected data, provided and cared for study patients, processed statistical analyses and composed the manuscript. ARL contributed to the conception and design of the work, and revised the work critically for important intellectual content. HMT contributed to the conception and design of the work, and revised the work critically for important intellectual content. TTT contributed to the conception and design of the work, conducted immunological analyses and revised the work critically for important intellectual content. SS-R contributed to the conception and design of the work, and revised the work critically for important intellectual content. EBV contributed to the conception and design of the work, conducted immunological analyses, and revised the work critically for important intellectual content. AM contributed to the conception and design of the work, and revised the work critically for important intellectual content. SW contributed to the conception and design of the work, supervised invitation of all participants and revised the work critically for important intellectual content. JA contributed to the conception and design of the work, supervised invitation of all participants and revised the work critically for important intellectual content. IASA contributed to the conception and design of the work, and revised the work critically for important intellectual content. ØT contributed to the conception and design of the work, and revised the work critically for important intellectual content. TH contributed to the conception and design of the work, and revised the work critically for important intellectual content. TB contributed to the conception and design of the work, and revised the work critically for important intellectual content. MK-M contributed to the conception and design of the work, and revised the work critically for important intellectual content. HFH contributed to the conception and design of the work, and revised the work critically for important intellectual content. JTA served as scientific advisor, processed pharmacological analyses and revised the work critically for important intellectual content. LAM contributed to the conception and design of the work, processed immunological analyses and revised the work critically for important intellectual content. AS contributed to the conception and design of the work, supervised analyses regarding healthy subjects and revised the work critically for important intellectual content. EGC contributed to the conception and design of the work, supervised the study and revised the work critically for important intellectual content. JTV contributed to the conception and design of the work, processed immunological analyses, supervised the study and revised the work critically for important intellectual content. FL-J contributed to the conception and design of the work, processed immunological analyses, supervised the study and revised the work critically for important intellectual content. GON contributed to the conception and design of the work, supervised the study and revised the work critically for important intellectual content. FL-J and GON are shared last authors.
Funding: This study has received funding from the South-Eastern Health authority of Norway (grant number is not applicable) and from the Coalition for Epidemic Preparedness Innovations (grant number is not applicable). This study has received research grant (thru ARL) from Sanofi (no grant number is available).
Competing interests: ARL research fundings from Sanofi. SW has received speaker honoraria from and served on scientific advisory boards for Biogen, Janssen-Cilag, Sanofi and Novartis. ØT has received speaker honoraria from and served on scientific advisory boards for Biogen, BMS, Jansen, Sanofi, Merck and Novartis. TH has received speaker honoraria and/or unrestricted research grants from Biogen, Merck, Roche, Novartis, Sanofi and Bristol-Myers Squibb, and participated in clinical trials organised by Merck, Sanofi and Roche. TB has received unrestricted research grants from Biogen Idec and Sanofi Genzyme. MK-M has received unrestricted research grants to his institution; scientific advisory board and speaker honoraria from Biogen, Merck, Novartis, Roche and Sanofi, and has participated in clinical trials organised by Biogen, Merck, Novartis, Roche and Sanofi. HFH has received honoraria for lecturing or advice from Biogen, Merck, Roche, Novartis and Sanofi. AS is shareholder of Age Labs, a molecular diagnostics company that discovers, develops and commercialises diagnostic tests for the early detection of age-related diseases. EGC has received honoraria for lecturing and advice from Biogen, BMS, Janssen, Merck, Novartis, Roche and Sanofi.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Norwegian Regional Ethical Committee (200 631 and 2021/8504). All participants gave informed consent before taking part in the study. This study and all authors followed the World Medical Association’s Declaration of Helsinki.
References
- 1. Reder AT, Centonze D, Naylor ML, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs 2021;35:317–30. 10.1007/s40263-021-00804-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedli C, Diem L, Hammer H, et al. Negative SARS-CoV2-antibodies after positive COVID-19-PCR nasopharyngeal swab in patients treated with anti-CD20 therapies. Ther Adv Neurol Disord 2021;14:17562864211016641. 10.1177/17562864211016641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol 2021;78:699–708. 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-Modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021;89:780–9. 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020;77:1079–88. 10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spelman T, Forsberg L, McKay K, et al. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler 2021;13524585211026272:13524585211026272. 10.1177/13524585211026272 [DOI] [PubMed] [Google Scholar]
- 7. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019;321:175–87. 10.1001/jama.2018.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med 2020;133:1380–90. 10.1016/j.amjmed.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020;77:184. 10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reyes S, Cunningham AL, Kalincik T, et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J Neuroimmunol 2021;357:577627. 10.1016/j.jneuroim.2021.577627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021;80:1312–6. 10.1136/annrheumdis-2021-220461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021;14:17562864211012835. 10.1177/17562864211012835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holter JC, Pischke SE, de Boer E, et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A 2020;117:25018–25. 10.1073/pnas.2010540117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021;39:4423–8. 10.1016/j.vaccine.2021.05.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerrieri S, Lazzarin S, Zanetta C. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: an initial real-life experience. J Neurol 2021:1–5. 10.1007/s00415-021-10663-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni L, Ye F, Cheng M-L, et al. Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 2020;52:971–7. 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]