Abstract
Background
Many patients in sub-Saharan Africa whom a diagnosis of tuberculosis is considered are subsequently not diagnosed with tuberculosis. The proportion of patients this represents, and their alternative diagnoses, have not previously been systematically reviewed.
Methods
We searched four databases from inception to 27 April 2020, without language restrictions. We included all adult pulmonary tuberculosis diagnostic studies from sub-Saharan Africa, excluding case series and inpatient studies. We extracted the proportion of patients with presumed tuberculosis subsequently not diagnosed with tuberculosis and any alternative diagnoses received. We conducted a random effects meta-analysis to obtain pooled estimates stratified by passive and active case finding.
Results
Our search identified 1799 studies, of which 18 studies (2002–2019) with 14 527 participants from 10 African countries were included. The proportion of patients with presumed tuberculosis subsequently not diagnosed with tuberculosis was 48.5% (95% CI 39.0 to 58.0) in passive and 92.8% (95% CI 85.0 to 96.7) in active case-finding studies. This proportion increased with declining numbers of clinically diagnosed tuberculosis cases. A history of tuberculosis was documented in 55% of studies, with just five out of 18 reporting any alternative diagnoses.
Discussion
Nearly half of all patients with presumed tuberculosis in sub-Saharan Africa do not have a final diagnosis of active tuberculosis. This proportion may be higher when active case-finding strategies are used. Little is known about the healthcare needs of these patients. Research is required to better characterise these patient populations and plan health system solutions that meet their needs.
PROSPERO registration number
CRD42018100004.
Keywords: tuberculosis, respiratory infection, clinical epidemiology
Key messages.
What is the key question?
What are the numbers and nature of alternative final diagnoses among patients with presumed tuberculosis in sub-Saharan Africa?
What is the bottom line?
Nearly half of all patients with presumed tuberculosis in sub-Saharan Africa are subsequently found not to have tuberculosis, with few receiving any alternative diagnoses.
Why read on?
Patients with symptoms suggestive of tuberculosis who may eventually receive an alternative diagnosis represent a major unmet need in sub-Saharan Africa; requiring better characterisation through research to develop health system solutions to meet their needs.
Introduction
The differential access to high-quality diagnostics experienced in most low-income middle-income countries (LMICs) illustrate important and growing global health disparities.1 Diagnostic tests are often not affordable or designed for application in LMICs and can, therefore, represent a barrier to high-quality healthcare access.1 Access to accurate diagnostics for a range of diseases is a cornerstone of high-quality patient care, enabling appropriate timely management, inclusive of transmission control in the case of communicable disease. Pulmonary tuberculosis (TB) is a highly prevalent poverty-related communicable disease that lays bare many of the diagnostic challenges faced in LMICs, not least because of non-specific symptoms at presentation.2
Patients with presumed TB are adults or children evaluated for active TB with suggestive signs and symptoms, such as cough, fever, night sweats, weight loss, haemoptysis and fatigue. While sputum culture remains the bacteriological reference standard for TB diagnostics, it is a costly, lengthy process and in LMICs is usually only available in central reference laboratories. At local clinics, a reliance on smear microscopy is being replaced by molecular diagnostics such as Xpert MTB/RIF and Xpert MTB/RIF Ultra nucleic acid amplification tests.3 Despite these advances, only 57% of global TB cases are bacteriologically confirmed, the rest are clinically diagnosed with negative or no bacteriological testing and notified to WHO as such. Whereas in high-income settings, 80% of TB cases are confirmed bacteriologically.4 The WHO describes the use of both passive and active case-finding strategies to detect TB cases.2 Passive case finding relies on symptomatic patients seeking medical care by presenting to health services, whereas active case finding involves community-based screening of patients who would not otherwise seek healthcare.
A proportion of patients with presumed TB are found not to have tuberculosis, following both bacteriological and clinical investigation. This proportion is likely to depend on tuberculosis prevalence, case-finding strategies (passive or active) and other context-specific factors such as access to alternative diagnostics. A community study in Malawi demonstrated that only 10%–20% of patients presenting to primary care with a persistent cough had TB.5 More recent observational data from The Gambia6 showed that nearly half of all patients with presumed TB did receive a final diagnosis of TB. A range of alternative diagnoses—predominantly respiratory—were described, but importantly, non-respiratory diagnoses such as heart failure, malignancy and renal failure were also noted. Furthermore, in 36% of patients not diagnosed with TB, no alternative diagnosis was made. Minimal healthcare was afforded to these patients beyond screening for TB and HIV.
The burden of ill health in patients with presumed TB subsequently found not to have TB and their ongoing engagement with health systems has been largely overlooked. While national guidelines exist for patients that receive a negative sputum smear microscopy result, these focus on further elucidating active TB cases rather than exploring alternative diagnoses.7 8 The rapid rise of non-communicable disease—including chronic respiratory diseases1—in TB endemic areas, means patients presenting with presumed TB may increasingly have alternative health issues that require investigation and management, once TB is ruled out.
The aim of this study was to undertake a systematic review and meta-analysis of the evidence describing the number and nature of alternative final diagnoses among patients with presumed TB in sub-Saharan Africa (sSA).
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis of the evidence in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidance.9 We searched Ovid Medline, Embase, Cumulative Index of Nursing and Allied Health Literature (CINAHL) and the Cochrane library. The search strategy involved Medical Subject Heading and free text terms relating to the concepts of WHO tuberculosis symptoms (such as “chronic cough”, “fever” and “weight loss”), diagnostics (such as “diagnos*”, “sensitivity” and “specificity”), TB and used filters for North,10 East,11 South,12 West13 and Central Africa.14 The full Medline search strategy is provided in online supplemental data 1 search strategy and was modified for other databases. Human studies that met the inclusion criteria from inception to 27 April 2020 were included. No language restrictions were applied.
thoraxjnl-2021-217663supp001.pdf (59.8KB, pdf)
We included all studies (Diagnostic, Cohort and Observational) conducted in sSA enrolling adult ( 15 years old) patients with presumed TB presenting with symptoms (cough>2 weeks or any one of cough, fever, weight loss, night sweats or haemoptysis). Duplicate articles, research on non-human subjects, in-patient settings, articles reporting exclusively paediatric, extrapulmonary, pregnant, prison or diabetic populations, and any studies irrelevant to TB and diagnostics not set in sSA were excluded. Narrative reviews, case reports, case series and studies reporting only smear microscopy diagnostics or screening with chest radiographs as opposed to symptoms were also excluded.
We screened citations of relevant articles and systematic reviews to identify additional studies. All articles identified by the initial search underwent title and abstract screening. Full-text review of potentially relevant articles was conducted. This was performed by two independent reviewers (SJ, FD-D), where a third reviewer (CM) was called on if a consensus could not be reached. If multiple studies used the same dataset or populations, we included the most comprehensive study with the largest number of participants and excluded the others. Multi-site studies were included where data from sSA sites were individually extractable from the total number of participants.
Data analysis
Data extraction was performed by two independent reviewers (SJ and FD-D) and compared, disagreements were resolved in the first instance by discussion and a third reviewer (CM) called on if consensus could not be reached. A piloted standardised data extraction form was used to collect information from all eligible studies. All non-English language studies were translated using an online document translator.15
For each eligible study, we extracted the year of publication, first authors name, mean or median age, proportion of male participants, study country, study setting (general or district hospital, local health centre or community), total number of participants eligible and included, diagnostic test used (culture or GeneXpert), number of patients with and without a diagnosis of TB disease (Bacteriologically confirmed or clinical) and their HIV rates, where available. Specific details of alternative diagnoses made, and their management were extracted. WHO Global Health Observatory data provided TB and HIV incidence estimates in-country during the years studies were undertaken and if they spanned more than a year the higher annual value used.
Included studies risk of bias was evaluated using a tool specifically for prevalence studies developed by the Joanna Briggs Institute.16 Each study was independently assessed according to ten items of methodological quality (online supplemental data 2 JBI Risk of Bias Table).
thoraxjnl-2021-217663supp002.pdf (65.1KB, pdf)
We used WHO case definitions for TB case reporting. These are bacteriologically confirmed TB cases and clinically diagnosed TB cases. All study participants included were tested for tuberculosis therefore clinically diagnosed tuberculosis cases in this review include patients with negative bacteriological results only and not patients that have not undergone testing. Bacteriologically confirmed TB refers to sputum culture positivity in all but one study6 that used Xpert MTB/RIF.
All data analyses were done using R (V.4.0.2) and the metafor package V.2.4–0 (online supplemental data 3 Statistical Analysis). We stratified random effects meta-analyses of the proportion of patients with presumed TB found not to have TB by passive or active case finding, and whether cases found passively included clinically diagnosed cases. Meta-regression was used to assess the association between the proportion of patients with presumed TB subsequently found not to have TB and the proportion of clinically diagnosed TB cases, as well as with matched country-year estimates of per capita TB incidence and HIV prevalence.
thoraxjnl-2021-217663supp003.pdf (1.7MB, pdf)
Results
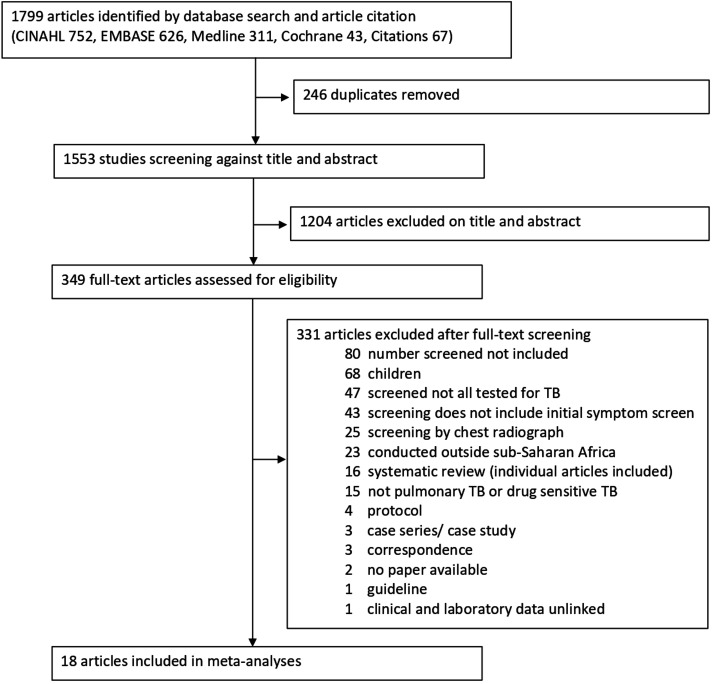
Our search yielded 1799 articles (64 identified from systematic review references and three through citation). A total of 246 duplicate articles were removed (figure 1). After screening abstracts and titles, we excluded 1204 articles that were not relevant. After screening full texts, we excluded an additional 331 articles that did not meet the eligibility criteria. Therefore, 18 articles with 14 527 participants from 10 African countries were included in this systematic review and meta-analysis.
Figure 1.
Study selection. TB, tuberculosis.
No studies were excluded following a risk of bias assessment (online supplemental data). All studies included reported 70% minimum study population coverage for TB diagnostic testing. Theron et al 17 and Ling et al 18 reported consecutive presumptive TB patient recruitment of 480 over 4 years and 398 over 5 years, respectively. It was unclear how sampling was performed (breaks during sampling or sampled on certain days) and clinic sizes were not stated that could account for the long study periods with relatively low recruitment numbers.
Passive case-finding studies
There were seven studies including (table 1)6 17 19–23 and five studies not including (table 2)18 24–27 clinically diagnosed TB cases that used passive case-finding strategies. Of the five studies (table 2) not including clinically diagnosed TB cases, only Dorman et al 25 did not document whether a clinical assessment was performed. Ling et al,18 Lawson et al,27 Hanrahan et al 26 and Cuevas et al 24 did perform a clinical assessment but reported no cases of clinically diagnosed TB. The proportion of patients with presumed TB subsequently found not to have TB increased with declining numbers of clinically diagnosed TB cases (p<0.0001).
Table 1.
Tuberculosis studies meeting inclusion criteria using passive case finding including clinically diagnosed tuberculosis cases
| Study title | Study type | Country | Age (median, IQR) |
Male (%) | Setting | Presumptive TB (included/ eligible) | Diagnosed with tuberculosis | Not tuberculosis | |||||
| Laboratory (bacteriological) |
Clinical | Total (%) |
HIV | Total (%) |
HIV | ||||||||
| Boehme et al (2011)19 | Feasibility, diagnostic accuracy and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study | Cross-sectional Study | South Africa | 36, 29–46 |
51 | Health centre | 1968/1968 | 473 | 824 | 1297 (66) |
NR | 671 (34) |
NR |
| Uganda | 32 26–38 |
54 | General hospital | 307/307 | 146 | 17 | 163 (53) |
NR | 144 (47) |
NR | |||
| Bruchfeld et al (2002)20 | Evaluation of outpatients with suspected pulmonary tuberculosis in a high HIV prevalence setting in Ethiopia: Clinical, Diagnostic and epidemiological characteristics | Cross-sectional study | Ethiopia | 33† | 56.3† | General hospital | 493/509 | 168 | 113 | 281 (57) |
148/281 | 212 (43) |
73 /212 |
| Jayasooriya et al (2019)6 | The burden of non-TB lung disease presenting to TB clinics in the Gambia: Preliminary data in the Xpert MTB/rif era | Cross-sectional Study | The Gambia | 40†
28–47 |
50† | Research clinic | 233/239 | 114 | 17 | 131 (56) |
17/131 | 102 (44) |
12 /102 |
| Munyati et al (2005)21 | Chronic cough in primary healthcare attendees, Hasrare, Zimbabwe: diagnosis and impact of HIV infection | Cross-sectional Study | Zimbabwe | 33 | 48 | Health centre | 544/550 | 184 | 50 | 234 (43) |
207/234 | 310 (57) |
247 /310 |
| Nliwasa et al (2016)22 | The Sensitivity and Specificity of Loop-Mediated Isothermal Amplification (LAMP) Assay for Tuberculosis Diagnosis in Adults with Chronic Cough in Malawi | Cross-sectional Study | Malawi | 32 25–41 |
48 | Health centre | 233/273 | 53 | 3 | 56 (24) |
24/56 | 177 (76) |
97 /177 |
| Reither et al (2010)23 | Evaluation of diagnos TB AG, a flow-through immunoassay for rapid detection of pulmonary tuberculosis | Cross-sectional Study | Tanzania | 36 | 47.4 | Research clinic | 171/202 | 45 | 33 | 78 (46) |
51/78 | 93 (54) |
50 /93 |
| Theron et al (2011)17 | Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high prevalence setting | Cross-sectional Study | South Africa | 36 18–83* |
68 | Health centre | 480/496 | 141 | 182 | 323 (67) |
46/323 | 157 (33) |
84 /157 |
*(Range).
†Not TB patients.
NR, not recorded; TB, tuberculosis.
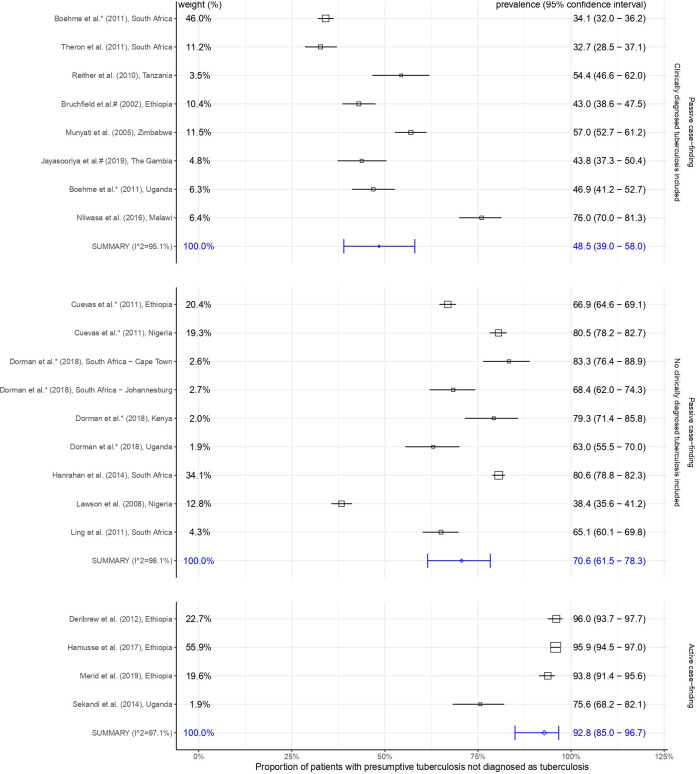
Figure 2 shows included studies and summary estimates grouped by passive and active case finding. Passive case-finding studies including clinically diagnosed TB cases (table 1) are shown in the top section of figure 2 with estimates ordered by this proportion. The summary proportion of patients with presumed TB subsequently found not to have TB was lower in passive case-finding studies that included clinically diagnosed TB cases (table 1) compared with those that did not (table 2), 48.5% (95% CI 39.0% to 58.0%) vs 70.6% (95% CI 61.5% to 78.3%) (figure 2). Heterogeneity was high (Iˆ2>95% for all estimates). Meta-regressions with HIV prevalence, TB incidence, calendar year and country group did not find significant associations with our outcome (see statistical analyses online supplemental data 3).
Figure 2.
Random effects meta-analyses of the proportion of patients with presumptive tuberculosis not diagnosed as tuberculosis. The weight, listed on the left-hand side is the percentage of the total inverse variance associated with a study in each analysis. Prevalence (95% CI) of patients not diagnosed as tuberculosis is listed on the right-hand side. Studies are stratified by passive or active case finding. Passive case-finding studies including clinically diagnosed tuberculosis are shown with estimates ordered by this proportion.
Table 2.
Tuberculosis (TB) studies meeting inclusion criteria using passive case finding not including clinically diagnosed TB cases
| Study title | Study type | Country | Age (median, IQR) |
Male (%) | Setting | Presumptive TB (included/ eligible) | Diagnosed with TB | Not TB | |||||
| Laboratory (bacteriological) |
Clinical | Total (%) |
HIV | Total (%) |
HIV | ||||||||
| Cuevas et al (2011)24 | A multicountry non-inferiority cluster randomised trials of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis | Cluster randomised trial | Ethiopia | 33.7* (±14.1) |
52.8 | Health centre | 1770/1909 | 586 | 0 | 586 (33) |
0/586 | 1184 (67) |
NR |
| Nigeria | 34.4* (±10.7) |
51.9 | Health centre | 1196/1238 | 233 | 0 | 233 (19) |
0/233 | 963 (81) |
NR | |||
| Dorman et al (2018)25 | Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study | Cross-sectional study | South Africa (Cape Town) | 41, 34–49 |
41 | District hospital | 152/152 | 27 | NR | 27 (18) |
NR | 125 (82) |
NR |
| South Africa (Johannesburg) | 34, 30–43 |
63 | District hospital | 234/234 | 74 | NR | 74 (32) |
NR | 160 (68) |
NR | |||
| Kenya | 33, 26–44 |
51 | District hospital | 135/135 | 28 | NR | 28 (21) |
NR | 107 (79) |
NR | |||
| Uganda | 30, 26–39 |
64 | District hospital | 181/181 | 67 | NR | 67 (37) |
NR | 114 (63) |
NR | |||
| Hanrahan et al (2014)26 | Xpert MTB/RIF as a measure of sputum bacillary burden. Variation by HIV status and immunosuppression | Cross-sectional study | South Africa | 37, 29–46 |
38 | Health centre | 2091/2406 | 406 | 0 | 406 (19) |
NR | 1685 (81) |
NR |
| Lawson et al (2008)27 | Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria | Cross-sectional study | Nigeria | 33* (±10) |
61 | District hospital | 1186/1321 | 731 | 0 | 731 (62) |
329† /625 |
455 (38) |
217† /377 |
| Ling et al (2011)18 | Are interferon-gamma release assays useful for diagnosing active tuberculosis in a high-burden setting? | Cross-sectional study | South Africa | 40* (±12) |
66 | Health centre | 395/500 | 138 | 0 | 138 (35) |
43 /138 |
257 (65) |
65 /257 |
*Age, mean (±SD).
†Not all tested, denominator.
NR, not recorded.
Active case-finding studies
There were four active case-finding studies without any clinically diagnosed TB cases (table 3). Three studies were conducted in Ethiopia reporting clinical assessments, but no clinically diagnosed TB cases found.28–31 No clinical assessments were reported by Sekandi et al in Uganda.31
Table 3.
Tuberculosis (TB) studies meeting inclusion criteria using active finding not including clinically diagnosed TB case
| Study title | Study type | Country | Age (median, IQR) |
Male (%) | Setting | Presumptive TB (included/ eligible) |
Diagnosed with TB | Not TB | |||||
| Laboratory (bacteriological) |
Clinical | Total (%) |
HIV | Total (%) |
HIV | ||||||||
| Deribew et al (2012)28 | Prevalence of pulmonary TB and spoligotype pattern of Mycobaterium tuberculosis among TB suspects in a rural community in Southwest Ethiopia | Cross-sectional study | Ethiopia | 41* (±16.2) |
39.3 | Community | 428/482 | 17 | 0 | 17 (4) |
NR | 411 (96) |
NR |
| Hamusse et al (2017)29 | Prevalence and Incidence of Smear-Positive Pulmonary Tuberculosis in the Hetosa District of Arsi Zone, Oromia Regional State of Central Ethiopia | Cross-sectional study | Ethiopia | 33.3* †
(±16) |
51† | Community | 1041/1041 | 43 | 0 | 43 (4) |
0/43 | 998 (96) |
NR |
| Merid et al (2019)30 | Population-based screening of pulmonary tuberculosis utilising community health workers in Ethiopia | Cross-sectional study | Ethiopia | 36 29–48 |
35 | Health Centre | 544/544 | 34 | 0 | 34 (6) |
0/31†‡ | 510 (94) |
NR |
| Sekandi et al (2014)31 | Yield of undetected tuberculosis and HIV coinfection from active case finding in urban Uganda | Cross-sectional study | Uganda | 24 20–30 |
37.2 | Community | 160/199 | 39 | NR | 39 (24) |
13/39 | 121 (76) |
32 |
*Age, mean (±SD).
†Age and Male (%) of community screened.
‡Not all tested.
NR, not reported.
Figure 2 illustrates that active case-finding studies had high proportions of patients with presumed TB subsequently found not to have TB, 92.8% (95% CI 85.0% to 96.7%) (table 3, figure 2).
Smear negative studies
A further two articles included patients with presumed TB that were already smear negative on microscopy (table 4). Affolabi et al 32 did not include and Huerga et al 33 included clinically diagnosed TB cases, with 89% and 61% of patients with presumed TB subsequently found not to have TB, respectively.
Table 4.
Tuberculosis (TB) studies of smear negative participants meeting inclusion criteria
| Study title | Study type | Country | Age (median, IQR) |
Male (%) | Setting | Presumptive TB (included/ eligible) |
Diagnosed with TB | Not TB | |||||
| Laboratory (bacteriological) |
Clinical | Total (%) |
HIV | Total (%) |
HIV | ||||||||
| Affolabi et al (2011)32 | Smear-negative, culture-positive pulmonary tuberculosis among patients with chronic cough in Cotonou, Benin | Cross-sectional Study | Benin | NR | NR | General Hospital | 207/251 | 22 | 0 | 22 (11) |
10/22 | 185 (89) |
81/185 |
| Huerga et al (2012)33 | Performance of the 2007 WHO Alorithm to Diagnose Smear-Negative Pulmonary Tuberculosis in a HIV Prevalent Setting | Cross-sectional Study | Kenya | 34 (26-48) |
37.1 | District Hospital | 380/380 | 61 | 89 | 150 (39) |
230 (61) |
||
NR, not reported.
Alternative diagnoses
Five studies reported diagnoses other than active TB (table 5).6 20 21 26 33 There were insufficient data available to analyse aetiology and prevalence as stated in the protocol. Two studies described non-TB mycobacteria and one Pneumocystis jirovecii pneumonia as the only alternative diagnoses.20 26 33 Jayasooriya et al 6 and Munyati et al 21 described a range of diagnoses which were predominantly respiratory, but importantly non-respiratory diagnoses such as heart failure, malignancy and renal failure were noted. Neither study performed spirometry. Four out of the five studies reported management of patients with presumed TB subsequently found not to have TB, two stating as clinically indicated. Notably, Affolabi et al 32 and Huerga et al 33 reported giving empirical antibiotics to all patients subsequently found not to have active TB amounting to mass administration of antibiotics to 207 and 380 patients respectively. Out of 18, 10 (55%) studies recorded historical TB episodes, and none recorded the number of times individuals had undergone previous TB testing.
Table 5.
Tuberculosis studies handling and reporting of patients with presumed tuberculosis found not to have tuberculosis
| Country | Diagnoses | Management | History of tuberculosis | Previous tuberculosis testing | WHO estimated incidence (year of study) |
||
| Tuberculosis (per 100 000) |
HIV (per 1000) |
||||||
| Affolabi et al (2011)32 | Benin | NR | 15 days erythromycin | NR | NR | 71 | 0.69 |
| Boehme et al (2011)19 | South Africa | NR | NR | NR | NR | 1260 | 10.29 |
| Uganda | NR | NR | NR | NR | 213 | 3.55 | |
| Bruchfeld et al (2002)20 | Ethiopia | 8 pneumocystis pneumonia | NR | 66* | NR | NR | 1.79 |
| Cuevas et al (2011)24 | Ethiopia | NR | NR | NR | NR | 296 | 0.44 |
| Nigeria | NR | NR | NR | NR | 219 | 0.79 | |
| Deribew et al (2012)28 | Ethiopia | NR | NR | NR | NR | 282 | 0.41 |
| Dorman et al (2018)25 | South Africa (Cape Town) | NR | NR | 59 | NR | 805 | 5.45 |
| South Africa (Johanesburg) | NR | NR | 55 | NR | 805 | 5.45 | |
| Kenya | NR | NR | 20 | NR | 348 | 1.17 | |
| Uganda | NR | NR | 15 | NR | 201 | 1.89 | |
| Hamusse et al (2017)29 | Ethiopia | NR | NR | NR | NR | 224 | 0.25 |
| Hanrahan et al (2014)26 | South Africa | 9 non-tuberculous mycobacteria | NR | NR | NR | 1200 | 8.67 |
| Huerga et al (2012)33 | Kenya | 11 non-tuberculous mycobacteria | 5 days amoxicillin | 92 | NR | 566 | 2.22 |
| Jayasooriya et al (2019)6 | The Gambia | 2 malignancy: 2 lung 1 haematological |
Clinically indicated | 16* | NR | 162 | 1.07 |
| 32 other respiratory tract infections | |||||||
| 8 pneumonia | |||||||
| 4 asthma | |||||||
| 2 pleural effusions | |||||||
| 1 lung abscess | |||||||
| 10 heart failure | |||||||
| 2 structural heart disease | |||||||
| 1 ischaemic heart disease | |||||||
| 2 chronic renal failure | |||||||
| 43 unknown | |||||||
| Lawson et al (2008)27 | Nigeria | NR | NR | NR | NR | 219 | 0.91 |
| Ling et al (2011)18 | South Africa | NR | NR | NR | NR | 1200 | 8.67 |
| Merid et al (2019)30 | Ethiopia | NR | NR | 151* | NR | 177 | 0.2 |
| Munyati et al (2005)† 21 | Zimbabwe | 178 other respiratory tract infections | Clinically indicated | 97 | NR | 607 | 8.67 |
| 87 bacterial pneumonia | |||||||
| 34 fibrotic lung disease: 28 post-tuberculous disease 2 idiopathic diffuse fibrosis | |||||||
| 26 asthma | |||||||
| 8 pneumocystis pneumonia | |||||||
| 5 cryptococcosis | |||||||
| 15 heart failure | |||||||
| 5 malignancy: 3 Kaposi sarcoma 1 primary bronchus 1 metastatic breast | |||||||
| 16 unknown | |||||||
| Nliwasa et al (2016)22 | Malawi | NR | NR | NR | NR | 261 | 3.2 |
| Reither et al (2010)23 | Tanzania | NR | NR | NR | NR | 492 | 2.75 |
| Sekandi et al (2014)31 | Uganda | NR | NR | NR | NR | 217 | 3.7 |
| Theron et al (2011)17 | South Africa | NR | NR | 158 | NR | 1270 | 11.82 |
*History of tuberculosis in participants without tuberculosis,
†Participants diagnosed with multiple conditions.
NR, not reported.
Discussion
Our findings demonstrate that almost half of patients with presumed TB in sSA were not given a final diagnosis of active TB. While this proportion varied according to study, it was not predicted by country incidence of TB or HIV. The few included studies that used active case-finding strategies had much lower proportions of patients with presumed TB with a final diagnosis of TB than those that used passive case finding. Only five of the identified studies attempted to characterise patients with presumed TB who were subsequently found not to have TB by reporting alternative diagnoses.6 20 21 26 33 Of these studies, only two reported a range of alternative diagnoses.6 21 In both of these studies, clinical judgement, rather than a standardised approach, was used to decide on investigations performed, and no spirometry was conducted.6 21 Just over half of included studies captured prior histories of TB and none indicated how many times patients had been previously tested for TB.
In the passive case-finding studies that included clinically diagnosed patients, the proportion of patients with presumed TB subsequently found not to have TB was inversely associated with the fraction of clinically diagnosed TB cases. While this could imply overdiagnosis of active TB through reliance on clinical judgement, it is important to note that many LMICs have high rates of active TB.4 This does highlight a need for improved point of care diagnostics for both TB and other respiratory pathogens. The lack of access to high-quality health systems and diagnostics in sSA means there is likely to be a high burden of unrecognised diseases of all causes and unmet clinical need in the general population.34 Therefore, patients with presumed TB—symptomatic by definition—risk having the true causes of their symptoms neglected if they are not due to active TB.6 21 The implications for missing active TB are clear, yet those of incorrectly labelling people as having active TB and/or missing other health conditions also need to be taken into consideration. For example, patients with non-communicable chronic respiratory diseases such as chronic obstructive airway disease, asthma and bronchiectasis are also likely to present to the health system with a chronic cough, requiring ongoing management. This is not only a missed opportunity for clinical engagement; patients who receive an incorrect diagnosis or are discharged without any follow-up may become reluctant to seek care in the future.
The higher proportions of patients found not to have TB in active case-finding studies is likely to be due to the difference in study population from those identified in passive case-finding studies. In addition, most active case-finding studies reported only bacteriologically confirmed TB cases. A WHO-commissioned systematic review reported general population community-based active case-finding studies set in sSA.35 These studies only used bacteriological (often smear) diagnoses of TB cases, and none reported any clinical diagnoses of TB. When we compared active with passive case-finding studies that also reported only bacteriologically confirmed TB cases, the former still had a higher proportion of patients with presumed TB subsequently found not to have TB. These findings imply that active case-finding strategies encounter more community members with unidentified health issues that have non-specific symptoms similar to those of active TB. A retrospective review of radiological findings from a Kenyan TB prevalence survey identified a wide variety of abnormalities unrelated to active TB in those that were not classified as having TB.36 Systematic active screening of high-risk groups is a central component of the WHO End Tuberculosis Strategy and the aforementioned systematic review suggests that community-based active case finding might be effective at detecting active TB early.35 However, the emphasis on active case-finding strategies in sSA should take into consideration patients with presumed TB subsequently found not to have TB, as they are likely to represent a large proportion of those with positive initial symptom screens. Improving the ability of local health systems to manage patients without TB, alongside making appropriate diagnoses of TB disease is imperative.
A history of TB is important for assessing the risk of active TB in patients with presumed TB. Recording and reporting TB history in future research is essential as it is necessary to fully interpret results, particularly with increasing use of Xpert MTB/RIF and Xpert MTB/RIF Ultra. Patients with presumed TB subsequently found not to have TB will include some of the estimated 155 million patients globally alive today post-TB.37 Recognition of history of TB could also help identify them allowing for the provision of ongoing care. Long-term effects, such as increased all-cause mortality post disease38 and post-TB lung disease,39 could start to be addressed.
Two included studies used mass administration of empirical antibiotics to several hundreds of patients with presumed TB subsequently not diagnosed with TB. With increasing antimicrobial resistance recognised as one of the biggest public health challenges of our time, nuanced strategies to mitigate against administering unnecessary antibiotics are vital. The lack of adequate point of care diagnostics, for both respiratory pathogens and TB alongside unavailable alternative management strategies can drive indiscriminate use of antimicrobials. Strategies such as the Practical Approach to Lung Health (PAL) have demonstrated that better integrated respiratory care can reduce antimicrobial usage in LMICs.
Our findings are also of importance when considering paediatric TB. The nature of limited diagnostics and well recognised high proportions of empirical TB treatment in paediatrics add further complexity. Distinguishing TB from other respiratory infections in children is an important area of ongoing research, and the development of easily applicable paediatric TB diagnostic tests able to do just that remains critical.
This work raises ethical issues around the inclusion of patients in research studies conducted in settings where limited primary care is available. Non-communicable chronic respiratory diseases caused an estimated 3.9 million deaths in 2017,40 of which a disproportionately high burden is seen in LMICs.1 Furthermore, the prevalence of TB has declined over time in many settings. It is critical that the care afforded as a minimum to symptomatic patients screening out of TB studies in settings with limited healthcare should be taken into consideration during research planning, offering, for example, in this case follow-up for patients subsequently found not to have TB until an alternative diagnosis is found. This will require improved collaboration between researchers and health system actors as well as greater consideration of the study participant’s health needs.
There are limitations to our review. We acknowledge that the meta-analytical portion was limited by substantial heterogeneity observed across studies. While summary values should, therefore, be treated with caution their general size indicates potentially important unmet needs in sSA communities. We found only two studies with a stated objective to describe patients with presumed TB subsequently found not to have TB. Most studies were cross-sectional and designed to capture patients with active TB. Therefore, understandably data on those essentially screening out of the study may not be as comprehensive as for those that were diagnosed with active TB and included as final study participants. In particular, we highlight that where data was not recorded, it does not always equate to not being performed and the cross-sectional nature of the studies meant there was limited follow-up. However, this absence of data further supports our conclusion that there is a critical lack of reported data on patients with presumed TB subsequently found not to have TB.
This systematic review of the literature highlights that at least half of all patients with presumed TB attending services in sSA are not given a diagnosis of active TB; many not receiving any alternative diagnoses. In sSA, 1.4 million TB cases were notified in 2019, our data suggest that this figure represents only half of all patients with symptoms consistent with presumptive TB. It is critical we address this by characterising the clinical needs among these hitherto neglected patients, in order to plan appropriate health system solutions. Future studies should explore patient experiences to better understand how these influence subsequent care-seeking behaviours and health system engagement. Generating such data would help facilitate integration of services for non-communicable chronic respiratory diseases with TB programmes.
Acknowledgments
We thank the NIHR Global Health Research Unit on Lung Health and tuberculosis in Africa at LSTM - 'IMPALA' for helping to make this work possible. In relation to IMPALA (grant number 16/136/35) specifically: This research was funded by the National Institute for Health Research (NIHR) (IMPALA, grant reference 16/136/35) using UK aid from the UK Government to support global health research.
Footnotes
Twitter: @de_shami, @mscihta, @BAwokola
Contributors: SJ (guarantor) formulated the research questions with input from BK and KMSJ, CB formulated the search strategy SJ, FDD screened articles and data extracted with input from FC. PJD synthesised data with input from SJ All authors (SJ, FDD, CB, JB, BA, CM, BK, FC, PJD & KM) contributed to data interpretation and drafting the manuscript.
Funding: KM is the director of the NIHR Global Health Research Unit on Lung Health and tuberculosis in Africa at LSTM (16/136/35). PD was supported by a fellowship from the UK Medical Research Council (MR/P022081/1); this UK-funded award is part of the EDCTP2 programme supported by the European Union. SJ was supported by an NIHR Clinical Lectureship Award.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Extraction data are available on github.https://github.com/petedodd/NotTB.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Meghji J, Mortimer K, Agusti A, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021;397:928–40. 10.1016/S0140-6736(21)00458-X [DOI] [PubMed] [Google Scholar]
- 2.Systematic screening for active tuberculosis: principles and recommendations. Geneva. 2013. https://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf. https://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf [PubMed]
- 3. Horne DJ, Kohli M, Zifodya JS, et al. Xpert MTB/RIF and Xpert MTB/RIF ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2019;6:CD009593. 10.1002/14651858.CD009593.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO . Global tuberculosis report: executive summary, 2020. [Google Scholar]
- 5. Banda HT, Thomson R, Mortimer K, et al. Community prevalence of chronic respiratory symptoms in rural Malawi: implications for policy. PLoS One 2017;12:e0188437. 10.1371/journal.pone.0188437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayasooriya S, Jobe A, Badjie S, et al. The burden of non-TB lung disease presenting to TB clinics in The Gambia: preliminary data in the Xpert ® MTB/Rif era. Public Health Action 2019;9:166–8. 10.5588/pha.19.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oshi DC, Chukwu JN, Nwafor CC, et al. Diagnosis of smear-negative tuberculosis in Nigeria: do health care workers adhere to the National guidelines? Int J Mycobacteriol 2014;3:163–7. 10.1016/j.ijmyco.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 8. Tafuma TA, Burnett RJ, Huis In 't Veld D. National guidelines not always followed when diagnosing smear-negative pulmonary tuberculosis in patients with HIV in Botswana. PLoS One 2014;9:e88654. 10.1371/journal.pone.0088654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell S. A filter to Retrieve studies related to Northern Africa from the OVID Medline database. Edmonton, AB: University of Alberta, 2017. [Google Scholar]
- 11. Campbell S. A filter to Retrieve studies related to eastern Africa from the OVID Medline database. Edmonton, AB: University of Alberta, 2018. [Google Scholar]
- 12. Campbell S. A filter to Retrieve studies related to southern Africa from the OVID Medline database. Edmonton, AB: University of Alberta, 2017. [Google Scholar]
- 13. Campbell S. A filter to Retrieve studies related to Western Africa from the OVID Medline database. Edmonton, AB: University of Alberta, 2017. [Google Scholar]
- 14. Campbell S. A filter to Retrieve studies related to middle Africa from the OVID Medline database. Edmonton, AB: University of Alberta, 2017. [Google Scholar]
- 15. DocTranslator . Available: https://www.onlinedoctranslator.com/en/
- 16. Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015;13:147–53. 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 17. Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011;184:132–40. 10.1164/rccm.201101-0056OC [DOI] [PubMed] [Google Scholar]
- 18. Ling DI, Pai M, Davids V, et al. Are interferon-gamma release assays useful for diagnosing active tuberculosis in a high-burden setting? Eur Respir J 2011;38:649–56. 10.1183/09031936.00181610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. The Lancet 2011;377:1495–505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruchfeld J, Aderaye G, Palme IB, et al. Evaluation of outpatients with suspected pulmonary tuberculosis in a high HIV prevalence setting in Ethiopia: clinical, diagnostic and epidemiological characteristics. Scand J Infect Dis 2002;34:331–7. 10.1080/00365540110080025 [DOI] [PubMed] [Google Scholar]
- 21. Munyati SS, Dhoba T, Makanza ED, et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin Infect Dis 2005;40:1818–27. 10.1086/429912 [DOI] [PubMed] [Google Scholar]
- 22. Nliwasa M, MacPherson P, Chisala P, et al. The sensitivity and specificity of loop-mediated isothermal amplification (lamp) assay for tuberculosis diagnosis in adults with chronic cough in Malawi. PLoS One 2016;11:e0155101. 10.1371/journal.pone.0155101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reither K, Saathoff E, Jung J, et al. Evaluation of Diagnos TB AG, a flow-through immunoassay for rapid detection of pulmonary tuberculosis. Int J Tuberc Lung Dis 2010;14:238–40. [PubMed] [Google Scholar]
- 24. Cuevas LE, Yassin MA, Al-Sonboli N, et al. A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med 2011;8:e1000443. 10.1371/journal.pmed.1000443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018;18:76–84. 10.1016/S1473-3099(17)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanrahan CF, Theron G, Bassett J, et al. Xpert MTB/RIF as a measure of sputum bacillary burden. variation by HIV status and immunosuppression. Am J Respir Crit Care Med 2014;189:1426–34. 10.1164/rccm.201312-2140OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawson L, Yassin MA, Thacher TD, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis 2008;40:30–5. 10.1080/00365540701509899 [DOI] [PubMed] [Google Scholar]
- 28. Deribew A, Abebe G, Apers L, et al. Prevalence of pulmonary TB and spoligotype pattern of Mycobacterium tuberculosis among TB suspects in a rural community in Southwest Ethiopia. BMC Infect Dis 2012;12:54. 10.1186/1471-2334-12-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamusse S, Demissie M, Teshome D, et al. Prevalence and incidence of smear-positive pulmonary tuberculosis in the Hetosa district of Arsi zone, Oromia regional state of central Ethiopia. BMC Infect Dis 2017;17:214. 10.1186/s12879-017-2321-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Merid Y, Mulate YW, Hailu M, et al. Population-based screening for pulmonary tuberculosis utilizing community health workers in Ethiopia. Int J Infect Dis 2019;89:122–7. 10.1016/j.ijid.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 31. Sekandi JN, List J, Luzze H, et al. Yield of undetected tuberculosis and human immunodeficiency virus coinfection from active case finding in urban Uganda. int j tuberc lung dis 2014;18:13–19. 10.5588/ijtld.13.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Affolabi D, Akpona R, Odoun M, et al. Smear-negative, culture-positive pulmonary tuberculosis among patients with chronic cough in Cotonou, Benin. Int J Tuberc Lung Dis 2011;15:67–70. [PubMed] [Google Scholar]
- 33. Huerga H, Varaine F, Okwaro E, et al. Performance of the 2007 WHO algorithm to diagnose smear-negative pulmonary tuberculosis in a HIV prevalent setting. PLoS One 2012;7:e51336. 10.1371/journal.pone.0051336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the sustainable development goals era: time for a revolution. The Lancet Glob Health 2018;6:e1196–252. 10.1016/S2214-109X(18)30386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burke RM, Nliwasa M, Feasey HRA, et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health 2021;6:e283–99. 10.1016/S2468-2667(21)00033-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mungai BN, Joekes E, Masini E, et al. 'If not TB, what could it be?' chest X-ray findings from the 2016 Kenya tuberculosis prevalence survey. Thorax 2021;76:607–14. 10.1136/thoraxjnl-2020-216123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dodd PJ, Yuen CM, Jayasooriya SM, et al. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis 2021;21:984–92. 10.1016/S1473-3099(20)30919-1 [DOI] [PubMed] [Google Scholar]
- 38. Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019;19:1129–37. 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 39. Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020;75:269–78. 10.1136/thoraxjnl-2019-213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. GBDCoD C. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217663supp001.pdf (59.8KB, pdf)
thoraxjnl-2021-217663supp002.pdf (65.1KB, pdf)
thoraxjnl-2021-217663supp003.pdf (1.7MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Extraction data are available on github.https://github.com/petedodd/NotTB.