Abstract
Objective
A portable, low-field MRI system is now Food and Drug Administration cleared and has been shown to be safe and useful in adult intensive care unit settings. No neonatal studies have been performed. The objective is to assess our preliminary experience and assess feasibility of using the portable MRI system at the bedside in a neonatal intensive care unit (NICU) at a quaternary children’s hospital.
Study design
This was a single-site prospective cohort study in neonates ≥2 kg conducted between October and December 2020. All parents provided informed consent. Neonates underwent portable MRI examination in the NICU with support equipment powered on and attached to the neonate during the examination. A paediatric radiologist interpreted each portable MRI examination. The study outcome variable was percentage of portable MRI examinations completed without artefacts that would hinder diagnosis. Findings were compared between portable MRI examinations and standard of care examinations.
Results
Eighteen portable, low-field MRI examinations were performed on 14 neonates with an average age of 29.7 days (range 1–122 days). 94% (17 of 18) of portable MRI examinations were acquired without significant artefact. Significant intracranial pathology was visible on portable MRI, but subtle abnormalities were missed. The examination reads were concordant in 59% (10 of 17) of cases and significant pathology was missed in 12% (2 of 17) of cases.
Conclusion
This single-centre series demonstrated portable MRI examinations can be performed safely with standard patient support equipment present in the NICU. These findings demonstrate that portable MRI could be used in the future to guide care in the NICU setting.
Trial registration number
Keywords: intensive care units, neonatal; Magnetic Resonance Imaging; neurology; paediatrics
The authors describe their single center NICU experience in use of a portable MRI. They report these scans are feasible in a sub-population on NICU babies, and are superior to bed side head ultrasound in detecting ischemic lesions. Images obtained are of lower resolution than conventional MRI scans.
WHAT IS ALREADY KNOWN ON THIS TOPIC
A portable, low-field MRI system is now cleared by the Food and Drug Administration and has been shown to be safe and diagnostically useful in adult intensive care unit settings. No studies of this technology have been performed in neonates.
WHAT THIS STUDY ADDS
This study demonstrates the capability of portable MRI to obtain neonatal brain imaging at the bedside in the neonatal intensive care unit.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study demonstrates that it is safe and possible to image neonates using portable low-field MRI at bedside in the neonatal intensive care unit. Since technical feasibility has been demonstrated, further studies can focus on imaging optimisation and diagnostic efficacy of portable MRI to evaluate the patient populations and pathologies where this technology could have the most clinical impact.
Introduction
Neonatal brain imaging is performed for various clinical indications including hypoxic ischaemic injury, haemorrhage, periventricular leucomalacia, congenital brain malformations and hydrocephalus.1 2 Head ultrasound is a portable screening modality. Conventional MRI (C-MRI; 1.5 or 3 Tesla) is a diagnostic modality with increased conspicuity of different brain pathologies.1 2 However, C-MRI requires transport of neonates to dedicated rooms1 2 and commonly used neonatal intensive care unit (NICU) support equipment (eg, incubators, monitors, etc) is not MR conditional, which increases complexity for scanning neonates with C-MRI.3
Recently, a low-field portable MRI (pMRI) system (Hyperfine, Guilford, Connecticut, USA) was cleared by the Food and Drug Administration for neonatal head imaging. This system operates using a static magnetic field strength of 0.064 T. Portable MRI has fewer limitations on patient support equipment that can be present in the room during pMRI examinations.4–6 Several studies have demonstrated the safe operation, feasibility and diagnostic utility of the pMRI system in the adult ICU setting4 5 7 and a paediatric study demonstrated that brain volumes obtained using the pMRI system were similar to those measured using C-MRI.8 However, to date, there has been no published report of a pMRI system being used in the NICU setting.
The primary objective of our study was to describe our preliminary experience with pMRI and assess the feasibility of using pMRI at the bedside in a level IV NICU. The secondary objective was to demonstrate safe operation and evaluate image quality of the pMRI system with support equipment nearby.
Methods
This is an investigator-initiated cohort study conducted in the NICU at a quaternary paediatric medical centre. This study was registered with ClinicalTrials.gov.
pMRI system
The pMRI device is 140 cm tall and 86 cm wide and weighs 630 kg (figure 1). The 5-gauss boundary around the scanner extends in a circle (radius=79 cm). The head coil is 26 cm long, 26 cm high and 20 cm wide. The pMRI was powered using a standard 15 A, 110 V wall outlet. The decibel level while scanning ranges from 60 to 80 db. Scan sequences were controlled using a tablet computer interface (iPad Pro, second generation and third generation; Apple, Cupertino, California, USA).4 Scan parameters are listed in table 1.
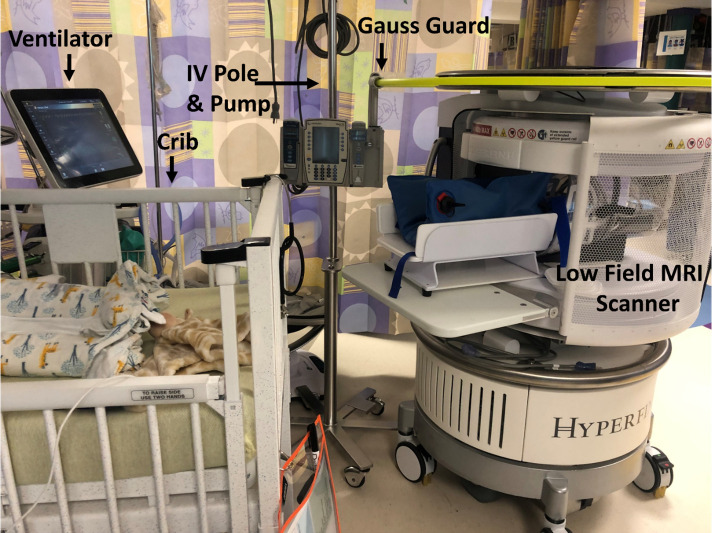
Figure 1.
Photograph of a typical set-up of patient support equipment for NICU present during portable MRI system examinations performed in the NICU. Note that the portable MRI system is located near the neonate’s crib and the neonate would be transferred to the cradle for scanning. The yellow ring shows the Gauss Guard that delineates the 5-gauss area associated with the portable MRI system. In this photo, a crib, ventilator and intravenous (IV) infusion pump, and IV pole (ie, standard device, not MR conditional) have been placed in their typical positions during neonate scanning. The custom neonatal cradle and an immobilisation device (blue equipment) are shown in their typical positions during MRI scanning of neonates’ brains. NICU, neonatal intensive care unit.
Table 1.
Portable MRI sequence scanning parameters
| Sequence name | Scanning parameters | Scan time (min) |
| T1W fast spin echo (FSE) | Repetition time (TR), 1500 ms; time to echo (TE), 6 ms; inversion time (TI), 300 ms; 1.5×1.5×5 mm resolution; 36 slices | 4:52 |
| T2W FSE | TR, 2200 ms; TE, 253 ms; 1.5×1.5×5 mm resolution; 36 slices | 5:54 |
| T2W FLAIR FSE | TR, 4000 ms; TE, 228 ms; TI, 1400 ms; 1.6×1.6×5 mm resolution; 36 slices | 9:00 |
| DWI FSE | TR, 1000 ms; TE, 100 ms; b=0, 800 s/mm2; 2.4×2.4×6 mm resolution; 30 slices | 9:27 |
DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; T1W, T1-weighted; T2W, T2-weighted.
pMRI system safety and quality check
Prior to scanning, the safety of and effects on image quality from support equipment present within the 5-gauss area of the pMRI system were assessed. To test for safety, we slowly moved support equipment closer to the pMRI system to ensure that each device was not attracted by the scanner, posing a risk of a projectile. The tested items included a standard (ie, non-MR conditional) intravenous (IV) stand 811 Hitch-N-Pal IV pole (Pryor Products, Oceanside, California, USA), Servo-U ventilator (Maquet, Sweden), Alaris 8100 IV infusion pump (BD, Franklin Lakes, New Jersey, USA), Carescape B450 physiological monitor (General Electric, Milwaukee, Wisconsin, USA), monitoring leads (3M, Saint Paul, Minnesota, USA), Giraffe Bedded medical warmers (Ohmeda Medical, Clark, New Jersey, USA) and crib (Hard, Overland Park, Kansas, USA). After initial projectile risk testing, we placed an IV pole, ventilator and physiological monitor within the 5-gauss area and scanned a quality control (QC) phantom (Hyperfine, Guilford, Connecticut, USA) with and without the support equipment operating. An MRI physicist and radiologist reviewed the two sets of images (ie, with and without operating devices in the 5-gauss area) in a blinded fashion to identify the presence of artefacts.
Study subjects
Between October 2020 and December 2020, all neonates in our NICU who underwent head imaging (ultrasound, CT or C-MRI) were screened for eligibility using the hospital’s electronic medical record. Neonates who needed additional sedation for pMRI examinations were on extracorporeal membrane oxygenation or had medical implants were excluded. Neonates in state custody and those who were isolated due to COVID-19 were also excluded. Neonates who weighed less than 2 kg were excluded due to concerns about temperature regulation during scanning. Parents of eligible neonates were approached for consent.
pMRI examinations
A pMRI was acquired within 24 hours of clinical head imaging. The pMRI system was positioned in each neonate’s room to minimise disruption of support equipment. Neonates were swaddled, provided with ear protection and positioned within the head coil. The neonates were placed on a custom-designed, neonatal MRI cradle (Hyperfine, Guilford, Connecticut, USA) to position them in the scanner. Throughout the MRI examination, vital signs were monitored. An MRI technologist with 12 years of experience (ALR) monitored the patient for gross motion during the examination and terminated the examination if the movement would likely result in too much artefact. Two research staff members with backgrounds as radiological technologists and a nurse were present during the examination. The average preparation time for a scan was 10 min.
Statistical descriptions and analysis
The pMRIs were assessed by a paediatric radiologist with 12 years of experience (SSC). Images were assessed for significant artefact (yes/no) and images without artefact (undesired signal in the MRI) were interpreted for key findings. The radiologist was provided the neonate’s age and the same clinical history for the pMRI examination that was provided for the standard of care (SOC) examination. The radiologist was blinded to the interpretation of the SOC examinations. The primary outcome variable was the percentage of pMRI examinations completed without significant artefact. This variable was defined as acquiring all four planned pMRI pulse sequences without artefact that would hinder diagnostic interpretation.
The SOC examinations’ key findings were obtained from the radiology reports for those studies. In the instances of discrepancies between the pMRI report and SOC ultrasound reports, an effort was made to obtain C-MRI results as a reference standard to resolve the difference. The clinical diagnosis for each neonate was obtained from the discharge summary.
Results
pMRI system safety and quality check
Figure 1 shows the pMRI system in a NICU room surrounded by the typical support equipment. Note that the 5-gauss area is indicated by a yellow ring (ie, the Gauss Guard) on top of the scanner. Importantly, of the devices that underwent evaluation, none of the NICU support devices were attracted by the magnet when placed within the 5-gauss line. On the quality check, there were no artefacts visible on the images obtained using the QC phantom, with and without support equipment present and operating within the 5-gauss area.
Study subjects
Ninety-seven neonates met the inclusion criteria and 47 neonates were excluded for the following reasons: weight under 2 kg (n=21), additional sedation required (n=12), implanted devices and/or extracorporeal membrane oxygenation (n=9), COVID-19 isolation (n=3) or in state custody (n=2). Of the remaining 50 neonates, 25 were not enrolled because the parents or legally authorised representative either declined participation in the study (n=10) or were unable to be approached for consent due to COVID-19 restrictions (n=15).
The parents of 25 neonates gave consent for participation and the neonates were enrolled in the study. Eleven of the enrolled neonates were unable to be scanned during the 24-hour window: three subjects were discharged from the NICU, three subjects were placed on electroencephalograms, two subjects had rapid responses, two subjects had C-MRI examinations on the weekend and one subject was undergoing phototherapy. Fourteen neonates had pMRI examinations with an average gestational age of 35 weeks, 1 day (range 24 weeks, 0 days–40 weeks, 0 days) at the time of the examination and were 29.7 (range 1–122) days old. Gender was split evenly (girls: n=7). All subjects underwent at least 1 pMRI examination, one subject underwent 3 pMRI examinations and two subjects underwent 2 pMRI examinations, resulting in a total of 18 examinations. The indications for SOC head imaging are listed in table 1.
pMRI examinations
Patient monitors, IV pumps and ECG leads were present during 100% of examinations. Cribs were present during 67% (n=12) of examinations and radiant warmers during 33% (n=5) of examinations. Ventilators were present during 17% (n=3) of examinations. Some equipment was within the 5-gauss line during imaging. Importantly, there were no MRI-related adverse events during the pMRI examinations.
Example MRIs obtained using the pMRI systems are shown along with the corresponding images from SOC head imaging performed within 24 hours of the pMRI examination (figures 2 and 3). One examination was terminated due to gross motion during the pMRI scan.
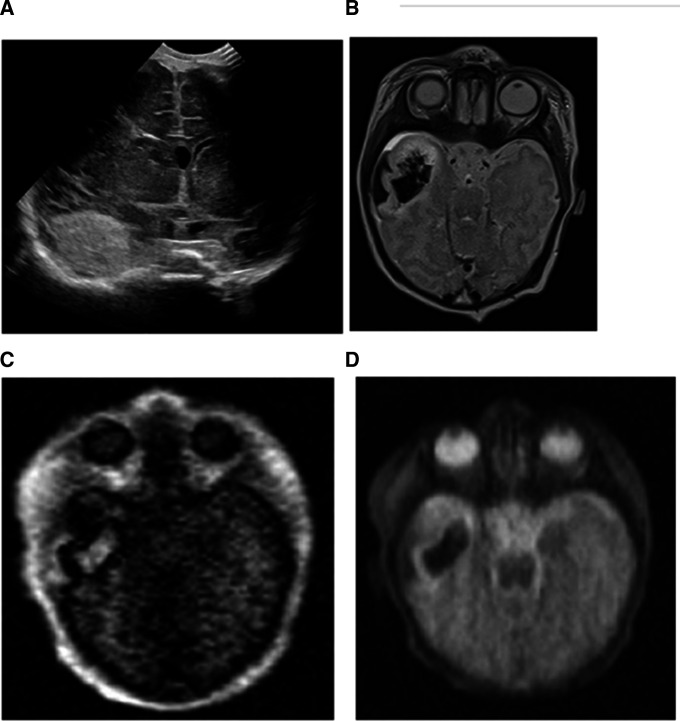
Figure 2.
(A) Five-day-old baby boy with intraparenchymal haemorrhage in the anterior right temporal lobe on an ultrasound examination. (B) The conventional MRI examination demonstrated the intraparenchymal and subpial haemorrhage well on the T2-weighted images. The portable MRIs also showed the extent of the intraparenchymal and subpial haemorrhage in the right temporal lobe on the T1-weighted (C) and T2-weighted images (D).
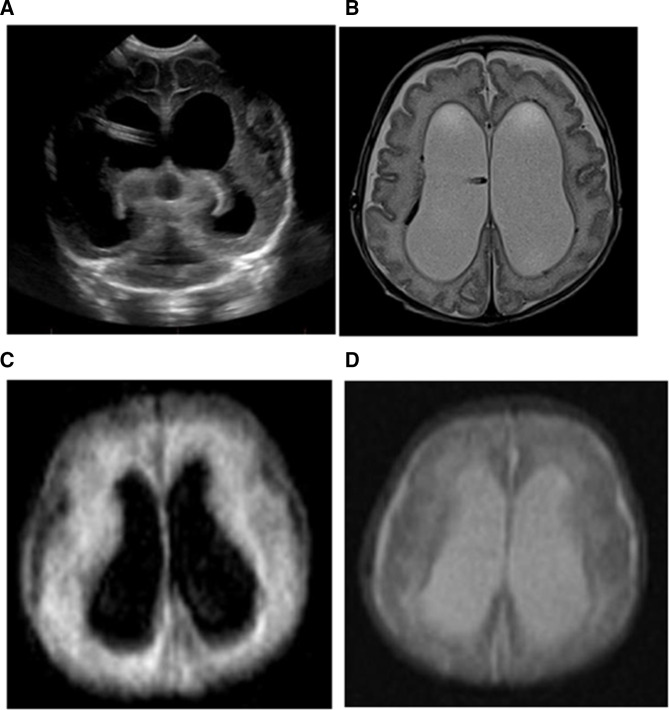
Figure 3.
(A) An 88-day-old baby girl with hydrocephalus with dilated lateral ventricles with an intraventricular catheter in place shown on head ultrasound. (B) The conventional MRI examination showed severe dilation of the bilateral lateral ventricles on the T2-weighted images with hemosiderin staining of the right lateral ventricular lining. This portable MRI also showed similar dilation of the bilateral lateral ventricles on FLAIR (C) and T2-weighted images (D). The hemosiderin staining of the ventricular lining is not imaged well on the portable MRIs. FLAIR, fluid-attenuated inversion recovery.
Statistical descriptions and analysis
Ninety-four per cent of pMRI examinations (17 of 18) were successfully completed without artefacts that would impair image interpretation. Some zipper (radiofrequency interference) artefact was noted on 53% (9 of 17) of examinations, but this artefact did not impair diagnostic information.
Table 2 lists the key findings for each neonate on the SOC imaging and the pMRI examination. Large infarcts and haemorrhages were clearly visible on the pMRI examinations. Ventricular volumes and extra-axial spaces appeared to be well evaluated with pMRI. Fifty-nine per cent (10 of 17) of the pMRI interpretations contained the same information as the SOC imaging reads and these patients either had large haemorrhages, hydrocephalus or were normal. Twelve per cent (2 of 17) of the pMRI examinations had major discrepancies, with pMRI missing the underlying brain tumour causing a large haemorrhage and pMRI missing some subcentimetre infarcts. Twenty-nine per cent (5 of 17) reads had only minor discrepancies. Of note, pMRI was more effective in neonate #8 in assessing posterior fossa pathology compared with ultrasound (table 1). When compared with C-MRI, subtle findings, such as cerebellar and brainstem hypoplasia and smaller subcentimetre areas of deep grey ischaemia, were missed on the pMRIs. This was especially true of findings on diffusion-weighted imaging and T1 images.
Table 2.
Neonatal subject demographics and scan results
| ID | Gestational age (weeks, days)+sex | Clinical diagnosis | SOC imaging | Age at scan (days) | Weight during scan (kg) | SOC key findings | Portable MRI key findings |
| 1 | 27, 2 F | Achondroplasia | CT | 77 | 2.590 | Mild decrease in white matter volume Hypoplastic pons and cerebellum |
Mild decrease in white matter volume |
| C-MRI | 105 | 3.440 | Mild decrease in white matter volume Hypoplastic pons and cerebellum Prior small cerebellar haemorrhages |
Mild decrease in white matter volume | |||
| 2 | 38, 4 M | HIE | C-MRI | 5 | 3.790 | Multifocal restricted diffusion: cortex, periventricular white matter and deep grey structures | Restricted diffusion: corpus callosum and periventricular white matter |
| 3 | 39, 2 F | Congenital heart defect | US | 1 | 3.14 | Normal | Normal |
| 4 | 24, 0 F | Intraventricular haemorrhage | US | 93 | 2.98 | Stable prior right grade 3 and left grade 4 IVH | Prior left greater than right IVH likely left grade 4 and right grade 3 No new bleed |
| US | 99 | 3.030 | Stable prior right grade 3 and left grade 4 IVH | Stable ventriculomegaly from prior IVH No new bleed |
|||
| C-MRI | 108 | 3.350 | Not Applicable Scan aborted due to motion |
||||
| 5 | 38, 2 M | Seizures and intracranial haemorrhage | C-MRI | 5 | 3.928 | Right temporal lobe and subpial haemorrhage | Right temporal lobe and subpial haemorrhage |
| 6 | 40, 0 M | Congenital heart defect | C-MRI | 4 | 2.990 | Normal | Normal |
| 7 | 28, 1 F | Intraventricular haemorrhage | US | 88 | 2.735 | Evolving bilateral intraventricular haemorrhage Stable ventriculomegaly |
Prior left greater than right IVH likely left and right grade 3 No new bleed Right shunt in place |
| US | 95 | 2.94 | Evolving bilateral intraventricular haemorrhage Stable ventriculomegaly |
Similar hydrocephalus Right shunt in place |
|||
| 8 | 37, 0 M | Myelomeningocele | US | 6 | 2.880 | Mild prominence of the lateral and third ventricles and Chiari II Subsequent MRI demonstrated no Chiari* |
Mild hydrocephalus No Chiari |
| 9 | 40, 3 M | Congenital heart defect | C-MRI | 2 | 3.5 | Small foci of restricted diffusion: right periventricular white matter | Normal |
| 10 | 37, 5 M | Congenital heart defect | C-MRI | 3 | 2.7 | Normal | Normal |
| 11 | 38, 3 F | Transient tachypnoea of the newborn with possible seizures | C-MRI | 3 | 3.485 | Normal | Normal |
| 12 | 39, 6 F | Neonatal stroke causing seizures | C-MRI | 4 | 3.052 | Multifocal restricted diffusion: left parietal and occipital lobes and smaller foci in the left periventricular white matter, basal ganglia and midbrain | Large area of restricted diffusion in left posterior frontal, parietal and occipital lobes |
| 13 | 28, 0 M | Myofibromatosis | US, CT, C-MRI | 122 | 4.235 | Large right supratentorial brain tumour | Complex large right supratentorial haemorrhage |
| 14 | 36, 0 F | HIE | C-MRI | 4 | 2.00 | Normal | Normal |
Bold indicates major discrepancies between SOC imaging and portable MRI.
*Note that in patients 2, 5, 6 and 9–12 also had head ultrasounds during their hospital course, but these ultrasounds were not within 24 hours of the portable MRI examination.
C-MRI, conventional MRI; F, female; HIE, hypoxic ischaemic encephalopathy; IVH, intraventricular haemorrhage; M, male; SOC, standard of care; US, ultrasound.
Discussion
Our preliminary experience demonstrated that pMRI brain imaging can be obtained in the NICU setting without artefacts that impair image interpretation. Some images had zipper artefact, likely caused by the radiofrequency shield remaining partly open during scanning to accommodate lines and tubes. Additionally, pMRI examinations were possible without having to remove patient support equipment. The pMRI examinations were concordant with the SOC examinations on large haemorrhages and large areas of ischaemia but not accurate for smaller findings.
Our preliminary experience in the NICU is similar to what has been published for this pMRI system in the adult ICU settings.5 9 Portable MRI examinations are feasible in both settings. The adult-based literature reported that pMRI has an 80% sensitivity and 97% specificity for intracranial haemorrhage. There were too few haemorrhage cases in our study to accurately assess sensitivity and specificity. Similar to the adult studies, pMRI examinations required much less staff and much less of their time compared with C-MRI examinations. The set-up time for pMRI was only 10 min, whereas preparing and transporting NICU patients to the C-MRI scanner and back can take over 1 hour in addition to the scanning time.
When the pMRI scanner was received at our medical centre, it was noted that it would be impossible to scan neonates in the NICU while adhering to the manufacturer’s recommendations in the instructions for use, which indicated that all support equipment must remain outside of the 5-gauss line. In general, tubing and lines (eg, IV tubing, ventilator, etc) are limited in length. Accordingly, the support equipment must be near the neonate. In consultation with our local MRI physicist, the importance of the 5-gauss value is that it is the fringe field area for which the general public is restricted from entering because of possible hazards of conventional magnetic field in relation to the operation of cardiac pacemakers and other active implants.10 11 It was not defined to denote a line of safety for items containing from ferromagnetic material.10 Therefore, we decided to test safety and image quality when support equipment was present within the 5-gauss area during operation of the pMRI system.
Although we tested equipment within the 5-gauss area for safety and effects on image quality, our results cannot be generalised to other institutions that may use different equipment. Our expectation is that other hospitals implementing pMRI examinations in the NICU would face similar challenges associated with the limited length of lines and tubes, necessitating the equipment being placed within the 5-gauss area during scanning. This limitation might be best addressed by the manufacturer since the main concern with the 5-gauss area restriction is possible malfunctioning of certain active implants (eg, pacemakers).12
Our study demonstrated that pMRI examinations are feasible in neonates, but there are limitations on the diagnostic utility as some of the pMRI key findings were discordant with C-MRI key findings. For small ischaemic regions, this could be due to the spatial resolution of the sequences and partial volume averaging (slice thicknesses of 5–6 mm). It is likely that most of the missed findings on pMRI were due to low signal-to-noise within the MRIs. However, many of the clinically important key findings were concordant. If this is validated over a larger dataset, then pMRI could be useful as a neonatal brain screening test. The pMRI system could have an important role in head imaging for neonates who are too sick to transport. It could also have a role in screening examinations for pathology that is often poorly imaged by head ultrasound such as posterior fossa haemorrhage and periventricular leucomalacia.13
Currently, pMRI is limited compared with C-MRI for diagnosis of subtle neonatal brain pathology due to its lower signal-to-noise and spatial resolution, which is an inherent limitation of the lower field strength and the limited power for the time-varying gradients. Compared with head ultrasound, pMRI is of similar diagnostic ability for ventricle size and hydrocephalus, but superior for early ischaemia and posterior fossa pathology (table 2). Therefore, pMRI could be a valuable tool in low-income countries where C-MRI availability is scarce and head ultrasound is a diagnostic test as well as a screening test.6 Thus, pMRI could potentially increase health equity by increasing MRI access and decreasing healthcare disparities between low-income versus high-income countries.14 Additionally, these units are an order of magnitude less expensive than C-MRI systems, can be plugged into a standard wall outlet and do not require specialised staff to operate.8 Of note, scan times are similar between the two systems with the standard brain examination times of 30–40 min.
Limitations
This was a small, single-site investigation of 14 neonates. Scanning of the neonates required them to be removed from their bed. The study was not powered appropriately to assess diagnostic accuracy; however, we are continuing to collect data to power such a study. Our study also had inter-rater differences because the C-MRI studies were read by different radiologists compared with the pMRI studies. There are still a significant number of infants in the NICU who would not qualify to be evaluated using the pMRI system under the device’s instructions for use, including those weighing less than 2 kg and those requiring equipment that is not acceptable for use with C-MRI. Additional studies will be needed to determine if criteria can be safely broadened.
Conclusion
Our study demonstrated that pMRI examinations are feasible in the NICU. This technology holds promise, but technical challenges limit its current clinical capabilities. Future studies could focus on optimising pulse sequences for neonatal imaging and formally measuring the diagnostic accuracy of this pMRI system for different neonatal pathologies. Finally, larger multicentre studies are needed to document generalisability.
Footnotes
Contributors: MS designed the data collection instruments, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. AR designed the data collection instruments, collected data, and reviewed and revised the manuscript. HHH, CRN, ASH, MGF, NSA and JTP helped to design the study, collected data, and reviewed and revised the manuscript. SSC conceptualised and designed the study, coordinated and supervised data collection, carried out the initial analyses, critically reviewed the manuscript for important intellectual content and is the guarantor of this work.
Funding: This work was supported by the Children’s Mercy Research Institute.
Competing interests: The study was investigator initiated and no funding was provided by the company. HHH and JTP are both employed by Hyperfine.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by Children’s Mercy Kansas City Institutional Review Board (STUDY00001356). Participants gave informed consent to participate in the study before taking part.
References
- 1. Tkach JA, Hillman NH, Jobe AH, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol 2012;42:1347–56. 10.1007/s00247-012-2444-9 [DOI] [PubMed] [Google Scholar]
- 2. Tkach JA, Merhar SL, Kline-Fath BM, et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol 2014;202:W95–105. 10.2214/AJR.13.10613 [DOI] [PubMed] [Google Scholar]
- 3. Serai SD, Ho M-L, Artunduaga M, et al. Components of a magnetic resonance imaging system and their relationship to safety and image quality. Pediatr Radiol 2021;51:716–23. 10.1007/s00247-020-04894-9 [DOI] [PubMed] [Google Scholar]
- 4. Mazurek MH, Cahn BA, Yuen MM, et al. Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage. Nat Commun 2021;12:5119. 10.1038/s41467-021-25441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheth KN, Mazurek MH, Yuen MM, et al. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol 2021;78:41–7. 10.1001/jamaneurol.2020.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geethanath S, Vaughan JT. Accessible magnetic resonance imaging: a review. J Magn Reson Imaging 2019;49:e65–77. 10.1002/jmri.26638 [DOI] [PubMed] [Google Scholar]
- 7. Turpin J, Unadkat P, Thomas J, et al. Portable magnetic resonance imaging for ICU patients. Crit Care Explor 2020;2:e0306. 10.1097/CCE.0000000000000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deoni SCL, Bruchhage MMK, Beauchemin J, et al. Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage 2021;238:118273. 10.1016/j.neuroimage.2021.118273 [DOI] [PubMed] [Google Scholar]
- 9. Abbasi J. Portable MRI-Coming to the ICU? JAMA 2020;324:1386. 10.1001/jama.2020.19612 [DOI] [PubMed] [Google Scholar]
- 10. ACR . ACR manual on Mr safety. 1.0 ed, 2020. [Google Scholar]
- 11. Bhachu DS, Kanal E. Implantable pulse generators (pacemakers) and electrodes: safety in the magnetic resonance imaging scanner environment. J Magn Reson Imaging 2000;12:201–4. [DOI] [PubMed] [Google Scholar]
- 12. Shellock FG. Magnetic resonance: bioeffects, safety, and patient management. 10, 1994: 101–21. [Google Scholar]
- 13. Lowe LH, Bailey Z. State-Of-The-Art cranial sonography: Part 2, pitfalls and variants. AJR Am J Roentgenol 2011;196:1034–9. 10.2214/AJR.10.6203 [DOI] [PubMed] [Google Scholar]
- 14. Shen FX, Wolf SM, Bhavnani S, et al. Emerging ethical issues raised by highly portable MRI research in remote and resource-limited international settings. Neuroimage 2021;238:118210. 10.1016/j.neuroimage.2021.118210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Data available upon reasonable request.