Abstract
The term neoaortoiliac system (NAIS) was coined by Clagett in 1993 to describe the use of the deep veins in the thigh to replace the aorta and iliac arteries in the setting of graft infection. Since that time, the NAIS procedure has been used to treat a wide array of both infectious and noninfectious conditions affecting the aortoiliac segment. In this article, we present a 10-step description of the NAIS procedure to treat an aortoduodenal fistula in a patient with an infected endovascular aneurysm repair.
The neoaortoiliac system (NAIS) was developed by Clagett et al1 at the University of Texas Southwestern Medical School more than 30 years ago in response to the need for a better way to treat aortic graft infections. Since the mid-1950s, most aortic graft infections were treated with complete graft excision, aortic ligation, and extra-anatomic bypass.2 These procedures were plagued by multiple mechanisms of failure, including reinfection and/or thrombosis of the extra-anatomic graft, and the fortunately rare, although catastrophic, aortic stump blowout.3 At about the same time, Nevelsteen in Belgium began similar investigation of the use of the superficial femoral vein in deep arterial infections.4,5 Both Clagett and Nevelsteen relied on pioneering work by Martin Schulman at SUNY Stony Brook, who published a series of femoral-popliteal bypasses using the femoral vein in 1987.6 Importantly, Schulman noted that late venous stasis changes and ulceration did not occur and ankle swelling was only slightly worse than after saphenous vein harvest. Encouraged by their initial results, the UT Southwestern group used the NAIS procedure with increasing enthusiasm to treat a variety of conditions over the next two decades.7, 8, 9 Ali et al10 published the first long-term results in 2009. The primary patency of the NAIS was 81%, secondary patency was 91%, and limb salvage was 89% at 6 years. Reinfection was observed in only 5% which was a significant improvement over competing in situ conduits, including rifampin-soaked polyester or cryopreserved allograft.10,11 The chief drawbacks of the procedure are the duration and technical complexity. Ali et al at the University of Arkansas for Medical Sciences later showed that staging the NAIS procedure by preparing the veins for harvest on 1 day followed by arterial reconstruction on the following day was far better tolerated by both the patient and the operating team and was associated with no adverse consequences.12 Other lessons learned included the early initiation of elemental enteral feeding through a gastrojejunostomy (G-J) tube, early tracheostomy to improve ventilator management, and shorter antibiotic courses that avoid the issues associated with central venous access and the induction of drug resistance.
In this article, we present our 10-step technique for performing the NAIS procedure in a patient with a secondary aortoduodenal fistula after endovascular aneurysm repair (EVAR). The procedure is equally well-suited for the management of primary aortic infection, primary aortoduodenal fistula, and aortic infection involving conventional open aortoiliofemoral synthetic grafts. The NAIS can also be considered in situations that have not historically performed well after conventional synthetic aortofemoral grafting, such as in younger women with gracile aortoiliofemoral vessels.13 In some circles, the NAIS procedure has gained an undeserved reputation for being too long and arduous in comparison to alternative in situ aortic reconstruction with synthetic graft or cryopreserved allograft. We believe that, with careful preoperative preparation, the NAIS procedure can be accomplished expeditiously with excellent results by any surgeon performing aortic surgery on a regular basis.
Preoperative considerations
Before the operation, it is particularly important to develop a disciplined process for reviewing the planned procedure. Based on work by Mitchell et al14 and her interviews with numerous surgical educators, we recommend structured preoperative planning that focuses on five phases—facts, anatomy, rationale, 10 steps, and crisis management.
Facts
It goes without saying that the surgeon must be thoroughly familiar with the facts of the case. A detailed review of prior surgical procedures should include reading previous operative notes and viewing prior imaging. The abdomen and groins are examined with attention to the location of all prior incisions and possible incisional hernias, masses, and tenderness. Peripheral pulses, Doppler signals, and ankle-brachial indices are recorded. The legs are examined for the presence of wounds, edema, and subjective assessment of compartment pressures for postoperative comparison. Preoperative laboratory work includes an assessment of nutritional status including albumin and prealbumin. Blood cultures should be obtained before antibiotic administration if possible.
Anatomy
Detailed knowledge of patient-specific anatomy is critical to successful completion of the NAIS procedure. The computed tomography angiogram is the diagnostic test of choice in the evaluation of possible aortic infection. All preoperative imaging should be reviewed with attention to the location, number and patency of visceral and renal arteries, presence of significant venous anomalies such as retroaortic or circumaortic renal veins, duplication of the inferior vena cava, and the presence of aortocaval fistulae. The relationship of the duodenum to the aorta may impact planned gastrointestinal reconstruction. All synthetic grafts should be assessed for signs of infection and incorporation. Preoperative bilateral venous ultrasound examination is used to evaluate the suitability of the femoral veins for use. In the best case scenario, the femoral veins are 7 to 8 mm in diameter and free of acute thrombus or chronic venous scarring related to prior deep vein thrombosis. Clinical evidence of significant chronic venous hypertension (presence of venous stasis ulcers, severe lipodermatosclerosis, venous claudication) represents a relative contraindication to ipsilateral femoral vein harvest that should be weighed against the benefit of an autogenous conduit.
Rationale
It is axiomatic that, before beginning the operation, the surgeon reviews the rationale for the selected procedure and considers risks and benefits of alternative options based on meaningful data. The surgeon should be confident that the planned procedure is clearly indicated and superior to other alternatives. (The NAIS procedure requires particular attention to detail, patience, stamina, and creativity on the part of the operating team and second guessing in the middle of the procedure is generally counterproductive.)
Ten steps
We recommend that the surgical team, including vascular and general surgeons, anesthesiologists, surgical technicians, and circulating nurses, rehearse the planned steps of the procedure. The team should be prepared to remain intact as a unit during critical phases of the operation, such as during aortic cross-clamping. Team members are reminded that distractions should be minimized, including unnecessary conversations and excessive traffic in the room. Operator fatigue can impact the success of the operation and planned breaks by all members of the team are encouraged.
Crisis management
The surgeon should review the likely problems and key decision points that may arise during the conduct of the operation and consider potential solutions. We like to ask the question “what would I do if…?” before surgery, much like a fighter pilot prepares for a mission.15
Staging the NAIS
We usually stage the operation on 2 consecutive days. On the first day, the femoral veins are exposed and the side branches are suture ligated and divided, but the axial veins themselves are left in continuity to maintain flow until they are formally harvested the next day. The patient is monitored in the intensive care unit overnight and returned to the operating room for a first start the following day. By staging the operation, the most complex portions of the operation, which involve obtaining aortic control, removing the infected graft and repairing the duodenum occur when the surgical team is well-rested and attentive. Although some groups prefer to enlist the services of two discrete surgical teams working simultaneously in close quarters in the abdomen and harvesting vein, we have found that temporal staging over 2 days is preferable.12
Ten STEPS: NAIS for aortoduodenal fistula after EVAR
Step 1: Prologue (day 1)
The patient is positioned supine with the arms extended and the legs slightly frog-legged (Table). The following tasks should be performed: induction of anesthesia, insertion of central venous access with a large bore sheath, insertion of radial arterial line, insertion of urinary catheter, placement of gastric decompression tube (either oral or nasal based on surgeon preference), application of body warmers, setting appropriate room temperature to avoid hypothermia (68F-72F). Blood should be available for transfusion with blood warmers available. We prefer to use the cell saver because it provides dependable high-volume suction, although we do not routinely transfuse cell-saver blood unless absolutely necessary because of the risk of a contaminated transfusion. It should be noted that warming blankets and pads should be turned off during aortic cross-clamping to avoid cutaneous thermal injury. In patients with hydronephrosis or hydroureter, ureteral stents are used to achieve the dual objectives of providing decompression of the collecting system and facilitating identification of the ureters during retroperitoneal dissection.
Table.
The 10 steps for using the neoaortoIliac system (NAIS) to treat an endovascular aortic aneurysm repair (EVAR) complicated by aortoduodenal fistula
| Step 1: Prologue (day 1) |
| Step 2: Deep vein preparation (day 1) |
| Step 3: Abdominal exploration (day 2) |
| Step 4: Aortic exposure |
| Step 5: Deep vein harvest and graft creation |
| Step 6: Opening the aortoduodenal fistula |
| Step 7: Aortic endograft excision |
| Step 8: Aortic reconstruction using deep vein |
| Step 9: Managing the duodenum |
| Step 10: Finishing touches |
Step 2: Deep vein preparation (day 1)
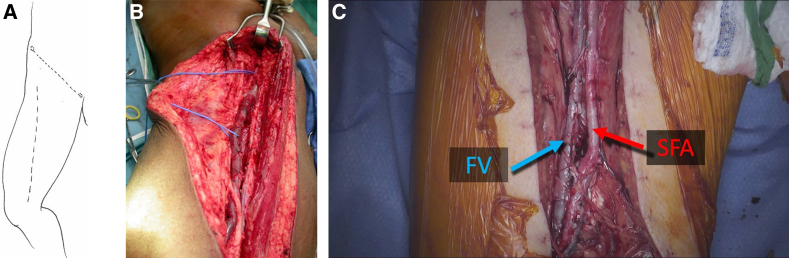
The patient is prepped from the xiphoid to the feet bilaterally. A longitudinal incision is made extending from the upper thigh to near the medial condyle along the course of the sartorius muscle (Fig 1). The dissection is continued with electrocautery through the subcutaneous tissue. The deep fascia overlying the femoral vessels is opened in the upper one-third of the thigh. The femoral vein is identified and completely exposed longitudinally along the anterior aspect before attempting circumferential control. Care is taken to avoid injury or excessive traction on the superficial femoral artery (SFA). The saphenous nerve accompanies the SFA and femoral vein in the proximal thigh and then courses thru the adductor canal to join the saphenous vein near the knee. The required length of the exposed vein is determined by the planned reconstruction. The femoral vein may be duplicated through all or part of its course. The junction with the deep femoral vein is identified and must be preserved to avoid severe postoperative edema. Dissection may be carried distally as far as the knee joint, but may be associated with more post operative edema than if dissection is terminated at the adductor canal. Small tributaries are ligated with 4-0 silk and larger side-branches are suture ligated with 5-0 polypropylene suture. Side branches are left relatively long (>5 mm) to prevent sutures from popping off with arterial pressure. As the vein courses through the adductor canal, the number and complexity of side branches requires particular attention to avoid injury to the femoral vein or SFA.
Fig 1.
(A) The dotted line shows the course of the sartorious muscle, which serves as a guide for the skin incision. (B) The thigh incision extends from near the anterior superior iliac spine to the medial condyle. (C) The right femoral vein (FV) and the adjacent superficial femoral artery (SFA) are labelled. The number and complexity of venous tributaries increases as the vein traverses the adductor canal. Small side-brances are ligated with 4-0 silk and larger tributaries (>3-4 mm) are suture-ligated with 5-0 polypropylene.
If the distal anastomoses are planned at the femoral level, the leg incisions may be extended proximally to the inguinal ligament. The common femoral artery, deep femoral artery, and SFA are exposed. Care is taken to avoid injury to the deep femoral artery and the medial femoral circumflex artery because these arteries provide blood supply to the gracilis muscle, which may be used if muscle flap coverage of infection involving the femoral vessels is encountered. Once the vein is adequately exposed and all relevant tributaries ligated and divided, the wound is loosely closed with skin staples over a 19F fluted suction drain. Postoperatively, sequential compression stockings are used to reduce the risk of venous thrombosis. Patients may be extubated after stage one but remain in the surgical intensive care unit overnight.
Step 3: Abdominal exploration (day 2)
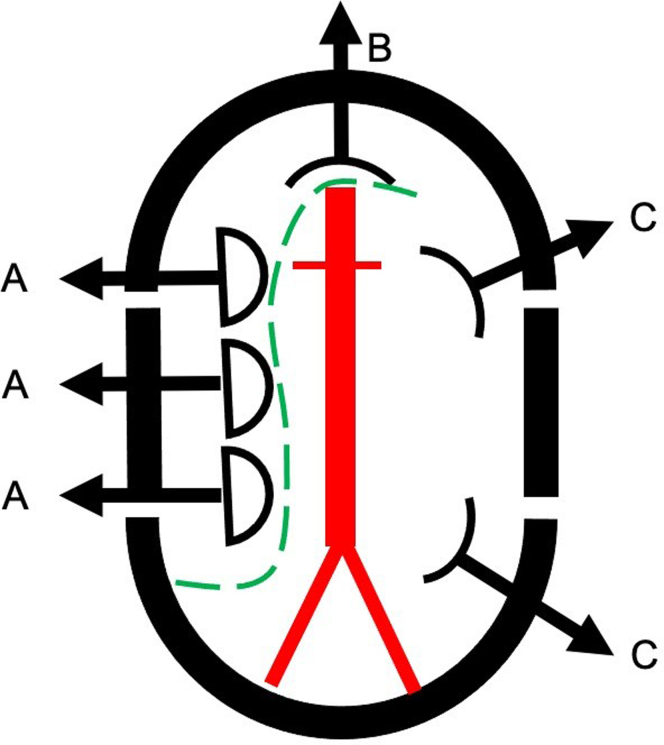
The patient is returned to the operating room early on day 2. After anesthesia is induced, the patient is prepped from the xiphoid to the feet bilaterally. The mid abdomen is aligned with the table break to allow flexion of the table. An Ioban abdominal adhesive pouch (3M, Saint Paul, MN) is positioned so that both the abdominal and femoral incisions are included. A long midline abdominal incision is used with care to avoid incisional hernias. Intra-abdominal adhesions are divided sharply before setting up the Bookwalter retractor. The operating table is gently flexed to decrease the depth of the abdominal exposure and the Bookwalter segmented ring is positioned close to but not in contact with the abdominal wall. The small bowel is retracted to the right and the transverse colon is retracted cranially and carefully packed away in an S-shaped configuration (Fig 2).
Fig 2.
The segmented Bookwalter ring is preferred. The retractors are arranged as illustrated. Three malleable retractors (A) are placed on the right side and retract the small bowel and right colon. (B) A straight, long retractor is used elevate the pancreas and retract the superior mesenteric artery and transverse colon. (C) Two short body wall retractors are used to compress and retract the left kidney and sigmoid colon. The green dotted line indicates the position of a single marked towel that controls the cecum, small bowel, and transverse colon.
Step 4: Aortic exposure
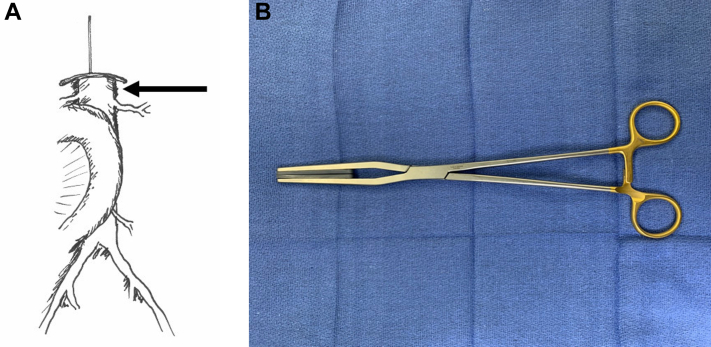
In the setting of an aortoduodenal fistula or erosion, the fourth portion of the duodenum is typically firmly adherent to the anterior surface of the aorta and should be left undisturbed until proximal aortic control is established. The selection of the position of the proximal aortic clamp is the first critical intraoperative decision. If there is suprarenal fixation of the endograft (eg, Cook Zenith, Cook Medical, Bloomington, IN) then aortic control must be obtained at the level of the diaphragm. If the endograft does not have suprarenal fixation (eg, Gore Excluder, W. L. Gore & Associates, Flagstaff, AZ), it is usually possible to expose the suprarenal aorta through a small window above the duodenum by gentle elevation of the pancreas and superior mesenteric artery (Fig 3). The left renal vein may be divided and suture-ligated to improve exposure of the aorta taking care to preserve the gonadal and adrenal tributaries. (We never try to reanastomose the left renal vein because the ends are very difficult to approximate after transection.) The dissection of the aorta proximal to the renal arteries is limited to creating small channels on each side of the aorta sufficient to accommodate the padded jaws of a straight aortic clamp (eg, Fogarty SoftJaw) (Fig 3, B). Care should be taken to avoid injury to small accessory renal or adrenal arteries that may not be identified on preoperative imaging but which can be the source of troublesome bleeding. (Although theoretically aortic balloon occlusion can be considered, in practice it is usually excessively cumbersome and much less appealing in reality than it seems on a whiteboard).
Fig 3.
(A) The fourth portion of the duodenum is adherent to the anterior surface of the aorta/graft in the setting of aortoduodenal fistula and should generally be left undisturbed until proximal aortic control can be established. If the endograft does not have a suprarenal component, it is usually possible to obtain aortic control with a vertical suprarenal aortic clamp. (B) Soft jaw aortic clamp (V Mueller, Berlin, Germany).
In the setting of an infected aortic endograft, the external and internal iliac arteries are exposed and controlled. On the right side, this goal is accomplished by simply dividing the peritoneum overlying these vessels in continuity with exposure of the aorta. Care should be taken to avoid injury to the iliac veins and ureter which usually crosses the distal third of the common iliac artery. (We frequently employ ureteral stents in the setting of infected aortoiliac or aortofemoral grafts to prevent inadvertent ureteral injury.) On the left side, it may be preferable to mobilize the sigmoid colon medially to expose the left internal and external iliac arteries.
Step 5: Deep vein harvest and graft creation
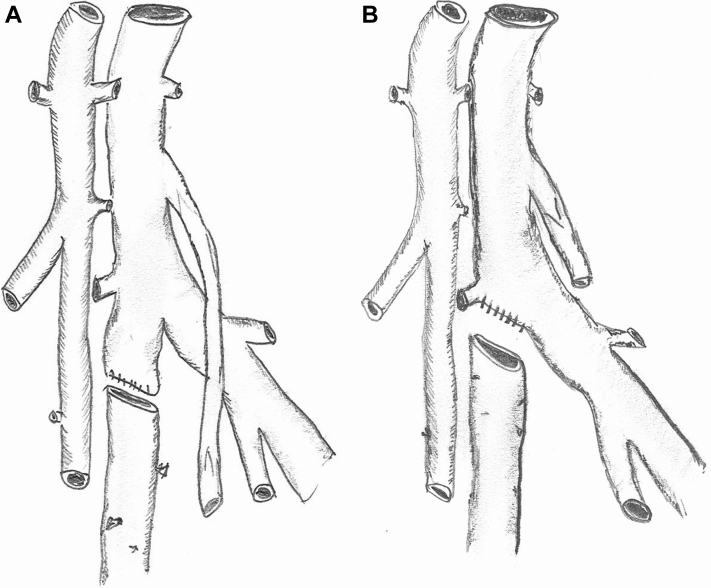
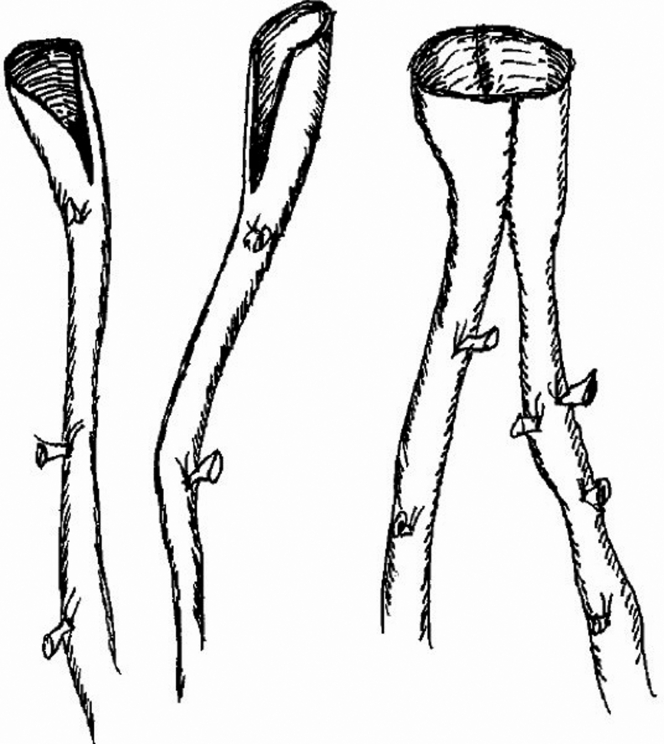
At this point, it is usually possible to determine the configuration of the planned arterial reconstruction and prepare the vein grafts. An umbilical tape or silk suture is used to measure the total length of femoral vein that will be required for arterial reconstruction. Each femoral vein is suture ligated and divided at the caudal end first. At the cephalad end, the femoral vein is suture ligated flush with the deep vein in a tapered fashion to avoid a blind pouch that could lead to venous thrombosis (Fig 4). Given the potential size mismatch between the aneurysmal aorta and a single femoral vein, we usually use a pantaloon vein graft composed of two symmetrical veins (Figs 5 and 6). If the aorta is too large for a pantaloon graft, there are a number of other options, including decreasing the circumference with an anterior aortic arterioplasty (Fig 5). (For patients with occlusive disease, especially women with smaller caliber vessels, a single femoral vein can typically be anastomosed directly to the aorta in an end-to-end configuration and end-to-side at the common femoral artery level. In these cases, an asymmetrical bifurcated graft is created by a cross-over graft arising from a convenient site on the donor graft limb.)
Fig 4.
(A) The long, blind stump of the femoral vein at the junction with the profunda femoris vein should be avoided. (B) Preferred configuration eliminates the blind stump and reduces the risk of deep vein thrombosis.
Fig 6.
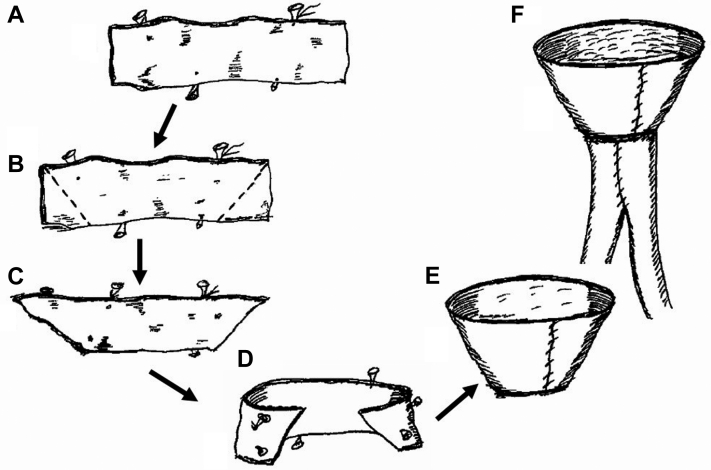
(A) A truncated cone can be created with a short segment of spare vein to greatly increase the circumference of the neoaortoiliac system (NAIS) conduit. A short segment of available donor vein is opened longitudinally. (B) The ends are trimmed to create a (C) trapezoid. The ends of the trapezoid (D) are sutured together (E) to create a collar, which is attached (F) to the pantaloon graft.
Fig 5.
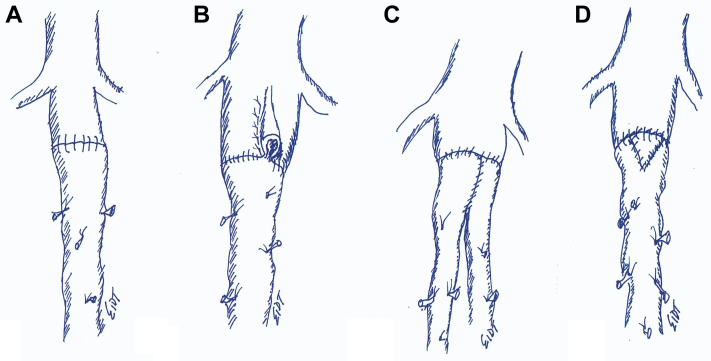
Modifications that can be used to obtain an appropriate size-match between the femoral vein (FV) and the aorta. (A) The most common configuration is a straight end-to-end anastomosis between the FV and the aorta. (B) Longitudinal aortoplasty. (C) The pantaloon configuration is used to accommodate a larger range of aortic diameters. (D) A V-shaped venoplasty can be used to increase the circumference of the FV graft.
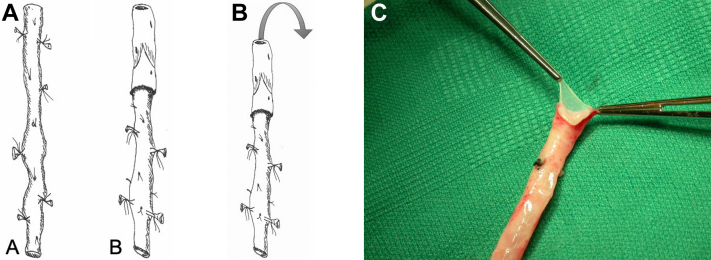
To create the simple pantaloon graft on the back-table, each vein segment is completely inverted and the valves are excised under direct vision with fine scissors (Fig 7). The largest end of each vein is positioned to create a single common channel (Fig 8). A longitudinal venotomy is made in each vein for a length of approximately 1.5 to 2.0 cm. The apex of each venotomy is sutured together with 5-0 monofilament polypropylene suture. The suture is continued up both sides of the V-shaped anastomosis and tied. Traction sutures may be used to simplify the procedure. The vein grafts are tested for leaks by gentle hydrodilation. It is critical to have the vein graft fully prepared and ready for immediate use before exposing the aortoduodenal fistula to reduce the duration of suprarenal clamping. (It is almost always necessary to place the aortic clamp in a suprarenal location, even if preoperative imaging suggests an adequate infrarenal neck. A suprarenal clamp allows the surgeon to safely obtain circumferential mobilization of the infrarenal segment of the aorta which simplifies the aortic anastomosis.)
Fig 7.
(A) The deep vein is usually used in a nonreversed translocated configuration. (B) The vein is completely inverted. (C) The valves are lysed under direct vision with scissors. Some surgeons prefer to avoid inversion and use a valvulotome to lyse the valves.
Fig 8.
To create a pantaloon graft, a 1.5- to 2.0-cm venotomy is made in the larger end of two veins. Beginning at the apex of the venotomy, the two sides are sutured using 5-0 monofilament suture to create a single lumen that can accommodate most aortas.
Step 6: Opening the aortoduodenal fistula
Before mobilizing the duodenum, the aortic clamp is positioned at the level of the suprarenal aorta, but not clamped. We usually enlist an assistant to stabilize the clamp firmly against the vertebral column should urgent aortic control be required. The fourth portion of the duodenum is mobilized and the fistula between the aorta, and the duodenum is exposed (Fig 9). It is uncommon to encounter catastrophic bleeding with exposure of the anterior surface of the aorta unless there is a significant endoleak. Direct finger pressure is usually sufficient to control bleeding from the anterior surface of the aorta. The hole in the bowel is easily recognized by the bile staining and the pouting edges of the duodenal mucosa. The duodenotomy should be closed temporarily with a simple purse-string suture pending definitive repair after vascular reconstruction is complete.
Fig 9.
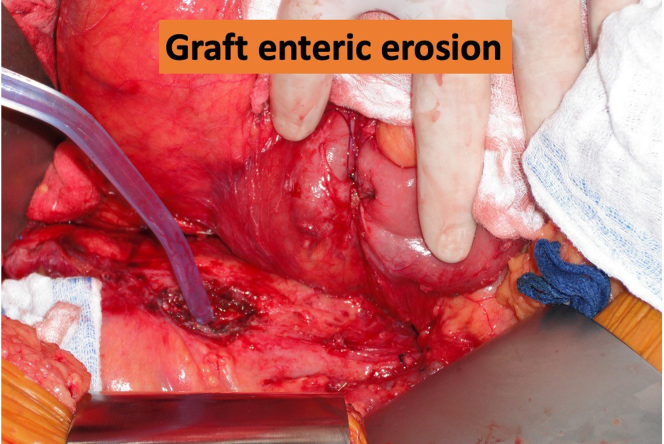
In the intraoperative photo, the head is to the right. The duodenum has been mobilized by the surgeon’s hand and the duodenal erosion has been temporarily closed with a purse-string suture. The tip of the suction is probing a full-thickness erosion in the anterior surface of the aorta that contains the infected endograft.
Step 7: Aortic endograft extraction
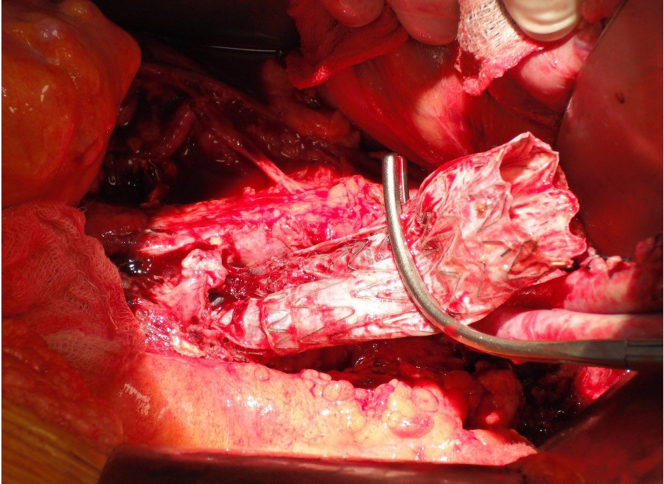
Systemic heparin is administered at a dose of 100 U/kg to achieve an activated clotting time of more than 200 seconds. Mannitol is frequently administered before aortic clamping.16 The aorta and iliac arteries are cross clamped. The aorta is opened longitudinally and the infected endograft is completely removed, including both iliac limbs (Figs 10 and 11). A variety of techniques have been proposed to remove suprarenal components including the use of reconstraining hoops, rings, and sleeves, or piecemeal removal with wire cutters.17 In cases with robust suprarenal fixation, it may be appropriate to leave the metallic suprarenal component and incorporate it into the proximal anastomosis, but we completely excise all endograft fabric because of its capacity to harbor infectious agents. Forceful extraction of barbed suprarenal components should be condemned owing to the risk of catastrophic injury to the visceral aorta. Samples of the aortic wall are submitted for Gram staining and cultures, including fungal. Obviously infected or necrotic tissue should be debrided, but we do not completely excise the aortic wall if it seems to be viable because it serves as an important protective barrier around the deep vein graft. In the setting of severe suppurative infection, the endograft will slide out without any resistance. In some cases, the endograft body and, more commonly, the limbs may be somewhat incorporated and require gentle traction for extraction. We do not attempt to preserve the endograft iliac limbs and suture directly to them in the setting of graft infection as one might in the setting of noninfectious, progressive aortic enlargement owing to endoleak.
Fig 10.
The infected endograft is completely excised including removal of both iliac limbs.
Fig 11.
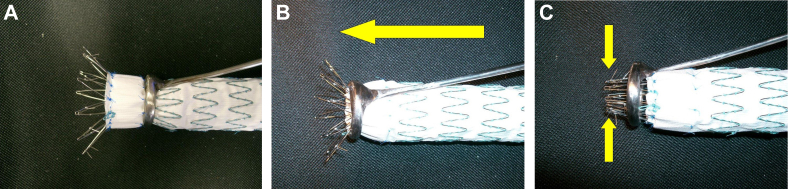
These custom-made endograft extractors were designed specifically to remove the suprarenal component of selected endografts. (A) The ring is positioned around the body of the graft and (B) advanced in a cranial direction until the suprarenal component is (C) completely disengaged from the aortic wall. Others have suggested the use of the barrel of an appropriate syringe in a similar maneuver. (See17 for use of syringe barrel technique for safe removal of an aortic endograft with suprarenal fixation.)
Step 8: Aortic reconstruction using deep vein
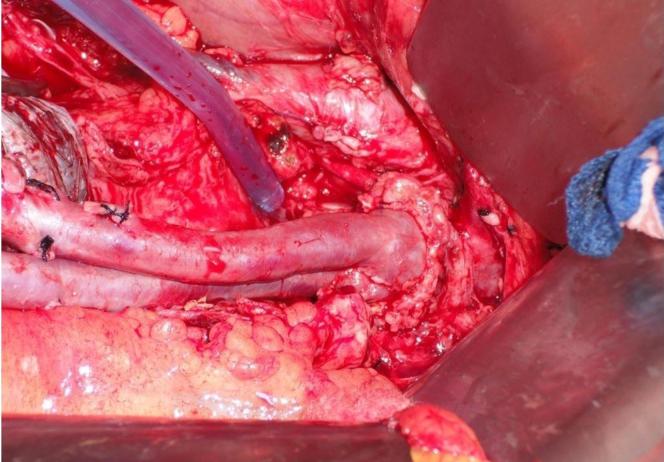
The pantaloon graft limbs are oriented to avoid a confluence of suture lines in the middle (6 o’clock) of the back wall; the limbs are over and under rather than side by side (Fig 12). The vein graft is remarkably adaptable to a wide range of aortic diameters and significant suture line bleeding is uncommon. 4-0 polypropylene suture on a small needle (RB-1) is preferred but a larger needle (eg, SH or MH) and suture combination may be necessary, depending on the thickness of the aortic wall and surrounding inflammation. Once the proximal anastomosis is complete, the limbs are pressurized and checked for leaks. The distal anastomoses are typically performed at the external iliac level in an end-of-vein to side-of-artery configuration. The common iliac arteries are oversewn with 3-0 sutures.
Fig 12.
The completion photo shows the proximal anastomosis of a deep vein pantaloon neoaortoiliac system (NAIS). Note the legs are intentionally rotated to a vertical orientation to reduce the risk of suture line bleeding in the middle of the back wall.
Step 9: Managing the duodenum
We typically meet with the general surgery team preoperatively and review the options for duodenal repair (primary repair vs duodenal resection), the importance of preserving the omentum for vascular graft coverage and the need for G-J tube placement. The most important objective in managing the duodenal injury is to achieve complete mobilization of the affected bowel and meticulous closure of the duodenotomy. (A postoperative fistula in a patient that does not have an infected aorta can be easily managed with percutaneous drainage and parenteral nutrition. But in the vascular patient, a leak from the gastrointestinal reconstruction is an ominous event that is commonly lethal.) If there is any difficulty performing a primary repair of the duodenum, it is preferable to resect the third and fourth portions of the duodenum and perform a primary anastomosis between the jejunum and the second part of the duodenum. A G-J tube is usually inserted under direct vision and the tip of the catheter is positioned in the proximal jejunum distal to the duodenal repair to allow early initiation of elemental tube feeding. Nutritional support is essential in these ill patients and a positive nitrogen balance is critical to the restoration of immune function and wound healing.
Step 10: Finishing touches
At the end of the procedure, all suture lines are inspected for bleeding. The most common causes of suture line bleeding are (1) loose suture, (2) excessive distance between bites (ie, a pleat), and (3) needle hole lacerations in the aortic wall owing to not following the curvature of the needle. The extremity circulation is checked. Most patients do not have palpable pedal pulses at this point but should have audible Doppler signals. The femoral pulses should be palpable, and the procedure should not be terminated until an investigation of absent femoral pulses is completed. If there is any indication that the compartments of the lower leg have elevated intracompartmental pressures (ie, they feel tight), a four-compartment fasciotomy is appropriate. Significant predictors of the need for fasciotomy are preoperative lower extremity ischemia and the length of harvested femoral vein.18 The abdomen is irrigated vigorously with warm saline to minimize adverse consequences of enteric spillage. An omental flap is inserted to create a barrier between the gastrointestinal tract and the vascular reconstruction.19 The abdomen is closed in routine fashion unless there are concerns for abdominal compartment syndrome, in which case the open abdomen is managed with a negative pressure dressing.
Postoperative care
Broad-spectrum antibiotics are started after culture samples have been obtained. Initially, we recommend the combination of intravenous linezolid, piperacillin/tazobactam, and micafungin for broad coverage and virtual absence of nephrotoxicity. Subsequent antibiotic coverage is based on culture results and continued for a period of at least 6 weeks. Because all synthetic, infected material is excised, life-long suppressive antibiotics are not typically required after the initial course is completed.
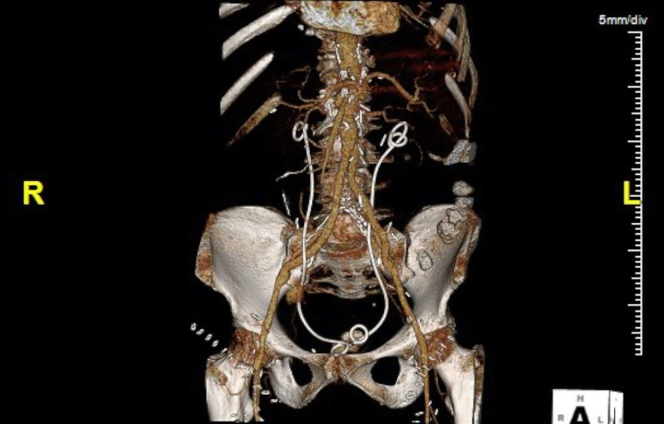
Postoperative cross-sectional imaging is obtained during the initial hospitalization, 4 to 6 weeks after discharge, and then annually (Fig 13). Sequential compression stockings are applied to the lower legs and the foot of the bed is elevated to reduce postoperative edema. Pharmacological deep vein thrombosis prophylaxis is started 12 to 24 hours postoperatively. Enteral feedings with an elemental diet are started in the early postoperative period through the jejunostomy port and gastric port is left open to gravity to prevent gastric dilatation. The drains in the thigh wounds are left in place until the output is less than 30 mL/24 hours, which may take several days owing to the inevitable disruption of lymphatic channels with vein harvesting.
Fig 13.
This postoperative computed tomography scan shows a completed NAIS procedure for infected abdominal endograft. Note ureteral stents in place.
Conclusions
Aortoduodenal fistula after EVAR is an uncommon but ominous diagnosis that requires expert surgical care to achieve a satisfactory outcome. With careful preparation, the NAIS is an effective and durable treatment for aortoduodenal fistula and a variety of other aortic conditions. Postoperative reinfection is uncommon and venous morbidity is remarkably mild in most cases. We hope that this 10-step outline can be used by novice and veteran surgeons alike in managing these complex patients.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Clagett G.P., Bowers B.L., Lopez-Viego M.A., Rossi M.B., Valentine R.J., Myers S.I., et al. Creation of a neo-aortoiliac system from lower extremity deep and superficial veins. Ann Surg. 1993;218:239–249. doi: 10.1097/00000658-199309000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schramel R.J., Creech O. Effects of infection and exposure on synthetic arterial prostheses. AMA Arch Surg. 1959;78 doi: 10.1001/archsurg.1959.04320020093013. [DOI] [PubMed] [Google Scholar]
- 3.Seeger J.M., Pretus H.A., Welborn M.B., Ozaki C.K., Flynn T.C., Huber T.S. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg. 2000;32:451–461. doi: 10.1067/mva.2000.109471. [DOI] [PubMed] [Google Scholar]
- 4.Nevelsteen A., Lacroix H., Suy R. The superficial femoral vein as autogenous conduit in the treatment of prosthetic arterial infection. Ann Vasc Surg. 1993;7:556–560. doi: 10.1007/BF02000150. [DOI] [PubMed] [Google Scholar]
- 5.Nevelsteen A., Lacroix H., Suy R. Autogenous reconstruction with the lower extremity deep veins: an alternative treatment of prosthetic infection after reconstructive surgery for aortoiliac disease. J Vasc Surg. 1995;22:129–134. doi: 10.1016/s0741-5214(95)70106-0. [DOI] [PubMed] [Google Scholar]
- 6.Schulman M.L., Badhey M.R., Yatco R. Superficial femoral—popliteal veins and reversed saphenous veins as primary femoropopliteal bypass grafts: a randomized comparative study. J Vasc Surg. 1987;6:1–10. doi: 10.1067/mva.1987.avs0060001. [DOI] [PubMed] [Google Scholar]
- 7.Clagett G.P. Fischer’s Mastery of Surgery. 6th ed. Wolters-Kluwer Health; Philadelphia, PA: 2012. Creation of a neoaortoiliac system (NAIS procedure) for the treatment of infected aortic grafts. [Google Scholar]
- 8.Chung J., Clagett G.P. Neoaortoiliac System (NAIS) procedure for the treatment of the infected aortic graft. Semin Vasc Surg. 2011;24:220–226. doi: 10.1053/j.semvascsurg.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Simmons C.D., Ali A.T., Foteh K., Abate M.R., Smeds M.R., Spencer H.J., et al. Unilateral inline replacement of infected aortofemoral graft limb with femoral vein. J Vasc Surg. 2017;65:1121–1129. doi: 10.1016/j.jvs.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 10.Ali A.T., Modrall J.G., Hocking J., Valentine R.J., Spencer H., Eidt J.F., et al. Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg. 2009;50:30–39. doi: 10.1016/j.jvs.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Beck A.W., Murphy E.H., Hocking J.A., Timaran C.H., Arko F.R., Clagett G.P. Aortic reconstruction with femoral-popliteal vein: graft stenosis incidence, risk and reintervention. J Vasc Surg. 2008;47:36–43. doi: 10.1016/j.jvs.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Ali A.T., McLeod N., Kalapatapu V.R., Moursi M.M., Eidt J.F. Staging the neoaortoiliac system: feasibility and short-term outcomes. J Vasc Surg. 2008;48:1125–1130. doi: 10.1016/j.jvs.2008.06.067. discussion: 1130-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith S.T., Timaran C.H., Hayes D.J., Arko F.R., Modrall J.G., Valentine R.J., et al. RR22. Neoaortoiliac System (NAIS) for failed aortic reconstructions: a durable solution in young patients. J Vasc Surg. 2009;49:S53. [Google Scholar]
- 14.Mitchell E.L., Arora S., Moneta G.L., Kret M.R., Dargon P.T., Landry G.J., et al. A systematic review of assessment of skill acquisition and operative competency in vascular surgical training. J Vasc Surg. 2014;59:1440–1455. doi: 10.1016/j.jvs.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Eidt J.F. The aviation model of vascular surgery education. J Vasc Surg. 2012;55:1801–1809. doi: 10.1016/j.jvs.2012.01.080. [DOI] [PubMed] [Google Scholar]
- 16.Teter K., Rockman C.B., Patel V.I., Chang H., Jacobowitz G.R., Gelb B., et al. Mannitol use is renal protective in patients with chronic kidney disease requiring suprarenal aortic clamping. J Vasc Surg. 2021;74:e214–e215. doi: 10.1016/j.avsg.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Koning O.H.J., Hinnen J.W., van Baalen J.M. Technique for safe removal of an aortic endograft with suprarenal fixation. J Vasc Surg. 2006;43 doi: 10.1016/j.jvs.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Modrall J.G., Sadjadi J., Ali A.T., Anthony T., Welborn M.B., Valentine R.J., et al. Deep vein harvest: predicting need for fasciotomy. J Vasc Surg. 2004;39:387–394. doi: 10.1016/j.jvs.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Oderich G.S., Bower T.C., Cherry K.J., Panneton J.M., Sullivan T.M., Noel A.A., et al. Evolution from axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J Vasc Surg. 2006;43:1166–1174. doi: 10.1016/j.jvs.2006.02.040. [DOI] [PubMed] [Google Scholar]