Abstract
The search for appropriate vaccine candidates and drug targets against onchocerciasis has so far been confronted with several limitations due to the unavailability of biological material, appropriate molecular resources, and knowledge of the parasite biology. To identify targets for vaccine or chemotherapy development we have undertaken two approaches. First, cDNA expression libraries were constructed from life cycle stages that are critical for establishment of Onchocerca volvulus infection, the third-stage larvae (L3) and the molting L3. A gene discovery effort was then initiated by random expressed sequence tag analysis of 5,506 cDNA clones. Cluster analyses showed that many of the transcripts were up-regulated and/or stage specific in either one or both of the cDNA libraries when compared to the microfilariae, L2, and both adult stages of the parasite. Homology searches against the GenBank database facilitated the identification of several genes of interest, such as proteinases, proteinase inhibitors, antioxidant or detoxification enzymes, and neurotransmitter receptors, as well as structural and housekeeping genes. Other O. volvulus genes showed homology only to predicted genes from the free-living nematode Caenorhabditis elegans or were entirely novel. Some of the novel proteins contain potential secretory leaders. Secondly, by immunoscreening the molting L3 cDNA library with a pool of human sera from putatively immune individuals, we identified six novel immunogenic proteins that otherwise would not have been identified as potential vaccinogens using the gene discovery effort. This study lays a solid foundation for a better understanding of the biology of O. volvulus as well as for the identification of novel targets for filaricidal agents and/or vaccines against onchocerciasis based on immunological and rational hypothesis-driven research.
Onchocerciasis, or river blindness, is the second leading cause of infectious blindness in humans. According to the World Health Organization, an estimated 18 million people are infected with the parasite, with over 1 million at risk of visual impairment (79). Ivermectin was shown to be both safe and effective in the treatment of onchocerciasis and has become the drug of choice for mass distribution (79). However, ivermectin is only effective against microfilariae released into the skin, and prolonged annual ivermectin therapy of up to 10 to 15 years is required for clearance of onchocerciasis from a human population (63). The potential development of drug-resistant strains of the parasite also demands the identification of alternative drug candidates for onchocerciasis control (67). The number of suitable targets for chemotherapy that have been identified in filarial and other parasitic nematodes is low, due in part to an inadequate understanding of the basic biology of these parasites. Ivermectin, as well as the other commonly used drugs, does not exploit known targets in the filarial parasites and was discovered by chance. Previous research has centered on important metabolic processes such as energy metabolism and nucleotide synthesis (75). However, nonmetabolic processes are also important either for parasite survival within the host or for propagation. Filarial nematodes do not multiply in the definitive host but molt, grow, and mature for a period following infection, after which they devote their energy almost entirely to microfilaria production. None of the proteins involved in these processes have yet been explored as possible drug targets.
An additional tool in the control of onchocerciasis would be the development of a prophylactic vaccine. One essential step in the development of immunoprophylaxis is the identification and immunochemical characterization of potential vaccine candidates that play a role in stimulating protective host immunity. There is mounting evidence that naturally acquired immunity against Onchocerca volvulus infection can occur in humans (20). Additionally, work in animal models suggests that the protective immune responses are directed at incoming infective third-stage larvae (L3) (37, 45, 62, 72). Interestingly, studies from animal models of filarial infections suggest that protective immune responses may inhibit the growth, development, and molting of the L3 to L4 (19, 37, 72). This suggests that molting L3 (mL3) proteins as well as excretory-secretory (ES) products are an important source of protective antigens (19, 46). Serum samples from O. volvulus putatively immune (PI) individuals and protected animals recognized similar antigens present only in day 2 extracts and ES products of molting larvae (32).
Due to the paucity of parasite material, construction of cDNA expression libraries and molecular cloning approaches are important methods for isolating and characterizing protein antigens. Immunoscreening of cDNA libraries, constructed from adult worms (18) and more recently from L3 (SAW94WL-OvL3), using polyclonal antibodies has resulted in the identification of more than 50 O. volvulus antigens (http://helios.bto.ed.ac.uk/mbx/fgn/OnchoNet/onchotable1.html). About 10 of the proteins are also present in larval stages of O. volvulus, some of which have been shown to confer partial protection against L3 challenge in surrogate rodent models of onchocercal infections (34, 39, 70, 71). However, there is still a need for the identification of novel larval proteins, particularly from the mL3 stage, that may prove to be better candidates for protective immunity alone or in combination with other molecules.
In an attempt to identify, clone, and characterize novel drug targets and vaccine candidates from the infective and molting larval stages of O. volvulus, we developed a bipartite program. (i) An expressed sequence tag (EST) project was undertaken to survey gene expression in both L3 and mL3 stages of the parasite life cycle. The thousands of new genes cloned in this effort and their expression profiles have been used to identify a set of potentially interesting vaccine and drug target candidates. (ii) The mL3 cDNA expression library was screened using a pool of sera from O. volvulus PI individuals. We describe the results of this effort, which has led to the identification of potential targets for drug and vaccine development and provided new information about genes that are highly expressed at these critical stages of the parasite life cycle.
MATERIALS AND METHODS
O. volvulus cDNA library construction.
All parasite material was prepared in the Tropical Medicine Research Station, Kumba, Cameroon. L3 were obtained from flies 7 days after infection with skin microfilariae. To obtain molting larvae, freshly dissected L3 were cultured in vitro in the presence of a 1:1 mixture of Iscove's modified Dulbecco's medium and NCTC-135, 20% fetal calf serum, and antibiotic-antimycotic solution (Life Technologies, Gaithersburg, Md.) for 3 days at 37°C. Larvae were collected after 1, 2, or 3 days in culture, washed in Tris-EDTA buffer and then snap-frozen in liquid nitrogen. Ultrastructural examination by electron microscopy confirmed that these cultured larvae had started the molting process, as the separation between the cuticle of L3 and the newly synthesized cuticle of L4 was evident in some of the cross sections (data not shown).
Total RNA was isolated separately from 9,000 vector-derived L3 and 6,000 mL3. The mRNA from each parasite stage was fractionated using the MicroFast Track mRNA isolation kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Individual unidirectional cDNA libraries were constructed using the Lambda Uni-ZAP XR cDNA synthesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions with the exception that drop dialysis against double-distilled water was performed instead of ethanol precipitation. Following ligation into the vector, the mixture was packaged using Gigapak III Gold Packaging Extract (Stratagene). Dilutions of the primary library were then plated out, and the titer and percentage of nonrecombinants were determined. The L3 cDNA library (SAW94WL-OvL3) had 1.8 × 105 independent recombinants with an average insert size of 900 bp. The mL3 cDNA library (SL96MLW-OvmL3) had 1.1 × 106 independent recombinants with an average insert size of 1,200 bp.
PCR and sequence analysis of ESTs.
Randomly selected individual plaques from the L3 and mL3 cDNA libraries were transferred into 50 μl of SM buffer (0.1 M NaCl, 50 mM Tris-HCl [pH 7.5], 8 mM MgCl2, 0.01% gelatin). To amplify the individual cDNA inserts, PCR was carried out on 5-μl aliquots of each plaque with T3 and T7 vector primers. Thirty cycles of amplification were performed under the following conditions: 94°C for 30 s, 55°C for 30 s, and 72°C for 60 s. The PCR products were purified and then sequenced using the 5′ SK vector primer and a 9600 Thermal Cycler (PE Biosystems, Foster City, Calif.). Purified sequencing reactions were resuspended in loading buffer and run on a 377 automated DNA sequencer (PE Biosystems). Sequences were edited to remove the vector sequences and poly(A) tails, and 2,935 L3 and 2,571 mL3 EST sequences were then submitted to the GenBank EST database.
Clustering and analysis of O. volvulus ESTs.
All the sequences used in this analysis are publicly available through the National Center for Biotechnology Information dbEST (http://www.ncbi.nlm.nih.gov/irx/dbST/dbest_query.html) or the nonredundant databases. Before clustering, clones representing rRNA or contaminating Escherichia coli DNA were removed from the starting datasets by BLASTN comparison against the fully sequenced Brugia malayi 18S, 28S rRNA (M. L. Blaxter, personal communication) and the E. coli genome, respectively. All clones with BLAST probability scores of e−50 or less to either of these datasets were eliminated from the subsequent analysis. EST clusters were then generated using a process that incrementally compares EST sequences against a local database using the BLASTN algorithm (1). If an EST did not match any other sequence in the database with a BLASTN probability score of e−50 or less, it was then given a unique cluster number, beginning with OVC00001, and added to the database. When an EST matched one or more ESTs already in the database it was assigned to that corresponding cluster. If two or more clusters had to be merged, the cluster with the lowest number took precedence. Each cluster, along with its member ESTs and abundance profiles were exported into a FileMaker Pro database (Filemaker, Inc., Santa Clara, Calif.) Consensus sequences for the clusters were generated using PHRAP (provided courtesy of P. Green with minor modifications by S. J. Jones) or AssemblyAlign (Oxford Molecular, Oxford, United Kingdom). The major open reading frames for consensus sequences were then predicted using MacVector (Oxford Molecular) and analyzed using PSORT (54) (http://psort.nibb.ac.jp/) to determine if the predicted amino acid sequence contained a potential secretory leader (SECL+). In addition, ESTs or consensus sequences were used in BLASTX or TBLASTX searches of Genpep (GenBank's nonredundant protein database [http://www.ncbi.nlm.nih.gov:80/blast]), wormpep19 (>19,000 genes predicted from the Caenorhabditis elegans genome [http://www.sanger.ac.uk/Projects/C_elegans /wormpep/]), and a database constructed from clustered ESTs sequenced from the closely related lymphatic filarial nematode B. malayi to identify homology with other known genes. Clusters representing genes that are possible drug targets (such as proteinases and receptors) or otherwise interesting were identified using text-based searches of dbEST (http://www.ncbi.nlm.nih.gov/irx/dbST /dbest_query.html).
Immunoscreening of the O. volvulus mL3 cDNA expression library using a pool of sera from PI individuals.
A pool of sera from individuals living in Ecuador and Liberia who were identified and classified as putatively immune to O. volvulus infection (29, 54) was used to immunoscreen 106 PFU of the amplified O. volvulus mL3 cDNA according to standard procedures. Prior to screening the libraries, and in order to reduce redundancy, the serum pool was depleted of antibodies by affinity chromatography against recombinant O. volvulus antigens previously cloned and characterized in S. Lustigman's laboratory. Recombinant antigens which were isolated by immunoscreening of L3, L4, or adult female cDNA libraries using rabbit anti-L3 and -L4 (Ov9M, Ov-10-1, and Ov6-5) or PI sera from Liberia and Ecuador (Ov-CPI-2, Ov-GRP-1, Ov-RAL-2, Ov-API-1, Ov-ALT-1, Ov-GBP-2, Ov-FBA-1, Ov-ASP-1, Ov-CPL-1, OvB8, OvB95, and Ov103) were used for depletion as described (32). pBluescript plasmid DNA from the mL3 immunoreactive clones was isolated by the rapid in vivo excision protocol according to the manufacturer's instructions (Stratagene). Plasmid DNA was then purified using the Concert Rapid Plasmid Purification System (Life Technologies). Nucleotide sequences were obtained using an ABI 373XL sequencer (PE Biosystems). Sequence analysis was performed using the Vector NTI Suite (Informax Inc., Bethesda, Md.) software and World Wide Web interface.
Expression of recombinant proteins.
The open reading frames of the cDNA clones isolated by immunoscreening were inserted into the multiple-cloning site of the pProEX HT expression vector (Life Technologies), designed for the expression of hexahistidine-tagged fusion proteins in E. coli DH5α. Following induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), bacterial cells were harvested and lysed in B-PER reagent (Pierce, Rockford, Ill.). Both soluble and insoluble E. coli fractions were tested for the presence of corresponding recombinant protein by Western blot analysis using the India HisProbe-HRP kit (Pierce). Recombinant proteins were purified either under nondenaturing conditions using the Xpress System Protein Purification (Invitrogen), or by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis using the PrepCell (Bio-Rad, Hercules, Calif.) according to manufacturers' instructions.
ELISA.
Individual human serum samples from subjects living in the onchocerciasis endemic region of Kumba, Cameroon, were analyzed for reactivity with recombinant antigens by enzyme-linked immunosorbent assay (ELISA). Subjects who were positive for microfilariae in skin biopsies, with symptomatic or nonsymptomatic infections, and had never been previously treated for onchocerciasis were categorized as infected individuals (INF) (n = 21). Individuals who were negative for skin microfilariae, with no clinical history of infection, and who were negative in a PCR-based assay for an O. volvulus specific tandem repeat DNA in their skin biopsies (83) were classified as PI (n = 21) (74). ELISA was performed as described (36) using 50 to 100 ng of purified recombinant protein per well and a serum dilution of 1:200. The serum samples were blocked with E. coli extract (400 μg/ml; Promega, Madison, Wis.) prior to incubation with the antigen. Bound antibodies were detected by reaction with a 1:3,000 dilution of horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG) (Zymed, San Francisco, Calif.) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma, St. Louis, Mo.). Absorbance was measured at 450 nm (SpectraMax 190 ELISA Reader; Molecular Devices, Sunnyvale, Calif.). The control sera used were from healthy New York resident blood donors. Differences in the immunoglobulin levels between groups were compared using the two-tailed non-parametric Mann-Whitney U test. Results were considered statistically significant if the P value was less than 0.05.
RESULTS
Characteristics of the O. volvulus cDNA libraries.
After filtering for contaminating rRNA and E. coli sequences, analysis of 2,554 ESTs from the L3 cDNA library showed that 28% were novel to the public databases, 64% had significant similarity to proteins in GenBank, and 57% had significant similarity to ESTs sequenced from the lymphatic filarial nematode B. malayi. Of the ESTs, 40% had significant similarities to proteins in wormpep19 (>19,000 proteins predicted from the C. elegans genome), and 7% had similarities only to proteins in wormpep19. Analysis of 2,081 EST sequences from the mL3 cDNA library showed that 26% were novel to public databases, 63% had significant similarity to proteins in GenBank, and 56% had significant similarity to ESTs sequenced from B. malayi. 55% of the ESTs had significant similarities to proteins in wormpep19, and 11% had similarities only to proteins in wormpep19.
Gene expression profiles in O. volvulus L3 and mL3 larvae.
Analysis of the clustered O. volvulus ESTs and cross-comparison of the ESTs from the L3 and mL3 libraries revealed a differential distribution of ESTs in both datasets, as summarized in Table 1. We found genes within the present O. volvulus database that are differentially expressed and/or up-regulated in the L3 and mL3 stages relative to other larval (microfilaria and L2) and adult stages. Some of these genes encode previously characterized structural and housekeeping proteins found in all metazoans, such as actin, 26S proteasome subunits, and histones. Others are active components in the metabolism of the parasite such as glyceraldehyde-3-phosphate dehydrogenase and fructose-bisphosphate aldolase. Although these genes are not expected to be stage specific, the high metabolic activity of the L3 and mL3 may require their up-regulation during these stages of development relative to the adult stages. Several up-regulated genes in L3 and/or mL3 were previously characterized as antigens such as Ov-RAL-2, OvL3-1, beta-galactoside-binding lectin, and onchocystatin (7, 41, 47, 56). The biological or immunological functions of these genes in the parasite-host relationship have been the subject of much discussion. However, their high representation in these datasets supports the overall hypothesis that they are playing an important role in some aspect of the biology of L3 and/or mL3, such as adaptation to the host environment. The other L3 and/or mL3 up-regulated clusters represent genes that have not been characterized in filariae and many are either completely novel or only have similarity to genes predicted in the C. elegans genome (11).
TABLE 1.
Stage-specific or up-regulated genes in the L3 and mL3 EST datasetsa
| Categoryg | Clusterb | Representative EST | Cluster profile
|
Homology(ies) | |||
|---|---|---|---|---|---|---|---|
| Ac | Bd | Ce | Df | ||||
| UR or PSS (L3) | OVC00048 | SWOvL3CAN54C08SK | 358 | 5.70 | 317 | 20 | 18-kDa larval antigen, Ov64, Ov-alt-1, Ov-alt-2 |
| OVC00142 | SWOvL3CAN54A03SK | 70 | 1.11 | 59 | 9 | Onchocystatin, OvMBP/10, Ov7, Ov-cpi-2 | |
| OVC00237 | SWOvL3CAN54C06SK | 51 | 0.81 | 50 | 1 | Venom allergen antigen homologue, B93-RP, Ov-asp-1 | |
| OVC00109 | SWOvL3CAN52G06SK | 27 | 0.43 | 27 | 0 | Bm-alt-2 (B. malayi) | |
| OVC00092 | SWOvL3CAN60A11SK | 25 | 0.39 | 25 | 0 | Novel (SECL+) | |
| OVC00762 | SWOvL3CAN62B11SK | 20 | 0.31 | 17 | 3 | Cuticular collagen, Bm-col-2 (B. malayi) | |
| OVC00099 | SWOvL3CAN29A04SK | 17 | 0.27 | 16 | 1 | Troponin (B. malayi) | |
| OVC00339 | SWOvL3CAN28B11SK | 17 | 0.27 | 15 | 2 | Troponin, F42E11.4 (C. elegans) | |
| OVC00916 | SWOvL3CAN54F09SK | 16 | 0.25 | 16 | 0 | Cathepsin l-cysteine proteinase, Ov-cpl-1 | |
| OVC00079 | SWOvL3CAN62C06SK | 13 | 0.20 | 11 | 1 | Similar to M02D8.1 (C. elegans) | |
| OVC00634 | SWOvL3CAN46D10 | 10 | 0.15 | 8 | 2 | Globin (C. briggsae) | |
| OVC00496 | SWOvL3CAN50E11SK | 9 | 0.14 | 8 | 0 | Beta-galactoside-binding lectin, Ov-gbp-1 | |
| OVC00072 | SWOvL3CAN45D10 | 8 | 0.12 | 5 | 1 | Fructose-bisphosphate aldolase, Ov-fba | |
| OVC00485 | SWOvL3CAN57E02SK | 7 | 0.11 | 7 | 0 | Actin 1, Ov-act-1 | |
| OVC00704 | SWOvL3CAN01D09SK | 7 | 0.11 | 7 | 0 | LIM domain protein, OvL3-1 | |
| OVC01107 | SWOvL3CAN02H03SK | 7 | 0.11 | 5 | 1 | Novel | |
| OVC00129 | SWOvL3CAN61B04SK | 6 | 0.09 | 6 | 0 | Novel | |
| OVC00469 | SWOvL3CAN50A03SK | 6 | 0.09 | 5 | 1 | 26S subunit S12 | |
| OVC00712 | SWOvL3CAN60A04SK | 6 | 0.09 | 5 | 1 | Novel | |
| OVC00380 | SWOvL3CAN01H08SK | 5 | 0.08 | 5 | 0 | Cuticle collagen, F08G5.4 (C. elegans) | |
| OVC00707 | SWOvL3CAN52D10SK | 5 | 0.08 | 4 | 1 | Novel protein recognized by PI serum (SECL+) | |
| OVC00807 | SWOvL3CAN62A04SK | 5 | 0.08 | 5 | 0 | Cuticular collagen, Bm-col-2 (B. malayi) | |
| OVC00951 | SWOvL3CAN53C03SK | 5 | 0.08 | 4 | 1 | Peptidylglycine alpha-hydroxylating monooxygenase (Drosophila melanogaster) | |
| OVC01003 | SWOvL3CAN51H08SK | 5 | 0.08 | 5 | 0 | Novel | |
| OVC01239 | SWOvL3CA721SK | 5 | 0.08 | 4 | 1 | Novel (SECL+) G-, F-, P-, and Y-rich protein | |
| UR or PSS (mL3) | OVC00032 | SWOv3MCA1365SK | 85 | 1.35 | 6 | 79 | Cuticle collagen, F58F6.1 (C. elegans) |
| OVC00144 | SWOv3MCA756SK | 29 | 0.46 | 2 | 26 | Cuticle collagen, R09A8.4 (C. elegans) | |
| OVC00017 | SWOv3MCA025SK | 22 | 0.35 | 0 | 19 | Small serine proteinase inhibitor, Ov-spi-1 | |
| OVC00246 | SWOv3MCA2020SK | 22 | 0.35 | 0 | 22 | CUT-1 cuticlin (Ascaris lumbricoides) | |
| OVC00156 | SWOv3MCAM01G07SK | 12 | 0.19 | 0 | 12 | Novel (SECL+) | |
| OVC00572 | SWOv3MCAM03A04 | 12 | 0.19 | 0 | 11 | Novel (SECL+) | |
| OVC00441 | SWOv3MCA1532SK | 9 | 0.14 | 0 | 9 | CUT-1-like cuticlin (A. lumbricoides) | |
| OVC00130 | SWOv3MCAM07F08SK | 7 | 0.11 | 0 | 7 | Novel (SECL+) | |
| OVC00510 | SWOv3MCA1970SK | 7 | 0.11 | 0 | 7 | Ostionectin-like protein, Ov-ost-1 (C. elegans) | |
| OVC00699 | SWOv3MCAM11G07SK | 6 | 0.09 | 0 | 6 | Cuticle collagen, F17C8.2 (C. elegans) | |
| OVC00322 | SWOv3MCA717SK | 5 | 0.08 | 0 | 5 | Cystathionine gamma-lyase, F22B8.6 (C. elegans) | |
| OVC00364 | SWOv3MCAM02A11SK | 5 | 0.08 | 1 | 4 | SAR1 RAS-related protein, ZK180.4 (C. elegans) | |
| OVC00465 | SWOv3MCA218SK | 5 | 0.08 | 1 | 4 | Tumor protein homologue, Bm-tph-1 (B. malayi) | |
| OVC00480 | SWOv3MCA622SK | 5 | 0.08 | 0 | 5 | Neuromodulin-like, ZK1290.8 (C. elegans) (SECL+) | |
| OVC00811 | SWOv3MCA135SK | 5 | 0.08 | 1 | 4 | Similar to T27C4.1 (C. elegans) | |
| UR (L3, mL3) | OVC00039 | SWOvL3CAN03H02SK | 133 | 2.12 | 89 | 37 | Cuticle collagen col-34, F36A4.10 (C. elegans) |
| OVC00025 | SWOvL3CAN05H09SK | 76 | 1.21 | 32 | 44 | L3 larval antigen B66-RP, Ov-agy-1, Ov-agy-2 | |
| OVC00036 | SWOvL3CA252SK | 65 | 1.03 | 29 | 35 | Cuticle collagen, ZC373.7 (C. elegans) | |
| OVC00084 | SWOv3MCA249SK | 25 | 0.39 | 13 | 10 | Glyceraldehyde-3-phosphate dehydrogenase, Ov-gpd-1 | |
| OVC00082 | SWOv3MCA1047SK | 23 | 0.36 | 16 | 6 | Actin-1A, Ov-act-1a | |
| OVC00505 | SWOv3MCAM03H10 | 18 | 0.28 | 8 | 10 | Galectin-3 (laminin-binding protein), Ov87, Ov-gbp-2 | |
| OVC00411 | SWOvL3CAN09D04 | 16 | 0.25 | 4 | 12 | Heat shock protein 12.6, C14B9.1 (C. elegans) | |
| OVC00482 | SWOv3MCA1520SK | 16 | 0.25 | 9 | 6 | Novel (SECL+) | |
| OVC00332 | SWOvL3CAN14G06 | 13 | 0.20 | 9 | 2 | Glutamic-rich protein, F53A9.10 (C. elegans) | |
| OVC00413 | SWOvL3CAN18A04 | 11 | 0.17 | 4 | 7 | Similar to R07E5.13 (C. elegans) | |
| OVC00461 | SWOvL3CAN53A07SK | 11 | 0.17 | 6 | 3 | Hypodermal antigen, Ov17, Ov-ral-2 | |
| OVC00238 | SWOvL3CAN10G08 | 10 | 0.15 | 7 | 3 | Cuticle collagen 2(F) (B. pahangi) | |
| OVC00295 | SWOvL3CAN58B02SK | 9 | 0.14 | 7 | 2 | Novel | |
| OVC00340 | SWOvL3CAN09C03 | 9 | 0.14 | 5 | 4 | Histone H3.3, F45E1.6 (C. elegans) | |
| OVC00650 | SWOv3MCAM03G02 | 9 | 0.14 | 4 | 4 | EF-hand calcium binding protein, F43C9.2 (C. elegans) | |
| OVC00667 | SWOvL3CAN11E10 | 9 | 0.14 | 7 | 2 | Two LIM-like binding domains, T04C9.4 (C. elegans) | |
| OVC00005 | SWOv3MCA003SK | 8 | 0.12 | 2 | 6 | Similar to aquaporin-1 Tc-aqp-1 (T. canis) | |
| OVC00028 | SWOvL3CAN45D05 | 8 | 0.12 | 3 | 4 | Transthyretin-like family, Y5F2A.1 (C. elegans) (SECL+) | |
| OVC00037 | SWOvL3CAN12B09 | 8 | 0.12 | 3 | 4 | Similar to C34E7.4 (C. elegans) (SECL+) | |
| OVC00171 | SWOvL3CA1139SK | 8 | 0.12 | 3 | 4 | GST 2 (PI class) Ov-gst-2 | |
| OVC00291 | SWOvL3CAN50H05SK | 7 | 0.11 | 4 | 3 | Cuticular collagen, F17C8.2 (C. elegans) | |
| OVC00106 | SWOvL3CAN63C12SK | 7 | 0.11 | 5 | 2 | ATP synthase complex f subunit-like protein (C. elegans) | |
| OVC00432 | SWOvL3CAN06B08SK | 7 | 0.11 | 4 | 3 | Similar to C16C10.11 (C. elegans) | |
| OVC00103 | SWOv3MCA057SK | 5 | 0.08 | 2 | 3 | Cuticular collagen, T28C6.4 (C. elegans) | |
| OVC00793 | SWOvL3CA309SK | 5 | 0.08 | 3 | 2 | Similar to C53A5.1 (C. elegans) (SECL+) | |
Of the 4,635 L3 and mL3 ESTs, 2.4% (corresponding to 111 individual ESTs grouped in 10 clusters) are L3 specific and 2.2% (corresponding to 103 ESTs in 10 clusters) are mL3 specific, while 25% (corresponding to 1,164 ESTs in 45 clusters) are present in both L3 and mL3 EST datasets.
Each cluster has at least five individual ESTs, >85% of which have been sequenced from the L3-mL3 datasets with the remaining 15% being ESTs from other stages (microfilariae, L2, and adult male and female).
Total number of ESTs within the cluster.
Percentage of the O. volvulus ESTs in the dataset.
Number of ESTs from the L3 dataset in the cluster.
Number of ESTs from the mL3 dataset in the cluster.
UR or PSS (L3), up-regulated or potentially stage-specific genes expressed in the L3; UR or PSS (mL3), up-regulated or potentially stage-specific genes expressed in mL3; UR (L3, mL3), up-regulated genes expressed in the L3 and mL3.
Two of the most highly expressed genes in the L3 (in clusters OVC00048 and OVC00109) are homologues of the B. malayi abundant larval transcripts (Bm-alt-1 and Bm-alt-2) and the 18-kDa antigen of Dirofilaria immitis and Acanthocheilonema viteae (23, 25, 61). The more abundant of these genes, in cluster OVC00048, encodes the two O. volvulus antigens Ov-ALT-1 and Ov-ALT-2 (39). Although the gene of the cluster OVC00109 is related to Bm-alt-2 it is a novel member of this family that has not yet been characterized. The function of these gene products is unknown. The D. immitis alt-1 gene (Di-alt-1) has been shown to be an ES component that may also be expressed on the surface of the mL3 (23). Families of other alt-like genes have been found in B. malayi and O. volvulus, most of which are specifically expressed in only the L3 or mL3. The cluster OVC00025 derives from an unrelated abundant larval transcript gene family encoding the Ov-B66 antigen (GenBank accession number AAB69626) which we previously cloned from the L3 cDNA library by immunoscreening with PI sera. A set of Brugia homologues was identified as differentially expressed vector-derived L3 genes (Bm-alt-3 and Bm-alt-4) (5; W. F. Gregory, personal communication). They are unrelated to the Bm-alt-1 and Bm-alt-2 and thus were renamed agy-1 and agy-2 (for abundant glycine- and tyrosine-rich proteins) based on their amino acid composition. In both species these genes appear to be expressed in a stage-specific manner, being abundant transcripts in the L3 and mL3 with expression ending before the completion of the L3-L4 molt (25).
The cluster OVC00237 derives from a gene family that was also recently cloned and whose products were identified as antigens in O. volvulus and B. malayi. It belongs to a nematode gene family initially identified in Ancylostoma as a component of secretions of activated L3 larvae called ancylostoma secreted protein (ASP) (41, 42) and another hookworm glycoprotein called neutrophil inhibition factor (NIF) which binds to the CD11b/CD18 receptor (53). Recent surveys of gene expression in Necator americanus adults and Toxocara canis infective L3 identified a dozen additional redundant ASP-like genes (15, 73). Comparison with the recently completed C. elegans genome revealed that it also possesses a large family of these genes (11, 15). The ASPs are related to a number of other vertebrate and invertebrate proteins, including helothermine venom proteins from lizards, sperm and testis proteins from mammals, pathogenesis-associated proteins from plants, and venom allergen antigen 5 from insects (27, 28). We have identified three different ASP-like proteins within the Onchocerca EST datasets (designated Ov-ASP, for activation-associated secreted protein), one of which we had previously isolated by immunoscreening of the L3 cDNA expression library (OvB93-RP [GenBank accession number AAB69625]). The expression profiles of these genes in B. malayi, T. canis, and hookworms indicate that this protein family may be important in establishing and maintaining infection in the mammalian hosts (15, 73).
Structural components of the cuticle are also abundantly expressed in L3 and mL3 relative to other larval and adult stages. This was not unexpected, as waves of collagen synthesis have been shown to precede each molt in C. elegans (38). Eleven of the abundant L3 and mL3 clusters represent distinct collagen genes. Six of these genes have differential expression profiles found only in either the L3 or the mL3 EST dataset. Some of these collagen genes may be specific components of the L4 cuticle, as they have not been seen in our other larval or adult datasets. Interestingly, the cuticlin and osteonectin-like genes are restricted to the mL3 dataset, indicating that they may be utilized later in the formation of the L4 cuticle than some of the collagens that are synthesized in the infective L3. A variety of enzymes are also involved in the processing, remodeling, and cross-linking of these cuticle components, and they represent extremely attractive drug targets because of their central role in parasite development (48, 59). Very few of these proteins that are potentially involved in molting have been cloned or characterized. Cysteine proteinases have been implicated in the O. volvulus L3-to-L4 molting process (49). One of the most abundantly expressed proteins in the L3 dataset is a cathepsin L-like cysteine proteinase. Its role in the molting process and parasite transition from the vector to the mammalian host is currently under investigation (D. B. Guiliano, J. McKerrow, X. Hong, and S. Lustigman, personal communication).
Nine of the abundant gene clusters have significant hits only to anonymous predicted genes from C. elegans (11). Two of these abundant gene clusters, OVC00650 (EF hand calcium-binding domains) and OV00667 (LIM metal binding domains), have matches to short functional domains that may indicate their potential function. Three others have SECL+s and thus could be novel secretory products that are involved in the molting process. The cluster OVC00480 appears to be expressed in only the mL3. C. elegans is a very tractable model system which could be exploited as a tool to study the biological functions of such proteins that may represent novel nematode-specific gene families (8). Twelve of the abundant gene clusters in both L3 and mL3 datasets were completely novel, with no significant similarity to any protein in the GenBank database, and thus are possibly novel parasite-specific molecules. The predicted open reading frames of six of these clusters also have SECL+s.
Genes of interest identified by the EST initiative.
Not all genes that are attractive for vaccine or drug development are stage specific or produce abundant transcripts. If a particular gene or class of genes is believed to be an appropriate target based on studies done in other systems, their homologues in O. volvulus can be identified within the EST dataset (including the approximately 2,000 clusters that have only one EST) (Table 2). The categories of genes listed below encode proteins that are important for the development and survival of the parasite within the host. Not surprisingly, we were also able to identify ESTs corresponding to several immunodominant proteins that had previously been cloned from other O. volvulus cDNA expression libraries by immunoscreening (http://helios.bto.ed.ac.uk/mbx/fgn/OnchoNet/onchotable1.html), some of which are also being pursued for diagnostic and vaccine development purposes.
TABLE 2.
Genes of potential interest identified by the EST initiative
| Category | Cluster | Representative EST | Gene | Totala | L3/mL3b | Homology(ies) |
|---|---|---|---|---|---|---|
| Genes involved in regulation of the immune response | OVC01428 | SWOvL3CAN61D08SK | Ov-mif-1 | 9 | 2/0 | Macrophage migration inhibitory factor homologue (B. malayi) |
| OVC00958 | SWOvL3CA187SK | Ov-mif-2 | 1 | 1/0 | Macrophage migration inhibitory factor homologue, C52E4.2 (C. elegans) | |
| OVC00496 | SWOvL3CAN50E11SK | Ov-gbp-1 | 9 | 8/0 | Beta-galactoside-binding lectin | |
| OVC00505 | SWOv3MCAM03H10 | Ov-gbp-2 | 18 | 8/10 | Galectin binding protein, Ov87 | |
| Proteinases | OVC00916 | SWOvL3CAN54F09SK | Ov-cpl-1 | 16 | 16/0 | Cathepsin L (D. immitis and B. pahangi) |
| OVC02102 | SWOv3MCAM03H03 | Ov-cpb-1 | 1 | 0/1 | Cathepsin B, F26E4.3 (C. elegans) | |
| OVC01179 | SWOvL3CA420SK | 2 | 2/0 | Metalloproteinase with TSP-like repeats, R151.5 (C. elegans) | ||
| Proteinase inhibitors | OVC00784 | SWOv3MCA640SK | Ov-spn-1 | 3 | 3/0 | Serpin (serine proteinase inhibitor) (B. malayi) |
| OVC00017 | SWOv3MCA025SK | Ov-spi-1 | 22 | 0/19 | Small serine proteinase inhibitors (A. suum) | |
| OVC00142 | SWOvL3CAN54A03SK | Ov-cpi-2 | 70 | 59/9 | Onchocystatin, Ov7 | |
| OVC01075 | SWOvL3CAN12H04 | Ov-cpi-1 | 4 | 4/0 | Cystatin homologue (B. malayi) | |
| OVC00100 | SWOv3MCA1851SK | Ov-api-1 | 11 | 0/8 | Pepsin inhibitor, Ov33 immunodominant antigen | |
| Antioxidant or detoxification enzymes | OVC00018 | SWOvL3CAN38F12 | Ov-tpx-2 | 93 | 39/43 | TPX |
| OVC00419 | SWOv3MCA780SK | Ov-tpx-3 | 8 | 2/4 | TPX | |
| OVC01115 | SWOv3MCAM02B04SK | Ov-gst-1 | 5 | 0/3 | GST 1 | |
| OVC00171 | SWOvL3CA1139SK | Ov-gst-2 | 8 | 3/4 | GST 2 | |
| OVC00056 | SWOvL3CAN12H07 | Ov-sod-1 | 22 | 12/6 | Cytoplasmic SOD (Cu/Zn) | |
| OVC02959 | SWOvL3CA395SK | Ov-sod-2 | 14 | 9/5 | Secreted SOD (Cu/Zn) | |
| OVC01526 | SWOv3MCA2030SK | Ov-sod-3 | 1 | 0/1 | SOD (Mn) | |
| OVC00681 | SWOvL3CAN01A05SK | Ov-lgl-1 | 2 | 2/0 | Lactolyglutathione lyase | |
| Nuclear hormone receptors | OVC00074 | SWOv3MCA2080SK | 3 | 1/1 | Steroid-thyroid-retinoic nuclear hormone receptor, F33D4.1 (C. elegans) | |
| OVC01135 | SWOv3MCAM02C09SK | 1 | 0/1 | Thyrotropin receptor, C50H2.1 (C. elegans) | ||
| Neurotransmitter receptors | OVC00899 | SWOvL3CAN61H03SK | 2 | 1/1 | Acetylcholine receptor alpha-7 chain, C33G3.3 (C. elegans) | |
| OVC00451 | SWOv3MCA1116SK | 1 | 0/1 | Ligand-gated ionic channel, T21F2.1 (C. elegans) | ||
| OVC01287 | SWOv3MCA1813SK | 1 | 0/1 | GABA and glycine receptors, C27H5.5 (C. elegans) | ||
| OVC01230 | SWOv3MCA175SK | 1 | 0/1 | Ionotropic GABA receptor subunit, UNC-49B.1 (C. elegans) | ||
| Developmental genes | OVC01723 | SWOvL3CAN12G11 | Ov-mom-5 | 1 | 1/0 | Secreted frizzled related protein sFRP-2, T23D8.1, mom-5 (C. elegans) |
| OVC00701 | SWOv3MCA127SK | Ov-sma-2 | 1 | 0/1 | MAD protein, SMA-2, dwarfin (C. elegans) | |
| OVC00763 | SWOv3MCA131SK | 1 | 0/1 | Quiescin, bone-derived growth factor, F47B7.2 (C. elegans) | ||
| Cyclophilins | OVC00488 | SWOv3MCA625SK | Ov-cyp-5 | 3 | 0/3 | Cyclophilin isoform 5 (C. elegans) |
| OVC01004 | SWOv3MCA1486SK | Ov-cyp-10 | 1 | 0/1 | Cyclophilin isoform 10 (C. elegans) | |
| OVC00001 | SWOv3MCA002SK | Ov-cyp-4 | 1 | 0/1 | Cyclophilin 4 | |
| OVC01099 | SWOvL3CAN53F06SK | Ov-cyp-2 | 1 | 1/0 | Cyclophilin 2 (O. volvulus) and cyclophilin isoform 3 (cyp-3) (C. elegans) | |
| OVC01257 | SWOv3MCAM04F09SK | 3 | 1/1 | Cyclophilin-33B (Homo sapiens) and Y116A8.34 (C. elegans) |
Total numbers of ESTs.
Individual representations of ESTs in the L3 and mL3 datasets.
(i) Genes involved in the regulation of the immune response.
In surveying the ESTs from the L3 and mL3 cDNA libraries, we have been able to identify several genes whose products show similarity to proteins utilized by the immune system and thus may be involved in the manipulation of host immune responses. These include two homologues of macrophage migration inhibitory factor, O. volvulus mif-1 and mif-2 (Ov-mif-1 and Ov-mif-2). The Brugia homologue of Ov-mif-1 has been shown to be secreted and have a cytokine-like activity (60), but its exact role in the manipulation of the immune response is still unknown. Galectins are beta-galactoside-binding lectins found in a variety of organisms, including nematodes. A galectin (Ov-gbp-2) that had been previously isolated by immunoscreening of the O. volvulus L3 library with PI sera (40) was identified in our EST survey and found to be up-regulated (18 ESTs and in L3 and mL3 stages only). Another galectin (Ov-gbp-1) which had been previously isolated by immunoscreening of an O. volvulus adult female cDNA library using loiasis patient sera was shown to specifically bind IgE (and not IgG) in a lactose-inhibitable manner, suggesting its role in the pathophysiology of filarial infections (41).
(ii) Proteinases.
Proteolytic enzymes have been identified as drug targets and vaccine candidates in a variety of disease systems. Three different putative proteinase genes have been identified from the L3 and mL3 EST sequences. The highly expressed Ov-cpl-1 (16 ESTs in L3 only) shows high homology to cathepsin L-like enzymes, whereas the Ov-cpb-1 is similar to a large family of C. elegans cathepsin B-like enzymes. Cathepsin L-like enzymes have been isolated from a variety of nematodes, including C. elegans, D. immitis, Haemonchus contortus, T. canis, and Ancylostoma caninum. The D. immitis, Brugia pahangi, and O. volvulus cathepsins have been shown to play a vital role in molting (Guiliano et al., unpublished data). The third proteinase identified, encoded by a gene of cluster OVC00100, is similar to a large family of astacin-like metalloproteinases from C. elegans with tsp (thrombospondin and properdin)-like repeats. Metalloproteinases have been shown to be involved in molting in some nematodes. A 44-kDa zinc-metalloprotease in H. contortus was shown to be responsible for the digestion of the ring region of the L2 cuticle before molting (24). D. immitis and B. pahangi metallopeptidases were also shown to be intimately associated with molting as well as activities that might facilitate larval migration (31, 64).
(iii) Proteinase inhibitors.
Specific protein inhibitors of proteinases have been isolated from filarial nematodes and were suggested to play a role in the inhibition of enzymes secreted from host immune cells (81, 82), blocking of antigen processing (W. F. Gregory, personal communication) and control of endogenous proteinases involved in parasite development (47). We identified five proteinase inhibitors in the L3 and mL3 EST datasets. Two are cystatin-like cysteine proteinase inhibitors (encoded by Ov-cpi-1 and Ov-cpi-2). The Ov-cpi-2 gene product was originally cloned as an antigen, onchocystatin, from O. volvulus (47) and has also been recently characterized in B. malayi (25) and A. viteae (26). While Ov-cpi-2 is transcribed throughout the parasite life cycle, it is clearly up-regulated in O. volvulus L3 (59 ESTs out of 70 are only in L3 [Tables 1 and 2]), where it was proposed to play a role in molting (47). The B. malayi cpi-2 is present in the ES products of larval and adult parasites, and the recombinant protein inhibits antigen processing (W. F. Gregory, personal communication), while Av17 was shown to directly inhibit T-cell proliferation (26). The serine proteinase inhibitor (Ov-spn-1) of the serpin family has a homologue in B. malayi (Bm-spn-1) that was shown to be highly expressed in L3 but not in L4 or adults and to be present in the ES products, where it is believed to interact with serum proteins (81). The second serine proteinase inhibitor (Ov-spi-1) belongs to a novel family of low-molecular-weight inhibitors originally isolated from Ascaris suum (3), where they are thought to be involved in protecting the nematodes from host trypsin and chymotrypsin activities. The Ov-spi-1 gene appears to be up-regulated in the mL3 (19 ESTs out of 22), and its function in O. volvulus is currently under investigation (D. Guiliano and S. Lustigman, unpublished data). Other members of this family have recently been identified in Anisakis simplex and A. suum (55) as well as in the C. elegans genome (11). An up-regulated aspartyl protease inhibitor (Ov-api-1) in mL3 was previously characterized as an immunodominant antigen (Ov33) in O. volvulus (77). Ov-spi-1 and Ov-api-1 genes code for part of a family of proteinase inhibitors originally characterized from Ascaris and which has not been seen outside of the phylum Nematoda (3, 51). These inhibitors could support the identification of synthetic molecules to specifically inhibit the endogenous or exogenous corresponding enzymes, and thus interfere with the function of these molecules during development in the host.
(iv) Antioxidant and detoxification enzymes.
Several genes encoding antioxidant and detoxification enzymes (three superoxide dismutases [SOD], two glutathione-S-transferases [GST], and two thioredoxin peroxidases [TPX]) have already been cloned using PCR approaches and are well characterized in O. volvulus (69). All of these genes are represented in the L3 and mL3 EST datasets, of which Ov-tpx-2, Ov-sod-1, and Ov-sod-2 are abundantly expressed in both L3 and mL3 (82, 18, and 14 ESTs, respectively). These antioxidant and detoxification enzymes are believed to play a role in protecting the nematodes from host immune effector mechanisms and are being pursued as drug targets (69).
(v) Nuclear hormone receptors.
Nuclear hormone receptors have been implicated in several important aspects of nematode biology, including sex determination (9), dauer formation (2), molting (44), and early development and reproductive functions (80). Human hormone receptors have been targets of rational drug design programs which aim to develop compounds that can control hormone receptor activity (52). The Macrofil Chemotherapy Project of the World Health Organization has identified nematode nuclear hormone receptor receptors as potential drug targets and is interested in developing reagents that will interfere with their function. Two nuclear hormone receptors have been identified in the L3 and mL3 EST datasets, potential steroid-thyroid-retinoic and thyrotropin receptors. Both have putative orthologues in C. elegans, which could be then used as a model system to help determine their function during development of the nematode and test possible antagonists (43).
(vi) Neurotransmitter receptors.
Drugs such as avermectin and levamisole that interfere with neurotransmitter receptors are already used as nematicides, making them attractive for developing novel chemotherapeutic agents. Although not abundant, four distinct potential neurotransmitter receptors have been identified in the L3 and mL3 EST datasets (acetylcholine, ligand-gated ionic channel, GABA-glycine, and ionotropic GABA receptors). All of these have homologues or orthologues in C. elegans. Only OVC01230, which is a subunit of ionotrophic GABA receptor UNC-49, has a characterized C. elegans mutant, with an uncoordinated phenotype (4).
(vii) Developmental genes.
Single ESTs in the L3 and mL3 datasets have been identified which encode proteins whose homologues are involved in C. elegans early development. OVC01723 is related to C. elegans mom-5, a secreted frizzled protein which is part of the wnt signaling pathway and controls cell fate in the developing embryo (66). The OVC00701 gene cluster is a homologue of C. elegans sma-2, a member of the dwarfin family of proteins, which are part of a transforming growth factor β signal transduction pathway (68). Because of the experimental limitations in the manipulation of parasitic nematodes, there have been very few studies investigating their developmental biology at the molecular level. C. elegans as a model metazoan and nematode can offer insights into nematode developmental biology and thus new opportunities in the discovery of strategies of interrupting the parasite life cycle.
(viii) Cyclophilins.
Cyclophilins are a diverse group of proteins that were originally characterized because they were the target of the immunosuppressive drug cyclosporin A. Five distinct cyclophilins were identified in the L3 and mL3 EST datasets, all having orthologues in C. elegans. In filariae, several cyclophilins and their cyclosporin sensitivities have been described (50, 57). Three of the onchocerca cyclophilins have not been previously characterized in filariae (those in clusters OVC00488, OVC01004, and OVC01257). Cyclophilins are currently being pursued as targets for novel anthelminthics (13, 14). In nematodes, cyclophilins have also been implicated in the processing of cuticle components (57, 58) and are therefore important for the development of the parasite in the host.
Isolation of immunoreactive clones from the O. volvulus mL3 cDNA library.
Eight distinct genes were isolated by immunoscreening with depleted PI sera, two of which had been previously isolated in other laboratories: the O. volvulus repetitive antigen (10) and the intermediate filament antigen (12). Following GenBank database similarity searches, the other six proteins were classified as either novel O. volvulus proteins with similarity to other known proteins (to dynein [Ov-DLC-1], calcium-binding protein [Ov-CBP-1], and guanosine 5′-monophosphate oxidoreductase [Ov-GMR-1]) or as novel O. volvulus proteins with no similarity to other known proteins, designated novel immunogenic proteins (NIP) (Ov-NIP-1, Ov-NIP-2, and Ov-NIP-3). Without the immunoscreening results, none of these antigens would have been selected for immunological studies since they all appear to be rare transcripts as depicted by their corresponding representation in the EST datasets (0 to 2 ESTs). Additionally, some are conserved and ubiquitous proteins and three of them are completely novel.
(i) Novel O. volvulus immunogenic proteins with similarity to known proteins.
The Ov-dlc-1 cDNA (GenBank accession number AF153718) encodes a 9.3-kDa protein that shows extensive similarity to the cytoplasmic dynein light chain (DLC) (47). Dyneins are highly conserved proteins involved in various types of microtubule-based intracellular transport and motility and also in changing or maintaining the spatial distribution of the cytoskeletal structure (6). A comparison of Ov-DLC-1 with other DLCs shows 75% identity with Schistosoma mansoni DLC-1 (30), 73% identity with the S. mansoni DLC-2 (42), 94% identity with human DLC (17), 99% identity with a predicted C. elegans DLC (GenBank accession number Q22799), and 100% identity with B. malayi DLC (GenBank accession number AA542793). Despite the high degree of identity between the parasite and the human primary amino acid sequences (73%), the S. mansoni DLC (Sm-DLC-2, Sm10) was described as a T-cell-stimulating antigen associated with protective immunity in humans (42). Dynein and other structural housekeeping genes such as calponin, tropomyosin, and paramyosin have also been demonstrated to be of immunological importance in onchocerciasis (33, 35, 71). No EST corresponding to Ov-dlc-1 was found in the present O. volvulus EST database (9,000 ESTs as of February 2000).
The Ov-cbp-1 cDNA (GenBank accession number AF153720 [OVC02120]) encodes a 62-kDa protein with seven classical EF-hand calcium-binding domains. This protein does not show significant similarity to previously described EF-hand calcium-binding proteins present in the database and may represent a novel member of this family. Interestingly, Ov-CBP-1 shows 60% identity to a predicted C. elegans calcium-binding protein (CBP-1 [K01A2.11b]), and both of them have putative N-terminal signal peptides and are therefore probably secreted. There is one molting larval EST (SWOv3MCAM07A01SK) corresponding to this cDNA in the current EST database.
An alignment of the deduced primary amino acid sequence of the Ov-gmr-1 cDNA (GenBank accession number AF153721 [OVC01489]) shows extensive identities with the two known GMP reductases from other nematodes: C. elegans (73%) and A. suum (81%), as well as the human protein (63%). One corresponding EST sequence (SWOvL3CAN62B09SK) was identified in the L3 EST dataset.
(ii) Novel O. volvulus immunogenic proteins.
A TBLASTX analysis revealed that three cDNA clones isolated by immunoscreening contained no homologues in the current public database. The cDNA clone designated Ov-nip-1 (GenBank accession number AF153719 [OVC00547]) encodes a highly basic (pI = 11.2) 32-kDa protein rich in serine, arginine, and lysine. The N-terminal portion of the protein is predominantly serine and arginine rich (58%) while the C-terminal region is lysine rich (38%). Two ESTs corresponding to this cDNA (SWOvL3CAN16C05SK and SWOv3MCA657SK) have been identified. This protein contains four classical (29) and six bipartite (65) nuclear localization signals. The Ov-nip-2 cDNA (GenBank accession number AF153723 [OVC00949]) encodes a 48-kDa protein with one potential N glycosylation site. The cDNA has a corresponding EST (SWOv3MCA915SK) in the mL3 EST dataset. The Ov-nip-3 cDNA (GenBank accession number AF153722 [OVC00115]) encodes a small protein (15 kDa) with a potential signal peptide. The cDNA has three corresponding EST sequences in the mL3 dataset (SWOv3MCAM07G11SK, SWOv3MCA061SK, and SWOv3MCAM11B11SK). Based on their novelty and immunogenicity, these proteins all represent a very attractive subset whose potentials as vaccinogens are currently being pursued.
Antibody responses to O. volvulus recombinant proteins.
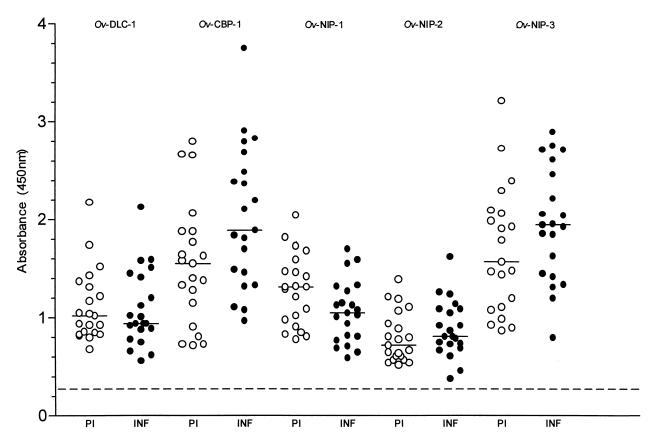
Serum samples from PI and INF individuals were tested against the recombinant Ov-DLC-1, Ov-NIP-1, Ov-NIP-2, Ov-NIP-3, and Ov-CBP-1 (Fig. 1). The mean optical density (OD) of three normal human serum samples plus three times the standard deviation (OD = 0.3) determined the cut-off level. Sera from 100% of the PI and the INF individuals recognized the recombinant proteins. The levels of recognition by both groups were high for all antigens (median ODs between 0.72 and 1.89). Only the response of the INF individuals to the Ov-CBP-1 protein (OD = 1.89) was significantly higher than in the PI group (OD = 1.55; P = 0.03). Difficulties in expressing recombinant Ov-GMR did not allow us to determine the immunogenicity of this protein in both groups.
FIG. 1.
Comparison of total IgG responses of the PI (n = 21) and INF (n = 21) individuals to recombinant Ov-DLC-1, Ov-CBP-1, Ov-NIP-1, Ov-NIP-2, and Ov-NIP-3 proteins. Spots represent the OD of each individual serum sample, and bars represent median values in each group. The cut-off level of all the assays (broken line) was the mean OD of three healthy human sera plus three times the standard deviation. Analysis was done by the two-tailed nonparametric Mann-Whitney U test, and results were considered statistically significant if the P value was less than 0.05.
DISCUSSION
The search for filarial drug targets and/or vaccine candidates has so far been based largely on one of the following approaches: (i) immunoscreening of cDNA expression libraries using patient sera or monoclonal antibodies, (ii) two-dimensional Western blots using parasite protein extracts and polyspecific patient sera followed by protein sequencing and cloning, or (iii) PCR amplification of genes based on rational (hypothesis-driven) design. With the inception of the filarial genome projects and the vast amount of information contained therein, an entirely new subset of genes have been identified as possible parasite target molecules which may not have been discovered using only the above methods. The resources of the recently completed C. elegans genome (11) are being exploited as a model for analysis of filarial genes, aimed at cataloging the complete inventory of such proteins as immunogens (for vaccine development) and drug targets (for chemotherapy) as well as virulence and developmental factors (21, 78). Several important genes, like transporters, receptors, proteinases, antioxidants, and abundant and developmentally regulated transcripts, have been identified in B. malayi and T. canis using such a gene discovery approach (5, 25, 73). Unlike in B. malayi, where the complete genome and gene expression profile are being investigated, only the EST approach is being undertaken in O. volvulus using specific life cycle stages of the parasite. In the present study, we have employed a combination of two strategies to identify potential vaccine candidates and drug targets in the filarial parasite O. volvulus: analysis of 4,635 L3 and mL3 ESTs and a selective immunoscreening of the O. volvulus mL3 cDNA library using a distinct source of human sera. These two complementary approaches facilitated the identification of potentially interesting genes which can be pursued for development of vaccines and/or drug targets against onchocerciasis based on their immunogenicity, up-regulation in larval stages, and/or predicted biological features.
The EST analysis approach (Table 1) has revealed an important group of genes that are developmentally up-regulated in L3 and/or mL3, as well as proteins that are entirely novel and may be specific to O. volvulus and thus associated with infection and host-parasite interactions. Other proteins have similarity only to proteins predicted from the C. elegans genome, representing an additional group of nematode proteins that can be more easily studied using C. elegans as a model organism. In particular, with the availability of RNA interference (RNAi) technology (22) the function and importance of the C. elegans homologues for survival and/or development could be rapidly assessed. Many of these proteins from both groups have potential signal peptides and are thus presumably destined for secretion, making them another attractive subset, since ES products have been demonstrated to be valuable candidates for vaccine development in filarial infections (32). In the EST initiative, we have identified, among several others, four previously uncharacterized neurotransmitter receptors, three proteinases, two nuclear hormone receptors, and three novel cyclophilins. Compounds such as ivermectin that interrupt neurotransmission are already in use as anthelminthics, and there is a great deal of information about their effects on nematodes and their efficacy in human infections. However, very few of their targets have been cloned and characterized, and the EST sequencing approach could offer interesting starting points for drug development. Additionally, the presence of homologues of O. volvulus proteins in C. elegans would allow its use as a model organism for the development of compounds that may interfere with the function of these proteins as therapeutic targets.
The immunoscreening approach directly identified parasite immunogens that otherwise would not have been identified as potential vaccinogens using the EST approach. None of these antigens would have been selected for immunological studies, as they either are absent or have only one to three ESTs within the datasets. In addition, some of them are conserved and/or ubiquitous proteins, and three of them are completely novel. Some protective novel antigens identified by immunoscreening of L3 or adult worm cDNA libraries are also not yet represented in the EST datasets (http://helios.bto.ed.ac.uk/mbx/fgn/OnchoNet/onchotable1.html). The ELISA data indicate that the five antigens isolated from the mL3 cDNA library were strongly recognized by sera from both the PI and the INF groups (Fig. 1). As the concept of concomitant immunity has been enunciated with regard to human filarial infections (16, 74), we propose that the comparable immune responses towards the cloned antigens by the PI and INF individuals indicate that these proteins may be participating in protective responses in both groups. None of the recombinant onchocerca antigens identified so far to be protective in the mouse model were found to be uniquely recognized by the PI. The recombinant proteins corresponding to the clones isolated by the immunoscreening approach will have to be tested in the O. volvulus mouse diffusion chamber model to confirm their relevance in conferring protection against infection.
The strength of using the dual strategy of EST analysis and immunoscreening is that both approaches have effectively uncovered potential vaccine candidates and drug targets in O. volvulus. To increase the value of our findings, additional research will be required to confirm the importance of the clones identified by the EST approach. (i) Potent inhibitors of previously characterized drug targets in other systems, whose homologues have been identified in this study, can be used to screen for their effect on O. volvulus worms or an appropriate model. (ii) Plaques expressing recombinant proteins that are L3- and/or mL3-specific (based on EST analysis) can be tested with sera from protected hosts to identify those that are immunogenic and therefore more associated with protective immunity. (iii) Extracts can be used to deplete sera in order to preselect for antibodies directed against larva-specific antigens. The depleted sera can then be used on selected cDNA expression arrays to rapidly identify larva-specific genes and simultaneously assess the immunogenicity of clones of interest. Interestingly, some of the highly expressed stage-specific and/or up-regulated genes in the L3 library have already been shown to be antigenic (ALT, ASP, CPI-2) (39, 47, 70). (iv) Plaque cellular proliferation assays can also be performed using arrays of such genes designed to select those that are able to induce particular cytokine responses in PI versus INF individuals. (v) Selected genes could be subcloned into mammalian expression vectors and then used for DNA immunization in the O. volvulus mouse diffusion chamber model (45). The combination of molecular and immunological approaches has thus provided us with tools that will be used in our continuing effort to elucidate a proactive method of combating onchocerciasis.
ACKNOWLEDGMENTS
We thank Mark Blaxter, Judith Allen, and Rick Maizels for their helpful comments on the manuscript. We also thank Roselle Hoffmaster, Michelle Mondoux, Lou Ann Bierwert, and Susan Haynes for their technical assistance.
This work was financially supported in part by the grants from The Edna McConnell Clark Foundation and by grant RO1 AI 42328-02 from the National Institutes of Health. D.B.G. was funded by the United Kingdom Medical Research Counsel.
M.L.-Z., W.T., and D.B.G. contributed equally to this work.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antebi A, Culotti J G, Hedgecock E M. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development. 1998;125:1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- 3.Babin D R, Peanasky R J, Goos S M. The isoinhibitors of chymotrypsin/elastase from Ascaris lumbricoides: the primary structure. Arch Biochem Biophys. 1984;232:143–161. doi: 10.1016/0003-9861(84)90530-7. [DOI] [PubMed] [Google Scholar]
- 4.Bamber B A, Beg A A, Twyman R E, Jorgensen E M. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaxter M L, Raghavan N, Ghosh I, Guiliano D, Lu W, Williams S A, Slatko B, Scott A L. Genes expressed in Brugia malayi infective third stage larvae. Mol Biochem Parasitol. 1996;77:77–93. doi: 10.1016/0166-6851(96)02571-6. [DOI] [PubMed] [Google Scholar]
- 6.Bloom G S. Motor proteins for cytoplasmic microtubules. Curr Opin Cell Biol. 1992;4:66–73. doi: 10.1016/0955-0674(92)90060-p. [DOI] [PubMed] [Google Scholar]
- 7.Bradley J E, Tuan R S, Shepley K J, Tree T I, Maizels R M, Helm R, Gregory W F, Unnasch T R. Onchocerca volvulus: characterization of an immunodominant hypodermal antigen present in adult and larval parasites. Exp Parasitol. 1993;77:414–424. doi: 10.1006/expr.1993.1101. [DOI] [PubMed] [Google Scholar]
- 8.Burglin T R, Lobos E, Blaxter M L. Caenorhabditis elegans as a model for parasitic nematodes. Int J Parasitol. 1998;28:395–411. doi: 10.1016/s0020-7519(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 9.Carmi I, Kopczynski J B, Meyer B J. The nuclear hormone receptor SEX-1 is an X-chromosome signal that determines nematode sex. Nature. 1998;396:168–173. doi: 10.1038/24164. [DOI] [PubMed] [Google Scholar]
- 10.Catmull J, Zhang D, Ruggiero F, Copeman D B, Miller D J. Identification and characterisation of a novel repetitive antigen from Onchocerca spp. Mol Biochem Parasitol. 1994;63:49–57. doi: 10.1016/0166-6851(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 11.The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 12.Chandrashekar R, Curtis K C, Weil G J. Molecular characterization of a parasite antigen in sera from onchocerciasis patients that is immunologically cross-reactive with human keratin. J Infect Dis. 1995;171:1586–1592. doi: 10.1093/infdis/171.6.1586. [DOI] [PubMed] [Google Scholar]
- 13.Chappell L H, Wastling J M. Cyclosporin A: antiparasite drug, modulator of the host-parasite relationship and immunosuppressant. Parasitology. 1992;105:S25–S40. doi: 10.1017/s0031182000075338. [DOI] [PubMed] [Google Scholar]
- 14.Cully D F, Vassilatis D K, Liu K K, Paress P S, Van der Ploeg L H, Schaeffer J M, Arena J P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 15.Daub J, Loukas A, Pritchard D I, Blaxter M. A survey of genes expressed in the adults of the human hookworm, Necator americanus. Parasitology. 2000;120:171–184. doi: 10.1017/s0031182099005375. [DOI] [PubMed] [Google Scholar]
- 16.Day K P, Gregory W F, Maizels R M. Age-specific acquisition of immunity to infective larvae in a bancroftian filariasis endemic area of Papua New Guinea. Parasite Immunol. 1991;13:277–290. doi: 10.1111/j.1365-3024.1991.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 17.Dick T, Ray K, Salz H K, Chia W. Cytoplasmic dynein (ddlc1) mutations cause morphogenetic defects and apoptotic cell death in Drosophila melanogaster. Mol Cell Biol. 1996;16:1966–1977. doi: 10.1128/mcb.16.5.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donelson J E, Duke B O, Moser D, Zeng W L, Erondu N E, Lucius R, Renz A, Karam M, Flores G Z. Construction of Onchocerca volvulus cDNA libraries and partial characterization of the cDNA for a major antigen. Mol Biochem Parasitol. 1988;31:241–250. doi: 10.1016/0166-6851(88)90154-5. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbiess W F, Apfel H, Meyer T F. Protective immunity linked with a distinct developmental stage of a filarial parasite. J Immunol. 1994;152:735–742. [PubMed] [Google Scholar]
- 20.Elson L H, Guderian R H, Araujo E, Bradley J E, Days A, Nutman T B. Immunity to onchocerciasis: identification of a putatively immune population in a hyperendemic area of Ecuador. J Infect Dis. 1994;169:588–594. doi: 10.1093/infdis/169.3.588. [DOI] [PubMed] [Google Scholar]
- 21.The Filarial Genome Project. Deep within the filarial genome: progress of the filarial genome project. Parasitol Today. 1999;15:219–231. doi: 10.1016/s0169-4758(99)01454-4. [DOI] [PubMed] [Google Scholar]
- 22.Fire A, Xu S, Montgomery M K, Kostas S A, Driver S E, Mello C C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 23.Frank G R, Tripp C A, Grieve R B. Molecular cloning of a developmentally regulated protein isolated from excretory-secretory products of larval Dirofilaria immitis. Mol Biochem Parasitol. 1996;75:231–240. doi: 10.1016/0166-6851(95)02534-0. [DOI] [PubMed] [Google Scholar]
- 24.Gamble H R, Purcell J P, Fetterer R H. Purification of a 44 kilodalton protease which mediates the ecdysis of infective Haemonchus contortus larvae. Mol Biochem Parasitol. 1989;33:49–58. doi: 10.1016/0166-6851(89)90041-8. [DOI] [PubMed] [Google Scholar]
- 25.Gregory W F, Blaxter M L, Maizels R M. Differentially expressed, abundant trans-spliced cDNAs from larval Brugia malayi. Mol Biochem Parasitol. 1997;87:85–95. doi: 10.1016/s0166-6851(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 27.Hawdon J M, Jones B F, Hoffman D R, Hotez P J. Cloning and characterization of Ancylostoma-secreted protein. A novel protein associated with the transition to parasitism by infective hookworm larvae. J Biol Chem. 1996;271:6672–6678. doi: 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- 28.Hawdon J M, Narasimhan S, Hotez P J. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol Biochem Parasitol. 1999;99:149–165. doi: 10.1016/s0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 29.Hicks G R, Raikhel N V. Protein import into the nucleus: an integrated view. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann K F, Strand M. Molecular identification of a Schistosoma mansoni tegumental protein with similarity to cytoplasmic dynein light chains. J Biol Chem. 1996;271:26117–26123. doi: 10.1074/jbc.271.42.26117. [DOI] [PubMed] [Google Scholar]
- 31.Hong X, Bouvier J, Wong M M, Yamagata G Y, McKerrow J H. Brugia pahangi: identification and characterization of an aminopeptidase associated with larval molting. Exp Parasitol. 1993;76:127–133. doi: 10.1006/expr.1993.1015. [DOI] [PubMed] [Google Scholar]
- 32.Irvine M, Johnson E H, Lustigman S. Identification of larval-stage-specific antigens on Onchocerca volvulus uniquely recognized by putative immune sera from humans and vaccination sera from animal models. Ann Trop Med Parasitol. 1997;91:67–77. doi: 10.1080/00034983.1997.11813113. [DOI] [PubMed] [Google Scholar]
- 33.Irvine M, Huima T, Prince A M, Lustigman S. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a highly immunogenic calponin-like protein. Mol Biochem Parasitol. 1994;65:135–146. doi: 10.1016/0166-6851(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins R E, Taylor M J, Gilvary N, Bianco A E. Characterization of a secreted antigen of Onchocerca volvulus with host-protective potential. Parasite Immunol. 1996;18:29–42. doi: 10.1046/j.1365-3024.1996.d01-10.x. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins R E, Taylor M J, Gilvary N J, Bianco A E. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. Proc Natl Acad Sci USA. 1998;95:7550–7555. doi: 10.1073/pnas.95.13.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson E H, Lustigman S, Kass P H, Irvine M, Browne J, Prince A M. Onchocerca volvulus: a comparative study of in vitro neutrophil killing of microfilariae and humoral responses in infected and endemic normals. Exp Parasitol. 1995;81:9–19. doi: 10.1006/expr.1995.1087. [DOI] [PubMed] [Google Scholar]
- 37.Johnson E H, Irvine M, Kass P H, Browne J, Abdullai M, Prince A M, Lustigman S. Onchocerca volvulus: in vitro cytotoxic effects of human neutrophils and serum on third-stage larvae. Trop Med Parasitol. 1994;45:331–335. [PubMed] [Google Scholar]
- 38.Johnstone I L, Barry J D. Temporal reiteration of a precise gene expression pattern during nematode development. EMBO J. 1996;15:3633–3639. [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph G T, Huima T, Lustigman S. Characterization of an Onchocerca volvulus L3-specific larval antigen, Ov-ALT-1. Mol Biochem Parasitol. 1998;96:177–183. doi: 10.1016/s0166-6851(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 40.Joseph G T, Huima T, Klion A, Lustigman S. A novel developmentally regulated galectin of Onchocerca volvulus. Mol Biochem Parasitol. 2000;106:187–195. doi: 10.1016/s0166-6851(99)00208-x. [DOI] [PubMed] [Google Scholar]
- 41.Klion A D, Donelson J E. OvGalBP, a filarial antigen with homology to vertebrate galactoside-binding proteins. Mol Biochem Parasitol. 1994;65:305–315. doi: 10.1016/0166-6851(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 42.Kohlstadt S, Couissinier-Paris P, Bourgois A, Bouchon B, Piper K, Kolbe H, Dessein A J. Characterization of a schistosome T cell-stimulating antigen (Sm10) associated with protective immunity in humans. Mol Biochem Parasitol. 1997;84:155–165. doi: 10.1016/s0166-6851(96)02787-9. [DOI] [PubMed] [Google Scholar]
- 43.Kostrouch Z, Kostrouchova M, Rall J E. Steroid/thyroid hormone receptor genes in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:156–159. doi: 10.1073/pnas.92.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostrouchova M, Krause M, Kostrouch Z, Rall J E. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development. 1998;125:1617–1626. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- 45.Lange A M, Yutanawiboonchai W, Lok J B, Trpis M, Abraham D. Induction of protective immunity against larval Onchocerca volvulus in a mouse model. Am J Trop Med Hyg. 1993;49:783–788. doi: 10.4269/ajtmh.1993.49.783. [DOI] [PubMed] [Google Scholar]
- 46.Lucius R, Textor G, Kern A, Kirsten C. Acanthocheilonema viteae: vaccination of jirds with irradiation-attenuated stage-3 larvae and with exported larval antigens. Exp Parasitol. 1991;73:184–196. doi: 10.1016/0014-4894(91)90021-n. [DOI] [PubMed] [Google Scholar]
- 47.Lustigman S, Brotman B, Huima T, Prince A M, McKerrow J H. Molecular cloning and characterization of onchocystatin, a cysteine proteinase inhibitor of Onchocerca volvulus. J Biol Chem. 1992;267:17339–17346. [PubMed] [Google Scholar]
- 48.Lustigman S, Brotman B, Huima T, Castelhano A L, Singh R N, Mehta K, Prince A M. Transglutaminase-catalyzed reaction is important for molting of Onchocerca volvulus third-stage larvae. Antimicrob Agents Chemother. 1995;39:1913–1919. doi: 10.1128/aac.39.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lustigman S, McKerrow J H, Shah K, Jing L, Huima H, Hough M, Brotman B. Cloning of a cysteine proteinase required for the molting of Onchocerca volvulus third-stage larvae. J Biol Chem. 1996;271:30181–30189. doi: 10.1074/jbc.271.47.30181. [DOI] [PubMed] [Google Scholar]
- 50.Ma D, Hong X, Raghavan N, Scott A L, McCarthy J S, Nutman T B, Williams S A, Carlow C K. A cyclosporin A-sensitive small molecular weight cyclophilin of filarial parasites. Mol Biochem Parasitol. 1996;79:235–241. doi: 10.1016/0166-6851(96)02654-0. [DOI] [PubMed] [Google Scholar]
- 51.Martzen M R, McMullen B A, Smith N E, Fujikawa K, Peanasky R J. Primary structure of the major pepsin inhibitor from the intestinal parasitic nematode Ascaris suum. Biochemistry. 1990;29:7366–7372. doi: 10.1021/bi00484a003. [DOI] [PubMed] [Google Scholar]
- 52.McDonnell D P, Vegeto E, Gleeson M A. Nuclear hormone receptors as targets for new drug discovery. Bio/Technology (New York) 1993;11:1256–1261. doi: 10.1038/nbt1193-1256. [DOI] [PubMed] [Google Scholar]
- 53.Moyle M, Foster D L, McGrath D E, Brown S M, Laroche Y, De Meutter J, Stanssens P, Bogowitz C A, Fried V A, Ely J A, Soule H R, Vlasuk G P. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem. 1994;269:10008–10015. [PubMed] [Google Scholar]
- 54.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen T T, Qasim M A, Morris S, Lu C C, Hill D, Laskowski M, Jr, Sakanari J A. Expression and characterization of elastase inhibitors from the ascarid nematodes Anisakis simplex and Ascaris suum. Mol Biochem Parasitol. 1999;102:79–89. doi: 10.1016/s0166-6851(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 56.Oberlander U, Adam R, Berg K, Seeber F, Lucius R. Molecular cloning and characterization of the filarial LIM domain proteins AvL3-1 and OvL3-1. Exp Parasitol. 1995;81:592–599. doi: 10.1006/expr.1995.1153. [DOI] [PubMed] [Google Scholar]
- 57.Page A P, Landry D, Wilson G G, Carlow C K. Molecular characterization of a cyclosporin A-insensitive cyclophilin from the parasitic nematode Brugia malayi. Biochemistry. 1995;34:11545–11550. doi: 10.1021/bi00036a030. [DOI] [PubMed] [Google Scholar]
- 58.Page A P, Winter A D. A divergent multi-domain cyclophilin is highly conserved between parasitic and free-living nematode species and is important in larval muscle development. Mol Biochem Parasitol. 1998;95:215–227. doi: 10.1016/s0166-6851(98)00096-6. [DOI] [PubMed] [Google Scholar]
- 59.Page A P. A highly conserved nematode protein folding operon in Caenorhabditis elegans and Caenorhabditis briggsae. Gene. 1999;230:267–275. doi: 10.1016/s0378-1119(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 60.Pastrana D V, Raghavan N, FitzGerald P, Eisinger S W, Metz C, Bucala R, Schleimer R P, Bickel C, Scott A L. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pogonka T, Oberlander U, Marti T, Lucius R. Acanthocheilonema viteae: characterization of a molt-associated excretory/secretory 18-kDa protein. Exp Parasitol. 1999;93:73–81. doi: 10.1006/expr.1999.4445. [DOI] [PubMed] [Google Scholar]
- 62.Prince A M, Brotman B, Johnson E H, Smith A, Pascual D, Lustigman S. Onchocerca volvulus: immunization of chimpanzees with X-irradiated third-stage (L3) larvae. Exp Parasitol. 1992;74:239–250. doi: 10.1016/0014-4894(92)90147-3. [DOI] [PubMed] [Google Scholar]
- 63.Richards F O, Miri E, Meredith S, Guderian R, Sauerbrey M, Remme H, Packard R, Ndiaye J M. Onchocerciasis. Bull W H O. 1998;76(Suppl. 2):147–149. [PMC free article] [PubMed] [Google Scholar]
- 64.Richer J K, Sakanari J A, Frank G R, Grieve R B. Dirofilaria immitis: proteases produced by third- and fourth-stage larvae. Exp Parasitol. 1992;75:213–222. doi: 10.1016/0014-4894(92)90181-9. [DOI] [PubMed] [Google Scholar]
- 65.Robbins J, Dilworth S M, Laskey R A, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 66.Rocheleau C E, Downs W D, Lin R, Wittmann C, Bei Y, Cha Y H, Ali M, Priess J R, Mello C C. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- 67.Sangster N. Pharmacology of anthelmintic resistance. Parasitology. 1996;113:S201–S216. doi: 10.1017/s0031182000077982. [DOI] [PubMed] [Google Scholar]
- 68.Savage C, Das P, Finelli A L, Townsend S R, Sun C Y, Baird S E, Padgett R W. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci USA. 1996;93:790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Selkirk M E, Smith V P, Thomas G R, Gounaris K. Resistance of filarial nematode parasites to oxidative stress. Int J Parasitol. 1998;28:1315–1332. doi: 10.1016/s0020-7519(98)00107-6. [DOI] [PubMed] [Google Scholar]
- 70.Taylor M J, Abdul-Wahab N, Jenkins R J, Bianco A E. Onchocerca volvulus larval antigen, OvB20, induces partial protection in a rodent model of onchocerciasis. Infect Immun. 1995;63:4417–4422. doi: 10.1128/iai.63.11.4417-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor M J, Jenkins R J, Bianco A E. Protective immunity induced by vaccination with Onchocerca volvulus tropomyosin in rodents. Parasite Immunol. 1996;18:219–225. doi: 10.1046/j.1365-3024.1996.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 72.Taylor M J, Van Es R P, Shay K, Folkard S G, Townson S, Bianco A E. Protective immunity against Onchocerca volvulus and Onchocerca lienalis infective larvae in mice. Trop Med Parasitol. 1994;45:17–23. [PubMed] [Google Scholar]
- 73.Tetteh K K, Loukas A, Tripp C, Maizels R M. Identification of abundantly expressed novel and conserved genes from the infective larval stage of Toxocara canis by an expressed sequence tag strategy. Infect Immun. 1999;67:4771–4779. doi: 10.1128/iai.67.9.4771-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turaga P S D, Tierney T J, Bennett K E, McCarthy M C, Simonek S C, Enyong P A, Moukatte D W, Lustigman S. Immunity to onchocerciasis: cells from putatively immune individuals produce enhanced levels of interleukin-5, gamma interferon, and granulocyte-macrophage colony-stimulating factor in response to Onchocerca volvulus larval and male worm antigens. Infect Immun. 2000;68:1905–1911. doi: 10.1128/iai.68.4.1905-1911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C C. Parasite enzymes as potential targets for antiparasitic chemotherapy. J Med Chem. 1984;27:1–9. doi: 10.1021/jm00367a001. [DOI] [PubMed] [Google Scholar]
- 76.Ward D J, Nutman T B, Zea-Flores G, Portocarrero C, Lujan A, Ottesen E A. Onchocerciasis and immunity in humans: enhanced T cell responsiveness to parasite antigen in putatively immune individuals. J Infect Dis. 1988;57:536–543. doi: 10.1093/infdis/157.3.536. [DOI] [PubMed] [Google Scholar]
- 77.Willenbucher J, Hofle W, Lucius R. The filarial antigens Av33/Ov33-3 show striking similarities to the major pepsin inhibitor from Ascaris suum. Mol Biochem Parasitol. 1993;57:349–351. doi: 10.1016/0166-6851(93)90212-g. [DOI] [PubMed] [Google Scholar]
- 78.Williams S A, Johnston D A. Helminth genome analysis: the current status of the filarial and schistosome genome projects. Filarial Genome Project. Schistosome Genome Project. Parasitology. 1999;118:S19–S38. doi: 10.1017/s0031182099004473. [DOI] [PubMed] [Google Scholar]
- 79.World Health Organization. WHO Expert Committee on Onchocerciasis Control. Onchocerciasis and its control. WHO technical report series 852. Geneva, Switzerland: World Health Organization; 1995. [PubMed] [Google Scholar]
- 80.Yates R A, Tuan R S, Shepley K J, Unnasch T R. Characterization of genes encoding members of the nuclear hormone receptor superfamily from Onchocerca volvulus. Mol Biochem Parasitol. 1995;70:19–31. doi: 10.1016/0166-6851(95)00018-v. [DOI] [PubMed] [Google Scholar]
- 81.Yenbutr P, Scott A L. Molecular cloning of a serine proteinase inhibitor from Brugia malayi. Infect Immun. 1995;63:1745–1753. doi: 10.1128/iai.63.5.1745-1753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zang X, Yazdanbakhsh M, Jiang H, Kanost M R, Maizels R M. A novel serpin expressed by blood-borne microfilariae of the parasitic nematode Brugia malayi inhibits human neutrophil serine proteinases. Blood. 1999;94:1418–1428. [PubMed] [Google Scholar]
- 83.Zimmerman P A, Guderian R H, Aruajo E, Elson L, Phadke P, Kubofcik J, Nutman T B. Polymerase chain reaction-based diagnosis of Onchocerca volvulus infection: improved detection of patients with onchocerciasis. J Infect Dis. 1994;169:686–689. doi: 10.1093/infdis/169.3.686. [DOI] [PubMed] [Google Scholar]