Abstract
Lethal shock can be associated with excessive secretion of cytokines such as tumor necrosis factor (TNF) and gamma interferon (IFN-γ). IFN-γ mediates macrophage activation and appears to be controlled by interleukin (IL)-12 and IL-18. To investigate the role of IL-18 in vivo, we generated IL-18-deficient mice by gene targeting. IL-18−/− mice showed decreased sensitivity towards lipopolysaccharide (LPS)-induced shock. LPS-induced IFN-γ production was abrogated, yet induction of IL-12 and TNF was not affected. Both wild-type and IL-18-deficient mice succumbed to LPS-induced lethal shock after sensitization with d-galactosamine. However, in marked contrast to LPS, the bacterial superantigen Staphylococcus aureus enterotoxin B (SEB) induced comparable serum levels of IFN-γ in IL-18+/+ and IL-18−/− mice, accompanied by an upregulation of cell surface markers CD14, CD122 (IL-2Rβ), and CD132 (IL-2Rγ) on peritoneal macrophages. Moreover, SEB injection rendered IL-18-deficient mice sensitive for subsequent challenge with LPS. The degree of sensitization was comparable to that in wild-type controls with respect to lethality. However, LPS-induced TNF levels in serum were significantly reduced in SEB-sensitized IL-18-deficient mice. These results imply that IL-18 plays an important role in induction of IFN-γ and lethality in response to LPS.
Interleukin-18 (IL-18), formerly known as gamma interferon (IFN-γ)-inducing factor, was initially identified as a strong inducer of IFN-γ production in mice treated with Propionibacterium acnes and lipopolysaccharide (LPS) (30, 31). Subsequently, IL-18 was shown to induce IFN-γ production from Th1 cells (31). Even B cells (43) and macrophages (29) could be brought to secrete IFN-γ by combined addition of IL-12 and IL-18. IL-18 was further defined as an important cofactor in enhancing the cytotoxic activity and proliferation of natural killer (NK) cells (17, 39, 42). IL-18 is primarily produced by dendritic cells (38), activated macrophages (31), and Kupffer cells (25) and appears to amplify Th1 development (35).
IL-18 is structurally related to the IL-1 family of cytokines (2), and its maturation and secretion are mediated by interleukin-1-beta-converting enzyme (ICE) (12, 14). ICE-knockout (ICE-KO) mice express neither mature IL-1α, IL-1β, nor IL-18 and exhibit a decreased sensitivity to LPS (20, 24). In contrast, IL-1β-deficient mice as well as IL-1RI-deficient mice, which are unresponsive to both IL-1α and IL-1β signaling, display normal sensitivity to LPS (8, 13, 44). Furthermore, ICE-KO mice show decreased LPS-induced IFN-γ production, although no alterations in IL-12 induction were observed (6, 12). These findings imply an important role of IL-18 in response to LPS, especially with respect to IFN-γ production.
Mice deficient for the IFN-γ receptor (IFN-γR) showed significantly decreased sensitivity to LPS, as determined by lethality and TNF production (4, 18). In accordance, liver injury caused by LPS was dramatically diminished in these mice. Furthermore, IFN-γ is critically involved in the pathogenesis of liver injury induced by concanavalin A (21). These findings imply a central role of IFN-γ in LPS-induced lethality and initiation and severity of liver injury. Similar to IFN-γ, IL-18 was also shown to be critical for LPS-induced liver injury in P. acnes-sensitized mice (31). Interestingly, IL-18 appeared to be essential during the sensitization to LPS mediated by P. acnes (37).
In this study, we generated IL-18-deficient (IL-18KO) mice to investigate the role of IL-18 during induction of and sensitization for lethal shock. IL-18KO mice displayed enhanced resistance to LPS. LPS-induced IFN-γ production was completely abrogated. In contrast, IL-18KO mice responded to the superantigen Staphylococcus aureus enterotoxin B (SEB) with IFN-γ production and could be sensitized by SEB for a subsequent challenge with LPS. These results indicate that sensitivity to LPS can be controlled by IL-18-dependent and -independent pathways. While IL-18 is critically involved during sensitization of mice to LPS by P. acnes (37), IL-18 is dispensable during sensitization mediated by superantigens.
MATERIALS AND METHODS
Cells.
E14.1 ES cells from 129Sv mice were maintained as described before (33).
Generation of IL-18-deficient mice.
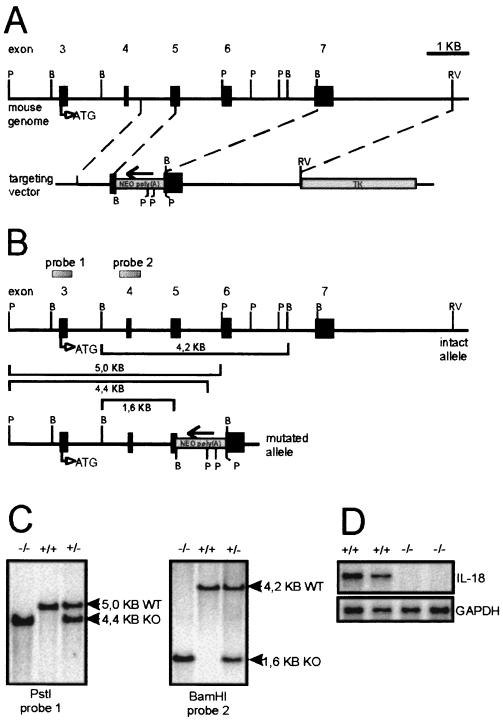
A genomic library (129/Sv/λDash2) was screened with a partial IL-18 cDNA clone. A genomic clone harboring a part of the murine IL-18 gene from exon 3 to exon 7 was isolated, subcloned into pBluescript vector (Stratagene), mapped, and partially sequenced (data not shown). The genomic structure was subsequently confirmed by the analysis described by Tone et al. (40). The targeting vector was constructed to replace a 3.3-kb genomic fragment with the neomycin resistance gene in pMC1neopolyA (Stratagene). The replaced genomic fragment contained a portion of exons 5 and 7 and all of exon 6 (Fig. 1). The neomycin gene cassette was flanked by the 0.84-kb 5′-homologous genomic fragment, which was generated by PCR, and the 3.2-kb 3′-homologous genomic BamHI-EcoRV fragment. Finally, the herpes simplex virus thymidine kinase gene (HSV-tk) cassette was ligated into the EcoRV site.
FIG. 1.
Targeted disruption of the mouse IL-18 gene. (A) Targeting vector. The upper thick line shows the IL-18 coding region of the mouse genome, with exons indicated as boxes and appropriate restriction enzyme sites labeled (B, BamHI; P, PstI; RV, EcoRV). The start codon of the IL-18 gene is indicated as ATG. To construct the targeting vector, NEO poly(A), a neomycin resistance gene cassette, was introduced at the indicated site, oriented as indicated by the arrow. TK, HSV tk gene. The thin lines represent the bacterial plasmid sequences. (B) Configuration of normal and mutated IL-18 alleles. The expected restriction fragment lengths are indicated, and the probes used for hybridization are depicted. (C) Southern blot analysis of DNAs. Genomic DNAs prepared from wild-type (WT, +/+), heterozygous (+/−), and IL-18KO (KO, −/−) mouse tails were digested with BamHI or PstI and hybridized with probe 1 or 2, as indicated. (D) Total RNA was prepared from untreated mouse livers (two mice each) and analyzed for IL-18 mRNA with an RNase protection assay. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
E14.1 ES cells were transfected with the NotI-linearized targeting vector as described before (33). G418- and gancyclovir-resistant clones were picked and screened by PCR for homologous recombination. Subsequently, positive PCR results were confirmed by genomic Southern blotting after digestion of ES cell DNA with BamHI and hybridization with a flanking probe (0.5-kb AccI-BglII fragment, indicated in Fig. 1 as probe 2). Single integration of the targeting vector was verified by Southern probing with the neo resistance cassette. Chimeric mice were generated as described before (33). Mice were housed in an animal facility under specific-pathogen-free conditions.
Injection protocols.
To study lethal shock induction, mice were injected intraperitoneally (i.p.) either with 20 mg of d-galactosamine (d-GalN; Roth, Karlsruhe, Germany) together with 2 μg of LPS (Escherichia coli O127:B8; Sigma, Deisenhofen, Germany), with LPS alone (dosages as indicated in Fig. 2B), or with 10 μg of SEB (Toxin Technologies, Sarasota, Fla.) and 5 h later with LPS (100 or 70 μg). To analyze cytokine production, mice were injected i.p. with either 500 or 1,000 μg of LPS.
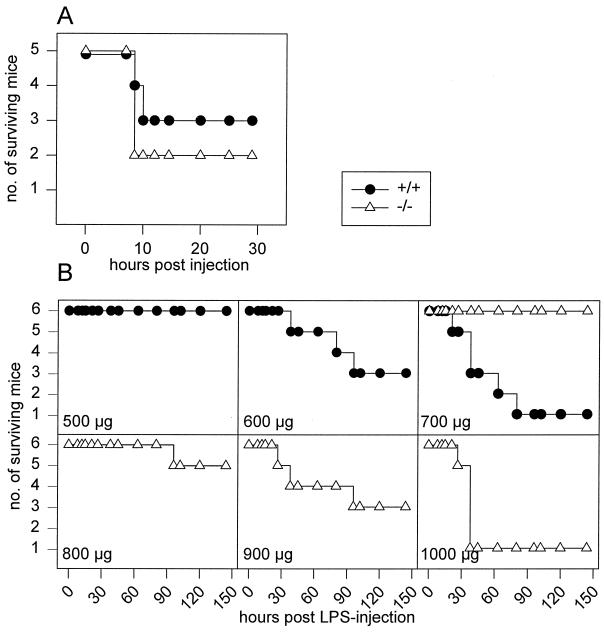
FIG. 2.
Enhanced resistance to LPS of IL-18KO mice. (A) d-GalN-dependent LPS shock. IL-18KO (KO) and wild-type (WT) mice (n = 5) were injected i.p. with 20 mg of d-GalN together with 2 μg of LPS, and lethality was monitored. (B) LPS shock model. Wild-type and IL-18KO mice (n = 6) were injected i.p. with increasing doses of LPS, and lethality was monitored.
Analysis for cytokine mRNA expression.
At different time points after injection, spleens were removed and frozen in liquid nitrogen. Subsequently, total RNA was extracted using Tri-Reagent (Sigma) following the protocol given by the manufacturer. The presence of mRNAs for the housekeeping gene L32 (protein from the large ribosomal subunit) and cytokines was determined with an RNase protection assay (Pharmingen, Hamburg, Germany) as described by the manufacturer.
Determination of cytokines.
For determination of cytokine release kinetics in serum, mice were killed at the indicated time points after treatment, and serum samples were harvested. Cytokine levels, except IL-18, were determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits (Pharmingen). IL-18 levels were determined with a rat anti-mouse IL-18 monoclonal antibody (clone 51817.111) and a goat anti-mouse IL-18 polyclonal biotinylated antibody from R&D Systems (Wiesbaden, Germany).
Flow cytometric analysis.
Peritoneal exudate cells (PEC) were harvested 12 h after i.p. SEB injection and kept through all staining procedures in phosphate-buffered saline with 2% (wt/vol) bovine serum albumin (Sigma, Deisenhofen, Germany). PEC were pretreated with 10% homologous mouse serum and subsequently labeled with specific fluorescein isothiocyanate- and R-phycoerythrin-coupled antibodies (Pharmingen). PEC were gated on F4/80 expression (murine macrophage-specific antigen) to define peritoneal macrophages, and fluorescence intensity was measured with a Coulter EPICS XL cytometer (Coulter, Hialeah, Fla.).
RESULTS
Generation of an IL-18-deficient mouse strain.
The murine IL-18 gene consists of seven exons (40; unpublished data). A targeting vector was designed to partially replace exons 5 and 7 and to replace exon 6 entirely with a neomycin resistance gene cassette. Homologous recombination of the IL-18 locus with the targeting vector was performed in E14.1 embryonic stem (ES) cells (Fig. 1A and B). Homologous recombination was detected in 2 of 47 clones that were resistant to both G418 and gancyclovir. After microinjection into C57BL/6 blastocysts, both targeted ES cell clones transmitted the disrupted gene successfully to germline, as determined by Southern blot analysis of mouse tail DNA (Fig. 1C). IL-18-deficient (IL-18KO) mice were born at the expected Mendelian ratio (45+/+:104+/−:62−/−). IL-18 mRNA was not detectable in the livers of IL-18KO mice, as determined by the RNase protection assay (RPA) (Fig. 1D). IL-18KO mice developed normally, were fertile, and showed no obvious abnormalities up to 52 weeks of age. Flow cytometry revealed a normal gross distribution of lymphocytes in the spleen, mesenteric lymph nodes, and thymus; however, the intensity of CD3 expression on T cells was reduced by about a third in these organs. Mean fluorescence intensity (MFI) values were 16.7 and 11.3 in the spleen, 35.9 and 22.1 in the mesenteric lymph nodes, and 20 and 15.3 in the thymus for the wild-type and IL-18KO mice, respectively (values are representative of two independent individuals each). In vitro antibody cross-linking of CD3 on T cells from IL-18KO mice revealed normal responsiveness with respect to IL-2 production and proliferation (data not shown).
LPS sensitivity of IL-18-deficient mice.
IL-18 was initially defined as a cytokine involved in hyperreactivity induced by LPS subsequent to sensitization with P. acnes (30, 31). In this study, we analyzed whether IL-18KO mice would display an altered reactivity to LPS without presensitization. Groups of six mice each were injected with increasing amounts of LPS, and lethality was monitored. The approximate 50% lethal dose (LD50) was 600 μg of LPS for littermate controls and 900 μg of LPS for IL-18KO mice; 90% lethality was achieved with 700 μg of LPS in wild-type controls and 1,000 μg of LPS in IL-18KO mice (Fig. 2B). Thus, IL-18-deficient mice tolerated a 50% higher LPS dose than wild-type mice.
We next tested whether d-GalN would sensitize IL-18KO mice to the lethal actions of LPS. d-GalN is known to impede the acute-phase response of liver cells, rendering these cells sensitive to TNF-mediated apoptosis (10, 11, 22). In this system, TNF levels in serum of about 0.5 ng/ml appear to be sufficient to cause lethality within 8 to 12 h (11, 23). When we administered d-GalN together with LPS, no difference in lethality between IL-18KO mice and littermate controls was observed. Both groups showed about 50% lethality under these experimental conditions (Fig. 2A). Thus, IL-18KO mice can be sensitized by d-GalN and show in this respect no alteration in response to LPS compared with wild-type mice.
To examine the enhanced resistance to LPS-induced shock of IL-18KO mice, we next analyzed cytokine mRNA expression and cytokine production induced by LPS. Mice were injected with 500 μg of LPS, and splenic mRNA was analyzed by an RNase protection assay (Fig. 3). We found induction of TNF mRNA in the spleens of IL-18KO mice compared with littermate controls, whereas no induction of IFN-γ mRNA was observed in the IL-18KO mice. Even when lethal doses (1,000 μg) of LPS were applied to IL-18KO mice, no induction of IFN-γ mRNA was recorded (Fig. 3A). We further determined mRNA expression kinetics for IL-12 p35, IL-12 p40, IL-10, IL-6, IL-1α, IL-1β, and IL-1 receptor antagonist. mRNAs for these cytokines were expressed in IL-18KO mice as in littermate controls (data not shown).
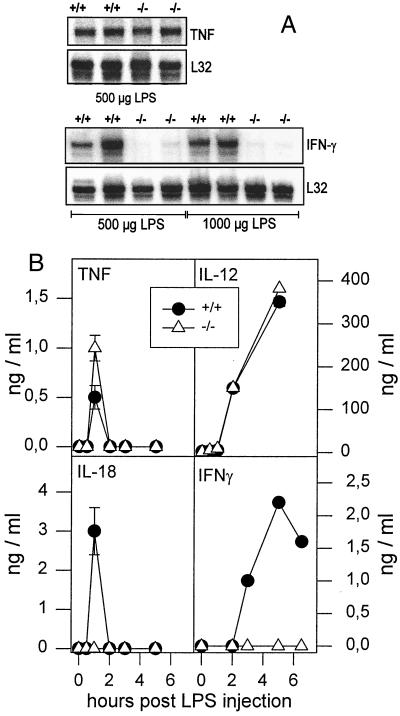
FIG. 3.
Altered cytokine pattern in IL-18KO mice after LPS treatment. (A) Wild-type (+/+) and IL-18KO (−/−) mice (n = 2) mice were injected i.p. with 500 or 1,000 μg of LPS, as indicated. Spleens were harvested after 1 h (TNF) or 5 h (IFN-γ), and RNase protection assays with total splenic RNA were performed. For standardization of the loaded probe amount, we used the mRNA signal of the housekeeping gene L32 (protein L32 from the large ribosomal subunit). (B) Wild-type and IL-18KO mice were injected i.p. with 500 μg of LPS. Serum samples were taken at the indicated times after LPS administration. Each cytokine concentration was determined by ELISA. Results are the mean values for three mice per time point for IL-18 and TNF and at least one mouse per time point for IL-12 and IFN-γ. Error bars represent the standard deviation (n = 3).
Corresponding serum levels of IL-12 p40, IL-18, IFN-γ, and TNF were also determined (Fig. 3B). As expected, no IL-18 was detectable in the serum of IL-18KO mice, whereas in wild-type controls, IL-18 peaked after 1 h and returned to background levels 2 h after LPS administration. The serum kinetics of TNF were similar, but slightly increased levels were measured in IL-18KO mice. IL-12 p40 expression and kinetics were identical in IL-18KO mice and in wild-type littermate controls (Fig. 3B). The most remarkable difference was found with IFN-γ. No IFN-γ was detectable in the serum of IL-18KO mice after LPS administration, while wild-type controls mounted a strong response that peaked after 5 h (Fig. 3B). Since IL-12 p40 levels in serum and expression of mRNAs for IL-12 p35 and IL-12 p40 were not changed in IL-18KO mice, the results imply that IL-18 is an important cytokine for induction and control of IFN-γ production after challenge with LPS.
Sensitization to LPS by superantigen acts independently of IL-18.
We have previously shown that superantigens like SEB sensitize mice to LPS for a defined period of time (16). Administration of SEB leads to a cyclosporine A-sensitive induction of IFN-γ production, presumably via SEB-mediated activation of Vβ8 T-cell receptor (TCR)-expressing cells. Blocking IFN-γ with neutralizing monoclonal antibodies abrogated the sensitizing effects of SEB (16). Since IL-18KO mice did not induce IFN-γ in response to LPS and since IFN-γ is crucial for SEB-mediated sensitization, we analyzed whether SEB would sensitize IL-18KO mice to LPS-mediated shock. IL-18KO mice and wild-type controls were primed with SEB and challenged with LPS 8 h later. The dose of LPS used was nonlethal in nonsensitized mice (data not shown). However, after sensitization with SEB, IL-18KO mice as well as wild-type littermates showed the same lethality after challenge with LPS (Fig. 4A). Thus, sensitization to LPS by SEB appears to function even in the absence of IL-18. When we measured IFN-γ production in IL-18KO mice induced with SEB 5 and 8 h after SEB administration, we detected identical IFN-γ levels in serum from IL-18KO mice and wild-type controls (Fig. 4C). We have shown previously that SEB administration in addition upregulates expression of CD14, IL-2Rβ, and IL-2Rγ chains on F4/80+ macrophages within PEC (16). For this reason, the upregulation of these surface markers on PEC can be used as an indicator to determine the magnitude of macrophage activation induced by SEB. After SEB administration, both wild-type and IL-18KO mice upregulated these surface markers on peritoneal macrophages (Fig. 4B). Collectively, these findings imply that SEB triggered the release of IFN-γ from activated Vβ8-TCR+ T cells and that subsequent activation of macrophages is independent of IL-18.
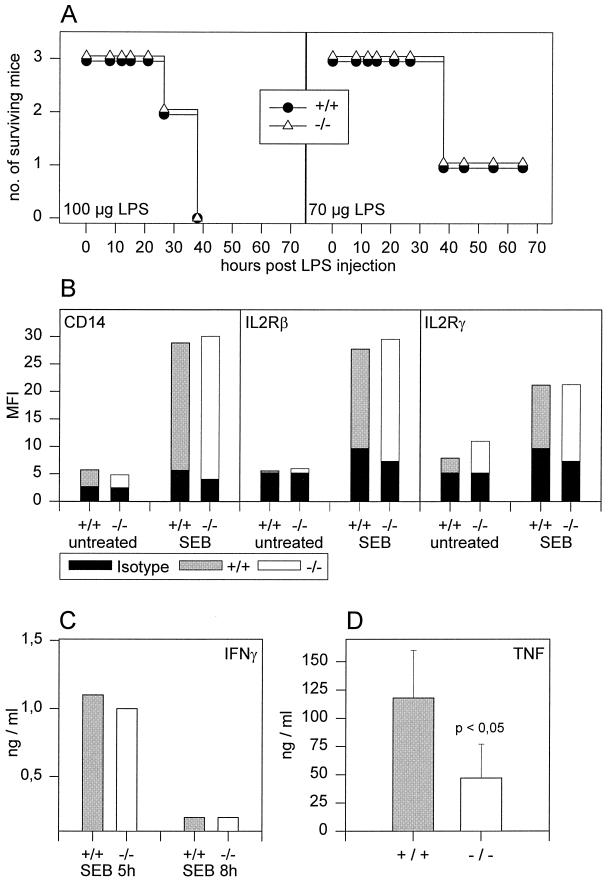
FIG. 4.
IL-18KO mice react normally to SEB. Wild-type (+/+) and IL-18KO (−/−) mice were injected i.p. with 10 μg of SEB. (A) Eight hours after priming, mice were challenged with the indicated doses of LPS, and lethality was monitored. (B) PEC were harvested 12 h after SEB treatment and labeled for F4/80, CD14, IL-2Rβ, and IL-2Rγ. After gating on F4/80+ cells, MFI values for surface markers were measured as indicated. Solid bars indicate MFI values after labeling with isotype control antibodies. (C) At 5 and 8 h after SEB i.p. injection, serum samples were harvested, and IFN-γ concentrations were determined by ELISA. (D) One hour after LPS (100 μg) challenge of SEB-primed mice, serum samples were harvested, and TNF levels were determined by ELISA. Error bars represent the standard deviation (n = 4).
Sakao et al. have shown that in the absence of IL-18, TNF expression in mice sensitized with P. acnes and challenged with LPS is increased up to 10-fold (37). To determine whether IL-18 would influence TNF expression after sensitization with SEB, we determined TNF levels in the serum of IL-18KO mice and wild-type controls after SEB priming and subsequent LPS challenge (Fig. 4D). Although peak TNF levels in response to LPS challenge were markedly enhanced after SEB sensitization in both IL-18KO and wild-type mice (compare Fig. 4D and 3B), the degree of augmentation was reduced in IL-18KO mice. In contrast to sensitization with P. acnes, potentiation of TNF expression after LPS challenge in SEB-sensitized mice seems to be only partially dependent on IL-18.
DISCUSSION
In the present report, we have generated and characterized mice deficient in IL-18 and studied their response to LPS and superantigen in models of lethal shock. IL-18KO mice were fertile, developed normally, and showed no obvious signs of disease up to 52 weeks in age. These findings imply that IL-18 may not be involved in crucial developmental processes of the mouse. Furthermore, this strongly suggests that IL-18 is not critically important for limiting the overgrowth of normal bacterial flora. However, in response to LPS, IL-18KO mice displayed decreased sensitivity to LPS and lack of IFN-γ production and yet a preserved TNF induction.
The in vivo response to LPS is not uniform but is critically dependent on the status of the immune system itself. Phases of LPS hyporeactivity (LPS tolerance) as well as LPS hyperreactivity have been identified (10, 26, 34, 45). While the former is mechanistically poorly understood yet, the latter has received profound attention. It has been recognized that although many different agents might induce hyperreactivity to LPS, the underlying mechanisms might be quite similar. Bacteria such as P. acnes (19) and other gram-positive and gram-negative bacteria (19, 26), bacterial components such as LPS itself (Shwartzman reaction) (32), and bacterial products such as superantigens (16) induce hyperreactivity to LPS. Common to most inducers of hyperreactivity to LPS is their profound ability to stimulate IFN-γ production, which has been defined as a key mediator of induction of hyperreactivity to LPS (4, 5, 32). For example, IFN-γ is a key mediator in the classical systemic Shwartzman reaction in both the sensitization and challenge phases (32). Several cytokine-deficient mouse strains are known to be resistant to the lethal actions of LPS (for a review, see reference 15). Of interest, IFN-γR-KO mice were shown to display enhanced resistance to LPS. Furthermore, clinical changes caused by LPS, such as weight loss and liver injury, were dramatically decreased in these mice (4). These findings imply a central role for IFN-γ in the response to LPS, especially in lethal shock. The failure of IL-18KO mice to produce IFN-γ after challenge with LPS and their decreased sensitivity to the lethal action of LPS further underline the central role of IFN-γ.
Production of IFN-γ itself, however, can be modulated by regulatory cytokines such as IL-12 and IL-18. IL-12 has the capacity to induce IFN-γ production and to act synergistically with IL-18 (31). B cells and macrophages were shown to produce IFN-γ only after simultaneous stimulation with IL-12 and IL-18 (29, 43), whereas T cells and NK cells have been reported not to require both cytokines to produce IFN-γ (3, 9, 17, 41). The relative significance of these cytokines during induction of LPS hyperreactivity might therefore depend on the nature of the stimulus and/or the target cell. After challenge with LPS, the expression of mRNAs for IL-12 p35 and IL-12 p40 and the level of IL-12 p40 in serum in IL-18KO mice compared with their wild-type controls. This might indicate that IL-18 contributes significantly to induction of IFN-γ by IL-12. Interestingly, recent experiments in caspase-1-deficient mice resulted in a similar conclusion (7). The mechanism by which IL-18 exerts this effect is not known, but one might speculate that IL-18 interferes with functional IL-12 receptor expression or IL-12-dependent signal cascades within the target cells.
In contrast to sensitization by bacteria or their products mediated via cytokines, mice can also be sensitized to LPS challenge by the chemical compound d-GalN (11). This agent induces transcriptional arrest in hepatocytes, sensitizing these cells to TNF-mediated apoptosis (1, 23, 28). It is generally accepted that in d-GalN-sensitized mice, TNF alone is sufficient to induce severe liver cell apoptosis and thus death (11, 22, 27, 33, 36). d-GalN-treated IL-18KO mice produced equal amounts of TNF after challenge with LPS and were as susceptible as control mice. These observations lead us to conclude that the impaired IFN-γ production in LPS-challenged IL-18KO mice is responsible for the increased LPS resistance observed in the absence of d-GalN. This conclusion is in accordance with previous reports showing the significance of IL-18 during the LPS challenge phase after P. acnes sensitization (31).
We further studied the effect of IL-18 on superantigen-mediated sensitization to LPS (16). In vivo administration of SEB sensitizes mice for a defined period of time to LPS (superantigen-mediated Shwartzman-like reaction). This sensitization is strictly dependent on T-cell-derived IFN-γ (16). Here we show that induction of IFN-γ by SEB and subsequent sensitization to LPS are not significantly altered in IL-18KO mice. No differences from wild-type mice were found with respect to expression of CD14, IL-2Rβ, and IL-2Rγ on peritoneal macrophages and to LPS-induced lethality. This indicates that reduced reactivity to LPS in nonsensitized IL-18KO mice cannot be due to a generalized suppression of macrophages. Furthermore, IFN-γ release by superantigen-activated T cells seems to be independent of IL-18. In terms of augmentation of TNF levels, sensitization efficacy might be reduced in IL-18KO mice (Fig. 4D). Whether this reflects a bystander role for IL-18 during SEB-mediated sensitization is currently under investigation.
In conclusion, we investigated the role of IL-18 during the immune response to bacterial products such as endotoxin (LPS) and superantigens (SEB). Our results demonstrate that IL-18 is important for the expression of LPS-induced IFN-γ and thus influences the response to LPS. In contrast, activation of T cells with a superantigen (SEB) and subsequent IFN-γ production act independently of IL-18.
ACKNOWLEDGMENTS
We thank Sylvia Bendigs, Ulrike Huffstadt, Susanne Weiss, Agnes Fütterer, Evi Schaller, and Karin Mink for excellent technical assistance.
This work was supported by a grant from BMBF “Sepsisverbund.”
REFERENCES
- 1.Bachmann W, Harms E, Hassels B, Henninger H, Reutter W. Studies on rat liver plasma membrane: altered protein and lipid metabolism after injection of d-galactosamine. Biochem J. 1977;166:455–462. doi: 10.1042/bj1660455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazan J F, Timans J C, Kastelein R A. A newly defined interleukin-1? Nature. 1996;379:591. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 3.Cai G, Kastelein R A, Hunter C A. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol. 1999;29:2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Car B D, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon gamma receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty G M, Lange J R, Langstein H N, Alexander H R, Buresh C M, Norton J A. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol. 1992;149:1666–1670. [PubMed] [Google Scholar]
- 6.Fantuzzi G, Puren A J, Harding M W, Livingston D J, Dinarello C A. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1beta-converting enzyme (caspase-1)-deficient mice. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 7.Fantuzzi G, Reed D A, Dinarello C A. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Investig. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fantuzzi G, Zheng H, Faggioni R, Benigni F, Ghezzi P, Sipe J D, Shaw A R, Dinarello C A. Effect of endotoxin in IL-1beta-deficient mice. J Immunol. 1996;157:291–296. [PubMed] [Google Scholar]
- 9.Fehniger T A, Shah M H, Turner M J, VanDeusen J B, Whitman S P, Cooper M A, Suzuki K, Wechser M, Goodsaid F, Caligiuri M A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 10.Freudenberg M A, Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization of the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 13.Glaccum M B, Stocking K L, Charrier K, Smith J L, Willis C R, Maliszewski C, Livingston D J, Peschon J J, Morrissey P J. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 14.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S S. Activation of interferon-gamma inducing factor mediated by interleukin 1-beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez-Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 16.Hochholzer P, Blank C, Bendigs S, Wagner H, Heeg K. Superantigen-induced Shwartzman-like reaction: critical role of interferon-gamma. In: Faist E, editor. The immune consequences of trauma, shock and sepsis. Bologna, Italy: Monduzzi Editore; 1997. pp. 479–482. [Google Scholar]
- 17.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 18.Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katschinski T, Galanos C, Coumbos A, Freudenberg M A. Gamma interferon mediates Propionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect Immun. 1992;60:1994–2001. doi: 10.1128/iai.60.5.1994-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuida K, Lippke J A, Ku G, Harding M W, Livingston D J, Su M S S, Flavell R A. Altered cytokine export and apoptosis in mice deficient in interleukin-1b converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 21.Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leist M, Gantner F, Bohlinger I, Germann P G, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-α requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 24.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, Towne E, Tracey D, Wardwell S, Wei F Y, Wong W, Kamen R, Seshadri T. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 25.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 26.Matsuura M, Galanos C. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect Immun. 1990;58:935–937. doi: 10.1128/iai.58.4.935-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang G Z, Takahashi K, Tamada Y, Yokochi T. Apoptotic cell death in the response of d-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun. 1996;64:734–738. doi: 10.1128/iai.64.3.734-738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata Y, Tanabe F, Akita K, Torigoe K, Okura T, Fukuda S. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 32.Ozmen L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Gately M, Garotta G. Interleukin 12, interferon gamma and tumor necrosis factor alpha are the key mediators of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeffer K, Matsuyama T, Kündig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T W. Mice deficient for the 55 kD tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 34.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk H D. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor β. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 36.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R M, Steinmetz M, Bluethmann H. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 37.Sakao Y, Takeda K, Tsutsui H, Kaisho T, Nomura F, Okamura H, Nakanishi K, Akira S. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int Immunol. 1999;11:471–480. doi: 10.1093/intimm/11.3.471. [DOI] [PubMed] [Google Scholar]
- 38.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk A H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 40.Tone M, Thompson S A J, Tone Y, Fairchild P J, Waldmann H. Regulation of IL-18 (IFN-gamma-inducing factor) gene expression. J Immunol. 1997;159:6156–6163. [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 43.Yoshimoto T, Okamura H, Tagawa Y I, Iwakura Y, Nakanishi K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann K J, Conn C A, Soszynski D, Grabiec C, Trumbauer M E, Shaw A, Kostura M J, Stevens K, Rosen H, North R J, Chen H Y, Tocci M J, Kluger M J, van der Ploeg L H T. Resistance to fever induction and impaired acute-phase response in interleukin-1beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler-Heitbrock H W. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]