Abstract
Background
Progressive scoliosis in neuromuscular patients often requires a long instrumented spinal fusion. Previous studies have shown larger intraoperative blood loss in these patients than those with adolescent idiopathic scoliosis (AIS), but the total blood loss composed of visible and hidden blood loss has not been described in this patient population. The aim of our study was to investigate the bleeding characteristics and hidden blood loss related to spinal fusion in neuromuscular scoliosis (NMS) as compared to AIS patients.
Methods
A retrospective cohort study with prospective data collection of NMS patients undergoing segmental pedicle screw instrumentation at a university hospital between 2009 and 2021. A comprehensive statistical analysis was performed regarding intra- and postoperative blood loss compared to patient characteristics such as age and diagnosis. Hidden blood loss was estimated and compared to the total blood loss. We standardized amount of bleeding with patient weight and fusion level for further analysis. Consecutive AIS patients served as a control population. Eighty-one consecutive patients with NMS (mean age 15.2 years, 37 females) were included and their bleeding characters were compared with 199 AIS patients (mean age 15.8 years, 143 females). The primary outcome was total blood loss including intraoperative, 24-hour drain output and hidden blood loss. Secondary outcome measures included requirement for blood transfusion.
Results
Neuromuscular patients had a significantly larger total blood loss with mean values of 1914 mL in the NMS and 1357 mL in the AIS groups, respectively (p<0.001). The hidden blood loss was also significantly larger in NMS than in AIS group (566 ± 533 mL vs. 398 ±411 mL, p=0.0332). Neuromuscular scoliosis was also associated with significantly greater weight and levels of fused adjusted intraoperative bleeding (1.79 ml/kg/fused level in NMS vs. 0.87 ml/kg/fused level in AIS vs. p< 0.0001) and hidden blood loss (1.00 ml/kg/levels fused vs. 0.65 ml/kg/fused levels, p=0.053). NMS diagnosis was also a risk factor for greater hidden blood loss in multivariable analysis (p=0.0011). 24-hour drain output was similar in the NMS and AIS groups. Male gender was a risk factor for greater hidden blood loss in the NMS group (p=0.0429). Fifty-nine (73%) NMS and 27 (14%) AIS patients received allogenic red blood cell infusions perioperatively (p<0.001).
Conclusions
Hidden blood loss constitutes one-third of total blood loss in children undergoing segmental pedicle screw instrumentation for neuromuscular scoliosis. Hidden blood loss is significantly greater in the neuromuscular as compared with idiopathic scoliosis. Hidden blood loss should be considered in the perioperative management of NMS.
Keywords: Scoliosis, Spinal fusion, Bleeding, Blood loss, Neuromuscular scoliosis, Idiopathic scoliosis, Pediatric
Introduction
Approximately 25% of the pediatric scoliosis patients have a neuromuscular diagnosis [1]. Indications for surgical treatment of neuromuscular scoliosis (NMS) include major curvature progression, loss of spinal balance, and pelvic obliquity affecting sitting balance and/or posture [2], [3], [4]. The literature has described different indications for pelvic fixation in neuromuscular scoliosis [5,6]. Non-ambulatory children with neuromuscular scoliosis typically undergo spinal fusion extending from upper thoracic spine to pelvis [7], [8], [9], [10]
Blood loss is an important factor to acknowledge when performing posterior spinal fusion (PSF) in pediatric patients, as intraoperative bleeding up to 100% of the patient's blood volume has been described in the literature [11]. Voluminous bleeding might be caused by the high vascularity of the paraspinal muscles, epidural venous plexus, posterior column osteotomies, or bony bleeding during pedicle screw placement. Excessive intraoperative blood loss has been associated with clotting abnormalities, hypothermia, and a higher need for blood transfusions. Allogenic blood transfusions increase the risk for postoperative infection and delays recovery [11], [12], [13]. The underlying diagnosis is an important factor affecting the blood loss [11,14,15]. Patients with NMS have a higher risk of intra- and postoperative complications associated with surgical treatment than patients with adolescent idiopathic scoliosis (AIS) [1,[16], [17], [18]].
Total blood loss is composed of measurable blood loss such as intraoperative hemorrhage and drain output [19,20], and hidden blood loss, defined as blood accumulated intra- and postoperatively into the surrounding tissues and the surgical area [21], [22], [23]. Risk factors for higher intraoperative bleeding include more severe spinal deformity, longer surgical time, and more extensive fusion. However, the differences between patient groups and the characteristics of the patients are not entirely known [[11], [12], [13],[24], [25], [26], [27], [28]]. Gelatin matrix with human thrombin [19], use of plasma blade [29] and anterior approach only [11], has been suggested to reduce intraoperative blood loss [11,15,[29], [30], [31]]. Postoperative 24 hour drain output often equals the intraoperative blood loss in adolescents undergoing segmental pedicle screw instrumentation for AIS [19,20].
The hidden blood loss (HBL) was first introduced by Sehat and al. in 2000 [32], and has since grown its interest in spine surgery research [21,33,34]. The HBL is thought to be caused by hemolysis, extravasation of blood into the tissues of the surgical site and subfascial hemorrhage [35], [36], [37]. The studies have shown hidden blood loss as a significant part of the total postoperative bleeding [21,22,35,36] and it has even exceeded the intraoperative blood loss in some patients undergoing PSF for AIS [23]. Intraoperative blood loss is known to be larger in neuromuscular scoliosis patients as compared to idiopathic, but their bleeding characteristics in terms of total blood loss including drain output and hidden blood loss remain poorly understood.
The aim of our study was to determine the bleeding characteristics and especially hidden blood loss in children with neuromuscular scoliosis undergoing segmental pedicle screw instrumentation. We hypothesized that postoperative drain output and hidden blood loss (per levels fused) would be significantly larger in NMS than in AIS.
Material and methods
We conducted a retrospective cohort study with consecutive children undergoing segmental pedicle screw instrumentation for neuromuscular scoliosis at our University Hospital between 2009 and 2021. The data was prospectively collected in our institutional pediatric spine register. The findings were compared to a consecutive cohort of patients with adolescent idiopathic scoliosis undergoing pedicle screw instrumentation during the same time period. Ethical committee approval was obtained for the study (ETMK 96/1801/2020).
Preoperative clinical examination and testing were standardized including upright (sitting in neuromuscular and standing in AIS) posteroanterior and lateral radiographs of the spine. All AIS patients underwent MRI of the spine prior to surgery. Laboratory testing included a standard coagulation profile (thrombocytes, activated partial thromboplastin time [aP-TT], international normalized ratio [INR], thrombin time [TT]), blood count including hemoglobin (Hb), electrolytes, creatinine, c-reactive protein (CRP), creatine kinase (CK), procalcitonin (PCT), and Ferritin.
Anesthetic management
Surgical and anesthetic management were standardized. Anesthesia was given by a consultant anesthesiologists qualified in pediatric anesthesia. Patients received total intravenous anesthesia, including propofol, remifentanil and dexmedetomidine infusion. Normothermia was maintained throughout the procedure. A mean arterial pressure of 65-75 mmHg was maintained during the surgery and 24 hours postoperatively; noradrenaline infusion was used if needed. All patients received an intravenous bolus of tranexamic acid (30mg/kg, max 1500mg) before the incision followed by an infusion (10mg/kg/h, max 500mg/h) during the procedure.
Preoperative surgical planning for implant placement and the need for posterior column osteotomies was done. In NMS non-ambulatory patients were fused from upper thoracic level T2/T3 to pelvis. Ambulatory patients without pelvic obliquity were fused to level L4 or L5, and patients with pelvic obliquity to S2AI or iliac screws. In AIS the selection for lowest instrumented vertebra was made according to Lenke classification [38].
Surgical protocol
The surgery was performed in a prone position, exposing the posterior elements subperiosteally with monopolar electrocautery, including careful hemostasis using bipolar and temporary packing. The spinal deformity was corrected using bilateral segmental pedicle screw instrumentation (6.35 Legacy, Solera 6.0, Medtronics Spinal and Biologics, Memphis, Tennessee, USA; Mesa2, Stryker Spine, USA). Pedicle screws were inserted using free-hand technique [39,40], with verification of screw placement using C-arm and later when that became available, using O -arm [41]. Correction of spinal deformity was performed using double rod cantilever, direct translation and segmental compression/distraction. Autograft from facetectomies and osteotomies and bone from a morselized femoral head allograft in the neuromuscular scoliosis group were applied on decorticated posterolateral bony elements to obtain spinal fusion. Patients with AIS received local autograft with bone graft extenders (Tricalcium phosphate and Nanostim, Medtronic; iFactor, Cerapedics). Gelatin matrix with human thrombin (2-4 × 5 mL, Floseal, Baxter) was routinely used to limit bleeding from cancellous bone, pedicle screw channels, and epidural space when performing posterior column osteotomies. Postoperative wound closure was carefully performed by the senior and attending surgeon in layers including running suture for fascia (Vicryl 1), subcutaneous tissue (subdermal, Vicryl 2-0), and skin always with intradermal running suture (Monocryl 3-0). All patients received a single closed suction subfascial drain (Hemovac Ch 14, Zimmer Biomet, Warsaw, Indiana, US). The operations were performed by a single experienced pediatric orthopedic spine surgeon (IH), together with another attending pediatric orthopedic surgeon.
Neurophysiological monitoring was conducted routinely at specific operational time points (incision, full exposure, after pedicle screw insertion, correction completed, and after wound closure) and every 20 minutes intraoperatively. All patients received cefuroxime and vancomycin as antibiotic prophylaxis at induction and three doses postoperatively. The hemoglobin threshold level for allogenic red blood cell transfusion was 80 g/L. Fresh frozen plasma was given if the blood loss reached 50% of the estimated blood volume of the patient. Platelets were infused if blood loss exceeded 100% of the blood volume. The estimated blood volume of the patient was calculated using the formula 70ml/kg x weight (kg).
Intraoperative bleeding was estimated by measuring the amount of blood suctioned from the surgical site and absorbed into the surgical drapes, with the subtraction of the amount of fluid used for irrigation. The amount of perioperative bleeding 24-hour postoperative drain output and the intra- and postoperative transfusion rate were used as bleeding markers. Cell saver was utilized for intraoperative blood salvage in 86% of the NMS patients, and 50% of the AIS patients, and the autologous blood was returned to the patients. Subfascial Ch14 closed suction drain was routinely removed 24 hours postoperatively. A combined bleeding marker was also applied to estimate the total bleeding.
Outcomes
The primary outcome was total blood loss. Total measured blood loss included intraoperative blood loss and 24-hour drain output. Total blood loss included intraoperative blood loss, 24-hour drain output, and calculated hidden blood loss. Secondary outcomes included autologous (cell saver) and allogenic blood transfusion rate (red blood cell units, fresh frozen plasma, and thrombocytes).
Statistical analysis
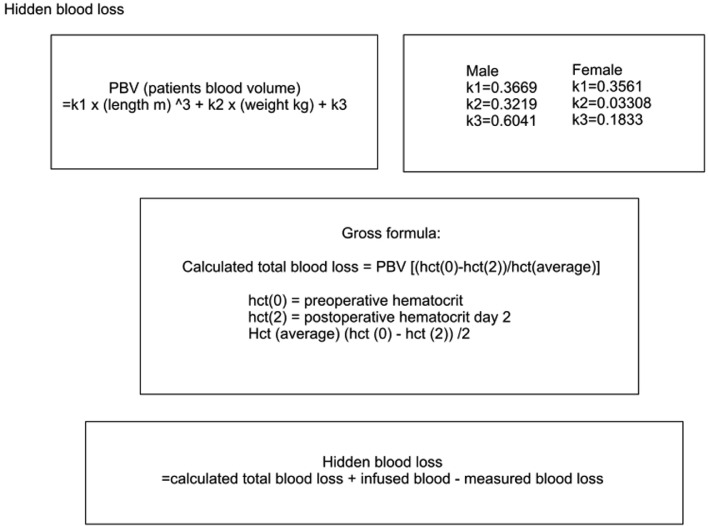
Comprehensive statistical analysis was performed with JMP Pro 16.2. Linear fit was used for suitable data and nonparametric tests were applied for nonparametric data. Hidden blood loss was calculated according to principles demonstrated in previous publications and visualized in Fig. 1. The pre- and postoperative hematocrit levels and estimated patient blood volume are utilized to calculate an approximate of the total blood loss. The amount of infused blood is added, and the measured blood loss is subtracted from this estimated total blood loss in order to find out the hidden blood loss [21,35,42,43]. Total bleeding was calculated combining intraoperative blood loss, drain output and hidden blood loss. The amount of bleeding was also divided with the patient weight and number of fused levels for further analysis (ml/kg/fused level).
Fig. 1.
Results
Baseline characteristics
Our data included 81 consecutive children with NMS and their findings were compared to 199 adolescents with AIS (Table 1). One NMS patient was excluded based on a complication that led to reoperation within the first postoperative day. Mean age ± SD of the patients was 15.5 ± 2.5 years and 180 (64%) of the patients were female. Operative time was significantly longer in the NMS than in the AIS group (mean 4.2±1.2 hours vs. 3.1±0.9 hours, p<0.001). Posterior column osteotomies were performed for a total of 39 NMS patients and 49 AIS patients, on average 3.49 ± 0.25 in NMS, and 2.96 ± 0.22 in AIS, the difference of which did not have statistical significance p = 0.1920.
Table 1.
Clinical characteristics of the study groups.
| NMS (N=81) | AIS (N=199) | p value* | |
|---|---|---|---|
| Age | 15.2 ± 3.4 | 15.6 ± 2.1 | p = 0.201 |
| Gender (M/F) | 44/37 | 56/143 | p<0.001 |
| Major curve, degrees Preoperatively Postoperatively |
72 ± 18 20 ± 12 |
52 ± 8 12 ± 5 |
p<0.001 p<0.001 |
| Pelvic instrumentation, n (%) | 63 (77) % | 0/0 | |
| Osteotomies, n, mean ± SD | 39, 3.49 ± 0.25 | 49, 2.96 ± 0.22 | 0.1920 |
| Intraoperative bleeding (mL) | 1085 ± 1049 | 554 ± 349 | p< 0.001 |
| Drain output (mL) | 566 ± 208 | 489 ± 188 | p =0.0294 |
| Hidden blood loss (mL) | 566 ± 533 | 398 ± 411 | p = 0.0332 |
| Total measured bleeding (mL) | 1348 ± 1066 | 965 ± 453 | p <0.001 |
| Total bleeding (mL) | 1914 ± 1006 | 1358 ± 544 | p < 0.001 |
| Posterior time (h) | 4.2 ± 0.13 | 3.08 ± 0.07 | p < 0.001 |
Values are Mean ± SD for continuous variables and numbers (percentages) for categorical variables.
Statistical comparisons were performed using Mann-Whitney U -test for continuous variables and x2-test for categorical variables.
Intraoperative blood loss
Intraoperative blood loss was significantly larger in the NMS than in the AIS group (mean ± SD 1085 ± 1049 mL vs. 554 ± 349 mL, p <0.001). This difference persisted when adjusted for weight and the number of levels fused (mean 1.79 vs. 0.87 ml/kg/levels fused, p<0.001).
Drain output
The mean 24 hour drain output was significantly larger in the NMS than in the AIS group, with means of 566 ± 208 mL and 489 ml±188 mL (p=0.0294) (Table 1). When adjusted for weight and number of levels fused, NMS patients tended to bleed slightly more, but this difference did not reach statistical significance (0.87±0.36 mL/kg/fused level vs. 0.78±0.35 mL/kg/fused level, p=0.1645).
Hidden blood loss
The hidden blood loss was significantly larger in NMS than in AIS group (566 ± 533 mL vs. 398 ±411 mL, p=0.0332) (Table 1). When adjusted for patient weight and fusion level, this difference reached borderline significance (1.00 ml/kg/levels fused vs. 0.65 ml/kg/fused levels, p=0.053).
Total blood loss
Neuromuscular patients had a significantly larger total blood loss with mean values of 1914 ± 1006 mL in the NMS and 1358 ±544 mL in the AIS group (p<0.0001). The adjusted total blood loss was significantly higher in NMS than in AIS group (2.67 mL/kg/level vs. 2.03 mL/kg/level, p <0.001). The hidden blood loss was 31% of total blood loss in the NMS patients and 27% in the AIS group (p=0.3055).
Variables explaining bleeding characters
In the NMS group male patients had significantly greater hidden blood loss than female patients (mean 1.13 ± 0.17 ml/kg/level, 0.84 ± 0.19 ml/kg/level, respectively, p=0.0429). Similarly, in the AIS group male patients had significantly greater intraoperative blood loss (p= 0.014). Drain output (ml/kg/level) did not differ between the genders in either group. Age or the number of osteotomies did not have any significant correlation with bleeding outcome parameters. In both NMS and AIS groups longer operative time correlated to greater intraoperative blood loss (ml/kg/levels fused, p<0.001 for both comparisons), while it did not have any association with hidden blood loss. The preoperative main curve Cobb angle correlated positively to total bleeding in NMS, p=0.0473. This finding was not seen in AIS.
Multivariable regression analysis
Multivariable regression analysis was performed in order to control for demographic differences in NMS versus AIS patients in bleeding outcomes in ml/kg/level of fusion. The patients’ diagnosis (NMS/AIS), sex, age and preoperative major curve Cobb angle degree were included as effects in the multivariable analysis, as the patient weight and fusion level were already included in the adjusted amount of bleeding in ml/kg/level. Regarding intraoperative, total measured and total bleeding, preoperative major curve degree was a significant risk factor, p<0.001 for each. Diagnosis was a significant risk factor for hidden blood loss, p=0.0011. There were no significant risk factors for drainage bleeding in this multivariable analysis.
Need for blood products
Red blood cell transfusions were significantly more often needed in the NMS group (p=0.0017), Table 2. The same tendency was noted in platelets as 7 patients in NMS group received platelet transfusion compared to none in the AIS group (p<0.001). Similarly, NMS patients received significantly more fresh frozen human plasma (42/81, 52% for NMS and 31/199, 16% for AIS, p<0.001). A majority of the NMS patients (55/64, 86%) received intraoperative autologous blood transfusion compared to the AIS group (86/171, 47%). The adjusted amount of Cell-Saver autologous blood transfusion (ml/kg/fused levels) received was significantly higher in the NMS than in the AIS group (p <0.001).
Table 3.
Regression analysis.
| bleeding ml/kg/level | NMS vs AIS | sex | age | main curve | Multivariable |
|---|---|---|---|---|---|
| Intraoperative blood loss | <0.001 | 0.006 | - | <0.001 | Main curve <0.001 |
| Drainage bleeding | - | - | - | - | |
| Total measured bleeding | 0.0395 | 0.0565 | <0.001 | Main curve <0.001 |
|
| Hidden blood loss | 0.0524 | - | - | - | NMS vs AIS 0.0011 |
| Total blood loss | <0.001 | 0.0171 | - | <0.001 |
Main curve <0.001 |
P-values from regression analysis concerning patient demographics. Left side, univariable analysis, right side multivariable analysis.
Table 2.
Need for blood transfusion in the study groups.
| NMS (N=81) |
AIS (N=199) |
P value+ | |
|---|---|---|---|
| Allogenic RBC N (%) ml/kg |
59 (73%) 18.2 ± 21.2 |
27 (14%) 9.1 ± 7.1 |
p < 0.001 p = 0.0017 |
| Thrombocytes N (%) ml/kg |
7 (9%) 23.9 ± 22.8 |
0 (0%) |
p < 0.001 p < 0.001 |
| Fresh frozen plasma N (%) ml/kg |
42 (52%) 15.4 ± 13.7 |
31 (16%) 7.0 ± 3.47 |
p < 0.001 p<0.001 |
| Autologous RBC infusion N (%) ml/kg |
55 (86%) 5.5 ± 6.9 |
86 (47%) 2.8 ± 1.6 |
p < 0.001 p < 0.001 |
Values are Mean ± SD for continuous variables and numbers (percentages) for categorical variables.
*Statistical comparisons were performed using Mann-Whitney U -test for continuous variables and x2-test for categorical variables.
Discussion
Based on our study hidden blood loss is a significant source of bleeding in children undergoing segmental pedicle screw instrumentation for NMS, representing one-third of total calculated blood loss. Hidden blood loss was significantly larger in the NMS than in the AIS group. In accordance with previous studies NMS patients had significantly larger intraoperative blood loss [11,13,14,44], but similar amount of 24 hour drain output. In the current study the number of fused levels had no significant impact on the total perioperative bleeding in NMS group on the contrary as described in several previous studies [12,13,15]. Longer operative time was associated with greater blood loss.
There was no correlation between fusion level and greater intraoperative or hidden blood loss in NMS patients in the current study, but fusion level correlated with significantly higher drainage bleeding in NMS patients. The impact of fusion levels to the perioperative bleeding has been described in the literature previously in scoliosis patients undergoing spinal fusion. Meert et al. (2002) and Cristante et al. (2014) described fusion level as an independent risk factor for greater blood loss in combined NMS/AIS dataset, but no comparison between AIS and NMS patients was demonstrated [12,15]. Jia et al. (2017) found a significant difference comparing groups of massive (estimated intraoperative blood loss [EBL]/total blood volume [TBV] > 30%) and minor blood loss (EBL/TBV < 30%) to fusion level on a dataset consisting of solely NMS patients. They described level means of 11.2 ± 1.84 and 12.24 ± 1.19 for minor and major blood loss respectively [13]. In comparison, in our dataset NMS patients have had more levels fused (mean + SD 16.6 ± 1.4) and 80% (65/81) of our NMS patients had a fusion of 15 to 17 vertebrae.
When adjusting the HBL for patient weight and fusion level the difference between groups resulted only borderline significance. We calculated the difference with adjusting the hidden blood loss only with patient weight and the difference between AIS and NMS was again statistically significant, 16.4 ± 1.3 ml/kg in NMS and 7.1 ± 0.77 ml/kg in AIS, p=0.0003, NMS patients bleed significantly more, which might explain the borderline significance, as hidden blood loss might not correlate to fusion level.
Comparison to previous data
Hidden blood loss constitutes a significant proportion of total bleeding after posterior instrumented spinal fusion [[21], [22], [23],35]. In the study of Wang et al. [21] hidden blood loss accounted over 50% of total blood loss in AIS patients. In our study, the hidden blood loss in NMS patients constituted one third of total blood loss, which is also clinically relevant in typically small sized neuromuscular patients with associated comorbidities. Hidden blood loss was significantly larger in the NMS than in the AIS group, and the difference between the two diagnostic groups was also seen in the multivariable regression analysis, p=0.0011. Almost 87% of the NMS patients needed allogenic red blood cell infusion. Despite larger intraoperative blood loss, drain output was similar in the NMS and AIS patients. In the neuromuscular patients, the posterior spinal exposure extends from the upper thoracic spine to the pelvis. It is therefore possible, that a single subfascial drain might not be sufficient to evacuate all postoperative bleeding and therefore hidden blood loss becomes larger in the neuromuscular group.
In previous studies male gender has been associated with greater bleeding especially in AIS [45] but also in NMS group [24]. In the current study, the male patients in the NMS group demonstrated larger hidden blood loss and in the AIS group they had larger intraoperative blood loss. Age was not significantly associated with the bleeding in the current study. Toombs et al. (2013) and Jia et al. (2017) described a positive correlation between bleeding and older age in neuromuscular scoliosis patients undergoing spinal fusion [13,24]. Our analyses were adjusted for patient weight, which might explain this difference as younger patients tend to be smaller.
According to predetermined criteria for blood transfusion patients, the NMS patients required significantly more often both autogenic and allogenic red blood cell infusion as well as fresh frozen plasma. Similar results have also previously been described in the literature [15].
As the hidden blood loss plays a role in postoperative bleeding after posterior spinal fusion in NMS patients, the minimization of HBL should be considered. The previous literature suggest fusionless strategies or diminishing the implant density to reduce the hidden blood loss in correction of scoliosis, [23,[46], [47], [48]] yet these methods have been mainly researched with adults [47,48] or patients with idiopathic scoliosis [23,46]. Miladi et al. recently suggested that ‘bipolar technique’ including upper thoracic instrumentation and pelvic fixation reduces both operative time and blood loss, while it remains unclear how sustainable this technique remains without spinal fusion [49]. This technique likely will reduce also hidden blood loss. Controllable risk factors for hidden blood loss in NMS patients might include the optimization of laboratory levels, nutrition status or other risk factors already preoperatively. Yet, they remain unclear, which leaves open question for further studies.
Strengths and limitations
The strengths of our study are a relatively large consecutive number of NMS patients whose results were compared with AIS patients undergoing similar type of operation (segmental pedicle screw instrumentation) using prospectively collected data from a pediatric spine register. Anesthetic management was standardized including tranexamic acid bolus and infusion as previously published [50].To our knowledge, this is the largest study cohort from a single center with standardized surgical and anesthetic protocol assessing bleeding characteristics in NMS patients. This is the first paper to report hidden blood loss in NMS patients.
We have chosen to remove the subcutaneous drain routinely after 24 hours as up to 18% of drains will be contaminated, and the frequency of contaminated drain tips markedly increases after 24 hours [51]. It is possible that continued bleeding occurs after this time point and therefore hidden blood loss was calculated.
The main limitation in our study setting is its retrospective design based on a prospective pediatric spine database. Spinal fusions in NMS and AIS patients are very different in nature. The NMS patients are generally smaller and have more comorbidities. The surgery is often more extensive in the NMS patients, with longer fusion and fusion to pelvis.
Conclusion
Hidden blood loss constitutes one-third of total blood loss in children undergoing segmental pedicle screw instrumentation for neuromuscular scoliosis, and hidden blood loss is a significantly larger in these patients as compared to AIS. As the neuromuscular patients typically have smaller blood volume, and this significant source of bleeding should be considered in the perioperative management of these patients. Majority (86%) of patients with NMS needed autogenous or allogenic blood transfusion.
Funding
VS has received grants from Vappu Uuspään säätiö, Finnish Pediatric Research foundation and Turku University research funding. Dr. Syvänen from the Clinical Research Institute HUCH, and Dr. Raitio from the Paulo Foundation. Dr. Helenius has received scientific funding from Finnish Paediatric Research Foundation and Finska Läkaresällskapet.
Declarations of Competing Interest
One or more of the authors declare financial or professional relationships on ICMJE-NASSJ disclosure forms.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: VS: Grants: Vappu Uuspään säätiö (Fee not disclosed); Finnish Pediatric Research foundation (Fee not disclosed); Turku University (Fee not disclosed). AR: Nothing to disclose. IH: All support for the present manuscript (e.g., funding, provision of study materials, medical writing, article processing charges, etc: Medtronic (Scientific research funding to institutions); Stryker (Scientific research funding to institutions); Nuvasive (Scientific research funding to institutions); Cerapedics (Scientific research funding to institutions). Consulting fees: Medtronic (Fee not disclosed): Globus (Fee not disclosed) LH: Nothing to disclose. JS: Grants: Clinical Research institute HUCH (Fee not disclosed).
References
- 1.Reames DL, Smith JS, Fu KM, Polly DW, Jr., Ames CP, Berven SH, et al. Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the Scoliosis Research Society Morbidity and Mortality database. Spine. 2011;36(18):1484–1491. doi: 10.1097/BRS.0b013e3181f3a326. [DOI] [PubMed] [Google Scholar]
- 2.Helenius IJ, Viehweger E, Castelein RM. Cerebral palsy with dislocated hip and scoliosis: what to deal with first? J Child Orthop. 2020;14(1):24–29. doi: 10.1302/1863-2548.14.190099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy NA, Firth S, Jorgensen T, Young PC. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What's the difference? J Pediatr Orthop. 2006;26(2):216–220. doi: 10.1097/01.bpo.0000206516.61706.6e. [DOI] [PubMed] [Google Scholar]
- 4.Sarwark J, Sarwahi V. New strategies and decision making in the management of neuromuscular scoliosis. Orthop Clin North Am. 2007;38(4):485–496. doi: 10.1016/j.ocl.2007.07.001. v. [DOI] [PubMed] [Google Scholar]
- 5.Modi HN, Suh SW, Song HR, Yang JH, Jajodia N. Evaluation of pelvic fixation in neuromuscular scoliosis: a retrospective study in 55 patients. Int Orthop. 2010;34(1):89–96. doi: 10.1007/s00264-008-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCall RE, Hayes B. Long-term outcome in neuromuscular scoliosis fused only to lumbar 5. Spine. 2005;30(18):2056–2060. doi: 10.1097/01.brs.0000178817.34368.16. [DOI] [PubMed] [Google Scholar]
- 7.El-Hawary R, Chukwunyerenwa C. Update on evaluation and treatment of scoliosis. Pediatr Clin North Am. 2014;61(6):1223–1241. doi: 10.1016/j.pcl.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 8.El-Bromboly Y, Hurry J, Padhye K, Johnston C, McClung A, Samdani A, et al. The effect of proximal anchor choice during distraction-based surgeries for patients with nonidiopathic early-onset scoliosis: a retrospective multicenter study. J Pediatr Orthop. 2021;41(5):290–295. doi: 10.1097/BPO.0000000000001784. [DOI] [PubMed] [Google Scholar]
- 9.Wishart BD, Kivlehan E. Neuromuscular scoliosis: when, who, why and outcomes. Phys Med Rehabil Clin N Am. 2021;32(3):547–556. doi: 10.1016/j.pmr.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Tondevold N, Lastikka M, Andersen T, Gehrchen M, Helenius I. Should instrumented spinal fusion in nonambulatory children with neuromuscular scoliosis be extended to L5 or the pelvis? Bone Joint J. 2020;102-B(2):261–267. doi: 10.1302/0301-620X.102B2.BJJ-2019-0772.R2. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J. 2004;13(Suppl 1):S6–17. doi: 10.1007/s00586-004-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cristante AF, Borges PA, Barbosa AR, Letaif OB, Marcon RM, Barros Filho TE. Predictive factors for perioperative blood transfusion in surgeries for correction of idiopathic, neuromuscular or congenital scoliosis. Clinics. 2014;69(10):672–676. doi: 10.6061/clinics/2014(10)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia R, Li N, Xu BY, Zhang W, Gu XP, Ma ZL. Incidence, influencing factors, and prognostic impact of intraoperative massive blood loss in adolescents with neuromuscular scoliosis: a STROBE-compliant retrospective observational analysis. Medicine. 2017;96(11):e6292. doi: 10.1097/MD.0000000000006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edler A, Murray DJ, Forbes RB. Blood loss during posterior spinal fusion surgery in patients with neuromuscular disease: is there an increased risk? Paediatr Anaesth. 2003;13(9):818–822. doi: 10.1046/j.1460-9592.2003.01171.x. [DOI] [PubMed] [Google Scholar]
- 15.Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine. 2002;27(19):2137–2142. doi: 10.1097/00007632-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 16.Cognetti D, Keeny HM, Samdani AF, Pahys JM, Hanson DS, Blanke K, et al. Neuromuscular scoliosis complication rates from 2004 to 2015: a report from the Scoliosis Research Society Morbidity and Mortality database. Neurosurg Focus. 2017;43(4):E10. doi: 10.3171/2017.7.FOCUS17384. [DOI] [PubMed] [Google Scholar]
- 17.Toll BJ, Samdani AF, Janjua MB, Gandhi S, Pahys JM, Hwang SW. Perioperative complications and risk factors in neuromuscular scoliosis surgery. J Neurosurg Pediatr. 2018;22(2):207–213. doi: 10.3171/2018.2.PEDS17724. [DOI] [PubMed] [Google Scholar]
- 18.Rumalla K, Yarbrough CK, Pugely AJ, Koester L, Dorward IG. Spinal fusion for pediatric neuromuscular scoliosis: national trends, complications, and in-hospital outcomes. J Neurosurg Spine. 2016;25(4):500–508. doi: 10.3171/2016.2.SPINE151377. [DOI] [PubMed] [Google Scholar]
- 19.Helenius I, Keskinen H, Syvanen J, Lukkarinen H, Mattila M, Valipakka J, et al. Gelatine matrix with human thrombin decreases blood loss in adolescents undergoing posterior spinal fusion for idiopathic scoliosis: a multicentre, randomised clinical trial. Bone Joint J. 2016;98-B(3):395–401. doi: 10.1302/0301-620X.98B3.36344. [DOI] [PubMed] [Google Scholar]
- 20.Helenius L, Gerdhem P, Ahonen M, Syvanen J, Jalkanen J, Perokorpi T, et al. Postoperative outcomes of pedicle screw instrumentation for adolescent idiopathic scoliosis with or without subfascial wound drain: a multicentre randomized clinical trial. Bone Joint J. 2022 doi: 10.1302/0301-620X.104B9.BJJ-2022-0391.R1. In press. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Liu J, Song X, Luo M, Chen Y. Hidden blood loss in adolescent idiopathic scoliosis patients undergoing posterior spinal fusion surgery: a retrospective study of 765 cases at a single centre. BMC Musculoskelet Disord. 2021;22(1):794. doi: 10.1186/s12891-021-04681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Ding W, Zhao R, Yang S. Risk factors of total blood loss and hidden blood loss in patients with adolescent idiopathic scoliosis: a retrospective study. Biomed Res Int. 2022;2022 doi: 10.1155/2022/9305190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolz J. Hidden blood loss in adolescent idiopathic scoliosis surgery. Orthop Traumatol Surg Res. 2022 doi: 10.1016/j.otsr.2022.103216. Kolz JM, et al. [DOI] [PubMed] [Google Scholar]
- 24.Toombs C, Verma K, Lonner BS, Feldman D, Errico T. Preliminary analysis of factors associated with blood loss in neuromuscular scoliosis surgery. Bull Hosp Jt Dis. 2018;76(3):207–215. (2013) [PubMed] [Google Scholar]
- 25.Liu HN, Zhang XJ, Li JX, Guo D, Bai YS, Yao ZM, et al. [Predictors for perioperative blood loss in pediatric patients with congenital scoliosis] Zhonghua Yi Xue Za Zhi. 2020;100(25):1962–1966. doi: 10.3760/cma.j.cn112137-20191201-02614. [DOI] [PubMed] [Google Scholar]
- 26.Maio M, Carvalho A, Pinho A, Serdoura F, Veludo V. What factors can influence massive blood loss in the surgical treatment of neuromuscular scoliosis? Rev Bras Ortop. 2020;55(2):181–184. doi: 10.1055/s-0039-3400527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang CYK, Kamath VHD, Cheung PWH, Cheung JPY. Predictive factors for intraoperative blood loss in surgery for adolescent idiopathic scoliosis. BMC Musculoskelet Disord. 2021;22(1):225. doi: 10.1186/s12891-021-04104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Xiao H, Wang R, Huang Y. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine. 2013;38(4):350–355. doi: 10.1097/BRS.0b013e31826c63cb. [DOI] [PubMed] [Google Scholar]
- 29.Piazzolla A, Bizzoca D, Solarino G, Parato C, Moretti B. Plasma technology reduces blood loss in adolescent idiopathic scoliosis surgery: a prospective randomized clinical trial. Global Spine J. 2020 doi: 10.1177/2192568220928344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardesty CK, Gordon ZL, Poe-Kochert C, Son-Hing JP, Thompson GH. Bipolar sealer devices used in posterior spinal fusion for neuromuscular scoliosis reduce blood loss and transfusion requirements. J Pediatr Orthop. 2018;38(2):e78–e82. doi: 10.1097/BPO.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 31.Kieser DC, Thakar C, Cunningham G, Vidakovic H, Hammer N, Nnadi C. The value of a modified Wiltse approach for deformity correction in neuromuscular scoliosis. Int J Spine Surg. 2020;14(2):170–174. doi: 10.14444/7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–155. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 33.Mima Y, Yagi M, Suzuki S, Tsuji O, Nagoshi N, Okada E, et al. Hidden blood loss in extreme lateral interbody fusion for adult spinal deformity. J Orthop Sci. 2022 doi: 10.1016/j.jos.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Ren Z, Li S, Sheng L, Zhuang Q, Li Z, Xu D, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: a retrospective study. Medicine. 2017;96(42):e8233. doi: 10.1097/MD.0000000000008233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. 2013;13(8):877–881. doi: 10.1016/j.spinee.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Ogura Y, Dimar Ii, JR, Gum JL, Crawford CH, 3rd, Djurasovic M, Glassman SD, et al. Hidden blood loss following 2- to 3-level posterior lumbar fusion. Spine J. 2019;19(12):2003–2006. doi: 10.1016/j.spinee.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Miao K, Ni S, Zhou X, Xu N, Sun R, Zhuang C, et al. Hidden blood loss and its influential factors after total hip arthroplasty. J Orthop Surg Res. 2015;10:36. doi: 10.1186/s13018-015-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenke L, Edwards CC, Bridwell KH. The Lenke classification of adolescent idiopathic scoliosis: how it organizes curve patterns as a template to perform selective fusions of the spine. Spine. 2003;28:S199–S207. doi: 10.1097/01.BRS.0000092216.16155.33. [DOI] [PubMed] [Google Scholar]
- 39.Kuklo TR, Lenke LG, O'Brien MF, Lehman RA, Jr., Polly DW, Jr., Schroeder TM. Accuracy and efficacy of thoracic pedicle screws in curves more than 90 degrees. Spine. 2005;30(2):222–226. doi: 10.1097/01.brs.0000150482.26918.d8. [DOI] [PubMed] [Google Scholar]
- 40.Helenius L, Diarbakerli E, Grauers A, Lastikka M, Oksanen H, Pajulo O, et al. Back pain and quality of life after surgical treatment for adolescent idiopathic scoliosis at 5-year follow-up: comparison with healthy controls and patients with untreated idiopathic scoliosis. J Bone Joint Surg Am. 2019;101(16):1460–1466. doi: 10.2106/JBJS.18.01370. [DOI] [PubMed] [Google Scholar]
- 41.Saarinen AJ, Suominen EN, Helenius L, Syvanen J, Raitio A, Helenius I. Intraoperative 3D imaging reduces pedicle screw related complications and reoperations in adolescents undergoing posterior spinal fusion for idiopathic scoliosis: a retrospective study. Children. 2022;9(8) doi: 10.3390/children9081129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 43.Bourke DL, Smith TC. Estimating allowable hemodilution. Anesthesiology. 1974;41(6):609–612. doi: 10.1097/00000542-197412000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Jain A, Njoku DB, Sponseller PD. Does patient diagnosis predict blood loss during posterior spinal fusion in children? Spine. 2012;37(19):1683–1687. doi: 10.1097/BRS.0b013e318254168f. [DOI] [PubMed] [Google Scholar]
- 45.Ialenti MN, Lonner BS, Verma K, Dean L, Valdevit A, Errico T. Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(4):372–376. doi: 10.1097/BPO.0b013e3182870325. [DOI] [PubMed] [Google Scholar]
- 46.Laumonerie P, Tibbo ME, Kerezoudis P, Langlais T, de Gauzy JS, Accadbled F. Influence of the sublaminar band density in the treatment of Lenke 1 adolescent idiopathic scoliosis. Orthop Traumatol Surg Res. 2020;106(7):1269–1274. doi: 10.1016/j.otsr.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Mathew S, Larson AN, Potter DD, Milbrandt TA. Defining the learning curve in CT-guided navigated thoracoscopic vertebral body tethering. Spine Deform. 2021;9(6):1581–1589. doi: 10.1007/s43390-021-00364-w. [DOI] [PubMed] [Google Scholar]
- 48.Wolff S, Habboubi K, Sebaaly A, Moreau PE, Miladi L, Riouallon G. Correction of adult spinal deformity with a minimally invasive fusionless bipolar construct: preliminary results. Orthop Traumatol Surg Res. 2019;105(6):1149–1155. doi: 10.1016/j.otsr.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Gaume M, Gerard P, Khouri N, Glorion C, Dubousset J, Miladi L. Long-term outcomes of ilio-sacral screws in minimally invasive bipolar fusionless technique for neuromuscular scoliosis: a retrospective study in 167 patients. Arch Orthop Trauma Surg. 2022 doi: 10.1007/s00402-021-04332-x. [DOI] [PubMed] [Google Scholar]
- 50.Helenius LL, Oksanen H, Lastikka M, Pajulo O, Loyttyniemi E, Manner T, et al. Preemptive pregabalin in children and adolescents undergoing posterior instrumented spinal fusion: a double-blinded, placebo-controlled, randomized clinical trial. J Bone Joint Surg Am. 2020;102(3):205–212. doi: 10.2106/JBJS.19.00650. [DOI] [PubMed] [Google Scholar]
- 51.Drinkwater CJ, Neil MJ. Optimal timing of wound drain removal following total joint arthroplasty. J Arthroplasty. 1995;10(2):185–189. doi: 10.1016/s0883-5403(05)80125-1. [DOI] [PubMed] [Google Scholar]