Abstract
Nearly a quarter of the total number of deaths in the world are caused by unhealthy living or working environments. Therefore, we consider it significant to introduce the effect of a widely distributed component of air/water/food-source contaminants, polycyclic aromatic hydrocarbons (PAHs), on the human body, especially on immunity in this review. PAHs are a large class of organic compounds containing two or more benzene rings. PAH exposure could occur in most people through breath, smoke, food, and direct skin contact, resulting in both cellular immunosuppression and humoral immunosuppression. PAHs usually lead to the exacerbation of autoimmune diseases by regulating the balance of T helper cell 17 and regulatory T cells, and promoting type 2 immunity. However, the receptor of PAHs, aryl hydrocarbon receptor (AhR), appears to exhibit duality in the immune response, which seems to explain some seemingly opposite experimental results. In addition, PAH exposure was also able to exacerbate allergic reactions and regulate monocytes to a certain extent. The specific regulation mechanisms of immune system include the assistance of AhR, the activation of the CYP-ROS axis, the recruitment of intracellular calcium, and some epigenetic mechanisms. This review aims to summarize our current understanding on the impact of PAHs in the immune system and some related diseases such as cancer, autoimmune diseases (rheumatoid arthritis, type 1 diabetes, multiple sclerosis, and systemic lupus erythematosus), and allergic diseases (asthma and atopic dermatitis). Finally, we also propose future research directions for the prevention or treatment on environmental induced diseases.
Keywords: Immunity, Polycyclic aromatic hydrocarbons, Lymphocytes, Autoimmune diseases
Highlights
-
•
PAH exposure occurred in most people through breath, smoke, food, and skin contact.
-
•
PAH exposure could exacerbate autoimmune diseases and allergic reactions.
-
•
PAHs affect the immune system mainly through T cells, monocytes, type 2 immunity, and so on.
-
•
Potential regulation modes include CYP-ROS axis, intracellular calcium, and others.
-
•
The impacts of PAHs on cancer, autoimmune and allergic diseases are introduced.
1. Introduction
In recent years, the role of environmental pollution on human health has attracted enhanced interest globally. World Health Organization (WHO) estimated that 12.6 million people died due to unhealthy living or working environments in 2012 worldwide, among which almost half of the deaths were attributed to non-communicable diseases caused by air pollution [1]. This data accounted for nearly a quarter of the total number of deaths in the world. Environmental risk factors such as air/water/soil pollution, chemical hazards, climate change, and ultraviolet radiation have caused more than 100 diseases and injuries [1]. About smoking, WHO has reported that tobacco killed 3.3 million users and people exposed to second-hand smoke from lung-related conditions in 2017, including more than 60 000 children aged under 5 who died of lower respiratory infections caused by second-hand smoke [2]. Similarly, Tobacco use and secondhand smoke exposure are also reported as major causes of cardiovascular disease, contributing to approximately 10% of all cardiovascular deaths globally [3]. Air pollution is one of the major environmental health risk factors, causing almost four to nine million deaths worldwide, especially with the increased human exposure to a major component of air pollution, particulate matter (PM) [4,5]. Many epidemiological studies have shown that high levels of air pollution, in particular, PM, would be harmful to human health, including inducing or worsening autoimmune diseases [[6], [7], [8]]. Due to its small particle size and large specific surface area, PM2.5 (PM between 0.1 and 2.5 mm) becomes a hotbed for toxic substances [9], of which polycyclic aromatic hydrocarbons (PAHs) [10] are one of the most important substances. In addition to air, PAHs are also present in pollutant water, soil, animals, and plants.
2. What are PAHs
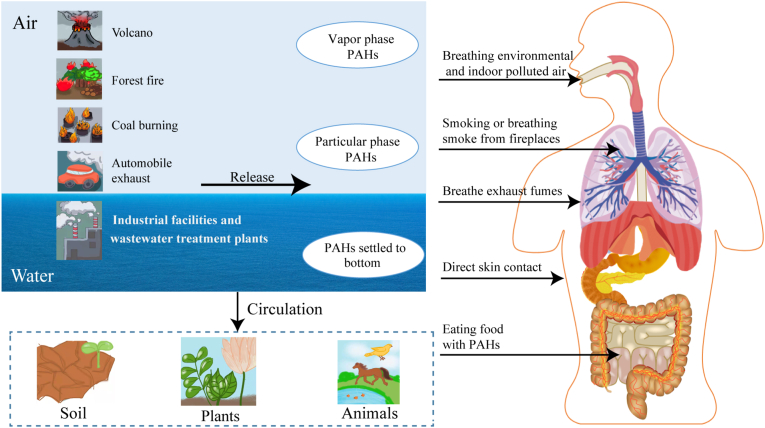
PAHs consist of over 100 different organic compounds containing at least two fused or condensed benzene rings in various configurations produced by incomplete combustion of organic matters [11]. PAHs could enter the air mainly through the release of volcanoes, forest fires, coal burning, and automobile exhaust, and enter the water through discharges from industrial and wastewater treatment plants (Fig. 1) [12]. PAHs in the air exist in the gas phase or are adsorbed into the airborne particulate matter. Generally, low-weight PAH with two, three, or four rings tend to exist in the vapor phase and are considered less toxic, but they could react with other air pollutants to produce more toxic substances [13,14]. High-weight PAHs with more than three rings do not vaporize, so they mainly occur in the particulate phase in the air [15]. In addition, the concentration of PAHs in the vapor phase increases in high-temperature, generally in summer or tropical regions, while in winter or arctic regions, the particular PAHs dominates [16,17]. Especially in winter, high levels of PAHs were also observed in urban areas or densely populated areas [18]. With the circulation of air and water, PAHs will also appear in soil, animals, and plants (Fig. 1) [19].
Fig. 1.
Pathways of human exposure to polycyclic aromatic hydrocarbons (PAHs).
PAHs enter the air primarily through volcanic eruptions, forest fires, coal burning, and automobile exhaust, and enter into the water through emissions from industry and sewage treatment plants, where they may sink to the seafloor. Airborne PAHs can exist in the gas phase or be adsorbed in suspended particulates in the air. PAHs also appear in soil, plants, and animals as the air and water circulate.
PAHs in these environments can all contact humans in a variety of ways, including breathing the environmental and indoor polluted air, smoking or inhaling smoke from fireplaces, eating foods that contain PAHs, and direct exposure to contaminated soil. Occupational exposures can also occur when workers inhale exhaust fumes.
The general population is exposed to PAHs mainly from polluted air from the environment, tobacco smoke, fireplace smoke, or consuming food contaminated with PAHs [20] (Fig. 1). Individuals may also be exposed to PAHs from contaminated soil through ingestion or direct skin contact (Fig. 1) [21]. Occupational exposure may occur when workers breathe exhaust fumes (Fig. 1). Therefore, most people would frequently be exposed to PAHs in different ways not limited to breathing. Luckily, there has existed a widely used urinary biomarker for assessing PAH exposure in human beings, 1-Hydroxypyrene [[22], [23], [24], [25]].
3. How PAHs influence the human body
The influence of PAHs on human body was mainly associated with the concentration and toxicity of PAHs, plus the degree and route of exposure (e.g., the length of time and e.g., inhalation or dermal contact) [20]. The carcinogenicity, teratogenicity, and genetic toxicity of PAHs have been thoroughly discussed [26]. Therefore, this review focuses on the impact of PAHs on human immune functions.
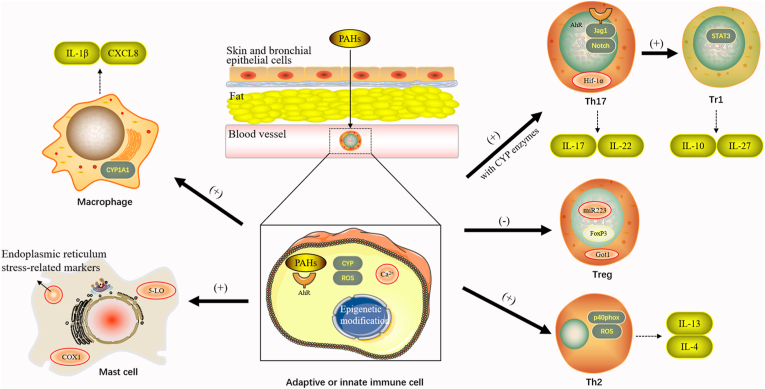
Since the skin and respiratory tracts are constantly in contact with various pollutants at the main barrier surfaces, PAHs tend to be concentrated in the skin and bronchial epithelial cells (Fig. 2) [27,28]. Due to the high lipophilicity of PAHs, they can easily penetrate the epithelial barrier, accumulate and retain with the lipids, which leads to the chronic activation of aryl hydrocarbon receptor (AhR) (Fig. 2) [29,30].
Fig. 2.
The mechanisms of polycyclic aromatic hydrocarbons on human immune cells.
PAHs contact mainly with the skin and respiratory tract, and tend to be concentrated in the skin and bronchial epithelial cells. Due to the high lipophilicity of PAHs, they readily penetrate the epithelial barrier, accumulate slowly in fat, and eventually persist for a long time.
In general, AhR signaling is needed in both adaptive and innate immune cells, in addition to different intracellular mechanisms, including cytochrome P450 (CYP)- reactive oxygen species (ROS) axis and intracellular calcium mobilization. The study of epigenetic mechanisms has also become increasingly diverse.
(1) PAHs can induce Th17 differentiation. The possible intracellular mechanisms include the Ahr-Jag1-Notch signaling pathway and the involvement of the glycogenesis regulator Hif-1α. The secretion of Th17 cytokines, including IL-17 and IL-22, was also increased. Th17 cells can be transformed by AhR into type 1 T regulatory (Tr1) cells that produce the immunosuppressive cytokine IL-10. Conversely, in Tr1 cells, IL-27 was able to increase the AhR expression through a Stat3-driven mechanism.
(2) PAH exposure is associated with impaired Treg function, downregulation of Foxp3, and increased methylation in the Foxp3 promoter region. PM2.5 promotes the expression of Got1, resulting in hypermethylation of the Foxp3 locus, thereby inhibiting Treg differentiation. Changes in miR223 were also associated with lower Treg levels.
(3) PAHs lead to increased expression of Th2-related markers and elevated levels of secreted IL-4 and IL-13. PAHs induce oxidative stress pathways that generate ROS. ROS production may also be caused by increased expression of p40phox, a member of the membrane NADPH oxidase complex.
(4) PM up-regulated the level of CYP 1A1, thereby affecting the activation of human monocytes. CXCL8 production was induced, as was IL-1β secretion by monocytes and activated macrophages.
(5) Exposure to PAHs results in enhanced mast cell signaling, degranulation, mediator and cytokine release, and allergic responses in vivo. Increased expression of several endoplasmic reticulum stress-related markers was also observed.
PAH is one of the relatively less potent AhR ligands, compared with some other PM components such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like compounds [31]. AhR, the cellular target of PAH, is a ligand-activated transcription factor [32]. AhR signaling is needed in both adaptive and innate immune cells at their key checkpoints. The “PM-PAH-AhR” chain has been directly studied in recent years, that is, PM adsorbed PAHs trigger AhR-dependent cellular changes [33]. The properties of PMs depending on PAH content and the interaction of air pollution with AhR signaling in target tissues will ultimately define the toxicity of pollutants. Targets of AhR genes generally include xenobiotic metabolic phase Ⅰ and Ⅱ enzymes, such as cytochrome P450 (CYP), nicotinamide adenine dinucleotide phosphate (NADPH), and UDP-glucuronosyltransferase 1A6, along with other signaling pathways such as nuclearfactor-κB (NF-κB), Jagged 1 (Jag1), nuclear factor erythroid 2-related factor 2 (NRF2), estrogen receptor signaling, etc. [34,35].
PAH exposure would lead to reactive oxygen species (ROS) formation and the oxidative damage. One possible pathway is the AhR-dependent CYP induction, including CYP1A1, CYP1A2, and CYP1B1 [36]. It was experimentally confirmed that AhR-dependent induction of CYP1A is a main source of ROS formation in TCDD-treated liver cells [37]. Taking an example of oxidative damage, skin exposure to PAHs leads to the formation of ROS, contributing to the development of inflammatory skin diseases. ROS formation can affect epidermal barrier function directly by interfering with keratinocyte differentiation and indirectly by stimulating MAPK signaling and downstream nuclear factor-κB (NF-κB) and AP-1 transcription factors in keratinocytes to induce proinflammatory enzymes and cytokines [38,39].
The AhR signaling pathway also regulates the antioxidant responses. NRF2 is a transcription factor that protects against oxidative stress through binding to antioxidant response elements that induce antioxidative enzymes. Both AhR and NRF2 signaling pathways respond to environmental and endogenous stressors. Recent studies suggested the junctions and cross-regulations between the two pathways provided an integrated response to environmental stressors [40]. The activation of NRF2 was recently shown after treatment with TCDD and diesel emission samples [41]. Furthermore, NRF2-dependent mechanisms may be involved in dioxin-induced chloracne, a hallmark skin disease specific and sensitive to dioxin exposure [42]. Treatment of epidermal keratinocytes with the antifungal agent ketoconazole induces nuclear translocation of NRF2 and downstream quinone oxidoreductase (a member of NADPH family) expression in an AhR-dependent manner [43]. Studies on PAH-induced immunotoxicity in fish have suggested different intracellular mechanisms, such as PAH metabolism of (CYP), binding to AhR, and mobilization of intracellular calcium [44]. An individual PAH, benzo[a]pyrene was able to upregulate intracellular calcium in lymphocytes and monocytes, possibly through the CYP 1A1 metabolic signaling. Meanwhile, PAHs can also upregulate intracellular calcium without CYP 1A1. Increased intracellular calcium concentrations of immunocytes may lead to changes in the expression of immune markers and thus changes in immune status [45].
Immuno-suppression was indicated to be the most common effect after PAH exposure and was suggested to be a mechanism of PAH-induced cancer and the increased sensitivity of individuals to the development of infectious diseases [46]. The decrease in humoral immunity is due to the modification of leukocyte membranes caused by activated lipid peroxidation (LPO), a process that ROS targets cell membranes and induces autocatalytic oxidation, which could also lead to decreased activity of lymphocyte enzymes and reduced number of spleen cells [47,48]. PAHs were observed to reduce the number of splenic B lymphocytes, inhibit antibody production, and decrease the number of antigen-specific responder B cells [49,50]. For cellular immunity, exposure to PAHs may cause thymus atrophy, because continuous aryl hydrocarbon receptor (AhR) activation could affect the proliferation of thymocytes, thereby decreasing T lymphocytes in the thymus. This process is likely to be mediated by the premature migration of CD4–CD8– double-negative T cells from the thymus to the peripheral lymphoid organs [51] and the induction of AhR-dependent transcription factor KLF2 [52]. In total, PAHs can affect T cells, B cells, mast cells, monocytes, dendritic cells, and macrophages with, at least partly, different mechanisms (Fig. 2).
3.1. The overall process on T cells
Recently, PAH exposure was found to be associated with the inhibition of T cell proliferation and the dose-dependent inhibition of IL-2, IL-10, IL-17A, and IFNγ secretion (Table 1) [53]. From the peripheral blood mononuclear cells (PBMC) of almost 200 men who participated in Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh, Lauer et al. [54] found that PAH adducts were inversely correlated with T and T memory cell phenotypes. Additionally, at higher levels of PAH-DNA adducts, the percentage of Th cells decreased significantly; at lower levels, the percentages of Th1 and Th2 cell populations first decreased and then increased. A non-monotonic decrease in the percentage of T helper 17 (Th17) cells with PAH adducts was also observed (Table 1). AhR is expressed in nearly all CD4+ T cells, most in Th17, type 1 regulatory T cells (Tr1), and regulatory T cells (Treg), relatively less in Th1 and Th2 [55,56]. O'Driscoll et al. hypothesized that the degree and duration of AhR activation process would have a dual effect on T cell response and the subsequent CYP enzymes, resulting in the inhibition or enhancement of autoimmunity [57]. This explained why some AhR ligands were able to improve autoimmune diseases, while others aggravated diseases. To support this viewpoint, there was an experiment showing that the degree and duration of AhR activation, rather than ligand-specific responses, could affect the observed immune status [58]. In this experiment using the graft-versus-host disease model, when AhR was activated to the normalized TCDD CYP 1A1 mRNA induction levels, all tested AhR ligands improved disease; when AhR was exposed to lower levels of AhR ligands, Th17 cells increased, leading to disease deterioration. This experiment has challenged the conventional idea that AhR regulates the differentiation of Th17 and Treg in a ligand-specific manner, that is, exogenous ligands (e.g., PAHs) promote immunosuppression, while endogenous ligands (e.g., FICZ) facilitate Th17 response, thereby intensifying immune response [55,59].
Table 1.
The effects of PAHs on T cell populations and monocytes.
| cell type | General trends | proportion in PBMC | cellular activitiesa | iconic molecule |
|---|---|---|---|---|
| T cells (in total) | dual effect | ↓ | proliferation: (-) | IL-2/10/17A, IFN-γ |
| Th17 | (+) at low-dose of PAHs (-) at high-dose of PAHs | ↓ | differentiation: (+) proliferation: (+) | IL-17 |
| Tr1 | (+) | - | differentiation: (+) | IL-10 |
| Treg | (-) | - | inhibition (-) | Foxp3 |
| Th1 | (+)/(-)b | non-monotonic | - | IL-10, IFN-g |
| Th2 | (+) | non-monotonic | - | CCR8, IL-4/13/19 |
| monocytes | (+) | ↑ | Activated | IL-1b |
Abbreviations: PAH, polycyclic aromatic hydrocarbon; PBMC, peripheral blood mononuclear cells; IL, interleukin; IFN, interferon; Th17, T helper 17 cells; Tr1, type 1 regulatory T cells; Treg, regulatory T cells; Th1, T helper 1 cells; Th2, T helper 2 cells.
Effects of PAHs at pathogenic doses on cell proliferation or differentiation.
Due to the relatively less AhR on Th1, high-dose PAHs may activate Th1 responses. However, some cytokines (IFN-g) are increased and some (IL-10) are decreased.
3.2. PAHs enhance autoimmunity by upregulating Th17 cells
Specific to Th17, early in 2013, either individual PAH or mixtures containing PAHs were proved to enhance Th17 differentiation (Table 1) [60]. Later, AhR ligands such as PAHs contained in cigarette smoke were shown to exacerbate arthritis and increase Th17 cells in animal models [61]. O'Driscoll et al. found that environmental PM samples enhanced Th17 differentiation in an AHR-dependent manner [62]. The same team also demonstrated the synthetic PAH mixture that existed in PM of natural environment enhanced Th17 differentiation through AHR and/or CYP metabolism [63]. Similarly, some kinds of synthetic PAH mixtures were found to enhance Th17 differentiation only without CYP enzymes, indicating that inhibiting the CYP enzymes could prevent the decomposition of some active ingredients (Fig. 2) [63].
PM2.5 in the city was shown to trigger Th17 cell polarization mediated by dendritic cell (DC) activation in an AhR-dependent manner [64]. PM2.5 activated the AhR and elevated levels of inflammatory markers related to enhanced DC activation, differentiating naive T cells into Th17 [64]. This effect of DC-mediated IL-17 elevation and enhanced autoimmune/allergic responses has also been demonstrated in PM-treated mice [65]. In vivo, the secretion of Th17 cytokines (including IL-17) depends on the activation of AhR signaling cascade in DCs by PAHs. For instance, components of the Notch pathway including Jag1 are targets of PM in human monocytes and murine dendritic cells. Afterward, the binding of activated AhR to the promoter of Jag1 could activate the subsequent Ahr-Jag1-Notch signaling pathway and increase the release of Th17 cytokines, thereby enhancing autoimmunity (Fig. 2) [65]. Intracellular deletion of AhR prevented PM-mediated increase of IL-17 and the exacerbation of allergic response, re-emphasizing the critical role of DCs and AhR [66]. Interestingly, a recent study has suggested that the production of IL-17 may also be regulated by the AhR in epithelial cells through innate lymphoid cells (ILCs) [67]. Additionally, the increase in IL-22 levels was also observed both in mice [59] and in humans [68].
Free radicals associated with combustion-generated particles were recently reported to activate AhR and induce Th17 cytokines, resulting in Th17 polarization and IL-17-related pneumonia, that is, the ROS-mediated Th17 polarization is partially activated by AhR [69]. Given that AhR can be regulated by a distal NF-κB-binding element located on AhR gene promoter [70], Vogel et al. speculated that ROS induced AhR expression through NF-κB signaling [33].
3.3. PAHs regulate the balance of Th17 and regulatory T cells
It has been reported that PAH exposure was related to impaired Treg function [71], Foxp3 downregulation [72], and the increasing methylation of Foxp3 promoter region (Table 1) [73]. The regulation of Treg and Th17 balance is critical in environmentally induced autoimmune diseases. A recent in vivo study using AhR-deficient mice found that PM2.5 exposure led to an increase in Th17 differentiation and a decrease in Treg differentiation, depending on AhR but not related to the antigen-presenting cells [74]. Higher IL-17 and relatively lower Foxp3 levels in mice CD4+ T cells reflected a T cell/AhR dependent dysregulated balance between Th17 and Treg cells [74]. Furthermore, the authors found that in PM2.5-stimulated Th17 cells, both the expression levels of enzymes in the glycolytic pathway and the glycolytic rate were upregulated. Specifically, the regulatory factor of glycogenesis, Hif-1α, was observed to have a positive effect on Th17 differentiation. Whereas, Hif-1α seemed not to affect Treg differentiation, though Hif-1α in Treg could also be induced by PM2.5. This may indicate different AhR regulating mechanisms in Th17 versus Treg cells. Epigenetically, PM2.5 promoted Got1 expression through the combination of AhR and its promotor, leading to hypermethylation in the Foxp3 locus, as to inhibit Treg differentiation (Fig. 2) [74]. However, PM2.5 contains other non-PAH substances, so it still needs further exploration about the mechanism of how PAHs influence Treg cells. A significant increase in methylation of Foxp3 in Tregs and the subsequent decrease in Foxp3 expression levels were observed at multiple PAHs exposure time windows, as well as the sustainability of this effect [73]. Exposure to tobacco smoke in pregnant women has also been reported to be associated with miR223 in pregnant women and cord blood (Fig. 2) [75]. The changes in these miRNAs are thought to be related to lower Treg levels [75].
AhR was interestingly able to transform the established Th17 cells into Type 1 T regulatory (Tr1) cells (FOXP3−CD4+ T cells) that produce immunosuppressive cytokine IL-10 in allergic rhinitis (Fig. 2) [76,77]. Activin-A was also observed to co-opt the IRF4 and AhR signaling in CD4+ T cells, giving rise to the production of Tr1-like cells, which could restrain allergic reactions in a humanized mouse model [78]. It has also been proven that in Tr1 cells, IL-27 was able to boost their AhR expression through a Stat3-driven mechanism to prevent autoimmune disease [79] (Table 1).
3.4. The effect of AhR on monocyte fate
From the PBMC of almost 200 men who participated in HEALS in Bangladesh, CD14+ monocytes and CD14+ CD16+ cells (non-classical or intermediate monocytes) tended to increase non-monotonously after arsenic and PAHs exposure, with elevated IL-1b secretion in monocytes and activated macrophages (Fig. 2) [54,80]. Similarly, Den Hartigh et al. studied the effect of PM on human monocyte activation and concluded that PM exposure upregulated CYP 1A1 levels, while inhibition of AhR downregulated the expression of CYP 1A1 and inflammation reactions (Fig. 2) [81].
Macrophages have been indicated to be the main target of ultrafine particles in mouse lungs [82]. In the presence of local pneumonia, exposure of pulmonary macrophages to PAHs was likely to trigger the release of pro-inflammatory mediators into the circulation, which may result in a subsequent systemic inflammatory response, further exacerbating local pneumonia [83]. Likewise, in human macrophages, Benzo(a)pyrene (B(a)P) induced the production of CXCL8 by binding AhR to the CXCL8 promoter, thereby triggering the expression of neutrophil chemotactic factor (Fig. 2) [84]. These observations support that macrophages activated by air pollutants in an AhR-dependent manner are associated with neutrophilia and airway remodeling, which may be one of the mechanisms of the pathogenesis of severe asthma.
Regarding whether human monocytes differentiate into DCs or macrophages, AhR activation was amazingly found to promote monocytes to be directed towards DCs instead of macrophages though both DC and macrophages express AhR, indicating that AhR may be the determinant of the fate of monocytes under the stimulation of microenvironmental factors [85].
3.5. PAHs promote type 2 immunity and allergic reactions
In the same study mentioned in the section about the influence of PAHs on Treg, PAHs exposure was also found to upregulate Th2 type inflammation and downregulate certain Th1-related immune cytokines (IL-10), but elevate other Th1-related immune cytokines (IFN-g) [73]. Additionally, this study firstly showed an increase of Th2-related marker, CCR8 expression on CD4+ T cells after PAH exposure [73]. The observed increased expression of IL-4 and IL-13 [73], the main pro-inflammatory cytokines released by Th2 cells, are closely associated with atopic diseases because of their critical roles in B cell-induced IgE production [86,87]. In asthma patients, IL-19 has been identified as one of the most highly upregulated genes with PAH exposure [88], which has been shown to induce Th2 cytokines production [89].
Oxidative stress was suggested to affect the Th1/Th2 bias [90]. AhR ligands including PAHs were reported to induce the oxidative stress pathway, in which PAHs were metabolically activated by CYP to form reactive species, subsequently generating ROS [[91], [92], [93]]. These reactive metabolites modulate both pro- and anti-inflammatory signaling pathways [83]. The production of ROS may also be caused by the increased expression of p40phox, a member of the membrane NADPH oxidase complex (Fig. 2) [94]. Increased Th2 secretion was observed in the Dermatophagoides farina 1-sensitized mice co-exposed with B(a)P through the AhR-ROS axis [95]. In C3H/HEJ mice administered intratracheally (ovalbumin and/or B(a)P), low-dose B(a)P exposure was observed to enhance allergic airway inflammation partly by promoting Th2 response, while high-dose B(a)P may activate both Th1 and Th2 responses [96].
Exposure to PAHs in vivo may also further affect the differentiation of B cells and T helper cells by biasing the immune response toward Th2-specific distribution, conducive to the production of B cell-induced IgE and eosinophils [97]. A classic experiment showed that pyrene, an individual PAH, enhanced the allergic IgE response in mice [98], indicating PAHs can also stimulate inflammation responses through IgE and enhance allergic reactions [99]. However, early studies also demonstrated that treatment of bone marrow cultures with PAH would lead to apoptosis of pre-B and pro/pre-B cells [100], and that B(a)P could significantly inhibit the proliferation of activated human B cells at low doses (10−7 M) [101]. Still from the HEALS in Bangladesh, Burchiel et al. found the association of smoking with the significant increase in the percentage of CD19+ B cells and activated B cells, but large PAHs, such as B(a)P, are unlikely to be responsible for this phenomenon because B cell activation was still detected after correction for these components [102]. Hence, the studies researching relationships between PAHs and B cells have still been rare and vague, we strongly suggest further exploration on this proposition.
Exposure to B(a)P led to augmenting mast cell signaling, degranulation, the release of mediators and cytokines, and allergic reactions [103]. Two other studies also illustrated the importance of AhR ligands in adjusting mast cell responses that AhR ligands could promote the process of degranulation and IL-6 secretion [104,105]. Besides, AhR ligands were found to chronically alter cytokine gene expression, such as IL-6, in activated mast cells [104]. After chronic treatment with AhR agonists or antagonists, calcium influx and mast cell secretory responses were enhanced or inhibited, respectively [104]. The hypo-responsiveness of AhR-null mast cells to stimulation demonstrated the key role of AhR signaling in maintaining homeostasis for mast cells [103]. However, repeated exposure to FICZ instead inhibits MC degranulation. In an in vivo passive systemic hypersensitivity model, histamine release was exacerbated by a single dose of FICZ, but attenuated by repeated stimulation of AhR [105]. The mechanism of repeated AhR activation “desensitization” in mast cells may have some relevance to desensitization therapy in clinical practice [106]. To help delineate its mechanism, Sibilano et al. [105] observed a transient increase in cAMP production following a single exposure to FICZ, whose rapid increase is necessary for degranulation of mast cells, whereas cAMP was no longer upregulated after two exposures to FICZ. This suggested that inhibition of AhR function may be due to direct inhibition of adenylate cyclase or enhancement of phosphodiesterase. Besides, the expression of several endoplasmic reticulum stress-related markers was observed to increase after exposure of mast cells to AhR ligands [107]. Moreover, the potentiation of PAHs and the ROS induced by AhR signaling in mast cells can be partially inhibited by 5-lipoxygenase (5-LO) and COX1 inhibitors, instead of COX2 inhibitors, indicating that eicosanoid metabolites may play a functional role in amplifying the effects of PAHs [108].
3.6. Evasion of the immune surveillance in PAH-induced cancer
Early in 2008, the U.S. Environmental Protection Agency (USEPA) classified seven PAH compounds as possible human carcinogens. Soon later, “evasion of immune destruction” was considered a hallmark of cancer [109,110]. Based on the preliminary correlation between the carcinogenic and immunosuppressive potential of PAHs in animal studies [111,112], we briefly reviewed the possible immune system changes in PAH-induced cancers and concluded that the immunosuppressive function of certain PAHs may contribute to their carcinogenicity.
B(a)P exposure could result in a variety of different effects in diverse immune tissues, which may individually or collectively lead to reduced immune surveillance or immunosuppression of cancer cells. Animal experiments indicated that B(a)P could suppress the immune responses to certain tumor cells [113]. After the consistency was reported between carcinogenic and immunosuppressive chemicals (including PAHs) [111,112], Zaccaria et al. further addressed the relationship between the carcinogenic and immunosuppressive potential of multiple PAHs [114]. For other carcinogenic PAHs, this relationship is recommended to be further assessed through some immunosuppressive data directly related to cancer immune monitoring, for example, natural killer cell-mediated cytolysis. However, this kind of correlation does not reflect a causal relationship between the immunotoxicity of PAHs and their capabilities of causing tumors, nor does it show the similarity in the mechanism between immunosuppression and carcinogenicity. This association is only likely to indicate a common upstream event that leads to carcinogenesis and immunosuppression of certain PAHs (for example, metabolic activation).
In the study of Cyp1a1(−/−) mice [115], lack of CYP1A1 in the gastrointestinal tract was found to cause severe damage to the clearance of B(a)P. A large amount of B(a)P reaches the bone marrow and other distal immune tissues, leading to CYP1B1-mediated immunosuppression. In the case that when the daily oral B(a)P dose was 10 times lower, the deletion of the Cyp1a1 gene could cause gastrointestinal tract cancer. Removal of the two genes Cyp1a1 and Cyp1b1 could prevent CYP1B1-mediated immunosuppression after taking high-dose B(a)P. Low-dose B(a)P also did not cause gastrointestinal tract cancer but resulted in squamous cell carcinoma of the preputial gland duct. Therefore, these phenomena suggested that CYP1A1, as an enzyme, would bring about oral B(a)P-induced immunosuppression when deleted. When CYP1B1 is also missing, the serious impact of oral B(a)P on the immune system would be reduced. Similarly, the cancer-induced immune response gene Adamdec1 related to B(a) P exposure was found to be up-regulated by another PAH, dibenz[a,h]anthracene (DB(a, h)A), prompting the similarity of B(a)P and DB(a, h)A in a sense [116]. In the latest study of 2021, An individual PAH 7,12-dimethylbenzene[a]anthracene (DMBA) was orally administered to wild-type female mice treated with subcutaneous sustained-release medroxyprogesterone (MPA) pellets. Breast cancer subsequently developed and many of its associated immunobiological features appeared, including immune surveillance evasion, infrequent immune infiltration, and immunosuppression, as well as great resistance to immune checkpoint blockers targeting PD-1 [117]. It was previously demonstrated that DMBA-driven mammary carcinomas in mice displayed features similar to human hormone receptor (HR+) breast cancer (HR+BC) cells: enrichment with activating PIK3CA mutations and loss of PTEN [118]. Mouse HR+BC cell lines have showed their abilities to evade immune surveillance in their hosts of origin, indicating their limited antigenic or adjuvant properties to the immune system [119,120]. Similarly, this MPA/DMBA-driven mammary carcinomas also displayed similar histological and transcriptional features to human HR+BC, which emerges by evading lymphoid immune surveillance, is scarcely infiltrated by immune cells, and displays markers of local immunosuppression [117].
3.7. The potential relationships between PAHs and autoimmune diseases
Rheumatoid arthritis(RA) could be defined as synovitis with tissue damage resulting from the accumulation of inflammatory cells in joints [121]. Analyzing data from the National Health and Nutrition Examination Survey (NHANES) (2003–2014), PAH metabolites in urine and the interaction between PAH exposure and smoking have both been found to be positively correlated with the prevalence of late-stage RA [122]. In the subgroup analysis, the differences between RA and PAH metabolites in the urine of youth, women, middle-aged, obese, smoking, and drinking people were significant [123]. Additionally, the correlations were not the same among these subgroups, depending on the specific PAH metabolites [123]. However, more prospective studies are still necessary to figure out the potential role of PAHs on RA. In animal experiments, cigarette smoke containing PAHs was able to induce arthritis and increase the level of Th17 cells [61]. In the contrast, the AhR antagonist resveratrol attenuates the production of intestinal Treg cells and the anti-articular effects of sinomenine in arthritic mice [124].
Type 1 diabetes (T1D) is an autoimmune disease mediated by the destruction of insulin-secreting pancreatic β cells [125]. Exposure to 2-aminoanthracene (2AA), an individual PAH, was recently considered to be one of the factors that increase TID [126]. T1D development was enhanced by 2AA [126]. O'Driscoll concluded in his review that PM exposure may upregulate the incidence of T1D in children and worsen diabetes in adults [57].
Multiple sclerosis (MS) is a central nervous system (CNS) demyelinating inflammatory disease due to pathogenic T cells targeting myelin [127]. There has established a strong link between the risk of MS hospitalization or recurrence and PM10 concentration [[128], [129], [130], [131]]. In animal experiments, intranasal exposure to PM was found to increase T cell differentiation through AhR, worsening the severity of experimental autoimmune encephalomyelitis (EAE) [63]. The AhR pathway has been identified as a new way for PM to decrease Th1 responses in the CNS so as to reduce the severity of EAE [62]. Interestingly, there was an overall decrease in circulating AhR agonists in patients with relapsing-remitting MS, while there was an enhanced AhR agonist activity in patients with acute CNS inflammation such as clinically isolated syndromes or active MS [132]. The effect of laquinimod on alleviating MS is currently being evaluated. Genes related to the AhR pathway were induced in both EAE mice and naive mice treated with laquinimod, such as Cyp1a1 And Ahrr. Laquinimod treatment led to an increase in Treg and a decrease in effector T cells through the AHR pathway in EAE [133]. In a landmark study, bilayers of PAHs and single-wall carbon nanotubes were used for the detection of MS from exhaled breath [134]. This cross-reactive array has opened up a new field for the development of fast, cheap, and non-invasive diagnostic tools for MS. It can also distinguish MS at different stages and identify who will respond well to immunotherapy.
Systemic lupus erythematosus(SLE) is an autoimmune disease involving multiple organs induced by abnormal autoimmune responses of autoreactive T cells and autoantibodies to unknown autoantigens [135]. When analyzing the aspect of the new onset of SLE, a recent population-based cohort study conducted in Taiwan retrieved 682,208 individuals among the ages of 18–70 from the National Health Insurance Research Database and used satellites to estimate air pollution concentration from 2001 to 2010. It was found that the new diagnosis of SLE was positively correlated with exposure to several air pollutants, such as NO2, CO, and PM2.5 [136]. A meta-analysis on whether smoking increases the risk of the onset of SLE combined the evaluation of the effects of existing studies, showing an elevated risk caused by current smoking to a certain extent, but no elevated risk caused by the previous smoking [137]. Some studies also suggested that the risk of SLE disease activity would be significantly increased after PM exposure [138,139]. In an animal experiment, the enhanced AhR transcription signal was found to be related to the disease activity of SLE. By regulating the activity of AhR, The intensity of AhR signal related to disease progression and course in SLE mouse model could be changed [140]. Interestingly, a potential lupus-inducing drug, propranolol, induced stronger AhR activation in the peripheral blood mononuclear cells of SLE patients than of the control group, and signs of AhR activation were also shown in skin tissues related to lesion expression [141].
3.8. PAHs enhance allergic sensitization and aggravate allergic diseases
The Th2-type immune response that leads to tissue inflammation and remodeling has been considered a potential mechanism of allergic diseases [142]. A recent animal study has shown that PM enhances allergic sensitization by increasing Th2-mediated inflammation, including the recruitment of a large number of immune cells, mucosal material production, IgE gene expression, and Th2 cytokine expression, in which the PAH components specifically promote Th17 immune response through AhR activation [143]. Likewise, exposure to 1-nitropyrene (an individual PAH) during pregnancy was found to increase the susceptibility of offspring to allergic asthma [144].
In asthma, the immune response can be aggravated by PAH-activated AhR upregulating the transcription of Jag1 in alveolar macrophages, thereby increasing Th2/Th17 cytokines and suppressing Tregs [82]. High levels of Th17 may aggravate the severity of asthma, while Treg cells are considered to be beneficial to asthma patients. In asthmatic patients, the degree of FOXP3 methylation and the severity of functional impairment in peripheral blood Treg cells are positively related to the level of air pollution [71].
PAH exposure induces redox imbalance by activating AhR, finally developing and exacerbating asthma. Elevated ROS, endoplasmic reticulum stress, and mitochondrial dysfunction lead to the production of metabolites (such as eicosanoids), lipid peroxidation, and protein oxidation and/or nitrification. They can induce or amplify inflammatory responses, or make cells more sensitive to subsequent pollutant/allergen stimuli. In general, the PAH-ROS axis contributes to the exacerbation of asthma in genetically susceptible individuals and individuals exposed to pollutants with epigenetic modifications [108].
Transgenic mice that consistently express active AhR (AhR-CA mice) exhibited phenotypes closely related to the features of human atopic dermatitis, including pruritus hypersensitivity (allergic reaction), loss of skin barrier function, and excessive activation of Th2 immune response [145]. Interestingly, AD was described to be induced by air pollutants through AhR [146]. However, it is worth noting that AhR-activated drugs for the skin have been developed to treat AD. Recent phase 2 clinical trials of the AhR agonist tapinarof cream have shown encouraging preliminary results in AD [147,148]. In a phase IIb, double-blind, vehicle-controlled study [147], 191/247 randomized subjects received tapinarof cream 0.5%, 1%, or vehicle, once or twice daily for 12 weeks with 4-week follow-up. Significant improvements were observed for almost all outcome measures. In another double-blind, vehicle-controlled, randomized, 6-arm trial [148] (1:1:1:1:1:1), the rates of treatment success at week 12 were observed to be 53% (a concentration of 1% twice daily), 46% (a concentration of 1% once daily), 37% (a concentration of 0.5% twice daily), 34% (0.5% once daily), 24% (vehicle twice daily), and 28% (vehicle once daily), respectively. This phenomenon could be explained by the antioxidant function of tapinarof, which could upregulate epidermal differentiation complex molecules critical for maintaining skin barrier function, including filaggrin, involucrin, and keratin [149].
4. Conclusion
This review introduces the nature of PAH and its influence on the human body, specifically elaborating on its effects on different immune cells. In addition to the assistance of AhR, the activation of the CYP-ROS axis, and the recruitment of intracellular calcium, the studies of PAHs affecting the immune system through epigenetic mechanisms have also become increasingly diverse in recent years. At present, the research about effects on T cells has been relatively mature, while the research on other immune cells such as B cells and monocytes still needs to be further explored. The impacts of environmental pollution, especially PAHs, on human diseases have received increasing attention in recent years. Therefore, This review has also introduced the relationship between PAHs and several diseases such as cancer, autoimmune diseases (RA, T1D, MS, and SLE), and allergic diseases (asthma and AD), We recommend more research on the relationship between PAHs and the immune system and immune-related diseases, and look forward to more findings to prevent environmental pollution, or more specifically, the role of PAHs in inducing or exacerbating these diseases.
Credit author statement
Yang-yiyi Yu: Writing - Original Draft; Hui Jin: Writing - Review & Editing; Qianjin Lu: Conceptualization, Supervision.
Funding
This study was supported by the National Natural Science Foundation of China (No.81830097, No. 1874253, No.81830097), The 15th medium-term special grant of postdoctoral Science Foundation of China (2022T150742) , Natural Science Foundation of Hunan Province China (No. 2021JJ40837), Excellent postdoctoral innovative talents of Hunan province in 2020 (No. 2020RC2014), CAMS Innovation Fund for Medical Sciences (CIFMS) (No.2021-I2M-1-059), The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-RC320-003), and Special Program of National Natural Science Foundation of China (NO. 32141004). The authors did not have financial support or benefits from commercial resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Hui Jin, Email: jh2020@csu.edu.cn.
Qianjin Lu, Email: qianlu5860@pumcderm.cams.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Prüss-Ustün A., et al. World Health Organization; 2016. Preventing Disease through Healthy Environments: a Global Assessment of the Burden of Disease from Environmental Risks. [Google Scholar]
- 2.Garwood P. World Health Organization; 2019. WHO Highlights Huge Scale of Tobacco-Related Lung Disease Deaths. [Google Scholar]
- 3.Project, I.T.C., O. World Health, and F. World Heart, Cardiovascular Harms from Tobacco Use and Secondhand Smoke: Global Gaps in Awareness and Implications for Action. Waterloo, Ontario, Canada and Geneva, Switzerland.
- 4.Landrigan P.J., et al. The Lancet Commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. 10119. [DOI] [PubMed] [Google Scholar]
- 5.Burnett R., et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U. S. A. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope C.A.R., et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope C.A.R., Dockery D.W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 8.Miller F.W., et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a national Institute of environmental health sciences expert panel workshop. J. Autoimmun. 2012;39(4):259–271. doi: 10.1016/j.jaut.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunzli N., et al. Comparison of oxidative properties, light absorbance, total and elemental mass concentration of ambient PM2.5 collected at 20 European sites. Environ. Health Perspect. 2006;114(5):684–690. doi: 10.1289/ehp.8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Air quality guidelines for Europe. WHO Reg. Publ. Eur. Ser. 2000;(91):1–273. [PubMed] [Google Scholar]
- 11.Council C. 2008. Canadian Soil Quality Guidelines Carcinogenic and Other Polycyclic Aromatic Hydrocarbons (PAHs) [Google Scholar]
- 12.Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [PubMed]
- 13.Kameda and Takayuki Atmospheric chemistry of polycyclic aromatic hydrocarbons and related compounds. J. Health Sci. 2011;57(6):504–511. [Google Scholar]
- 14.Park J.-S., Wade T.L., Sweet S. Atmospheric distribution of polycyclic aromatic hydrocarbons and deposition to Galveston Bay, Texas, USA. Atmos. Environ. 2001;35(19):3241–3249. [Google Scholar]
- 15.Kameda Y., et al. Atmospheric polycyclic aromatic hydrocarbons: size distribution, estimation of their risk and their depositions to the human respiratory tract. Sci. Total Environ. 2005;340(1):71–80. doi: 10.1016/j.scitotenv.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Lai I.C., et al. Seasonal variation of atmospheric polycyclic aromatic hydrocarbons along the Kaohsiung coast. J. Environ. Manag. 2011;92(8):2029–2037. doi: 10.1016/j.jenvman.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Mohanraj R., Dhanakumar S., Solaraj G. ScientificWorldJournal; 2012. Polycyclic Aromatic Hydrocarbons Bound to PM 2.5 in Urban Coimbatore, India with Emphasis on Source Apportionment. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dat N.D., Chang M.B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 2017;609:682–693. doi: 10.1016/j.scitotenv.2017.07.204. [DOI] [PubMed] [Google Scholar]
- 19.Ciecierska M., Obiedzinski M.W. Polycyclic aromatic hydrocarbons in the bakery chain. Food Chem. 2013;141(1):1–9. doi: 10.1016/j.foodchem.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 20.ACGIH (American Conference of Governmental Industrial Hygienists). Polycyclic Aromatic Hydrocarbons (PAHs) Biologic Exposure Indices (BEI) Cincinnati. OH: American Conference of Governmental Industrial Hygienists.
- 21.Wang Y., et al. Polycyclic aromatic hydrocarbons (PAHs) in soils and vegetation near an e-waste recycling site in South China: concentration, distribution, source, and risk assessment. Sci. Total Environ. 2012;439:187–193. doi: 10.1016/j.scitotenv.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Jongeneelen F.J. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann. Occup. Hyg. 2001;45(1):3–13. [PubMed] [Google Scholar]
- 23.McClean M.D., et al. Urinary 1-hydroxypyrene and polycyclic aromatic hydrocarbon exposure among asphalt paving workers. Ann. Occup. Hyg. 2004;48(6):565–578. doi: 10.1093/annhyg/meh044. [DOI] [PubMed] [Google Scholar]
- 24.McClean M.D., et al. Using urinary biomarkers of polycyclic aromatic compound exposure to guide exposure-reduction strategies among asphalt paving workers. Ann. Occup. Hyg. 2012;56(9):1013–1024. doi: 10.1093/annhyg/mes058. [DOI] [PubMed] [Google Scholar]
- 25.Sobus J.R., et al. Comparing urinary biomarkers of airborne and dermal exposure to polycyclic aromatic compounds in asphalt-exposed workers. Ann. Occup. Hyg. 2009;53(6):561–571. doi: 10.1093/annhyg/mep042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K.H., et al. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013;60:71–80. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Chu I., et al. Skin reservoir formation and bioavailability of dermally administered chemicals in hairless Guinea pigs. Food Chem. Toxicol. 1996;34(3):267–276. doi: 10.1016/0278-6915(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 28.Alexandrov K., Rojas M., Satarug S. The critical DNA damage by benzo(a)pyrene in lung tissues of smokers and approaches to preventing its formation. Toxicol. Lett. 2010;198(1):63–68. doi: 10.1016/j.toxlet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Foth H., Kahl R., Kahl G.F. Pharmacokinetics of low doses of benzo[a]pyrene in the rat. Food Chem. Toxicol. 1988;26(1):45–51. doi: 10.1016/0278-6915(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 30.Kao J., Patterson F.K., Hall J. Skin penetration and metabolism of topically applied chemicals in six mammalian species, including man: an in vitro study with benzo[a]pyrene and testosterone. Toxicol. Appl. Pharmacol. 1985;81(3 Pt 1):502–516. doi: 10.1016/0041-008x(85)90421-1. [DOI] [PubMed] [Google Scholar]
- 31.Denison M.S., Nagy S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 32.De Groot D., et al. The AH Receptor in Biology and Toxicology. 2011. AHR ligands: promiscuity in binding and diversity in response. [Google Scholar]
- 33.Vogel C.F.A., et al. The aryl hydrocarbon receptor as a target of environmental stressors - implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nebert D.W. Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Prog. Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19(3):184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 36.Park J.Y., Shigenaga M.K., Ames B.N. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc. Natl. Acad. Sci. U. S. A. 1996;93(6):2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knerr S., et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cytochrome P450s alter the formation of reactive oxygen species in liver cells. Mol. Nutr. Food Res. 2006;50(4–5):378–384. doi: 10.1002/mnfr.200500183. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.W., et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016;6 doi: 10.1038/srep27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acheva A., Schettino G., Prise K.M. Pro-inflammatory signaling in a 3D organotypic skin model after low LET irradiation-NF-kappaB, COX-2 activation, and impact on cell differentiation. Front. Immunol. 2017;8:82. doi: 10.3389/fimmu.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohle C., Bock K.W. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem. Pharmacol. 2006;72(7):795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Vogel C.F.A., et al. Inflammatory marker and aryl hydrocarbon receptor-dependent responses in human macrophages exposed to emissions from biodiesel fuels. Chemosphere. 2019;220:993–1002. doi: 10.1016/j.chemosphere.2018.12.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panteleyev A.A., Bickers D.R. Dioxin-induced chloracne--reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 2006;15(9):705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji G., et al. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J. Invest. Dermatol. 2012;132(1):59–68. doi: 10.1038/jid.2011.194. [DOI] [PubMed] [Google Scholar]
- 44.Reynaud S., Deschaux P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: a review. Aquat. Toxicol. 2006;77(2):229–238. doi: 10.1016/j.aquatox.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 45.McLoone P., et al. The effect of exposure to crude oil on the immune system. Health implications for people living near oil exploration activities. Int. J. Environ. Health Res. 2019:1–26. doi: 10.1080/09603123.2019.1689232. [DOI] [PubMed] [Google Scholar]
- 46.Burchiel S.W., Luster M.I. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001;98(1):2–10. doi: 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]
- 47.Zhigacheva I.V., et al. Humoral immunity: polycyclic aromatic hydrocarbons and nitrosamines. Dokl. Biol. Sci. 2002;383:120–122. doi: 10.1023/a:1015381622241. [DOI] [PubMed] [Google Scholar]
- 48.Hannam M.L., et al. Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere. 2010;78(7):779–784. doi: 10.1016/j.chemosphere.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 49.De Jong W.H., et al. Detection of immunotoxicity of benzo[a]pyrene in a subacute toxicity study after oral exposure in rats. Toxicol. Sci. 1999;50(2):214–220. doi: 10.1093/toxsci/50.2.214. [DOI] [PubMed] [Google Scholar]
- 50.Vorderstrasse B.A., et al. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol. Appl. Pharmacol. 2001;171(3):157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- 51.Temchura V.V., et al. Role of the aryl hydrocarbon receptor in thymocyte emigration in vivo. Eur. J. Immunol. 2005;35(9):2738–2747. doi: 10.1002/eji.200425641. [DOI] [PubMed] [Google Scholar]
- 52.McMillan B.J., et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces premature activation of the KLF2 regulon during thymocyte development. J. Biol. Chem. 2007;282(17):12590–12597. doi: 10.1074/jbc.M611446200. [DOI] [PubMed] [Google Scholar]
- 53.Parvez F., et al. Assessment of arsenic and polycyclic aromatic hydrocarbon (PAH) exposures on immune function among males in Bangladesh. PLoS One. 2019;14(5):e0216662. doi: 10.1371/journal.pone.0216662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lauer F.T., et al. Changes in human peripheral blood mononuclear cell (HPBMC) populations and T-cell subsets associated with arsenic and polycyclic aromatic hydrocarbon exposures in a Bangladesh cohort. PLoS One. 2019;14(7):e0220451. doi: 10.1371/journal.pone.0220451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldhoen M., et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 56.Mascanfroni I.D., et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 2015;21(6):638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Driscoll C.A., Mezrich J.D. The aryl hydrocarbon receptor as an immune-modulator of atmospheric particulate matter-mediated autoimmunity. Front. Immunol. 2018;9:2833. doi: 10.3389/fimmu.2018.02833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrlich A.K., et al. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol. Sci. 2018;161(2):310–320. doi: 10.1093/toxsci/kfx215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quintana F.J., et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 60.van Voorhis M., et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talbot J., et al. Smoking-induced aggravation of experimental arthritis is dependent of aryl hydrocarbon receptor activation in Th17 cells. Arthritis Res. Ther. 2018;20(1):119. doi: 10.1186/s13075-018-1609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Driscoll C.A., et al. Ambient urban dust particulate matter reduces pathologic T cells in the CNS and severity of EAE. Environ. Res. 2019;168:178–192. doi: 10.1016/j.envres.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Driscoll C.A., et al. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part. Fibre Toxicol. 2018;15(1):35. doi: 10.1186/s12989-018-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castaneda A.R., et al. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol. Lett. 2018;292:85–96. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia M., et al. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J. Allergy Clin. Immunol. 2015;136(2):441–453. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyers J.L., et al. Environmental cues received during development shape dendritic cell responses later in life. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207007. e0207007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai T., et al. IL-17-producing ST2(+) group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J. Allergy Clin. Immunol. 2019;143(1):229–244. doi: 10.1016/j.jaci.2018.03.007. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trifari S., et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 69.Jaligama S., et al. Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 2018;15(1):20. doi: 10.1186/s12989-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogel C.F., et al. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J. Biol. Chem. 2014;289(3):1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nadeau K., et al. Ambient air pollution impairs regulatory T-cell function in asthma. J. Allergy Clin. Immunol. 2010;126(4):845–852. doi: 10.1016/j.jaci.2010.08.008. e10. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen K.D., Vanichsarn C., Nadeau K.C. Impaired IL-10-dependent induction of tolerogenic dendritic cells by CD4+CD25hiCD127lo/- natural regulatory T cells in human allergic asthma. Am. J. Respir. Crit. Care Med. 2009;180(9):823–833. doi: 10.1164/rccm.200905-0761OC. [DOI] [PubMed] [Google Scholar]
- 73.Hew K.M., et al. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin. Exp. Allergy. 2015;45(1):238–248. doi: 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun L., et al. Particulate matter of 2.5 mum or less in diameter disturbs the balance of TH17/regulatory T cells by targeting glutamate oxaloacetate transaminase 1 and hypoxia-inducible factor 1alpha in an asthma model. J. Allergy Clin. Immunol. 2020;145(1):402–414. doi: 10.1016/j.jaci.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Herberth G., et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J. Allergy Clin. Immunol. 2014;133(2):543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 76.Wei P., et al. An aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress the Th17 response in allergic rhinitis patients. Lab. Invest. 2014;94(5):528–535. doi: 10.1038/labinvest.2014.8. [DOI] [PubMed] [Google Scholar]
- 77.Gagliani N., et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523(7559):221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tousa S., et al. Activin-A co-opts IRF4 and AhR signaling to induce human regulatory T cells that restrain asthmatic responses. Proc. Natl. Acad. Sci. U. S. A. 2017;114(14):E2891–E2900. doi: 10.1073/pnas.1616942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apetoh L., et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopez-Castejon G., Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011;22(4):189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.den Hartigh L.J., et al. Endotoxin and polycyclic aromatic hydrocarbons in ambient fine particulate matter from Fresno, California initiate human monocyte inflammatory responses mediated by reactive oxygen species. Toxicol. Vitro. 2010;24(7):1993–2002. doi: 10.1016/j.tiv.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 82.Xia M., et al. A Jagged 1-Notch 4 molecular switch mediates airway inflammation induced by ultrafine particles. J. Allergy Clin. Immunol. 2018;142(4):1243–1256. doi: 10.1016/j.jaci.2018.03.009. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ovrevik J., et al. Activation of proinflammatory responses in cells of the airway mucosa by particulate matter: oxidant- and non-oxidant-mediated triggering mechanisms. Biomolecules. 2015;5(3):1399–1440. doi: 10.3390/biom5031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Podechard N., et al. Interleukin-8 induction by the environmental contaminant benzo(a)pyrene is aryl hydrocarbon receptor-dependent and leads to lung inflammation. Toxicol. Lett. 2008;177(2):130–137. doi: 10.1016/j.toxlet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Goudot C., et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity. 2017;47(3):582–596. doi: 10.1016/j.immuni.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Ingram J.L., Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 2012;130(4):829–842. doi: 10.1016/j.jaci.2012.06.034. quiz 843-4. [DOI] [PubMed] [Google Scholar]
- 87.Barnes P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meldrum K., et al. Bronchial epithelial innate and adaptive immunity signals are induced by polycyclic aromatic hydrocarbons. Toxicol. Res. 2016;5(3):816–827. doi: 10.1039/c5tx00389j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao S.C., et al. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J. Immunol. 2004;173(11):6712–6718. doi: 10.4049/jimmunol.173.11.6712. [DOI] [PubMed] [Google Scholar]
- 90.Riedl M.A., Nel A.E. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8(1):49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 91.Esser C., Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67(2):259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 92.Yang L., et al. Polycyclic aromatic hydrocarbons are associated with increased risk of chronic obstructive pulmonary disease during haze events in China. Sci. Total Environ. 2017;574:1649–1658. doi: 10.1016/j.scitotenv.2016.08.211. [DOI] [PubMed] [Google Scholar]
- 93.Cho Y.S., Moon H.B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol. Res. 2010;2(3):183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wada T., et al. Aryl hydrocarbon receptor modulates NADPH oxidase activity via direct transcriptional regulation of p40phox expression. Mol. Pharmacol. 2013;83(5):1133–1140. doi: 10.1124/mol.112.083303. [DOI] [PubMed] [Google Scholar]
- 95.Wang E., et al. Benzo(a)pyrene facilitates dermatophagoides group 1 (Der f 1)-induced epithelial cytokine release through aryl hydrocarbon receptor in asthma. Allergy. 2019;74(9):1675–1690. doi: 10.1111/all.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yanagisawa R., et al. Low-dose benzo[a]pyrene aggravates allergic airway inflammation in mice. J. Appl. Toxicol. 2016;36(11):1496–1504. doi: 10.1002/jat.3308. [DOI] [PubMed] [Google Scholar]
- 97.Karimi P., et al. Polycyclic aromatic hydrocarbons and childhood asthma. Eur. J. Epidemiol. 2015;30(2):91–101. doi: 10.1007/s10654-015-9988-6. [DOI] [PubMed] [Google Scholar]
- 98.Kanoh T., et al. Adjuvant activities of pyrene, anthracene, fluoranthene and benzo(a)pyrene in production of anti-IgE antibody to Japanese cedar pollen allergen in mice. J. Clin. Lab. Immunol. 1996;48(4):133–147. [PubMed] [Google Scholar]
- 99.Tsien A., et al. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B cells in vitro. Toxicol. Appl. Pharmacol. 1997;142(2):256–263. doi: 10.1006/taap.1996.8063. [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi K., et al. Induction of PreB cell apoptosis by 7,12-dimethylbenz[a]anthracene in long-term primary murine bone marrow cultures. Toxicol. Appl. Pharmacol. 1997;147(2):190–203. doi: 10.1006/taap.1997.8263. [DOI] [PubMed] [Google Scholar]
- 101.Allan L.L., et al. CYP1A1 in polycyclic aromatic hydrocarbon-induced B lymphocyte growth suppression. Biochem. Biophys. Res. Commun. 2006;342(1):227–235. doi: 10.1016/j.bbrc.2006.01.131. [DOI] [PubMed] [Google Scholar]
- 102.Burchiel S.W., et al. An increase in circulating B cells and B cell activation markers in peripheral blood is associated with cigarette smoking in a male cohort in Bangladesh. Toxicol. Appl. Pharmacol. 2019;384 doi: 10.1016/j.taap.2019.114783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Y., et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121(16):3195–3204. doi: 10.1182/blood-2012-08-453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maaetoft-Udsen K., et al. Aryl hydrocarbon receptor ligand effects in RBL2H3 cells. J. Immunot. 2012;9(3):327–337. doi: 10.3109/1547691X.2012.661802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sibilano R., et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J. Immunol. 2012;189(1):120–127. doi: 10.4049/jimmunol.1200009. [DOI] [PubMed] [Google Scholar]
- 106.Nowak-Wegrzyn A., Fiocchi A. Is oral immunotherapy the cure for food allergies? Curr. Opin. Allergy Clin. Immunol. 2010;10(3):214–219. doi: 10.1097/ACI.0b013e3283399404. [DOI] [PubMed] [Google Scholar]
- 107.Wang H.C., Zhou Y., Huang S.K. SHP-2 phosphatase controls aryl hydrocarbon receptor-mediated ER stress response in mast cells. Arch. Toxicol. 2017;91(4):1739–1748. doi: 10.1007/s00204-016-1861-1. [DOI] [PubMed] [Google Scholar]
- 108.Huang S.K., et al. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J. Thorac. Dis. 2015;7(1):23–33. doi: 10.3978/j.issn.2072-1439.2014.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Cavallo F., et al. The immune hallmarks of cancer. Cancer Immunol. Immunother. 2011;60(3):319–326. doi: 10.1007/s00262-010-0968-0. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stjernsward J. Effect of noncarcinogenic and carcinogenic hydrocarbons on antibody-forming cells measured at the cellular level in vitro. J. Natl. Cancer Inst. 1966;36(6):1189–1195. [PubMed] [Google Scholar]
- 112.Luster M.I., et al. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fund. Appl. Toxicol. 1992;18(2):200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 113.Kong L.Y., et al. Inhibition of lung immunity after intratracheal instillation of benzo(a)pyrene. Am. J. Respir. Crit. Care Med. 1994;150(4):1123–1129. doi: 10.1164/ajrccm.150.4.7921446. [DOI] [PubMed] [Google Scholar]
- 114.Zaccaria K.J., McClure P.R. Using immunotoxicity information to improve cancer risk assessment for polycyclic aromatic hydrocarbon mixtures. Int. J. Toxicol. 2013;32(4):236–250. doi: 10.1177/1091581813492829. [DOI] [PubMed] [Google Scholar]
- 115.Nebert D.W., et al. Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences--Cyp1 knockout mouse lines as a paradigm. Mol. Pharmacol. 2013;84(3):304–313. doi: 10.1124/mol.113.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Malik A.I., et al. Hepatic genotoxicity and toxicogenomic responses in MutaMouse males treated with dibenz[a,h]anthracene. Mutagenesis. 2013;28(5):543–554. doi: 10.1093/mutage/get031. [DOI] [PubMed] [Google Scholar]
- 117.Buque A., et al. MPA/DMBA-driven mammary carcinomas. Methods Cell Biol. 2021;163:1–19. doi: 10.1016/bs.mcb.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 118.Piscuoglio S., et al. The genomic landscape of male breast cancers. Clin. Cancer Res. 2016;22(16):4045–4056. doi: 10.1158/1078-0432.CCR-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamazaki T., et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 2020;21(10):1160–1171. doi: 10.1038/s41590-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 120.Rodriguez-Ruiz M.E., et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. OncoImmunology. 2019;8(11):e1655964. doi: 10.1080/2162402X.2019.1655964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feldmann M., Brennan F.M., Maini R.N. Rheumatoid arthritis. Cell. 1996;85(3):307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 122.Sun L., et al. Relationship between polycyclic aromatic hydrocarbons and rheumatoid arthritis in US general population. Sci. Total Environ. 2020;704 doi: 10.1016/j.scitotenv.2019.135294. NHANES 2003-2012. [DOI] [PubMed] [Google Scholar]
- 123.Li J., et al. Subgroup analysis of the relationship between polycyclic aromatic hydrocarbons and rheumatoid arthritis: data from the National Health and Nutrition Examination Survey, 2003-2014. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145841. [DOI] [PubMed] [Google Scholar]
- 124.Tong B., et al. Sinomenine induces the generation of intestinal Treg cells and attenuates arthritis via activation of aryl hydrocarbon receptor. Lab. Invest. 2016;96(10):1076–1086. doi: 10.1038/labinvest.2016.86. [DOI] [PubMed] [Google Scholar]
- 125.Pugliese A. Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 2017;127(8):2881–2891. doi: 10.1172/JCI94549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seise I., et al. Dietary ingestion of 2-aminoanthracene (2AA) and the risk for type-1 diabetes (T1D) J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2020;55(14):1638–1645. doi: 10.1080/10934529.2020.1830651. [DOI] [PubMed] [Google Scholar]
- 127.Compston A., Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 128.Oikonen M., et al. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. 2003;22(1):95–99. doi: 10.1159/000067108. [DOI] [PubMed] [Google Scholar]
- 129.Oikonen M.K., Eralinna J.P. Beta-interferon protects multiple sclerosis patients against enhanced susceptibility to infections caused by poor air quality. Neuroepidemiology. 2008;30(1):13–19. doi: 10.1159/000113301. [DOI] [PubMed] [Google Scholar]
- 130.Jeanjean M., et al. Ozone, NO2 and PM10 are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ. Res. 2018;163:43–52. doi: 10.1016/j.envres.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roux J., et al. Air pollution by particulate matter PM10 may trigger multiple sclerosis relapses. Environ. Res. 2017;156:404–410. doi: 10.1016/j.envres.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 132.Rothhammer V., et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol. Neuroimmunol. Neuroinflamm. 2017;4(4):e359. doi: 10.1212/NXI.0000000000000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaye J., et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2016;113(41):E6145–E6152. doi: 10.1073/pnas.1607843113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ionescu R., et al. Detection of multiple sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2011;2(12):687–693. doi: 10.1021/cn2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsokos G.C. Systemic lupus erythematosus. N Engl. J. Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 136.Jung C.R., et al. Long-term exposure to traffic-related air pollution and systemic lupus erythematosus in Taiwan: a cohort study. Sci. Total Environ. 2019;668:342–349. doi: 10.1016/j.scitotenv.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 137.Costenbader K.H., Karlson E.W. Cigarette smoking and systemic lupus erythematosus: a smoking gun? Autoimmunity. 2005;38(7):541–547. doi: 10.1080/08916930500285758. [DOI] [PubMed] [Google Scholar]
- 138.Fernandes E.C., et al. Exposure to air pollutants and disease activity in juvenile-onset systemic lupus erythematosus patients. Arthritis Care Res. 2015;67(11):1609–1614. doi: 10.1002/acr.22603. [DOI] [PubMed] [Google Scholar]
- 139.Alves A.G.F., et al. Influence of air pollution on airway inflammation and disease activity in childhood-systemic lupus erythematosus. Clin. Rheumatol. 2018;37(3):683–690. doi: 10.1007/s10067-017-3893-1. [DOI] [PubMed] [Google Scholar]
- 140.Shinde R., et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 2018;19(6):571–582. doi: 10.1038/s41590-018-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dorgham K., et al. Ultraviolet light converts propranolol, a nonselective beta-blocker and potential lupus-inducing drug, into a proinflammatory AhR ligand. Eur. J. Immunol. 2015;45(11):3174–3187. doi: 10.1002/eji.201445144. [DOI] [PubMed] [Google Scholar]
- 142.Barnes P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011;242(1):31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 143.Castaneda A.R., et al. Ambient particulate matter enhances the pulmonary allergic immune response to house dust mite in a BALB/c mouse model by augmenting Th2- and Th17-immune responses. Phys. Rep. 2018;6(18) doi: 10.14814/phy2.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu X., et al. Maternal 1-nitropyrene exposure during pregnancy increases susceptibility of allergic asthma in adolescent offspring. Chemosphere. 2020;243 doi: 10.1016/j.chemosphere.2019.125356. [DOI] [PubMed] [Google Scholar]
- 145.Turk B.U., et al. Air pollution, a possible risk factor for multiple sclerosis. Acta Neurol. Scand. 2020;141(5):431–437. doi: 10.1111/ane.13223. [DOI] [PubMed] [Google Scholar]
- 146.Hidaka T., et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017;18(1):64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 147.Paller A.S., et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021;84(3):632–638. doi: 10.1016/j.jaad.2020.05.135. [DOI] [PubMed] [Google Scholar]
- 148.Peppers J., et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019;80(1):89–98. doi: 10.1016/j.jaad.2018.06.047. e3. [DOI] [PubMed] [Google Scholar]
- 149.Smith S.H., et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Invest. Dermatol. 2017;137(10):2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.