Abstract
Objective
People with knee or hip osteoarthritis (OA) can experience comorbid lumbar spinal stenosis (LSS), but the impact on treatment outcomes is unknown. The aim of this study was to investigate associations between comorbid LSS symptoms and changes in pain, function, and quality of life following a patient education and exercise therapy program.
Design
This was a longitudinal analysis of 6813 participants in the Good Life with osteoArthritis in Denmark (GLA:D®) program; a structured patient education and exercise therapy program for knee and hip OA. Participants were classified as having comorbid LSS symptoms based on self-report symptom items. Linear mixed models were used to assess differences in change in pain, function, and quality of life outcomes (0 worst to 100 best) at 3- and 12-month follow-up.
Results
15% and 23% of knee and hip OA participants had comorbid LSS symptoms, respectively. Knee participants with comorbid LSS symptoms had smaller improvement in pain at 3-months (−1.7, 95% CI -3.3 to −0.1) and hip participants with comorbid LSS symptoms had greater improvement in function at 3- (2.5, 95% CI 0.5 to 5.0) and 12-months (3.8, 95% CI 0.9 to 6.6), when compared to those without LSS symptoms. These differences were not clinically significant and no differences in other outcomes were observed.
Conclusion
Knee or hip OA patients with comorbid LSS symptoms should expect similar improvements in knee- or hip-related pain, function, and quality of life outcomes when undergoing a patient education and exercise therapy program compared to those without LSS symptoms.
Keywords: Lumbar spinal stenosis, Knee osteoarthritis, Hip osteoarthritis, Comorbidity, Association
1. Introduction
Both degenerative lumbar spinal stenosis (LSS) and knee and hip osteoarthritis (OA) are prevalent conditions in the aging population [1,2]. Like knee and hip OA, LSS is a result of age-related changes to joint structures [1]. These changes narrow the spinal canal leading to compression and/or ischemia of the spinal nerves and the resultant clinical symptomology [1,3]. Symptomatic LSS presents as pain and numbness in the legs radiating from the spine, typically exacerbated with walking, standing, and spinal extension, and relieved with flexion of the lumbar spine [4,5].
The shared degenerative etiology between LSS, knee, and hip OA [6,7] has led to suggestions that LSS can be considered as lumbar spine OA with resultant neurological symptomology in one or both lower extremities [8]. Therefore, co-occurrence with knee or hip OA as part of a multi-joint or generalized OA presentation could be expected. A recent review found the prevalence of comorbid LSS was 17–54% (depending on case definition) in people with knee OA, but no studies reporting comorbid LSS in people with hip OA were identified [9]. However, comorbid symptomatic hip OA was found in 17–35% of people with LSS [9], supporting the notion that LSS and hip OA co-occur.
Previous studies suggest patients with comorbid LSS or other degenerative disorders of the lumbar spine may have worse outcomes after total knee or hip replacement [[10], [11], [12], [13], [14], [15]]. So far however, no studies have looked at how co-occurrence of LSS and OA might affect outcomes after commonly applied and recommended primary care treatments [9], such as patient education and exercise therapy [16]. This information is needed to determine if those with comorbid LSS represent a clinically distinct group of patients with knee or hip OA requiring additional or different treatment, or if they are likely to experience similar benefits from patient education and exercise therapy as those without coexisting LSS symptoms.
The Good Life with osteoArthritis in Denmark (GLA:D®) program – a structured primary care patient education and exercise therapy intervention for people with knee and hip OA – is well-suited to study the impact of comorbid LSS symptoms on patient outcomes. A previous GLA:D® study found up to 40% of participants with knee OA and 50% of participants with hip OA reported symptoms commonly attributed to LSS [17], but the impact on patient outcomes has not been evaluated. Therefore, the objective of this study was to investigate associations between comorbid LSS symptoms and changes in pain, function, and quality of life following participation in GLA:D® for knee and hip OA patients. We hypothesized that comorbid LSS symptoms would be associated with less improvement in outcomes.
2. Methods
2.1. Study design
This was a longitudinal analysis of registry data from the GLA:D® program for knee and hip OA. GLA:D® consists of two patient education sessions and 12 supervised group exercise sessions delivered over an 8-week period [18,19]. Detailed information, including the content of the education and exercise program, is available elsewhere [[18], [19], [20]].
This report conforms to the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) statement for reporting of observational studies [21]. Ethics approval of GLA:D® studies are not needed according to the ethics committee of the North Denmark Region. The GLA:D® registry has been approved by the Danish Data Protection Agency (SDU; 10.084). All participants provided informed consent prior to enrolment in the GLA:D® program. According to the Danish Data Protection Act, patient consent was not required for this study as personal data was processed exclusively for research and statistical purposes.
2.2. Participants
People seeking care due to knee or hip pain and/or functional limitations associated with OA are screened for eligibility for GLA:D® by enrolling clinicians. Participants are eligible if they: 1) do not have reasons other than OA for their joint symptoms; 2) do not have other symptoms that are more pronounced than their OA symptoms; and 3) can understand Danish.
Items assessing symptoms associated with LSS were included in the routinely collected baseline questions in the GLA:D® registry. In this study, participants enrolled in GLA:D® between January 2019 and November 2019 with completed baseline LSS symptom data were included, to ensure only participants completing the 8-week program prior to the COVID-19 pandemic (March 2020) were included. Two cohorts of participants were constructed at baseline: 1) primary knee complaint (knee OA cohort); and 2) primary hip complaint (hip OA cohort).
2.3. Comorbid LSS symptoms (exposure)
Participants were classified as having comorbid LSS symptoms if they reported: 1) “sometimes feeling pain or numbness in one/both legs or buttocks (meaning other symptoms than from the knee or hip joint)”; 2) at least one symptom-worsening activity (walking or standing for a while); at least one symptom-relieving activity (bending forwards; sitting; riding a bicycle; or bending over a shopping cart); 3) not having diabetes; and 4) were aged 60 years or older (see Supplementary File for full details on classification). The individual LSS symptom items are commonly used self-report items that distinguish other types of leg pain from LSS [22]. The age cut-point of 60 years or older was selected to decrease the probability of leg pain due to lumbar disc herniation and has been used in previous criteria for LSS [23,24]. The no diabetes criteria was included to ensure participants with diabetic neuropathy, an important differential diagnosis for symptomatic LSS in people over 60,23 were not included. Low back pain was not included in the LSS symptom definition because it is not considered a key feature of LSS or necessary for diagnosis, due to the fact many individuals with LSS do not report back pain [5,22,25]. The LSS symptom items and diabetic status were collected at baseline via electronic self-report questions and age was calculated using the 10-digit person-unique Central Person Registry number assigned to all people residing in Denmark.
2.4. Outcomes
The primary outcome was difference in mean change in pain between those with LSS symptoms and those without from baseline to immediately following completion of GLA:D® (approximately 3 months), and from baseline to 12 months. Change in pain was measured using the Knee injury and Osteoarthritis Outcome Score 12 item short form (KOOS-12) pain subscale [26] in the knee OA cohort, and by the Hip disability and Osteoarthritis Outcome Score 12 item short form (HOOS-12) pain subscale [27] in the hip OA cohort. On both measures, pain is assessed on four items and scored from 0 (worst) to 100 (best). Both measures have demonstrated adequate validity and reliability in people with knee or hip OA [26,27].
Secondary outcomes were the difference in mean change for those with LSS symptoms and those without in function and joint-related quality of life from baseline to 3- and 12-month follow-up. Change in function and quality of life was measured on the function and quality of life subscales of the KOOS-12 and HOOS-12 in the knee and hip cohorts, respectively. Function and quality of life subscales are scored 0 (worst) to 100 (best) on both measures [26,27]. All outcomes at each follow-up were collected by electronic self-report questionnaire.
2.5. Statistical analysis
2.5.1. Multiple imputation
Missing values for the outcomes at all time points and baseline covariates (confounders described below) were imputed using multiple imputation with chained equations [28], under the assumption of data missing at random. The imputation model included all outcomes at each time point, comorbid LSS symptom status (exposure), and the confounders in the main analyses (see main analyses below). Predictive mean matching was used to impute outcomes at all time points (continuous data). Missing covariate data was imputed using predictive mean matching for continuous variables, ordered logistic regression for ordered categorical variables, and logistic regression for binary variables. 40 imputed data sets were generated to approximate the proportion of missing observations for the primary outcome at 12-month follow-up [28].
2.5.2. Main analyses
The primary outcome (difference in mean change in pain scores from baseline to 3- and 12-month follow-up) was analysed using a linear mixed regression (restricted maximum likelihood estimation) separately for the knee and hip OA cohort. Comorbid LSS symptom status and follow-up time were entered as fixed effects and participants nested within clinics as random effects. Unadjusted and adjusted differences in change with 95% confidence intervals (CI) were combined across imputed data sets using Rubin's rules [29]. Models in the adjusted analyses were controlled for age (continuous), sex (male/female), BMI (continuous), education level (ordered categorical), symptom duration (continuous), additional joint (knee or hip) symptoms (yes/no), and comorbid back pain intensity (continuous). Potential confounders (described in Supplementary File) were selected based on previous literature investigating the association of these variables with GLA:D® outcomes [30,31], OA outcomes in general [[32], [33], [34]], and LSS [35,36].
The same analysis approach was used for the secondary outcomes (difference in mean change in function and quality of life scores from baseline to 3- and 12-month follow-up).
2.5.3. Sensitivity analyses
Two sensitivity analyses were conducted. First, the impact of missing data was explored by conducting a complete-case analysis where participants with missing outcome data at any time point were excluded. This was conducted for adjusted differences (same controlled covariates as main analysis) in mean change in pain, function, and quality of life scores in both cohorts separately.
Then the impact of the age cut-point (≥60 years) was investigated by repeating the analysis using an alternate age criterion, where a cut-point of 50 years or older was used. This alternate definition was tested since previous LSS diagnostic literature has suggested an age cut-point as low as 48 years old [37] and 50 years old has been used with similar LSS symptom items [38]. The same linear mixed regression analyses outlined in the main analyses were conducted, using comorbid LSS symptoms (based on alternate LSS definition) and follow-up time as fixed effects. Adjusted differences (same controlled covariates as main analysis) in mean change in pain, function, and quality of life scores were combined across imputed data sets using Rubin's rules [29], in both cohorts separately. All analyses were performed in Stata 17.0.
3. Results
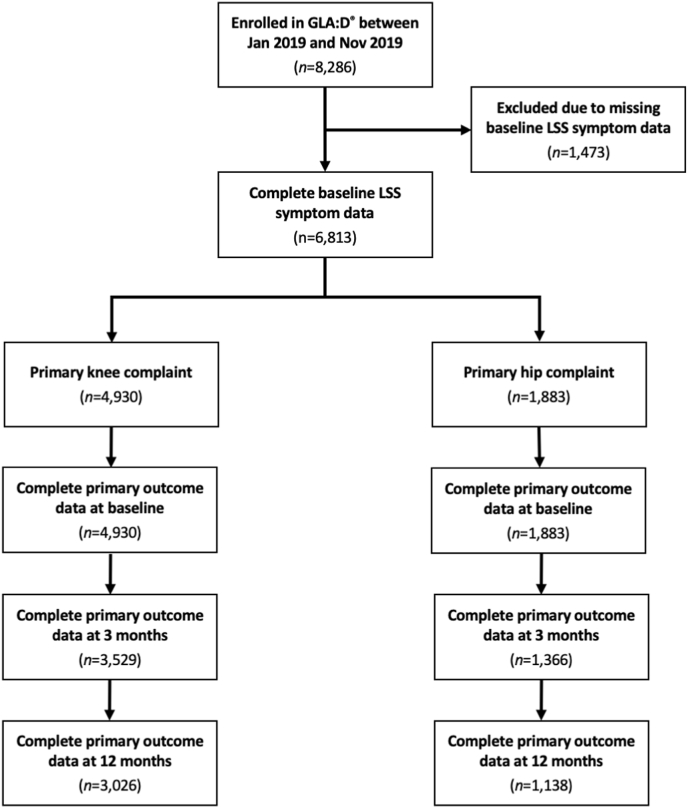
A total of 8286 participants were enrolled in GLA:D® during the study period (Fig. 1). 1473 were excluded due to missing baseline LSS symptom data. Excluded participants were similar to those included, but were slightly older and more often male (Supplementary Table S1).
Fig. 1.
Participant flow diagram.
A total of 4930 participants with knee OA and 1883 participants with hip OA were included. 15% of knee OA and 23% of hip OA participants had comorbid LSS symptoms. Cohort baseline characteristics by comorbid LSS symptom status are presented in Table 1, Table 2.
Table 1.
Knee osteoarthritis cohort baseline characteristics.
| Overall (n = 4930) | Comorbid LSS symptoms (n = 755) | No comorbid LSS symptoms (n = 4175) | |
|---|---|---|---|
| Age (years), mean (95% CI) | 65.7 (65.4–66.0) | 70.0 (69.5–70.4) | 64.9 (64.6–65.2) |
| Missing, n | 0 | 0 | 0 |
| Female, % (95% CI) | 69.6 (68.3–70.9) | 72.7 (69.5–75.9) | 69.0 (67.6–70.4) |
| Missing, n | 0 | 0 | 0 |
| BMI (kg/m2), mean (95% CI) | 29.0 (28.8–29.1) | 29.1 (28.7–29.4) | 29.0 (28.8–29.1) |
| Missing, n | 40 | 5 | 35 |
| Education level, % (95% CI) | |||
| Primary school | 18.6 (17.5–19.8) | 23.3 (20.3–26.5) | 17.7 (16.6–18.9) |
| Secondary school | 11.3 (10.4–12.2) | 9.8 (7.8–12.1) | 11.5 (10.6–12.5) |
| Short-term education | 20.3 (19.2–21.4) | 18.7 (16.0–21.6) | 20.6 (19.4–21.8) |
| Middle-term education | 38.7 (37.3–40.1) | 38.4 (34.9–42.0) | 38.8 (37.3–40.3) |
| Long-term education | 11.1 (10.2–12.0) | 9.8 (7.8–12.1) | 11.3 (10.3–12.3) |
| Missing, n | 7 | 0 | 7 |
| Symptom duration (months), mean (95% CI) | 32.3 (30.8–33.8) | 35.8 (31.6–40.0) | 31.7 (30.1–33.2) |
| Missing, n | 4 | 0 | 4 |
| Additional joint symptoms, % (95% CI) | 57.6 (56.2–59.0) | 67.4 (63.9–70.8) | 55.8 (54.3–57.3) |
| Missing, n | 1 | 0 | 1 |
| Comorbid back pain, mean (95% CI) | 2.5 (2.5–2.6) | 3.6 (3.4–3.8) | 2.3 (2.3–2.4) |
| Missing, n | 0 | 0 | 0 |
| KOOS-12 pain, mean (95% CI) | 50.0 (49.5–50.4) | 45.6 (44.5–46.6) | 50.7 (50.2–51.3) |
| Missing, n | 0 | 0 | 0 |
| KOOS-12 function, mean (95% CI) | 56.4 (55.9–57.0) | 50.2 (49.0–51.5) | 57.6 (57.0–58.1) |
| Missing, n | 0 | 0 | 0 |
| KOOS-12 quality of life, mean (95% CI) | 45.8 (45.4–46.3) | 42.0 (41.0–43.1) | 46.5 (46.0–47.0) |
| Missing, n | 0 | 0 | 0 |
n: number, BMI: body mass index (kg/m2), KOOS-12: Knee injury and Osteoarthritis Outcome Score 12 item short form (0–100, worst-best), Comorbid back pain: Numeric Pain Rating Scale (0–10, best-worst).
Table 2.
Hip osteoarthritis cohort baseline characteristics.
| Overall (n = 1883) | Comorbid LSS symptoms (n = 424) | No comorbid LSS symptoms (n = 1459) | |
|---|---|---|---|
| Age (years), mean (95% CI) | 66.8 (66.4–67.3) | 69.8 (69.1–70.4) | 66.0 (65.5–66.5) |
| Missing, n | 0 | 0 | 0 |
| Female, % (95% CI) | 69.7 (67.6–71.8) | 69.1 (64.7–73.5) | 69.9 (67.6–72.3) |
| Missing, n | 0 | 0 | 0 |
| BMI (kg/m2), mean (95% CI) | 27.2 (27.0–27.4) | 27.4 (27.0–27.9) | 27.1 (26.9–27.4) |
| Missing, n | 19 | 7 | 12 |
| Education level, % (95% CI) | |||
| Primary school | 17.8 (16.1–19.6) | 20.0 (16.3–24.2) | 17.2 (15.3–19.2) |
| Secondary school | 11.6 (10.2–13.1) | 12.5 (9.5–16.0) | 11.3 (9.7–13.0) |
| Short-term education | 20.7 (18.9–22.6) | 18.6 (15.0–22.7) | 21.2 (19.2–23.4) |
| Middle-term education | 38.8 (36.6–41.1) | 38.4 (33.8–43.3) | 38.9 (36.4–41.5) |
| Long-term education | 10.8 (9.5–12.3) | 10.3 (7.6–13.7) | 11.0 (9.4–12.7) |
| Missing, n | 5 | 0 | 5 |
| Symptom duration (months), mean (95% CI) | 27.6 (25.9–29.4) | 29.7 (25.3–34.1) | 27.0 (25.2–28.9) |
| Missing, n | 1 | 0 | 1 |
| Additional joint symptoms, % (95% CI) | 54.8 (52.5–57.1) | 58.3 (53.4–63.0) | 53.8 (51.2–56.4) |
| Missing, n | 0 | 0 | 0 |
| Comorbid back pain, mean (95% CI) | 3.1 (3.0–3.3) | 3.9 (3.6–4.2) | 2.9 (2.8–3.0) |
| Missing, n | 0 | 0 | 0 |
| HOOS-12 pain, mean (95% CI) | 49.1 (48.4–49.9) | 44.8 (43.5–46.2) | 50.4 (49.5–51.2) |
| Missing, n | 0 | 0 | 0 |
| HOOS-12 function, mean (95% CI) | 59.1 (58.2–60.0) | 53.1 (51.3–54.9) | 60.8 (59.8–61.8) |
| Missing, n | 0 | 0 | 0 |
| HOOS-12 quality of life, mean (95% CI) | 48.7 (48.0–49.5) | 44.6 (43.2–46.0 | 49.9 (49.0–50.8) |
| Missing, n | 0 | 0 | 0 |
n: number, BMI: body mass index (kg/m2), HOOS-12: Hip disability and Osteoarthritis Outcome Score 12 item short form (0–100, worst-best) Comorbid back pain: Numeric Pain Rating Scale (0–10, best-worst).
3.1. Missing outcome data
In both cohorts, all participants completed the primary and secondary outcomes at baseline.
In the knee OA cohort, the proportion of missing data was 28% and 39% at 3- and 12-month follow-up for all outcomes, respectively. Participants with missing primary outcome data at 12-months had a greater mean BMI and comorbid back pain intensity, were less educated, and had a smaller proportion of participants with comorbid LSS symptoms, but the differences were small (Supplementary Table S2).
In the hip OA cohort, the proportion of missing data was 27% and 40% at 3- and 12-month follow-up for all outcomes, respectively. Participants with missing primary outcome data at 12-months had a greater mean comorbid back pain intensity and were less educated, but the differences were small (Supplementary Table S3).
3.2. Primary outcome
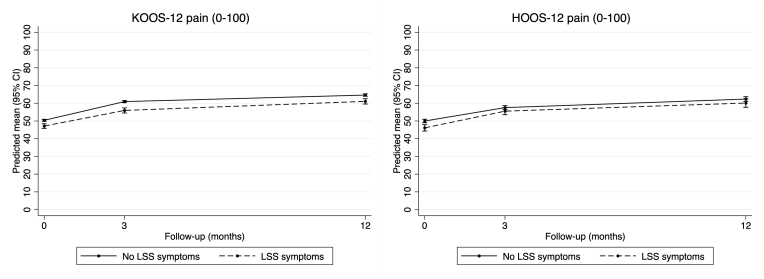
In the knee OA cohort, adjusted analysis (Table 3), pain scores improved over time regardless of LSS symptom status, while those with comorbid LSS symptoms had slightly worse scores at each time-point (Fig. 2). Participants with comorbid LSS symptoms had slightly smaller pain improvement from baseline to 3-month follow-up (−1.7, 95% CI -3.3 to −0.1), but no certain difference was observed between LSS groups at 12-month follow-up (−0.3, 95% CI -1.9 to 1.3). Unadjusted results were similar (Supplementary Table S4).
Table 3.
KOOS-12 subscale differences at baseline, and changes at 3- and 12-month follow-up in participants with knee osteoarthritis.
| Baseline score | 3-month follow-up | 12-month follow-up | |||
|---|---|---|---|---|---|
| KOOS-12 pain adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 50.4 (49.8–51.0) | 60.9 (60.3–61.6) | 10.5 (9.9–11.2) | 64.6 (63.9–65.3) | 14.2 (13.6–14.9) |
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms |
−3.2 (−4.7 to −1.8) |
−5.0 (−6.6 to −3.4) |
−1.7 (−3.3 to −0.1) |
−3.6 (−5.2 to −2.0) |
−0.3 (−1.9 to 1.3) |
| KOOS-12 function adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 57.1 (56.6–57.7) | 66.4 (65.8–67.1) | 9.3 (8.7–9.9) | 68.7 (68.1–69.4) | 11.6 (11.0–12.2) |
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms |
−5.0 (−6.5 to −3.5) |
−5.2 (−6.9 to −3.5) |
−0.2 (−1.8 to 1.4) |
−4.6 (−6.3 to −2.9) |
0.4 (−1.2 to 2.0) |
| KOOS-12 quality of life adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 46.3 (45.8–46.9) | 52.9 (52.3–53.5) | 6.6 (6.0–7.2) | 56.6 (56.0–57.3) | 10.3 (9.7–10.9) |
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms | −3.4 (−4.8 to −2.0) | −3.6 (−5.2 to −2.1) | −0.2 (−1.7 to 1.3) | −3.2 (−4.9 to −1.6) | 0.2 (−1.4 to 1.7) |
KOOS-12: Knee injury and Osteoarthritis Outcome Score 12 item short form (0–100, worst-best).
Fig. 2.
Changes in KOOS-12 and HOOS-12 pain scores at each time-point.
In the hip OA cohort adjusted analysis (Table 4), pain scores also improved over time regardless of LSS symptom status, while those with comorbid LSS symptoms had slightly worse scores at baseline, but not at 3- and 12-months (Fig. 2). There was no certain difference between groups in change in pain scores from baseline to 3-month (1.9, 95% CI -0.6 to 4.3) or 12-month follow-up (1.6, 95% CI -1.4 to 4.6). Unadjusted results were similar (Supplementary Table S5).
Table 4.
HOOS-12 subscale differences at baseline, and changes at 3- and 12-month follow-up in participants with hip osteoarthritis.
| Baseline score | 3-month follow-up | 12-month follow-up | |||
|---|---|---|---|---|---|
| HOOS-12 pain adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 50.0 (49.0–51.0) | 57.6 (56.4–58.7) | 7.6 (6.4–8.7) | 62.4 (44.3–47.9) | 12.4 (11.0–13.7) |
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms |
−3.9 (−5.9 to −1.8) |
−2.0 (−4.3 to 0.2) |
1.9 (−0.6 to 4.3) |
−2.3 (−5.2 to 0.6) |
1.6 (−1.4 to 4.6) |
| HOOS-12 function adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 60.3 (59.2–61.3) | 66.2 (65.0–67.3) | 5.9 (4.8–7.1) | 69.3 (68.0–70.6) | 9.0 (7.7–10.3) |
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms |
−5.4 (−7.5 to −3.2) |
−2.8 (−5.2 to −0.4) |
2.5 (0.5–5.0) |
−1.6 (−4.4 to 1.2) |
3.8 (0.9–6.6) |
| HOOS-12 quality of life adjusted model | Mean (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) | Mean (95% CI) | Mean change from baseline (95% CI) |
| No comorbid LSS symptoms (reference) | 49.7 (48.7–50.7) | 53.2 (52.2–54.3) | 58.6 (57.4–59.9) | ||
| Mean difference (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | Mean difference (95% CI) | Difference in change from baseline (95% CI) | |
| Comorbid LSS symptoms | −4.3 (−6.3 to −2.3) | −2.6 (−4.7 to −0.4) | 1.7 (−0.6 to 4.0) | −3.0 (−5.7 to −0.2) | 1.3 (−1.6 to 4.2) |
HOOS-12: Hip disability and Osteoarthritis Outcome Score 12 item short form (0–100, worst-best).
3.3. Secondary outcomes
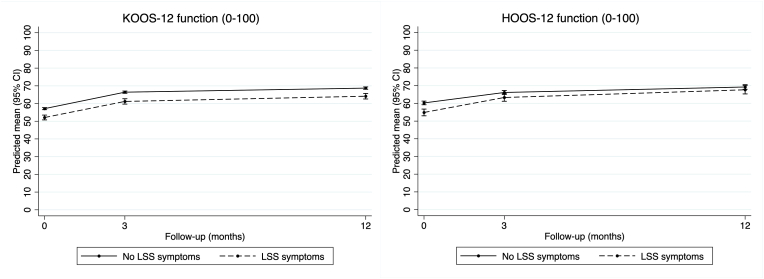
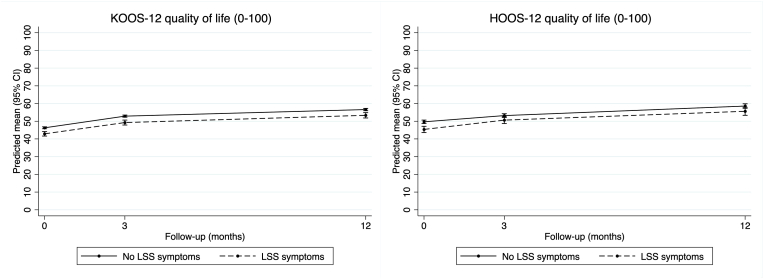
In the knee OA cohort adjusted analysis (Table 3), function scores improved over time regardless of LSS symptom status, while those with comorbid LSS symptoms had slightly worse scores at each time-point (Fig. 3). There was no certain difference between groups in change in function scores at 3-month (−0.2, 95% CI -1.8 to 1.4) or 12-month follow-up (0.4, 95% CI -1.2 to 2.0). Likewise, quality of life scores improved over time regardless of LSS symptom status, but those with comorbid LSS symptoms had slightly worse scores at each time-point (Fig. 4). There was no certain difference between groups in change in quality of life scores at 3-month (−0.2, 95% CI -1.7 to 1.3) or 12-month follow-up (0.2, 95% CI -1.4 to 1.7). Unadjusted results were similar (Supplementary Table S4).
Fig. 3.
Changes in KOOS-12 and HOOS-12 function scores at each time-point.
Fig. 4.
Changes in KOOS-12 and HOOS-12 quality of life at each time-point.
In the hip OA cohort adjusted analysis (Table 4) function scores improved over time regardless of LSS symptom status, but those with comorbid LSS symptom status had slightly worse scores at baseline and 3-months, but not at 12-months (Fig. 3). Participants with comorbid LSS symptoms had slightly greater improvement in function scores at 3-month (2.5, 95% CI 0.5 to 5.0) and 12-month follow-up (3.8, 95% CI 0.9 to 6.6). Likewise, quality of life scores improved over time regardless of LSS symptom status, but those with comorbid LSS symptoms had slightly worse scores at each time-point (Fig. 4). There was no certain difference between groups in change in quality of life scores at 3-month (1.7, 95% CI -0.6 to 4.0) or 12-month follow-up (1.3, 95% CI -1.6 to 4.2). Unadjusted results were similar (Supplementary Table S5).
3.4. Sensitivity analyses
The complete case analyses generally supported the main results for both cohorts. In the knee OA cohort (Supplementary Table S6), the slightly smaller improvement in pain from baseline to 3-month follow-up in participants with comorbid LSS symptoms was not confirmed (−1.5, 95% CI -3.1 to 0.1). All results were confirmed in the hip OA cohort (Supplementary Table S7).
The alternate age criteria analyses also generally supported the main results for both cohorts. Again, in the knee OA cohort (Supplementary Table S8), the slightly smaller improvement in pain from baseline to 3-month follow-up in participants with comorbid LSS symptoms was not confirmed (−1.1, 95% CI -2.5 to 0.3). All results were confirmed in the hip OA cohort (Supplementary Table S9).
4. Discussion
These are the first findings exploring the impact of comorbid LSS symptoms in people with knee or hip OA on outcomes in a primary care treatment program. Even though comorbid LSS symptoms were associated with smaller improvement in pain at 3-months in participants with knee OA and greater improvement in function at 3- and 12-months in participants with hip OA, the differences were small and unlikely to be clinically relevant. Therefore, our findings suggest that patients with knee or hip OA can expect similar benefits in key outcomes when participating in a structured patient education and exercise therapy program, regardless of the presence of comorbid LSS symptoms. However, participants with comorbid LSS symptoms should expect worse, albeit small, absolute pain, function, and quality of life scores before and after the program, despite similar levels of improvement.
We found that 15% of patients with knee OA had comorbid LSS symptoms. These patients had slightly worse baseline scores for pain, function, and quality of life, but experienced similar improvements in all outcomes, except for smaller pain improvement from baseline to 3-months. The magnitude of this difference (1.7 points on a 100-point scale) is unlikely to be clinically relevant. While no studies have investigated what constitutes a meaningful between-group difference on the KOOS-12 pain subscale, a minimum clinically important within-group change of at least 15 points following joint replacement surgery [39] suggests the observed difference in our study is far from meaningful. The absence of a clinically or statistically significant association at the 3-month follow-up in both sensitivity analyses further supports this interpretation.
We found a higher proportion of patients with hip OA had comorbid LSS symptoms than in the knee OA cohort, but we observed similar results in the hip OA cohort. Here, those with comorbid LSS symptoms had worse baseline scores for pain, function, and quality of life, but experienced similar improvements in all outcomes. We did find that those with hip OA and comorbid LSS symptoms had greater improvements in function at both follow-up points. This difference may be attributed to a lower baseline function score and thus greater potential for improvement, but the magnitude of this difference (2.5 points at 3-months and 3.8 points at 12-months on a 100 point scale) is also not likely clinically meaningful, since the minimum meaningful within-group change on the HOOS-12 function subscale is estimated to be at least 24 points [39]. The small difference in functional improvement was confirmed in both sensitivity analyses, supporting the notion that no clinically significant difference exists.
Few studies have explored the impact of co-occurring LSS on treatment outcomes in people with knee or hip OA. Clinical reports suggest that comorbid LSS can negatively affect outcomes after total hip replacement [40], but provide insufficient data to assess these claims. We are aware of only one study comparing outcomes after total knee replacement between patients with and without comorbid LSS [10]. Like in our study, patients with comorbid LSS improved after surgery, but had significantly worse function and objective scores compared to those without comorbid LSS [10]. These findings match the general trends observed in other studies assessing the impact of other degenerative lumbar spinal disorders on total knee and hip replacement outcomes [[11], [12], [13], [14], [15]]. We are not aware of any other studies investigating the impact of LSS on outcomes for non-surgical interventions, which is problematic considering that most patients with knee or hip OA rely on non-surgical interventions for their OA [41] and the likely differential impacts on surgical and non-surgical interventions. Studies investigating the impact of comorbid LSS on treatment outcomes, both surgical and non-surgical, are needed to better inform expectations and clinical decision-making for patients with knee and hip OA.
The main limitation of this study is our case definition for LSS symptoms. Currently, there is no consensus criterion standard for the diagnosis of LSS [1,5,25] and thus, varying LSS case definitions have been used in previous studies [42], none of which have been validated. The specific symptom items used in this study were derived from a review of commonly used self-report LSS screening items [22] and include those most likely to identify LSS [25]. We expect that the term “numbness” in the symptom items is distinct and therefore noticeably different for people with knee or hip OA, therefore improving one's ability to differentiate LSS symptoms from knee- or hip-related symptoms. We also classified all individuals with diabetes as not having LSS symptoms to prevent leg diabetic neuropathy symptoms from being attributed to LSS, and used an age cut-point of 60 years or older to reduce the chance of patients having leg symptoms related to lumbar disc herniation and not LSS. Our definition also closely resembles those used in a recent trial and prevalence study [43,44] and our findings were confirmed in the sensitivity analysis using an alternate age cut-point. An ideal case definition should also include imaging confirmation, but our approach represents a pragmatic real-world primary care approach sufficient for the first estimates in this field.
There is also a potential selection bias in GLA:D® participants, whereby those with more severe LSS are excluded from the program since GLA:D® clinicians are trained to enrol patients with knee or hip dominant symptoms and not those with other more pronounced symptoms, such as from comorbid LSS. This bias is likely to underestimate the impact of comorbid LSS on outcomes. There was also a large proportion of missing outcome data, which is an unavoidable consequence of real-world implementation programs like GLA:D®. However, results were similar for the imputed and complete case analyses and participants with missing data were relatively similar to those with complete outcome data, increasing confidence in our results. Due to potential unmeasured confounding, between group differences may be smaller than our observed results. For example, inclusion of additional parameters related to LSS, such as number of stenotic levels (based on imaging) and LSS symptom duration or severity, may alter our findings. Finally, we were unable to assess if comorbid LSS is a moderator of the effectiveness of a patient education and exercise therapy program due to the single-arm design of this study.
Strengths of this study include the pragmatic and resource-efficient approach to providing the first data investigating associations between comorbid LSS symptoms in a large, real-world primary care sample. Future research should examine the relationship between comorbid LSS symptoms and outcomes across the range of treatments, both surgical and non-surgical, recommended for knee and hip OA. Existing datasets, such as surgical registries, could be explored for this purpose. However, these datasets will likely suffer from similar LSS case definition limitations as our study. Therefore, all future research will benefit from a standardized LSS definition. Ideally, subgroup powered randomized trials will help determine if comorbid LSS is a moderator of treatment effect if observational designs show a potential effect.
5. Conclusion
Our findings suggest patients with knee or hip OA and comorbid LSS symptoms do not differ on knee- or hip-related pain, function, and quality of life status compared to those without LSS symptoms and should expect similar improvements with treatment. Therefore, patient education and exercise therapy programs designed for people with knee or hip OA are a viable option for those who also have LSS symptoms.
Author contributions
Study conception and design: Young, Hartvigsen, Kongsted, Roos, Ammendolia, Jensen.
Recruitment of patients: Roos, Skou, Grønne.
Acquisition of data: Roos, Skou, Grønne.
Analysis and/or interpretation of data: Young, Kongsted, Hartvigsen, Jensen.
Drafting the article or revising it critically for important intellectual content: Young, Kongsted, Hartvigsen, Jensen, Roos, Ammendolia, Skou, Grønne.
Final approval of the article: Young, Kongsted, Jensen, Hartvigsen, Roos, Ammendolia, Kongsted, Skou, Grønne.
All authors had full access to all the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the funding source
This study was supported by postdoctoral funding support for JJY from the Danish Foundation for Chiropractic Research and Post-graduate Education.
The initiation of GLA:D® Denmark was partly funded by the Danish Physiotherapy Association's fund for research, education and practice development; the Danish Rheumatism Association; and the Physiotherapy Practice Foundation.
Declaration of competing interest
JJY has none to declare.
JH is co-founder of GLA:D® Back, a not-for profit initiative hosted at University of Southern Denmark aimed at implementing clinical guidelines for back pain in clinical practice. He has received personal fees from TrustMe-Ed and LearnPhysio.
EMR is the developer of the Knee injury and Osteoarthritis Outcome Score (KOOS) and several other freely available patient-reported outcome measures and co-founder of GLA:D®, a not-for profit initiative hosted at University of Southern Denmark aimed at implementing clinical guidelines for osteoarthritis in clinical practice.
CA has none to declare.
AK is co-founder of GLA:D® Back and is financially supported by an unrestricted grant from the Foundation for Chiropractic Research and Post-graduate Education and has received personal fees from TrustMe-Ed and Physical Pod unrelated to this work.
STS has received personal fees from Munksgaard and TrustMe-Ed outside the submitted work. Furthermore, he is co-founder of GLA:D®.
DTG has none to declare.
RKJ has none to declare.
Acknowledgements
The authors would like to thank the clinicians and patients involved in collecting data for GLA:D®. STS is currently funded by a program grant from Region Zealand (Exercise First) and two grants from the European Union's Horizon 2020 research and innovation program, one from the European Research Council (MOBILIZE, grant agreement No 801790) and the other under grant agreement No 945377(ESCAPE).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2022.100324.
Contributor Information
James J. Young, Email: jyoung@health.sdu.dk.
Alice Kongsted, Email: akongsted@health.sdu.dk.
Jan Hartvigsen, Email: jhartvigsen@health.sdu.dk.
Ewa M. Roos, Email: eroos@health.sdu.dk.
Carlo Ammendolia, Email: carlo.ammendolia@sinaihealth.ca.
Søren T. Skou, Email: stskou@health.sdu.dk.
Dorte T. Grønne, Email: dgronne@health.sdu.dk.
Rikke Krüger Jensen, Email: rikkekruger@kiroviden.sdu.dk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jensen R.K., Harhangi B.S., Huygen F., Koes B. Lumbar spinal stenosis. BMJ. 2021;373:n1581. doi: 10.1136/bmj.n1581. [DOI] [PubMed] [Google Scholar]
- 2.Safiri S., Kolahi A.A., Smith E., Hill C., Bettampadi D., Mansournia M.A., et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020;79(6):819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi S. Pathophysiology, diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J. Orthoped. 2014;5(2):134. doi: 10.5312/wjo.v5.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook C.J., Cook C.E., Reiman M.P., Joshi A.B., Richardson W., Garcia A.N. Systematic review of diagnostic accuracy of patient history, clinical findings, and physical tests in the diagnosis of lumbar spinal stenosis. Eur. Spine J. 2020;29(1):93–112. doi: 10.1007/s00586-019-06048-4. [DOI] [PubMed] [Google Scholar]
- 5.Tomkins-Lane C., Melloh M., Lurie J., Smuck M., Battié M.C., Freeman B., et al. Consensus on the clinical diagnosis of lumbar spinal stenosis: results of an international Delphi study. Spine. 2016;41(15):1239–1246. doi: 10.1097/BRS.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellhorn A.C., Katz J.N., Suri P. Osteoarthritis of the spine: the facet joints. Nat. Rev. Rheumatol. 2013;9(4):216–224. doi: 10.1038/nrrheum.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goode A.P., Carey T.S., Jordan J.M. Low back pain and lumbar spine osteoarthritis: how are they related? Curr. Rheumatol. Rep. 2013;15(2):305. doi: 10.1007/s11926-012-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Luca K., Chiarotto A., Cicuttini F., Creemers L., de Schepper E., Ferreira P.H., et al. Consensus for statements regarding a definition for spinal osteoarthritis for use in research and clinical practice: a Delphi study. Arthritis Care Res. 2021 doi: 10.1002/acr.24829. (Published ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Young J.J., Jensen R.K., Hartvigsen J., Roos E.M., Ammendolia C., Juhl C.B. Prevalence of multimorbid degenerative lumbar spinal stenosis with knee or hip osteoarthritis: a systematic review and meta-analysis. BMC Muscoskel. Disord. 2022;23(1):177. doi: 10.1186/s12891-022-05104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pivec R., Johnson A., Naziri Q., Issa K., Mont M., Bonutti P. Lumbar spinal stenosis impairs function following total knee arthroplasty. J. Knee Surg. 2012;26(1):59–64. doi: 10.1055/s-0032-1313754. [DOI] [PubMed] [Google Scholar]
- 11.Chang C.B., Park K.W., Kang Y.G., Kim T.K. Coexisting lumbar spondylosis in patients undergoing TKA: how common and how serious? Clin. Orthop. Relat. Res. 2014;472(2):710–717. doi: 10.1007/s11999-013-3298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eneqvist T., Nemes S., Brisby H., Fritzell P., Garellick G., Rolfson O. Lumbar surgery prior to total hip arthroplasty is associated with worse patient-reported outcomes. Bone Joint Lett. J. 2017;99-B(6):759–765. doi: 10.1302/0301-620X.99B6.BJJ-2016-0577.R2. [DOI] [PubMed] [Google Scholar]
- 13.Ellenrieder M., Bader R., Bergschmidt P., Fröhlich S., Mittelmeier W. Coexistent lumbar spine disorders have a crucial impact on the clinical outcome after total hip replacement. J. Orthop. Sci. 2015;20(6):1046–1052. doi: 10.1007/s00776-015-0764-y. [DOI] [PubMed] [Google Scholar]
- 14.Prather H., Van Dillen L.R., Kymes S.M., Armbrecht M.A., Stwalley D., Clohisy J.C. Impact of coexistent lumbar spine disorders on clinical outcomes and physician charges associated with total hip arthroplasty. Spine J. 2012;12(5):363–369. doi: 10.1016/j.spinee.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Londhe S.B., Shah R.V., Patwardhan M., Doshi A.P., Londhe S.S., Subhedar K., et al. Study of patients with bilateral knee osteoarthritis undergoing total knee replacement procedure with coexisting lumbar spondylosis symptoms. Asian Spine J. 2021;15(6):825–830. doi: 10.31616/asj.2020.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Young J.J., Hartvigsen J., Roos E.M., Ammendolia C., Kongsted A., Skou S.T., et al. Symptoms of lumbar spinal stenosis in people with knee or hip osteoarthritis or low back pain: a cross-sectional study of 10,234 participants in primary care. Osteoarthritis Cartilage. 2021;29(11):1515–1520. doi: 10.1016/j.joca.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Skou S.T., Roos E.M. Good Life with osteoArthritis in Denmark (GLA:DTM): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Muscoskel. Disord. 2017;18(1):72. doi: 10.1186/s12891-017-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ageberg E., Nilsdotter A., Kosek E., Roos E.M. Effects of neuromuscular training (NEMEX-TJR) on patient-reported outcomes and physical function in severe primary hip or knee osteoarthritis: a controlled before-and-after study. BMC Muscoskel. Disord. 2013;14(1):232. doi: 10.1186/1471-2474-14-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos E.M., Barton C.J., Davis A.M., McGlasson R., Kemp J.L., Crossley K.M., et al. GLA:D to have a high-value option for patients with knee and hip arthritis across four continents: Good Life with osteoArthritis from Denmark. Br. J. Sports Med. 2018;52(24):1544–1545. doi: 10.1136/bjsports-2017-098904. [DOI] [PubMed] [Google Scholar]
- 21.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R.K., Lauridsen H.H., Andresen A.D.K., Mieritz R.M., Schiøttz-Christensen B., Vach W. Diagnostic screening for lumbar spinal stenosis. Clin. Epidemiol. 2020;12:891–905. doi: 10.2147/CLEP.S263646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konno S., Hayashino Y., Fukuhara S., Kikuchi S., Kaneda K., Seichi A., et al. Development of a clinical diagnosis support tool to identify patients with lumbar spinal stenosis. Eur. Spine J. 2007;16(11):1951–1957. doi: 10.1007/s00586-007-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genevay S., Courvoisier D.S., Konstantinou K., Kovacs F.M., Marty M., Rainville J., et al. Clinical classification criteria for neurogenic claudication caused by lumbar spinal stenosis. The N-CLASS criteria. Spine J. 2018;18(6):941–947. doi: 10.1016/j.spinee.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 25.de Schepper E.I.T., Overdevest G.M., Suri P., Peul W.C., Oei E.H.G., Koes B.W., et al. Diagnosis of lumbar spinal stenosis: an updated systematic review of the accuracy of diagnostic tests. Spine. 2013;38(8):E469–E481. doi: 10.1097/BRS.0b013e31828935ac. [DOI] [PubMed] [Google Scholar]
- 26.Gandek B., Roos E.M., Franklin P.D., Ware J.E. A 12-item short form of the Knee injury and Osteoarthritis Outcome Score (KOOS-12): tests of reliability, validity and responsiveness. Osteoarthritis Cartilage. 2019;27(5):762–770. doi: 10.1016/j.joca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Gandek B., Roos E.M., Franklin P.D., Ware J.E. A 12-item short form of the Hip disability and Osteoarthritis Outcome Score (HOOS-12): tests of reliability, validity and responsiveness. Osteoarthritis Cartilage. 2019;27(5):754–761. doi: 10.1016/j.joca.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 28.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat. Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 29.Rubin D. John Wiley & Sons, Ltd; New York: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 30.Johnsen M.B., Roos E., Grønne D.T., Bråten L.C.H., Skou S.T. Impact of educational level and employment status on short-term and long-term pain relief from supervised exercise therapy and education: an observational study of 22 588 patients with knee and hip osteoarthritis. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perruccio A.V., Roos E.M., Skou S.T., Grønne D.T., Davis A.M. Factors influencing pain response following GLA:D® patient education and supervised exercise in males and females with hip osteoarthritis. Arthritis Care Res. 2022 doi: 10.1002/acr.24954. (Published ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Calders P., Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;47(6):805–813. doi: 10.1016/j.semarthrit.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Perruccio A.V., Power J.D., Evans H.M.K., Mahomed S.R., Gandhi R., Mahomed N.N., et al. Multiple joint involvement in total knee replacement for osteoarthritis: effects on patient-reported outcomes. Arthritis Care Res. 2012;64(6):838–846. doi: 10.1002/acr.21629. [DOI] [PubMed] [Google Scholar]
- 34.Perruccio A.V., Fitzpatrick J., Power J.D., Gandhi R., Rampersaud Y.R., Mahomed N.N., et al. Sex-modified effects of depression, low back pain, and comorbidities on pain after total knee arthroplasty for osteoarthritis. Arthritis Care Res. 2020;72(8):1074–1080. doi: 10.1002/acr.24002. [DOI] [PubMed] [Google Scholar]
- 35.Aalto T.J., Malmivaara A., Kovacs F., Herno A., Alen M., Salmi L., et al. Preoperative predictors for postoperative clinical outcome in lumbar spinal stenosis: systematic review. Spine. 2006;31(18):E648–E663. doi: 10.1097/01.brs.0000231727.88477.da. [DOI] [PubMed] [Google Scholar]
- 36.Sobottke R., Herren C., Siewe J., Mannion A.F., Röder C., Aghayev E. Predictors of improvement in quality of life and pain relief in lumbar spinal stenosis relative to patient age: a study based on the Spine Tango registry. Eur. Spine J. 2017;26(2):462–472. doi: 10.1007/s00586-015-4078-8. [DOI] [PubMed] [Google Scholar]
- 37.Cook C., Brown C., Michael K., Isaacs R., Howes C., Richardson W., et al. The clinical value of a cluster of patient history and observational findings as a diagnostic support tool for lumbar spine stenosis: patient history and findings for LSS. Physiother. Res. Int. 2011;16(3):170–178. doi: 10.1002/pri.500. [DOI] [PubMed] [Google Scholar]
- 38.Konno S., Kikuchi S., Tanaka Y., Yamazaki K., Shimada Y., Takei H., et al. A diagnostic support tool for lumbar spinal stenosis: a self-administered, self-reported history questionnaire. BMC Muscoskel. Disord. 2007;8(1):102. doi: 10.1186/1471-2474-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soh S.E., Harris I.A., Cashman K., Heath E., Lorimer M., Graves S.E., et al. Minimal clinically important changes in HOOS-12 and KOOS-12 scores following joint replacement. J. Bone Joint Surg. Am. 2022 doi: 10.2106/JBJS.21.00741. (Published ahead of print) [DOI] [PubMed] [Google Scholar]
- 40.Prather H., Dillen L. Links between the hip and the lumbar spine (hip spine syndrome) as they relate to clinical decision making for patients with lumbopelvic pain. Pharm. Manag. PM R. 2019;11(S1):64–72. doi: 10.1002/pmrj.12187. [DOI] [PubMed] [Google Scholar]
- 41.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 42.Genevay S., Atlas S.J., Katz J.N. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine. 2010;35(7):803–811. doi: 10.1097/BRS.0b013e3181bc9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williamson E., Boniface G., Marian I.R., Dutton S.J., Garrett A., Morris A., et al. The clinical effectiveness of a physiotherapy delivered physical and psychological group intervention for older adults with neurogenic claudication: the BOOST randomised controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2022;77(8):1654–1664. doi: 10.1093/gerona/glac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson E., Sanchez Santos M.T., Morris A., Garrett A., Conway O., Boniface G., et al. The prevalence of back and leg pain and the cross-sectional association with adverse health outcomes in community dwelling older adults in England. Spine. 2021;46(1):54–61. doi: 10.1097/BRS.0000000000003719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.