Abstract
Background:
To determine the relationship between iron exposure and the development of bronchopulmonary dysplasia (BPD).
Methods:
A secondary analysis of the PENUT Trial dataset. The primary outcome was BPD at 36 weeks gestational age and primary exposures of interest were cumulative iron exposures in the first 28-days and through 36 weeks gestation. Descriptive statistics were calculated for study cohort characteristics with analysis adjusted for the factors used to stratify randomization.
Results:
Of 941 patients, 821 (87.2%) survived to BPD evaluation at 36 weeks, with 332 (40.4%) diagnosed with BPD. The median cohort gestational age was 26 weeks and birth weight 810g. In the first 28-days, 76% of infants received enteral iron and 55% parenteral iron. The median supplemental cumulative enteral and parenteral iron intakes at 28-days were 58.5 mg/kg and 3.1 mg/kg respectively and through 36 weeks’ 235.8 mg/kg and 3.56 mg/kg respectively. We found lower volume of red blood cell transfusions in the first 28 days after birth and higher enteral iron exposure in the first 28 days after birth to be associated with lower rates of BPD.
Conclusion:
We find no support for an increased risk of BPD with iron supplementation.
Introduction:
Bronchopulmonary dysplasia (BPD) affects as many as 68% of infants born at extremely low gestational ages (22–28 weeks) and very low birth weight (≤ 1500g).1 BPD is the most common chronic lung disease of childhood and its incidence has slowly been increasing over the past 20 years, despite advances in maternal and neonatal care.2–3 The etiology of BPD is multifactorial and includes developmental changes in the lung that start before birth as well as lung injury and repair mechanisms after birth.4 These include well described hypotheses about mechanisms of oxidative stress contributing to the development of lung injury and BPD.5–7 Preterm infants may be especially sensitive to diseases exacerbated by oxidative stress given their immature antioxidant defenses.
Iron is essential for the metabolism of all living cells and is critically important for normal brain development.8–9 Eighty percent of fetal iron accretion occurs in the third trimester and for extremely low gestational age newborns (ELGAN) delivered between 24–27 weeks’ gestation much of this important time has been missed.10 As a result, postnatal iron supplementation is essential and current recommendations are to supplement preterm infants 2–3 mg/kg/day starting between 2 weeks and 2 months of age.11–12 Prior randomized trials found early (beginning at 2 weeks of age) iron supplementation compared with late (beginning at 2 months) supplementation was beneficial in improving serum ferritin and hemoglobin levels, as well as reducing iron deficiency,13–14 though a subsequent meta-analysis suggested uncertainty regarding long-term neurodevelopmental benefits.15 Despite this, given the prioritization of iron for erythropoiesis, added supplementation in the setting of erythropoiesis-stimulating agents is clearly of importance.16
Preterm infants are frequently treated with blood transfusions and iron supplementation, and both are potential sources of non-transferrin bound iron (NTBI). NTBI represents the free iron present in plasma that is not bound to transferrin, heme, or other iron binding proteins. This NTBI is a potentially significant source of free radicals and tissue oxidative stress.17 One form of NTBI, known as labile plasma iron, has the ability to engage in reduction-oxidation reactions (Brissot, Biochemica et Biophysica Acta, 2012).18 Prior retrospective studies have found a relationship between red blood cell transfusions and risk of development of BPD, however clear mechanistic links have not been discerned.19–23 It has previously been demonstrated that enteral iron supplementation for one week in healthy very low birthweight (VLBW) infants does not increase certain markers of oxidative stress,24 and that iron supplementation up to 12mg/kg/day is also not associated with increased markers of oxidative stress.25 However, an increased risk of BPD has also been described with higher cumulative dose of supplemental enteral iron in VLBW infants.26 Ultimately, due to the multifactorial etiology of BPD and the difficulty in dissecting out related clinical risk factors in critically ill neonates, limitations may exist in providing attribution to individual clinical conditions or exposures towards development of BPD.
The Preterm Erythropoietin Neuroprotection (PENUT) Trial randomized ELGANs to high dose erythropoietin or placebo in the setting of recommended iron supplementation guidelines for all sites.27 We sought to utilize the PENUT Trial cohort to further study the relationship between iron supplementation and development of BPD. We hypothesized that infants with greater administration of supplemental iron would have a greater risk of developing BPD.
Methods:
We conducted a secondary analysis using prospectively collected data from a multicenter, randomized placebo-controlled, double masked trial investigating the neuroprotective effect of high-dose erythropoietin in extremely preterm infants.27 The PENUT Trial randomized infants 24 to 27 weeks’ gestation to receive erythropoietin or placebo and enrolled 941 infants from 19 sites throughout the United States. Exclusion criteria were known major life-threatening anomalies, known or suspected chromosomal anomalies, disseminated intravascular coagulopathy, twin-to-twin transfusion, a hematocrit level above 65%, hydrops fetalis, or known congenital infection. Our study cohort included all participants in the PENUT Trial surviving through 36 weeks’ 6 days gestation who had respiratory assessments at 28 days and at 36 weeks’.
Standard PENUT Trial iron therapy guidelines included supplementation at 3 mg/kg/day when subjects reached enteral intake of 60 mL/kg/day with escalation to 6 mg/kg/day when enteral intake reached 100 mL/kg/day. Dose titration was based on serum ferritin or zinc protoporphyrin-to-heme ratio (ZPP/H) at 14 and 42 days of age with maximal enteral iron dose of 12 mg/kg/day. If subjects were not receiving 60 mL/kg/d of enteral feeds by study day 8, parenteral iron (iron sucrose or dextran) was initiated at a dose of 1.5 mg/kg/dose twice a week until goal feedings were achieved. Similarly, if infants had feedings held at a later time, parenteral iron was administered if no feedings were given for a period of 7 days or greater. While following iron therapy guidelines was recommended, it was not mandated as part of the PENUT Trial protocol. No transfusion guidelines were in place for the PENUT Trial.
We defined the primary outcome of BPD as the use of any supplemental oxygen at 36 weeks of postmenstrual age, consistent with the PENUT Trial definition and did not stratify by BPD severity. We defined the primary exposures of iron supplementation for two time periods – the first 28 days after birth and from birth through 36 weeks’ gestational age. Iron supplementation was calculated in three ways: total cumulative, total cumulative with weight adjustment, and total cumulative with weight and time adjustment. For total cumulative supplementation, all doses were summed. For total cumulative with weight adjustment, each supplementation was divided by the weight of the baby at the time of supplementation and then summed. If weight was not measured on any given day, the previously recorded weight was utilized. For total cumulative with weight and time adjustment, the weight adjusted measure was further standardized by dividing by the time from birth to BPD assessment. All three measurements are described below for both time periods.
Descriptive statistics (medians, inter-quartile range (IQR), mean, standard deviation (SD), frequency, and percentage) are shown for characteristics of the study cohort. Clinical and demographic characteristics were compared by BPD status using generalized estimating equation (GEE) models with robust standard errors to account for within sibship correlation.28 Each analysis adjusted for the factors used to stratify randomization (gestational age at birth, recruitment site, and treatment assignment). We then used GEE models to evaluate the relationship between enteral and parenteral iron, and BPD diagnosis at 36 weeks. Adjusted odds ratios (OR) and corresponding confidence intervals (CIs) were calculated from the models. A case complete analysis was performed as missingness was 3% or less for exposures of interest. All statistical analyses were conducted using R version 4.0.2.
All PENUT Trial patients had informed consent obtained and the PENUT Trial approved by their local institutional review boards27. This study was determined to be exempt by the Methodist Healthcare IRB, San Antonio, TX.
Results:
Of 941 patients enrolled in the PENUT Trial, 832 (88.4%) survived through 36 weeks with 821 (87.2%) having a documented BPD evaluation (Supplemental Figure S1). Patient cohort characteristics are shown in Table 1. The median birth weight was 810 grams (IQR 670, 950 grams) and median gestational age was 26 weeks (IQR 25, 27 weeks). Antenatal steroid exposure was documented in 745 (91%) with 653 (80%) receiving intubation in the delivery room and 577 (70%) receiving surfactant administration on the day of birth. In the first 28-days after birth 94% of infants received iron, with 76% of infants receiving enteral iron and 55% receiving parenteral iron (Table 2). Overall, 341 (42%) of infants received only enteral iron during their hospital stay. The median enteral and parenteral iron intakes at 28 days were 58.5 mg/kg (IQR 27.7, 94.6 mg/kg) and 3.1 mg/kg (IQR 1.8, 5.8 mg/kg) respectively and through 36 weeks’ 235.8 mg/kg (IQR 151.9, 329.7 mg/kg) and 3.6 mg/kg (IQR 2.6, 7.0 mg/kg) respectively. Evaluating serum ferritin status when measured at 14 ± 3 days and 42 ± 3 days found no significant differences between patients with and without BPD at 36 weeks (Table 3). The median transfusion volumes were 29 mL (IQR 12, 55 mL) for 37 mL/kg (IQR 15, 72 mL/kg) at 28 days and 45 mL (IQR 15, 85 mL) for 47 mL/kg (IQR 16, 95 mL/kg) at 36 weeks (Table 2).
Table 1:
Characteristics of the patient cohort
| Patient characteristics | n (%) |
|---|---|
| n=821 | |
| Birth weight, median g (IQR) | 810 (670, 950) |
| Small for gestational age | 118 (14%) |
| Gestational age, median (IQR) | 26 (25, 27) |
| 24 weeks | 189 (23%) |
| 25 weeks | 210 (26%) |
| 26 weeks | 200 (24%) |
| 27 weeks | 222 (27%) |
| Female sex | 396 (48%) |
| Race | |
| Black | 219 (26%) |
| White | 546 (67%) |
| Other | 52 (7%) |
| Antenatal steroids given | 745 (91%) |
| Delivery room intubation | 653 (80%) |
| Chorioamnionitis | 102 (12%) |
| Surfactant on day of birth | 577 (70%) |
| Caffeine on day of birth | 561 (68%) |
Table 2:
Enteral iron and transfusion exposure within the cohort
| Iron exposure at 28 days | Enteral | Parenteral |
|---|---|---|
| Receipt of any iron supplementation, n (%) | 623 (76%) | 454 (55%) |
| Total cumulative iron exposure among all infants, median mg (IQR) | 34.7 (2.6, 78.8) | 1.2 (0, 3) |
| Total cumulative iron exposure among all infants, median mg/kg (IQR) | 39.5 (2.6, 82.8) | 1.5 (0, 3.5) |
| Total cumulative iron exposure among only infants receiving supplementation, median mg (IQR) | 55 (24.5, 93.3) | 2.9 (1.6, 4.8) |
| Total cumulative iron exposure among only infants receiving supplementation, median mg/kg (IQR) | 58.5 (27.7, 94.6) | 3.10 (1.8, 5.8) |
| Duration of supplementation among infants receiving supplementation, median d (IQR) | 13 (7, 18) | 2 (2, 4) |
| Iron exposure at 36 weeks | ||
| Receipt of any iron supplementation, n (%) | 798 (97%) | 474 (58%) |
| Total cumulative iron exposure among all infants, median mg (IQR) | 334 (202.7, 458.4) | 1.3 (0, 3.9) |
| Total cumulative iron exposure among all infants, median mg/kg (IQR) | 231.3 (144.4, 327.6) | 1.5 (0, 4.4) |
| Total cumulative iron exposure among infants receiving supplementation, median mg (IQR) | 342.9 (213.6, 466) | 3.1 (1.8, 6.6) |
| Total cumulative iron exposure among infants receiving supplementation, median mg/kg (IQR) | 235.8 (151.9, 329.7) | 3.6 (2.6, 7.0) |
| Duration of supplementation among infants receiving supplementation, median d (IQR) | 51 (37, 60) | 3 (2, 5) |
| RBC transfusions | ||
| Receipt of any packed red blood cell transfusions, n (%) | 670 (82%) | |
| Total cumulative volume transfused among all infants, median mL (IQR) at 28 days | 29 (12, 55) | |
| Total cumulative volume transfused among all infants, median mL/kg (IQR) at 28 days | 37 (15, 72) | |
| Total cumulative volume transfused among all infants, median mL (IQR) at 36 weeks | 45 (15, 85) | |
| Total cumulative volume transfused among all infants, median mL/kg (IQR) at 36 weeks | 47 (16, 95) | |
Table 3:
Serum iron markers of iron status among PENUT Trial patients with and without BPD at 36 weeks PMA.
| BPD (n=332) | No BPD (n=489) | p-value | |
|---|---|---|---|
| 14-day ferritin (median, IQR) | 227 (105, 382)a | 191 (91, 348)b | 0.11 |
| 42-day ferritin (median, IQR) | 154 (61, 341)c | 129 (56, 265)d | 0.23 |
n=254;
n=392;
n=87;
n=144
Measurements recorded if taken at 14 ± 3 days and 42 ± 3 days. For infants with multiple measurements within a given window, the mean was utilized.
The measurements were log transformed, using a generalized estimating equations model adjusting for site, gestational age, and erythropoietin exposure to provide a p-value.
At 36 weeks’ gestation, 332 infants (40.4%) of the PENUT Trial cohort had a diagnosis BPD. The incidence of BPD is shown by quartiles of supplemental enteral and parenteral iron exposure in Figure 1. Analysis of the relationship between individual risk factors and BPD were adjusted for gestational age at birth, recruitment site, and PENUT Trial treatment arm (erythropoietin or placebo). Increasing enteral iron supplementation through 28 days was associated with a lower risk of BPD (OR for BPD per 1 mg/kg/day increase in enteral iron of 0.89; CI 0.80–0.99) but at 36 weeks was no longer statistically significant (OR 1.02, CI 0.93–1.12) (Table 4). There was no association between parenteral iron supplementation and increased risk of BPD. Greater mean volume of red blood cell (RBC) transfusion was associated with an increased risk of BPD (OR for BPD per 1 ml/day increase in RBC transfusion of 1.20, CI 1.02–1.41 at 28 days but not at 36 weeks (OR 1.04, CI 0.86–1.26). Receipt of erythropoietin was not associated with any increased risk in BPD.
Figure 1. The relationship between iron exposure in the first 28-days and diagnosis of bronchopulmonary dysplasia (BPD) at 36 weeks’.
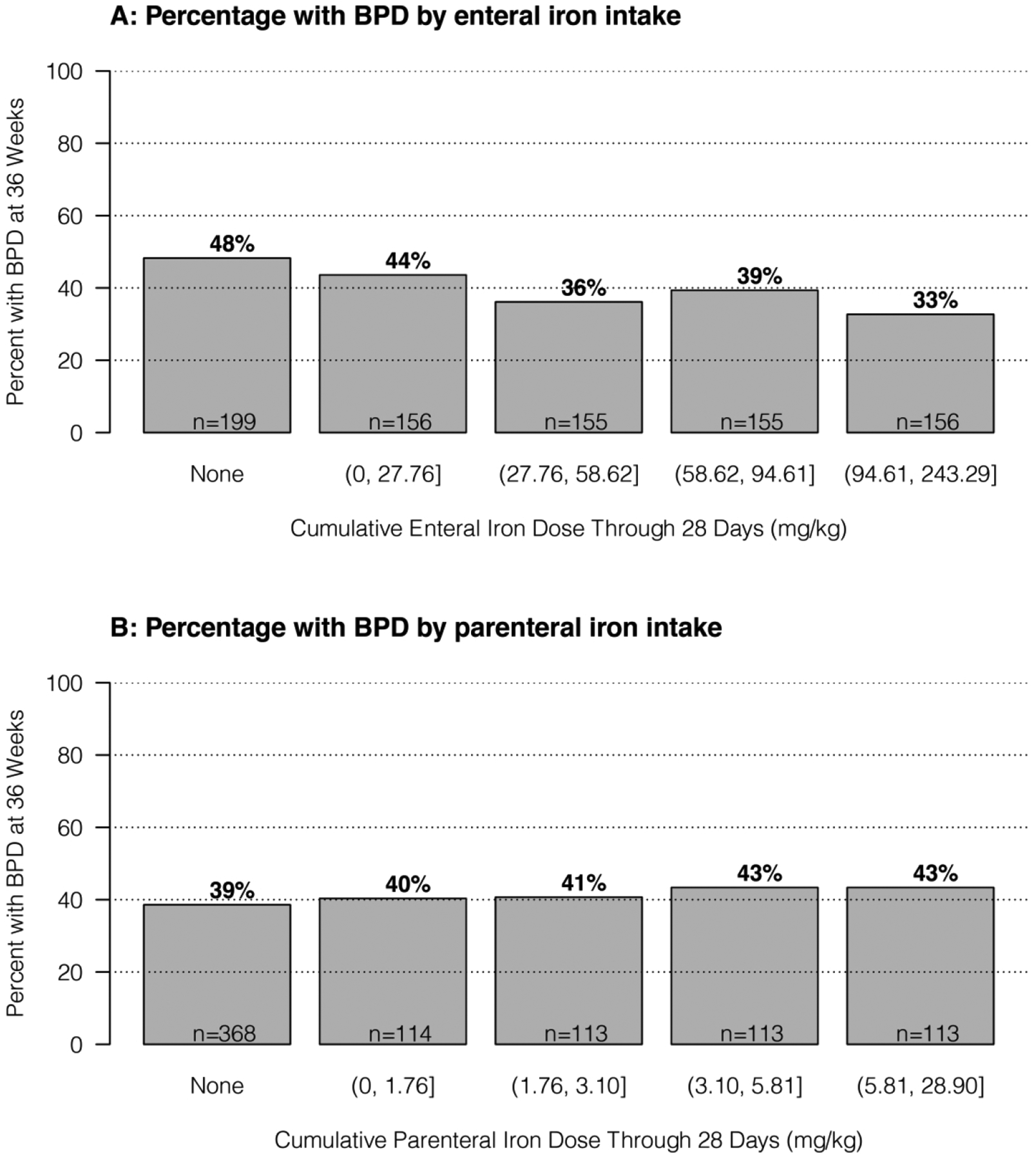
In both Figure 1A (enteral iron exposure) and 1B (parenteral iron exposure), the analyzed cohort was separated into those patients who had no iron exposure with the remainder divided into quartiles based on total iron exposure. Groups are ordered from lowest exposure (far left, no iron) to greatest exposure (far right, greatest quartile of exposure). An inverse relationship exists between enteral iron exposure and diagnosis of BPD at 36 weeks’ (1A) whereas a weak direct relationship exists between escalating parenteral iron exposure and diagnosis of BPD at 36 weeks’ (1B).
Table 4:
Multivariable analysis of risk factors for bronchopulmonary dysplasia
| Factor | BPD (n=332) | No BPD (n=489) | aOR (95%) CI | Change in odds per |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| Received ≥ 1 dose of antenatal steroids, n (%) | 292 (88%) | 453 (93%) | 0.67 (0.38, 1.16) | yes vs. no |
| Female sex, n (%) | 159 (48%) | 237 (48%) | 1.02 (0.75, 1.38) | yes vs no |
| Black race*, n (%) | 80 (25%) | 130 (27%) | 0.85 (0.58, 1.22) | yes vs no |
| Birth weight, mean g (sd) | 794 (189) | 823 (185) | 0.95 (0.86, 1.05) | 100 g increase |
| Small for gestational age, n (%) | 49 (15%) | 69 (14%) | 1.06 (0.70, 1.61) | yes vs no |
| Delivery Room Intubation, n (%) | 265 (80%) | 388 (79%) | 0.79 (0.59, 1.07) | yes vs. no |
| Chorioamnionitis, n (%) | 31 (11%) | 71 (15%) | 0.80 (0.51, 1.25) | yes vs. no |
| Surfactant on DOB, n (%) | 237 (71%) | 340 (70%) | 1.01 (0.71, 1.42) | yes vs. no |
| Caffeine on DOB, n (%) | 235 (71%) | 326 (67%) | 1.16 (0.82, 1.64) | yes vs. no |
| Enteral Iron Exposure | ||||
| Mean mg/kg/day (sd) through 28 days | 1.49 (1.50) | 1.83 (1.62) | 0.89 (0.80, 0.99) | 1 mg/kg/day increase |
| Mean mg/kg/day (sd) through 36 weeks | 3.09 (1.85) | 3.25 (1.87) | 1.02 (0.93, 1.12) | 1 mg/kg/day increase |
| IV Iron Exposure | ||||
| Mean mg/kg/day (sd) through 28 days | 0.10 (0.13) | 0.08 (0.12) | 1.05 (0.92, 1.20) | 0.1 mg/kg/day increase |
| Mean mg/kg/day (sd) through 36 weeks | 0.05 (0.07) | 0.04 (0.06) | 1.07 (0.84, 1.38) | 0.1 mg/kg/day increase |
| Packed Red Blood Cell Transfusions | ||||
| Mean ml/day (sd) at 28 days | 1.52 (1.24) | 0.91 (1.23) | 1.20 (1.02, 1.41) | 1 ml/day increase |
| Mean ml/day (sd) at 36 weeks | 0.81 (0.72) | 0.53 (0.86) | 1.04 (0.86, 1.26) | 1 ml increase |
| PENUT Trial Treatment Arm | ||||
| Received erythropoietin | 172 (52%) | 243 (50%) | 1.14 (0.85, 1.54) | yes vs. no |
The multivariable regression model was adjusted for recruitment site, gestational age at birth, and randomization assignment.
Black race compared to white or other race.
Differences in enteral and parenteral iron supplementation by participating site is shown in Figure 2.
Figure 2: Variation in enteral iron exposure in the first 28-days by recruitment site.
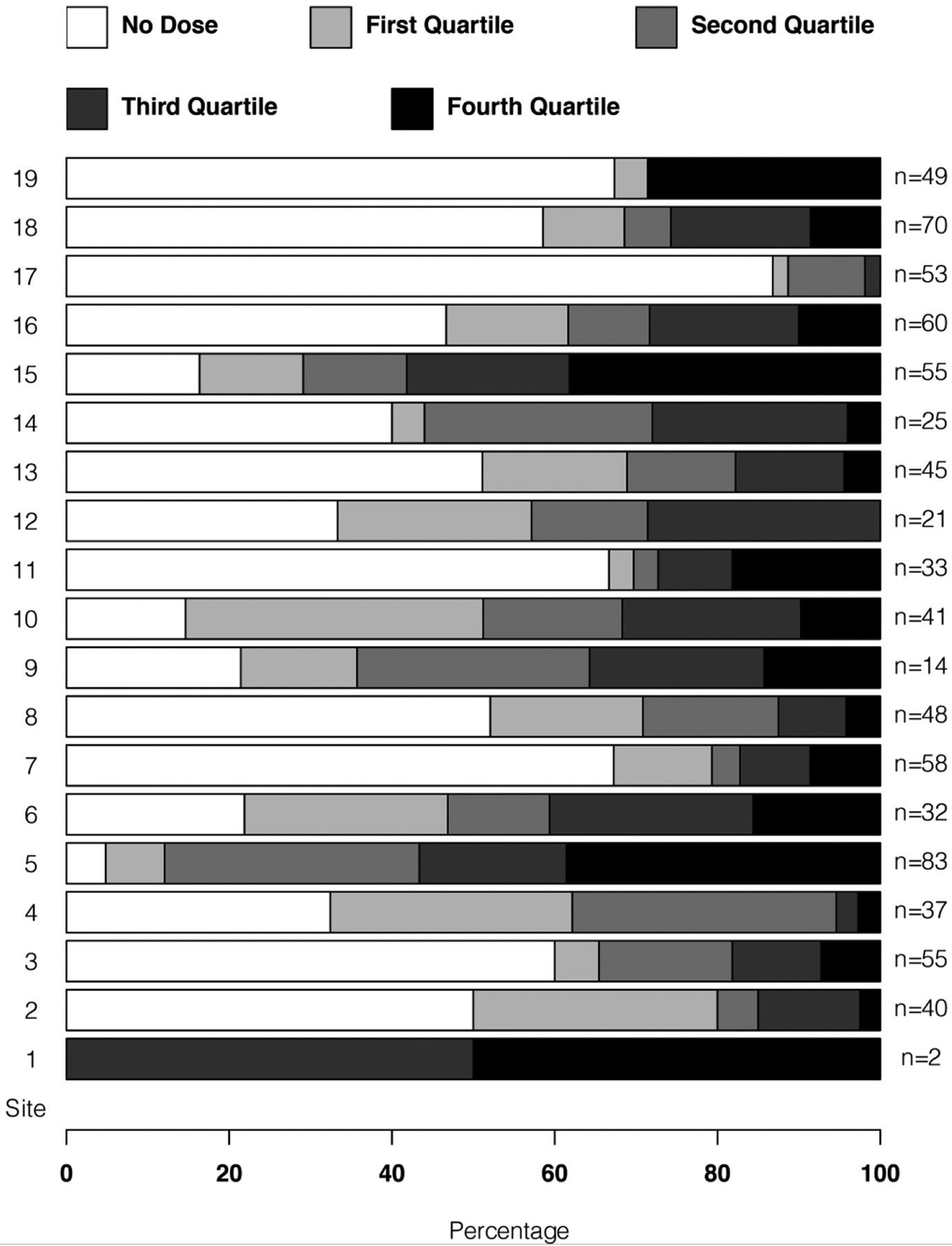
The study cohort was separated into those patients who had no enteral iron exposure in the first 28-days with the remaining patients divided into quartiles by total exposure in mg per kg. Deidentified PENUT Trial sites (1 through 19) are noted on the far left and number of patients recruited at each site at the far right.
Significant site to site variation in exposure to enteral iron is seen in the first 28-days, with some sites (#3, #7, #11, 17) having a majority of recruited patients not receiving any enteral iron in the first 28-days after birth.
Discussion:
In this secondary analysis of the PENUT Trial, we find an inverse relationship among ELGANs between enteral iron intake in the first 28-days after birth and diagnosis of BPD at 36 weeks’ gestation. While these results are in contrast to the conclusion from an earlier observational cohort study,26 they align with findings from two prior studies that did not report an increase in biomarkers of oxidative stress in preterm infants after treatment with short-term high-dose enteral iron supplementation.24–25 We further did not find a relationship intravenous iron exposure and the development of BPD. These data indicate that enteral and intravenous iron supplementation are unlikely to significantly contribute to the pathogenesis of BPD.
The primary route of iron exposure in our cohort was enteral, with measured enteral iron substantially exceeding parenteral iron through the first 28 days after birth and from birth through 36 weeks gestational age. The most preterm infants in the PENUT Trial cohort experienced both the longest hospital course and the greatest inherent risk for developing BPD but also had the highest overall enteral iron dose exposure, similar to any prescribed nutrient. Despite this, we note a trend towards an inverse relationship between early enteral iron exposure and rate of BPD. This may be related to otherwise well preterm infants initiating enteral iron supplementation sooner and therefore receiving higher cumulative enteral iron exposure compared to those who have delayed initiation of iron. Earlier or more rapid enteral iron supplementation advancement may be a surrogate for other reduced BPD risk factors. The PENUT Trial’s dataset does not contain information regarding timing of enteral feed initiation, time to full enteral feeds, or intake of other micronutrients, so we are unable to directly answer this question.
In the context of the PENUT Trial’s protocolized iron supplementation guidelines, it is possible that in many cases the observed lower supplemental enteral iron delivered to infants with BPD is reflective of a more complicated clinical course with delayed or interruption of established enteral feeds. The finding of infants with BPD receiving more blood transfusion volume in the first 28 days is also likely an additional surrogate marker of severity of illness. This finding is consistent with prior observational studies22, 26, 29 but different from previous randomized control trials evaluating liberal versus restrictive red blood cell transfusion guidelines that did not find an increased risk of BPD with higher transfusion volumes,30–33 suggesting that the observational studies might be biased by residual confounding.
Given that the pathophysiology of BPD is a complex interaction of antenatal and postnatal exposures coupled with injury and repair mechanisms, it is likely that individual patients have different exposure risks that contribute to their development of BPD.4 Many of the biologic and environmental influences that contribute to the development of BPD have occurred in the neonate well before the typical timing for initiation of enteral nutritional supplements.34 Even with the protocolized iron regimen in the PENUT Trial, routine enteral iron supplementation was typically not started until infants reached 60 mL/kg/day of feeds, during which time many pathologic changes may have already occurred in the lung of the preterm infant. Iron supplementation is essential for normal brain development and preterm infants are both at risk for iron deficiency and iron overload.8, 35 Therefore it is important that decisions regarding timing of initiation and optimal dose of iron supplementation should be directed by close monitoring of biomarkers of iron sufficiency. Of note, prior work linking enteral iron supplementation to the development of BPD did not involve the routine monitoring of iron parameters.26 Our routine monitoring of serum ferritin or ZnPP/H levels may have prevented the development of iron excess.
Strengths of this study include the large patient cohort of ELGAN from multiple centers across the United States. Additionally, these infants were supplemented with iron based on guidelines with dose titration based on biomarkers of iron status. The early initiation and relatively high maximum enteral iron dose of 12 mg/kg/day allowed us to investigate of a greater range of total iron exposures. Limitations to this study include the heterogeneity of dosing across sites which may reflect differences in fidelity to iron supplementation guidelines, a reflection of differences in severity of illness between sites, or both. PENUT Trial sites did not have a standard transfusion protocol, which led to significant variability to transfusion exposures, another potential source of inconsistency across sites. Further, as a secondary analysis of the original data, the PENUT trial was not designed to detect factors causative for BPD. Lastly, this study did not assess any biomarkers of oxidative stress.
In conclusion, we do not find evidence that iron administration has an association with the subsequent development of BPD. Given the continued rise in incidence and long-term complications of this disease, there is a continued need to further evaluate medical, environmental, and nutritional factors that may impact BPD risk and be targets for future interventional studies.
Supplementary Material
Impact:
Prior studies and biologic plausibility raise the possibility that iron administration could contribute to the pathophysiology of oxidant-induced lung injury and thus bronchopulmonary dysplasia in preterm infants.
For 24 to 27 week premature infants, this study finds no association between total cumulative enteral iron supplementation at either 28-days or 36-weeks postmenstrual age and the risk for developing bronchopulmonary dysplasia.
Financial Support:
NINDS U01NS077955 and U01NS077953
Footnotes
Consent Statement: Patient consent was obtained for the original PENUT Trial enrollment but was not required for this secondary study.
Trial registration number: NCT01378273. https://clinicaltrials.gov/ct2/show/NCT01378273
Publisher's Disclaimer: Disclaimer: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of the Navy, or the Department of Defense, or the US Government.
Disclosures Statement: None
Added individual patient consent was not required for this study, which utilized deidentified patient data. All patient families consented for enrollment in the PENUT Trial.
Data Availability Statement:
The datasets analyzed for this study are available through the NINDS Archived Clinical Research Datasets at https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets.
References:
- 1.Stoll BJ, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah PS, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32(2):132–138. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA. 2015;314(10):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jobe AH. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am J Perinatol.2016;33(11):1076–1078. [DOI] [PubMed] [Google Scholar]
- 5.Saugstad OD. Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinat Med. 2010;38(6):571–577. [DOI] [PubMed] [Google Scholar]
- 6.Perrone S, et al. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxid Med Cell Longev. 2018;2018:7483062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177–183. [DOI] [PubMed] [Google Scholar]
- 8.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165. [DOI] [PubMed] [Google Scholar]
- 9.Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61(6):933–952. [DOI] [PubMed] [Google Scholar]
- 10.Widdowson EM, Spray CM. Chemical development in utero. Arch Dis Child. 1951;26(127):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker RD, Greer FR. Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126(5):1040–1050. [DOI] [PubMed] [Google Scholar]
- 12.Agostoni C, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91. [DOI] [PubMed] [Google Scholar]
- 13.Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics. 2000;106(4):700–706. [DOI] [PubMed] [Google Scholar]
- 14.Joy R, et al. Early versus late enteral prophylactic iron supplementation in preterm very low birth weight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F105–F109. [DOI] [PubMed] [Google Scholar]
- 15.Mills RJ, Davies MW. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst Rev. 2012;(3):CD005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddappa AM, et al. High Prevalence of Iron Deficiency Despite Standardized High-Dose Iron Supplementation During Recombinant Erythropoietin Therapy in Extremely Low Gestational Age Newborns. J Pediatr. 2020;222:98–105.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M, Ramavataram DVSS. Non Transferrin Bound Iron: Nature, Manifestations and Analytical Approaches for Estimation. Ind J Clin Biochem 2012; 27(4): 322–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochemica et Biophysica Acta. 2012; 1820:403–410 [DOI] [PubMed] [Google Scholar]
- 19.Cooke RW, Drury JA, Yoxall CW, James C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr. 1997;156(1):47–50. [DOI] [PubMed] [Google Scholar]
- 20.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity?. Med Hypotheses. 2006;66(2):355–364. [DOI] [PubMed] [Google Scholar]
- 21.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155(3):331–37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Huang X, Lu H. Association between red blood cell transfusion and bronchopulmonary dysplasia in preterm infants. Sci Rep. 2014;4:4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghirardello S, et al. Effects of Red Blood Cell Transfusions on the Risk of Developing Complications or Death: An Observational Study of a Cohort of Very Low Birth Weight Infants. Am J Perinatol. 2017;34(1):88–95. [DOI] [PubMed] [Google Scholar]
- 24.Braekke K, et al. Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. J Pediatr. 2007;151(1):23–28. [DOI] [PubMed] [Google Scholar]
- 25.Miller SM, McPherson RJ, Juul SE. Iron sulfate supplementation decreases zinc protoporphyrin to heme ratio in premature infants. J Pediatr. 2006;148(1):44–48. [DOI] [PubMed] [Google Scholar]
- 26.Patel RM, et al. Enteral iron supplementation, red blood cell transfusion, and risk of bronchopulmonary dysplasia in very-low-birth-weight infants. Transfusion. 2019;59(5):1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juul SE, et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N Engl J Med. 2020;382(3):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 29.Murphy T, Chawla A, Tucker R, Vohr B. Impact of Blood Donor Sex on Transfusion-Related Outcomes in Preterm Infants. J Pediatr. 2018;201:215–220. [DOI] [PubMed] [Google Scholar]
- 30.Bell EF, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirpalani H, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307. [DOI] [PubMed] [Google Scholar]
- 32.Franz AR, et al. Effects of Liberal vs Restrictive Transfusion Thresholds on Survival and Neurocognitive Outcomes in Extremely Low-Birth-Weight Infants: The ETTNO Randomized Clinical Trial. JAMA. 2020;324(6):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirpalani H, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383(27):2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and Perinatal Determinants of Lung Health and Disease in Early Life: A National Heart, Lung, and Blood Institute Workshop Report. JAMA Pediatr. 2016;170(5):e154577. [DOI] [PubMed] [Google Scholar]
- 35.Rao R, Georgieff MK. Iron therapy for preterm infants. Clin Perinatol. 2009;36(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed for this study are available through the NINDS Archived Clinical Research Datasets at https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets.


