Abstract
Strains of enterotoxigenic Escherichia coli that express K88 fimbriae are among the most common causes of diarrhea in young pigs. Adhesion of bacteria to receptors on intestinal epithelial cells, mediated by K88 fimbriae, is the initial step in the establishment of infection. Three antigenic variants of K88 fimbriae exist in nature: K88ab, K88ac, and K88ad. K88ac is the most prevalent and may be the only variant of significance in swine disease. Each K88 fimbrial variant is composed of multiple antigenic determinants. Some of these determinants are shared among the three variants and may be referred to as conserved epitopes, whereas others are unique to a specific variant and may be referred to as variable epitopes. In this study, monoclonal antibodies (MAbs) specific to either variable or conserved epitopes of K88ac fimbriae were produced. The specificity of each MAb was tested by enzyme-linked immunosorbent and immunoblot assays. Fab fragments were prepared from these MAbs and were tested for their ability to block the binding of K88-positive bacteria and purified fimbriae to porcine enterocyte brush border vesicles and purified K88 receptors, respectively. The purified receptors were intestinal mucin-type sialoglycoproteins (IMTGP) isolated from porcine enterocytes (A. K. Erickson, D. R. Baker, B. T. Bosworth, T. A. Casey, D. A. Benfield, and D. H. Francis, Infect. Immun. 62:5404–5410, 1994). Fab fragments prepared from MAbs specific for variable epitopes blocked the binding of bacteria to brush borders and of fimbriae to IMTGP. However, those from MAbs specific for a conserved epitope did not. These observations indicate that the receptor-binding domain of a K88ac fimbria is contained, at least in part, within the antigenically variable epitopes of that fimbria. Epitope mapping for one of the MAbs, which recognizes a linear epitope on K88ac fimbriae, indicated that this MAb binds to the region from amino acid no. 64 to no. 107 on the major subunit of K88ac fimbriae.
Strains of enterotoxigenic Escherichia coli that express K88 fimbriae are an important cause of diarrhea in newborn and weaned piglets. The K88 fimbriae mediate adhesion of bacteria to receptors on porcine intestinal epithelial cells, which is the initial step in the establishment of enteric infection. Fimbriae are nonflagellular, filamentous adhesins arrayed over the surface of the bacterium (29). Three serological variants of K88 exist in nature: K88ab, K88ac, and K88ad (14, 22, 32, 37). However, K88ac is by far the most common variant associated with diarrheal disease in pigs (18, 39). Each antigenic variant of K88 exhibits uniqueness in its hemagglutinating properties with respect to erythrocytes from various species (2, 4). In addition, each variant exhibits uniqueness in the specificity of its binding to porcine enterocytes (1, 3, 4, 36). Bijlsma et al. (3) identified five phenotypes of pigs with regard to the adhesion of K88+ E. coli to enterocyte brush borders. Those phenotypes (and their associated fimbria-binding specificities) were A (K88ab, K88ac, and K88ad), B (K88ab and K88ac), C (K88ab and K88ad), D (K88ad), and E (no fimbriae). In a subsequent investigation, an additional phenotype, F (K88ab), was identified (1).
K88 fimbriae are composed of multiple copies of the major fimbrial protein subunit, FaeG, and one copy of a minor subunit, FaeC (24). The minor subunit is located mainly at the fimbrial tip (33, 34). Removal of this protein subunit does not alter fimbria-binding activity, suggesting that the adhesive domain of K88 resides within the major fimbrial subunit (2). The genes encoding the major subunits of the three K88 variants have been sequenced and exhibit a high degree of variant-to-variant homology (K88ab to K88ac, 92%; K88ab to K88ad, 87%; K88ac to K88ad, 88%) (11, 15, 16, 27). The differences that exist between major subunit proteins in deduced amino acid sequence are scattered throughout the subunit but tend to cluster in the center of the molecule (14, 27).
The K88 variants contain multiple antigenic determinants, some of which are shared by all three variants (conserved determinants [e.g. K88a]) and others of which are not (variable determinants [e.g., K88b, K88c, and K88d]) (7, 27). Efforts to correlate serological differences between the variants with differences in amino acid sequence have been few. Furthermore, the location of the receptor-binding epitope is uncertain, as the results of various studies concerning that epitope have been interpreted in ways that conflict with each other. Wilson and Hohmann (40) produced K88 variant-specific antisera (K88ab and K88ac) which blocked binding of homologous fimbriae to porcine enterocytes. However, such antisera did not block binding of the reciprocal K88 variant to porcine enterocytes. These results were interpreted to suggest that the receptor-binding domain of the fimbria was contained within its antigenically variable epitope. However, Parry and Porter (35) reported that antisera raised to K88ab and K88ac fimbriae were cross-reactive with the reciprocal fimbria type and blocked binding of the reciprocal and homologous fimbrial variants to porcine enterocytes. Jacobs and his colleagues (26) enzymatically digested K88ab fimbriae and from that digest isolated peptides that inhibited K88ab's hemagglutinating activity and ability to adhere to porcine enterocytes. The inhibiting peptides were Ser-Leu-Phe and Ala-Ile-Phe. Both tripeptides corresponded to peptide stretches contained within conserved regions of the major subunits of the three K88 variants. Jacobs et al. (25) modified the gene encoding the K88 fimbrial adhesin by oligonucleotide-directed site-specific mutagenesis, resulting in the replacement of the phenylalanine by serine at several positions, including two corresponding with the peptide stretches mentioned above. The substitution resulting in the change of Ser-148–Leu–Phe-150 to Ser-148–Leu–Ser-150 caused a dramatic decrease in the capacity of K88ab fimbriae to adhere to cavia erythrocytes. The mutant fimbriae were somewhat thinner and less abundant on the surface of bacteria than were wild-type fimbriae but were otherwise similar in appearance. Jacobs and his colleagues proposed that Ser-148–Leu–Phe-150, which is conserved among all K88 variants, is an essential part of the receptor-binding site of K88 fimbriae. By substituting various parts of the major subunit genes of K88ab for K88ac genes and vice versa, Bakker et al. (2) constructed hybrid K88 fimbriae. Regions of peptide or individual amino acids thought to be involved in the formation of antigenically variable determinants were located by use of monoclonal antibodies (MAbs). Hemagglutination was used to correlate receptor-binding domains with variable antigenic determinants. A switch in antigenic specificity of the hybrid fimbria from K88ab to K88ac was accompanied by a switch in hemagglutination specificity from that characteristic of K88ab to that characteristic of K88ac. From these results, the investigators concluded that there was an overlap between the receptor-binding site and serotype-specific antigenic determinants.
Several putative receptors for K88 adhesins have been identified on porcine epithelial cells. These include intestinal mucin-type glycoproteins (IMTGP), which bind K88ab and K88ac (8, 9); porcine enterocyte transferrin (19), which binds K88ab; and a neutral glycosphingolipid (21), which binds K88ad. The presence of IMTGP has been correlated with piglet susceptibility to enterotoxigenic colibacillosis (13). The IMTGP have been purified to homogeneity (8), and the characterization and availability of this type of receptor in pure form makes more definitive identification of the receptor-binding epitopes of K88 adhesins possible. Because each identified porcine enterocyte K88 receptor differs with regard to the K88 variant fimbria that binds to it, it is obvious that the receptor-binding domains of the K88 variants must have at least some unique binding characteristics. Therefore, it appears likely that those amino acids, or peptide stretches unique to each variant, are contained within the receptor-binding domains of these fimbriae. The objective of the present study was to determine whether antigenically unique determinants of K88 fimbriae contribute to the receptor-binding domains of these molecules. MAbs to variable and conserved epitopes of K88ac were prepared, and Fab fragments were purified therefrom. Fab fragments specific for variable epitopes of K88 blocked the binding of K88+ E. coli to porcine enterocyte brush borders and the binding of isolated K88 fimbriae to IMTGP. Fab fragments from a MAb specific for a conserved epitope of K88ac had no such effect. These results suggest that the receptor-binding domain of K88ac is contained, at least in part, within antigenically variable epitopes of the major fimbrial subunit of K88ac. Epitope mapping for one MAb indicated that it bound to the region from amino acid no. 64 to no. 107 on the major K88ac fimbrial subunit.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The following K88-fimbriated E. coli strains were used: 1476 (K12:K88ac) (8), 263 (O8:K87:K88ab) (1), Morris (O8:K87:K88ad) (5), and 3030-2 (O157:K87:K88ac) (9). All strains were cultured on sheep blood agar (Columbia base) from stock frozen in liquid nitrogen and were passaged a maximum of two times on the same medium. Cultures were incubated for 18 h at 37°C before use.
K88 fimbria extraction and purification.
The K88 fimbriae were extracted from K88-positive E. coli strains, purified, and biotinylated as previously described (9). Purity of fimbrial preparations was determined on sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) gels.
MAb production.
Purified K88ac fimbriae from the K12:K88ac E. coli strain 1476 were suspended in phosphate-buffered saline (pH 7.4; PBS) and were emulsified in an equal volume of Freund's complete adjuvant. Female BALB/c mice were inoculated intraperitoneally with 0.2 ml of the emulsion, containing 500 μg of protein. Three weeks later, inoculations were repeated, using Freund's incomplete adjuvant in place of Freund's complete adjuvant. Two weeks after the second inoculation, mice were intravenously given 125 μg of fimbriae suspended in 50 μl of PBS. Mice were sacrificed 2 or 3 days after the intravenous inoculation, and their spleens were removed for the collection of lymphocytes. Lymphocytes were fused with NS-1 myeloma cells as described previously (11). Resultant hybridoma cells were propagated and screened by indirect enzyme-linked immunosorbent assay (ELISA) as described below for the production of MAbs reactive with K88 fimbriae. The cells in culture plate wells containing antibodies reactive with K88 were cloned by limiting dilution in 96-well microtiter plates and were screened again for K88 antibody production. The K88 variant specificity of antibodies was determined by indirect ELISA as described below, and antibodies were characterized as either variant specific (anti-K88c) or reactive with all three K88 variants (anti-K88a). MAbs from various hybridoma lines were isotyped with the use of an antibody isotyping kit (ICN, Costa Mesa, Calif.), and cells from selected immunoglobulin G1 (IgG1)-producing cell lines were injected intraperitoneally into pristane-primed BALB/c mice for the production of ascitic fluid as described previously (11).
Indirect ELISA for screening hybridoma culture supernatants.
Hybridoma culture supernatants were screened for K88 antibody, using purified K88ab, K88ac, and K88ad fimbriae from E. coli strains 263, 3030-2, and Morris, respectively. Microtiter plates (96-well Immulon-I; Dynatech Laboratories, Chantilly, Va.) were coated with 2.5 μg of K88ab, 0.6 μg of K88ac, or 0.6 μg of K88ad adhesin (the amount of antigen required for optimum signal) in 0.05 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight. After three washes with PBS–0.05% Tween 20, hybridoma culture supernatant in twofold serial dilutions was added to plate wells and incubated for 45 min at 37°C with rotational agitation at 50 rpm. The plates were then washed as before, peroxidase-conjugated goat anti-mouse immunoglobulins (Pierce, Rockford, Ill.) were added, and the plates were again incubated. Following another washing, bound peroxidase was detected using the chromogenic peroxidase substrate 2,2′-azinobis(3-ethyl-benzthiazoline-6-sulfonic acid). Color change was assessed spectrophotometrically at 405 nm, using an EL340 ELISA reader (Bio-TEK Instruments Inc., Winooski, Vt.).
MAb purification and characterization and Fab preparation.
The protein concentration of ascites containing anti-K88a or anti-K88c MAb was quantified by the Lowry method (31), and antibodies were purified by protein G column chromatography as described by the manufacturer (Pharmacia, Piscataway, N.J.). The specificity of MAbs was determined by indirect ELISA as described above. Purified MAbs were enzymatically cleaved to yield Fab fragments, using a commercial kit (Immunopure Fab preparation kit; Pierce). The completeness of separation of Fab fragments from undigested MAbs was assessed using nondenaturing SDS-PAGE separation and the Coomassie blue staining detection method (23).
Western blot test for MAb specificity for variant (K88c) or conserved (K88a) antigenic epitopes.
The specificity of MAbs was confirmed by Western blotting using purified K88ab, K88ac, and K88ad fimbriae. Protein separation by SDS-PAGE was done as described for the assessment of fimbria purity, except that 1.5 μg of the K88 fimbrial preparation was loaded per lane. Fimbriae were electrophoretically transferred from SDS-PAGE gels to nitrocellulose membranes. The membranes were incubated with the purified MAb (0.34 μg in 2 ml) for 60 min and were washed three times with PBS-Tween. With each wash, membranes were incubated for 10 min with rotational agitation at 50 rpm. The membranes were then incubated in 2 ml of peroxidase-conjugated goat anti-mouse immunoglobulin (Pierce) diluted 1:1,000 and were again washed as described above. Peroxidase bound to membranes was detected using 3,3-diaminobenzidine as described previously (9).
Direct-competition ELISA to determine whether individual MAbs were specific for the same epitope.
Direct-competition assays were conducted using ELISAs as described above, except for modifications made for the competition assay as described elsewhere (38). Competition curves (percent competition) were calculated using the following formula: (1 − absorbance in presence of unlabeled MAb/absorbance in absence of unlabeled MAb) × 100.
Bacterial adherence inhibition assay.
The Fab fragments of each MAb studied were tested for the ability to inhibit the binding of K88ac-positive E. coli to brush border vesicles prepared from porcine enterocytes. Pig enterocyte brush border vesicles were prepared and purified as described previously (1). Only brush borders adhesive to bacterial cells expressing the K88ac antigenic variant were used, including those of phenotype A (binding all three variants) and phenotype B (binding K88ab and K88ac but not K88ad). Fab fragment preparations were each adjusted to the same concentration (0.4 μg/μl in PBS at pH 7.4) and were serially diluted (twofold increments). Fab fragments (500 μl) at each concentration were mixed with 50 μl of a suspension of E. coli strain 3030-2 (1.5 × 108 CFU/ml). Mixtures of bacteria and Fab fragments were incubated at 37°C for 45 min with rotational agitation at 50 rpm. Then 50 μl of brush border suspension (3.93 μg/μl) in the presence of d-mannose (final concentration of 2.5%) was added and was incubated for 15 min at room temperature with rotational agitation at 140 rpm. Brush borders were examined at a magnification of ×400 by phase-contrast microscopy for adherent bacteria. Fab fragments were replaced with bovine serum albumin (BSA) in negative control preparations. The number of bacteria adhering to 40 individual brush border vesicles was determined at each Fab concentration, and the average number of bacteria per brush border was calculated.
Fimbrial adherence inhibition assay using solubilized brush borders.
Fab fragments from each MAb under investigation were tested for the ability to inhibit the binding of K88ac fimbriae to IMTGP. Porcine enterocyte brush borders containing IMTGP-1 and -2 (apparent molecular masses of 210 and 240 kDa, respectively) were solubilized, and proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose as described previously (9). Concurrently, Fab fragments (200 μg in 1 ml of PBS) were incubated with biotinylated K88ac adhesins (0.6 μg in 2 ml of PBS-Tween) for 45 min at 37°C. This mixture was used in place of biotinylated K88ac adhesins for incubation with the brush border proteins on nitrocellulose strips in the biotinylated-adhesin overlay assay (9). BSA was used in place of MAbs as negative controls.
Fimbrial adherence inhibition assay using purified K88 receptors.
Fab fragments from each MAb under investigation were tested by ELISA for the ability to inhibit the binding of K88 fimbriae to purified IMTGP-1 and -2. The IMTGP were purified as described previously (20). To determine Fab inhibitory activity relative to protein concentration, purified receptors were first immobilized on plates by applying 0.5 μg of the material in 100 μl of 0.05 M carbonate-bicarbonate buffer (pH 9.6), followed by incubation at 4°C overnight. Plates were then washed three times with PBS-Tween. Fab fragments (42.5 ng/μl in PBS, pH 7.4) were diluted twofold serially. One hundred microliters of each dilution was mixed with 50 μl of biotinylated K88ac adhesins (0.6 μg/μl in PBS-Tween) and then incubated at 37°C for 45 min with rotational agitation at 50 rpm. BSA was used in place of Fab fragments as a negative control. The Fab–biotinylated-adhesin mixtures were placed in the microtiter plate wells containing immobilized IMTGP and were incubated at 37°C for 45 min with agitation. After the removal of unbound reagents by washing of ELISA plates as described above, 100 μl (0.43 μg/ml in PBS-Tween) of horseradish peroxidase-streptavidin was placed in each plate well and was incubated for 30 min at room temperature. The plate wells were again washed, and the bound peroxidase was detected by reaction with 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) as described above for other ELISAs. The percentage of inhibition was determined by the following equation: (1 − absorbance in presence of MAb/absorbance in absence of MAb) × 100.
PCR amplification of different segments of the K88ac faeG gene.
Portions of the K88ac (faeG) gene were PCR amplified from pDB88-102 (2), which contains genes faeC to faeH of the K88 operon. PCR reagents were purchased from PE Applied Biosystems (Foster City, Calif.). The PCR was performed in 50 μl of reaction mixture containing 0.3 mM deoxynucleoside triphosphate, 0.6 μM concentrations of both primers, 0.75 mM MgCl2, and 1.25 U of Taq DNA polymerase. Different sets of primers were designed to amplify corresponding segments of the faeG gene according to the published sequence of the K88ac major fimbrial subunit gene (GenBank accession number M35954) (27). The amplification proceeded through three linked programs. To amplify the gene segment coding the peptide stretch from amino acid no. −17 (the negative number indicates that the amino acid is located in the signal peptide sequence, which is upstream from the fimbrial protein sequence) to amino acid no. 129, the primer sequences were 5′-CCAAGCTTTCTGATTGCACTGGCAATTGC-3′ and 5′-CGGATCCTTAAGATGCATTCACTTTCACTGA-3′. The program used was 4 cycles of 96°C for 1.5 min, 60°C for 1 min, and 72°C for 0.5 min and 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 0.5 min, followed by 72°C for 5 min. For amplification of the gene segment for the peptide stretch from amino acid no. −17 to no. 63, the primer sequences were 5′-CCAAGCTTTCTGATTGCACTGGCAATTGC-3′ and 5′-GTTCCCGGGGCCTAACAAAATTGGCTTATT-3′. The program was the same as the previous program, except that 65°C was used as the annealing temperature. For amplification of the gene segment for the peptide stretch from amino acid no. 64 to no. 129, the primer sequences were 5′-GGAAGCTTCCGAACCAAAGAAGCATTTGCT and 5′-CGGATCCTTAAGATGCATTCACTTTCACTGA-3′. The program was the same as that previously used, except that 62°C was used as the annealing temperature. For amplification of the gene segment for the peptide stretch from amino acid no. 64 to no. 107, the primer sequences were 5′-GGAAGCTTCCGAACCAAAGAAGCATTTGCT-3′ and 5′-CCCGGGTGCTAAACCTTTTTTATTAG-3′. The program was the same as the previous programs, except that 57°C was used as the annealing temperature.
Cloning of the PCR product into the pBAD-TOPO expression vector.
The PCR products were purified by 1% SeaKem GTG agarose (FMC Bioproducts, Rockland, Maine) gel electrophoresis using Tris-acetate-EDTA buffer. The specific DNA band was then excised, and the DNA was eluted using the Ultrafree-DA DNA extraction kit (Millipore, Bedford, Mass.) as described by the manufacturer. The PCR product was cloned into the pBAD-TOPO vector followed by transformation into competent E. coli Topo 10 cells provided in the pBAD-TOPO cloning kit (Invitrogen, Carlsbad, Calif.) as described by the manufacturer. Positive clones were selected by PCR amplification to ensure that the correct gene was cloned into the vector. The cloned gene was then expressed by adding d-arabinose at a final concentration of 0.2% as an inducer for expression as described by the manufacturer.
Western blot test for the identification of the faeG gene fragment expression product reactive with MAb 36/41.
After expression, bacteria were pelleted by centrifugation and were then lysed by B-PER II bacterial extraction reagent as described by the manufacturer (Pierce). The expressed proteins were separated on a 17% Tricine gel (17), transferred to a nitrocellulose membrane, and then screened by Western blotting for identification by MAb 36/41 as described above. Untransformed host cells were used as a negative control. The expression of a protein by fragments of the faeG gene whose products were not recognized by MAb 36/41 was verified by the appearance of a unique band of the predicted mass in Coomassie blue-stained Tricine gel preparations. It was further verified by identification of the protein in Western blots stained with horseradish peroxidase-conjugated V5 antibodies used according to the supplier's directions (Invitrogen).
RESULTS
MAb production and characterization.
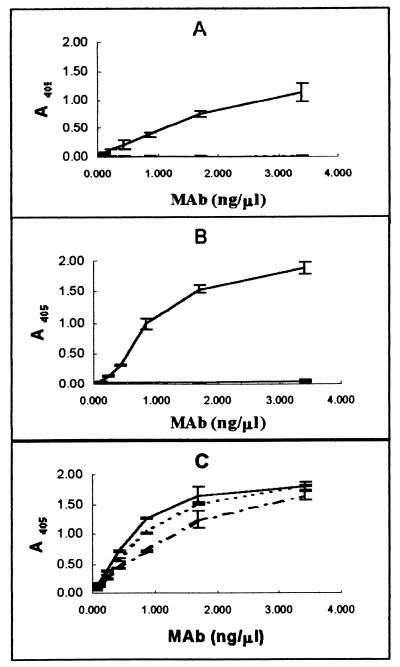
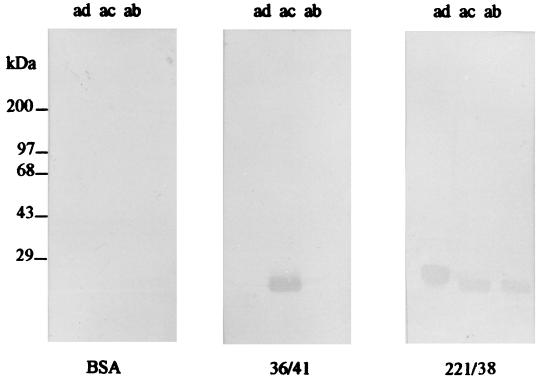
Preparations of K88 adhesins purified from E. coli strains and used for production of hybridomas exhibited a high degree of purity. The only Coomassie blue-staining bands observed in SDS-PAGE gels to which 2.5 μg of K88 adhesin preparation had been applied had an apparent molecular mass of 27.5 kDa, consistent with that of the K88 major protein subunit (11). Three hybridoma cell lines resulting from the fusion of myeloma cells with lymphocytes from K88-immunized mice were selected for use in this study, based on their production of high-avidity IgG1 antibodies with specificity for K88. Ascites from hybridoma cell lines 30/17 and 36/41 bound K88ac but not K88ab or K88ad adhesins when tested by ELISA (Fig. 1). This indicates that these two MAbs bound to variable antigenic domains on K88ac. By contrast, ascites from hybridoma cell line 221/38 bound all three K88 fimbrial adhesins, indicating that it bound a conserved domain of the K88ac fimbria. In Western blotting MAb 36/41 bound K88ac adhesin exclusively, whereas 221/38 bound all three K88 variants, suggesting that 36/41 recognizes a linear epitope in the variable region of the K88ac adhesin and that 221/38 recognizes a linear epitope in a region conserved among the three K88 variants (Fig. 2). MAb 30/17 did not bind K88 adhesins in Western blot preparations (data not shown), suggesting that its binding is conformationally dependent.
FIG. 1.
The ability of MAbs 30/17 (A), 36/41 (B), and 221/38 (C) to bind to K88ac (——), K88ab (–––), and K88ad (–·–·–) was determined by indirect ELISA as described in Materials and Methods. MAbs 30/17 and 36/41 bind to K88ac but not to K88ab or K88ad. MAb 221/38 binds to all three K88 variants.
FIG. 2.
Determination by Western blotting of the specificity of MAbs with regard to K88ab, K88ac, and K88ad adhesins. K88ab, K88ac, and K88ad adhesin antigens were separated by SDS-PAGE and transferred to nitrocellulose membranes. These membranes were probed with MAb 36/41 or 221/38 or BSA, followed by incubation with peroxidase-conjugated goat anti-mouse IgG as described in Materials and Methods. Molecular mass standards are indicated on the left. MAb 36/41 binds to K88ac fimbriae specifically, and MAb 221/38 binds to all three variants of K88 fimbriae.
Effect of anti-K88 MAbs on K88ac-mediated bacterial adhesion to phenotype A and B pig brush borders.
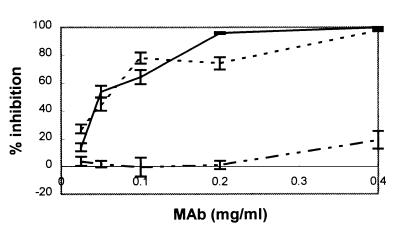
Fab fragments prepared from MAbs 30/17 and 36/41 exhibited the ability to inhibit binding of K88ac+ E. coli to porcine phenotype A brush borders in a concentration-dependent manner (Fig. 3). At 0.4 mg/ml, Fab fragments from these MAbs completely blocked binding of K88ac+ E. coli to those brush borders. The blocking action of these Fab fragments indicates that the antibodies bind at or near the receptor-binding site. Fab fragments from MAb 221/38 exhibited minimal inhibition of K88ac+ E. coli binding at a Fab concentration of 0.4 mg/ml. Minimal interference of K88 binding by this MAb indicates that it binds to an epitope distant from the adhesin binding site. Results from the test of phenotype B brush borders were essentially the same as those for phenotype A brush borders (data not shown).
FIG. 3.
Effect of Fab fragments of variant-specific (K88c) and variant-cross-reactive (K88a) MAbs on the binding of bacteria to brush borders. The ability of MAbs 30/17 (–––), 36/41 (——), and 221/38 (–··–··–) to inhibit bacterial adhesion is presented as the percentage of inhibition relative to a BSA control. K88ac-specific MAbs 30/17 and 36/41 blocked binding of E. coli K88ac bacteria to porcine brush borders, but K88a-specific MAb 221/38 did not.
Effect of anti-K88 MAbs on the binding of K88 fimbriae to IMTGP in the biotinylated-adhesin overlay assay.
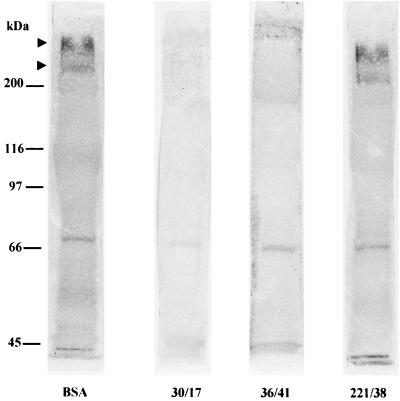
When 200 μg each of Fab fragments of MAbs 30/17 and 36/41 was incubated with biotinylated K88ac fimbriae in the biotinylated-adhesin overlay assay, they completely blocked the binding of that adhesin to IMTGP (Fig. 4). The binding of biotinylated K88ac to other brush border membrane glycoproteins to which it typically binds was also blocked by the Fab fragments of these MAbs. A 75-kDa brush border protein bound the horseradish peroxidase-streptavidin directly (9) and was stained prominently regardless of membrane treatment. The Fab fragments from MAb 221/38 did not block the binding of biotinylated K88ac fimbriae to IMTGP when used under the same conditions under which MAbs 30/17 and 36/41 were used.
FIG. 4.
Inhibition of the binding of K88ac fimbriae to IMTGP K88 receptors by Fab fragments of K88 MAbs. Brush border proteins were separated by SDS–7% PAGE and transferred to nitrocellulose membranes. The separated proteins were incubated with biotinylated K88ac adhesins that had been preincubated with MAb 30/17, 36/41, or 221/38 or BSA. The arrowheads indicate the positions of the IMTGP. Molecular mass standards are indicated on the left. Two K88ac-specific MAbs (30/17 and 36/41) inhibited K88ac adhesin binding to IMTGP, whereas K88a-specific MAb (221/38) and BSA did not inhibit binding under the same conditions.
Effect of anti-K88 MAbs on the binding of K88 fimbriae to purified IMTGP in an ELISA.
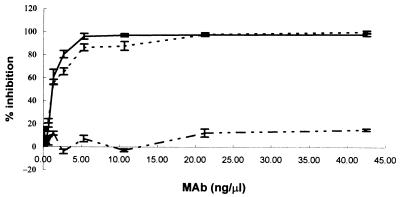
To further test the inhibitory activity of K88ac-specific MAbs on the binding of K88ac fimbriae to IMTGP, purified IMTGP-1 and -2 were immobilized on ELISA plates and the ability of each MAb to block K88ac-IMTGP interactions was tested. Fab fragments of MAbs 30/17 and 36/41 almost completely blocked binding of biotinylated K88ac fimbriae to IMTGP. However, Fab fragments of MAb 221/38 blocked binding of K88ac to IMTGP only minimally (Fig. 5). The inhibition of binding by MAbs 36/41 and 30/17 was Fab fragment concentration dependent, reaching 100% at 42.5 ng/μl for MAb 30/17 and 97.9% for MAb 36/41. The maximal inhibition of K88ac binding achieved by MAb 221/38 was 15%.
FIG. 5.
Effect of K88 variant-specific (K88ac) and variant-cross-reactive (K88a) MAbs on K88ac fimbria binding to IMTGP K88 receptors. Twofold serial dilutions of Fab fragments of MAbs 30/17 (–––), 36/41 (——), and 221/38 (–··–··–) were incubated with biotinylated K88ac adhesins. Then each mixture was placed in microtiter plate wells coated with purified receptors. The percent inhibition of the binding of K88ac to IMTGP was determined as described in Materials and Methods. Results are the means of three replications of the experiment. K88ac-specific MAbs 30/17 and 36/41 blocked K88ac fimbriae from binding to IMTGP in a dose-dependent manner, whereas K88a-specific MAb 221/38 did not.
Epitope mapping for MAb 36/41.
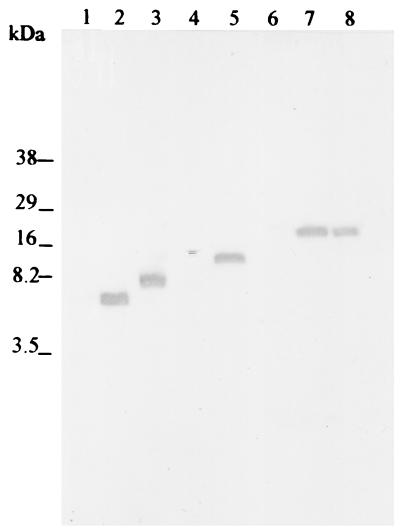
To identify the region of the fimbria to which the neutralizing MAb bound, each half of the whole fimbrial gene, faeG, was amplified by PCR and cloned into a pBAD-TOPO vector, followed by expression and screening of the expressed product as described in Materials and Methods. Then the cloned half of the faeG gene encoding the protein recognized by the MAb was split into two parts of similar size, and the procedure was repeated. This cycle of gene division, amplification, expression, and screening of the expressed product for identification by the MAb was repeated until the smallest MAb-recognized peptide product was identified. In the initial subdivision, the expressed peptide stretch from amino acid no. −17 to no. 129 of the K88ac major fimbrial subunit was recognized by MAb 36/41 in a Western blot assay. With subdivision of the gene segment corresponding to the peptide stretch from amino acid no. −17 to no. 129, the peptide stretch from amino acid no. 64 to no. 129 was recognized by MAb 36/41 in Western blotting, while the peptide stretch from amino acid no. −17 to no. 63 was not. The existence of faeG fragment expression products for peptide stretches not recognized by MAb 36/41 was verified on Coomassie blue-stained Tricine gels and on Western blots probed with anti-V5-horseradish peroxidase antibody. Subdivision of the gene segment for the peptide stretch from amino acid no. 64 to no. 129 into equal parts followed by expression of the gene segments failed to produce a product recognizable by MAb 36/41 (data not shown). However, the expressed product for the gene fragment corresponding to the amino acid stretch from no. 64 to no. 107 was recognized by MAb 36/41 (Fig. 6). This region was the minimal product recognized by MAb 36/41 and contains eight amino acids that differ among the three K88 variants.
FIG. 6.
Western blotting for screening by MAb 36/41 of peptides expressed by different segments of the K88ac faeG gene. PCR products of different segments of the K88ac faeG gene were cloned into a pBAD-TOPO vector, followed by expression of the gene segments in E. coli Topo 10 host cells. Whole cells were lysed, and peptides were separated on a 17% tricine gel and transferred to nitrocellulose membranes. The expressed peptides were screened for identification by MAb 36/41. Lane 1, E. coli host cell lysate; lane 2, cell lysate containing the FaeG peptide stretch from amino acid no. 64 to no. 107; lane 3, cell lysate containing the FaeG peptide stretch from amino acid no. 64 to no. 129; lane 4, cell lysate containing the FaeG peptide stretch from amino acid no. −17 to no. 63; lane 5, cell lysate containing the FaeG peptide stretch from amino acid no. −17 to no. 129; lane 6, cell lysate containing the FaeG peptide stretch from amino acid no. 130 to no. 242; lane 7, cell lysate containing the whole faeG gene; lane 8, wild-type K88ac fimbriae.
DISCUSSION
Our objective in conducting this study was to identify the region of the K88ac major fimbrial subunit responsible for fimbrial binding to receptors on porcine intestinal epithelial brush borders. In identifying that region, it was expected that we could more clearly determine the evolutionary strategies that result in antigenic change in the K88 fimbriae. Results of this study indicate that antigenic diversity in the K88 fimbriae is at least in part a result of structural differences in the adhesive domain of the major fimbrial subunit, FaeG. Structural differences resulting in antigenic diversity were likely selected to modify adhesin specificity and to improve the binding strength of the fimbria. As indicated above, the 44-amino-acid segment identified in this study as a part of the receptor-binding domain of K88ac contains eight amino acids that differ between that fimbria and either K88ab or K88ad. Perhaps one or several of these amino acids are responsible for specificity differences between K88ac and either K88ab or K88ad. From their analysis of antigenic and adhesive characteristics of K88 using nucleic acid substitutions in the faeG gene, Bakker et al. (2) concluded, as did we, that there is an overlap between the receptor-binding site of K88 fimbriae and serotype-specific antigenic determinants of those fimbriae. However, the correlation made by Bakker and coworkers between receptor-binding domains and antigenic epitopes was based on hemagglutination activity, not binding of fimbriae to porcine enterocytes. Despite their modification of hemagglutination specificity through mutagenesis, Bakker and his colleagues observed no changes in porcine enterocyte brush border binding. This lack of observed change in enterocyte binding specificity is not surprising, as most porcine brush borders that bind to either K88ab or K88ac contain receptors that bind both fimbrial variants (1, 5, 13). Those receptors include IMTGP (5, 13) and another receptor tentatively called bcd that has not yet been isolated or fully characterized (5, 13). Only if Bakker et al. had selected brush borders that bound K88ab but not K88ac (phenotype C or F brush borders) (1) would they have been able to determine whether their fimbrial modification resulted in a change in brush border binding specificity. Phenotype C and F brush borders likely possess the transferrin reported by Grange and Mouricout (19) to be exclusively a receptor for K88ab fimbriae.
Observations made in the present study suggest that the receptor-binding domain of the K88ac fimbria contains linear and conformational-dependent determinants, both within an antigenically variable portion of FaeG. The presence of a linear epitope within the antigenically variable domain of K88ac was previously reported (37), although its contribution to receptor binding was not assessed. Other portions of the major fimbrial subunit protein may also contribute to its adhesive function. The observations of Jacobs et al. (26), that tripeptides corresponding to amino acid stretches in a conserved antigenic epitope of K88ab block the binding of that fimbria to porcine brush borders, suggest that at least one conserved epitope contributes to receptor binding. It seems logical that the receptor-binding domain should contain both conserved and variable elements, as the receptor binding specificities of the three K88 variants appear to be unique but not entirely different from each other. K88ab and K88ac both bind IMTGP, and all three variants appear to bind the receptor that is tentatively called bcd. However, K88ab uniquely binds an enterocyte transferrin (19), and K88ad uniquely binds an enterocyte-neutral glycosphingolipid (20). Thus, the structure of the receptor-binding domain is sufficiently conserved for some cross-reactivity in binding to occur despite obvious evidence of uniqueness. The three identified receptors each contain galactose and N-acetylglucosamine, apparently in terminal positions. Galactose moieties appear to be critical to receptor binding (20, 21). However, each receptor exhibits uniqueness with regard to at least one other sugar (12). The presence of different sugars in these receptors may affect the way the common sugar molecules are displayed, thus affecting how they are recognized by the K88 fimbria variants. Mutations that result in a modification of amino acid sequence in variable portions of FaeG undoubtedly have been selected to exploit different receptors. Amino acid changes resulting in receptor specificity switching remain to be determined but may be among those that differ between K88ac and either K88ab or K88ad in the amino acid stretch recognized by MAb 36/41.
The present study focuses on an adhesin responsible for the binding of K88ac fimbriae to receptors contained within porcine enterocyte brush border membranes. The existence of another adhesin that mediates fimbrial binding to a receptor located elsewhere cannot be ruled out by this study. The three K88 variants share an identical fimbria tip subunit whose function has not been determined (7). However, tip subunits associated with some other fimbriae have adhesive activity (28, 30). If the tip subunit of K88 is adhesive, its specificity must be identical for the three variants. In addition, the receptor to which it binds must be somewhere other than in brush borders, because MAb 36/41 blocks the binding of K88ac to porcine enterocyte brush borders of phenotypes A and B, the only brush borders to which K88ac binds (1). It has been suggested by other investigators that the mucus covering the intestinal epithelium contains glycoproteins to which K88 fimbriae bind and that these glycoproteins may serve as receptors for the adherence of bacteria to that mucus (6, 10). The mucus glycoproteins identified were different from the receptors previously identified on porcine enterocyte brush border membranes (10). If the fimbrial tip subunit is adhesive, it may bind to mucus glycoproteins or perhaps to receptors found on the epithelium in another part of the gastrointestinal tract or in an alternative host. Assessment for receptors of biological relevance in mucus would require use of intact intestinal tissues, perhaps unwashed to retain the mucus sheet. Assessment of K88ac fimbriae for a second adhesin that bound to a receptor in mucus could be accomplished by testing bacterial binding following blockade by MAb 36/41.
In summary, we report production of two MAbs that are specific for antigenically variable determinants of the K88ac fimbria of E. coli and block binding of K88ac fimbriae to porcine enterocyte brush borders and to IMTGP, a characterized K88ab and K88ac receptor isolated from porcine enterocytes. Epitope mapping with one of the MAbs indicated that it bound within the region from amino acid no. 64 to no. 107 of FaeG, the major subunit of the K88ac fimbria. These results suggest that the receptor-binding domain of K88ac is, at least in part, contained within antigenically variable determinants of the fimbria.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support for this work through USDA grant 94-02409, NSF Cooperative agreement EP5-9720642, and the South Dakota Agricultural Experiment Station.
We thank Frits K. de Graaf for providing us with the plasmid construct pDB 88-102. We also thank Raymond Rowland and Chris Chase for helpful advice.
REFERENCES
- 1.Baker D R, Billey L O, Francis D H. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123–132. doi: 10.1016/s0378-1135(96)01277-1. [DOI] [PubMed] [Google Scholar]
- 2.Bakker D, Willemsen P T J, Simons L H, van Zijderveld F G, de Graaf F K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol Microbiol. 1992;6:247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 3.Bijlsma I G W, de Nijs A, van der Meer C, Frik J F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982;37:891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijlsma S G M, Frik J F. Haemagglutination patterns of the different variants of Escherichia coli K88 antigen with porcine, bovine, guinea pig, chicken, ovine, and equine erythrocytes. Res Vet Sci. 1987;43:122–123. [PubMed] [Google Scholar]
- 5.Billey L O, Erickson A K, Francis D H. Multiple receptors on porcine intestinal epithelial cells of the three variants of Escherichia coli K88 fimbrial adhesin. Vet Microbiol. 1997;59:203–212. doi: 10.1016/s0378-1135(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 6.Conway P L, Welin A, Cohen P S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990;58:3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykes C W, Halliday I J, Read M J, Hobden A N, Harford S. Nucleotide sequences of four variants of the K88 gene of porcine enterotoxigenic Escherichia coli. Infect Immun. 1985;50:279–283. doi: 10.1128/iai.50.1.279-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson A K, Baker D R, Bosworth B T, Casey T A, Benfield D A, Francis D H. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994;62:5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson A K, Willgohs J A, McFarland S Y, Benfield D A, Francis D H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesin of Escherichia coli and correlation of these glycoproteins with the adhesive phenotype. Infect Immun. 1992;60:983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang L, Gan Z, Marquardt R R. Isolation, affinity purification, and identification of piglet small intestine mucosa receptor for enterotoxigenic Escherichia coli K88ac+ fimbriae. Infect Immun. 2000;68:564–569. doi: 10.1128/iai.68.2.564-569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foged N T, Klemm P, Elling F, Jorsal S E, Zeuthen J. Monoclonal antibodies to K88ab, K88ac, and K88ad fimbriae from enterotoxigenic Escherichia coli. Microb Pathog. 1986;1:57–69. doi: 10.1016/0882-4010(86)90032-x. [DOI] [PubMed] [Google Scholar]
- 12.Francis D H, Erickson A K, Grange P A. K88 adhesins of enterotoxigenic Escherichia coli and their porcine enterocyte receptors. Adv Exp Med Biol. 1999;473:147–154. doi: 10.1007/978-1-4615-4143-1_13. [DOI] [PubMed] [Google Scholar]
- 13.Francis D H, Grange P A, Zeman D H, Baker D R, Sun R, Erickson A K. Expression of mucin-type glycoprotein K88 receptors strongly correlates with piglet susceptibility to K88+ enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect Immun. 1998;66:4050–4055. doi: 10.1128/iai.66.9.4050-4055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaastra W, Amstru-Pedersen P. Serologic variants of the K88 antigen. In: Lark D L, Normark S, editors. Protein-carbohydrate interactions in biological systems. London, England: Academic Press Inc.; 1986. pp. 95–102. [Google Scholar]
- 15.Gaastra W, Mooi F R, Stuitje A R, de Graaf F K. The nucleotide sequence of the gene encoding the K88ab protein subunit of porcine enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 1981;12:41–46. [Google Scholar]
- 16.Gaastra W, Klemm P, de Graaf F K. The nucleotide sequence of the K88ad protein subunit of porcine enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 1983;18:177–183. [Google Scholar]
- 17.Gallagher S R. One-dimensional SDS gel electrophoresis of proteins. In: Coligan J E, Dunn B M, Ploegh H L, Speischer D W, Wingfield P T, editors. Current protocols in protein science. New York, N.Y: John Wiley & Sons; 1995. pp. 10.1.1–10.1.34. [Google Scholar]
- 18.Gonzalez E A, Vazquez F, Ignacio Garabal J, Blanco J. Isolation of K88 antigen variants (ab, ac, ad) from porcine enterotoxigenic Escherichia coli belonging to different serotypes. Microbiol Immunol. 1995;39:937–942. doi: 10.1111/j.1348-0421.1995.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 19.Grange P A, Mouricout M A. Transferrin associated with the porcine intestinal mucosa is a receptor specific for K88ab fimbriae of Escherichia coli. Infect Immun. 1996;64:606–610. doi: 10.1128/iai.64.2.606-610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grange P A, Erickson A K, Anderson T J, Francis D H. Characterization of the carbohydrate moiety of intestinal mucin-type sialoglycoprotein receptors for the K88ac fimbrial adhesin of Escherichia coli. Infect Immun. 1998;66:1613–1621. doi: 10.1128/iai.66.4.1613-1621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange P A, Erickson A K, Levery S B, Francis D H. Identification of an intestinal neutral glycosphingolipid as a phenotype-specific receptor for the K88ad fimbrial adhesin of Escherichia coli. Infect Immun. 1999;67:165–172. doi: 10.1128/iai.67.1.165-172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinée P A M, Jansen W H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979;23:700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow E, Lane D. Antibodies, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 628–637. [Google Scholar]
- 24.Hoschutzky H, Buhler T, Ahrens R, Jann K. Function and molecular architecture of E. coli adhesins. In: Wadstrom T, Makela P H, Svennerholm A M, Wolfwatz H, editors. Molecular pathogenesis of gastrointestinal infections. New York, N.Y: Plenum Press; 1991. pp. 71–78. [Google Scholar]
- 25.Jacobs A A C, Roosendaal B, van Breemen J F L, de Graaf F K. Role of phenylalanine 150 in the receptor-binding domain of the K88 fibrillar subunit. J Bacteriol. 1987;169:4907–4911. doi: 10.1128/jb.169.11.4907-4911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs A A C, Venema J, Leeven R, van Pelt-Heerschap H, de Graaf F K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987;169:735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josephsen J, Hansen F, de Graaf F K, Gaastra W. The nucleotide sequence of the K88ac fimbriae of porcine enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 1984;25:301–306. [Google Scholar]
- 28.Khan A S, Schifferli D M. A minor 987P protein different from the structural fimbrial subunit is the adhesin. Infect Immun. 1994;62:4233–4243. doi: 10.1128/iai.62.10.4233-4243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krogfelt K A, Meldal M, Klemm P. K88 fimbrial antigens: identification of antigenic determinants by the use of synthetic peptides. Microb Pathog. 1987;2:465–472. doi: 10.1016/0882-4010(87)90053-2. [DOI] [PubMed] [Google Scholar]
- 30.Lindberg F, Lund B, Johansson L, Normark S. Localization of the receptor-binding adhesin at the tip of the bacterial pilus. Nature. 1987;328:84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- 31.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Mooi F R, de Graaf F K. Molecular biology of fimbriae of enterotoxigenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:119–138. doi: 10.1007/978-3-642-70586-1_7. [DOI] [PubMed] [Google Scholar]
- 33.Mooi F R, van Buuren M, Koopman G, Roosendaal B, de Graaf F K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984;159:482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oudega B, de Graaf M, de Boer L, Bakker D, Vader C E M, Mooi F R, de Graaf F K. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol Microbiol. 1989;3:645–652. doi: 10.1111/j.1365-2958.1989.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 35.Parry S, Porter P. Immunological aspects of cell membrane adhesion demonstrated by porcine enteropathogenic Escherichia coli. Immunology. 1978;34:41–49. [PMC free article] [PubMed] [Google Scholar]
- 36.Rapacz J, Hasler-Rapacz J. Polymorphism and inheritance of swine small intestinal receptors mediating adhesion of three serological variants of Escherichia coli-producing K88 pilus antigen. Anim Genet. 1986;17:305–321. doi: 10.1111/j.1365-2052.1986.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 37.Thiry G, Clippe A, Scarcez T, Petre J. Cloning of DNA sequences encoding foreign peptides and their expression in the K88 pili. Appl Environ Microbiol. 1989;55:984–993. doi: 10.1128/aem.55.4.984-993.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Zijderveld F G, Anakotta J, Brouwers R A M, van Zijderveld A M, Bakker D, de Graaf F K. Epitope analysis of the F4 (K88) fimbrial antigen complex of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1990;58:1870–1878. doi: 10.1128/iai.58.6.1870-1878.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westerman R B, Mills K W, Phillips R M, Fortner G W, Greenwood J M. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988;26:149–150. doi: 10.1128/jcm.26.1.149-150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson M R, Hohmann A W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974;10:776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]