Abstract
Biofilm-associated bacteria, especially ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.), are a serious challenge worldwide. Due to the lack of discovery of novel antibiotics, in the past two decades, it has become necessary to search for new antibiotics or to study synergy with the existing antibiotics so as to counter life-threatening infections. Nature-derived compounds/based products are more efficient than the chemically synthesized ones with less resistance and lower side effects. In this descriptive review, we discuss the most promising therapeutics for the treatment of ESKAPE-related biofilms. The first aspect includes different types of natural agents [botanical drugs, essential oils (EOs), antimicrobial peptides, bacteriophages, and endolysins] effective against ESKAPE pathogens. The second part of the review deals with special references to EOs/essential oil components (EOCs) (with some exclusive examples), mode of action (via interfering in the quorum-sensing pathways, disruption of biofilm and their inhibitory concentrations, expression of genes that are involved, other virulence factors), existing in literature so far. Moreover, different essential oils and their major constituents were critically discussed using in vivo models to target ESKAPE pathogens along with the studies involving existing antibiotics.
Keywords: antibiotics, biofilm, ESKAPE, essential oils, quorum-sensing, synergy, toxicity
Introduction
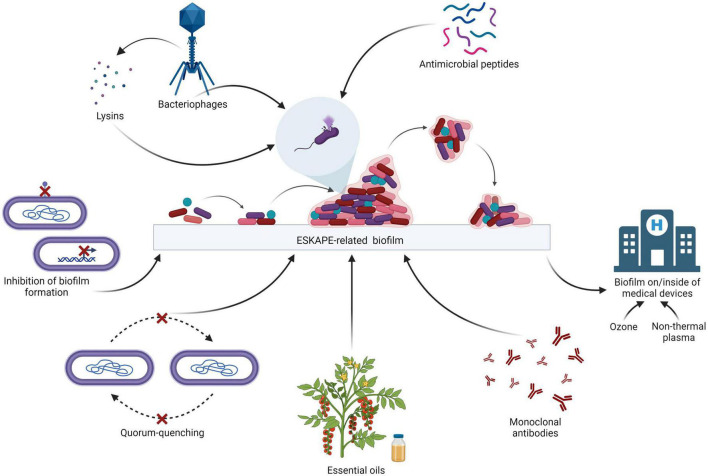
The ESKAPE bacteria are a group of opportunistic pathogens consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These bacteria represent a global threat from a clinical point of view since they are generally multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan drug-resistant (PDR). In particular, E. faecium is a Gram-positive generally inhabiting the human gastrointestinal tract which may lead to several diseases such as bacteremia, endocarditis, and neonatal meningitis. S. aureus is a Gram-positive bacterium colonizing humans at the level of the skin and the upper respiratory tract (nostrils) which maybe involved in skin infections, as well as pneumonia and sepsis. K. pneumoniae is a Gram-negative that is part of the normal microbiota of humans (skin and digestive system) which may cause respiratory and urinary infections, as well as bacteremia and liver abscess. A. baumannii is a ubiquitous Gram-negative bacterium which may lead to respiratory and urinary infections. P. aeruginosa is a ubiquitous Gram-negative causing several infections, including respiratory (especially in cystic fibrosis patients), urinary and skin infections (generally after a burn injury). Last, the genus Enterobacter comprises Gram-negative bacteria that may be natural commensals of the human gastrointestinal tract which may be involved in urinary, respiratory and soft skin infections. Due to the resistance of the ESKAPE bacteria to a broad range of antibiotics, there are severe challenges in the treatment of their infections, especially when biofilms are involved. In fact, bacteria inside the biofilms are about 1,000 times more resistant to antimicrobials as compared to planktonic cells (Patil et al., 2021). Consequently, there is an urgent need to develop new weapons to fight these pathogens, with particular emphasis on the eradication of their biofilms (Panda et al., 2021). Several strategies are being explored around the world in order to treat ESKAPE-related biofilms (Sahoo et al., 2021; Figure 1). A broad variety of proteins are involved in biofilm structuration, making them attractive targets to inhibit biofilm formation. For instance, the Acinetobacter baumannii outer membrane protein A (OmpA), involved in the virulence of this bacterium, plays a role in the formation of the biofilm: by down-regulating the expression of this protein through synthetic small compounds inhibiting the ompA promoter, the in vitro formation of biofilm is also affected (Na et al., 2021). Another example is the Pseudomonas aeruginosa carbohydrate-binding protein Lectin A (LecA) which is involved in the generation of the biofilm matrix: this molecule may be targeted by LecA synthetic inhibitors in order to impair the structure of the biofilm (Siebs et al., 2022).
FIGURE 1.
Therapeutic strategies for the treatment of the ESKAPE biofilms (adapted from “Biofilm Formation Cycle”, by BioRender.com, 2022).
For the establishment of biofilm, bacteria have to communicate among each other by means of quorum-sensing. The inhibition of quorum-sensing, i.e., quorum-quenching, is one of the promising strategies to impair biofilm formation. For instance, the P. aeruginosa acylase PvdQ is able to cleave the acyl homoserine lactones of A. baumannii which are the mediators of quorum-sensing in this pathogen, affecting bacterial communication and, as a consequence, impairing biofilm formation in vitro (Vogel et al., 2022).
Other strategies used to target biofilms involve immunotherapy. Besides vaccines (active immunotherapy), monoclonal antibodies (passive immunotherapy) may be exploited to fight the ESKAPE-related biofilms. For example, a monoclonal antibody which targets alginate produced by P. aeruginosa, an exopolysaccharide involved in biofilm structuration and protection of the bacterium from the host immune system, could be used. Indeed, when treated in vitro with this antibody, P. aeruginosa showed impairment in the formation of biofilm (Gao et al., 2020).
In general, it is important to consider both drug reuse/resensitization and drug repurposing. Belonging to the first category, carboxylic acid salts derived from the fluoroquinolone norfloxacin have a higher activity on the ESKAPE pathogens compared to the parent molecule and are active on their biofilms, particularly on the Gram-negative ones (Lowrence et al., 2018). Ethyl bromopyruvate, in spite of being a derivative of anticancer agent 3-bromo-pyruvic acid, can be used for drug repurposing (Kumar et al., 2019). In fact, it is effective on planktonic ESKAPE bacteria and on Staphylococcus aureus biofilms, and hence is a good example of drug repurposing (Kumar et al., 2019).
Some of the most promising therapeutics for the treatment of the ESKAPE-related biofilms are antimicrobial peptides, bacteriophages, bacteriophage-encoded products, and natural products such as essential oils (EOs) to eradicate them. It is crucial to consider the treatment of the biofilms of the ESKAPE pathogens on/inside of medical devices since they are sources of nosocomial infections, before implementing the treatment options. For instance, non-thermal plasma (NTP) is a partially ionized gas characterized by both antimicrobial and antibiofilm activity on the ESKAPE pathogens that can be used for the disinfection of medical devices as well as hospital surfaces. NTP is more effective on Gram-negative bacteria compared to Gram-positive bacteria (Scholtz et al., 2021). Besides NTP, ozone can be used in order to eradicate ESKAPE-related biofilms from medical tools (Ibáñez-Cervantes et al., 2022).
This descriptive review includes the discussion of the most promising therapeutics for the treatment of ESKAPE-related biofilms. The first aspect covers different types of natural products effective against ESKAPE pathogens. The second part of the review deals with special references to EOs/essential oil components (EOCs) with some exclusive examples, mode of action, and synergy studies. Moreover, different EOs and their major constituents, as well as in vivo models to target ESKAPE pathogens were critically discussed.
Natural molecules against ESKAPE pathogens
Nature has provided a vast source of therapeutic agents along with a wide range of modern drugs which are in current use. These drugs are obtained from traditional medicinal plants as the diversity of biologically active molecules in these plants make them a potent source of medicines (Kothari et al., 2010; Tiwari et al., 2015). It has been reported that approximately 80% of the world’s population, specifically in developing countries, depend on medicinal plants to fulfill their primary health care needs (Akinsulire et al., 2007). The plant secondary metabolites like alkaloids, flavonoids, terpenes, saponins, tannins, etc., possess different medicinal properties (Mors et al., 2000; Tiwari, 2016).
The prolonged hospital stays have resulted in increased risk of medical expenses and mortality due to hospital-acquired infections which majorly occur in immunocompromised patients because of the exposure to ESKAPE pathogens. Therefore, in order to combat the rapid evolution of disease-causing pathogens, finding new antimicrobials is essential. Numerous secondary metabolites, or phytochemicals, that have the ability to prevent disease are known to be produced by plants. The main benefits of plant-derived products make them viable options for medical treatments because of their potential efficacy and minimal to no negative side effects (Pal and Shukla, 2003). Therefore, the discussion of use of such chemicals and extracts produced from plants to combat ESKAPE pathogens is quite significant. Many efficacious drugs can be produced for disease eradication by using these bioactive compounds. About 80% of the total population which majorly includes developing nations is dependent on natural products. Synthetic drugs are gaining popularity these days due to their time effectiveness, refined quality, cost-effectiveness, and quick effect (Ekor, 2014). However, many natural products–derived compounds are in various stages of drug development and have already highlighted the importance as well as the versatility of natural products as a future of novel drug development. Plants are a vital resource for novel drug development and other pharmacologically active compounds it has been observed that there are many drugs that are developed directly or indirectly from plants. According to World Health Organization (WHO), 11% of 252 drugs are considered basic and essential during the start of the 21st century and these drugs exclusively originated from the flowering plant (Veeresham, 2012). Some recent studies against biofilms provide the best example of natural products being effective and safe like synthetic halogenated furanone molecule, a secondary metabolite derivative, is generated from natural furanone produced by the Australian macroalga Dilsea pulchra. This substance can hinder bacterial signaling mechanisms and swarm cell movement. Additionally, it was proposed that D. pulchra furanones and AHL molecules’ structural similarity affects how putative regulatory proteins interact with AHL molecules by binding competitively to the receptor. In ecologically relevant concentrations, furanone prevents the surface aggregation characteristics of relevant ecological microorganisms. Other common examples of plant-based drugs are Tannic acid, Endolysins (PlyC), and Epigallocatechin gallate (EGCG) which result in the cleavage of peptidoglycan (Payne et al., 2013).
Proteins including enzymes and transporters are the main target of the herbal compounds. Additionally, these active substances could attach to or block the location where the pathogenic elements bind. These herbal medicines can also impact the behavior of biomolecules, or even their expression in a disease-causing condition. By utilizing cutting-edge techniques, their target and method of action can also be determined, hence, lowering the chance of protein conformations that could cause diseases in the future. Plants can be metabolically engineered to produce more antimicrobial chemicals, which may pave the way for the discovery of new therapeutics. Active substances found in plants which change the expression or shape of proteins that cause illness are proved to be effective against drug-resistant microbes (Table 1). Consequently, they can be useful for creating a brand-new medicine to treat illnesses. To develop phytoconstituents as medicines targeting protein conformation, a thorough biochemical and biophysical investigation is required. Before being employed as medicines, phytoconstituents must be examined for their absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics, pharmacophore mapping, effectiveness, and safety. However, for screening the antimicrobial compounds extracted from various plants against a wide range of Gram-positive as well as Gram-negative bacteria, the most commonly adopted methods are minimum inhibitory concentration (MIC), disk diffusion assay, and colony forming unit (CFU). According to the literature survey, it was found that few compounds were highly active against Gram-negative bacteria while some other compounds showed high activity against the Gram-positive bacteria.
TABLE 1.
Plant active compounds and their significant efficacy against various resistant pathogens.
| Active compound | Plant (common name) | Botanical name | Active against | MIC (μg/ml) | References |
| Hexahydroxy diphenoyl ester vescalagin | Purple loosestrife | Lythrum salicaria L. | Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus | NA NA 62 |
Becker et al., 2005; Guclu et al., 2014 |
| Geraniol and terpinol-4-ol | Sugandhakokila | Cinnamomum glaucescens | MERSA | 369 | Panda et al., 2020 |
| Diosgenin, smilagenin, β-sitosterol and hydroxy-tyrosol | Kumarika | Smilax zeylanica | MERSA | 220 | Panda et al., 2020 |
| Propylene glycol | N/A | Syzygium praecox | MERSA | 1,019 | Panda et al., 2020 |
| Tetradecanal and hexadecanoic aicid | Charcoal tree | Trema orientalis | MDR-S | 369 | Panda et al., 2020 |
| β-Amyrine, aerosolic acid, Betulinic acid | Java cedar | Bischofia javanica | MDR-S | 234 | Panda et al., 2020 |
| Myricitrin, mearnsatin-3-O-β-D gluco-pyrnoside | Ceylon olive | Elaeocarpus serratus | MDR-S | 2,768 | Panda et al., 2020 |
| Methyl tridecanoate, arborinone, conferamide | Climbing acacia | Acacia pennata | MERSA | 369 | Panda et al., 2020 |
| Terpenoids and steroids | Long-leaf varnish tree | Holigarna caustica | MERSA | 369 | Panda et al., 2020 |
| α-pinene, methyl salicylate, β- cyclocitrol | Orange Jessamine | Murraya Pennicula | MERSA | 406 | Panda et al., 2020 |
| Glycosides and saponins | Buddha coconut | Plarygota alata | MDR-S | 550 | Panda et al., 2020 |
| No phytochemical reported till now | Lavender scallops | Kalanchoe fedtschenkoi | Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas areugenosa | 256 | Richwagen et al., 2019 |
| Saponins, polyphenols, tannins, anthrocynins, triterpines | Coastal golden leaf | Bridelia mircrantha | Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas arugenosa, MERSA, | 128 | Ngane, 2019 |
| glucopyranoside | Bichoo | Martynia annua | Klebsiella pneumoniae, Acinetobacter baumannii, Enterrococcus faecalis, Staphylococcus aureus | 256 | Khan et al., 2018 |
| Methanol | Stiff Bottlebrush | Collistemon rigidus | MERSA | N/A | Subramani et al., 2017 |
| Aloe—emodine, coniine and lupeol | Bitter Aloe | Aloe ferox |
Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae |
310 | Ghuman et al., 2016 |
| Ellagic acid | Japanese rose | Rosa rugosa Thunb. | A. baumannii | 250 | Miyasaki et al., 2013 |
| Terchebulin Chebulagic acid Chebulinic acid Corilagin |
Black- or chebulic myrobalan | Terminalia chebula Retz. | A. baumannii | 500 1,000 62.5 1,000 |
Miyasaki et al., 2013 |
| Norwogonin Baicalin Baicalein |
Chinese skullcap | Scutellaria baicalensis Georgi | A. baumannii | 128 NA NA |
Chan et al., 2007; Miyasaki et al., 2013 |
| Eugenol | Clove | Syzygium aromaticum (L.) Merr. & L.M.Perry | A. baumannii | 1,250 | Johny et al., 2010; Pelletier, 2012 |
| Trans-cinnamaldehyde | Cinnamon (Dalchini) | Cinnamomum verum J.Presl | A. baumannii | 310 | Johny et al., 2010; Pelletier, 2012 |
| Carvacro | Oregano | Origanum vulgare L. | Biofilms of S. aureus, A. baumannii |
0.015–0.031% v/v 310 |
Nostro et al., 2007; Johny et al., 2010; Pelletier, 2012 |
| Thymol | Thyme | Thymus adamovicii Velen. | A. baumannii, Staphylococcus epidermidis biofilms | NA 0.031%, v/v 0.031–0.062%, V/V |
Nostro et al., 2007; Johny et al., 2010; Pelletier, 2012 |
| Curcumin | Turmeric | Curcuma longa L. |
S. aureus, A. baumannii |
125–250 4 (while EGCG is present) |
De et al., 2009; Mun et al., 2013; Betts and Wareham, 2014 |
| Epigallocatechin gallate (EGCG) | Green tea | Camellia sinensis (L.) Kuntze |
S. aureus, A. baumannii |
100 312–625 |
Kono et al., 1997; Zhao et al., 2002; Osterburg et al., 2009; Gordon and Wareham, 2010; Betts and Wareham, 2014 |
| Epicatechin | Green tea | C. sinensis | A. baumannii | NA | Betts et al., 2011 |
| Theaflavin | Black tea | C. sinensis | A.baumannii | NA NA |
Betts et al., 2011 |
| (+)-Lyoniresinol-3 alpha-O-beta-D-glucopyranoside | Chinese boxthorn | Lycium chinense Mill. |
A. baumannii, S. aureus, Enterococcus faecalis |
NA NA NA |
Chan et al., 2011 |
| Paeonol | Moutan Peony | Paeonia × suffruticosa Andrews |
A. baumannii, S. aureus, E. faecalis |
NA NA NA |
Chan et al., 2011 |
| Berberine | Coptidis Rhizoma | Coptis chinensis Franch. |
A. baumannii, S. aureus, E. faecalis |
30 | Chan et al., 2011 |
| Berberine | Desert barberry | *Berberis fremontii | S. aureus | NA | Stermitz et al., 2000 |
| Honokiol, Magnolol | Cloudforest mangolia | Magnolia macrophylla var. dealbata (Zucc.) D.L.Johnson |
P. aeruginosa, A. baumannii |
NA | Jacobo-Salcedo et al., 2011 |
| α-Elemene, δ-elemene, furanosesquiterpenes | Myrrh | Commiphora myrrha (Nees) Engl. |
Klebsiella pneumoniae, P. aeruginosa, A. baumannii |
6,250 6,250 2,500 |
Masoud and Gouda, 2012 |
| p-Coumaric acid, ascorbic acid, pyrocatechol, cinnamic acid | Aloe vera | Aloe barbadensis Mill. |
S. aureus, Streptococcus sp., P. aeruginosa, K. pneumoniae |
NA | Lawrence et al., 2009 |
| Allyl methyl disulfide, diallylsulfide, diallyltrisulfide, allyl methyl trisulfide, diallyldisulfide, diallyltetrasulfide | Garlic | Allium sativum L. |
P. aeruginosa, K. pneumoniae, A. baumannii |
3,120 1,250 3,120 |
Khadri et al., 2010 |
| Stigmasterol, nimbiol, sugiol, 4-cymene, α-terpinene, terpinen-4-ol | Neem | Azadirachta indica A.Juss. |
S. aureus, Enterococci, Klebsiella sp., P. aeruginosa |
1,000 500 2,000 1,000 |
Fabry et al., 1998; Nand et al., 2012 |
| Gossypetin, hibiscetin, quercetin, sabdaretin, delphinidin 3-O-sambubioside and cyanidin 3-O-sambubioside | (Indian sorrel/Rose mallow) | *Hibiscus subdarifa Rottb. |
K. pneumoniae, Enterobacter aerogens, Enterobacter cloacae |
1,024 1,024 256 |
Djeussi et al., 2013; Rebelo, 2014 |
| Quercetin-7-0-B-Dxylopyranoside, 7-baueren-3-acetate | Baobab/Gorakh imli | Adansonia digitata L. |
K. pneumoniae E. aerogens E. cloacae |
1,024 1,024 1,024 |
Djeussi et al., 2013 |
*Unresolved name, NA, MIC information is not available.
Antimicrobial peptides
One of the most promising alternatives for the treatment of the ESKAPE-related biofilms are the antimicrobial peptides (AMPs). AMPs are positively-charged peptides generally made up of 10–15 amino acids. They are found in all the living organisms and are involved in the innate immunity (Patil et al., 2021). Generally, AMPs are broad-spectrum and cause the osmotic lysis of bacterial cells by permeabilizing their membranes, due to their amphipathic nature. Since this mechanism of action differs from that of antibiotics, resistance to AMPs can be more difficult to achieve (Panda et al., 2021).
Despite their potential, they rarely reach the market mostly due to problems such as low solubility, cytotoxicity, loss of activity (after administration), and susceptibility to proteolysis (Rodríguez et al., 2021; Sarkar et al., 2021). Synthetic molecular evolution can be exploited to evolve the AMPs that may overcome these limitations (Chen et al., 2022). The pipeline starts from parent peptides characterized by a strong antimicrobial activity on both Gram-positive and Gram-negative bacteria in vitro and, at the same time, ineffectiveness in vivo due to the impediments previously cited. These peptides are modified in order to construct a library of evolved peptides. Then, In vitro assays are performed to down-select candidate molecules based on solubility, cytotoxicity and peptide inactivation, besides their antimicrobial activity. Rational variation follows the down-selection step. As an example of an effective evolved AMP, D-CONGA showed a good activity on the ESKAPE pathogens in the planktonic form. Moreover, this peptide dramatically reduced P. aeruginosa viability within the biofilm in vitro (Starr et al., 2020).
In the context of synthetic AMPs, antimicrobial peptoids are synthetic oligomers that mimic AMPs and are resistant to proteolysis since their backbone is based on nitrogen atoms rather than carbon atoms. Not only their chemical structure is crucial to define the antimicrobial activity, but also their propensity to self-assemble in a physiological environment: among the most interesting therapeutic peptoids, TM peptoids that form bundled or ellipsoidal structures show better antimicrobial and antibiofilm activity against the ESKAPE pathogens compared to the TM peptoids which are not able to properly self-assemble or are characterized by worm-like assemblies. As an example of biologically active TM peptoids, the TM1 peptoid inhibits the growth of planktonic ESKAPE pathogens and affects the formation of their biofilms in vitro (Nielsen et al., 2022).
Bacteriophages and bacteriophage endolysins
Besides AMPs, bacteriophages (phages) and bacteriophage-encoded products can be employed as therapeutics for the treatment of the ESKAPE-related biofilms. Virulent phages are viruses that naturally infect bacterial hosts, eventually causing their lysis. As this interaction is specific, dysbiosis may be prevented. Importantly, they can be used to target antibiotic-resistant strains and are able to eradicate the biofilms of the ESKAPE pathogens. Noteworthy, phages are able to degrade the biofilm matrix (including capsular polysaccharides) via specific enzymes that may promote the access of antibiotics to the deeper regions of the biofilms. Since bacteria can develop resistances against phages, phage cocktails may be used in order to overcome this problem, which also broadens the host range (targeting different strains of the target bacterium). In this context, PA4 is a recently described phage belonging to the Myoviridae family which reduces the biomass of P. aeruginosa biofilms, impairing the viability of the bacterium in vitro (Camens et al., 2021). Similarly, some of the recently characterized vB_SauM kayviruses are able to affect the vitality of S. aureus within the biofilm, reducing the pathogen biomass in vitro (Kaźmierczak et al., 2022).
During the lytic cycle of the bacteriophage within the bacterial host, lysins play a fundamental role, since they digest the peptidoglycan layer of the bacterial cell wall, causing the lysis of the cell and the release of the phage progeny. One of the features of lysins is that they are generally narrow-spectrum, which means that the beneficial bacteria belonging to the patient’s microbiota are not compromised once administered. Not only lysins are active on planktonic bacteria, but also on biofilms. Efforts have been made in recent years specially to target Gram-negative bacteria. Due to the low permeability of the outer membrane of these bacteria, lysins are not able to reach their target. In this context, LysECD7 is a promising recombinant lysin that shows a promising activity on the Gram-negative ESKAPE pathogens, both in the planktonic form and in the biofilm state, even if a significantly higher lysin concentration is needed for a marked effect on the latter; this lysin is able to cross the outer membrane without any delivery system or additives (Vasina et al., 2021).
Interestingly, lysins encoded by phages infecting Gram-negative bacteria may be characterized by an amphipathic region able to permeabilize the outer membrane after administration, allowing the peptidoglycan layer to be affected. These amphipathic components may be isolated and employed as cationic peptides. PaP1 is a peptide derived from the PlyPa01 lysin (isolated from a phage infecting P. aeruginosa) characterized by membrane-acting properties and modified in order to increase the net charge of the peptide, as well as its antibacterial properties. When tested, PaP1 was found active on the ESKAPE pathogens in both monospecies and polymicrobial populations (A. baumannii, P. aeruginosa and S. aureus) and eradicates P. aeruginosa mature biofilms in vitro (Heselpoth et al., 2022).
Essential oils
Natural products can be a source of compounds which are active on both the planktonic and sessile forms of bacteria (Da Silva et al., 2017). Plant-derived EOs are a reservoir of volatile, hydrophobic secondary metabolites which may show a broad antimicrobial and antibiofilm activity against microbial pathogens, including the ESKAPE bacteria (El-Tarabily et al., 2021). The antimicrobial properties of these molecules are due to the fact that medicinal and aromatic plants naturally synthetize them in order to respond to various stresses, including microbial attacks. Noteworthy, EOs have a low potential for the development of microbial resistance due to their complex nature with multiple bioactive compounds, that leads to a multi-target activity which is not in the case of conventional antibiotics (Angane et al., 2022).
The mechanisms of action of these compounds are diverse and may be related to the target of either the bacterial virulence factors or the drug resistance mechanisms that characterize these pathogens (Vasconcelos et al., 2018). Belonging to the first category, these metabolites can inhibit biofilm formation and quorum-sensing while, in the second case, they can inhibit the function of efflux pumps and plasmid-mediated resistance (possibly by causing the loss of the plasmid carrying the resistance gene or by interfering with the transfer of the R-plasmid itself to a recipient cell). In the latter cases, EOs can be used in combination with known antibiotics since they mediate re-sensitization of the bacteria to the drug: in this way, the problem of antibiotic resistance can be addressed. It is important to highlight that these metabolites can also have bactericidal properties since they are lipophilic and can alter the membrane permeability, possibly causing membrane disruption (Álvarez-Martínez et al., 2021). An interesting application of EOs is related to their use in the disinfection of medical devices as well as hospital surfaces, preventing hospital-associated infections. As an example of EOs applications in the treatment of the ESKAPE biofilm, P. aeruginosa biofilm formation can be impaired in vitro by using EOs extracted from Mediterranean plants such as Foeniculum vulgare and Ridolfia segetum (Artini et al., 2018).
Anti-biofilm activities of essential oils and/or components
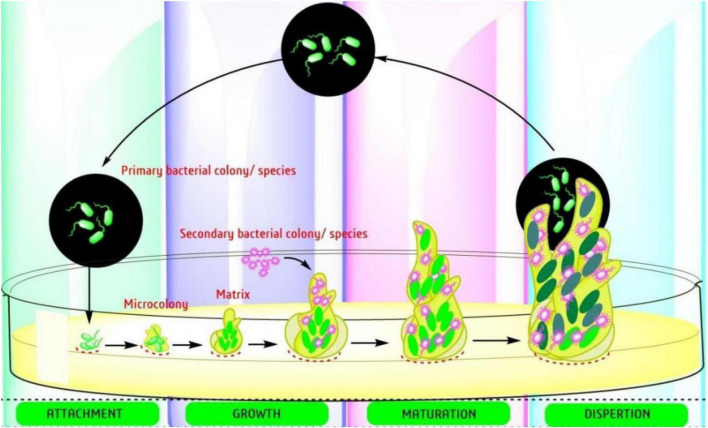
Biofilm-producing bacteria are typically resistant to antimicrobials and there is an urgent need for new approaches to fill the gap (Jouneghani et al., 2020; Sahoo et al., 2021). The formation of biofilm involves a complex mechanism with several targeted factors. There are several reports already published on the steps involved in development of biofilm (adhesion, microcolonies formation, and maturation) (Figure 2) and the targets starting with the adhesion to mature biofilm. The easiest way to prevent the formation is initiated by attachment, which involves the interaction between adhesive substances and receptors on the host surface. Table 2, provides a list of EOs and their components with anti-biofilm activity against ESKAPE pathogens. The list involves complex oils like Cassia, cinnamon, clove, eucalyptol, lavender, lemon, marjoram, orange, oregano, peppermint, Peru balsam, rosemary, tea, and thyme oil. Moreover, several individual components were also reported including carvacrol, cinnamaldehyde, citral, citronellol, eugenol, Linalool, linalyl acetate, menthol, pulegone, thymol, α-terpineol, and terpinen-4-ol (Table 2). Most detailed studies involved the Gram-positive bacteria S. aureus, with genes [icaD/icaA, (intracellular gene)], biofilm-associated protein (bap), and several controlling genetic loci, e.g., sarA luxS, and agr quorum sensing (QS) (Yadav et al., 2015). Beside S. aureus, the Gram-negative bacteria P. aeruginosa is well studied with its mode of action.
FIGURE 2.
Steps involved in the development of bacterial biofilms (Sahoo et al., 2021).
TABLE 2.
Antibacterial activities of essential oils/essential oil components against ESKAPE pathogen.
| Essential oils and constituent(s) | Pathogenic bacteria | MIC values; (references) |
MBIC; (references) |
| Cinnamomum cassia (L.) J.Presl. | P. aeruginosa | –; (Condò et al., 2020) |
0.2%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Coriandrum sativum L. | A. baumannii | 1–4 μl/ml; (Duarte et al., 2013) |
4 μl/ml; (Duarte et al., 2013) |
| Cymbopogon flexuosus (Nees ex Steud.) W.Watson | S. aureus, MRSA | 0.5–4 mg/ml; (Piasecki et al., 2021) |
0.06% v/v; (Adukwu et al., 2012) |
| Cymbopogon nardus (L.) Rendle | S. aureus | – | 0.5 mg/ml; (Pontes et al., 2019) |
| Mentha piperita L. | 28 clinic strains of S. aureus | 64–256 μg/ml; (Li et al., 2011) |
– |
|
Origanum vulgare L. |
S. aureus | – | 10 μl/ml; (Dos Santos Rodrigues et al., 2018) |
| Perilla frutescens L. Britton | S. aureus, MRSA | 0.4 μl/ml; (Qiu et al., 2011) |
– |
| Plectranthus amboinicus (Lour.) Spreng. | S. aureus | 0.25 mg/ml; (Vasconcelos et al., 2017) |
0.5 mg/ml; (Vasconcelos et al., 2017) |
| Syzygium aromaticum (L.) Merr. & L.M.Perry | S. aureus | 85.4 μl/ml; (Kačániová et al., 2021) |
0.106 mg/ml; (Budri et al., 2015) |
| S. aromaticum | P. aeruginosa | 223.3 μl/ml; (Kačániová et al., 2021) |
1.3% v/v; (Kavanaugh and Ribbeck, 2012) |
| Cassia oil | S. aureus | 0.3%, v/v; (Kavanaugh and Ribbeck, 2012) |
0.6%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Cassia oil | P. aeruginosa | 0.2%, v/v; (Kavanaugh and Ribbeck, 2012) |
0.4%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Cinnamon oil | K. pneumoniae, S. aureus | >1.6 mg/ml 3.2 mg/ml; (Prabuseenivasan et al., 2006) |
S. aureus (1 mg/ml) (Cui et al., 2016) |
| Clove oil |
P. aeruginosa; K. pneumoniae and S. aureus |
>1.6 mg/ml; >6.4 mg/ml; (Prabuseenivasan et al., 2006) |
3.2%, sub MIC, biofilm of P. aeruginosa; (Husain et al., 2013) |
| Clove oil | S. aureus | 1.2%, v/v; (Kavanaugh and Ribbeck, 2012) |
1.6%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Lavendor oil | S. aureus | 0.8%, v/v; (Budzyńska et al., 2011) |
1.6%, v/v; (Budzyńska et al., 2011) |
| Lavendor oil | A. baumannii | 10.5–13.0 μl/ml; (Sienkiewicz et al., 2014) |
>5%, v/v (P. aeruginosa); (Kavanaugh and Ribbeck, 2012) |
| Lemon oil |
K. pneumoniae; P. aeruginosa, S. aureus |
>12.8 mg/ml; (Prabuseenivasan et al., 2006) |
K. pneumoniae (170 μl/L); (Sahal et al., 2016) |
| Lemon Balm oil | S. aureus | 0.1%, v/v; (Budzyńska et al., 2011) |
0.4%, v/v; (Budzyńska et al., 2011) |
| Orange oil | K. pneumoniae, P. aeruginosa; S. aureus | ≥12.8 mg/ml >6.4 mg/ml; (Prabuseenivasan et al., 2006) |
– |
| Oregano oil | S. aureus | 0.062%, v/v; (Nostro et al., 2007) | 0.5%, v/v; (Nostro et al., 2007) |
| Peru Balsam oil | S. aureus | 2.5%, v/v; (Kavanaugh and Ribbeck, 2012) |
3.5%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Peru Balsam oil | P. aeruginosa | 2.5%, v/v; (Kavanaugh and Ribbeck, 2012) |
3.5%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Rosemary oil |
K. pneumoniae, P. aeruginosa; S. aureus |
>6.4 mg/ml; >12.8 mg/ml; (Prabuseenivasan et al., 2006) |
– |
| Tea tree oil | S. aureus | 0.4%, v/v; (Budzyńska et al., 2011) |
0.8%, v/v; (Budzyńska et al., 2011) |
| Thyme oil | S. aureus | 0.5%, v/v; (Kavanaugh and Ribbeck, 2012) |
1.6%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Allicin | MSSA and MRSA | 32–64 μg/ml; (Leng et al., 2011) |
80 μg/ml; (Zhang et al., 2022) |
| Carvacrol | S. aureus | 0.35–2.80 mg/ml; (Rosato et al., 2010) |
0.25 mg/ml; (Vasconcelos et al., 2017) |
| Carvacrol | S. aureus | 0.02%, v/v; (Yadav et al., 2015) |
0.04%, v/v; (Yadav et al., 2015) |
| Carvacrol | S. aureus | 0.015%, v/v; (Nostro et al., 2007) |
0.25%, v/v; (Nostro et al., 2007) |
| Carvacrol | S. aureus | 0.3 μl/ml; (Souza et al., 2013) |
200 μl/L; (Espina et al., 2017) |
| Carvacrol | Group A Streptococci | 64–256 μg/ml; (Magi et al., 2015) |
E. faecalis (0.01%, v/v); (Campana and Baffone, 2018) |
| Cinnamaldehyde | P. aeruginosa | 0.1%, v/v; (Kavanaugh and Ribbeck, 2012) |
0.2%, v/v; (Kavanaugh and Ribbeck, 2012) |
| Cinnamaldehyde | P. aeruginosa | – | 11.8 mM; (Topa et al., 2018) |
| Cinnamaldehyde | S. aureus | – | 0.199 mg/ml; (Budri et al., 2015) |
| Citral | S. aureus, MRSA | 0.5 mg/ml; (Porfírio et al., 2017) |
500 μl/L; (Espina et al., 2017) |
| Citronellol | S. aureus | 0.35–1.40 mg/ml; (Rosato et al., 2007) |
0.5 mg/ml; (Piasecki et al., 2021) |
| Curcumin | P. aeruginosa | 62.5 μg/ml; (Adamczak et al., 2020) |
100 μg/ml; (Packiavathy et al., 2014) |
| Eucalyptol | S. aureus | 2.80–5.60 mg/ml; (Rosato et al., 2007) |
256 μg/ml; (Hendry et al., 2009) |
| Eugenol | S. aureus | 0.04%, v/v; (Yadav et al., 2015) |
0.08%, v/v; (Yadav et al., 2015) |
| Eugenol | S. aureus (26 strains) | 128–512 μg/ml; (Qiu et al., 2010) |
0.237 mg/ml; (Budri et al., 2015) |
| Geraniol | S. aureus | 0.08–1.40 mg/ml; (Rosato et al., 2007) |
0.25 mg/ml; (Pontes et al., 2019) |
| Geranium |
K. pneumoniae, P. aeruginosa, S. aureus |
12.8 to >12.8 mg/ml; (Prabuseenivasan et al., 2006) |
– |
| Limonene | MRSA | – | 200 μl/L; (Espina et al., 2015) |
| Linalool | S. aureus | 0.2–2.5 mg/ml; (Sonboli et al., 2005) |
A. baumannii (8 μl/ml); (Alves et al., 2016) |
| Linalool | S. aureus | 0.19%, v/v; (Budzyńska et al., 2011) |
0.78%, v/v; (Budzyńska et al., 2011) |
| Linalyl acetate | S. aureus | 0.19%, v/v; (Budzyńska et al., 2011) |
0.19%, v/v; (Budzyńska et al., 2011) |
| Marjoram | S. aureus | 0.125% v/v; (El-Shenawy et al., 2015) |
Mixed biofilm (0.25 and 20 mg/ml); (Kerekes et al., 2019) |
| Menthol | S. aureus, MRSA | >2 mg/ml; (Qiu et al., 2010) |
0.64–1.98 mg/ml; (Kifer et al., 2016) |
| Peppermint | S. aureus | 0.5% (v/v); (Xiao et al., 2020) |
0.5 mg/ml; (Kang et al., 2019) |
| Pulegone | S. aureus | 3.75 to >15 mg/ml; (Sonboli et al., 2006) |
– |
| Thymol | S. aureus | 0.7–1.40 mg/ml; (Rosato et al., 2007) |
0.5 μl/ml; (Ceylan and Ugur, 2015) |
| Thymol | S. aureus | 0.031%, v/v; (Nostro et al., 2007) |
0.25%, v/v; (Nostro et al., 2007) |
| Triacetin | S. aureus | 22.40 mg/ml; (Rosato et al., 2007) |
– |
| α- terpineol | S. aureus | 0.19%, v/v; (Budzyńska et al., 2011) |
0.38%, v/v; (Budzyńska et al., 2011) |
| Terpinen-4-ol | S. aureus | 0.19%, v/v; (Budzyńska et al., 2011) |
0.19%, v/v; (Budzyńska et al., 2011) |
–, data not available; MIC, minimum inhibitory concentration; MBIC, minimum biofilm inhibitory concentration; MSSA, methicillin susceptible S. aureus; MRSA, methicillin resistant S. aureus.
Essential oil and quorum sensing—The language of bacteria
The two most studied bacteria are S. aureus and P. aeruginosa. Two most common oils, viz. clove oil (Husain et al., 2013) and peppermint oil (Husain et al., 2015), were tested to see their effects in reducing P. aeruginosa QS regulated biofilm formation. Authors observed a strong effect of both oils along with menthol as a constituent in inhibiting biofilm formation (84%), swarming migration (81%), production of virulence factors like LasB elastase activity (80%), protease activity (76%), chitinase activity (78%), and pyocyanin production (85%) (Table 3). A similar observation was also verified with eugenol on two MDR P. aeruginosa. Moreover, the authors found additional virulence factors such as rhamnolipid (57%), and pyoverdine (69%), which are responsible for microbial cell adhesion (Rathinam et al., 2017). Another report is also available by Al-Shabib et al. (2017) on the effect of eugenol to reduce the QS-regulated production of similar virulence factors in P. aeruginosa PAO1. In P. aeruginosa, curcumin (1 μg/ml) inhibits the formation of signaling molecules (Rudrappa and Bais, 2008). Tea oil was also observed to be effective in controlling biofilm formation in S. aureus, with interesting findings by transcriptome analysis as 104 genes downregulated while 200 genes were upregulated. Many of these genes are linked to biofilm formation, e.g., sarA gene (encodes the DNA-binding protein SarA) which is downregulated, and responsible for biofilm (Zhao et al., 2018).
TABLE 3.
Mode of action of select EOs/EOC against ESKAPE pathogen.
| Essential oils | Pathogen | Mode of action | References |
| Chenopodium ambrosioides/α-Terpinene | S. aureus (IS-58) | Inhibition of EPs (tetK) | Limaverde et al., 2017 |
| C. ambrosioides/α-Terpinene | S. aureus (1199B/1199) | Inhibition of EPs (norA) | Salehzadeh et al., 2018 |
| C. verum | K. pneumoniae | Induction of oxidative stress and oxidation/DBM | Yang et al., 2019 |
| C. cyminum | K. pneumoniae | Loss of plasmid integrity | Derakhshan et al., 2008 |
| Dodartia orientalis | S. aureus | DBM | Wang et al., 2017 |
| Dorema aucheri Bioss | P. aeruginosa PAO1 | QS-inhibition, reduction on virulence factors (RVF) (pyoverdine and elastase production), and the transcription of lasI | Sepahi et al., 2015 |
| Ferula asafoetida L. | P. aeruginosa PAO1 | QS-inhibition, RVF (pyocyanin, pyoverdine, elastase production), and the transcription of lasI | Sepahi et al., 2015 |
|
Melaleuca alternifolia (tea tree oil) |
P. aeruginosa NCTC 6749 | DBM and metabolic events | Cox et al., 2001 |
| Melaleuca alternifolia | Methicillin-resistant S. aureus, Carbapenem-resistant K. pneumoniae, A. baumannii, and P. aeruginosa | DBM (structural integrity and membrane permeabilization) | Oliva et al., 2018 |
| Tea tree oil | S. aureus | Cell division inhibition | Reichling et al., 2002 |
| Mentha piperita L. | S. aureus | Inhibition of α-toxin production (ITP) | Li et al., 2011 |
| Mentha piperita L. | P. aeruginosa PAO1 | QS-RVF (elastase, proteases, pyocyanin and chitinase) | Husain et al., 2015 |
| Ocimum gratissimum | P. aeruginosa and S. aureus | Change in membrane permeability (CMP) | Hyldgaard et al., 2012 |
|
Origanum vulgare (oregano oil) |
P. aeruginosa and S. aureus | Bacterial enzyme inhibition and reduce bacterial lipase and coagulase activity | Lambert et al., 2001 |
| Rosmarinus officinalis/1,8-cineol | MDR K. pneumoniae | CMP | Moreno et al., 2015 |
| Rosmarinus officinalis/Eucalyptol | MDR A. baumannii and P. aeruginosa | Inhibition of EPs | Saviuc et al., 2016 |
| Origanum vulgare L. | S. aureus | ITP | De Souza et al., 2010 |
| Perilla frutescens L. Britton | S. aureus, MRSA | ITP | Qiu et al., 2011 |
| Syzygium aromaticum/Eugenol | MRSA | Anti-QS and anti-biofilm | Al-Shabib et al., 2017 |
| Syzygium aromaticum | P. aeruginosa | QS- Inhibit swarming motility | Khan et al., 2009 |
| Thymus vulgaris/thymol | S. aureus | Inhibition of EPs (norA) | Salehzadeh et al., 2018 |
| Essential oil components | |||
| Allicin | MSSA and MRSA | ITP | Leng et al., 2011 |
| Carvacrol | S. aureus | DBM | Di Pasqua et al., 2007 |
| Carvacrol | S. aureus | Inhibit enterotoxin production completely | Souza et al., 2013 |
| Carvacrol | MRSA | Anti-QS and anti-biofilm | Sharifi et al., 2018 |
| Carvacrol | S. aureus | Changes in fatty acid composition | Noumi et al., 2018 |
| Carvacrol | P. aeruginosa | QS-Inhibition (pyocyanin production, biofilm) | Tapia-Rodriguez et al., 2017 |
| Carvone | MRSA | Disruption and separation of the cytoplasmic contents by R-cat | Oosterhaven et al., 1995 |
| Cinamoldehyde | Carbapenem resistant A. baumannii | Anti-biofilm | Mohamed et al., 2018 |
| Cinamoldehyde | P. aeruginosa PA01 | QS-Inhibition 7-fold of the lasR level, and pyocyanin production | Ahmed et al., 2019 |
| Curcumin | P. aeruginosa PA01 | QS-Inhibition 2-fold of elastase activity | Rudrappa and Bais, 2008 |
| Curcumin | P. aeruginosa PA01 | QS-Inhibition (alginate and prodigiosin production) | Packiavathy et al., 2014 |
| Eugenol | S. aureus | Inhibit enterotoxin and α-hemolysin production significantly | Qiu et al., 2010 |
| Farnesol and nerolidol | S. aureus | Cytoplasm disruption (alteration of integrity and permeability, inhibition of cell respiration, K+ leakage) | Cox et al., 2000; Inoue et al., 2004 |
| 6-Gingerol | P. aeruginosa | QS-Inhibition (biofilm formation, 53%), RVF (exoprotease, pyocyanin and rhamnolipid) | Kim et al., 2015 |
| Linalyl acetate | S. aureus | DBM and leakage of intracellular materials | Trombetta et al., 2005; Mahizan et al., 2019 |
| Linalyl anthranilate | Carbapenemase-producing K. pneumoniae | DBM and leakage of intracellular materials | Yang et al., 2021 |
| Menthol | S. aureus | DBM and leakage of intracellular materials | Trombetta et al., 2005; Mahizan et al., 2019 |
| Menthol | S. aureus, MRSA | ITP | Qiu et al., 2010 |
| Menthol | P. aeruginosa PA01 | QS-Inhibition, Anti-biofilm, RVF (protease activity, elastase activity, chitinase activity, pyocyanin production, swarming motility, EPS production) | Husain et al., 2015 |
| β-pinene | E. faecalis | Anti-biofilm | Negreiros et al., 2016 |
| Thymol | S. aureus | DBM and leakage of intracellular materials | Trombetta et al., 2005; Mahizan et al., 2019 |
| Thymol | MRSA | Anti-biofilm and anti-quorum sensing | Sharifi et al., 2018 |
| Thymol | S. aureus; MRSA | Inhibit α-hemolysin, enterotoxin production | Qiu et al., 2011 |
| α- and γ-terpinene | S. aureus | DBM | Mahizan et al., 2019 |
| Terpinene-4-ol | MRSA | Anti-QS and anti-biofilm | Perez et al., 2019 |
| Terpinene-4-ol | S. aureus | Formation of multilamellar, mesosome-like structures | Carson et al., 2002; Reichling et al., 2002 |
| Zingerone | P. aeruginosa PA01 | QS-Inhibit protease production | Kumar et al., 2015 |
CMP, change in membrane permeability; DBM, disruption of bacterial membrane; Eps, extracellular polymeric substances; ITP, inhibition of α-toxin production; QS, quorum sensing.
Eugenol is also very effective in reducing the formation of biofilm in methicillin-resistant S. aureus (MRSA) (MIC: 0.04%) (Yadav et al., 2015). With the help of RT-qPCR tests, it was confirmed that eugenol (0.5 × MIC) reduced sarA, seA and icaD expression. Further, the gene expression studies showed why eugenol is strong enough to reduce the biofilm since these genes are related to biofilm e.g. regulatory gene (sarA), enterotoxin gene (seA), and adhesion gene (icaD) (Yadav et al., 2015). The authors also studied the effects of eugenol in in vivo experiments using an otitis media rat model. Eugenol (0.02%) bears a significant reduction of the bacterial colonization without any biofilms in comparison to the control tympanic bulla which was filled with cell debris and biofilm (Yadav et al., 2015). Another study also evidenced a significant reduction of biofilm in MRSA at sub-MIC concentration (Al-Shabib et al., 2017).
Several monoterpenes (citral, MIC = 500 μl/L; carvacrol, MIC = 200 μl/L; (+)-limonene, MIC = 5,000 μl/L), also showed reduction of biofilm formation in S. aureus at sub-MIC concentration. Among them, carvacrol is reported to be highly potent with the lowest concentration of 10 μl/L up to 80% reduction (Espina et al., 2015). In P. aeruginosa, cinnamaldehyde is able to reduce the production of AHLs probably due to hydrophobic interaction and strong bonding between cinnamaldehyde and LasI (Chang et al., 2014).
Eradication of established biofilms
Most of the biofilm studies are performed using spectrophotometer for absorption analysis performed using biofilm specific dyes. These assays indicate only the inhibition of biofilms and do not indicate the capability of the compound to eradicate an already established biofilm. All ESKAPE pathogens are biofilm-associated and, due to their intrinsic resistance, they are difficult to treat. Table 2 shows a list of selected essential oils/essential oil components and their activity against ESKAPE pathogens in both planktonic (MIC values) and biofilm cells (Minimum Biofilm Inhibitory Concentration, MBIC). Most studies are found to be effective against S. aureus followed by P. aeruginosa while limited studies have been performed on A. baumannii and K. pneumoniae. In most cases, it is observed that the MIC value is not sufficient to inhibit biofilm, while in few cases even 100 fold could is not sufficient to treat established biofilm. Coelho and Pereira (2013) tested the three most common oils, e.g., cinnamon, tea, and palm rosa on established biofilm of P. aeruginosa. The authors noticed a sufficient reduction of the bacterial population (5 log10 to 2.5 log10 CFU/cm2) and compared it with the standard antibiotic ciprofloxacin (Coelho and Pereira, 2013). Another interesting study by Lu et al. (2018) established the removal of preformed biofilm against both S. aureus and P. aeruginosa biofilms when treated with the oregano oil (carvacrol as a major constituent) (Lu et al., 2018). The experiment was further validated by an in vivo mouse model (third-degree burn wound infection) treated with the oregano oil (10 mg/ml for 3 days), resulting in a reduction of the 3 log10 steps population (Lu et al., 2018). Moreover, authors also detected changes in the cell structure, with disruption of biofilm under electron microscopy. This kind of study is highly valuable as it shows the direct effect of EOs/EOC and can distinguish the destruction or eradication of established biofilm.
Bactericidal toxic effects of essential oils and essential oil components on biofilms
Only limited oils/components were reported to be bactericidal. Eugenol is one of the major components that act very effectively at higher concentrations, at 12.8% (v/v), with 91.6% on the inhibitions of biofilms formed by MDR S. aureus. In comparison to eugenol, thyme oil also showed similar antibiofilm activity. The highest biofilm reduction (88% inhibition of S. aureus JSA10) was observed at 12.8% v/v (Jafri et al., 2014). Both S. aureus and P. aeruginosa were inhibited significantly when treated with cinnamaldehyde, a major constituent of Cinnamomum zeylanicum and Cinnamomum cassia. An interesting observation made by the authors is that the inhibition is not due to its anti-QS effect, but to its cytotoxic effects (Firmino et al., 2018).
Synergistic potentials of essential oils/essential oil components along with antibiotics against multidrug-resistant ESKAPE pathogens
In the past decade, there has been an increase in the research on the synergetic potentials of clinical antibiotics in combination with essential oils or individual components. We have reviewed various publications on the ESKAPE pathogens and summarized (Table 4) the synergistic effects of essential oils and antibiotics.
TABLE 4.
Synergistic effects of Essential oils and antibiotics against ESKAPE pathogens.
| EOs/EOC | Antibiotics | Bacterial strains | Method(s) | Outcome | Mechanism of action | References |
| Artemisia herba-alba Asso | Chloramphenicol | E. aerogenes EA27 | Microdilution method (MDM) | 4-fold reduction in MIC | Alteration of outer membrane (OM), lipopolysaccharide structure (LPS) | Fadli et al., 2016 |
| Aniba rosaeodora | Gentamicin | A. baumannii | Checkerboard assay (CBA) | FICI = 0.11 | – | Rosato et al., 2007 |
| Cymbopogon citratus (DC.) Stap | Chloramphenicol | E. aerogenes EA27 | MDM | 4-fold reduction in MIC | Alteration of OM-LPS | Fadli et al., 2016 |
| Cinnamomum zeylanicum | Amikacin | Acinetobacter sp., | CBA | Additive effect | – | Guerra et al., 2012 |
| Coriandrum sativum | Chloramphenicol, Ciprofloxacin, Gentamicin |
A. baumannii | CBA | FICI = 0.047 to 0.375 | – | Duarte et al., 2012 |
| Croton zehntneri Pax & K.Hoffm | Norfloxacin | S. aureus SA 1199B | Change in inhibition zone by EO gaseous contact (IZGC) | IZGC = 39.5% | Inhibition of efflux pumps activity/expression (IEPA/E) | Coutinho et al., 2010 |
| Helichrysum italicum (Roth) G.Don | Chloramphenicol | E. aerogenes EA27 | MDM | 8-fold reduction in MIC | IEPA/E | Lorenzi et al., 2009 |
| Helichrysum italicum (Roth) G.Don | Chloramphenicol | A. baumannii AP1 | MDM | 8-fold reduction in MIC | IEPA/E | Lorenzi et al., 2009 |
| Lippia microphylla Cham. | Norfloxacin | S. aureus SA 1199B | IZGC | IZGC = 39.5% | IEPA/E | Coutinho et al., 2011 |
| Myrtus communis | Ciprofloxacine, polymixin B | A. baumannii | CBA | FICI = 0.047–0.375 | – | Aleksic et al., 2014 |
| Origanum vulgare L | Tetracycline | S. aureus I-58 | MDM | 4-fold reduction in MIC | IEPA/E | Cirino et al., 2014 |
| Pelargonium graveolens | Gentamicin | A. baumannii, S. aureus | CBA | FICI = 0.11 | – | Rosato et al., 2007 |
| P. graveolens | Norfloxacin | S. aureus | CBA | FICI = 0.11 | – | Rosato et al., 2007 |
| Thymus broussonetii Bois | Cefixime | MDR P. aeruginosa | CBA, Cell lysis assay (CLA) | FICI = 0.5 | Disruption of bacterial membrane (DBM) | Fadli et al., 2012 |
| T. broussonetii | Cefixime | MDR S. aureus | CBA; CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. broussonetii | Ciprofloxacin | MDR P. aeruginosa | CBA, CLA | FICI = 0.14 | DBM | Fadli et al., 2012 |
| T. broussonetii | Ciprofloxacin | MDR S. aureus | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. broussonetii | Gentamycin | MDR E. clocae | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. broussonetii | Gentamycin | MDR P. aeruginosa | CBA, CLA | FICI = 0.28 | DBM | Fadli et al., 2012 |
| T. broussonetii | Gentamycin | MDR S. aureus | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. broussonetii | Pristinamycin | MDR K. pneumoniae | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. broussonetii | Pristinamycin | MDR S. aureus | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| Thymus maroccanus Ball | Chloramphenicol | A. baumannii AP1 | MDM | 8 to 16 -fold reduction in MIC | Alteration of OM- LPS | Fadli et al., 2011 |
| T. maroccanus | Chloramphenicol | P. aeruginosa PA124 | MDM | 4 to 8 -fold reduction in MIC | IEPA/E | Fadli et al., 2011 |
| Thymus riatarum Humbert & Maire | Chloramphenicol | E. aerogenes EA27 | MDM | 16 -fold reduction in MIC | IEPA/E | Fadli et al., 2011 |
| T.riatarum | Ciprofloxacin | MDR E. clocae | CBA, CLA | FICI = 0.37 | DBM | Fadli et al., 2012 |
| T. riatarum | Ciprofloxacin | MDR K. penumoniae | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. riatarum | Ciprofloxacin | MDR P, aeruginosa | CBA, CLA | FICI = 0.15 | DBM | Fadli et al., 2012 |
| T. riatarum | Ciprofloxacin | MDR S. aureus | CBA, CLA | FICI = 0.26 | DBM | Fadli et al., 2012 |
| T. riatarum | Gentamycin | MDR E. clocae | CBA, CLA | FICI = 0.19 | DBM | Fadli et al., 2012 |
| T. riatarum | Gentamycin | MDR K. penumoniae | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. riatarum | Gentamycin | MDR K. P. aeruginosa | CBA, CLA | FICI = 0.18 | DBM | Fadli et al., 2012 |
| T. riatarum | Gentamycin | MDR S. aureus | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. riatarum | Pristinamycin | MDR E. cloacae | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| T. riatarum | Pristinamycin | MDR S. aureus | CBA, CLA | FICI = 0.5 | DBM | Fadli et al., 2012 |
| Zataria multiflora | Vancomycin | S. aureus | CBA | FICI = 0.32 | – | Mahboubi and Bidgoli, 2010 |
| Carvacrol | Ampicillin | S. aureus | CBA | FICI < 0.3 | – | Palaniappan and Holley, 2010 |
| Carvacrol | Bacitracin | S. aureus | CBA | FICI < 0.3 | – | Palaniappan and Holley, 2010 |
| Carvacrol | Nitrofurantoin | K. oxytoca | CBA | FICI ≤ 0.5 | – | Zhang et al., 2011 |
| Carvacrol | Ciprofloxacin | S. aureus | MDM, CLA | 8-fold reduction in MIC | DBM | Fadli et al., 2012 |
| Carvacrol | Ciprofloxacin | K. pneumoniae | MDM, CLA | 4-fold reduction in MIC | DBM | Fadli et al., 2012 |
| Carvacrol | Ciprofloxacin | E. cloacae | MDM, CLA | 4-fold reduction in MIC | DBM | Fadli et al., 2012 |
| Carvacrol | Tetracycline | S. aureus I-58 | MDM | 2-fold reduction in MIC | IEPA/E | Cirino et al., 2014 |
| Eugenol | Vancomycin, β-lactams | E. aerogenes, P. aeruginosa | MDM, CBA | – | DBM | Hemaiswarya and Doble, 2009 |
| Geraniol | Chloramphenicol | E. aerogenes EA27 | MDM | 256-fold reduction in MIC | IEPA/E | Lorenzi et al., 2009 |
| Thymol | Tetracycline | S. aureus I-58 | MDM | 2-fold reduction in MIC | IEPA/E | Cirino et al., 2014 |
CBA, checkerboard assay; CLA, cell lysis assay; IZGC, change in inhibition zone by EO gaseous contact; OM, outer membrane, LPS, lipopolysaccharide structure; IEPA/E, inhibition of efflux pumps activity/expression; DBM, disruption of bacterial membrane.
In combination with chloramphenicol, several essential oils viz. Artemisia herba-alba, Cymbopogon citratus, Helichrysum italicum, and Thymus riatarum showed synergistic effects against Enterococcus aerogenes EA27 (overexpressing the AcrAB-TolC efflux system) (Lorenzi et al., 2009; Fadli et al., 2014, 2016). Moreover, available literature suggests that H. italicum and Thymus maroccanus act synergetically against A. baumannii AP1 (reduced OprD expression) (Lorenzi et al., 2009; Fadli et al., 2011). Geraniol is the major constituent in most of these oils which in combination with chloramphenicol showed synergistic effects E. aerogenes EA27 with 256-fold reduction in MIC (Lorenzi et al., 2009). Authors also concluded that the mechanism of action is due to inhibition of efflux pumps activity/expression (AcrAB-TolC efflux system) (Table 3). Similarly, EOs- Thymus broussonetii and Thymus riatarum, showed synergistic effects when combined with ciprofloxacin, against MDR P. aeruginosa, MDR S. aureus and MDR K. penumoniae Fractional inhibitory Concentration Index (FICI < 0.5) (Fadli et al., 2012). The major constituents among both essential oils are carvacrol which was also observed to be in synergism with ciprofloxacin showing 8-fold reduction in MIC. The mechanism of action is due to the disruption of the bacterial membrane (Fadli et al., 2012). Moreover, carvacrol has also been observed to be synergistic with other antibiotics, such as nitrofurantoin against Klebsiella oxytoca (Zhang et al., 2011), and tetracycline against MDR S. aureus (Cirino et al., 2014; Table 3). Gentamicin is a commonly used antibacterial agent for synergy studies in several MDR bacteria, such as A. baumannii, S. aureus, E. clocae, P. aeruginosa, and K. penumoniae. Gentamicin in combination with EOs such as Pelargonium graveolens, T. broussonetii, and T. riatarum showed synergism by action on disruption of bacterial membrane (FICI < 0.5) (Fadli et al., 2012). Fadli et al. (2012), also studied the same essential oils in combination with the antibiotic Pristinamycin against S. aureus, E. clocae, P. aeruginosa, and K. penumoniae and recorded synergistic effects (FICI = 0.5). Other major components include thymol and eugenol, whose synergistic effects were tested in combination with tetracycline (Cirino et al., 2014) and vancomycin (Hemaiswarya and Doble, 2009), respectively.
Most studies focused on bacteria S. aureus and P. aeruginosa while few studies involved A. baumannii, Enterobacter, and Klebsiella. Most essential oils are complex in nature, but few studies also followed up on the major components, e.g., carvacrol, eugenol, geraniol, and thymol. Owing to the complexity, it is important to know the mechanism of action that is responsible for synergistic effects. Table 3 describes the mechanism of actions in which mostly the disruption of bacterial membrane and inhibition of efflux pump activity/expression was observed to be the most common mode. Few studies also provide insights into the changes occuring in lipopolysaccharide structure and alteration of the outer membrane. Due to a complex mixture of components, most EOs exhibit antibacterial activities against both Gram-positive and Gram-negative pathogens. Moreover, the target bacteria and mode of action are different, as a result, the chances of resistance probability are less (Rai et al., 2017; Subramani et al., 2017). In most cases, the mode of mechanism acts as a membrane disruption, and as a result, leakage of cell content as well as coagulation of the cytoplasm. Moreover, other mechanisms involve their ability to inhibit the bacterial efflux pumps, metabolic pathways, antibiofilm, and anti-quorum sensing activity (Table 4). Moreover, due to the multiple bioactivities, such as antioxidant, anti-inflammatory, and wound healing properties, additional benefits may be observed during the treatment of ESKAPE pathogens (Subramani et al., 2017).
In vivo analysis of antimicrobial properties of essential oils against ESKAPE pathogens
Biofilm-forming bacteria are commonly involved in infection of skin wounds impairing the reparative process due to prolongation of inflammatory phase (Macedo et al., 2021). Thus, the microbial infection can lead to chronic wound development (Rahim et al., 2016). In this section we discuss the application of EOs and EOCs in the treatment of wound infections provoked by biofilm-forming bacteria (Table 5) (A. baumannii, P. aeruginosa, and S. aureus).
TABLE 5.
In vivo antimicrobial properties of essential oils against ESKAPE pathogens.
| Bacteria | Essential oil/essential oil compound | Main compounds | Formulation | Model | References |
| A. baumannii | Pimenta dioica (L.) Merr. (Myrtaceae) | β-Myrcene (44.1%), 1,8-cineol (18.8%), Limonene (11.7%), Eugenol (8.6%) | EO was dissolved in sweet almond oil. | Excisional wounds in mice. | Ismail et al., 2020 |
| Pimenta racemose (Mill.) J.W.Moore (Myrtaceae) | β-Myrcene (39.6%), Eugenol (31%), Limonene (15.5%) | EO was dissolved in sweet almond oil. | Excisional wounds in mice. | Ismail et al., 2020 | |
| Eugenol | – | Eugenol was dissolved in sweet almond oil. | Excisional wounds in mice. | Ismail et al., 2020 | |
| Origanum vulgare (Lamiaceae) | α-Thujene, α-pinene, octanone, terpinene, p-cymene, carvacrol and thymol | – | Excisional wounds in Wistar rats. | Amini et al., 2019 | |
| P. aeruginosa | Cinnamomum zeylanicum Blume (Lauraceae) | Cinnamaldehyde (the main constituent), eugenol, coumarin, and O-methoxycinnamaldehyde. | Nanostructured lipid carrier gel containing the EO at 2% or 4% | Third-degree burn in male Sprague-Dawley rats. | Wen et al., 2021 |
| Cinnamaldehyde | – | – | Excisional wounds in female Swiss mice | Ferro et al., 2019 | |
| S. aureus | Anethum graveolens (Apiaceae) | α-Phellandrene (47.3%), p-cymene (18.5%) and carvone (14.1%) | Ointments containing the EO at 2 or 4% | Excisional wounds in BALB/c mice | |
| Cymbopogon citratus (Poaceae) | Citral (38.66%), Myrcene (13.78%), Nerol (2.90%) | Carbopol gel with the EO 1% | Excisional wounds in Wistar rats | Oliveira et al., 2019 | |
| Oliveria decumbens (Apiaceae) | Thymol (50.1%), γ -terpinene (20.7%), and p-cymene (17.6%) | Cream formulation containing O. decumbens and P. graveolent | Excisional wounds in BALB/c mice | Mahboubi et al., 2016 | |
| Pelargonium graveolent (Geraniaceae) | β-Citronellol (39.3%) and geraniol (23.6%) | ||||
| Trachyspermum ammi (L.) Sprague (Apiaceae). | Cymene (ρ) (36.64%), Terpinene (γ-) (35.98%), Thymol (18.14%) | Core-shell electrospun nanofibers containing the EO. | Excisional wounds in Sprague-Dawley male rats | Zare et al., 2021 | |
| S. aureus and P. aeruginosa | T.carum carvi L. (Apiaceae) | Carvone (56.9%), Limonene (36.1%) | EO encapsulated in nanostructured lipid carriers | Excisional wounds in BALB/c mice | Tazehjani et al., 2021 |
| Cinnamomum verum J.Presl (Lauraceae) | Cinnamic aldehyde (54.1%), α-copaene (12.3%), and styrene benzebe, ethenyle (7%) | Ointments containing the EO at 2 and 4%. | Excisional wounds in BALB/c mice | Seyed Ahmadi et al., 2019 | |
| Mentha × piperita L. (Lamiaceae): | Menthol (39.80%), mentone (19.55%), neomenthol (8.82%) | Ointments containing the EO at 2, 4, or 8% | Excisional wounds in BALB/c mice | Modarresi et al., 2019 | |
| EO encapsulated in nanostructured lipid carriers | Excisional wounds in BALB/c mice | Ghodrati et al., 2019 | |||
| Mentha pulegium L. (Lamiaceae) | Pulegone (72.18%), piperitenone (24.04%) | EO encapsulated in nanostructured lipid carriers | Excisional wounds in BALB/c mice | Khezri et al., 2019 | |
| Rosmarinus officinalis L. (Lamiaceae) | 1,8-Cineole and α-Pinene. | nanostructured lipid carrier gel containing the EO at 2% and 4%. | Excisional wounds in BALB/c mice | Khezri et al., 2019 | |
| Salvia officinalis L. (Lamiaceae) | cis-Thujone (26.8%), camphor (16.4%), trans-thujone (14.1%) and 1,8-cineole (10.8%) | Ointments containing the EO at 2 and 4%. | Excisional wounds in BALB/c mice | Farahpour et al., 2020 | |
| Satureja sahendica Bornm (Lamiaceae) | Carvacrol, thymol, γ-terpinene, p-cymene | Ointments containing the EO at 1, 2, and 4%. | Excisional wounds in BALB/c mice | Omarizadeh et al., 2021 | |
| Zataria multiflora Boiss (Lamiaceae) | Thymol (52.90%), p-cymene (9.10), γ-terpinene (8.10%) and carvacrol (6.80%) | Ointments containing the EO. | Excisional wounds in BALB/c mice | Farahpour et al., 2020 |
Acinetobacter baumannii
Some studies have examined the antimicrobial effects of EOs against A. baumannii using mammalian models. One study showed the evaluation of the EO extracted from Origanum vulgare (Lamiaceae) in A. baumannii-infected wounds in Wistar rats. The EO enhanced the healing and reduced the growth in tissue and wound secretions. The EO is mainly composed of alpha thujene, alpha-pinene, octanone, terpinene, p-cymene, carvacrol, and thymol (Amini et al., 2019).
Other research examined the in vitro and in vivo antimicrobial effects of EOs from Pimenta dioica (L.) Merr. and Pimenta racemose (Mill.) J.W.Moore (Myrtaceae) against A. baumannii (Premachandran and Murthy, 2022). The EOs showed in vitro antimicrobial and antibiofilm actions against a range of A. baumannii strains. The authors selected the leaf oils for in vivo assay using male mice. The excisional wounds were infected by two successive additions of 10 μl of A. baumannii suspensions (107 and 108 CFU/ml). The animals were treated with the EOs and eugenol dissolved in sweet almond oil (5.2 and 2 μg/ml). Interestingly, the administration of Pimenta EOs had the highest action than eugenol. The authors did not report data about the wound repair (Ismail et al., 2020).
Pseudomonas aeruginosa
Trans-cinnamaldehyde is an EOC with reported antivirulence and antimicrobial action against P. aeruginosa. The in vivo antimicrobial effects of Trans-cinnamaldehyde were analyzed in wounds contaminated by P. aeruginosa (30 μl of 1.5 × 108 CFU/ml). Female Swiss mice (4 months old) were treated by the EOC solution in Dimethylsulfoxide (DMSO). The daily administration of trans-cinnamaldehyde modulated the host inflammatory response and inhibited the bacterial growth. Interestingly, the authors reported that the EOC actions were mediated by Transient Receptor Potential Ankyrin 1 (TRPA1) (Ferro et al., 2019).
The barks of Cinnamomum zeylanicum (Lauraceae) bear an EO mainly composed of Cinnamaldehyde, eugenol, coumarin, and O-methoxycinnamaldehyde (Alizadeh Behbahani et al., 2020). Wen et al. (2021) developed and characterized topical nanostructured lipid carrier (NLC) gel loaded with C. zeylanicum bark EO using thermosensitive Poloxamer 407 as a gelling agent. The effectiveness of this formulation was evaluated in a model of third-degree burn in male Sprague-Dawley rats. Each wound received an injection of P. aeruginosa suspension (∼7.5 × 105 CFU/ml). The daily administration inhibited the growth of P. aeruginosa at the wound tissue and enhanced the skin repair (Wen et al., 2021).
Staphylococcus aureus
The EOs from Oliveria Decumbens (Apiaceae) and Pelargonium Graveolens (Geraniaceae) have been reported as effective against these bacteria (Mahboubi et al., 2011). These EOs were incorporated in a topical ointment and the antimicrobial effects were evaluated on MRSA-infected wounds in male Balb/c mice. In this model, the infection was established by the application of sutures containing MRSA strain. After 6 hour, the treatment was initiated and was provided three times a day. The treatment with the herbal formulation containing both EO reduced the bacterial load in the wound, with values similar to those observed in the positive control (mupirocin ointment). In addition, the EOs-incorporated ointment enhanced the healing process by collagen deposition. The major compounds detected in the EOs were thymol and β-citronellol for O. decumbens and P. graveolent, respectively (Mahboubi et al., 2016).
Anethum graveolens L. (Apiaceae) is popularly known as dill and its essential oil (DEO) is reported as an antimicrobial agent (Singh et al., 2005). The effects of ointments (90%, 5% hard paraffin and 5%) containing DEO at 2 or 4% were evaluated in excisional wounds infected by MRSA in male BALB/c mice (nine-weeks-old). The wounds were infected with 50 μl of MRSA suspension (5 × 107CFU/wound) and treated daily with the ointment. The topical administration of DEO-ointments promoted the wound contraction and reduced the bacterial burden. Histological analysis revealed that these effects were associated with the improvement of re-epithelialization, angiogenesis and collagen deposition. The animals treated with DEO-ointments also showed higher expressions of Bcl-2, p53, caspase-3, VEGF, and FGF-2 in comparison to the untreated mice. The major compounds of DEO were α-phellandrene, p-cymene and carvone (Manzuoerh et al., 2019).
The EO from Cymbopogon citratus is another example of EO which reported in vivo anti-S. aureus activity. In this case, the EO was incorporated in carbopol at 1% and the formulation was applied in S. aureus-infected wounds (0.5 × 108 CFU/wound) performed in male Wistar mice (3–4 months). The EO used is mainly composed of Citral (38.66%), Myrcene (13.78%), and Nerol (2.90%). The treatment with C. citratus EO reduced the bacterial load with the same effectiveness as vancomycin (Oliveira et al., 2019).
Zare et al. (2021) developed Core-shell electrospun nanofibers containing the EO from Trachyspermum ammi (L.) Sprague (Apiaceae) and evaluated them in wounds contaminated by S. aureus. In this case, the author used Sprague-Dawley male rats (7–8 weeks old) and the wounds were infected with 1,000 μl of S. aureus suspension (1–1.5 × 108 CFU/ml). The major compounds detected in the EO were Cymene (ρ) (36.64%), Terpinene (γ) (35.98%), Thymol (18.14%). The formulation showed high efficacy in reducing the growth of S. aureus in the wounds and promoted the healing process (Zare et al., 2021).
Staphylococcus aureus and Pseudomonas aeruginosa
Mentha × piperita L. (Lamiaceae) is source of an EO (known as PEO) with antibiofilm and antimicrobial actions against S. aureus and P. aeruginosa (Husain et al., 2015; Chraibi et al., 2021). The in vivo antimicrobial effects of ointments containing the PEO at 2, 4 and 8% were investigated. In this case, wounds in male BALB/c mice (12–14 weeks old) were infected by 25 × 107 units of S. aureus and P. aeruginosa. The topical treatment with PEO-containing ointments reduced the bacterial load and the expression of some inflammatory mediators (TNF-α, VEGF and FGF-2), while the genes for CCL2, CXCL1, IL-1β, TGF-β1, and IL-10 were upregulated. On the other hand, the PEO-treated wounds showed faster wound contraction than control animals, due to the increase in fibroblasts migration and collagen synthesis (Modarresi et al., 2019). PEO loaded in nanostructured lipid carriers also exhibited efficacy in this model (Ghodrati et al., 2019).
Similarly, the antimicrobial effects of ointments prepared with Cinnamon verum essential oil (2 and 4%) were tested in wounds infected by S. aureus and P. aeruginosa. The ointments were prepared using soft yellow paraffin. The excisional wounds were contaminated with 107 CFU of each bacterium. C. verum EO-containing ointment accelerated the tissue repair by inhibiting the inflammation and increasing the collagen deposition, keratin synthesis and the expression of key genes (IGF-1, FGF-2, and VEGF expression). The animals treated with C. verum EO exhibited higher antioxidant power. The major compounds presented in the used EO were Cinnamic aldehyde (54.1%), α-copaene (12.3%), and styrene benzebe, ethenyle (7%) (Seyed Ahmadi et al., 2019).
Other EOs that were efficient for the treatment of wounds with mixed infections by S. aureus and P. aeruginosa including those obtained from Carum carvi L. (Apiaceae) (Tazehjani et al., 2021), Mentha pulegium L. (Lamiaceae) (Khezri et al., 2019), Rosmarinus officinalis L. (Lamiaceae) (Khezri et al., 2019), Salvia officinalis L. (Lamiaceae) (Farahpour et al., 2020), Satureja sahendica Bornm (Lamiaceae) (Omarizadeh et al., 2021), Zataria multiflora Boiss (Lamiaceae) (Farahpour et al., 2020).
Mechanism of resistance, safety and application of essential oils
The present review deals with the recent development of EOs/EOCs targeting biofilm amongst ESKAPE pathogens. Table 3 has elaborated the mode of action of selected EOs/EOCs but the gathered information is not sufficient and still, several efforts needed further studies on the mechanism of actions of individual EOs for a better understanding of the EO’s anti-biofilm mechanisms. In general majority of EO’s mechanisms of action to inhibit the formation of biofilm as well as to eradicate matured biofilm involves the action of EOs by inhibiting QS mechanisms, as well as interacting with the EPS matrix. Words and phrases like “all-natural” are safe, not correct that includes diffusing essential oils. Like other plant compounds safety of essential oils also depends largely on dosage. Many of the reported essential oils are reported to have allergic reactions to the skin as well as “hormone-related health complications” https://www.cnet.com/health/are-essential-oils-actually-safe/. Lu et al. (2018) studied the efficacy of oregano oil against several isolated bacteria with multidrug resistance as well as in in vivo burn infection model (P. aeruginosa PA01 and S. aureus USA 300). An interesting observation was found that MDR strains could not regain resistance even up to 20 passages when tested at sub-lethal concentrations. The effect of oregano oils on the skin of mice is prominent without any side effects (skin histologically or genotoxicity). Another experiment on mice model infected with S. aureus (ATCC #14775), aids in the survival of one-third treated population for thirty days compared to the control (all dead within 7 days) when treated with Origanum oil (Preuss et al., 2005). Yang et al. (2019), studied effects of Artemisia vestita oil on mice model infected with Streptococcus pyogenes and observed significant improvement on respiratory function of lungs as well as biochemical parameters of blood without any noticeable toxicity. Table 5, provides details of several in vivo studies infected with ESKAPE pathogens and most of these essential oils are effective without any side effects (Amini et al., 2019; Ferro et al., 2019; Oliveira et al., 2019; Tazehjani et al., 2021).
On the other hand, EOs are used for human kinds since ancient times. Many of the EOs are widely used in food and cosmetic as well as pharmaceutical industries without any toxicity. Indeed, it is necessary to study complete toxicity so as to make the best use of beneficial effects. The prime rewards of essential oil are its vast collection of aromatic plants (≈3000, of which 300) are known to be safe for humans by the U.S. FDA (Lu et al., 2018). Most of these essential oils are reported to possess antioxidant and antimicrobial activity, so as to consider green antimicrobials (economically low cost in crude form, biocompatible, less toxic without any harm to the environment and most importantly less resistance toward antibiotics). All these characteristics perfectly suit for an alternative effective solution for tackling antimicrobial resistance for ESKAPE pathogens. Moreover, certain limitation still needs to be addressed and more research needs to address including the “stability, selectivity, bio-availability, biocompatibility or any possible non-target or toxic effects on the human body or any type of allergy” (Yu et al., 2020).
Conclusions, challenges, and future perspectives
Compared to the planktonic form of the ESKAPE bacteria, those found within biofilms are estimated to be up to 1,000 times less sensitive to antimicrobials. To make this scenario worse, antibiotic resistance characterizing these pathogens is an ever-growing global threat. In order to address these problems, several strategies are being explored all over the globe especially to eradicate the biofilms of these bacteria. Some strategies include antimicrobial peptides and peptoids, bacteriophages and bacteriophage-encoded products such as endolysins, compounds able to impair biofilm formation (inhibiting either biofilm structuration or the quorum-sensing mechanisms regulating the formation of the biofilm) and immunotherapies (both active and passive), also drug reuse/resensitization and drug repurposing. Despite the potential of these strategies, the most important challenge that must be faced is the possible development of bacterial antibiotic resistance. This problem may be addressed by combining these strategies among each other, as well as with conventional antibiotics. For instance, one of the most promising alternative approaches is the use of EOs since their components naturally possess antimicrobial and antibiofilm activity. EOs can mediate resensitization of the bacteria to currently available antimicrobials by inhibiting antibiotic resistance mechanisms; another example is connected to the capability of phages to disrupt the matrix of the biofilm, making it permeable to antibiotics. New combinations of different approaches must be investigated as well, in order to broaden the therapeutic options for eradication of ESKAPE-biofilm mediated infections.
Author contributions
SP and VT: conceptualization and design and proofread of the final version. SP, SS, AB, EF, MK, LS, and VT: data curation. SP, LS, and VT: data analysis. SP, SB, SS, AB, EF, MK, LS, and VT: writing the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
SP was thankful to the RUSA 2.0 for supporting Centre of Excellence in Environment, Climate Change and Public Health (ECCPH), Utkal University, India.
Funding Statement
SB was funded by the Italian Ministry of Education, University and Research (MIUR) (Dipartimenti di Eccellenza, Program 2018–2022) to Department of Biology and Biotechnology, “L. Spallanzani”, University of Pavia (SB) and by the FWO Biofilm community (W000921N). EF receives a scholarship from Higher Education Personnel Improvement Coordination (CAPES-Brazil) and LS has research productivity grant from the National Council for Scientific and Technological Development (CNPq–Brazil). VT would like to thank the Indian Council of Medical Research, India (ICMR/AMR/Adhoc/291/2022-ECD-II) for funding.
Abbreviations
AMPs, antimicrobial peptides; CBA, checkerboard assay; CFU, colony forming unit; CLA, cell lysis assay; EGCG, epigallocatechin gallate; EOs, essential oils; EOCs, essential oil components; ESKAPE, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.; DEO, dill and its essential oil; DMSO, dimethyl sulfoxide; FICI, fractional inhibitory concentration index; HAI, hospital-acquired infections; IEPA/E, inhibition of efflux pumps activity/expression; IZGC, inhibition zone by EO gaseous contact; LecA, lectin A; LPS, lipopolysaccharide structure; OM, outer membrane; OmpA, outer membrane protein A; QS, quorum sensing; MBIC, minimum biofilm inhibitory concentration; MDM, microdilution method; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MSSA, methicillin susceptible S. aureus; MRSA, methicillin resistant S. aureus; NTP, non-thermal plasma; XDR, extensively drug-resistant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adamczak A., Ożarowski M., Karpiński T. M. (2020). Curcumin, a natural antimicrobial agent with strain-specific activity. Pharmaceuticals 13:153. 10.3390/ph13070153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adukwu E., Allen S. C., Phillips C. A. (2012). The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 113 1217–1227. 10.1111/j.1365-2672.2012.05418.x [DOI] [PubMed] [Google Scholar]
- Ahmed S. A., Rudden M., Smyth T. J., Dooley J. S., Marchant R., Banat I. M. (2019). Natural quorum sensing inhibitors effectively downregulate gene expression of Pseudomonas aeruginosa virulence factors. Appl. Microbiol. Biotechnol. 103 3521–3535. 10.1007/s00253-019-09618-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinsulire O. R., Aibin I., Adenipekun T., Adelowotan T., Odugbemi T. (2007). In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr. J. Tradit. Complement. Altern. Med. 4 338–344. 10.4314/ajtcam.v4i3.31227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksic V., Mimica-Dukic N., Simin N., Nedeljkovic N. S., Knezevic P. (2014). Synergistic effect of Myrtus communis L. essential oils and conventional antibiotics against multi-drug resistant Acinetobacter baumannii wound isolates. Phytomedicine 21 1666–1674. 10.1016/j.phymed.2014.08.013 [DOI] [PubMed] [Google Scholar]
- Alizadeh Behbahani B., Falah F., Lavi Arab F., Vasiee M., Tabatabaee Yazdi F. (2020). Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid. Based Complement. Alternat. Med. 2020:5190603. 10.1155/2020/5190603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabib N. A., Husain F. M., Ahmad I., Baig M. H. (2017). Eugenol inhibits quorum sensing and biofilm of toxigenic MRSA strains isolated from food handlers employed in Saudi Arabia. Biotechnol. Biotechnol. Equip. 31 387–396. 10.1080/13102818.2017.1281761 [DOI] [Google Scholar]
- Álvarez-Martínez F., Barrajón-Catalán E., Herranz-López M., Micol V. (2021). Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 90:153626. 10.1016/j.phymed.2021.153626 [DOI] [PubMed] [Google Scholar]
- Alves S., Duarte A., Sousa S., Domingues F. C. (2016). Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 32 155–165. 10.1080/08927014.2015.1133810 [DOI] [PubMed] [Google Scholar]
- Amini M., Habibi G., Rakei S., Heidari S., Taheri H., Esfahani R. B., et al. (2019). Growth Control of Acinetobacter baumannii in Infected wound by oregano essential oil in rats. J. Res. Med. Dent. Sci. 7 136–139. [Google Scholar]
- Angane M., Swift S., Huang K., Butts C. A., Quek S. Y. (2022). Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 11:464. 10.3390/foods11030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artini M., Patsilinakos A., Papa R., Božović M., Sabatino M., Garzoli S., et al. (2018). Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against Pseudomonas aeruginosa. Molecules 23:482. 10.3390/molecules23020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H., Scher J. M., Speakman J.-B., Zapp J. (2005). Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia 76 580–584. 10.1016/j.fitote.2005.04.011 [DOI] [PubMed] [Google Scholar]
- Betts J. W., Wareham D. W. (2014). In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 14 1–5. 10.1186/1471-2180-14-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts J., Kelly S., Haswell S. (2011). Antibacterial effects of theaflavin and synergy with epicatechin against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents 38 421–425. 10.1016/j.ijantimicag.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Budri P. E., Silva N. C., Bonsaglia E. C., Júnior A. F., Júnior J. A., Doyama J. T., et al. (2015). Effect of essential oils of Syzygium aromaticum and Cinnamomum zeylanicum and their major components on biofilm production in Staphylococcus aureus strains isolated from milk of cows with mastitis. J. Dairy Sci. 98 5899–5904. 10.3168/jds.2015-9442 [DOI] [PubMed] [Google Scholar]
- Budzyńska A., Wickowska-Szakiel M., Sadowska B., Kalemba D., Różalska B. (2011). Antibiofilm activity of selected plant essential oils and their major components. Pol. J. Microbiol. 60:35. 10.33073/pjm-2011-005 [DOI] [PubMed] [Google Scholar]
- Camens S., Liu S., Hon K., Bouras G. S., Psaltis A. J., Wormald P. J., et al. (2021). Preclinical development of a bacteriophage cocktail for treating multidrug resistant Pseudomonas aeruginosa Infections. Microorganisms 9:2001. 10.3390/microorganisms9092001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana R., Baffone W. (2018). Carvacrol efficacy in reducing microbial biofilms on stainless steel and in limiting re-growth of injured cells. Food Control 90 10–17. [Google Scholar]
- Carson C. F., Mee B. J., Riley T. V. (2002). Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 46 1914–1920. 10.1128/AAC.46.6.1914-1920.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan O., Ugur A. (2015). Chemical composition and anti-biofilm activity of Thymus sipyleus BOISS. subsp. sipyleus BOISS. var. davisianus RONNIGER essential oil. Arch. Pharm. Res. 38 957–965. 10.1007/s12272-014-0516-0 [DOI] [PubMed] [Google Scholar]
- Chan B. C.-L., Lau C., Jolivalt C., Lui S.-L., Ganem-Elbaz C., Paris J.-M., et al. (2011). Chinese medicinal herbs against antibiotic-resistant bacterial pathogens. Sci. Against Microb. Pathog. Communica. Curr. Res. Technol. Adv. 2 773–781. [Google Scholar]
- Chan P.-C., Huang L.-M., Lin H.-C., Chang L.-Y., Chen M.-L., Lu C.-Y., et al. (2007). Control of an outbreak of pandrug-resistant Acinetobacter baumannii colonization and infection in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 28 423–429. 10.1086/513120 [DOI] [PubMed] [Google Scholar]
- Chang C.-Y., Krishnan T., Wang H., Chen Y., Yin W.-F., Chong Y.-M., et al. (2014). Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 4:7245. 10.1038/srep07245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Bepler T., Pepper K., Fu D., Lu T. K. (2022). Synthetic molecular evolution of antimicrobial peptides. Curr. Opin. Biotechnol. 75:102718. 10.1016/j.copbio.2022.102718 [DOI] [PubMed] [Google Scholar]
- Chraibi M., Fadil M., Farah A., Lebrazi S., Fikri-Benbrahim K. (2021). Antimicrobial combined action of Mentha pulegium, Ormenis mixta and Mentha piperita essential oils against S. aureus, E. coli and C. tropicalis: Application of mixture design methodology. LWT 145:111352. 10.1016/j.lwt.2021.111352 [DOI] [Google Scholar]
- Cirino I. C. S., Menezes-Silva S. M. P., Silva H. T. D., De Souza E. L., Siqueira-Júnior J. P. (2014). The essential oil from Origanum vulgare L. and its individual constituents carvacrol and thymol enhance the effect of tetracycline against Staphylococcus aureus. Chemotherapy 60 290–293. 10.1159/000381175 [DOI] [PubMed] [Google Scholar]
- Coelho F. A. B. L., Pereira M. O. (2013). “Exploring new treatment strategies for Pseudomonas aeruginosa biofilm infections based on plant essential oils,” in Microbial pathogens and strategies for combating them: Science, technology and education, Vol. 1 ed. Méndez-Vilas A. (Badajoz: Formatex Research Center; ), 83–89. [Google Scholar]
- Condò C., Anacarso I., Sabia C., Iseppi R., Anfelli I., Forti L., et al. (2020). Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat. Prod. Res. 34 567–574. 10.1080/14786419.2018.1490904 [DOI] [PubMed] [Google Scholar]
- Coutinho H. D., Rodrigues F. F., Nascimento E. M., Costa J. G., Falcão-Silva V. S., Siqueira-Júnior J. P. (2011). Synergism of gentamicin and norfloxacin with the volatile compounds of Lippia microphylla Cham.(Verbenaceae). J. Essent. Oil Res. 23 24–28. 10.1080/10412905.2011.9700443 [DOI] [Google Scholar]
- Coutinho H., Matias E., Santos K., Tintino S., Souza C., Guedes G., et al. (2010). Enhancement of the norfloxacin antibiotic activity by gaseous contact with the essential oil of Croton zehntneri. J. Young Pharm. 2 362–364. 10.4103/0975-1483.71625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S., Mann C., Markham J. (2001). Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 91 492–497. 10.1046/j.1365-2672.2001.01406.x [DOI] [PubMed] [Google Scholar]
- Cox S., Mann C., Markham J., Bell H. C., Gustafson J., Warmington J., et al. (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88 170–175. 10.1046/j.1365-2672.2000.00943.x [DOI] [PubMed] [Google Scholar]
- Cui H., Li W., Li C., Vittayapadung S., Lin L. (2016). Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 32 215–225. 10.1080/08927014.2015.1134516 [DOI] [PubMed] [Google Scholar]
- Da Silva L. C., Da Silva M. V., Correia M. T. D. S. (2017). New Frontiers in the search of antimicrobials agents from natural products. Lausanne: Frontiers Media. 10.3389/fmicb.2017.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza E. L., De Barros J. C., De Oliveira C. E. V., Da Conceição M. L. (2010). Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 137 308–311. 10.1016/j.ijfoodmicro.2009.11.025 [DOI] [PubMed] [Google Scholar]
- De R., Kundu P., Swarnakar S., Ramamurthy T., Chowdhury A., Nair G. B., et al. (2009). Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 53 1592–1597. 10.1128/AAC.01242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan S., Sattari M., Bigdeli M. (2008). Effect of subinhibitory concentrations of cumin (Cuminum cyminum L.) seed essential oil and alcoholic extract on the morphology, capsule expression and urease activity of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 32 432–436. 10.1016/j.ijantimicag.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Di Pasqua R., Betts G., Hoskins N., Edwards M., Ercolini D., Mauriello G. (2007). Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 55 4863–4870. 10.1021/jf0636465 [DOI] [PubMed] [Google Scholar]
- Djeussi D. E., Noumedem J. A., Seukep J. A., Fankam A. G., Voukeng I. K., Tankeo S. B., et al. (2013). Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Alterna. Med. 13 1–8. 10.1186/1472-6882-13-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Rodrigues J. B., De Souza N. T., Scarano J. O. A., De Sousa J. M., Lira M. C., De Figueiredo R. C. B. Q., et al. (2018). Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT 93 293–299. 10.1016/j.lwt.2018.03.052 [DOI] [Google Scholar]
- Duarte A. F., Ferreira S., Oliveira R., Domingues F. C. (2013). Effect of coriander oil (Coriandrum sativum) on planktonic and biofilm cells of Acinetobacter baumannii. Nat. Prod. Commun. 8 673–678. 10.1177/1934578X1300800532 [DOI] [Google Scholar]
- Duarte A., Ferreira S., Silva F., Domingues F. (2012). Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 19 236–238. 10.1016/j.phymed.2011.11.010 [DOI] [PubMed] [Google Scholar]
- Ekor M. (2014). The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4:177. 10.3389/fphar.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shenawy M. A., Baghdadi H. H., El-Hosseiny L. S. (2015). Antibacterial activity of plants essential oils against some epidemiologically relevant food-borne pathogens. Open Public Health J. 8 30–34. 10.2174/1874944501508010030 [DOI] [Google Scholar]
- El-Tarabily K. A., El-Saadony M. T., Alagawany M., Arif M., Batiha G. E., Khafaga A. F., et al. (2021). Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 28 5145–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina L., Berdejo D., Alfonso P., García-Gonzalo D., Pagán R. (2017). Potential use of carvacrol and citral to inactivate biofilm cells and eliminate biofouling. Food Control 82 256–265. 10.1016/j.foodcont.2017.07.007 [DOI] [Google Scholar]
- Espina L., Pagán R., López D., García-Gonzalo D. (2015). Individual constituents from essential oils inhibit biofilm mass production by multi-drug resistant Staphylococcus aureus. Molecules 20 11357–11372. 10.3390/molecules200611357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry W., Okemo P. O., Ansorg R. (1998). Antibacterial activity of East African medicinal plants. J. Ethnopharmacol. 60 79–84. [DOI] [PubMed] [Google Scholar]
- Fadli M., Bolla J.-M., Mezrioui N.-E., Pagès J.-M., Hassani L. (2014). First evidence of antibacterial and synergistic effects of Thymus riatarum essential oil with conventional antibiotics. Ind. Crops Prod. 61 370–376. [Google Scholar]
- Fadli M., Chevalier J., Saad A., Mezrioui N.-E., Hassani L., Pages J.-M. (2011). Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 38 325–330. 10.1016/j.ijantimicag.2011.05.005 [DOI] [PubMed] [Google Scholar]
- Fadli M., Pagès J.-M., Mezrioui N.-E., Abbad A., Hassani L. (2016). Artemisia herba-alba Asso and Cymbopogon citratus (DC.) Stapf essential oils and their capability to restore antibiotics efficacy. Ind. Crops Prod. 89 399–404. [Google Scholar]
- Fadli M., Saad A., Sayadi S., Chevalier J., Mezrioui N.-E., Pagès J.-M., et al. (2012). Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection–bacteria and their synergistic potential with antibiotics. Phytomedicine 19 464–471. 10.1016/j.phymed.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Farahpour M. R., Pirkhezr E., Ashrafian A., Sonboli A. (2020). Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 128:110120. 10.1016/j.biopha.2020.110120 [DOI] [PubMed] [Google Scholar]
- Ferro T. A., Souza E. B., Suarez M. A., Rodrigues J. F., Pereira D. M., Mendes S. J., et al. (2019). Topical application of cinnamaldehyde promotes faster healing of skin wounds infected with pseudomonas aeruginosa. Molecules 24:1627. 10.3390/molecules24081627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firmino D. F., Cavalcante T. T. A., Gomes G. A., Firmino N. C. S., Rosa L. D., De Carvalho M. G., et al. (2018). Antibacterial and antibiofilm activities of cinnamomum sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018:7405736. 10.1155/2018/7405736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Zhai G., Wang H., Lu L., Xu J., Zhu J., et al. (2020). Protective effects of anti-alginate monoclonal antibody against Pseudomonas aeruginosa infection of HeLa cells. Microb. Pathog. 145:104240. 10.1016/j.micpath.2020.104240 [DOI] [PubMed] [Google Scholar]
- Ghodrati M., Farahpour M. R., Hamishehkar H. (2019). Encapsulation of peppermint essential oil in nanostructured lipid carriers: In-vitro antibacterial activity and accelerative effect on infected wound healing. Colloids Surf. A Physicochem. Eng. Asp. 564 161–169. [Google Scholar]
- Ghuman S., Ncube B., Finnie J. F., Mcgaw L. J., Coopoosamy R. M., Van Staden J. (2016). Antimicrobial activity, phenolic content, and cytotoxicity of medicinal plant extracts used for treating dermatological diseases and wound healing in KwaZulu-Natal, South Africa. Front. Pharmacol. 7:320. 10.3389/fphar.2016.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N. C., Wareham D. W. (2010). Antimicrobial activity of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against clinical isolates of Stenotrophomonas maltophilia. Int. J. Antimicrob. Agents 36 129–131. 10.1016/j.ijantimicag.2010.03.025 [DOI] [PubMed] [Google Scholar]
- Guclu E., Genc H., Zengin M., Karabay O. (2014). Antibacterial activity of Lythrum salicaria against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Annu. Res. Rev. Biol. 4 1099–1105. [Google Scholar]
- Guerra F. Q. S., Mendes J. M., Sousa J. P. D., Morais-Braga M. F., Santos B. H. C., Melo Coutinho H. D., et al. (2012). Increasing antibiotic activity against a multidrug-resistant Acinetobacter spp by essential oils of Citrus limon and Cinnamomum zeylanicum. Nat. Prod. Res. 26 2235–2238. 10.1080/14786419.2011.647019 [DOI] [PubMed] [Google Scholar]
- Hemaiswarya S., Doble M. (2009). Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 16 997–1005. [DOI] [PubMed] [Google Scholar]
- Hendry E., Worthington T., Conway B. R., Lambert P. (2009). Antimicrobial efficacy of eucalyptus oil and 1, 8-cineole alone and in combination with chlorhexidine digluconate against microorganisms grown in planktonic and biofilm cultures. J. Antimicrob. Chemother. 64 1219–1225. 10.1093/jac/dkp362 [DOI] [PubMed] [Google Scholar]
- Heselpoth R. D., Euler C. W., Fischetti V. A. (2022). PaP1, a broad-spectrum lysin-derived cationic peptide to treat polymicrobial skin infections. Front. Microbiol. 13:817228. 10.3389/fmicb.2022.817228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain F. M., Ahmad I., Asif M., Tahseen Q. (2013). Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 38 835–844. 10.1007/s12038-013-9385-9 [DOI] [PubMed] [Google Scholar]
- Husain F. M., Ahmad I., Khan M. S., Ahmad E., Tahseen Q., Khan M. S., et al. (2015). Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 6:420. 10.3389/fmicb.2015.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyldgaard M., Mygind T., Meyer R. L. (2012). Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 3:12. 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Cervantes G., Cruz-Cruz C., Durán-Manuel E. M., Loyola-Cruz M. Á, Cureńo-Díaz M. A., Castro-Escarpulli G., et al. (2022). Disinfection efficacy of ozone on ESKAPE bacteria biofilms: Potential use in difficult-to-access medical devices. Am. J. Infect. Control. 10.1016/j.ajic.2022.03.037 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Shiraishi A., Hada T., Hirose K., Hamashima H., Shimada J. (2004). The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 237 325–331. 10.1016/j.femsle.2004.06.049 [DOI] [PubMed] [Google Scholar]
- Ismail M. M., Samir R., Saber F. R., Ahmed S. R., Farag M. A. (2020). Pimenta oil as a potential treatment for Acinetobacter baumannii wound infection: In vitro and in vivo bioassays in relation to its chemical composition. Antibiotics 9:679. 10.3390/antibiotics9100679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Salcedo M. D. R., Gonzalez-Espindola L. A., Alonso-Castro A. J., Gonzalez-Martinez M. D. R., Domínguez F., Garcia-Carranca A. (2011). Antimicrobial activity and cytotoxic effects of Magnolia dealbata and its active compounds. Nat. Prod. Commun. 6 1121–1124. [PubMed] [Google Scholar]
- Jafri H., Husain F. M., Ahmad I. (2014). Antibacterial and antibiofilm activity of some essential oils and compounds against clinical strains of Staphylococcus aureus. J. Biomed. Ther. Sci. 1 65–71. [Google Scholar]
- Johny A. K., Darre M., Donoghue A., Donoghue D., Venkitanarayanan K. (2010). Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 19 237–244. [Google Scholar]
- Jouneghani R. S., Castro A. H. F., Panda S. K., Swennen R., Luyten W. (2020). Antimicrobial activity of selected banana cultivars against important human pathogens, including candida biofilm. Foods 9:435. 10.3390/foods9040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kačániová M., Galovičová L., Borotová P., Valková V., Ïúranová H., Kowalczewski P. Ł, et al. (2021). Chemical composition, in vitro and in situ antimicrobial and antibiofilm activities of Syzygium aromaticum (Clove) essential oil. Plants 10:2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Jin W., Wang J., Sun Y., Wu X., Liu L. (2019). Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. Lwt 101 639–645. [Google Scholar]
- Kavanaugh N. L., Ribbeck K. (2012). Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 78 4057–4061. 10.1128/AEM.07499-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaźmierczak N., Grygorcewicz B., Roszak M., Bochentyn B., Piechowicz L. (2022). Comparative assessment of bacteriophage and antibiotic activity against multidrug-resistant Staphylococcus aureus biofilms. Int. J. Mol. Sci. 23:1274. 10.3390/ijms23031274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerekes E. B., Vidács A., Takó M., Petkovits T., Vágvölgyi C., Horváth G., et al. (2019). Anti-biofilm effect of selected essential oils and main components on mono-and polymicrobic bacterial cultures. Microorganisms 7:345. 10.3390/microorganisms7090345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadri S., Boutefnouchet N., Dekhil M. (2010). Antibacterial activity evaluation of Allium sativum essential oil compared to different Pseudomonas Aeruginosa strains in eastern Algeria. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 11 421–428. [Google Scholar]
- Khan M. F., Tang H., Lyles J. T., Pineau R., Mashwani Z.-U.-R., Quave C. L. (2018). Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Fronti. Pharmacol. 9:815. 10.3389/fphar.2018.00815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. S. A., Zahin M., Hasan S., Husain F. M., Ahmad I. (2009). Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 49 354–360. 10.1111/j.1472-765X.2009.02666.x [DOI] [PubMed] [Google Scholar]
- Khezri K., Farahpour M. R., Mounesi Rad S. (2019). Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 47 980–988. 10.1080/21691401.2019.1582539 [DOI] [PubMed] [Google Scholar]
- Kifer D., Mužinić V., Klarić M. Š. (2016). Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 69 689–696. 10.1038/ja.2016.10 [DOI] [PubMed] [Google Scholar]
- Kim H., Lee S., Byun Y., Park H. (2015). 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci. Rep. 5:8656. 10.1038/srep08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K., Tatara I., Takeda S., Arakawa K., Shirotani T., Okada M., et al. (1997). Antibacterial activity of epigallocatechin gallate against Helicobacter pylori: Synergistic effect with Plaunotol. J. Infect. Chemother. 3 170–172. [Google Scholar]
- Kothari V., Shah A., Gupta S., Punjabi A., Ranka A. (2010). Revealing the antimicrobial potential of plants. Int. J. Biosci. Technol. 3 1–20. [Google Scholar]
- Kumar A., Boradia V. M., Thakare R., Singh A. K., Gani Z., Das S., et al. (2019). Repurposing ethyl bromopyruvate as a broad-spectrum antibacterial. J. Antimicrob. Chemother. 74 912–920. 10.1093/jac/dky555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar L., Chhibber S., Kumar R., Kumar M., Harjai K. (2015). Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 102 84–95. 10.1016/j.fitote.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Lambert R., Skandamis P. N., Coote P. J., Nychas G. J. (2001). A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 91 453–462. [DOI] [PubMed] [Google Scholar]
- Lawrence R., Tripathi P., Jeyakumar E. (2009). Isolation, purification and evaluation of antibacterial agents from Aloe vera. Braz. J. Microbiol. 40 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng B.-F., Qiu J.-Z., Dai X.-H., Dong J., Wang J.-F., Luo M.-J., et al. (2011). Allicin reduces the production of α-toxin by Staphylococcus aureus. Molecules 16 7958–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dong J., Qiu J.-Z., Wang J.-F., Luo M.-J., Li H.-E., et al. (2011). Peppermint oil decreases the production of virulence-associated exoproteins by Staphylococcus aureus. Molecules 16 1642–1654. 10.3390/molecules16021642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaverde P. W., Campina F. F., Da Cunha F. A., Crispim F. D., Figueredo F. G., Lima L. F., et al. (2017). Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 109 957–961. 10.1016/j.fct.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Lorenzi V., Muselli A., Bernardini A. F., Berti L., Pagès J.-M., Amaral L., et al. (2009). Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 53 2209–2211. 10.1128/AAC.00919-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrence R., Ramakrishnan A., Sundaramoorthy N., Shyam A., Mohan V., Subbarao H., et al. (2018). Norfloxacin salts of carboxylic acids curtail planktonic and biofilm mode of growth in ESKAPE pathogens. J. Appl. Microbiol. 124 408–422. 10.1111/jam.13651 [DOI] [PubMed] [Google Scholar]
- Lu M., Dai T., Murray C. K., Wu M. X. (2018). Bactericidal property of oregano oil against multidrug-resistant clinical isolates. Front. Microbiol. 9:2329. 10.3389/fmicb.2018.02329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo D., Almeida F., Wanderley M., Ferraz M., Santos N., López A., et al. (2021). Usnic acid: From an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem. Rev. 20 609–630. [Google Scholar]
- Magi G., Marini E., Facinelli B. (2015). Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 6:165. 10.3389/fmicb.2015.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboubi M., Bidgoli F. G. (2010). Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine 17 548–550. 10.1016/j.phymed.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Mahboubi M., Feizabadi M. M., Khamechian T., Kazempour N., Zadeh M. R., Sasani F., et al. (2016). The effect of Oliveria decumbens and Pelargonium graveolens on healing of infected skin wounds in mice. World J. Plast. Surg. 5:259. [PMC free article] [PubMed] [Google Scholar]
- Mahboubi M., Kazempour N., Farzin N. (2011). Antimicrobial activity of Pelargonium graveolens and Oliveria decumbens extracts against clinical isolates of Staphylococcus aureus. J. Biol. Act. Prod. Nat. 1 105–111. [Google Scholar]
- Mahizan N. A., Yang S.-K., Moo C.-L., Song A. A.-L., Chong C.-M., Chong C.-W., et al. (2019). Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules 24 2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzuoerh R., Farahpour M. R., Oryan A., Sonboli A. (2019). Effectiveness of topical administration of Anethum graveolens essential oil on MRSA-infected wounds. Biomed. Pharmacother. 109 1650–1658. 10.1016/j.biopha.2018.10.117 [DOI] [PubMed] [Google Scholar]
- Masoud E., Gouda H. (2012). Effect of some natural plant extracts against gram negative bacteria in Njran Area, Saudi Arabia. Egypt. Acad. J. Biol. Sci. 4 85–92. [Google Scholar]
- Miyasaki Y., Rabenstein J. D., Rhea J., Crouch M.-L., Mocek U. M., Kittell P. E., et al. (2013). Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS One 8:e61594. 10.1371/journal.pone.0061594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modarresi M., Farahpour M.-R., Baradaran B. (2019). Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 27 531–537. 10.1007/s10787-018-0510-0 [DOI] [PubMed] [Google Scholar]
- Mohamed S. H., Salem D., Azmy M., Fam N. S. (2018). Antibacterial and antibiofilm activity of cinnamaldehyde against carbapenem-resistant Acinetobacter baumannii in Egypt: In vitro study. J. Appl. Pharm. Sci. 8 151–156. [Google Scholar]
- Moreno S., Galván E., Vázquez N., Fiorilli G., Guido P. C. (2015). “Antibacterial efficacy of Rosmarinus officinalis phytochemicals against nosocomial multidrug-resistant bacteria grown in planktonic culture and biofilm,” in The battle against microbial pathogens: Basic science, technological advances and educational programs. 1st edition, (Badajoz: Formatex; ), 3–8. [Google Scholar]
- Mors W. B., Do Nascimento M. C., Pereira B. M. R., Pereira N. A. (2000). Plant natural products active against snake bite—the molecular approach. Phytochemistry 55 627–642. 10.1016/s0031-9422(00)00229-6 [DOI] [PubMed] [Google Scholar]
- Mun S.-H., Joung D.-K., Kim Y.-S., Kang O.-H., Kim S.-B., Seo Y.-S., et al. (2013). Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 20 714–718. 10.1016/j.phymed.2013.02.006 [DOI] [PubMed] [Google Scholar]
- Na S.-H., Jeon H., Oh M.-H., Kim Y.-J., Chu M., Lee I.-Y., et al. (2021). Therapeutic effects of inhibitor of ompA expression against carbapenem-resistant Acinetobacter baumannii strains. Int. J. Mol. Sci. 22:12257. 10.3390/ijms222212257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nand P., Drabu S., Gupta R. K. (2012). Insignificant anti-acne activity of Azadirachta indica leaves and bark. J. Pharm. Negat. Results 3 29–33. [Google Scholar]
- Negreiros M. O., Pawlowski Â, Zini C. A., Soares G. L., Motta A. S., Frazzon A. P. (2016). Antimicrobial and antibiofilm activity of Baccharis psiadioides essential oil against antibiotic-resistant Enterococcus faecalis strains. Pharm. Biol. 54 3272–3279. 10.1080/13880209.2016.1223700 [DOI] [PubMed] [Google Scholar]
- Ngane R. A. N. (2019). Antibacterial activity of methanol extract and fractions from stem Bark of Bridelia micrantha (Hochst.) Baill.(Phyllanthaceae). EC Pharmacol. Toxicol. 7 609–616. [Google Scholar]
- Nielsen J. E., Alford M. A., Yung D. B. Y., Molchanova N., Fortkort J. A., Lin J. S., et al. (2022). Self-assembly of antimicrobial peptoids impacts their biological effects on ESKAPE bacterial pathogens. ACS Infect. Dis. 8 533–545. 10.1021/acsinfecdis.1c00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostro A., Roccaro A. S., Bisignano G., Marino A., Cannatelli M. A., Pizzimenti F. C., et al. (2007). Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 56 519–523. 10.1099/jmm.0.46804-0 [DOI] [PubMed] [Google Scholar]
- Noumi E., Merghni A., Alreshidi M. M., Haddad O., Akmadar G., De Martino L., et al. (2018). Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for evaluating anti-quorum sensing activity of Melaleuca alternifolia essential oil and its main component terpinen-4-ol. Molecules 23:2672. 10.3390/molecules23102672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Costantini S., De Angelis M., Garzoli S., Božović M., Mascellino M. T., et al. (2018). High potency of melaleuca alternifolia essential oil against multi-drug resistant gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules 23:2584. 10.3390/molecules23102584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J. B., Teixeira M. A., Paiva L. F. D., Oliveira R. F. D., Mendonça A. R. D. A., Brito M. J. A. D. (2019). In vitro and in vivo antimicrobial activity of Cymbopogon citratus (DC.) Stapf. against Staphylococcus spp. isolated from newborn babies in an intensive care unit. Microb. Drug Resist. 25 1490–1496. 10.1089/mdr.2018.0047 [DOI] [PubMed] [Google Scholar]
- Omarizadeh K., Farahpour M. R., Alipour M. (2021). Topical administration of an ointment prepared from Satureja sahendica essential oil accelerated infected full-thickness wound healing by modulating inflammatory response in a mouse model. Wounds 33 321–328. [PubMed] [Google Scholar]
- Oosterhaven K., Poolman B., Smid E. (1995). S-carvone as a natural potato sprout inhibiting, fungistatic and bacteristatic compound. Ind. Crops Prod. 4 23–31. [Google Scholar]
- Osterburg A., Gardner J., Hyon S., Neely A., Babcock G. (2009). Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG). Clin. Microbio. Infect. 15 341–346. 10.1111/j.1469-0691.2009.02710.x [DOI] [PubMed] [Google Scholar]
- Packiavathy I. A. S. V., Priya S., Pandian S. K., Ravi A. V. (2014). Inhibition of biofilm development of uropathogens by curcumin–an anti-quorum sensing agent from Curcuma longa. Food Chem. 148 453–460. 10.1016/j.foodchem.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Pal S. K., Shukla Y. (2003). Herbal medicine: Current status and the future. Asian Pac. J. Cancer Prev. 4 281–288. [PubMed] [Google Scholar]
- Palaniappan K., Holley R. A. (2010). Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 140 164–168. [DOI] [PubMed] [Google Scholar]
- Panda S. K., Buroni S., Tiwari V., Da Silva L. C. N. (2021). Insights into new strategies to combat biofilms. Front. Microbiol. 12:742647. 10.3389/fmicb.2021.742647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S. K., Das R., Lavigne R., Luyten W. (2020). Indian medicinal plant extracts to control multidrug-resistant S. aureus, including in biofilms. S. Afr. J. Bot. 128 283–291. [Google Scholar]
- Patil A., Banerji R., Kanojiya P., Saroj S. D. (2021). Foodborne ESKAPE biofilms and antimicrobial resistance: Lessons learned from clinical isolates. Pathog. Glob. Health 115 339–356. 10.1080/20477724.2021.1916158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. E., Martin N. R., Parzych K. R., Rickard A. H., Underwood A., Boles B. R. (2013). Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 81 496–504. 10.1128/IAI.00877-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. P. (2012). Effect of plant-derived molecules on Acinetobacter baumannii biofilm on abiotic surfaces. Honors Scholar Theses 257. [Epub ahead of print]. [Google Scholar]
- Perez A. P., Perez N., Lozano C. M. S., Altube M. J., De Farias M. A., Portugal R. V., et al. (2019). The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine 57 339–351. 10.1016/j.phymed.2018.12.025 [DOI] [PubMed] [Google Scholar]
- Piasecki B., Biernasiuk A., Skiba A., Skalicka-Woźniak K., Ludwiczuk A. (2021). Composition, Anti-MRSA activity and toxicity of essential oils from Cymbopogon species. Molecules 26:7542. 10.3390/molecules26247542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes E. K. U., Melo H. M., Nogueira J. W. A., Firmino N. C. S., De Carvalho M. G., Catunda Júnior F. E. A., et al. (2019). Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 28 633–639. 10.1007/s10068-018-0502-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfírio E. M., Melo H. M., Pereira A. M. G., Cavalcante T. T. A., Gomes G. A., Carvalho M. G. D., et al. (2017). In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World J. 2017:4962707. 10.1155/2017/4962707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabuseenivasan S., Jayakumar M., Ignacimuthu S. (2006). In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 6:39. 10.1186/1472-6882-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premachandran M. S., Murthy P. S. (2022). Ethnobotanical, phytochemical, pharmacological properties and applications of Pimenta dioica L. J. Essent. Oil Res. 34 216–232. 10.1080/10412905.2022.2032423 [DOI] [Google Scholar]
- Preuss H. G., Echard B., Dadgar A., Talpur N., Manohar V., Enig M., et al. (2005). Effects of essential oils and monolaurin on Staphylococcus aureus: In vitro and in vivo studies. Toxicol. Mech. Methods 15 279–285. 10.1080/15376520590968833 [DOI] [PubMed] [Google Scholar]
- Qiu J., Feng H., Lu J., Xiang H., Wang D., Dong J., et al. (2010). Eugenol reduces the expression of virulence-related exoproteins in Staphylococcus aureus. Appl. Environ. Microbiol. 76 5846–5851. 10.1128/AEM.00704-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Zhang X., Luo M., Li H., Dong J., Wang J., et al. (2011). Subinhibitory concentrations of perilla oil affect the expression of secreted virulence factor genes in Staphylococcus aureus. PLoS One 6:e16160. 10.1371/journal.pone.0016160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim K., Qasim M., Rahman H., Khan T., Ahmad I., Khan N., et al. (2016). Antimicrobial resistance among aerobic biofilm producing bacteria isolated from chronic wounds in the tertiary care hospitals of Peshawar, Pakistan. J. Wound Care 25 480–486. 10.12968/jowc.2016.25.8.480 [DOI] [PubMed] [Google Scholar]
- Rai M., Paralikar P., Jogee P., Agarkar G., Ingle A. P., Derita M., et al. (2017). Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 519 67–78. 10.1016/j.ijpharm.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Rathinam P., Vijay Kumar H., Viswanathan P. (2017). Eugenol exhibits anti-virulence properties by competitively binding to quorum sensing receptors. Biofouling 33 624–639. 10.1080/08927014.2017.1350655 [DOI] [PubMed] [Google Scholar]
- Rebelo J. M. D. C. C. (2014). Pétalas da Flor de Açafrão (Crocus sativus L.): Valorização de um subproduto. Ph.D. dissesrtation. Porto: Faculdade de Farmácia da Universidade do Porto. [Google Scholar]
- Reichling J., Harkenthal M., Geiss H., Hoppe-Tichy T., Saller R. (2002). Electron microscopic and biochemical investigations on the antibacterial effects of Australian tea tree oil against Staphylococcus aureus. Curr. Top. Phytochem. 5 77–84. [Google Scholar]
- Richwagen N., Lyles J. T., Dale B. L., Quave C. L. (2019). Antibacterial activity of Kalanchoe mortagei and K. fedtschenkoi against ESKAPE pathogens. Front. Pharmacol. 10:67. 10.3389/fphar.2019.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A. A., Otero-González A., Ghattas M., Ständker L. (2021). Discovery, optimization, and clinical application of natural antimicrobial peptides. Biomedicines 9:1381. 10.3390/biomedicines9101381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato A., Piarulli M., Corbo F., Muraglia M., Carone A., Vitali M., et al. (2010). In vitro synergistic action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 17 3289–3295. 10.2174/092986710792231996 [DOI] [PubMed] [Google Scholar]
- Rosato A., Vitali C., De Laurentis N., Armenise D., Milillo M. A. (2007). Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 14 727–732. 10.1016/j.phymed.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Rudrappa T., Bais H. P. (2008). Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 56 1955–1962. 10.1021/jf072591j [DOI] [PubMed] [Google Scholar]
- Sahal G., Avcioglu N. H., Bilkay I. S. (2016). Higher biofilm formation by multi-drug resistant K. pneumoniae and K. rhinoscleromatis strains and effects of lemon and ginger essential oils on biofilm formation. Indian J. Pharm. Educ. Res. 50 S82–S88. [Google Scholar]
- Sahoo A., Swain S. S., Behera A., Sahoo G., Mahapatra P. K., Panda S. K. (2021). Antimicrobial Peptides derived from insects offer a novel therapeutic option to combat biofilm: A review. Front. Microbiol. 12:661195. 10.3389/fmicb.2021.661195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehzadeh A., Hashemi Doulabi M. S., Sohrabnia B., Jalali A. (2018). The effect of thyme (Thymus vulgaris) extract on the expression of norA efflux pump gene in clinical strains of Staphylococcus aureus. J. Genet. Resour. 4 26–36. [Google Scholar]
- Sarkar T., Chetia M., Chatterjee S. (2021). Antimicrobial peptides and proteins: From nature’s reservoir to the laboratory and beyond. Front. Chem. 9:432. 10.3389/fchem.2021.691532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviuc C., Gheorghe I., Coban S., Drumea V., Chifiriuc M.-C., Banu O., et al. (2016). Rosmarinus officinalis essential oil and eucalyptol act as efflux pumps inhibitors and increase ciprofloxacin efficiency against Pseudomonas aeruginosa and Acinetobacter baumannii MDR strains. Rom. Biotechnol. Lett. 21:11783. [Google Scholar]
- Scholtz V., Vaňková E., Kašparová P., Premanath R., Karunasagar I., Julák J. (2021). Non-thermal plasma treatment of ESKAPE pathogens: A review. Front. Microbiol. 12:737635. 10.3389/fmicb.2021.737635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepahi E., Tarighi S., Ahmadi F. S., Bagheri A. (2015). Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol. 53 176–180. 10.1007/s12275-015-4203-8 [DOI] [PubMed] [Google Scholar]
- Seyed Ahmadi S. G., Farahpour M. R., Hamishehkar H. (2019). Topical application of Cinnamon verum essential oil accelerates infected wound healing process by increasing tissue antioxidant capacity and keratin biosynthesis. Kaohsiung J. Med. Sci. 35 686–694. 10.1002/kjm2.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi A., Mohammadzadeh A., Zahraei Salehi T., Mahmoodi P. (2018). Antibacterial, antibiofilm and antiquorum sensing effects of Thymus daenensis and Satureja hortensis essential oils against Staphylococcus aureus isolates. J. Appl. Microbiol. 124 379–388. 10.1111/jam.13639 [DOI] [PubMed] [Google Scholar]
- Siebs E., Shanina E., Kuhaudomlarp S., Da Silva Figueiredo Celestino Gomes P., Fortin C., Seeberger P. H., et al. (2022). Targeting the central pocket of the Pseudomonas aeruginosa lectin LecA. ChemBioChem. 23:e202100563. 10.1002/cbic.202100563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz M., Głowacka A., Kowalczyk E., Wiktorowska-Owczarek A., Jóźwiak-Bębenista M., Łysakowska M. (2014). The biological activities of cinnamon, geranium and lavender essential oils. Molecules 19 20929–20940. 10.3390/molecules191220929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Maurya S., De Lampasona M., Catalan C. (2005). Chemical constituents, antimicrobial investigations, and antioxidative potentials of Anethum graveolens L. essential oil and acetone extract: Part 52. J. Food Sci. 70 M208–M215. 10.1111/j.1365-2621.2005.tb07190.x [DOI] [Google Scholar]
- Sonboli A., Eftekhar F., Yousefzadi M., Kanani M. R. (2005). Antibacterial activity and chemical composition of the essential oil of Grammosciadium platycarpum Boiss. from Iran. Zeitschrift für Naturforschung C 60 30–34. 10.1515/znc-2005-1-206 [DOI] [PubMed] [Google Scholar]
- Sonboli A., Mirjalili M. H., Hadian J., Ebrahimi S. N., Yousefzadi M. (2006). Antibacterial activity and composition of the essential oil of Ziziphora clinopodioides subsp. bungeana (Juz.) Rech. f. from Iran. Zeitschrift für Naturforschung C 61 677–680. 10.1515/znc-2006-9-1011 [DOI] [PubMed] [Google Scholar]
- Souza E., Oliveira C., Stamford T., Conceição M., Gomes Neto N. (2013). Influence of carvacrol and thymol on the physiological attributes, enterotoxin production and surface characteristics of Staphylococcus aureus strains isolated from foods. Braz. J. Microbiol. 44 29–36. 10.1590/S1517-83822013005000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr C. G., Ghimire J., Guha S., Hoffmann J. P., Wang Y., Sun L., et al. (2020). Synthetic molecular evolution of host cell-compatible, antimicrobial peptides effective against drug-resistant, biofilm-forming bacteria. Proc. Natl. Acad. Sci. U.S.A. 117 8437–8448. 10.1073/pnas.1918427117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermitz F. R., Lorenz P., Tawara J. N., Zenewicz L. A., Lewis K. (2000). Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5’-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. U.S.A. 97 1433–1437. 10.1073/pnas.030540597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani R., Narayanasamy M., Feussner K.-D. (2017). Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 7 1–15. 10.1007/s13205-017-0848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rodriguez M. R., Hernandez-Mendoza A., Gonzalez-Aguilar G. A., Martinez-Tellez M. A., Martins C. M., Ayala-Zavala J. F. (2017). Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 75 255–261. 10.1016/j.foodcont.2016.12.014 [DOI] [Google Scholar]
- Tazehjani D. A. J., Farahpour M. R., Hamishehkar H. (2021). Effectiveness of topical caraway essential oil loaded into nanostructured lipid carrier as a promising platform for the treatment of infected wounds. Colloids Surf. A Physicochem. Eng. Asp. 610:125748. 10.1016/j.colsurfa.2020.125748 [DOI] [Google Scholar]
- Tiwari V. (2016). In vitro engineering of novel bioactivity in the natural enzymes. Front. Chem. 4:39. 10.3389/fchem.2016.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Roy R., Tiwari M. (2015). Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front. Microbiol. 6:618. 10.3389/fmicb.2015.00618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topa S. H., Subramoni S., Palombo E. A., Kingshott P., Rice S. A., Blackall L. L. (2018). Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 164 1087–1097. 10.1099/mic.0.000692 [DOI] [PubMed] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M. G., Venuti V., Cristani M., Daniele C., et al. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 49 2474–2478. 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos N., Croda J., Simionatto S. (2018). Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 120 198–203. 10.1016/j.micpath.2018.04.036 [DOI] [PubMed] [Google Scholar]
- Vasconcelos S. E. C. B., Melo H. M., Cavalcante T. T. A., Júnior F. E. A. C., De Carvalho M. G., Menezes F. G. R., et al. (2017). Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin-and vancomycin-resistant Staphylococcus aureus. BMC Complement. Altern. Med. 17:462. 10.1186/s12906-017-1968-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasina D. V., Antonova N. P., Grigoriev I. V., Yakimakha V. S., Lendel A. M., Nikiforova M. A., et al. (2021). Discovering the potentials of four phage endolysins to combat Gram-negative infections. Front. Microbiol. 12:748718. 10.3389/fmicb.2021.748718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeresham C. (2012). Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 3:200. 10.4103/2231-4040.104709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Jansen L., Setroikromo R., Cavallo F. M., Van Dijl J. M., Quax W. J. (2022). Fighting Acinetobacter baumannii infections with the acylase PvdQ. Microbes Infect. 24:104951. 10.1016/j.micinf.2022.104951 [DOI] [PubMed] [Google Scholar]
- Wang F., Wei F., Song C., Jiang B., Tian S., Yi J., et al. (2017). Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crops Prod. 109 358–366. [Google Scholar]
- Wen M. M., Abdelwahab I. A., Aly R. G., El-Zahaby S. A. (2021). Nanophyto-gel against multi-drug resistant Pseudomonas aeruginosa burn wound infection. Drug Deliv. 28 463–477. 10.1080/10717544.2021.1889720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Cui P., Shi W., Zhang Y. (2020). Identification of essential oils with activity against stationary phase Staphylococcus aureus. BMC Complement. Med. Ther. 20:99. 10.1186/s12906-020-02898-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. K., Chae S.-W., Im G. J., Chung J.-W., Song J.-J. (2015). Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS One 10:e0119564. 10.1371/journal.pone.0119564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-K., Yusoff K., Ajat M., Thomas W., Abushelaibi A., Akseer R., et al. (2019). Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PloS One 14:e0214326. 10.1371/journal.pone.0214326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-K., Yusoff K., Ajat M., Yap W.-S., Lim S.-H. E., Lai K.-S. (2021). Antimicrobial activity and mode of action of terpene linalyl anthranilate against carbapenemase-producing Klebsiella pneumoniae. J. Pharm. Anal. 11 210–219. 10.1016/j.jpha.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Tang J., Khare T., Kumar V. (2020). The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 140:104433. 10.1016/j.fitote.2019.104433 [DOI] [PubMed] [Google Scholar]
- Zare M. R., Khorram M., Barzegar S., Asadian F., Zareshahrabadi Z., Saharkhiz M. J., et al. (2021). Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 603:120698. 10.1016/j.ijpharm.2021.120698 [DOI] [PubMed] [Google Scholar]
- Zhang D., Hu H., Rao Q., Zhao Z. (2011). Synergistic effects and physiological responses of selected bacterial isolates from animal feed to four natural antimicrobials and two antibiotics. Foodborne Pathog. Dis. 8 1055–1062. 10.1089/fpd.2010.0817 [DOI] [PubMed] [Google Scholar]
- Zhang H., Li S., Cheng Y. (2022). Antibiofilm activity of allicin and quercetin in treating biofilm-associated orthopaedics infection. Appl. Biochem. Biotechnol. 1–7. 10.1007/s12010-022-03845-4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zhao W.-H., Hu Z.-Q., Hara Y., Shimamura T. (2002). Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 46 2266–2268. 10.1128/AAC.46.7.2266-2268.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Liu Z., Liu Z., Meng R., Shi C., Chen X., et al. (2018). Phenotype and RNA-seq-Based transcriptome profiling of Staphylococcus aureus biofilms in response to tea tree oil. Microb. Pathog. 123 304–313. 10.1016/j.micpath.2018.07.027 [DOI] [PubMed] [Google Scholar]