Abstract
The invasive capability of Treponema. pallidum is central to its infection process. Matrix metalloproteinases (MMPs), which are specifically inhibited by the tissue inhibitors of metalloproteinases (TIMPs), play a pivotal role in promoting pathogenic invasion by destroying tissue barriers within the body. This study aimed to explore the effect of T. pallidum protein Tp0136 on the balance of MMPs/TIMPs in human dermal vascular smooth muscle cells (HDVSMCs) and the related underlying mechanisms. A number of in vitro studies were conducted to access the impact of recombinant Tp0136 protein on the balance of MMPs/TIMPs in HDVSMCs. The involvement of the PI3K, MAPK, and NF-κB signaling pathways in this process was also investigated. Tp0136 induced the mRNA and protein expressions of MMP1 in HDVSMCs in a concentration-dependent way. In addition, MMP1/TIMP1 and MMP1/TIMP2 ratios were also increased. Furthermore, the study demonstrated that treatment of HDVSMCs with Tp0136 activated the PI3K, MAPK, and NF-κB signaling pathways. Inhibition of PI3K, JNK, P38, and NF-κB, suppressed MMP1 expression and reduced the induction of MMP1/TIMP1 and MMP1/TIMP2 ratios by Tp0136. These findings demonstrate that Tp0136 enhanced the expression of MMP1 involving the PI3K, MAPK, and NF-κB signaling pathways in HDVSMCs, and thus generated the unbalance of MMPs/TIMP, which could contribute to the early spread of T. pallidum and pathogenesis of syphilis.
Keywords: Treponema pallidum, Vascular smooth muscle cells, Matrix metalloproteinases, Tissue inhibitors of metalloproteinases, Imbalance
Treponema pallidum; Vascular smooth muscle cells; Matrix metalloproteinases; Tissue inhibitors of metalloproteinases, Imbalance.
1. Introduction
Syphilis, a sexually transmitted disease, which is chronic and systematic, is caused by Treponema pallidum (T. pallidum) [1]. If left untreated, it can damage the nervous systems and cardiovascular, potentially resulting in debilitation and death [2]. Even though penicillin is an effective treatment for syphilis, it still remains a significant public health problem, with an estimated annual incidence of 12 million [3]. T. pallidum begins disseminating and invading virtually all organ systems after penetrating through mucosal surfaces or skin abrasions at the site of initial infection. Furthermore, T. pallidum has been observed to infiltrate smooth muscle cells as well as the adjacent pericytes in dermal arterioles [4]. The invasive capability of T. pallidum is central to its infection process, however, little is known about the pathogenesis of T. pallidum infection.
Syphilis infection is characterized by the generation of a widespread immune-inflammatory response [5]. The classic histological manifestation of vascular inflammation in syphilis is a proliferation of blood vessels encircled by T. pallidum, macrophages, and lymphocytes [5], in association with cytokine release, degradation of the extracellular matrix (ECM), and increased permeability of blood vessels [6]. Recently, some clinical studies have elicited a correlation between increased vascular permeability and upregulation of the expression of matrix metalloproteinases (MMPs) in this disease [7]. MMPs are a family of zinc-dependent ECM remodeling endopeptidases that can degrade almost every component of the ECM [8, 9]. Tissue inhibitors of metalloproteinases (TIMPs) explicitly suppress the proteolytic activity of MMPs in the ECM [10]. A disturbance in the balance between MMPs and TIMPs activity has been implicated in altering ECM turnover, cellular behavior, and tissue remodeling [11].
A previous study has shown that the flagellins of T. pallidum have the ability to induce MMP9 and MMP13 production in keratinocytes through the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathways, which may contribute to the dermal inflammatory responses as well as promotion of pathogenic invasion by the destruction of tissue barriers [12]. In our prior investigation, we discovered a correlation between the expression of MMP1 and MMP9 and the activation of the MAPK and NF–B pathways. in T. pallidum stimulated THP1 cells [13]. In addition, a previous study examining angiogenesis in syphilis demonstrated that the induction of human umbilical vein endothelial cells by T. pallidum Tp47 protein caused an up-regulation of the expression of MMP1 and MMP10 and an imbalance of MMPs/TIMPs via the AKT, mTOR, and S6 pathways [14]. Therefore, based on the studies above stated, it can be suggested that the AKT, MAPK, and NF-κB signaling pathways are important for the regulation of MMP expression in various cell types.
T. pallidum protein Tp0136, a fibronectin-binding protein is known to induce fibroblasts to produce MMP9 [15]. In addition, Tp0136 mRNA transcript levels have been reported to be considerably higher when compared with the rest of the transcriptome during syphilis infection [16, 17], suggesting that Tp0136 might have a significant role in the process of T. pallidum infection. Based on this finding, we stipulated that Tp0136 would promote the expression of MMP or TIMP, which in turn might contribute to the imbalance of MMP/TIMP, which can induce T. pallidum invasion and dissemination from the site of entry.
Herein, we conducted a number of in vitro studies to access the impact of recombinant Tp0136 protein on MMP/TIMP balance in human dermal vascular smooth muscle cells (HDVSMCs). Additionally, we investigated the function of PI3K, MAPK, and NF-κB signaling pathways in this procedure.
2. Materials and methods
2.1. Cell culture
HDVSMCs purchased from ATCC (Manassas, VA, USA) were cultured in DMEM/F-12 supplemented with 20% fetal bovine serum (Gibco, CA, USA) at 37 °C in a 5% CO2 atmosphere. After the HDVSMCs reached 90% confluence, they were subcultured in a 6-well culture plate (NEST biotechnology Co., LTD, Wuxi, China) at a ratio of 1:2, until they reached 90% confluence. These cells were then suspended in serum-free DMEM/F-12 for use in the subsequent experiments.
2.2. Expression and purification of the recombinant T. pallidum protein Tp0136
According to the instructions in our earlier investigation, Tp0136 was expressed and purified [18]. Thereafter, the endotoxin was then eliminated as previously mentioned [19].
2.3. MMP and TIMP expression profile analysis
HDVSMCs were seeded in 20% FBS-DMEM/F12, and after reaching 90% confluence these were stimulated for 24 h with 10 μg/mL Tp0136 (using phosphate-buffered saline [PBS] as a control). The expression profile of MMPrelated proteins in the cell culture supernatant was analyzed using an MMP antibody array (AAH-MMP1, Ray-Biotech, Norcross, GA, USA), following the manufacturer's instructions. Further, the chemiluminescence signal was analyzed using the ImageJ software, and MMP protein signal intensities were adjusted to those of the PBS group.
2.4. Analysis of MMP1, MMP3, TIMP1 and TIMP2 mRNA expressions
HDVSMCs were stimulated by Tp0136 at different concentrations (0, 1, 2.5, 5, 7.5, and 10 μg/mL) for 24 h and by 10 μg/mL Tp0136 for different time durations (0, 3, 6, 12, 24, and 36 h). PBS-treated HDVSMCs were used as controls. A total RNA kit (TANGEN Biotech Co., Ltd., Beijing, China) was employed to extract total RNA from HDVSMCs. Thereafter, following the manufacturer's instructions, first-strand cDNA synthesis was performed on 1μg of the extracted RNA using a first-strand cDNA synthesis kit (TransGen Biotech Co., Ltd. Beijing, China). RT-PCR was utilized to measure the mRNA expression levels of MMP1, MMP3, TIMP1, and TIMP2 as previously stated [20]. Table 1 contains a list of the primers utilized in the RT-PCR analyses.
Table 1.
Primers used for real-time PCR analysis in this study.
| Gene | Primer sequences (5′-3′) | |
|---|---|---|
| mmp1 | Forward primer | AAAATTACACGCCAGATTTGCC |
| Reverse primer | GGTGTGACATTACTCCAGAGTTG | |
| mmp3 | Forward primer | CGGTTCCGCCTGTCTCAAG |
| Reverse primer | CGCCAAAAGTGCCTGTCTT | |
| timp1 | Forward primer | TGCGGATACTTCCACAGGTC |
| Reverse primer | GCATTCCTCACAGCCAACAG | |
| timp2 | Forward primer | AAGAGCCTGAACCACAGGTA |
| Reverse primer | GAGCCGTCACTTCTCTTGAT | |
| gapdh | Forward primer | GAGTCAACGGATTTGGTCGT |
| Reverse primer | GACAAGCTTCCCGTTCTCAG | |
2.5. Analysis of MMP1, MMP3, TIMP1 and TIMP2 protein expressions
A commercial ELISA kit was applied to quantify the MMP1, MMP3, TIMP1, and TIMP2 proteins in the culture supernatants of HDVSMCs in accordance with the manufacturer's instructions. MMP1 and TIMP1 ELISA kits were obtained from RayBiotech, Inc. (Norcross, GA, USA). The MMP3 and TIMP2 ELISA kits were obtained from Jianglai biological Co., Ltd. (Shanghai, China). This was followed by calculating the rates of MMP1/TIMP1, MMP1/TIMP2, MMP3/TIMP1, and MMP3/TIMP2.
2.6. Analysis of PI3K/MAPK/NF-κB signaling pathway activation in Tp0136-treated HDVSMCs
Cell lysates from the above experiments were collected and utilized for the detection of phosphorylated PI3K, AKT, JNK, P38, c-Jun, IκBα, and total IκBα by western blotting. To further verify whether Tp0136 triggered the activation of PI3K/MAPK/NF-κB signaling pathways in HDVSMCs, HDVSMCs were preincubated with the PI3K inhibitor LY294002, JNK inhibitor SP600125, P38 inhibitor SB203580, or NF-κB inhibitor BAY11-7082 for 1 h, and then stimulated with 10 μg/mL Tp0136 for 24 h. The expression levels of MMP1, MMP3, TIMP1, and TIMP2 were then analyzed. To investigate the relationship between PI3K/MAPK and NF-κB signaling pathways, HDVSMCs were preincubated with LY294002, SP600125, or SB203580 for 1 h. Next, the cells were stimulated with 10 μg/mL Tp0136 for 90 min, and the protein levels of phosphorylated and total IκBα were detected as described above.
2.7. Analysis of NF-κB p65 nuclear translocation in Tp0136-treated HDVSMCs
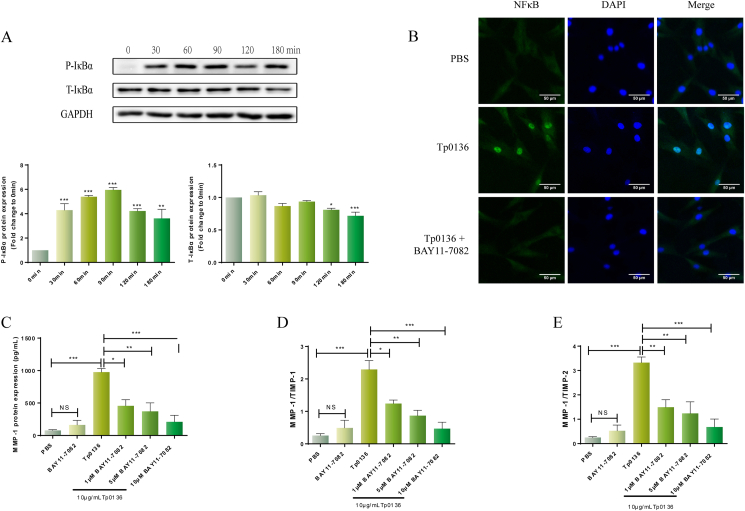
Nuclear translocation of the NF-κB p65 subunit in HDVSMCs was determined by using immunofluorescence staining. HDVSMCs were cultivated on a Millicell EZ Slide 4-well glass slide box and stimulated with Tp0136 at a concentration of 10 μg/mL for 1 h. Thereafter, cells were fixed and permeabilized for 15 min at room temperature with Triton X-100 (0.25%) in 4% formaldehyde. Then the slides with primary antibodies against NF-κB p65 (Cell Signaling Technology, Inc., MA, USA) were incubated at a dilution of 1:1000 overnight at 4 °C overnight, after that, cells were incubated for 2 h at room temperature with a FITC-conjugated anti-rabbit IgG secondary antibody (Abcam, Cambridge, USA) at a dilution of 1:800. Finally, the slides were treated with 4’,6-Diamidino-2-phenylindole (DAPI) for nuclear staining, and the translocation of p65 into the nucleus was observed using a confocal microscope (Zeiss Axio Observer LSM780, Oberkochen, Germany). HDVSMCs were treated with 10 μg/mL Tp0136 for 1 h after preincubating with LY294002, SP600125, SB203580, or BAY11-7082 for 1 h for the inhibition assay. Lastly, the analysis of NF-κB p65 nuclear translocation was performed as previously mentioned.
2.8. Statistical analysis
Statistical analysis was performed using SPSS, version 22.0 (SPSS Inc., Chicago, IL, USA). The results of the three independent experiments were expressed as means ± standard error of the means (SDs). The MMP antibody array's mean values for the PBS and Tp0136 groups were compared using the Paired Student's t-test. To compare the values among several groups, one-way ANOVA was employed. Statistical significance was set at P < 0.05.
3. Results
3.1. Effects of Tp0136 on the expression of MMPs and TIMPs in HDVSMCs
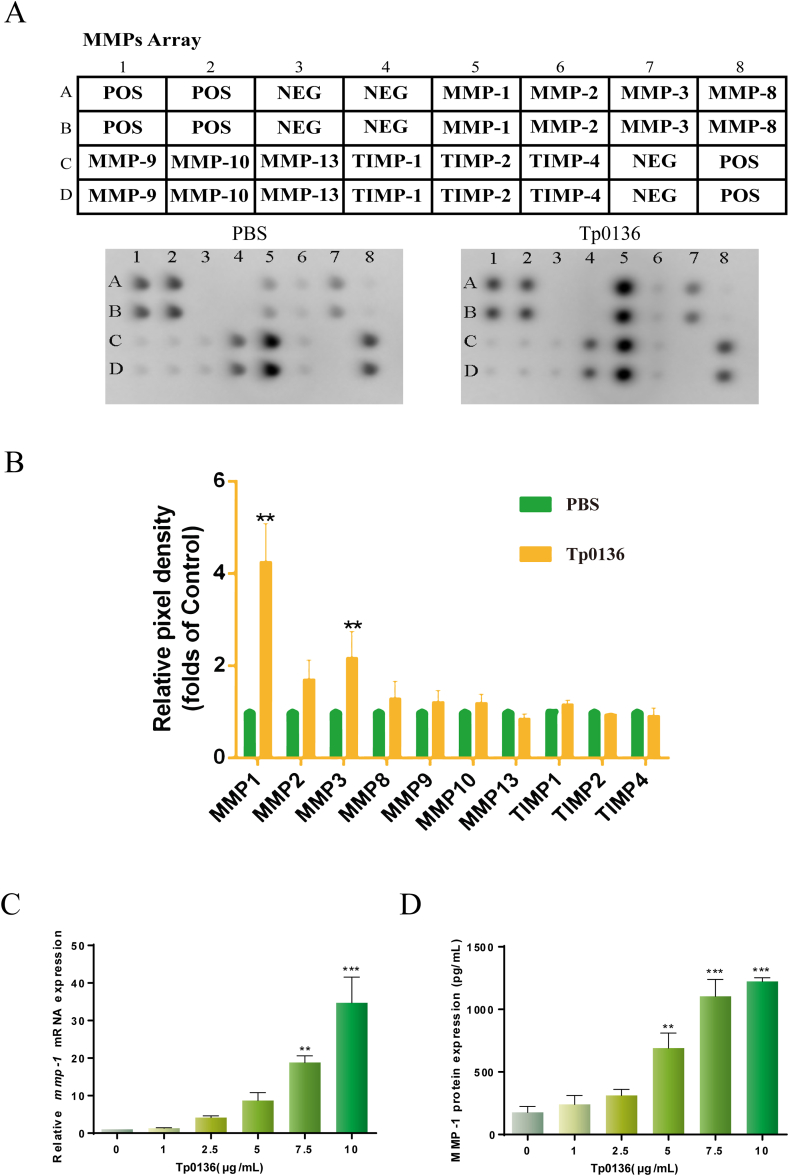
To determine the impact of Tp0136 on MMP and TIMP expression in HDVSMCs, the cells were treated with 10 μg/mL Tp0136 for 24 h, followed by an MMP antibody array analysis of MMPs and TIMPs secretion in the culture media (Figure 1A). It was observed that Tp0136 significantly induced the secretion of MMP1 and MMP3 in HDVSMCs compared to those in the PBS group (P < 0.01). TIMP1 and TIMP2 were highly expressed but the expression levels did not differ appreciably between the PBS and Tp0136 groups. In addition, MMP2, MMP8, MMP9, MMP10, MMP13, and TIMP4 were scarcely found (Figure 1B).
Figure 1.
Tp0136-induced expression of MMPs and TIMPs in HDVSMCs. (A and B) Expression profiles of MMPs and TIMPs based on the MMP array; (C) mRNA expressions of MMP1; (D) Protein expressions of MMP1. All values are represented as means ± SDs of triplicate samples and are representative of three independent experiments. The statistical significance of differences between the PBS and Tp0136 group data for the MMP array was analyzed using a paired t-test. One-way ANOVA was used to compare values among multiple groups (∗∗P < 0.01, ∗∗∗P < 0.001). MMP1: matrix metalloproteinase-1, MMP3: matrix metalloproteinase-3, TIMP1: tissue inhibitor of metalloproteinases-1, TIMP2: tissue inhibitor of metalloproteinases-2, POS: positive, NEG: negative.
To better understood if Tp0136 influenced the transcriptional and translational expression of MMP1, MMP3, TIMP1, and TIMP2 in HDVSMCs, we treated cells with various concentrations of Tp0136 (1, 2.5, 5, 7.5, and 10 μg/mL) for 24 h. It was noted that Tp0136 significantly increased MMP1 mRNA expression in a concentration-dependent way (Figure 1C). The mRNA expression levels of MMP1 were increased considerably at 7.5 μg/mL Tp0136 (P < 0.01) and reached a peak at 10 μg/mL Tp0136 (P < 0.001). In contrast, no significant differences were detected between the PBS and Tp0136 groups in the mRNA expression of MMP3, TIMP1, and TIMP2 (Figure S1). Furthermore, the protein expression of MMP1 was noted to be dependent on the concentration of Tp0136 and increased significantly at 5 μg/mL Tp0136 (P < 0.01) (Figure 1D). However, MMP3, TIMP1, and TIMP2 expression did not differ noticeably between the PBS and Tp0136 groups (Figure S1).
3.2. Effects of Tp0136 on the balance of MMPs/TIMPs in HDVSMCs
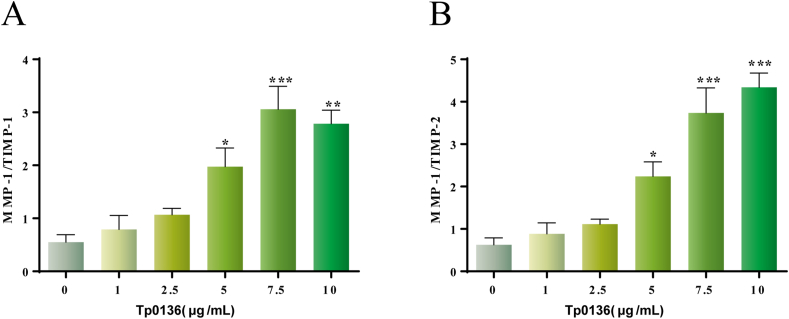
To clarify whether Tp0136 can cause the imbalance of MMPs/TIMPs, calculations were made to determine the ratios of MMP1/TIMP1, MMP1/TIMP2, MMP3/TIMP1, and MMP3/TIMP2. The results showed that the MMP1/TIMP1 and MMP1/TIMP2 ratios began to increase at 5 μg/mL Tp0136 (P < 0.05), and reached a maximal response at 7.5 μg/mL and 10 μg/mL Tp0136, respectively (P < 0.001) (Figure 2A and B). However, there were no differences in the MMP3/TIMP1 and MMP3/TIMP2 ratios among the two groups (P > 0.05) (Figure S2).
Figure 2.
Tp0136-induced imbalance of MMP and TIMP expression in HDVSMCs. (A) MMP1/TIMP1 ratio; (B) MMP1/TIMP2 ratio. The presented values are the means of triplicate trials; one-way ANOVA was used to compare values among multiple groups (∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001).
3.3. Tp0136-induced MMP/TIMP imbalance via PI3K signaling pathway in HDVSMCs
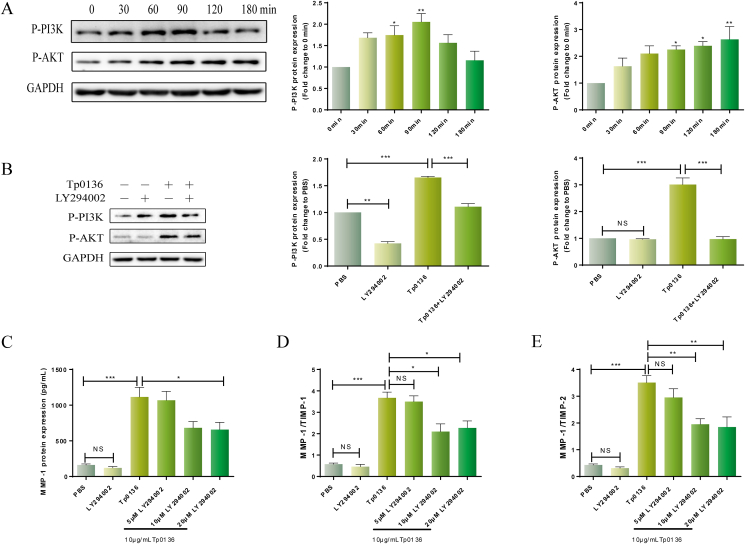
To explore the role of the PI3K signaling pathway in the MMP/TIMP imbalance caused by Tp0136, The phosphorylated protein levels of PI3K and AKT in HDVSMCs were measured following various time periods of stimulation with 10 μg/mL Tp0136. As shown in Figure 3A, phosphorylated PI3K protein was induced 30 min after treating cells with Tp0136 and increased markedly at 60 (P < 0.05) and 90 min (P < 0.01) post-treatment. In addition, the peak response of Tp0136 stimulated time-dependent phosphorylation of AKT protein was seen at 120 min (P < 0.01).
Figure 3.
Tp0136 activated the PI3K signaling pathway and promoted an MMP/TIMP imbalance in HDVSMCs. (A) Phosphorylated protein levels of AKT and PI3K induced by Tp0136; (B) Effect of PI3K inhibitors on Tp0136-induced phosphorylated AKT and PI3K expression; (C) Protein expression of MMP1; (D and E) The ratios of MMP1/TIMP1 and MMP1/TIMP2. One-way ANOVA was used to compare values among multiple groups (∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001). NS: not significant.
To identify the function of the PI3K signaling pathway in the MMP/TIMP imbalance caused by Tp0136, following a 1-h preincubation with the PI3K inhibitor LY294002, we stimulated the cells with Tp0136 for 60 min Figure 3B demonstrated that the phosphorylation of PI3K and AKT stimulated by Tp0136 was significantly weakened by LY294002 (P < 0.001). Thereafter, HDVSMCs were stimulated with Tp0136 for 24 h after receiving a 1-hour pretreatment with PI3K inhibitors. MMP1 production induced by Tp0136 was significantly inhibited as a result of pretreatment with LY294002 (P < 0.05) (Figure 3C). However, the protein expressions of MMP3, TIMP1, and TIMP2 did not differ significantly (P > 0.05) (Figure S3). In addition, the MMP1/TIMP1 and MMP1/TIMP2 ratios in the culture medium were considerably attenuated following pretreatment with LY294002 in comparison to those in the Tp0136 group. (P < 0.05) (Figure 3D and E). On the other hand, HDVSMCs preincubated with LY294002 did not demonstrate a lowering of the MMP3/TIMP1 or MMP3/TIMP2 ratio (P > 0.05) (Figure S3).
3.4. Tp0136-induced MMP/TIMP imbalance via MAPK signaling pathway in HDVSMCs
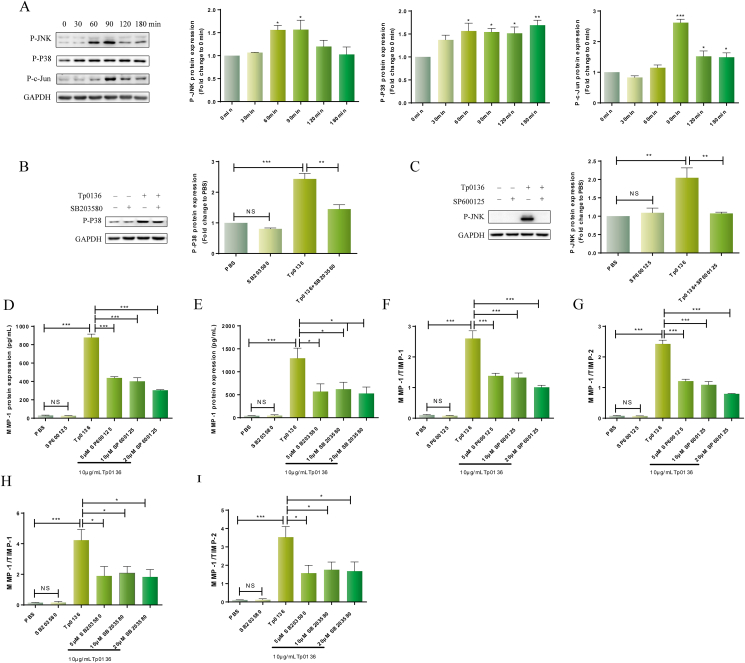
The MAPK signaling pathway is an important signal transmitter from the cell surface to the nucleus and plays a critical part in the regulation of gene expression and cytoplasmic functional activities in eukaryotic cells. The MAPK signaling pathway comprises the JNK and P38 subfamilies. To determine if Tp0136 promoted MAPK signaling pathway activation in HDVSMCs, the phosphorylation protein levels of JNK, P38, and c-Jun were directly examined after treatment of HDVSMCs with Tp0136 for different time durations as specified above. As shown in Figure 4A, the phosphorylation of JNK was significantly decreased at 60 min after treatment of cells with Tp0136 and peaked at 90 min (P < 0.05). Tp0136 stimulated P38 phosphorylation in a time-dependent way, with a significant response at 60 min. (P < 0.05), which was sustained at 120 min (P < 0.01). At 90 min, c-Jun phosphorylation substantially increased (P < 0.001), and at 120 min, it started to significantly decline (P < 0.05).
Figure 4.
Tp0136 activated the MAPK signaling pathway and promoted an MMP/TIMP imbalance in HDVSMCs. (A) Phosphorylated protein levels of JNK, P38 and c-Jun induced by Tp0136; (B and C) Effect of JNK and P38 inhibitors on Tp0136-induced phosphorylated JNK and P38 expression; (D and E) Protein expression of MMP1. (F–I) Ratios of MMP1/TIMP1, MMP1/TIMP2. One-way ANOVA was used to compare values among multiple groups (∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001). NS: not significant.
To investigate the role of the MAPK signaling pathway in the imbalance of MMP/TIMP imbalance induced by Tp0136, cells were first pre-incubated for 1 h with SP600125 (a JNK inhibitor) or SB203580 (a P38 inhibitor) and then stimulated with Tp0136 for 60 min; Tp0136-stimulated JNK and P38 phosphorylation were observed to be significantly inhibited (Figure 4B and C) (P < 0.01). Furthermore, Tp0136-induced MMP1 production was considerably reduced by pretreatmenting with SP600125 (P < 0.001) and SB203580 (P < 0.05) (Figure 4D and E). Similarly, pretreatment with SP600125 or SB203580 attenuated Tp0136-induced escalation in the ratios of MMP1/TIMP1 and MMP1/TIMP2 at 24 h (Figure 4F and I). However, SP600125 or SB203580 did not inhibit the expression of MMP3, TIMP1, TIMP2, or the ratio of MMP1/TIMP1 and MMP1/TIMP2 in HDVSMCs stimulated with Tp0136 (Figure S4).
3.5. Tp0136-induced MMP/TIMP imbalance via NF-κB signaling pathway in HDVSMCs
NF-κB is a nuclear transcription factor that is widely present in eukaryotes and regulates the transcription of multiple cytokines and protease genes. In the present study, phosphorylation of IκBα and the degradation of total IκBα (an indicator of NF-κB activation) were identified following stimulation of HDVSMCs for the specified durations with 10 g/mL Tp0136. Figure 5A shows that the expression of phosphorylated-IκBα became evident at 30 min (P < 0.001) after treatment with Tp0136 and peaked at 90 min (P < 0.01). IκBα degradation started at 120 min (P < 0.05) and was sustained at 180 min (P < 0.001) after initiating treatment. Furthermore, 60 min after treatment, Tp0136 clearly triggered NF–B p65 subunit translocation from the cytoplasm into the nucleus. This occurrence was attenuated by pretreatment with the NF–B inhibitor BAY11-7082 (Figure 5B).
Figure 5.
Tp0136 activated the NF-κB signaling pathway and promoted an MMP/TIMP imbalance in HDVSMCs. (A) Phosphorylated and total protein levels of IκBα induced by Tp0136; (B) Effect of NF-κB inhibitors on Tp0136-induced NF-κB p65 nuclear translocation; (C) Protein expressions of MMP1. (D and E) The ratios of MMP1/TIMP1, MMP1/TIMP2. One-way ANOVA was used to compare values among multiple groups (∗P < 0.05, ∗∗P < 0.01 and ∗∗∗P < 0.001). NS: not significant.
To ascertain the part played by the NF–B signaling pathway in the MMP/TIMP imbalance triggered by Tp0136, we used BAY11-7082, an NF-κB inhibitor. Pretreatment with this inhibitor led to a significant decrease in MMP1 protein expression in the Tp0136-induced HDVSMCs’ culture medium at 24 h (P < 0.05) (Figure 5C). Similarly, the ratios of MMP1/TIMP1 and MMP1/TIMP2 in HDVSMCs stimulated with Tp0136 were diminished by treatment with BAY11-7082 (P < 0.05) (Figure 5D and E). However, BAY11-7082 did not inhibit the expression of MMP3, TIMP1, TIMP2, and the values of MMP1/TIMP1 and MMP1/TIMP2 ratios in HDVSMCs stimulated with Tp0136 (Figure S5).
3.6. PI3K and MAPK signaling pathways mediated NF-κB activity in Tp0136-induced HDVSMCs
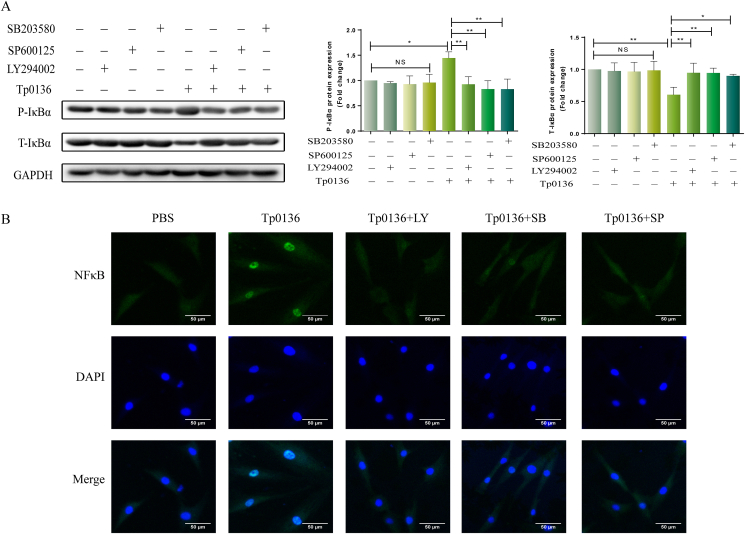
To further explore the relationships between PI3K/MAPK and NF-κB signaling pathways, HDVSMCs were incubated with PI3K and MAPK inhibitors (LY294002, SP600125, and SB203580) for 1 h. Thereafter, the IκBα phosphorylation and IκBα degradation were detected after the stimulation of the cells with 10 μg/mL Tp0136 for 90 min. Preincubation with LY294002, SP600125, or SB203580 substantially inhibited the phosphorylation of IκBα stimulated by Tp0136 and degradation of total IκBα. (P < 0.05) (Figure 6A). Likewise, Tp0136-induced translocation of NF-κB p65 subunit into the nucleus was attenuated by pretreatment with LY294002, SP600125, or SB203580 at 90 min post-treatment (Figure 6B).
Figure 6.
PI3K and MAPK signaling pathways mediated NF-κB activity in Tp0136-induced HDVSMCs. (A) Phosphorylation and total protein levels of IκBα; (B) NF-κB p65 subunit translocation into the nucleus. Values represent the mean ± SD of triplicate samples and are representative of three independent experiments (∗P < 0.05, ∗∗P < 0.01). LY: LY294002, SB: SB203580, SP: SP600125, NS: not significant.
4. Discussion
The invasive capability of T. pallidum is exemplified by the widespread disease manifestations associated with syphilis [21]. Shortly after inoculation, T. pallidum invades the local site of infection and its hematogenous dissemination occurs well before the appearance of the chancre. It has been suggested that in addition to contributing to the pathogenic invasion of T. pallidum from the infection site into the bloodstream, MMPs also play a role in the enhancement of vascular permeability. TIMPs inhibit the activity of MMPs via reversible blockage, leading to the formation of 1:1 stoichiometric complexes [22]. The balance of activities between MMPs and TIMPs is important for regulating both, physiological as well as pathological processes within the body, including wound healing, angiogenesis, and invasion [22, 23, 24]. In most infectious diseases, pathogens enhance the proteolytic activity of MMPs, causing a disruption of the MMP/TIMP balance. This results in the breakdown of the host's defense barrier by ECM degradation, thereby promoting the escape and diffusion of pathogens into surrounding structures [12, 24, 25, 26]. Prior articles have reported that T. pallidum flagellin induces not only the expression of inflammatory cytokines such as IL-6 and IL-8 but also the expression of MMP9 and MMP13 [12,27]. In addition, T. pallidum can stimulate human dermal fibroblasts to increase the synthesis of MMP1, which is essential for degrading type I collagen, the most abundant component of the human dermis [28]. In the current investigation, we discovered that Tp0136, a human fibronectin-binding protein [29], was able to induce the synthesis and secretion of MMP1 in HDVSMCs, resulting in an imbalance of MMPs/TIMPs involving the PI3K, MAPK, and NF-κB signaling pathways, suggesting that Tp0136 may play an important role in T. pallidum invasion. In addition, vascular smooth muscle cells are not passive spectators during the process of vascular angiogenesis, they may play a key role in vascular angiogenesis in functional and organic disorders and result in cardiovascular syphilis. Our previous study found that recombinant T. pallidum protein Tp47 imbalances MMP/TIMP in endothelial cells, which can stimulate angiogenesis [14]. By adjusting the matrix metalloproteinase/tissue inhibitor of metalloproteinase balance in endothelial cells, the recombinant Treponema pallidum protein Tp47 stimulates angiogenesis. Whether the Tp0136-induced imbalance of MMP/TIMP, observed in the present study, also plays a crucial role in vascular angiogenesis, and its possible larger role in the pathogenesis of cardiovascular syphilis needs to be investigated further.
It is well known that the expression and secretion of MMPs are regulated by cell signaling molecules, transcription factors, and post-transcriptional regulators. Previous articles have shown that the activation of PI3K, MAPK, and NF-κB pathways plays an important part in the upregulation of MMP expression in a variety of cell types [12, 13, 14, 30]. In the present study, we examined the relevant signaling pathways involved in the MMP/TIMP imbalance, induced by the Tp0136 protein. Our results indicated that PI3K (PI3K and AKT), MAPK (JNK, P38, and c-Jun), and NF-κB signaling pathways were activated in this procedure, and that the MMP/TIMP imbalance could be alleviated by the inhibitors of these pathways. In addition, Tp0136-induced phosphorylation of IκBα and the translocation of NF-κB p65 subunit into the nucleus were inhibited by PI3K, JNK, and P38 inhibitors, indicating that Tp0136-stimulated phosphorylation of IκBα and NF-κB translocation are mediated by the PI3K and MAPK signaling pathways.
Despite its several merits, our study had a few limitations. First, the recombinant Tp0136 expressed in Escherichia coli is not a natural structure of T. pallidum. Second, the imbalance of MMPs/TIMPs is a complicated process involving several interrelated factors. We found that both PI3K and MAPK signaling pathways were activated during Tp0136-induced MMP1/TIMP1 and MMP1/TIMP2 imbalance, and the relationship between PI3K and MAPK signaling pathways needs further exploration. Third, the current study lacks gain-of-function experiments, and future animal experiments are needed to confirm how T. pallidum infection is impacted by the imbalance of MMP/TIMP.
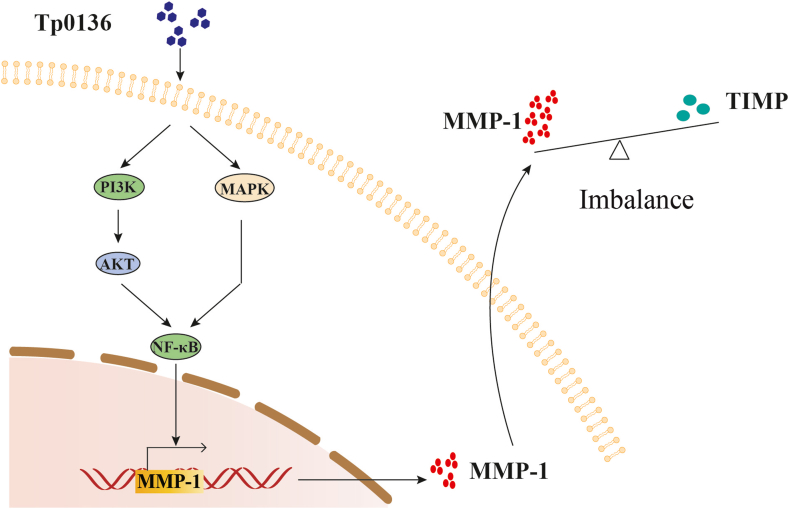
In conclusion, our study found that Tp0136 causes an imbalance of MMPs/TIMPs by increasing the expression of MMP1 through PI3K, MAPK, and NF-κB signaling pathways in HDVSMCs (Figure 7). This may contribute to the degradation of vascular ECM and pathogenic spread during T. pallidum infection. These findings offer novel insights into the molecular pathogenesis of T. pallidum infection.
Figure 7.
Schematic diagram of the proposed mechanism of Tp1036-induced imbalance of MMPs/TIMPs via PI3K, MAPK, and NF-κB signaling pathways in HDVSMCs.
Declarations
Author contribution statement
Chun-Xiang Cai: Performed the experiments; Wrote the paper.
Shu-Lian Li: Performed the experiments.
Hui-Ling Lin; Zi-Han Wei; Lin Xie: Contributed reagents, materials, analysis tools or data.
Li-Rong Lin; Tian-Ci Yang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Jian-Jun Niu: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
Dr Li-Rong Lin was supported by National Natural Science Foundation of China [82172331; 81972028; 81672094], Key Projects for Province Science and Technology Program of Fujian Province [2020D017].
Tian-Ci Yang was supported by National Natural Science Foundation of China [81973104; 81772260], Natural Science Foundation of Fujian Province [2021J02055].
Jian-Jun Niu was supported by Fujian Provincial Health Technology Project [2020CXB047].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at [URL].
Contributor Information
Li-Rong Lin, Email: linlirong@xmu.edu.cn.
Jian-Jun Niu, Email: niujianjun211@xmu.edu.cn.
Tian-Ci Yang, Email: yangtianci@xmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Denman J., Hodson J., Manavi K. Infection risk in sexual contacts of syphilis: a systematic review and meta-analysis. Journa. 2022;84:760–769. doi: 10.1016/j.jinf.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Gao K., Shen X., Lin Y., Zhu X.Z., Lin L.R., Tong M.L., Xiao Y., Zhang H.L., Liang X.M., Niu J.J., Liu L.L., Yang T.C. Origin of nontreponemal antibodies during Treponema pallidum infection: evidence from a rabbit model. Journa. 2018;218:835–843. doi: 10.1093/infdis/jiy241. [DOI] [PubMed] [Google Scholar]
- 3.Newman L., Rowley J., Vander Hoorn S., Wijesooriya N.S., Unemo M., Low N., Stevens G., Gottlieb S., Kiarie J., Temmerman M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. Journa. 2015;10 doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín-Ezquerra G., Fernandez-Casado A., Barco D., Jucglà A., Juanpere-Rodero N., Manresa J.M., de Almeida L.M., Rodríguez-Peralto J.L., Kutzner H., Cerroni L., Barranco C., Lloreta J., Requena L., Pujol R.M. Treponema pallidum distribution patterns in mucocutaneous lesions of primary and secondary syphilis: an immunohistochemical and ultrastructural study. Journa. 2009;40:624–630. doi: 10.1016/j.humpath.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Harman M., Vig D.K., Radolf J.D., Wolgemuth C.W. Viscous dynamics of Lyme disease and syphilis spirochetes reveal flagellar torque and drag. Journa. 2013;105:2273–2280. doi: 10.1016/j.bpj.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafond R.E., Lukehart S.A. Biological basis for syphilis. Journa. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabral-Pacheco G.A., Garza-Veloz I., Castruita-De la Rosa C., Ramirez-Acuña J.M., Perez-Romero B.A., Guerrero-Rodriguez J.F., Martinez-Avila N., Martinez-Fierro M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Journa. 2020;21 doi: 10.3390/ijms21249739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui N., Hu M., Khalil R.A. Biochemical and biological attributes of matrix metalloproteinases. Journa. 2017;147:1–73. doi: 10.1016/bs.pmbts.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor C., Vaidya S., Wadhwan V., Kaur G., Pathak A. Seesaw of matrix metalloproteinases (MMPs) Journa. 2016;12:28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- 10.Arpino V., Brock M., Gill S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Journa. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S., Allen E., Chung D., Weiss S.J. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. Journa. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang C., Xu M., Kuang X., Xiao J., Tan M., Xie Y., Xiao Y., Zhao F., Wu Y. Treponema pallidum flagellins stimulate MMP9 and MMP13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes. Journa. 2017;361:46–55. doi: 10.1016/j.yexcr.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Lin S.W., Gao Z.X., Lin L.R., Luo X., Liu L.L., Yang T.C. Treponema pallidum enhances human monocyte migration and invasion by dysregulating the MMP/TIMP balance. Journa. 2019;75 doi: 10.1016/j.intimp.2019.105744. [DOI] [PubMed] [Google Scholar]
- 14.Gao Z.X., Luo X., Liu L.L., Lin L.R., Tong M.L., Yang T.C. Recombinant Treponema pallidum protein Tp47 induces angiogenesis by modulating the matrix metalloproteinase/tissue inhibitor of metalloproteinase balance in endothelial cells. Journa. 2019;33:1958–1970. doi: 10.1111/jdv.15725. [DOI] [PubMed] [Google Scholar]
- 15.Luo X., Gao Z.X., Lin S.W., Tong M.L., Liu L.L., Lin L.R., Ke W.J., Yang T.C. Recombinant Treponema pallidum protein Tp0136 promotes fibroblast migration by modulating MCP1/CCR2 through TLR4. Journa. 2020;34:862–872. doi: 10.1111/jdv.16162. [DOI] [PubMed] [Google Scholar]
- 16.Smajs D., McKevitt M., Howell J.K., Norris S.J., Cai W.W., Palzkill T., Weinstock G.M. Transcriptome of Treponema pallidum: gene expression profile during experimental rabbit infection. Journa. 2005;187:1866–1874. doi: 10.1128/JB.187.5.1866-1874.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Lay B.D., Cameron T.A., De Lay N.R., Norris S.J., Edmondson D.G. Comparison of transcriptional profiles of Treponema pallidum during experimental infection of rabbits and in vitro culture: highly similar, yet different. Journa. 2021;17 doi: 10.1371/journal.ppat.1009949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo X., Lin S.W., Xu Q.Y., Ke W.J., Gao Z.X., Tong M.L., Liu L.L., Lin L.R., Zhang H.L., Yang T.C. Tp0136 targets fibronectin (RGD)/Integrin β1 interactions promoting human microvascular endothelial cell migration. Journa. 2020;396 doi: 10.1016/j.yexcr.2020.112289. [DOI] [PubMed] [Google Scholar]
- 19.Gao Z.X., Liu D., Liu L.L., Lin L.R., Tong M.L., Niu J.J., Yang T.C. Recombinant Treponema pallidum protein Tp47 promotes the migration and adherence of THP1 cells to human dermal vascular smooth muscle cells by inducing MCP1 and ICAM-1 expression. Journa. 2019;381:150–162. doi: 10.1016/j.yexcr.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M., Gong Q.Y., Lai S.S., Cheng Z.X., Chen Z.G., Zheng J., Peng B. Phenylalanine enhances innate immune response to clear ceftazidime-resistant Vibrio alginolyticus in Danio rerio. Journa. 2019;84:912–919. doi: 10.1016/j.fsi.2018.10.071. [DOI] [PubMed] [Google Scholar]
- 21.Lithgow K.V., Church B., Gomez A., Tsao E., Houston S., Swayne L.A., Cameron C.E. Identification of the neuroinvasive pathogen host target, LamR, as an dhesin Tp0751. Journa. 2020;5 doi: 10.1128/mSphere.00195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson H.W., Defamie V., Waterhouse P., Khokha R. TIMPs: versatile extracellular regulators in cancer. Journa. 2017;17:38–53. doi: 10.1038/nrc.2016.115. [DOI] [PubMed] [Google Scholar]
- 23.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Journa. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kübler A., Luna B., Larsson C., Ammerman N.C., Andrade B.B., Orandle M., Bock K.W., Xu Z., Bagci U., Mollura D.J., Marshall J., Burns J., Winglee K., Ahidjo B.A., Cheung L.S., Klunk M., Jain S.K., Kumar N.P., Babu S., Sher A., Friedland J.S., Elkington P.T., Bishai W.R. Mycobacterium tuberculosis dysregulates MMP/TIMP balance to drive rapid cavitation and unrestrained bacterial proliferation. Journa. 2015;235:431–444. doi: 10.1002/path.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W.L., Sheu J.R., Chen R.J., Hsiao S.H., Hsiao C.J., Chou Y.C., Chung C.L., Hsiao G. Mycobacterium tuberculosis upregulates TNF-α expression via TLR2/ERK signaling and induces MMP1 and MMP9 production in human pleural mesothelial cells. Journa. 2015;10 doi: 10.1371/journal.pone.0137979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grenier D., Uitto V.J., McBride B.C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Journa. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M., Xie Y., Jiang C., Xiao Y., Kuang X., Wen Y., Tan Y., Tan M., Zhao F., Zeng T., Wu Y. Treponema pallidum flagellins elicit proinflammatory cytokines from human monocytes via TLR5 signaling pathway. Journa. 2017;222:709–718. doi: 10.1016/j.imbio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Chung K.Y., Kim K.S., Lee M.G., Chang N.S., Lee J.B. Treponema pallidum induces uPregulation of interstitial collagenase in human dermal fibroblasts. Journa. 2002;82:174–178. doi: 10.1080/00015550260132442. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman M.B., McGill M.A., Pettersson J., Rogers A., Matejkova P., Smajs D., Weinstock G.M., Norris S.J., Palzkill T. A novel Treponema pallidum antigen, TP0136, is an outer membrane protein that binds human fibronectin. Journa. 2008;76:1848–1857. doi: 10.1128/IAI.01424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan C., Boyd D.D. Regulation of matrix metalloproteinase gene expression. Journa. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.