Highlights
-
•
Neighborhood-level factors are important in healthcare utilization after surgery.
-
•
Social factors can be reliably identified pre-op engendering targeted interventions.
-
•
Area of Deprivation Index predicts resource utilization following spine surgery.
Keywords: Socioeconomic status, Length of stay, Lumbar decompression, Healthcare policy, Economics, Healthcare utilization
Abstract
Background
In the context of increased attention afforded to hospital efficiency and improved but safe patient throughput, decreasing unnecessary hospital length of stay (LOS) is imperative. Given that lumbar spine procedures may be among a hospital's most profitable services, identifying patients at risk of increased healthcare resource utilization prior to surgery is a valuable opportunity to develop targeted pre- and peri-operative intervention and quality improvement initiatives. The purpose of the present investigation was to examine patient factors that predict prolonged LOS as well as discharge disposition following elective, posterior, lumbar spine surgery.
Methods
We employed a retrospective cohort analysis on 779 consecutive patients treated with lumbar surgery without fusion. Our primary outcome measures were extended LOS (three or more midnights) and discharge disposition. Patient sociodemographic, procedural, and discharge characteristics were adjusted for in our analysis. Sociodemographic variables included Area of Deprivation Index (ADI), a comprehensive metric of socioeconomic status, utilizing income, education, employment, and housing quality based on patient zip code. Multivariable logistic regression and ordinal logistic regression analyses were performed to assess whether covariates were independently predictive of extended LOS and discharge disposition, respectively.
Results
779 patients were studied, with a median age of 66 years (±15) and a median LOS of 1 midnight (range, 1-10 midnights). Patients in the most disadvantaged ADI quintile (adjusted odds ratio, aOR 2.48 95% CI 1.15-5.47), those who underwent a minimally-invasive or tubular retractor surgery (aOR 3.03 95% CI 1.02-8.56), those who had an intra-operative drain placed (aOR 4.46 95% CI 2.53-7.26), who had a cerebrospinal fluid leak (aOR 3.46 95% CI 1.55-7.58), who were discharged anywhere but home (aOR 17.11 95% CI 9.24-33.00), and those who were evaluated by physical therapy (aOR 7.23 95% CI 2.13-45.30) or OT (aOR 2.20 95% CI 1.13-4.22) had a significantly increased chance of an extended LOS. Preoperative opioid use was not associated with an increased LOS following surgery (aOR 1.12 95% CI 0.56-1.46). Extended LOS was not associated with post-discharge emergency department representation or unplanned readmission within 90 days following discharge (p=0.148). Patients who were older (aOR 1.99 95% CI 1.62-2.48), in higher quintiles on ADI (3rd quintile; aOR 1.90 95% CI 1.12-3.23, 4th quintile; aOR 1.79, 95% CI 1.05-3.05, 5th quintile; aOR 2.16 95% CI 1.26-3.75), who had a CSF leak (aOR 2.18 95% CI 1.22-3.86), or who had a longer procedure duration (aOR 1.38 95% CI 1.17-1.62) were more likely to require additional services or be sent to a subacute facility upon discharge.
Conclusions
Patient sociodemographics, along with procedural factors, and discharge disposition were all associated with an increased likelihood of prolonged LOS and resource intensive discharges following elective lumbar spine surgery. Several of these factors could be reliably identified pre-operatively and may be amenable to targeted preoperative intervention. Improving discharge disposition planning in the peri-operative period may allow for more efficient use of hospitalization and inpatient and post-acute resources.
Introduction1
As pay-for-performance and bundled care reimbursement structures become more prevalent, health systems face increasing financial pressure to deliver high quality care more efficiently and cost effectively [1,2]. Extended length of stay (LOS) and non-home discharges are associated with significant healthcare costs [3,4]. However, a small percentage of patients contribute to the majority of these costs—roughly 10% of patients contribute to 63% of all US healthcare expenditures [5,6]. Given that lumbar spine surgeries may be among a hospital's most profitable services [7] and that the volume of spine surgery performed is increasingly annually [8], identifying patients at risk of extended LOS and discharge to non-home destinations prior to surgery is a valuable opportunity to develop targeted pre- and peri-operative intervention, to improve quality and outcomes, and to decrease needless expense.
Prior research has identified patient age, baseline functional status, governmental forms of insurance, procedure duration, and surgical complications as risk factors for prolonged LOS and resource intensive discharges [4,[9], [10], [11], [12], [13], [14], [15], [16]]. However, these studies were largely performed on national databases which only provide coarse estimates of socioeconomic status (SES) through variables such as age, gender, race, and insurance status [9,[12], [13], [14],[17], [18], [19], [20]]. These measures may not adequately encapsulate a patient's true SES, which is affected by local, neighborhood-level factors [21]. One metric of area-level social determinants of health is Area of Deprivation index (ADI), a composite measure of income, education, employment, and housing quality [22], [23], [24]. Patients with an extended LOS and non-home discharges disproportionately contribute to costs of care [3,4,25], and thus recognizing these patients is critical.
It remains unclear how area-level metrics of deprivation, such as ADI, may affect outcomes and resource utilization following spine surgery. In patients undergoing and elective, posterior non-fusion lumbar surgery, we examined potential interactions between socioeconomic status, length of stay, complications, disposition at discharge, and unplanned re-engagement with the acute care setting within 90 days of surgery.
Material and methods
Study population
A single-center, retrospective, cohort analysis using electronic medical record (EMR) data from patients receiving lumbar surgery between April 1, 2015, and December 1, 2021, was performed. Using Current Procedural Terminology (CPT) codes, patients, 18 years and older, who underwent an elective posterior, non-endoscopic, lumbar laminectomy, discectomy, microdiscectomy, foraminotomy, and/or facetectomy, without fusion, were identified. This study was approved by the local Institutional Review Board (IRB, [BLINDED FOR REVIEW]). Patient consent was waived in accordance with adjudicating IRB policy.
Exclusion criteria
Patients with significant functional neurological disability that required pre-operative hospitalization were excluded as were patients admitted to the intensive care unit (ICU) at any point during their hospitalization so as to capture an elective cohort of patients. Patients admitted for emergency surgery (i.e. cauda equina syndrome) were excluded as were those who underwent multi-stage surgical interventions.
Covariates
We included sociodemographic, procedural, and hospitalization characteristics for our cohort and adjusted for them in our statistical analyses. Sociodemographic features included age and ADI, a comprehensive metric of socioeconomic status based on the patient's nine-digit zip code. Patient's nine-digit zip code is calculated from the street address present in their EMR. Patient's nine-digit zip code is then converted to an ADI using a publicly available database [26]. ADIs account for patient education, housing quality, income, and employment. The metric is a national percentile ranking; a score of 100 is most disadvantaged and a score of 1 is least disadvantaged. ADI was divided into quintiles as previously described in the literature [27], [28], [29].
Procedural covariates included surgeon experience, procedure day of the week, procedure duration, procedure start time, preprocedural opioid use, redo operation at the same site of a prior surgery, utilization of a tubular retractor system or minimally invasive surgery (MIS), drain placement, and intraoperative cerebrospinal fluid (CSF) leak. Hospital admission characteristics included discharge disposition and whether the patient was evaluated by physical therapy (PT) or occupational therapy (OT) specialists post-operatively.
Primary and secondary outcomes variables
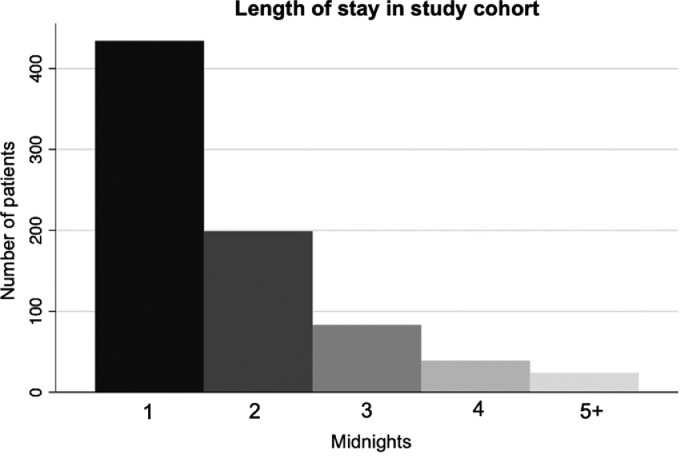
The primary outcome measure was extended LOS following surgery, defined as the 90th percentile or greater in the number of midnights admitted post-procedurally (three or more, Figure 1) [5,[30], [31], [32]]. Our secondary outcome variables were 90-day readmission or emergency department representation risk related to surgery, and discharge disposition.
Figure 1.
Entire patient population (N=779) and their length of stays following elective lumbar surgery.
Statistical analysis
Univariate analyses assessed unadjusted demographic, procedural, and discharge characteristics between patient cohorts. Generalized linear modeling through a multivariate logistic regression analysis then assessed factors independently predictive of a prolonged LOS. Post-hoc variance inflation factor (VIF) was calculated. All VIFs were less than 5. As a result, all variables were included in multiple logistic regression analysis evaluating whether our covariates independently predicted extended LOS. Pearson's chi-squared test assessed whether those who had an extended LOS were at increased risk of readmission or emergency department (ED) re-presentation within 90 days of hospital discharge.
Ordinal logistic regression was used to elucidate factors associated with discharge disposition. For this analysis, discharge to home served as a reference category versus either with discharge to home with services or to a skilled nursing facility (SNF) or acute rehabilitation, creating a scale of increasing dependence on medical assistance upon discharge. Less than 5% of data was missing from our patient cohort, thus statistical analyses were performed on a complete case basis. Odds ratios (OR) are reported as adjusted OR (aOR). Continuous variables were normalized for regression analyses. A p-value of <0.05 was considered statistically significant. Stata statistical software version 17 (StataCorp LP, College Station, Texas) and R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for data management, statistical analysis, and figure generation.
Results
Patient cohort and descriptive statistics
779 consecutive patients met inclusion criteria for the study (Table 1). The cohort had a median age of 68 years (IQR: 57, 77) with 650 (83.4%) identifying as white, 41 (5.3%) identifying as African-American, 65 (8.3%) as Hispanic, and 23 (3.0%) as another race. One-hundred and eighteen (15.1%) identified their primary language spoken as one other than English. Insurance statuses of the cohort included 402 (51.6%) with private insurance, 275 (35.3%) with Medicare, 77 (9.9%) with Medicaid, and 25 (3.2%) with another form of insurance. ADI was not normally distributed on a Shapiro-Wilk Test (p<0.001) with a rightward skew (towards less disadvantaged indices). One hundred and sixteen (14.9%) patients underwent reoperation after prior decompression at the same site, 42 (5.4%) underwent surgery with a tubular retractor system, 276 (35.4%) had a drain placed, 58 (7.5%) had an intraoperative a CSF leak, and 281 (36.1%) took an opioid medication pre-procedurally. The median case duration in minutes was 94 (IQR: 72, 131). Six-hundred and thirty-one (81.0%) patients were evaluated by PT and 82 (10.5%) were evaluated by OT.
Table 1.
Summary statistics of patient cohort (N=779).
| Age, median (IQR) | 68 (57, 77) |
| Race | |
| White | 650 (83.4%) |
| African-American | 41 (5.3%) |
| Hispanic | 65 (8.3%) |
| Other | 23 (3.0%) |
| English as primary language | 661 (96.4%) |
| Insurance status | |
| Private | 402 (51.6%) |
| Medicare | 275 (35.3%) |
| Medicaid | 77 (9.9%) |
| Other | 25 (3.2%) |
| Area of Deprivation Index, median (IQR) | 34 (23, 49) |
| Surgeon experience | |
| > 5 years | 595 (76.4%) |
| 2 – 5 years | 114 (14.6%) |
| < 1 year | 70 (9.0%) |
| Case duration in minutes, median (IQR) | 94 (72, 131) |
| 90-day emergency department visit (%) | 66 (8.5%) |
| 90-day readmission, frequency (%) | 57 (7.3%) |
IQR, interquartile range
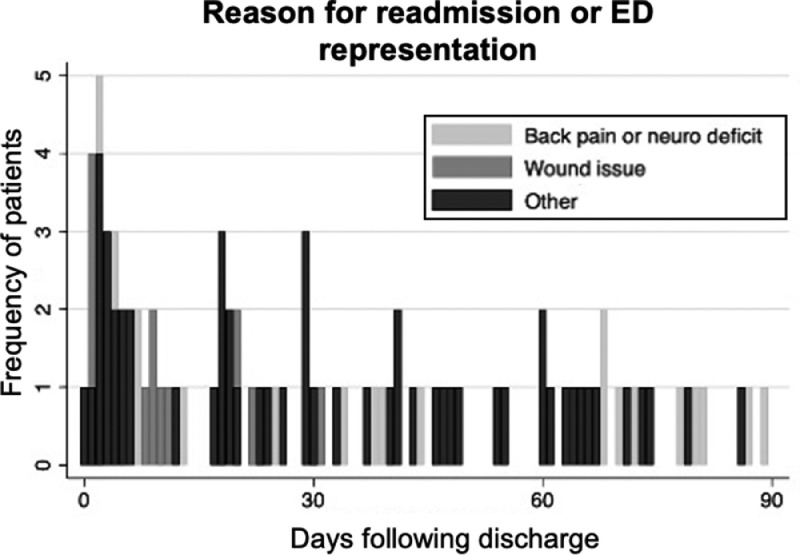
At discharge, 523 (67%) patients went home without services, 166 (21%) went home with services, and 88 (11%) went to a SNF or a rehabilitation facility (Supplemental Figure 1). Two (0.3%) patients were discharged to law enforcement. The most common reason for an ED visit or readmission following discharge was back pain or concern for a neurological deficit (n=46) followed by a wound issue (n=21). Fifty-six patients represented to the ED or were readmitted for a reason that was not back pain or concern for a neurological deficit or a wound issue (Figure 2).
Figure 2.
Ninety-day readmission or emergency department (ED) representation risk by reason. The most common reason for an ED visit or readmission following discharge was back pain or concern for a neurological deficit (n=46) followed by a wound issue (n=21). The remaining patients (n=56) came to the ED or were readmitted for another reason.
Extended LOS regression
Results from multivariable logistic regression (Table 2) demonstrated that patients in the most disadvantaged quintile on ADI (aOR 2.48; 95% CI 1.15-5.47; p=0.022), who underwent minimally invasive surgery (aOR 3.03; 95% CI 1.02-8.56; p=0.039), who had an intra-operative drain placed (aOR 4.46; 95% CI 2.53-7.26; p<0.001), who had a CSF leak (aOR 3.46; 95% CI 1.55-7.58; p=0.002), who were discharged anywhere but home (aOR 17.1; 95% CI 9.24-33.0; p<0.001), and who were evaluated by PT (aOR 7.23; 95% CI 2.13-45.3; p=0.008) or OT (aOR 2.20; 95% CI 1.13-4.22; p=0.018) had a significantly increased chance of an extended LOS. Patient LOS was not associated with surgeon years of experience.
Table 2.
Multivariable logistic regression for prolonged length of stay following elective lumbar surgery.
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.13 | 0.85 - 1.51 | 0.396 |
| Area of Deprivation Index (quintile) | |||
| 1st | Reference | – | – |
| 2nd | 1.78 | 0.87 - 3.72 | 0.118 |
| 3rd | 1.23 | 0.57 - 2.69 | 0.597 |
| 4th | 1.87 | 0.88 - 4.00 | 0.104 |
| 5th | 2.48 | 1.15 - 5.47 | 0.022 |
| Surgeon years experience | |||
| > 5 years | Reference | – | – |
| 2-5 years | 0.89 | 0.44 - 1.73 | 0.738 |
| < 1 year | 0.45 | 0.15 - 1.25 | 0.143 |
| Start time | 1.03 | 0.81 - 1.31 | 0.806 |
| Thursday-Sunday Surgery | 1.51 | 0.95 - 2.43 | 0.084 |
| Redo at same surgical site | 0.77 | 0.38 - 1.48 | 0.452 |
| Tubular retractors used | 3.03 | 1.02 - 8.56 | 0.039 |
| Drain placed | 4.46 | 2.53 - 7.26 | <0.001 |
| CSF leak | 3.46 | 1.55 - 7.58 | 0.002 |
| Opiate Naive | 1.12 | 0.56 - 1.46 | 0.655 |
| Procedure duration | 1.10 | 0.88 - 1.37 | 0.388 |
| Discharge not to home | 17.11 | 9.24 - 33.00 | <0.001 |
| Seen by PT | 7.23 | 2.13 - 45.30 | 0.008 |
| Seen by OT | 2.20 | 1.13 - 4.22 | 0.018 |
CSF, Cerebrospinal fluid; PT, Physical therapy; OT, Occupational therapy. p-Values in bold indicate significance at p<0.05.
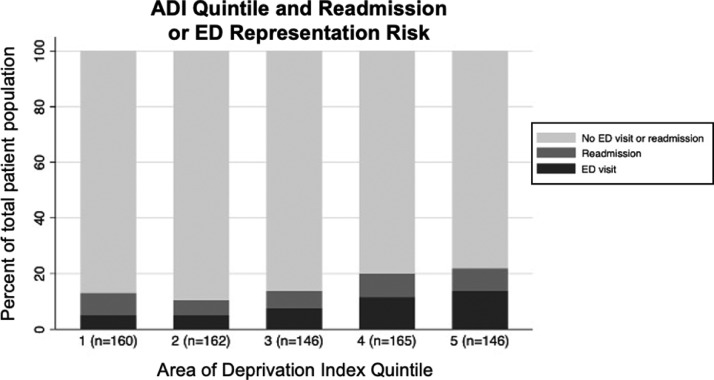
Preoperative opioid use was not associated with an increased risk of a prolonged length of stay following lumbar decompression surgery (aOR 1.12; 95% CI 0.56-1.46; p=0.655). There was no association identified between patients who had a prolonged LOS and 90-day readmission and ED representation (p=0.148, Table 3, Figure 3). The area under the receiver operating characteristic (ROC) curve for multivariate regression of extended LOS was 0.8709 (Supplemental Figure 2).
Table 3.
Patients who experienced an extended length of stay and 90-day emergency department representation or readmission risk.
| No ED Visit or Readmission within 90-days | ED Visit or Readmission within 90-days | Total | |
|---|---|---|---|
| No extended LOS (n, %) | 538 (82%) | 95 (77%) | 633 (81%) |
| Extended LOS (n, %) | 117 (18%) | 29 (23%) | 146 (19%) |
| Total (n, %) | 655 (100%) | 124 (100%) | 779 |
ED, emergency department; LOS, length of stay.
Figure 3.
Area of deprivation Index (ADI) in quintiles and 90-day readmission or emergency department (ED) representation risk.
Discharge disposition regression
Ordinal logistic regression results (Table 4) demonstrated that older patient age was significantly associated with a discharge disposition with greater medical support (aOR 1.99; 95% CI 1.62-2.48; p<0.001, Supplemental Figure 3). Increasing disadvantage on ADI was significantly associated with increased resource utilization following surgery (3rd quintile: aOR 1.90; 95% CI 1.12-3.23; p=0.017, 4th quintile: aOR 1.79; 95% CI 1.05-3.05; p=0.032, 5th quintile: aOR 2.16: 95% CI 1.26-3.75; p=0.005). Presence of a CSF leak (aOR 2.18; 95% CI 1.22-3.86; p=0.008) and longer procedure duration (aOR 1.38; 95% CI 1.17-1.62; p<0.001) were also significantly associated with increasing reliance on support services following discharge (Supplemental Figures 4, 5, 6). Two patients discharged to law enforcement were excluded from this analysis. Patients discharged to home were more likely than patients discharged to SNF or acute rehab to represent to the ED or be readmitted within 90-days following discharge after elective lumbar spine surgery (p=0.003, Table 5). Seven-hundred and seventy-seven patients were included in this analysis as two patients were discharged to law enforcement and were excluded.
Table 4.
Ordinal logistic regression for discharge disposition with discharge to home without services as a reference group.
| Odds Ratio | p-value | 95% CI | ||||
|---|---|---|---|---|---|---|
| Age | 1.99 | <0.001 | 1.62 - 2.48 | |||
| Area of Deprivation Index (quintile) | ||||||
| 1st | Reference | – | – | |||
| 2nd | 1.17 | 0.559 | 0.69 -1.99 | |||
| 3rd | 1.90 | 0.017 | 1.12 - 3.21 | |||
| 4th | 1.79 | 0.032 | 1.05 - 3.05 | |||
| 5th | 2.16 | 0.006 | 1.26 - 3.75 | |||
| Surgeon years experience | ||||||
| > 5 years | Reference | – | – | |||
| 2-5 years | 0.76 | 0.275 | 0.46 - 1.24 | |||
| < 1 year | 1.79 | 0.081 | 0.93 - 3.46 | |||
| Start time | 1.06 | 0.498 | 0.89 - 1.26 | |||
| Thursday-Sunday Surgery | 1.20 | 0.285 | 0.86 - 1.70 | |||
| Redo at same surgical site | 0.96 | 0.855 | 0.60 - 1.50 | |||
| Tubular retractors used | 1.44 | 0.363 | 0.64 - 3.13 | |||
| Drain placed | 1.09 | 0.637 | 0.76 - 1.57 | |||
| CSF leak | 2.18 | 0.008 | 1.22 - 3.86 | |||
| Opiate Naive | 0.87 | 0.432 | 0.62 - 1.23 | |||
| Procedure duration | 1.38 | <0.001 | 1.17 - 1.62 | |||
| Seen by PT | 19.56 | <0.001 | 7.13 - 80.83 | |||
| Seen by OT | 3.58 | <0.001 | 2.17 - 5.92 | |||
CSF, Cerebrospinal fluid; PT, Physical therapy; OT, Occupational therapy. p-Values in bold indicate significance at p<0.05.
Table 5.
Patient discharge disposition and 90-day emergency department representation or readmission risk.
| No ED Visit or Readmission within 90-days | ED Visit or Readmission within 90-days | % of patients by group with ED Visit or Readmission | Total | |
|---|---|---|---|---|
| Home without services (n, %) | 457 (70%) | 66 (54%); | 12.6 | 523 (67%) |
| Home with services (n, %) | 131 (20%) | 35 (29%) | 26.1 | 166 (21%) |
| SNF or acute rehab (n, %) | 67 (10%) | 21 (17%) | 23.9 | 88 (11%) |
| Total (n, %) | 655 (100%) | 122 (100%) | N=777 |
ED, emergency department; SNF, skilled nursing facility.
Discussion
Since spine surgery is one of the 17 reimbursement categories that account for half of Medicare spending [33], identifying populations requiring pre- and peri-operative intervention to prevent needless resource expenditure is critical. We sought to understand how neighborhood-level metrics of SES influence resource utilization in the post-operative period. We examined patients who underwent elective lumbar decompression at a large academic hospital. Individuals in the most disadvantaged groups were more likely to have a prolonged LOS or discharge home requiring assistance or discharge anywhere but home, after controlling for other sociodemographic, procedural, and hospital admission characteristics. These findings highlight the need to evaluate healthcare utilization and patient care in the context of neighborhood-level factors of the social determinants of health. Furthermore, quality improvement initiatives aimed at identifying patients at risk for disproportionate healthcare utilization in the post-operative period may also consider employing ADI or other more comprehensive metrics of SES.
Prior research has found that the area-level measure of disadvantage, ADI, is associated with disparities in hospital readmission risk [23,24], diabetes care [29], cancer screening [28], and drug related mortality [34]. We find that ADI may also be an important metric in understanding healthcare utilization following elective, posterior, lumbar spine surgery. Interestingly, those in the most disadvantaged ADI quintile were at heightened risk of an extended LOS. Also, patients in the three most disadvantaged quintiles were at risk of discharge to home with home services or to a SNF or a rehabilitation facility. These patients may benefit from targeted quality improvement protocols, such as Enhanced Recovery After Surgery (ERAS), prehab, or patient navigators, all of which may reduce inpatient LOS following spine surgery [35], [36], [37]. ERAS programs have been found to reduce LOS, accelerate the return of function, decrease costs, and improve post-operative pain [38]. Debono et al. (2019) [35] found that ERAS reduced LOS for anterior lumbar interbody fusion (6.06 ± 1.1 to 3.33 ± 0.8 days), anterior cervical discectomy and fusion (3.08 ± 0.9 to 1.3 ± 0.7), and posterior spinal fusion patients (6.7 ± 4.8 to 4.8 ± 2.3). This decrease in LOS did not lead to an increase in overall complications. Furthermore, the researchers found that in patients who underwent posterior fusion, those who received ERAS had lower rates of overall complications (p=0.02) as well as revision surgeries (p=0.03).
In addition, patient navigators have previously been shown to reduce LOS following elective surgery [39,40]. Patient navigators may also reduce the risk of hospital readmission in high-risk patients [41]. It follows that setting patients up with ERAS and patient navigators peri-operatively may reduce healthcare resource utilization. Patients may meet with a patient navigator during their pre-operative office visit. The navigator can understand how the patient's unique social pressures may act to prolong their LOS post-operatively and work to address them before their procedure. For example, if appropriate, the navigator may help the patient get pre-authorization if they are expected to require a SNF following discharge. This may help expedite their discharge and avoid the patient waiting in a hospital bed to get insurance authorization before they can be discharged. Future work should study whether there is a benefit of targeted interventions like ERAS and patient navigators in the most socially disadvantaged individuals.
Our work reinforces research performed by Salas-Vega et al. (2021) [42], who investigated extended LOS following lumbar laminectomy. Salas-Vega and colleagues found that patients with surgical complications, non-home discharge destinations, and surgery performed later in the week were more likely to have an extended LOS. Our results are similar; as expected, discharge disposition anywhere but home or with services, and surgical complications are associated with a longer LOS. However, we did not find that surgery performed later in the week was associated with prolonged LOS. In addition, the Salas-Vega et al. did not find that SES, using age, gender, race, and insurance status as covariates, predicted LOS although the researchers did not investigate ADI, specifically. Similarly, pre-operative opioid use did not predict an extended LOS or discharge to higher levels of care; results in the literature remain divided over whether preoperative opioid use increases the chance of an extended LOS following surgery [43,44].
As bundled payment structures become more prevalent, hospitals are incentivized to pre-emptively recognize patients at risk of extended LOS [1,2]. An extended LOS may result from the setup of home services or placement in a SNF or acute rehab facility [45,46]. This delay in discharge, after the patient is medically safe to leave the hospital, is an unnecessary driver of costs[47] and subjects the patient to risk of nosocomial conditions [48]. Identification of patients with a high propensity of requiring SNF or acute rehabilitation may engender anticipatory insurance prior authorization for a nonroutine discharge, which may lead to a decreased LOS, lower risk for nocosomial infection, cost savings, and improved patient experience.
Prior literature has identified age, functional status, and comorbidities as risk factor for nonroutine discharge following spine surgery [12,14]. Using the American College of Surgeons National Surgical Quality Improvement Program, Karhade et al. (2018)[49] identified age, gender, body mass index, surgery level, presence of fusion, functional status, comorbidities, and preoperative hematocrit level as predictors of nonroutine discharge following elective lumbar surgery. However, the only social determinants of health the researchers included in their model were age and gender. While they reported four models with successful prediction of nonroutine discharge, our current findings suggest neighborhood-level metrics of SES should be incorporated into machine learning algorithms.
The Affordable Care Act expanded the use of value-based or pay-for-performance approaches to health system reimbursement. One example is Medicare's Hospital Readmissions Reduction Program which penalizes hospitals for avoidable readmissions [50]. As of 2013 the Center for Medicare and Medicaid Services financially reprimands hospitals and providers with disproportionate readmission rates [51,52]. Thus, understanding drivers of readmission rates is imperative. The current literature suggests that poor functional status at baseline, impaired self-management skills, and living alone are associated with increased readmission risk [52,53]. While not the principal investigation of the present analyses, we found that patients discharged to healthcare facilities were less likely to be readmitted or re-present to the hospital following elective lumbar spine surgery. Prior research has reported similar observations following spine surgery, [54] although other studies have reported no association between discharge disposition and hospital readmission risk [52,55].
Given the high costs of SNF and acute rehabilitation facilities, [4,56] coupled with the increase in spine surgery incidence, [8] understanding the cost-benefit analysis of utilizing medical facilities following discharge is particularly salient as healthcare expenditure continues to rise [57]. A 2014 Cochrane systematic review concluded that more evidence in the form of randomized control trials to assess the utility of rehabilitation following lumbar disc herniation is needed [58]. Other studies suggest that the costs of these interventions may outweigh the benefits on patient clinical outcomes [59].
This study has limitations. Our sample size may not be large enough to identify all potential outcomes. Future multicenter, prospective studies with larger patient cohorts should aim to identify area-level socioeconomic forces and determine their influence on disproportionate healthcare utilization via extended LOS and discharge to non-home destinations. Furthermore, our data analysis was performed on a complete case basis. This was done as <5% of our data was missing [60]. In addition, no power analysis was performed in the present study. We instead chose to include all patients that had undergone elective posterior non-fusion lumbar spine surgery in the electronic medical record era at our institution (beginning in 2015). In the present study, we cannot prove the causality of any of the relationships, and instead, our analysis serves to inform future studies and hypothesis formation. For example, while patients that were seen by PT/OT were more likely to stay in the hospital longer, this is not necessarily because they were seen by these specialties. Future work may study if waiting on PT/OT clearance may delay Our data lack specifics of the type of SNF or acute rehabilitation to which patients were discharged and individuals may have received differential care, which might account for our finding that those discharged to SNF, or a rehabilitation facility were less likely to be readmitted or re-present to the hospital following elective lumbar spine surgery. While we cannot prove causality in the present analyses, we present one of the first analyses of LOS, discharge destination, and post-discharge unplanned encounters with acute care into how neighborhood-level metrics may influence following elective spine surgery and the potential importance of examining SES factors pre-operatively.
Conclusion
Patient sociodemographics, along with procedural factors, and discharge destination were associated with an increased chance of an extended LOS and disproportionate healthcare utilization in individuals having an elective, posterior lumbar spine surgery. Our analysis suggests that ADI, a neighborhood level metric of SES, may predict resource utilization in elective lumbar spine surgery. Several of these SES identified factors can be reliably identified pre-operatively and may be amenable to targeted preoperative intervention.
Declarations of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: MJH: Nothing to disclose. RAS: Nothing to disclose. JF: Nothing to disclose. HA: Nothing to disclose. PZS: Nothing to disclose. JFA: Nothing to disclose. JQC: Nothing to disclose. TN: Nothing to disclose. JSF: Nothing to disclose. AAO: Nothing to disclose. PS: Nothing to disclose. AET: Nothing to disclose. ZLG: Nothing to disclose. SAT: Nothing to disclose. RJW: Nothing to disclose.
LOS, length of stay; SES, socioeconomic status; ADI, area of deprivation index; EMR, electronic medical record; CPT, current procedural terminology; IRB, institutional review board; ICU, intensive care unit; MIS, minimally invasive surgery; CSF, cerebrospinal fluid; PT, physical therapy; OT, occupational therapy; VIF, variance inflation factor; ED, emergency department; SNF, skilled nursing facility; aOR, adjusted odds ratio; ROC, receiver operating characteristic; ERAS, enhanced recovery after surgery
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2022.100187.
Appendix. Supplementary materials
References
- 1.Kyeremanteng K, Robidoux R, D'Egidio G, Fernando SM, Neilipovitz D. An analysis of pay-for-performance schemes and their potential impacts on health systems and outcomes for patients. Crit Care Res Pract. 2019;2019 doi: 10.1155/2019/8943972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih T, Chen LM, Nallamothu BK. Will bundled payments change health care? Examining the evidence thus far in cardiovascular care. Circulation. 2015;131(24):2151–2158. doi: 10.1161/CIRCULATIONAHA.114.010393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan ME, Bradburn EH, Vernon TM, et al. Predictors of trauma high resource consumers in a mature trauma system. Am Surg. 2020;86(5):486–492. doi: 10.1177/0003134820919723. [DOI] [PubMed] [Google Scholar]
- 4.Passias PG, Poorman GW, Bortz CA, et al. Predictors of adverse discharge disposition in adult spinal deformity and associated costs. Spine J. 2018;18(10):1845–1852. doi: 10.1016/j.spinee.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Norbeck TB. Drivers of health care costs. A Physicians Foundation white paper - second of a three-part series. Mo Med. 2013;110(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- 6.Conwell L, Cohen J. Characteristics of persons with high medical expenditures in the U.S. civilian noninstitutionalized population, 2002. [webpage] Rockville, MD: Medical Expenditure Panel Survey; 2005 [cited 2022 June 25]; Available from: https://meps.ahrq.gov/data_files/publications/st73/stat73.pdf.
- 7.Robinson JC. Hospital market concentration, pricing, and profitability in orthopedic surgery and interventional cardiology. Am J Manag Care. 2011;17(6) [PubMed] [Google Scholar]
- 8.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 9.Elsamadicy AA, Koo AB, Kundishora AJ, et al. Impact of patient and hospital-level risk factors on extended length of stay following spinal fusion for adolescent idiopathic scoliosis. J Neurosurg: Pediatrics PED. 2019;24(4):469–475. doi: 10.3171/2019.5.PEDS19161. [DOI] [PubMed] [Google Scholar]
- 10.Pitter FT, Lindberg-Larsen M, Pedersen AB, Dahl B, Readmissions Gehrchen M. Length of stay, and mortality after primary surgery for adult spinal deformity: a 10-year danish nationwide cohort study. Spine. 2019;44(2):E107–EE16. doi: 10.1097/BRS.0000000000002782. [DOI] [PubMed] [Google Scholar]
- 11.Basques BA, Bohl DD, Golinvaux NS, Gruskay JA, Grauer JN. Preoperative factors affecting length of stay after elective anterior cervical discectomy and fusion with and without corpectomy: a multivariate analysis of an academic center cohort. Spine. 2014;39(12):939–946. doi: 10.1097/BRS.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGirt MJ, Parker SL, Chotai S, et al. Predictors of extended length of stay, discharge to inpatient rehab, and hospital readmission following elective lumbar spine surgery: introduction of the Carolina-Semmes Grading Scale. J Neurosurg Spine. 2017;27(4):382–390. doi: 10.3171/2016.12.SPINE16928. [DOI] [PubMed] [Google Scholar]
- 13.Ahn A, Phan K, Cheung ZB, White SJW, Kim JS, Cho SK-W. Predictors of discharge disposition following laminectomy for intradural extramedullary spinal tumors. World Neurosurg. 2019;123:e427–ee32. doi: 10.1016/j.wneu.2018.11.183. [DOI] [PubMed] [Google Scholar]
- 14.Di Capua J, Somani S, Lugo-Fagundo N, et al. Predictors for non-home patient discharge following elective adult spinal deformity surgery. Glob Spine J. 2017;8(3):266–272. doi: 10.1177/2192568217717971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mummaneni PV, Bydon M, Knightly J, et al. Predictors of nonroutine discharge among patients undergoing surgery for grade I spondylolisthesis: insights from the Quality Outcomes Database. J Neurosurg: Spine SPI. 2020;32(4):523–532. doi: 10.3171/2019.9.SPINE19644. [DOI] [PubMed] [Google Scholar]
- 16.Winkler EA, Yue JK, Birk H, et al. Perioperative morbidity and mortality after lumbar trauma in the elderly. Neurosurg Focus. 2015;39(4):E2. doi: 10.3171/2015.7.FOCUS15270. [DOI] [PubMed] [Google Scholar]
- 17.Hagan MJ, Pertsch NJ, Leary OP, et al. Influence of psychosocial and sociodemographic factors in the surgical management of traumatic cervicothoracic spinal cord injury at level I and II trauma centers in the United States. J Spine Surg. 2021;7(3):277–288. doi: 10.21037/jss-21-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adogwa O, Lilly DT, Khalid S, et al. Extended length of stay after lumbar spine surgery: sick patients, postoperative complications, or practice style differences among hospitals and physicians? World Neurosurg. 2019;123 doi: 10.1016/j.wneu.2018.12.016. e734-e9. [DOI] [PubMed] [Google Scholar]
- 19.Elsamadicy AA, Koo AB, Lee M, et al. Associated risk factors for extended length of stay following anterior cervical discectomy and fusion for cervical spondylotic myelopathy. Clin Neurol Neurosurg. 2020;195 doi: 10.1016/j.clineuro.2020.105883. [DOI] [PubMed] [Google Scholar]
- 20.Basques BA, Fu MC, Buerba RA, Bohl DD, Golinvaux NS, Grauer JN. Using the ACS-NSQIP to identify factors affecting hospital length of stay after elective posterior lumbar fusion. Spine. 2014;39(6):497–502. doi: 10.1097/BRS.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. EGEMS. 2016;4(3):1238. doi: 10.13063/2327-9214.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Area Deprivation Index [database on the Internet]. 2019 [cited 9/21/21].
- 23.Hu J, Kind AJH, Nerenz D. Area deprivation index predicts readmission risk at an urban teaching hospital. Am J Med Qual. 2018;33(5):493–501. doi: 10.1177/1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner RM, Coe NB, Qi M, Konetzka RT. Patient outcomes after hospital discharge to home with home health care vs to a skilled nursing facility. JAMA Intern. Med. 2019;179(5):617–623. doi: 10.1001/jamainternmed.2018.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible - the neighborhood Atlas. N Engl J Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain AM, Finney Rutten LJ, Wilson PM, et al. Neighborhood socioeconomic disadvantage is associated with multimorbidity in a geographically-defined community. BMC Public Health. 2020;20(1):13. doi: 10.1186/s12889-019-8123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurani SS, McCoy RG, Lampman MA, et al. Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurani SS, Lampman MA, Funni SA, et al. Association between area-level socioeconomic deprivation and diabetes care quality in us primary care practices. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.38438. e2138438-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fee C, Burstin H, Maselli JH, Hsia RY. Association of emergency department length of stay with safety-net status. JAMA. 2012;307(5):476–482. doi: 10.1001/jama.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffee EG, Arora VM, Matthiesen MI, Meltzer DO, Press VG. Health literacy and hospital length of stay: an inpatient cohort study. J Hosp Med. 2017;12(12):969–973. doi: 10.12788/jhm.2848. [DOI] [PubMed] [Google Scholar]
- 32.Schwam ZG, Ferrandino R, Kaul VZ, Wanna GB, Cosetti MK. Thirty-day readmission and prolonged length of stay in malignant otitis externa. Laryngoscope. 2020;130(9):2220–2228. doi: 10.1002/lary.28409. [DOI] [PubMed] [Google Scholar]
- 33.Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075–1077. doi: 10.1056/NEJMp1113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurani S, McCoy RG, Inselman J, et al. Place, poverty and prescriptions: a cross-sectional study using Area Deprivation Index to assess opioid use and drug-poisoning mortality in the USA from 2012 to 2017. BMJ Open. 2020;10(5) doi: 10.1136/bmjopen-2019-035376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of Enhanced Recovery After Surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. 2019;46(4):E6. doi: 10.3171/2019.1.FOCUS18669. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen PR, Jørgensen LD, Dahl B, Pedersen T, Tønnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24(2):137–148. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 37.Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(15 Suppl):3543–3552. doi: 10.1002/cncr.26264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elsarrag M, Soldozy S, Patel P, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus. 2019;46(4):E3. doi: 10.3171/2019.1.FOCUS18700. [DOI] [PubMed] [Google Scholar]
- 39.Jalilvand A, Suzo A, Hornor M, et al. Impact of care coaching on hospital length of stay, readmission rates, postdischarge phone calls, and patient satisfaction after bariatric surgery. Surg Obes Relat Dis. 2016;12(9):1737–1745. doi: 10.1016/j.soard.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawhney M, Teng L, Jussaume L, Costa S, Thompson V. The impact of patient navigation on length of hospital stay and satisfaction in patients undergoing primary hip or knee arthroplasty. Int J Orthop Trauma Nurs. 2021;41 doi: 10.1016/j.ijotn.2020.100799. [DOI] [PubMed] [Google Scholar]
- 41.Balaban RB, Galbraith AA, Burns ME, Vialle-Valentin CE, Larochelle MR, Ross-Degnan D. A patient navigator intervention to reduce hospital readmissions among high-risk safety-net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915. doi: 10.1007/s11606-015-3185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salas-Vega S, Chakravarthy VB, Winkelman RD, et al. Late-week surgery and discharge to specialty care associated with higher costs and longer lengths of stay after elective lumbar laminectomy. J Neurosurg Spine. 2021:1–7. doi: 10.3171/2020.11.SPINE201403. [DOI] [PubMed] [Google Scholar]
- 43.Cheah JW, Sing DC, McLaughlin D, Feeley BT, Ma CB, Zhang AL. The perioperative effects of chronic preoperative opioid use on shoulder arthroplasty outcomes. J Shoulder Elbow Surg. 2017;26(11):1908–1914. doi: 10.1016/j.jse.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Tank A, Hobbs J, Ramos E, Rubin DS. Opioid dependence and prolonged length of stay in lumbar fusion: a retrospective study utilizing the national inpatient sample 2003–2014. Spine. 2018;43(24):1739–1745. doi: 10.1097/BRS.0000000000002714. [DOI] [PubMed] [Google Scholar]
- 45.Porter ME. A strategy for health care reform–toward a value-based system. N Engl J Med. 2009;361(2):109–112. doi: 10.1056/NEJMp0904131. [DOI] [PubMed] [Google Scholar]
- 46.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 47.Smith AL, Kulhari A, Wolfram JA, Furlan A. Impact of insurance precertification on discharge of stroke patients to acute rehabilitation or skilled nursing facility. J Stroke Cerebrovasc Dis. 2017;26(4):711–716. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 48.Arefian H, Hagel S, Heublein S, et al. Extra length of stay and costs because of health care-associated infections at a German university hospital. Am J Infect Control. 2016;44(2):160–166. doi: 10.1016/j.ajic.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Karhade AV, Ogink P, Thio Q, et al. Development of machine learning algorithms for prediction of discharge disposition after elective inpatient surgery for lumbar degenerative disc disorders. Neurosurg Focus. 2018;45(5):E6. doi: 10.3171/2018.8.FOCUS18340. [DOI] [PubMed] [Google Scholar]
- 50.McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131(20):1796–1803. doi: 10.1161/CIRCULATIONAHA.114.010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaziri S, Cox JB, Friedman WA. Readmissions in neurosurgery: a qualitative inquiry. World Neurosurg. 2014;82(3):376–379. doi: 10.1016/j.wneu.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 52.Vasquez RA, Chotai S, Freeman TH, et al. Impact of discharge disposition on 30-day readmissions following elective spine surgery. Neurosurgery. 2017;81(5):772–778. doi: 10.1093/neuros/nyx114. [DOI] [PubMed] [Google Scholar]
- 53.Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26(1):71–77. doi: 10.3122/jabfm.2013.01.120107. [DOI] [PubMed] [Google Scholar]
- 54.Park C, Cook CE, Garcia AN, Gottfried ON. Discharge destination influences risks of readmission and complications after lumbar spine surgery in severely disabled patients. Clin Neurol Neurosurg. 2021;207 doi: 10.1016/j.clineuro.2021.106801. [DOI] [PubMed] [Google Scholar]
- 55.Minetos PD, Canseco JA, Karamian BA, et al. Discharge disposition and clinical outcomes after spine surgery. Am J Med Qual. 2021 doi: 10.1097/01.JMQ.0000753240.14141.87. [DOI] [PubMed] [Google Scholar]
- 56.Theologis AA, Lau D, Dalle-Ore C, Tsu A, Deviren V, Ames CP. Costs and utility of post-discharge acute inpatient rehabilitation following adult spinal deformity surgery. Spine Deform. 2021;9(3):817–822. doi: 10.1007/s43390-020-00251-w. [DOI] [PubMed] [Google Scholar]
- 57.Chernew ME, Hirth RA, Cutler DM. Increased spending on health care: how much can the United States afford? Health Aff. 2003;22(4):15–25. doi: 10.1377/hlthaff.22.4.15. [DOI] [PubMed] [Google Scholar]
- 58.Oosterhuis T, Costa LO, Maher CG, de Vet HC, van Tulder MW, Ostelo RW. Rehabilitation after lumbar disc surgery. Cochrane Database Syst Rev. 2014;2014(3) doi: 10.1002/14651858.CD003007.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paulsen RT, Sørensen J, Carreon LY, Andersen MØ. Cost-effectiveness of postoperative rehabilitation after surgery for lumbar disc herniation: an analysis based on a randomized controlled trial. J Neurosurg: Spine SPI. 2020;32(5):733–740. doi: 10.3171/2019.11.SPINE191003. [DOI] [PubMed] [Google Scholar]
- 60.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Method. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.