Abstract
Chalcone was prepared in a new route by reacting o-hydroxyacetophenone with 4-dimethylaminobenzaldehyde using piperidine as a catalyst. 3-Hydroxy-2-[4-(dimethylamino)phenyl] benzopyran-4-one were prepared by Algar-Flynn-Oyamada method by cyclization of chalcone using Hydrogen peroxide. A series of alkyl and ester derivatives of the flavonoid 3-hydroxy-2-[4-(dimethylamino)phenyl] benzopyran-4-one were prepared by reacting the above mentioned compound with different chemical reagents (Methyl iodide, Allyl bromide, Benzyl chloride, Bromoacetylcoumarin, Chloroacetamide, Chloroacetyl chloride, Phthalic anhydride, Maleic anhydride, Phthalimide, Cinnamoyl chloride) with potassium carbonate and acetone or DMF as a solvent. The physical and spectroscopic properties of the new compounds were studied by (FT-IR, 13C-NMR and 1H-NMR) spectral methods. The purity of the synthesized compounds were confirmed using TLC thin layer chromatography. The biological activity of some synthetic flavonoids (A2, A5, A7, A8, A9, A12) at two different concentrations (0.5 mg/ml, 0.25 mg/ml) were studied on three types of fungi: Aspergillus flavus, Acremonium strictum, Penicillium expansum. Some of this compounds showed high activity against the tested fungi.
Keywords: Aldol condensation, Oxidative cyclization, Chalcone, Flavonol, o-Alkyl flavone, Bromo-acetylcoumarin, Antifungal
Aldol condensation; Oxidative cyclization; Chalcone; Flavonol; o-Alkyl flavone; Bromo-acetylcoumarin; Antifungal.
1. Introduction
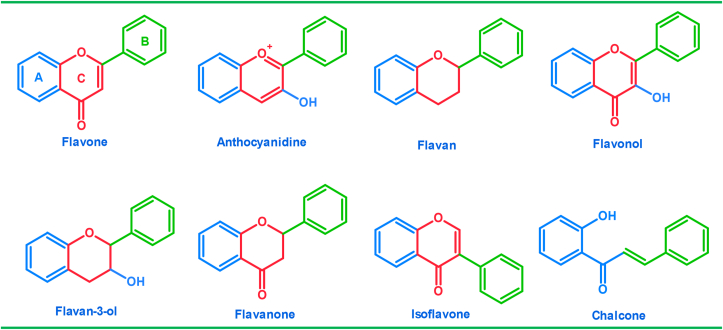
Flavonoids and their derivatives have gained the attention of biologists and chemists because they play a major role in nature. It has wide applications in the pharmaceutical field, as they are Antioxidants, Anticancers, Antidepressants, Antibacterials, Anti-inflammatory...etc [1, 2, 3, 4]. And in the field of the Food Industry (as coloring materials and sweeteners) [5]. In addition to being used in agriculture as insecticides [6]. It also gave a new insight into laboratory technology to explore the effectiveness of plant cell tissue implantation to produce chemical compounds similar to those of the mother plant [7]. Flavonoids are polyphenolic compounds with low molecular weight [8, 9], widely found in plants. It has biological activity [10] and play vital role in photosynthesis cells [11]. The structure of flavonoids consists of fifteen basic carbon atoms with a general formula C15H10O2 (C6–C3–C6). The basic nucleus of flavonoids is 2-phenyl benzo-γ-pyrone (2-phenyl chromone). Which consists of two Benzen rings (A and B), Figure 1 one of which is condensed with the Pyran ring (C) and the other is branched [11].
Figure 1.
Basic structures of flavonoids subclasses.
Flavonoids can be classified according to their sources [12]. Some of them are intermediates in the biosynthesis, such as: chalcones, flavanone, flavanone-3-ol, flavan-3,4-diol. The end products of biosynthesis are anthocyanins, flavones and flavonols. There are two other classes of flavonoids related to the aromatic ring (B): isoflavones: in which the aromatic ring (B) is at position C3, and neoflavonoids: in which the aromatic ring (B) is at position C4. Flavonoids possess antibacterial, antifungal, antiviral, anti-allergic, anti-inflammatory and antioxidant activity [1, 2, 3, 4]. Studies have shown that the activity of flavonoids against microbes depends on its chemical composition, which is affected in particular by the number and position of different functional groups such as: hydroxy, methoxy, halogens, nitro, methyl, cyano-substituted groups in the two aromatic rings (A, B) [13, 14, 15, 16]. Studies have shown that the substitution pattern at site 3 of the C-ring has a regulatory role on biological activity and metabolism.
Researcher Jayashree and his colleagues [17] were able to synthesize a series of ester derivatives of flavonoids by reacting the esterification of the –OH enolic group at the C3 position of the flavonoid 3-hydroxy-2-[4-(dimethylamino)phenyl] benzopyran-4-one with acid chlorides with pyridine as an intermediate.
American researchers at the University of California [18] were able to synthesize a series of 48 compounds from 3-O-alkyl quercetin derivatives through several stages in the laboratories of the Cancer Research Center.
In our research, we used the condensation method to prepare chalcone using an organic alkali (piperidine) as a catalyst through the reaction of o-hydroxyacetophenone and p-dimethylamino benzaldehyde. A series of alkyl and ester derivatives were prepared by reacting 3-hydroxyflavone with different alkyl halides (methyl iodide, allyl bromide, benzyl chloride, bromoacetylcoumarin, chloroacetamide) and carboxylic acid derivatives (phthalic anhydride, maleic anhydride, phthalimide, chloroacetyl chloride, cinnamoyl chloride).
2. Experimental
2.1. General information
All compounds were purified by recrystallization and chromatographically separated using Preparative-TLC. The course of the reactions were monitored by thin-layer chromatography (TLC) on aluminum plates coated with silica gel with a fluorescent indicator (DC-Fertigfolien ALUGRAM Xtra SIL G/UV254) and using a DESAGA-UVIS/254/366 nm UV lamp. Melting points were determined using the Stauart Electrothermeal Entineering LTD (measuring melting points up to 400 Cͦ). Infrared spectra were recorded using Bruker FT-IR alpha T within a potassium bromide disc. 13C-NMR and 1H-NMR spectra were recorded via a 400 MHz Bruker-Avance Spectrometer using (DMSO-d6) solvent with compound (TMS) as standard reference at the High Atomic Energy Authority – Damascus.
Chemical and Starting Materials: The solvents used were from different companies (Sigma Aldrich, Merck, Fluka, BDH) (absolute ethanol, dry acetone, N,N-dimethylformamide DMF, hexane, dry ether, ethyl acetate,...), the materials used are: o-Hydroxyacetophenone, 4-Dimethylaminobenz aldehyde, methyl iodide, benzyl chloride, allyl bromide, chloroacetyl chloride, chloroacetamide, thionyl chloride, phthalic anhydride, maleic anhydride, Hydrogen peroxide 35%, ethyl acetoacetate, Salicyl aldehyde, potassium hydroxide, potassium carbonate, concentrated hydrochloric acid from different companies (Merck, Fluka, BDH, Sigma Aldrich), Potato Dextrose Agar P.D.A (HIMEDIA).
2.2. Chemistry
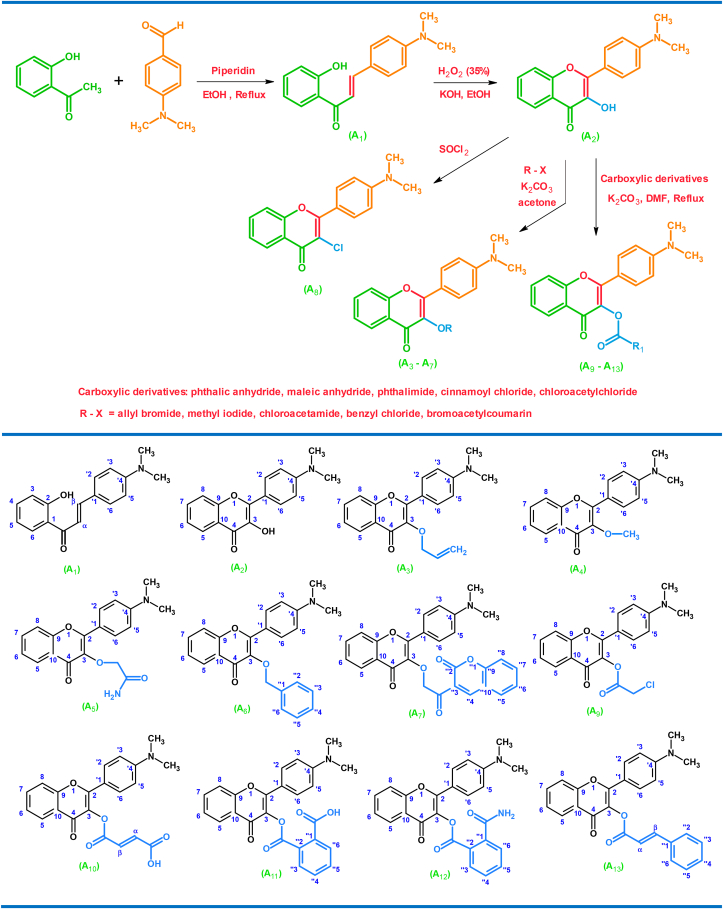
New flavonoid derivatives were synthesized from o-hydroxyacetophenone and 4-dimethylamino benzaldehyde according to the following scheme.
2.2.1. Preparation of some primary organic materials used in the synthesis of flavonoid derivatives
2.2.1.1. Preparation of 3-acetyl coumarin [19]
3-Acetylcoumarin was prepared according to the method used in reference [19]. Yield (95%). Melting point 120–122 Cͦ.
2.2.1.2. Preparation of 3-(bromoacetyl) coumarin [19]
3-Bromo-acetylcoumarin was prepared according to the method used in reference [19]. Yield (85%). Melting point 166–168 Cͦ.
2.2.1.3. Preparation of cinnamoyl chloride [18]
100 ml of (0.008 mol, 1.2 g) of cinnamic acid and 5–6 ml of thionyl chloride were added to a Erlenmeyer flask, stirred well at 50 Cͦ for 2 h. After the reaction were completed, the thionyl chloride were evaporated using a rotary evaporator and a colorless oily liquid were obtained, which is cinnamoyl chloride.
2.2.1.4. Preparation of phthalimide [20]
Phthalimide was prepared according to the method used in reference [19]. Yield (Rf = 0.25. Melting Point: 232–234 Cͦ.
2.2.2. Synthesis of the flavonoid 2-[4-(dimethylamino) phenyl]-3-hydroxy-4H-1-benzopyran-4-one (A2)
The flavonoid compound is synthesized from chalcone according to the following stages.
2.2.2.1. Synthesis of chalcone: (2E)-3-[4-(dimethylamino)phenyl]-1-(2-hydroxyphenyl) prop-2-en-1-one (A1)
This compound were previously prepared [21] using the aldol condensation method by condensa--tion of the compound o-hydroxyacetophenone with the compound 4-dimethylaminobenzaldehyde using NaOH as a catalyst and the yield were (94.2%). We used a new method to prepare this compound using an organic alkaloid (piperidine) according to the following [22]: (0.04 mol, 5.44 g) of o-hydroxyacetophenone and (0.04 mol, 5.96 g) of 4-Dimethylaminobenzaldehyde were added in 100 ml of absolute ethanol, 4–5 ml of piperidine were added to the previous solution. The mixture were refluxed for 5 h, the reaction monitored using TLC. After cooling the solution a precipitate were formed which were separated by filtration. 2 ml piperidine were added to the filtrate and refluxed for 4 h. The precipitate formed were filtered, washed with water and ethanol several times, and recrystallized with ethanol, resulting in a precipitate of Bright pink colour. The purity of the prepared compound were confirmed by TLC technology using the mixture (ether:hexane 2:3).
2.2.2.2. A1. (2E)-3-[4-(Dimethylamino)phenyl]-1-(2-hydroxyphenyl) prop-2-en-1-one
Bright pink solid; Yield: 80.9%; mp 178–180 Cͦ; Rf = 0.47 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 3650 (OH), 2890 (C–Haliphatic), 1596 (C O pyrone), 1432–1485 (C C), 1202 (C–O).
2.2.2.3. Synthesis of 2-[4-(dimethylamino) phenyl]-3-hydroxy-4H-1-benzopyran-4-one (A2) [21]
This compound was previously prepared according to the method used in reference [21].
2.2.2.4. A2. 2-[4-(Dimethylamino)phenyl]-3-hydroxy-4H-1-benzopyran-4-one
Bright yellow solid; Yield: 82%; mp 190–191 Cͦ; Rf = 0.36 (ether: hexane 2:3); IR (KBr, cm−1) ʋ: 3295 (OH), 2910 (C–Haliphatic), 1597 (C O pyrone), 1482–1558 (C C), 1152–1287 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 6.85–8.12 (m, 8H, aromatic ring), 9.19 (s, H, OH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.55 (2 CH3), 111.71 (C3′,5′), 118.35 (C8), 118.95 (C1′), 121.86 (C10), 123.70 (C6), 125.08 (C5), 129.39 (C2′,6′), 133.57 (C7), 137.72 (C3), 147.29 (C4′), 151.51 (C2), 154.72 (C9), 172.43 (C O pyron).
2.2.3. Synthesis of new alkyl derivatives of flavonoid (A2)
2.2.3.1. Synthesis of the compound 2-[4-(dimethylamino)phenyl]-3-[(prop-2-en-1-yl)oxy]-4H-1-benzopyran-4-one (A3) [23,24]
(0.00177 mol, 0.5 g) of the flavonoid compound (A2) were dissolved in 50 ml of dry acetone, an excess amount of potassium carbonate K2CO3 were added, while stirring well for 5 min, (0.00177 mol, 0.22 g) of Allyl bromide dissolved in 10 ml acetone were added dropwise, the reaction mixture were stirred for 48 h at 50–60 Cͦ. The mixture were poured into a beaker containing crushed ice and neuterlised with dilute hydrochloric acid, the formed precipitate were filtered, washed with water and then recrystallized with ethanol, resulting in a yellow precipitate. Its purity were confirmed by TLC using the mixture (ether:hexane 2:3).
2.2.3.2. A3. 2-[4-(Dimethylamino)phenyl]-3-[(prop-2-en-1-yl)oxy]-4H-1-benzopyran-4-one
Yellow solid; Yield: 78.9%; mp 76–78 Cͦ; Rf = 0.33 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 2820–2900 (C–Haliphatic), 1722 (C O pyrone), 1469–1594 (C C), 1100–1288 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 4.58 (d, 2H, O–CH2), 5.19–5.33 (dd, 2H, CH2), 5.97 (m, H, CH), 6.83–8.03 (m, 8H, aromatic ring); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.22 (CH3, 2 CH3), 72.46 (CH2), 111.72 (C3′,5′), 117.18 (C8), 118.34 ( CH2), 118.59 (C1′), 123.92 (C10), 125.19 (C6), 125.27 (C5), 130.13 (C2′,6′) 133.98 ( CH), 134.54 (C7),138.14 (C3), 152.15 (C4′), 154.92 (C9), 156.53 (C2), 173.69 (C O pyrone).
2.2.3.3. Synthesis of 2-[4-(dimethylamino)phenyl]-3-methoxy-4H-1-benzo pyran-4-one (A4) [23,24]
This compound were previously synthesized [17] via a methylation reaction of the flavonoid compound using dimethyl sulfate (CH3)2SO4 using potassium carbonate as catalyst and the yield were (68%).
We used a new method to prepare this compound according to the following [24, 25]: (0.00177 mol, 0.5 g) of the flavonoid compound (A2) were Dissolve in 50 ml of dry acetone, an excess of potassium carbonate K2CO3 were added and stirred well for 5 min, then (0.00177 mol, 0.25 g) of methyl iodide dissolved in 10 ml acetone were added dropwise and stirred well for 48 h at 50–60 Cͦ. The reaction mixture were poured into a beaker containing crushed ice and neuterlised with dilute hydrochloric acid, the formed precipitate were filtered, washed with water and then recrystallized in absolute ethanol, resulting in a yellow precipitate. Its purity were confirmed by TLC using the mixture (ether:hexane 2:3). After comparing the new method (using methyl iodide) and the previously used method (using dimethyl sulfate), the yield were higher according to the new method (82.8%).
2.2.3.4. A4. 2-[4-(Dimethylamino)phenyl]-3-methoxy-4H-1-benzopyran-4-one
Yellow solid; Yield: 82.8%; mp 123–125 Cͦ; Rf = 0.22 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 2900 (C–Haliphatic), 1593 (C O pyrone), 1467–1556 (C C), 1127–1198 (C–O).
2.2.3.5. Synthesis of 2-({2-[4-(dimethylamino) phenyl]-4-oxo-4H-1-benzopyran-3-yl} oxy) acetamide (A5) [23,24,25]
(0.00177 mol, 0.5 g) of the flavonoid compound (A2) were dissolved in 50 ml of N,N-dimethylformamide DMF, excess of potassium carbonate K2CO3 were added with good stirring for 5 min, then (0.00177 mol, 0.17 g) of chloroacetamide dissolved in 10 ml DMF were added to it dropwise under good stirring for 48 h at 50–60 Cͦ. The mixture were poured into a beaker containing crushed ice and neuterlised with dilute hydrochloric acid, the precipitate formed were filtered, washed with water and dried. The resulting flavonoid were separated by P-TLC using ethyl acetate mobile phase. A yellow precipitate is produced. Its purity were confirmed by TLC technology using the mixture (acetone:hexane 2:3).
2.2.3.6. A5. 2-({2-[4-(Dimethylamino)phenyl]-4-oxo-4H-1-benzo pyran-3-yl} oxy) acetamide (A5)
Yellow solid; Yield: 66.7%; mp 187–188 Cͦ; Rf = 0.37 (acetone:hexane 2:3); IR (KBr, cm−1) ʋ: 3417 (NH2), 2922 (C–Haliphatic), 1704 (C O amide), 1616 (C O pyrone), 1477–1548 (C C), 1115–1290 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 4.74 (s, 2H, O–CH2), 7.30 (s, 2H, NH2), 6.80–8.04 (m, 8H, aromatic ring); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.67 (CH3, 2 CH3), 68.51 (CH2), 111.69 (C3′,5′), 117.24 (C8), 118.93 (C1′), 123.81 (C10), 124.74 (C6), 127.35 (C5), 128.52 (C2′,6′), 134.25 (C7), 137.79 (C3), 151.89 (C4′), 155.17 (C9), 157.74 (C2), 169.52 (C O amide), 174.74 (C O pyron).
2.2.3.7. Synthesis of 3-(benzyloxy)-2-[4-(dimethyl amino) phenyl]-4H-1-benzopyran-4-one (A6) [23,24]
(0.00177 mol, 0.5 g) of the flavonoid compound (A2) were dissolved in 50 ml of dry acetone, excess of K2CO3 potassium carbonate were added and sti--rred well for 5 min, then (0.00177 mol, 0.23 g) of benzyl chloride dissolved in 10 ml acetone added to it dropwise with good stirring for 48 h at 50–60 Cͦ. The reaction mixture were poured into a beaker containing crushed ice and neuterlised with dilute hydrochloric acid. The flavonoid compound were extracted from the aqueous solution using n-hexane, the extraction process were repeated twice (2 × 40 ml) and the solvent were evaporated using a Rotary evaporator, the precipitate were washed with water and ethanol, resulting in a bright yellow precipitate. Its purity were confirmed by TLC technology using the mixture (ether:hexane 2:3).
2.2.3.8. A6. 3-(Benzyloxy)-2-[4-(dimethylamino)phenyl]-4H-1-benzo pyran-4-one
Yellow solid; Yield: 76.9%; mp 98–100 Cͦ; Rf = 0.36 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 2889 (C–Haliphatic), 1597 (C O pyrone), 1483–1560 (C C), 1191–1281 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 5.05 (s, 2H, O–CH2), 6.80–8.10 (m, 13H, aromatic ring), 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.66 (CH3, 2 CH3), 73.16 (CH2), 111.73 (C3′,5′), 117.13 (C8), 118.63 (C1′), 124.01 (C10), 125.31 (C6), 128.47 (C2″,4″,6″), 128.50 (C5), 128.71 (C2′,6′), 130.18 (C3″,5″), 134.04 (C7), 137.46 (C1″), 138.27 (C3), 152.19 (C4′), 154.96 (C9), 156.74 (C2), 173.74 (C O pyron).
2.2.3.9. Synthesis of 3-({[2-(4-Dimethylaminophenyl)-4-oxo-4H-1-benzopyran-3-yl]oxy}acetyl)-2H-1-benz opyran-2-one (A7) [23,24]
(0.00177 mol, 0.5 g) of the flavonoid compound (A2) were dissolve in 20 ml of N,N-dimethylformamide DMF, an excess of potassium carbonate K2CO3 were added, with good stirring for 5 min, then (0.00177 mol, 0.47 g) of bromo-acetylcoumarin dissolved in 5 ml DMF were added dropwise with continuous stirring for 72 h at 100 Cͦ. The mixture were poured into a beaker containing crushed ice and neuterlised with dilute hydrochloric acid. The formed precipitate were filtered, washed with water and then recrystallized with absolute ethanol, resulting in a brown precipitate. Its purity were confirmed by TLC technology using the mixture (acetone:hexane 2:3).
2.2.3.10. A7. 3-({[2-(4-Dimethylaminophenyl)-4-oxo-4H-1-benzo pyran-3-yl] oxy} acetyl)-2H-1-benzopyran-2-one
Brown solid; Yield: 76%; mp 278–280 Cͦ; Rf = 0.46 (acetone:hexane 2:3); IR (KBr, cm−1) ʋ: 2865–2956 (C–Haliphatic), 1715 (C O lactone), 1668 (C=O acetyl), 1606 (C=O pyrone), 1558 (C=C), 1115–1273 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 5.49 (s, 2H, O–CH2), 6.78–8.05 (m, 12H, aromatic ring), 8.56 (s, 1H, H4″); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.48 (CH3, 2 CH3), 68.04 (CH2), 111.82 (C3′,5′), 115.75 (C8″), 116.25 (C8), 118.35 (C10″), 119.55 (C1′), 122.51 (C10), 123.21 (C6), 125.30 (C5), 125.34 (C6″), 127.73 (C2′,6′), 127.87 (C5″), 129.33 (C7″), 130.70 (C3″) 135.49 (C7), 136.69 (C4″), 136.96 (C3), 150.71 (C4′), 152.84 (C9″), 155.87 (C9), 159.33 (C2), 160.09 (C=O lactone), 174.36 (C=O pyron), 192.87 (C=O ketone).
2.2.4. Synthesis of 3-chloro-2-[4-(dimethyl amino)phenyl]-4H-1-benzopyran-4-one (A8) [17]
10 ml of thionyl chloride SOCl2 were added to (0.00142 mol, 0.4 g) of flavonoid (A2), and the mixture were heated at 50–60 Cͦ for 4 h. The remaining thionyl chloride were evaporated, the precipitate were washed with water and ethanol, then recrystallized with absolute ethanol, resulting in a violet precipitate. Its purity were confirmed by TLC using the mixture (ether:hexane 2:3).
2.2.4.1. A8. 3-Chloro-2-[4-(dimethylamino)phenyl]-4H-1-benzopyran-4-one
Dark purple solid; Yield: 94%; mp 156–158 Cͦ; Rf = 0.15 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 1700 (C=O pyrone), 1430–1558 (C=C), 1020.85 (C–O), 755 (C–Cl); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.01 (s, 6H, 2 CH3), 6.84–8.13 (m, 8H, aromatic ring); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.62 (CH3, 2CH3), 111.29 (C3′,5′), 115.90 (C8), 119.67 (C1′), 124.04 (C10), 123.33 (C6), 125.92 (C5), 127.59 (C2′,6′), 134.93 (C7), 102.13 (C3), 151.03 (C4′), 155.72 (C9), 167.90 (C2), 178.14 (C=O pyron).
2.2.5. Synthesis of new ester derivatives of flavonoid (A2)
2.2.5.1. Synthesis of 2-[4-(dimethylamino) phenyl]-4-oxo-4H-1- benzopyran-3-yl chloro acetate (A9) [23,24]
(0.0035 mol, 1 g) of the flavonoid compound (A2) were Dissolved in 50 ml of dry acetone, an excess of potassium carbonate were added and stirred well for 5 min, then (0.0035 mol, 0.4 g) of Chloroacetyl chloride dissolved in 10 mL of acetone were added to it dropwise with continuous stirring for 48 h at room temperature. The resulting mixture were poured into a beaker containing crushed ice, the formed precipitate were filtered and washed withwater and ethanol, then recrystallized with absolute ethanol, resulting in a yellow precipitate. Its purity were confirmed by TLC using the mixture (ether:hexane 2:3).
2.2.5.2. A9. 2-[4-(Dimethylamino)phenyl]-4-oxo-4H-1-benzopyran-3-yl chloroacetate
Yellow solid; Yield: 75%; mp 155–157 Cͦ; Rf = 0.29 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 2888 (C–Haliphatic), 1778 (C=O ester), 1591 (C=O pyrone), 1478–1517 (C=C), 1118–1288 (C–O), 757 (C–Cl); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 4.27 (s, 2H, CH2), 6.85–8.11 (m, 8H, aromatic ring); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.13 (CH3, 2CH3), 42.45 (CH2), 112.04 (C3′,5′), 115.22 (C8), 118.91 (C1′), 121.84 (C10), 123.12 (C6), 125.43 (C5), 129.42 (C2′,6′), 133.54 (C3), 134.82 (C7), 151.53 (C4′), 152.71 (C2), 155.27 (C9), 165.64 (C=O ester), 170.47 (C=O pyron).
2.2.5.3. Synthesis of flavonoids derivatives (A10–A13) [23,24]
(0.001066 mol, 0.3 g) of the flavonoid compound (A2) were dissolve in 20 ml of DMF, an excess of potassium carbonate were added to it and stirred well for 5 min, then (Maleic anhydride or phthalic anhydride or phthalimide or cinnamoyl chloride (0.001066 mol) dissolved in 5 ml of DMF were added to it drop by drop and stirred under reflux for 24 h. The mixture were poured into a beaker containing crushed ice and the medium were neuterlised with diluted hydrochloric acid. The flavonoids were extracted from the aqueous phase using dichloromethane (3 × 30 ml). The organic extracts were collected, dried using anhydrous magnesium sulfate and the solvent were evaporated. The precipitate were recrystallized with absolute ethanol, resulting in a yellow precipitate. Its purity were confirmed by TLC using the mixture (ether:hexane 2:3).
2.2.5.4. A10. (2E)-4-({2-[4-(Dimethylamino)phenyl]-4-oxo-4H-1-benzopyran-3-yl}oxy)-4-oxobut-2-enoic acid
Dark yellow solid; Yield: 87.5%; mp 129–131 Cͦ; Rf = 0.18 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 3415 (OH), 2869–2907 (C–Haliphatic), 1723 (C=O ester), 1682 (C=O acid), 1605 (C=O pyrone), 1484–1553 (C=C), 1173–1276 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 6.47 (d, Hβ, CH), 6.65(d, Hα, CH), 6.81–8.04 (m, 8H, aromatic ring), 13.14 (s, H, COOH); 13C-NMR.
(100 MHz, DMSO-d6, δ ppm): 40.46 (CH3, 2 CH3), 111.72 (C3′,5′), 116.70 (C8), 119.39 (C1′), 121.76 (C10), 123.42 (C6), 125.60 (C5), 128.22 (C2′,6′), 133.28 (C3), 133.96 (CH), 134.01 (CH), 134.17 (C7), 147.36 (C4′), 153.64 (C2), 154.62 (C9), 157.13 (C=O ester), 167.10 (C=O acid), 174.49 (C=O pyron).
2.2.5.5. A11. 2-[({2-[4-(Dimethylamino)phenyl]-4-oxo-4H-1-benzopyran-3-yl}oxy) carbonyl]benzoic acid
Yellow solid; Yield: 77%; mp 146–148 Cͦ; Rf = 0.21 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 3415 (OH), 2910 (C–Haliphatic), 1724 (C=O ester), 1684 (C=O acid), 1606 (C=O pyrone), 1435–1536 (C=C), 1135–1232 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 6.81–8.27 (m, 12H, aromatic ring), 13.11 (s, H, COOH); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.53 (CH3, 2 CH3), 111.68 (C3′,5′), 116.80 (C8), 119.41 (C1′), 121.88 (C10), 123.45 (C6), 125.29 (C3″), 125.66 (C5), 128.22 (C2′,6′), 128.49 (C6″), 132.77 (C1″,2″), 133.31 (C3), 133.97 (C4″,5″), 134.08 (C7), 147.34 (C4′), 153.63 (C2), 153.48 (C=O ester), 154.68 (C9), 168.18 (C=O acid), 174.37 (C=O pyron).
2.2.5.6. A12. 2-[4-(Dimethylamino)phenyl]-4-oxo-4H-1-benzopyran-3-yl 2-carbamoyl benzoate
Yellow solid; Yield: 78%; mp 138–140 Cͦ; Rf = 0.24 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 3474 (NH2), 2856 (C–Haliphatic), 1723 (C=O ester), 1684 (C=O amid), 1605 (C=O pyrone), 1435–1535 (C=C), 1134–1276 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 6.82–8.19 (m, 12H, aromatic ring), 7.91 (s, 2H, NH2); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.44 (CH3, 2 CH3), 111.71 (C3′,5′), 116.72 (C8), 119.40 (C1′), 121.87 (C10), 123.44 (C6), 124.08 (C6″), 125.59 (C5), 128.21 (C2′,6′), 128.30 (C3″), 130.16 (C2″), 131.61 (C1″), 132.45 (C4″), 133.28 (C3), 134.11 (C5″), 134.12 (C7), 147.38 (C4′), 153.65 (C2), 154.67 (C9), 153.51 (C=O ester), 165.78 (C=O amid), 174.46 (C=O pyron).
2.2.5.7. A13. 2-[4-(Dimethylamino)phenyl-4-oxo-4H-1-benzopyran-3-yl (2E)-3-phenylprop-2-enoate
Yellow solid; Yield: 83.7%; mp 131–133 Cͦ; Rf = 0.26 (ether:hexane 2:3); IR (KBr, cm−1) ʋ: 2983–3026 (C–Haliphatic), 1723 (C=O ester), 1606 (C=O pyrone), 1448–1535 (C=C), 1158–1281 (C–O); 1H-NMR (400 MHz, DMSO-d6, δ ppm): 3.02 (s, 6H, 2 CH3), 6.44 (d, H, Hα), 7.63 (d, H, Hβ), 6.81–8.05 (m, 13H, aromatic ring); 13C-NMR (100 MHz, DMSO-d6, δ ppm): 40.45 (CH3, 2 CH3), 111.72 (C3′,5′), 115.31 (Cα), 116.73 (C8), 119.39 (C1′), 121.76 (C10), 123.41 (C6), 125.58 (C5), 127.79 (C4″), 128.13 (C2″,6″), 128.21 (C2′,6′), 128.77 (C3″,5″), 133.30 (C3), 134.21.
(C7), 134.90 (C1″), 147.35 (C4′), 147.83 (Cβ), 153.66 (C2), 154.62 (C9), 157.20 (C=O ester), 174.54 (C=O pyron).
2.3. Study of antifungal activity [26]
The anti-fungal activity of Six flavonoids were studied by Petri dish method against three types of fungi: Aspergillus flavus, Acremonium strictum, and Penicillium expansum This is according to Suarez-Jimenez et al. [26]. With some modifications to suit the research, in the Microbiology Laboratory under the supervision of Prof. Dr. Maysa Yazigi – Botany Department – Faculty of Science – Tishreen University. Different amounts of the compounds were dissolved in DMSO dimethyl sulfoxide before mixing with the agar nutrient medium (100 ml in P.D.A) whose temperature were 45 Cͦ to obtain the two concentrations (0.5 mg/ml, 0.25 mg/ml). The medium were poured into Petri dishes with a diameter of 9 cm and left to cool and solidify. A 5 mm disc were placed from the tips of the active fungi colony at the age of 7 days, each fungus separately, and placed on both ends of the Petri dish and at the two concentrations studied. The dishes were incubated at 25 ± 1 °C for 7 days, and control dishes were prepared in each experiment by culturing the mushrooms on P.D. A medium free of the chemical compound. Four replicates were performed for each compound and each concentration separately and for the control dishes as well, after which the average diameters of colony growth were calculated and then the percentage of inhibition were calculated from the following relationship:
where dc: is the average diameter of the control colonies, dt: is the average diameter of the treated colonies.
3. Results and discussion
3.1. Chemistry
In this research, the chalcone compound A1 was synthesized via new synthesise route with a high yield (80.9%). The cyclooxidation process were performed on A1 and we obtained the flavonoid compound A2 with a yield of 82%. By different reactions on compound A2, we obtained ten new flavonoid derivatives with different functional groups and yields ranging between (66.7–94%). The alkylation, esterification and chlorination reactions were carried out on the –OH enolic group, of which synthesis reactions were very few.
The colors of the resulting derivatives ranged between yellow and brown and their melting points varied between (76–78 Cͦ) for flavonoid A3 and (278–280 Cͦ) for A7 flavonoids. The low melting point of compound A3 is due to the presence of an unsaturated carbon chain that lowers its melting points. While compound A7 is characterized by a large molecular weight and the presence of ketone groups and a lactone ring. As for the two compounds A10 and A13, they are close in chemical structure and contain an unsaturated esteric group, their melting points are close. Compounds A11 and A12 are similar in chemical structure and melting points, where the melting point of compound A11, which has a carboxylic group, is slightly higher than the melting point of compound A12, which has an amide group. The lower melting points of the flavonoid derivatives (A3–A6, A8–A13) compared to compound A2 is due to the presence of alkyl groups and ester groups, while the basic compound A2 contains an –OH enolic group that increases its melting point.
The chlorination reaction using Thionyl chloride gave a high yield of 94%. It is generally known that the reaction of the alcoholic group with Thionyl chloride is very fast and takes place in a short time. Compound A2 was successfully reacted with bromoacetyl coumarin, despite the presence of a steric obstruction, as it took a long time to finish the reaction, and the yield was 76%. Figure 2 shows a general scheme for the synthesis of A3–A13 flavonoid derivatives. Table 1 includes the physical properties (melting point, sample color, yield, Molecular formula, molecular weight, Rf) of the new flavonoids (see Figure 2).
Figure 2.
Reaction scheme for the synthesis of alkyl and ester derivatives of flavonoids and their chemical structures.
Table 1.
Shows the physical properties of the new flavonoids.
| Compound | Yield% | Molecular formula | Color | m.w (g/mol) | m.p Cͦ | Rf |
|---|---|---|---|---|---|---|
| A1 | 80.9 | C17H17O2N | Bright pink | 267.3 | 178–180 | 0.47a |
| A2 | 82 | C17H15O3N | Bright yellow | 281.3 | 190–191 | 0.36a |
| A3 | 78.9 | C20H19O3N | Yellow | 321.3 | 76–78 | 0.33a |
| A4 | 82.8 | C18H17O3N | Yellow | 295.3 | 123–125 | 0.22a |
| A5 | 66.7 | C19H18O4N2 | Yellow | 339.3 | 187–188 | 0.37b |
| A6 | 76.9 | C24H21O3N | Yellow | 371.3 | 98–100 | 0.36a |
| A7 | 76 | C28H21O6N | Brown | 467.3 | 278–280 | 0.46b |
| A8 | 94 | C17H14O2NCl | Dark purple | 299.75 | 156–158 | 0.15a |
| A9 | 75 | C19H16O4NCl | Yellow | 357.78 | 155–157 | 0.19a |
| A10 | 87.5 | C21H17O6N | Dark Yellow | 379.36 | 129–131 | 0.18a |
| A11 | 77 | C25H19O6N | Yellow | 429.42 | 146–148 | 0.21a |
| A12 | 78 | C25H20O5N2 | Yellow | 428.43 | 138–140 | 0.24a |
| A13 | 83.7 | C26H21O4N | Yellow | 411.44 | 131–133 | 0.26a |
Ether:hexane 2:3.
Acetone: hexane 2:3.
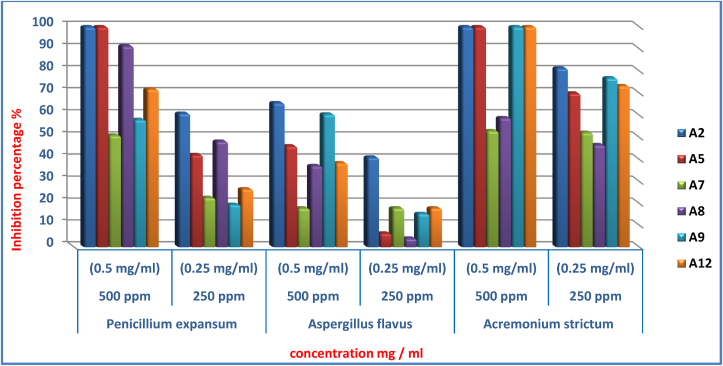
Figure 3.
Results of the biological activity of flavonoids (A2, A5, A7, A8, A9. A12) against fungal strains (Acremonium strictum, Aspergillus flavus, Penicillium expansum).
Infrared spectroscopy of the synthesized alkyl derivatives (A3, A4, A5, A6) revealed the disappearance of the absorption band characteristic of the (–OH) group of the basic flavonoid compound and the appearance of new bands belonging to the methylene (–CH2) group, the (C C) ethylene group and the (-NH2) amide which confirms the chemical structure of the prepared compounds. Spectroscopic analysis also revealed new absorption bands for compound (A7) belonging to the (C O) and (C C) ethylene groups in the lactone ring. Spectroscopic analysis of compound (A8) showed the disappearance of the absorption band characteristic of (–OH) and the appearance of a new absorption band belonging to the group (C–Cl). The spectroscopic analysis of the compounds (A9, A10, A11, A12, A13) showed the disappearance of the absorption band belonging to the (–OH) group and the emergence of.
new absorption bands belonging to the esteric, amide and acidic (C O) groups, which confirms the chemical structure of the new compounds, in addition to several other bands in the fields of Different spectra are shared by all spectra to some extent.
The spectroscopic analysis by (1H-NMR) spectroscopy showed multiple chemical shifts of different protons for all the prepared compounds, the most characteristic of which are the chemical shifts of the protons of the (–CH2) group and (–NH2) group in the amide group, as well as the chemical shifts of the allyl group protons, the benzyl group protons and the protons of the group (C C) ethylene, which confirms the chemical structure of the prepared flavonoids. As for the (13C-NMR) spectra of the prepared compounds, they showed multiple peaks, the most distinct of which are those belonging to the carbon of the methylene group and C C group in the unsaturated alpha-beta ester groups, and the allyl group carbons, as well as the carbon of the carbonyl group in both the amide group, the ester group and the lactone ring. In addition to other peaks Common to all prepared compounds.
3.1.1. Antifungal activity
The antifungal activity were tested on six flavonoids (A2, A5, A7, A8, A9, A12) against three strains of fungi: Acremonium strictum, Aspergillus flavus, and Penicillium expansum. The results shown in Table 2 showed that the compounds (A2, A5, A9, A12) had a high degree of inhibition for Acremonium strictum, where the percentage of inhibition was 100% at 500 ppm. And the least inhibited were (A7, A8), where the percentage of inhibiting them was (53%, 58.88%), respectively. While the effectiveness of compound A2 was higher at the concentration of 250 ppm, where the percentage of inhibition was 81.33%, and the lowest was the effectiveness of compound A8, where the percentage of inhibition was 46.66%.
Table 2.
Percentage inhibition of fungal strains (Acremonium strictum, Aspergillus flavus, Penicillium expansum) in the presence of different concentrations of synthetic flavonoids (A2, A5, A7, A8, A9. A12).
| Fungi Compound |
Acremonium strictum |
Aspergillus flavus |
Penicillium expansum |
|||
|---|---|---|---|---|---|---|
| 250 ppm (0.25 mg/ml) | 500 ppm (0.5 mg/ml) | 250 ppm (0.25 mg/ml) | 500 ppm (0.5 mg/ml) | 250 ppm (0.25 mg/ml) | 500 ppm (0.5 mg/ml) | |
| A2 | 81.33 | 100 | 41.02 | 65.64 | 60.87 | 100 |
| A5 | 70 | 100 | 6.41 | 46.15 | 42.15 | 100 |
| A7 | 52.22 | 53 | 17.94 | 17.94 | 22.74 | 50.98 |
| A8 | 46.66 | 58.88 | 4.1 | 37.17 | 48.23 | 91.37 |
| A9 | 76.88 | 100 | 15.38 | 60.51 | 19.60 | 58.03 |
| A12 | 73.33 | 100 | 17.94 | 38.46 | 26.66 | 71.76 |
The two compounds (A2, A5) showed a higher activity on the fungus Penicillium expansum at the concentration of 500 ppm, where the percentage of its inhibition was 100%, while the least effective was the compound A7, where the percentage of inhibition was 50.98%. As for the concentration of 250 ppm, the effectiveness of compound A2 was the highest, reaching 60.87%, while the lowest was the effectiveness of compound A9, where the percentage of inhibition was 19.60%.
All studied compounds showed medium activity on Aspergillus flavus, and the most inhibiting compound A2 at a concentration of 500 ppm reached 65.64%, while the least effective of compound A8 at a concentration of 250 ppm was 4.1%. The high activity of the compounds (A2, A5, A12) is due to the presence of functional groups (–OH, O C–NH2 aliphatic and aromatic), as it was found that the compound containing the aliphatic amide group was more effective on Penicillium expansum than the compound containing the aromatic amide group. Figure 3 shows the results of biological activity of flavonoids (A2, A5, A7, A8, A9, A12) against fungal strains (Acremonium strictum, Aspergillus flavus, Penicillium expansum).
4. Conclusions
Ten new flavonoid derivatives were synthesized in an easy way and with high yield (66.7–94%), including four alkyl derivatives, five ester derivatives and a new chlorine derivative. All reactions were carried out on the –OH enolic group at the C3 position of the Pyran ring. The course of the reactions was monitored by TLC thin layer chromatography. All compounds were purified by recrystallization and chromatographically separated using Preparative-TLC. The new compounds showed good thermal stability up to 280 Cͦ. The structure of the prepared compounds was confirmed by spectroscopic methods (FT-IR, 13C-NMR, 1H-NMR). The synthetic compounds showed moderate to good antifungal efficacy. Studying the structural relationship with biological activity on a wide range of fungi and comparing it with very important standard drugs that need follow-up later. The study is important for the synthesis of new flavonoid derivatives and their proposal as a broad-spectrum antifungal agent in the field of medical and pharmaceutical sciences.
Declarations
Author contribution statement
Hadi Aqel Khdera: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sawsan Saad, Farouk Kandil: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Aoula Moustapha: Analyzed and interpreted the data.
Funding statement
Hadi Akel Khdera was supported by Tishreen University - Faculty of Science - Department of Chemistry – Syria.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cheong H., Ryu S.Y., Oak M.H., Cheon S.H., Yoo G.S. Studies of structure activity relationship of flavonoids for the anti-allergic actions. Arch Pharm. Res. 1998;21:478–480. doi: 10.1007/BF02974647. [DOI] [PubMed] [Google Scholar]
- 2.García-Mediavilla V., Crespo I., Collado P.S., Esteller A., Sánchez-Campos S. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrich A.B. Flavonoid-membrane interactions: possible consequences for biological effects of some polyphenolic compounds 1. Acta Pharmacol. Sin. 2006;27:27–40. doi: 10.1111/j.1745-7254.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava N., Bezwada R. Indofine Chemical Company; Hillsborough, NJ: 2015. Flavonoids: the Health Boosters. White Paper. [Google Scholar]
- 6.Wink M. Encyclopedia of Plant and Crop Science. 2004. Phytochemical diversity of secondary metabolites; pp. 915–919. [Google Scholar]
- 7.Anand S. Various approaches for secondary metabolite production through plant tissue culture. Pharmacia. 2010;1:1–7. [Google Scholar]
- 8.Fernandez S.P., Wereowski C., Loscalzo L.M., Granger R.E., Johnston G.A.R., Paladini A.C., Marder M. Central nervous system depressant action of flavonoid glycosides. Eur. J. Pharmacol. 2006;539(3):168–176. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Heim K.E., Tagliaferro A.R., Bobliya D.J. Flavonoids antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 10.Hollman P.C.H., Katan M.B. Dietary flavonoids: intake, health effects and bioavailability. Food Chem. Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 11.Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarker S.D., Nahar L. John Wiley & Sons; England: 2007. Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry. [Google Scholar]
- 13.Choi J.S., Chung H.Y., Kang S.S., Jung M.J., Kim J.W., et al. The structure–activity relationship of flavonoids as scavengers of peroxynitrite. Ptr. 2002;16:232–235. doi: 10.1002/ptr.828. [DOI] [PubMed] [Google Scholar]
- 14.Cos P., Ying L., Calomme M., Hu J.P., Cimanga K., et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998;61:71–76. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.G., Liu B.G., Liang G.Z., Ning Z.X. Structure-activity relationship of flavonoids active against lard oil oxidation based on quantum chemical analysis. Molecules. 2009;14:46–52. doi: 10.3390/molecules14010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas O., Jakus J., Héberger K. Quantitative structure–antioxidant activity relationships of flavonoid compounds. Molecules. 2004;9:1079–1088. doi: 10.3390/91201079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jayashree b., anjum N.F., nayak Y., kumar V. Synthesis of substituted 3-hydroxy flavones for antioxidant and antimicrobial activity. Pharmacology. 2008;3:586–595. [Google Scholar]
- 18.Rajaram P., Jiang Z., Chen G., Rivera A., Phasakda A., Zhang Q., Zheng S.l., Wang G., Chen Q.H. Nitrogen-containing derivatives of O-tetramethylquercetin: synthesis and biological profiles in prostate cancer cell models. Bioorg. Chem. 2019 doi: 10.1016/j.bioorg.2019.03.047. S0045-2068(18).30979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonmeza F., Kurtb B.Z., Gazioglu I. Design, synthesis and docking study of novel coumarin ligands as potential selective acetylcholinesterase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32(1):285–297. doi: 10.1080/14756366.2016.1250753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel' S Textbook of Practical Organic Chemistry by Brian S. Furniss, Antony J. Hannaford, Peter W. G. Smith & Austin R. Tatchell; fifth ed.; page: 1065-1066.
- 21.patil v. c. Synthesis and in vitro antiplaque activity of chalcone, flavonol and flavanol derivatives. ijpsr. 2012;3(12):5006–5014. [Google Scholar]
- 22.Venkatesan P., Sumathi S. Piperidine mediated synthesis of N-heterocyclic chalcones and their antibacterial activity. J. Heterocycl. Chem. 2010;47:81. [Google Scholar]
- 23.Liu R., Zhang H., Yuan M. Synthesis and biological evaluation of apigenin derivatives as antibacterial and antiproliferative agents. Molecules. 2013;18:11496–11511. doi: 10.3390/molecules180911496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozłowska J., Potaniec B., arowska B.Z., Anioł M. Synthesis and biological activity of novel O-alkyl derivatives of naringenin and their oximes. Molecules. 2017;22(1485):1–14. doi: 10.3390/molecules22091485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Yu Y.L., Ma D., Zhang Z.Y., Liu X.H. Synthesis, telomerase inhibitory and anticancer activity of new 2-phenyl-4Hchromone derivatives containing 1,3,4-oxadiazole moiety. J. Enzym. Inhib. Med. Chem. 2021;36(1):345–361. doi: 10.1080/14756366.2020.1864630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez-Jiménez G., Cortez-Rocha M., Rosas-Burgos C., Burgos-Hernández A., Plascencia-Jatomea M., Cinco-Moroyoqui F. Vol. 25. 2007. Antifungal activity of plant methanolic extracts against Fusarium verticillioides (Sacc.) nirenb. And Fumonisin B1 production; pp. p134–142. Número 2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.