Abstract
Background
Type 1 diabetes mellitus (T1DM) is an autoimmune disease caused by an autoimmune response against pancreatic islet β cells. Increasing evidence indicates that specific microRNAs (miRNAs) from immune cells extracellular vesicles are involved in islet β cells apoptosis.
Methods
In this study, the microarray datasets GSE27997 and GSE137637 were downloaded from the Gene Expression Omnibus (GEO) database. miRNAs that promote islet β cells apoptosis in T1DM were searched in PubMed. We used the FunRich tool to determine the miRNA expression in extracellular vesicles derived from immune cells associated with islet β cell apoptosis, of which we selected candidate miRNAs based on fold change expression. Potential upstream transcription factors and downstream target genes of candidate miRNAs were predicted using TransmiR V2.0 and starBase database, respectively.
Results
Candidate miRNAs expressed in extracellular vesicles derived from T cells, pro-inflammatory macrophages, B cells, and dendritic cells were analyzed to identify the miRNAs involved in β cells apoptosis. Based on these candidate miRNAs, 25 downstream candidate genes, which positively regulate β cell functions, were predicted and screened; 17 transcription factors that positively regulate the candidate miRNAs were also identified.
Conclusions
Our study demonstrated that immune cell-derived extracellular vesicular miRNAs could promote islet β cell dysfunction and apoptosis. Based on these findings, we have constructed a transcription factor-miRNA-gene regulatory network, which provides a theoretical basis for clinical management of T1DM. This study provides novel insights into the mechanism underlying immune cell-derived extracellular vesicle-mediated islet β cell apoptosis.
Keywords: Type 1 diabetes mellitus, Immune cells, Extracellular vesicles, miRNA, β cells, Apoptosis
Graphical abstract
Type 1 diabetes mellitus; Immune cells; Extracellular vesicles; miRNA; β cells; Apoptosis.
1. Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease attributed to the interaction between genetic and environmental factors such as enterovirus (EV) infection. Recent studies have reported a link between EVs, such as the rotavirus (RV), and the coxsackievirus B (CVB) family and the development of T1DM [1, 2]. EV infection triggers an autoimmune response via inflammatory mediators and other factors; consequently, immune cells such as CD8+ T lymphocytes attack pancreatic β-cells, thereby decreasing their number [3] and impairing islet function, ultimately resulting in insufficient insulin secretion [4].
During the early stages of T1DM, due to the presence of autoantigens on the surface of β-cells, immune cells, especially T lymphocytes and pro-inflammatory macrophages, infiltrate the islet and release pro-inflammatory factors such as interleukin (IL)-β, tumor necrosis factor α (TNFα), and interferon gamma (IFN-γ). These pro-inflammatory cytokines bind to the corresponding β-cell receptors, thereby causing β-cells to release a series of chemokines, which in turn recruit more immune cells to the islet, thus forming a vicious cycle that eventually leads to β-cell death via apoptosis, ultimately resulting in insulin deficiency [3, 5]. To date, there have been no effective treatment strategies to reverse T1DM, and patients require life-long insulin treatment to maintain normal blood glucose levels [6].
Immune cells can release extracellular vesicles during an immune response [7, 8]. Extracellular vesicles, approximately 50–150 nm in diameter and originate from the late endoplasmic reticulum pathway, are secreted into the extracellular space during the fusion of multivesicular bodies with the plasma membrane [7]. Released extracellular vesicles can transport proteins, lipids, mRNAs, and non-coding RNAs from parent to recipient cells, thereby regulating the biological functions of recipient cells [8, 9, 10, 11, 12]. In T1DM, extracellular vesicles released from immune cells are transferred to islet β-cells where they activate intracellular signaling pathways, causing the release of cytokines and chemokines [8]; extracellular vesicles also promote the formation of immune complexes and immune cell-mediated islet β-cell damage. These findings suggest that extracellular vesicles may contribute to the progression of T1DM and are therefore potential early diagnostic markers and effective therapeutic targets.
MicroRNAs (miRNAs) are non-coding single-stranded RNA molecules of 18–25 nucleotides in length and contain some of the main extracellular vesicle components [9]. miRNAs are important regulators of post-transcriptional gene expression and inactivate their target mRNAs by binding to the 3′ UTR region. miRNAs play an important role in β-cell functions, such as proliferation, development, differentiation, functional maturation, and the regulation of insulin secretion [13, 14]. Guay et al. have reported that T lymphocytes release specific miRNAs-containing extracellular vesicles, which bind to islet β-cells and induce the release of chemokines, thereby exacerbating immune system-mediated islet β-cell attack [8].
Although several studies have evaluated the role of miRNAs in promoting islet β-cell apoptosis, limited number of studies have assessed the role of immune cell-derived extracellular vesicular miRNAs in islet β-cell apoptosis. Herein, we construct a transcription factor-miRNA-gene regulatory network in T1DM to provide potential targets for T1DM diagnosis and treatment.
2. Experimental materials and methods
2.1. Experimental design
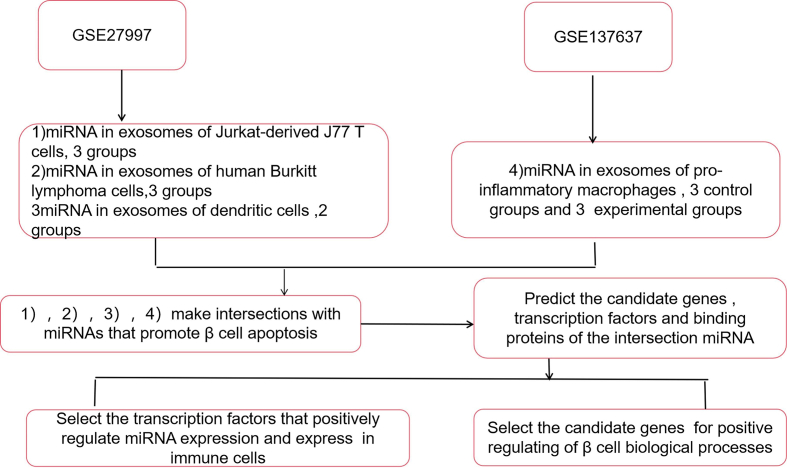
We chronologically searched the GEO database using the keywords, “lymphocyte,” “Exosome,” “miRNA,” and “macrophage.” The research type used was “matrix analysis of non-coding RNA,” and the tissue type was “Homo sapiens; ” the sample source was “extracellular vesicles.” We obtained two qualified datasets, GSE27997 and GSE137637. Using the GSE27997 dataset, the miRNA expression profiles of extracellular vesicles derived from T-, B-, and dendritic cells were analyzed. Conversely, using the GSE137637 dataset, the miRNA expression profiles of extracellular vesicles derived from pro-inflammatory macrophages following treatment with Treponema pallidum (experimental group) or not (control group) were analyzed. The fold change difference in expression of these miRNAs was >|1.5|. Subsequently, using Venn diagrams in the FunRich software [15], we generated intersections between miRNAs expressed in immune cell-derived extracellular vesicles and 17 miRNAs that reportedly promote β cell apoptosis; the latter were obtained from PubMed using the keywords, “microRNA,” “Diabetes,” and “apoptosis.” We identified miRNAs derived from T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages, and selected candidate miRNAs based on fold change difference in their expression by selecting those with significant differential expression; the transcription factors and candidate genes of these miRNAs were predicted using the MISIM V2.0 [16] and starBase [17] software packages, respectively. Finally, we conducted an extensive literature search in PubMed and identified transcription factors (TFs) expressed in immune cells, positively regulated candidate miRNAs, and candidate genes that positively regulate biological functions in islet β-cells. The flow diagram illustrated in Figure 1 depicts the study design.
Figure 1.
Flow diagram showing process the underlying identification of β cell apoptosis-inducing immune cell-derived extracellular vesicular miRNAs.
2.2. Data collection
We searched the GEO database (https://www.ncbi.nlm.nih.gov/gds/term=) using the keywords, “lymphocyte,” “exosome or Extracellular vesicle,” “miRNA,” and “macrophage”. The research type used was “Matrix analysis of non-coding RNA,” the tissue type was “Homo sapiens,” and the sample source was “exosome.” Finally, two datasets (GSE27997 and GSE137637) were obtained for subsequent data analysis. Detailed information on these datasets is listed in Table 1. The expression spectrum is provided in supplementary Tables S1–S4.
Table 1.
2.3. miRNAs present in immune cell-derived extracellular vesicles promote islet β-cell apoptosis
To identify the miRNAs involved in islet β-cell apoptosis, we conducted an extensive literature search in PubMed using the keywords, “microRNA,” “Diabetes,” and “apoptosis,” and selected 17 miRNAs that promote islet β-cell apoptosis during T1DM. FunRich software was used to analyze the intersection of these 17 miRNAs with miRNAs identified in extracellular vesicles derived from T cells, pro-inflammatory macrophages, dendritic cells, and B cells. The heatmap of miRNAs present at the insertion that were selected based on fold change difference in their expression level are shown in Figure 2A and B. Candidate miRNAs with significantly different expression levels were selected. To further clarify the regulatory relationship among candidate miRNAs, MISIM V2.0 was used to construct an miRNA-miRNA interaction network, and the sequences of each candidate miRNA were searched in the miRbase database. Based on the sequences obtained, miRNA-binding proteins were searched using the RBPDP analytical tool [18]. Furthermore, proteins in extracellular vesicles secreted by immune cells were confirmed using the EVpedia database [19]. Finally, miRNAs that stably existed in immune cell-derived extracellular vesicles that could promote islet β-cell apoptosis were identified.
Figure 2.
Heatmap of the candidate miRNAs. A. Heatmap of candidate miRNAs in T lymphocytes (GSM692621, GSM692622, and GSM692630), B lymphocytes (GSM692617, GSM692618, and GSM692629), and dendritic cells (GSM692625 and GSM692626); B. Heatmap of candidate miRNAs in macrophages; GSM4083583, GSM4083584, and GSM4083585 represent the control groups, while GSM4083590, GSM4083591, and GSM4083592 represent the experimental groups.
2.4. Prediction of candidate miRNAs, TFs, and candidate genes
The TransmiR V2.0 [20] database was used to predict the upstream TFs of each candidate miRNA, and the “NetWork” platform was used to identify TFs that could positively regulate candidate miRNAs. In addition, TFs predicted to positively regulate candidate miRNAs were searched in PubMed. Furthermore, TFs expressed in immune cells that could positively regulate the expression of candidate miRNAs were screened.
The starBase tool was used to predict the candidate target genes of identified miRNAs. To confirm these candidate genes, we integrated the potential target genes of candidate miRNAs in four immune cells and confirmed them in PubMed using relevant keywords. Finally, the candidate genes that positively regulate the biological functions of islet β-cells, such as cell proliferation and glucose-stimulated insulin secretion (GSIS), were identified.
3. Results
3.1. miRNAs present in immune cell-derived extracellular vesicles promote β-cell apoptosis
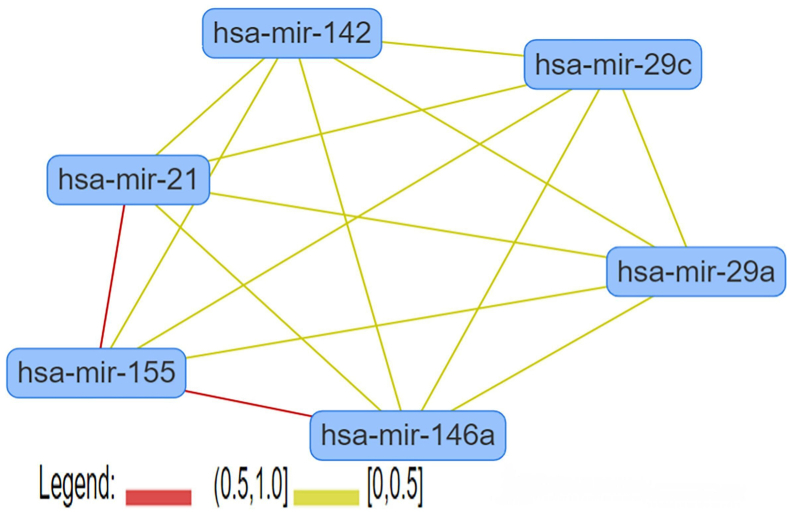
miRNAs play important roles in biological function of immune cell-derived extracellular vesicles; the level of some miRNAs were significantly changed following immunoreaction in immune cells using GEO database. After removing redundancies from the two datasets retrieved from the GSE27997 and GSE137637, we selected miRNAs in extracellular vesicles derived from T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages with fold change >|1.5| in their expression. In addition, we identified 17 miRNAs that promote islet β cell apoptosis in the context of T1DM (Table 2) based on previous studies. Upon analyzing the intersection between miRNAs in immune cell-derived extracellular vesicles and these 17 miRNAs (Supplementary Tables S5–S8), we obtained specific miRNAs and used them for subsequent analyses. Heat maps were drawn for these specific miRNAs based on their expression levels (Figure 2); miRNAs with high fold-change difference in their expression levels were identified as candidate miRNAs and included hsa-miR-142-3p, -142-5p, and -155-5p in extracellular vesicles derived from T lymphocytes, hsa-miR-21-5p, -155-5p, -29a-3p, -29b-3p, and -29c-3p in extracellular vesicles derived from B lymphocytes, hsa-miR-21-5p, -142-3p, and -142-5p in extracellular vesicles derived from dendritic cells, and hsa-miR-21-5p, -29a-3p, -29c-3p, and -146a-5p in extracellular vesicles derived from pro-inflammatory macrophages. Using candidate miRNAs, we constructed a miRNA-miRNA interaction network using MISIM V2.0 (http://www.lirmed.com/misim/allvsall) (Figure 3). All miRNA interactions were positively correlated (red and yellow lines in Figure 3). These miRNAs inhibited the expression of their target candidate genes and impaired the normal functioning of islet β cells. In addition, a strong correlation was observed between hsa-miR-21 and hsa-miR-155 (between 0.5 and 1), indicating that hsa-miR-21 and hsa-miR-155 have a stronger correlation with the occurrence of T1DM.
Table 2.
miRNAs known to promote β cell apoptosis.
| miRNA | Mechanism | References |
|---|---|---|
| hsa-miR-21-5p | pro-inflammatory cytokines induce miR-21 expression in human islet β cells by activating the transcription factor, NF-KB; miR-21 targets the pro-apoptotic mRNA, BCL2, thereby promoting islet β cell apoptosis | [23, 24, 79] |
| hsa-miR-34a | The P53 apoptotic pathway can be activated, thereby promoting β cell apoptosis. | [79] |
| hsa-miR-29a/b/c | miR-29 family RNA molecules promote β cell apoptosis by decreasing the expression levels of the anti-apoptotic protein, Mcl1 | [25] |
| hsa-miR-142-3p | It regulates the NF-KB signaling pathway and stimulates the expression of the chemokine gene, CCL2, thereby promoting islet β cell apoptosis | [8] |
| hsa-miR-142-5p | It regulates the NF-KB signaling pathway and stimulates the expression of the chemokine gene, CCL7, thereby promoting islet β cell apoptosis | [8] |
| hsa-miR-155 | hsa-miR-155 regulates the NF-KB signaling pathway and stimulates the expression of chemokine gene, CXCL10. In addition, increased hsa-miR-155 expression may reduce the expression levels of the anti-apoptotic gene, GLIS3, ultimately promoting islet β cell apoptosis | [2, 8] |
| hsa-miR-146a-5p | Human islet β cell exposure to the pro-inflammatory cytokines, IL-1β and TNF-α, increases miR-146 expression, resulting in islet β cell apoptosis and a decrease in glucose-induced insulin secretion | [13] |
|
Aside from miR-10b-3p, the levels of the other miRNAs are dysregulated following Coxsackievirus B5 injection. These miRNAs target various pattern recognition receptors (PRRs), such as IFHI1, TLR7, and TLR8, and induce pro-inflammatory cytokine production | [2] |
Figure 3.
miRNA-miRNA interaction network. All miRNA interactions were positively correlated, which noted as red and yellow lines; the red lines indicate stronger correlation than the yellow lines.
In extracellular vesicles, miRNAs bind to RNA-binding proteins to increase their stability during transport to target cells where they exert their effects on cells. Using the miRbase (https://www.miRbase.org/) and RBPDB (http://rbpdb.ccbr.utoronto.ca/) databases, we analyzed candidate miRNAs. hsa-miR-142-3p present in extracellular vesicles secreted by T lymphocytes bound to the SFRS1 protein, while hsa-miR-21-5p and hsa-miR-29b-3p in extracellular vesicles derived from B lymphocytes bound to the PABPC1 and ACO1 proteins, respectively; hsa-miR-21-5p and hsa-miR-29c-3p in extracellular vesicles derived from pro-inflammatory macrophages bound to the ELAVL1 protein. Candidate miRNAs in extracellular vesicles derived from dendritic cells remain to be evaluated as no protein was found in published databases that bound to them. Information regarding the binding of miRNAs to proteins in extracellular vesicles secreted by T lymphocytes, pro-inflammatory macrophages, and B lymphocytes is listed in Table 3. Based on the existing database, we identified no RNA-binding proteins that could bind to miRNAs in the dendritic cells derived extracellular vesicles. Moreover, the EVpedia database (http://evpedia.info/evpedia2_xe/) suggests that these proteins are present in extracellular vesicles containing specific miRNAs of the related immune cells. Therefore, we concluded that hsa-miR-21-5p, hsa-miR-29b, hsa-miR-29c-3p, and hsa-miR-142-3p stably existed in extracellular vesicles derived from immune cells and promote islet β cell apoptosis.
Table 3.
Binding situation between miRNAs and proteins in immune cell-derived extracellular vesicles.
| miRNA | RBP name | Matching sequence | Motif logo | |
|---|---|---|---|---|
| miRNAs that bind to proteins in extracellular vesicles derived from T cells | hsa-miR-142-5p | SFRS9 | AGCAC | 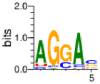 |
| KHDRBS3 | CAUAAA | / | ||
| hsa-miR-142-3p | Pum2 | UGUA | 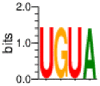 |
|
| ELAVL1 | GUUU | / | ||
| SFRS1 | UGGA | / | ||
| hsa-miR-155-5p | MBNL1 | UGCU | 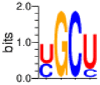 |
|
| YTHDC1 | UAAUGC |  |
||
| KHDRBS3 | GCUAAU | / | ||
| miRNAs that bind to proteins in extracellular vesicles derived from B lymphocytes | hsa-miR-155-5P | MBNL1 | UGCU | 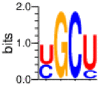 |
| YTHDC1 | UAAUGC | 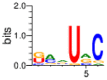 |
||
| KHDRBS3 | GCUAAU | / | ||
| hsa-miR-21-5p | PABPC1 | ACUGAUG |  |
|
| ELAVL1 | GUUG | / | ||
| hsa-miR-29a-3p | SFRS9 | AGCAC | 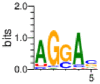 |
|
| RBMX | CCAU | 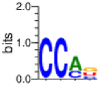 |
||
| hsa-miR-29b-3p | ACO1 | CAGUGU |  |
|
| SFRS9 | AGCAC | 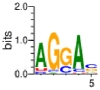 |
||
| RBMX | CCAU |  |
||
| hsa-miR-29c-3p | SFRS9 | AGCAC | 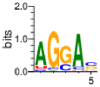 |
|
| RBMX | CCAU |  |
||
| FLAVL1 | AUUU | / | ||
| miRNAs that bind to proteins in extracellular vesicles derived from pro-inflammatory macrophages | hsa-miR-21-5p | PABPC1 | ACUGAUG |  |
| ELAVL1 | GUUG | / | ||
| hsa-miR-29a-3p | SFRS9 | AGCAC | 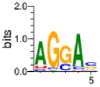 |
|
| RBMX | CCAU | 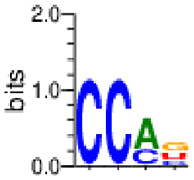 |
||
| hsa-miR-29c-3p | SFRS9 | AGCAC | 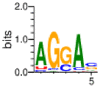 |
|
| RBMX | CCAU | 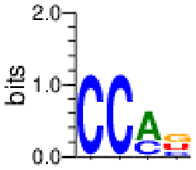 |
||
| ELAVL1 | AUUU | / | ||
| hsa-miR-146a-5p | RBMX | CCAU | 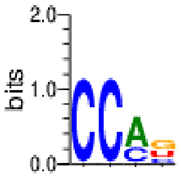 |
3.2. Transcriptional regulation of candidate miRNAs and their targeted effects on mRNAs
The level of some miRNAs of extracellular vesicles was significantly elevated following immunoreaction in immune cells, suggesting that the miRNA transcription was activated by special transcription factor in immune cells. Using the TransmiR V2.0 (http://www.cuilab.cn/transmir) database, we predicted the upstream transcription factors of candidate miRNAs to be hsa-miR-21, hsa-miR-29a, hsa-miR-29b, hsa-miR-29c, hsa-miR-142, hsa-miR-155, and hsa-miR-146a, and selected TFs that upregulated the candidate miRNAs.
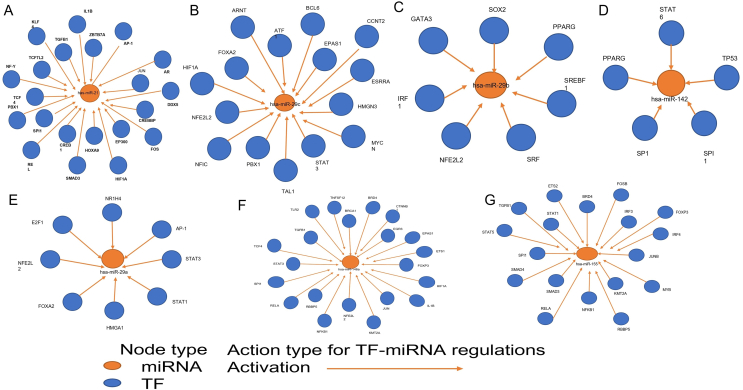
TF-miRNA regulatory networks are shown in Figure 4. Five transcription factors, Ap-1, JUN, KLF4, SMAD3, and TCF4, appeared to have a positive effect on hsa-miR-21-5p (Figure 4A); three transcription factors, EGR3, RELA, and STAT3, positively regulated hsa-miR-146a (Figure 4B); nine transcription factors, BRD4, ETS2, FOSB, FOXP3, IRF4, JUNB, NFKB1, SMAD3, and SMAD4, upregulated hsa-miR-155 (Figure 4C); two transcription factors, STAT3 and STAT1, regulated hsa-miR-29a (Figure 4D). Intriguingly, SMAD3 and STAT3 do not only regulate one miRNA type; thus, their roles in islet β cell apoptosis remain to be fully evaluated. Meanwhile, we observed no transcription factors that simultaneously upregulated the levels of hsa-miR-29b, hsa-miR-29c, and hsa-miR-142 in immune cells (Figure 4E–G). Detailed information on these transcription factors is presented in Table 4.
Figure 4.
TF-miRNA interaction network. A. hsa-miR-21 and its upstream transcription factors; TFs that could positively regulate hsa-miR-21 included AP-1, JUN, KLF4, SMAD3, and TCF4; B. has-miR-29c and its upstream transcription factors; C. hsa-miR-29b and its upstream transcription factors; D. hsa-miR-142 and its upstream transcription factors; E. hsa-miR-29a and its upstream transcription factors, STAT3 and STAT1; F. hsa-miR-146a and its upstream transcription factors; TFs that exerted positive regulatory effects on hsa-miR-146a included EGR3, FOXP3, RELA, and STAT3; G. hsa-miR-155 and its upstream transcription factors; TFs that exerted positive effects on hsa-miR-155 included BRD4, ETS2, FOSB, FOXP3, IRF4, JUNB, NFKB1, SMAD3, and SMAD4.
Table 4.
Upstream TFs of the candidate miRNAs in immune cells.
| TF | Candidate miRNA(s) | Expression in immune cell(s) | References |
|---|---|---|---|
| AP-1 | hsa-miR-21 | Regulates the polarization of pro-inflammatory macrophages | [26, 32] |
| JUN | hsa-miR-21 | M-CSF can promote its expression in macrophages, and JUN together with NF-KB promote the transformation of macrophages from M1 to M2 | [27, 31] |
| KLF4 | hsa-miR-21 | Regulates the polarization of pro-inflammatory macrophages | [28, 33] |
| SMAD3 | hsa-miR-21/hsa-miR-155 | It mediates macrophage phenotype and anti-inflammatory transformation, and acts synergistically with TGFβ to maintain CD4+ T cell immune tolerance | [29, 34, 45] |
| TCF4 | hsa-miR-21 | It regulates development and maintains normal physiological functions in dendritic cells | [30, 35] |
| EGR3 | hsa-miR-146a | EGR3 is expressed in bone pro-inflammatory macrophages; it binds to the ifngr1 promoter and inhibits its transcription under IFNβ stimulation, and this may be associated with anti-inflammatory effects | [42, 43] |
| FOXP3 | hsa-miR-155 | FOXP3 plays a role in FOXP3+/CD4+ regulatory T cell development | [46, 52] |
| RELA | hsa-miR-146a | RELA expression in pro-inflammatory macrophages promotes cytokine production, which is beneficial for immune function | [41, 44] |
| STAT3 | hsa-miR-146a/hsa-miR-29a | STAT3 plays a role in B cell development | [37, 38, 42] |
| BRD4 | hsa-miR-155 | It plays a role in the transcription of some specific genes associated with CD4+T cells | [47, 53] |
| ETS2 | hsa-miR-155 | It binds to the miR-155 promoter sequence and induces high miR-155 expression in B cells | [48] |
| FOSB | hsa-miR-155 | FOSB dimerizes with c-jun to induce T cell death | [49, 54] |
| IRF4 | hsa-miR-155 | It binds with SPI1 and BATF to form a transcription factor complex and plays an important role in the activation of B and T lymphocytes | [50] |
| JUNB | hsa-miR-155 | After TCR activation, JUNB expression and transcriptional activity increases in T cells | [49, 54] |
| NFKB1 | hsa-miR-155 | It plays a role in B cell maturation | [51, 56] |
| SMAD4 | hsa-miR-155 | It plays a role in the expression of the ligand, selectin, in CD4+T cells | [45, 55] |
| STAT1 | hsa-miR-29a | STAT1 indirectly regulates macrophage polarization in synergy with METTL3 | [36, 39] |
As for miRNAs in extracellular vesicles derived from T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages, which were selected based on fold change, starBase was used to predict their potential downstream candidate genes. Based on the data from previous studies, we preliminarily identified candidate genes associated with the biological functions of beta cells; we found eight downstream candidate genes, CDC42, SLC30A7, GNPNAT1, SMAD2, RICTOR, PELO, NNT, and RHEB, associated with miRNAs in T lymphocyte-derived extracellular. There were 13 downstream candidate genes, DHCR24, CAMTA1, FBXO28, CD59, GIT2, ZDHHC17, STAT3, FLOT2, ONECUT2, ARFRP1, OPA1, CDK6, and MTPN, associated with miRNAs in extracellular vesicles derived from pro-inflammatory macrophages. Meanwhile, there were nine downstream candidate genes, DHCR24, KDM5B, CAMTA1, CD59, DICER1, XRN1, HMGCR, CDK6, and MTPN, associated with miRNAs in B lymphocyte-derived extracellular vesicles (Table 5).
Table 5.
Target genes of the candidate miRNAs.
| miRNA | Target genes | Functions | Mechanism | References |
|---|---|---|---|---|
| hsa-miR-21/29a/29b/29c/146a/155 | DHCR24 | ↓β cell apoptosis | Inhibits the generation of reactive oxygen species (ROS) | [58] |
| hsa-miR-21/29a/29b/29c/146a/155 | CAMTA1 | Maintains β cell identity, and promotes insulin synthesis and secretion | Regulates NKX2-2 expression and promotes SLC2a2 and Mafa expression | [62] |
| hsa-miR-21/29a/29b/29c/146a/155 | CD59 | ↑glucose-induced insulin secretion (GSIS) | CD59 interacts with SNARE proteins to promote insulin secretion | [61] |
| hsa-miR-21/29a/29b/29c/155 | DICER1 | Affects β cell mass, insulin secretion, and β cell development | Processes pre-miRNA and guarantees the expression of functional miRNAs in β cells | [80] |
| hsa-miR-21/29a/29b/29c/155 | XRN1 | ↑insulin synthesis and protects β cells against cytokine-induced apoptosis | Promotes pro-apoptotic protein degradation | [59] |
| hsa-miR-142-3p/155 | PELO | ↑insulin synthesis and protects cells against cytokine-induced apoptosis | Promotes pro-apoptotic protein degradation | [59] |
| hsa-miR-21/29a/29b/29c/155 | HMGCR | ↑β cell mass and insulin secretion | Inhibits TAP/TAC activation | [80] |
| hsa-miR-142/155 | NNT | ↑insulin secretion | Increases the NADH/NAD + ratio under high glucose conditions and regulates the effects of Ca2+ on exocytosis | [64] |
| hsa-miR-21/29a/29b/29c/146a/155 | CDK6 | ↑β cell proliferation | Regulates the G1/S phase of the growth cycle in β cells | [77] |
| hsa-miR-21/29a/29b/29c/146a/155 | MTPN | Regulates insulin secretion | Controls F-actin formation and fuses the cyst with the plasma membrane | [65] |
| hsa-miR-21/29a/29c/146a/142 | FBXO28 | ↑β cell survival under diabetic conditions | Protects β cells against apoptosis | [81] |
| hsa-miR-21/29a/29c/146a | GIT2 | Affects insulin secretion and β cell mass | Controls insulin receptor-related signals | [66] |
| hsa-miR-21/29a/29c/146a | ZDHHC17 | ↑ β cell survival and glucose-induced insulin secretion | Inhibits the IL-1β-induced NF-KB signaling pathway and protects islet β cells against IL-1β-mediated β cell apoptosis | [67] |
| hsa-miR-21/29a/29c/146a | STAT3 | ↓β cell apoptosis and ↑β cell proliferation | Inhibits PTEN expression and promotes the phosphorylation of downstream AKTs, thereby triggering the transcription of β cell-related genes | [60] |
| hsa-miR-21/29a/29c/146a | ONECUT2 | ↑insulin secretion | Inhibits the expression of granulocyte proteins, thereby blocking the bioregulatory process of granulocyte protein-mediated insulin secretion inhibition | [25] |
| hsa-miR-21/29a/29c/146a | ARFRP1 | ↑insulin secretion | ARFRP1 associates with the Golgi-associated PDZ, coiled-coil motif-containing protein (GOPC), to regulate insulin secretion by controlling the localization of the SNAP25 protein on the plasma membrane | [68] |
| hsa-miR-21/29a/29c/146a | OPA1 | ↑insulin secretion | Maintains the integrity of the electron transport chain in the mitochondrion, thereby ensuring the production of ATP and providing energy for insulin secretion | [69] |
| hsa-miR-142-3p/155 | CDC42 | Regulates insulin secretion and expression | Promotes insulin secretion by inducing actin remodeling, and promotes insulin expression by phosphorylating ERK1/2 and NeuroD1 | [70] |
| hsa-miR-142/155 | SLC30A7 | Regulates insulin expression | Regulates insulin expression by regulating Mtf1 transcriptional activity | [82] |
| hsa-miR-142/155 | GNPNAT1 | ↑insulin secretion and may protect β cells | GNPNAT1 methylation may have a protective effect on beta cells and increases insulin secretion to meet up with increasing insulin demand | [71] |
| hsa-miR-142 | SMAD2 | Maintains β cell identity | Maintains β cell function and identity through the Tgfb2 pathway | [83] |
| hsa-miR-142 | RICTOR | ↑ β cell proliferation | RICTOR/mTORC2 downregulates FOXO1 and P27 expression by acting on PAKT-S437 | [78] |
| hsa-miR-21/142 | SIK2 | ↑insulin secretion | SIK2 is activated at high glucose levels, and phosphorylates P35 | [72] |
| hsa-miR-21/142 | SOX5 | ↑insulin secretion | Regulates the opening of calcium channels on the mitochondrial membrane and produces ATP synthase for the provision of energy for insulin secretion | [73] |
| hsa-miR-21/142 | TSHZ1 | Regulates β cell maturation and insulin secretion | Directly regulates CLEC16A or indirectly regulates CLE16A through PDX1 | [74] |
| hsa-miR-21/142 | ABCA1 | Regulates insulin secretion | Maintains normal cholesterol levels, thereby preventing lipid toxicity-induced dysregulation of normal pancreatic β cell function, thus ensuring normal insulin secretion | [76] |
| hsa-miR-21/142 | IDS | ↑GSIS | Promotes insulin secretion by Phosphorylating PKCα and MARCKS | [75] |
3.3. Construction of the diagram showing islet β cell apoptosis-promoting immune cell-derived extracellular vesicular miRNAs
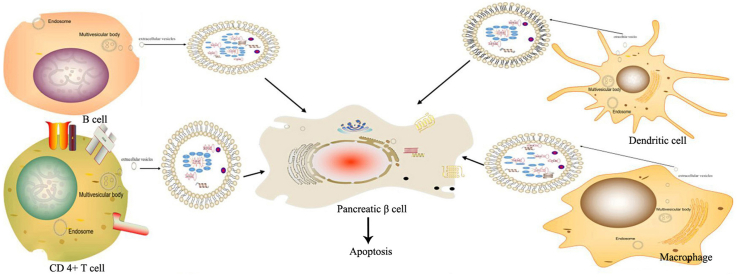
The immune cells included in this study were CD4+ T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages, all of which release cytokines and extracellular vesicles that contain specific miRNAs. In response to stimuli, cytokines are released by these immune cells and bind to cytokine receptors on islet β cells, thereby activating downstream transcription factors (e.g., NF-κB and STAT-1) that promote chemokine release. Immune cells are recruited to the islet β cell environment, thereby exposing islet β cells to a long-term inflammatory environment. miRNAs contained in extracellular vesicles can be positively regulated by upstream transcription factors, thereby increasing their expression. miR-155, miR-21, miR-146a, and miR-29a are transported by extracellular vesicles and transferred to β cells in their active form, where they bind to candidate genes that regulate cell function. Figure 5 illustrated the process of miRNA binding and their candidate genes in the cytoplasm of β cells. These candidate genes promote insulin secretion and islet β cell proliferation, and inhibit apoptosis. After miRNAs bind to their candidate genes, expression is inhibited, thereby causing islet β cell dysfunction, which decreases islet β cell count and decreases insulin secretion, and ultimately T1DM. In addition, in extracellular vesicles derived from B lymphocytes, miR-29b binds to the ACO1 protein, while in those derived from pro-inflammatory macrophages, miR-29c binds to the ELAVL1 protein. These miRNAs can exist stably in extracellular vesicles. Our results indicate that miR-155, miR-21, miR-146a, and miR-29a can regulate transcription factors, along with some target genes, which may play a role in T1DM progression through TFs-miRNA gene regulatory network.
Figure 5.
Immune cell-derived extracellular vesicular MicroRNAs induce pancreatic beta cell apoptosis.
4. Discussion
T1DM is a chronic disease prevalent worldwide, with significant deleterious effects on human health. Although the pathogenesis and treatment for T1DM have been extensively studied, the specific mechanism underlying islet β cell loss in T1DM remains unclear. Studies have reported that pattern recognition receptors (PRRs) (e.g., TLR3, TLR4, RIG, and MAD5) and cytokine receptors (IL-1R and TNFR, among others) are expressed on the surface of islet β cells [3]. PRRs can detect rotavirus nucleic acids [1, 2]. In addition, some pro-inflammatory cytokines (IL-β, TNF-α, and IFN-γ) bind to cytokine receptors, thereby activating transcription factors, such as NF-κB and STAT-1 [3], which induce the release of chemokines, such as CXCL10 and CCL2 [21,22], which in turn induce the recruitment of more immune cells to the islet, ultimately aggravating islet β cell apoptosis. Furthermore, MHC class I molecules expressed in islet β cells present autoantigens such as proinsulin, GAD65, and IA-2, which are recognized by CD8+ T cells. This process initiates islet β cell attack, which leads to progressive β cell loss [1]. Exogenous insulin supplementation is the conventional approach for T1DM treatment; however, miRNAs present in immune cell-derived extracellular vesicles can promote islet β cell apoptosis and serves as biomarkers for T1DM prevention and treatment.
miRNAs expressed in extracellular vesicles released by Jurakt cells, Raji cells, dendritic cells, and pro-inflammatory macrophages were analyzed, and the following eight miRNAs were found to promote islet β cell apoptosis: hsa-miR-142-3p, hsa-miR-142-5p, hsa-miR-155-5p, hsa-miR-21-5p, hsa-miR-29a-3p, hsa-miR-29b-3p, and hsa-miR-29c-3p. Guay et al. found that T lymphocyte-derived extracellular vesicles specifically contain miR-142-3p/-5p and miR-155: miR-142-3p is overexpressed in activated T lymphocytes and transferred to β cells where it promotes the expression of CCL2, CCL7, and CXCL10, which are involved in the chemokine signaling pathway [8]. In addition, miR-142-3p can regulate the expression of cytokine-encoding genes [8] which induces the formation an inflammatory environment by producing pro-inflammatory cytokines and facilitating immune cell recruitment. This leads to the impairment of normal islet β cell function and a reduction in islet β cell count. miR-21 can inhibit the transcription of the anti-apoptotic BCL2-encoding gene and increase caspase3 production, thereby promoting islet β cell apoptosis [23, 24]. During the early stages of diabetes, islet β cells are exposed to the inflammatory environment formed by pro-inflammatory cytokines; under these conditions, miR-29 overexpression inhibits the expression of the transcription factor, Onecut2, which is associated with defective insulin secretion. Furthermore, miR-29 overexpression-induced decrease in the levels of the antiapoptotic protein Mcl1 impairs mitochondrial function, cytochrome c release, and caspase3 activation, thereby accelerating islet β cell apoptosis [25].
Roggli et al. confirmed that following human islet β cell exposure to an inflammatory environment, miR-146a overexpression promoted islet β cell apoptosis and decreased GSIS levels; however, the specific mechanism underlying this process remains poorly understood [13].
TFs can regulate miRNA expression. Thus, we predicted a series of TFs that could positively regulate candidate miRNAs in the TransmiR V2.0 database. In addition, to confirm the accuracy of our data, we analyzed studies published in PubMed to verify these TFs. AP-1, JUN, KLF4, SMAD3, and TCF4 were found to promote miR-21 expression [26, 27, 28, 29, 30]. Based on the data obtained from our datasets, miR-21 was overexpressed in B lymphocytes, dendritic cells, and pro-inflammatory macrophages. By analyzing related literature, we confirmed that these miR-21-regulating TFs were expressed in at least one of these immune cells. AP-1, JUN, KLF4, and SMAD3 play a role in macrophage polarization [31, 32, 33, 34], while TCF4 plays a role in dendritic cell development and functional maintenance [35]. TFs, STAT1 and STAT3 can promote miR-29a expression [36, 37] in extracellular vesicles secreted by B lymphocytes and pro-inflammatory macrophages. STAT3 is required for B cell development [38], whereas STAT1 plays an indirect role in pro-inflammatory macrophage polymerization as METTL3 regulates pro-inflammatory macrophage polarization [39]; miR-146a is significantly overexpressed in extracellular vesicles derived from pro-inflammatory macrophages, while the TFs, EGR3, RELA, and STAT3 can upregulate miR-146a levels [40, 41, 42]. Both EGR3 and RELA are expressed in pro-inflammatory macrophages [38, 43, 44], while STAT3 is expressed in B lymphocytes [38]. Several upstream TFs, including JUNB, FOSB, BRD4, FOXP3, SMAD3, ETS2, IRF4, NFKB1, and SMAD4, regulate miR-155 expression [45, 46, 47, 48, 49, 50, 51]. Data obtained from our datasets indicate that miR-155 is overexpressed in extracellular vesicles derived from T and B lymphocytes. Literature was retrieved for these nine miR-155-regulating TFs to determine whether they were expressed in T or B cells. We concluded that the TFs JUNB, FOSB, BRD4, FOXP3, SMAD3, and SMAD4 are likely expressed in CD4+T cells [34, 52, 53, 54, 55], while ETS2 and NFKB1 are likely expressed in B cells [48, 56]. The complex between IRF4, SPI1, and BATF plays an important role in T and B cell activation [50]. By analyzing TFs, we confirmed that IRF4 is expressed in immune cells and positively regulates miRNAs promoting β cell apoptosis. T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages can release extracellular vesicles containing specific TFs and deliver their regulated miRNAs to islet β cells following stimulation by pro-inflammatory cytokines. These miRNAs enter β cells and modulate the expression of target genes that regulate cell biological functions, such as insulin secretion and cell development, thereby dysregulating normal biological functions in islet β cells and causing metabolic dysfunction.
Specific miRNAs present in extracellular vesicles derived from T lymphocytes, B lymphocytes, dendritic cells, and pro-inflammatory macrophages were selected based on their fold-change values; these included 10 miRNAs, such as hsa-miR-142 and hsa-miR-155. We predicted the candidate target genes of these miRNAs, and an intersection analysis of the obtained candidate genes and their screening based on their roles in islet β cells led to the identification of the appropriate downstream targeting genes for miR-155, miR-21, miR-29a, and miR-146a. Among other functions, these genes regulate insulin secretion and islet β cell proliferation and protect islet β cells against apoptosis, thereby maintaining normal β cell function [8, 24, 25, 57]. miRNAs can interfere with islet β cell function by acting on different genes; however, some genes are not targeted by only one miRNA. For example, DHCR24 can be targeted by miR-21, miR-29a, and miR-155 [58]. Apoptosis-induced loss of β cell mass is the main mechanism underlying T1DM, and ultimately leads to insufficient insulin secretion [5, 8]. The candidate genes, DHCR24, XRN1, PELO, and STAT3, can decrease islet β cell apoptosis by inhibiting reactive oxygen species generation and degrading pro-apoptotic proteins [58, 59, 60]. XRN1 and PELO protect islet β cells against cytokine-induced apoptosis [59]. Several candidate genes, including CAMTA1 and CD59, play important roles in the biological process underlying insulin secretion [25, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76]; CDK6, STAT3, and RICTOR participate in islet β cell proliferation [60, 77, 78]. Some candidate genes can regulate multiple biological processes in islet β cells; for instance, STAT3 can promote islet β cell proliferation and protect them against apoptosis [60]. This increases diversity and complexity of islet β cell regulation. Targeting multiple regulatory target genes may interrupt biological processes in islet β cells, affect islet β cell function, and alter the course of the disease. Therefore, these predicted candidate genes should be further evaluated to identify key genes that play a critical role in maintaining normal islet β cell function.
In this study, by integrating the datasets and microarray analysis, we determined the miRNA expression in immune cell-derived extracellular vesicles. We focused on immune cell-derived extracellular vesicular miRNAs capable of inducing islet β cell apoptosis. Among these, miR-155, miR-21, miR-146a, and miR-29a were found to regulate transcription factors as well as some target genes, which can form a TFs-miRNA gene regulatory network; thus, they may play a key role in the progression of T1DM. Immune cells are speculated to secrete extracellular vesicles containing specific miRNAs, which can be transferred to islet β cells, thereby inhibiting normal islet β cell biological function via inhibiting the expression of candidate genes, leading to disease aggravation.
This study has some limitations. First, some miRNAs that regulate islet β cell apoptosis during T1DM may have been omitted due to different retrieval methods, whereas for some other miRNAs, it remains to be determined whether the experimental results obtained in mice comparable to those in humans; therefore, these miRNAs warrant further investigated. Second, based on the results obtained from the database analyzed in this study, binding proteins were not identified for some immune cell-derived extracellular vesicular miRNAs that affect islet β cell apoptosis; however, this does not indicate that these miRNAs could not exist stably in immune cell-derived extracellular vesicles. Third, we found no key genes nor TFs that promoted islet beta cell apoptosis, thus could not construct a specific TF-microRNA gene regulatory network.
TFs and candidate genes identified in this study remain to be assessed to confirm the molecules involved in islet β cell apoptosis. Finally, the current data was obtained from cell lines, including B cells, macrophages, dendritic cell, CD 4+ T cells, but not CD 8+ T cells, hence further studies are warranted prior to clinical applications. Despite these limitations, this study provides a theoretical basis and research direction for the construction of the TF-miRNA gene regulatory network, along with novel insights into the diagnosis, treatment, and prevention of T1DM.
Declarations
Author contribution statement
Yueyang Yu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mengyin Li: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yuxuan Zhao: Contributed to reagents, materials, analysis tools or data.
Fangzhou Fan and Wenxiang Wu: Contributed to reagents, materials, analysis tools or data; Analyzed and interpreted the data.
Chunyu Bai and Yuhua Gao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Chunyu Bai was supported by National Natural Science Foundation of China, China [31972755], Shandong Provincial Natural Science Foundation, China [ZR2020KH031], Project of Shandong Province Higher Educational Youth Innovation Science and Technology Program, China [2019KJK010], Shandong Provincial Natural Science Foundation, China (Grant No. ZR2020KH031), Project of Shandong Province Higher Educational Youth Innovation Science and Technology Program (2019KJK010).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Yuhua Gao, Email: anngyh@126.com.
Chunyu Bai, Email: chunyu_bai@hotmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Harrison L.C., Perrett K.P., Jachno K., Nolan T.M., Honeyman M.C. Does rotavirus turn on type 1 diabetes? PLoS Pathog. 2019;15(10) doi: 10.1371/journal.ppat.1007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim K.W., Ho A., Alshabee-Akil A., Hardikar A.A., Kay T.W., Rawlinson W.D., Craig M.E. Coxsackievirus B5 infection induces dysregulation of microRNAs predicted to target known type 1 diabetes risk genes in human pancreatic islets. Diabetes. 2016;65(4):996–1003. doi: 10.2337/db15-0956. [DOI] [PubMed] [Google Scholar]
- 3.Eizirik D.L., Colli M.L., Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 4.Tomita T. Apoptosis of pancreatic β-cells in Type 1 diabetes. Bosn. J. Basic Med. Sci. 2017;17(3):183–193. doi: 10.17305/bjbms.2017.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eizirik D.L., Colli M.L. Revisiting the role of inflammation in the loss of pancreatic β-cells in T1DM. Nat. Rev. Endocrinol. 2020;16(11):611–612. doi: 10.1038/s41574-020-00409-6. [DOI] [PubMed] [Google Scholar]
- 6.Eizirik D.L., Pasquali L., Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat. Rev. Endocrinol. 2020;16(7):349–362. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 7.Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12(12):1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 8.Guay C., Kruit J.K., Rome S., Menoud V., Mulder N.L., Jurdzinski A., Mancarella F., Sebastiani G., Donda A., Gonzalez B.J., Jandus C., Bouzakri K., Pinget M., Boitard C., Romero P., Dotta F., Regazzi R. Lymphocyte-derived exosomal MicroRNAs promote pancreatic β cell death and may contribute to type 1 diabetes development. Cell Metabol. 2019;29(2):348–361.e6. doi: 10.1016/j.cmet.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Dumortier O., Hinault C., Van Obberghen E. MicroRNAs and metabolism crosstalk in energy homeostasis. Cell Metabol. 2013;18(3):312–324. doi: 10.1016/j.cmet.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 10.de Candia P., Torri A., Pagani M., Abrignani S. Serum microRNAs as biomarkers of human lymphocyte activation in health and disease. Front. Immunol. 2014;5:43. doi: 10.3389/fimmu.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvestro S., Chiricosta L., Gugliandolo A., Pizzicannella J., Diomede F., Bramanti P., Trubiani O., Mazzon E. Extracellular vesicles derived from human gingival mesenchymal stem cells: a transcriptomic analysis. Genes. 2020;11(2) doi: 10.3390/genes11020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestro S., Gugliandolo A., Chiricosta L., Diomede F., Trubiani O., Bramanti P., Pizzicannella J., Mazzon E. MicroRNA profiling of HL-1 cardiac cells-derived extracellular vesicles. Cells. 2021;10(2) doi: 10.3390/cells10020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roggli E., Britan A., Gattesco S., Lin-Marq N., Abderrahmani A., Meda P., Regazzi R. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010;59(4):978–986. doi: 10.2337/db09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osmai M., Osmai Y., Bang-Berthelsen C.H., Pallesen E.M., Vestergaard A.L., Novotny G.W., Pociot F., Mandrup-Poulsen T. MicroRNAs as regulators of beta-cell function and dysfunction. Diabetes Metab. Res. Rev. 2016;32(4):334–349. doi: 10.1002/dmrr.2719. [DOI] [PubMed] [Google Scholar]
- 15.Pathan M., Keerthikumar S., Ang C.S., Gangoda L., Quek C.Y., Williamson N.A., Mouradov D., Sieber O.M., Simpson R.J., Salim A., Bacic A., Hill A.F., Stroud D.A., Ryan M.T., Agbinya J.I., Mariadason J.M., Burgess A.W., Mathivanan S. FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics. 2015;15(15):2597–2601. doi: 10.1002/pmic.201400515. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Zhang S., Wan Y., Zhao Y., Shi J., Zhou Y., Cui Q. MISIM v2.0: a web server for inferring microRNA functional similarity based on microRNA-disease associations. Nucleic Acids Res. 2019;47(W1):W536–w541. doi: 10.1093/nar/gkz328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook K.B., Kazan H., Zuberi K., Morris Q., Hughes T.R. RBPDB: a database of RNA-binding specificities. Nucleic Acids Res. 2011;39(Database issue):D301–D308. doi: 10.1093/nar/gkq1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D.K., Lee J., Simpson R.J., Lötvall J., Gho Y.S. EVpedia: a community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin. Cell Dev. Biol. 2015;40:4–7. doi: 10.1016/j.semcdb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Tong Z., Cui Q., Wang J., Zhou Y. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019;47(D1):D253–d258. doi: 10.1093/nar/gky1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier J.J., Sparer T.E., Karlstad M.D., Burke S.J. Pancreatic islet inflammation: an emerging role for chemokines. J. Mol. Endocrinol. 2017;59(1):R33–r46. doi: 10.1530/JME-17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar S.A., Lee C.E., Victorino F., Nguyen T.T., Walters J.A., Burrack A., Eberlein J., Hildemann S.K., Homann D. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61(2):436–446. doi: 10.2337/db11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakhter A.J., Pratt R.E., Moore R.E., Doucette K.K., Maier B.F., DiMeglio L.A., Sims E.K. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia. 2018;61(5):1124–1134. doi: 10.1007/s00125-018-4559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims E.K., Lakhter A.J., Anderson-Baucum E., Kono T., Tong X., Evans-Molina C. MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells. Diabetologia. 2017;60(6):1057–1065. doi: 10.1007/s00125-017-4237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roggli E., Gattesco S., Caille D., Briet C., Boitard C., Meda P., Regazzi R. Changes in microRNA expression contribute to pancreatic β-cell dysfunction in prediabetic NOD mice. Diabetes. 2012;61(7):1742–1751. doi: 10.2337/db11-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita S., Ito T., Mizutani T., Minoguchi S., Yamamichi N., Sakurai K., Iba H. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J. Mol. Biol. 2008;378(3):492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Echevarría-Vargas I.M., Valiyeva F., Vivas-Mejía P.E. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0097094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S.B., Lin C.C., Farrugia M.K., McLaughlin S.L., Ellis E.J., Brundage K.M., Salkeni M.A., Ruppert J.M. MicroRNAs 206 and 21 cooperate to promote RAS-extracellular signal-regulated kinase signaling by suppressing the translation of RASA1 and SPRED1. Mol. Cell Biol. 2014;34(22):4143–4164. doi: 10.1128/MCB.00480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong X., Chung A.C., Chen H.Y., Meng X.M., Lan H.Y. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 2011;22(9):1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan F., Yue X., Han L., Shi Z., Yang Y., Pu P., Yao Z., Kang C. Genome-wide identification of TCF7L2/TCF4 target miRNAs reveals a role for miR-21 in Wnt-driven epithelial cancer. Int. J. Oncol. 2012;40(2):519–526. doi: 10.3892/ijo.2011.1215. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y., Qin J., Lan L., Li N., Wang C., He P., Liu F., Ni H., Wang Y. M-CSF cooperating with NFκB induces macrophage transformation from M1 to M2 by upregulating c-Jun. Cancer Biol. Ther. 2014;15(1):99–107. doi: 10.4161/cbt.26718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannemann N., Cao S., Eriksson D., Schnelzer A., Jordan J., Eberhardt M., Schleicher U., Rech J., Ramming A., Uebe S., Ekici A., Cañete J.D., Chen X., Bäuerle T., Vera J., Bogdan C., Schett G., Bozec A. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Invest. 2019;129(7):2669–2684. doi: 10.1172/JCI96832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenzweig J.M., Glenn J.D., Calabresi P.A., Whartenby K.A. KLF4 modulates expression of IL-6 in dendritic cells via both promoter activation and epigenetic modification. J. Biol. Chem. 2013;288(33):23868–23874. doi: 10.1074/jbc.M113.479576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delisle J.S., Giroux M., Boucher G., Landry J.R., Hardy M.P., Lemieux S., Jones R.G., Wilhelm B.T., Perreault C. The TGF-β-Smad3 pathway inhibits CD28-dependent cell growth and proliferation of CD4 T cells. Gene Immun. 2013;14(2):115–126. doi: 10.1038/gene.2012.63. [DOI] [PubMed] [Google Scholar]
- 35.Grajkowska L.T., Ceribelli M., Lau C.M., Warren M.E., Tiniakou I., Nakandakari Higa S., Bunin A., Haecker H., Mirny L.A., Staudt L.M., Reizis B. Isoform-specific expression and feedback regulation of E protein TCF4 control dendritic cell lineage specification. Immunity. 2017;46(1):65–77. doi: 10.1016/j.immuni.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt M.J., Philippidou D., Reinsbach S.E., Margue C., Wienecke-Baldacchino A., Nashan D., Behrmann I., Kreis S. Interferon-γ-induced activation of Signal Transducer and Activator of Transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell Commun. Signal. 2012;10(1):41. doi: 10.1186/1478-811X-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang A., Deng S., Chen X., Yu C., Du Q., Wu Y., Chen G., Hu L., Hu C., Li Y. miR-29a-5p/STAT3 positive feedback loop regulates TETs in colitis-associated colorectal cancer. Inflamm. Bowel Dis. 2020;26(4):524–533. doi: 10.1093/ibd/izz281. [DOI] [PubMed] [Google Scholar]
- 38.Bhansali R.S., Rammohan M., Lee P., Laurent A.P., Wen Q., Suraneni P., Yip B.H., Tsai Y.C., Jenni S., Bornhauser B., Siret A., Fruit C., Pacheco-Benichou A., Harris E., Besson T., Thompson B.J., Goo Y.A., Hijiya N., Vilenchik M., Izraeli S., Bourquin J.P., Malinge S., Crispino J.D. DYRK1A regulates B cell acute lymphoblastic leukemia through phosphorylation of FOXO1 and STAT3. J. Clin. Invest. 2021;131(1) doi: 10.1172/JCI135937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Liu Z., Tang H., Shen Y., Gong Z., Xie N., Zhang X., Wang W., Kong W., Zhou Y., Fu Y. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am. J. Physiol. Cell Physiol. 2019;317(4):C762–c775. doi: 10.1152/ajpcell.00212.2019. [DOI] [PubMed] [Google Scholar]
- 40.Sun X., Zhang J., Hou Z., Han Q., Zhang C., Tian Z. miR-146a is directly regulated by STAT3 in human hepatocellular carcinoma cells and involved in anti-tumor immune suppression. Cell Cycle. 2015;14(2):243–252. doi: 10.4161/15384101.2014.977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghose J., Bhattacharyya N.P. Transcriptional regulation of microRNA-100, -146a, and -150 genes by p53 and NFκB p65/RelA in mouse striatal STHdh(Q7)/Hdh(Q7) cells and human cervical carcinoma HeLa cells. RNA Biol. 2015;12(4):457–477. doi: 10.1080/15476286.2015.1014288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H.S., Sivachandran N., Lau A., Boudreau E., Zhao J.L., Baltimore D., Delgado-Olguin P., Cybulsky M.I., Fish J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013;5(7):1017–1034. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kearney S.J., Delgado C., Eshleman E.M., Hill K.K., O'Connor B.P., Lenz L.L. Type I IFNs downregulate myeloid cell IFN-γ receptor by inducing recruitment of an early growth response 3/NGFI-A binding protein 1 complex that silences ifngr1 transcription. J. Immunol. 2013;191(6):3384–3392. doi: 10.4049/jimmunol.1203510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittet L.A., Quinton L.J., Yamamoto K., Robson B.E., Ferrari J.D., Algül H., Schmid R.M., Mizgerd J.P. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am. J. Respir. Cell Mol. Biol. 2011;45(3):573–581. doi: 10.1165/rcmb.2010-0210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X., Mao Y., Zhu J., Meng F., Chen Q., Tao L., Li R., Fu F., Liu C., Hu Y., Wang W., Zhang H., Hua D., Chen W., Zhang X. TGF-β1 promotes colorectal cancer immune escape by elevating B7-H3 and B7-H4 via the miR-155/miR-143 axis. Oncotarget. 2016;7(41):67196–67211. doi: 10.18632/oncotarget.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown C.Y., Dayan S., Wong S.W., Kaczmarek A., Hope C.M., Pederson S.M., Arnet V., Goodall G.J., Russell D., Sadlon T.J., Barry S.C. FOXP3 and miR-155 cooperate to control the invasive potential of human breast cancer cells by down regulating ZEB2 independently of ZEB1. Oncotarget. 2018;9(45):27708–27727. doi: 10.18632/oncotarget.25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mensah A.A., Cascione L., Gaudio E., Tarantelli C., Bomben R., Bernasconi E., Zito D., Lampis A., Hahne J.C., Rinaldi A., Stathis A., Zucca E., Kwee I., Gattei V., Valeri N., Riveiro M.E., Bertoni F. Bromodomain and extra-terminal domain inhibition modulates the expression of pathologically relevant microRNAs in diffuse large B-cell lymphoma. Haematologica. 2018;103(12):2049–2058. doi: 10.3324/haematol.2018.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn S.R., Mangan N.E., Caffrey B.E., Gantier M.P., Williams B.R., Hertzog P.J., McCoy C.E., O'Neill L.A. The role of Ets2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J. Biol. Chem. 2014;289(7):4316–4325. doi: 10.1074/jbc.M113.522730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Y., Diao Z., Sun H., Li R., Qiu Z., Hu Y. MicroRNA-155 is involved in the remodelling of human-trophoblast-derived HTR-8/SVneo cells induced by lipopolysaccharides. Hum. Reprod. 2011;26(7):1882–1891. doi: 10.1093/humrep/der118. [DOI] [PubMed] [Google Scholar]
- 50.Diener C., Hart M., Kehl T., Rheinheimer S., Ludwig N., Krammes L., Pawusch S., Lenhof K., Tänzer T., Schub D., Sester M., Walch-Rückheim B., Keller A., Lenhof H.P., Meese E. Quantitative and time-resolved miRNA pattern of early human T cell activation. Nucleic Acids Res. 2020;48(18):10164–10183. doi: 10.1093/nar/gkaa788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covino D.A., Kaczor-Urbanowicz K.E., Lu J., Chiantore M.V., Fiorucci G., Vescio M.F., Catapano L., Purificato C., Galluzzo C.M., Amici R., Andreotti M., Gauzzi M.C., Pellegrini M., Fantuzzi L. Transcriptome profiling of human monocyte-derived macrophages upon CCL2 neutralization reveals an association between activation of innate immune pathways and restriction of HIV-1 gene expression. Front. Immunol. 2020;11:2129. doi: 10.3389/fimmu.2020.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohlhaas S., Garden O.A., Scudamore C., Turner M., Okkenhaug K., Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J. Immunol. 2009;182(5):2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W., Prakash C., Sum C., Gong Y., Li Y., Kwok J.J., Thiessen N., Pettersson S., Jones S.J., Knapp S., Yang H., Chin K.C. Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J. Biol. Chem. 2012;287(51):43137–43155. doi: 10.1074/jbc.M112.413047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumann S., Hess J., Eichhorst S.T., Krueger A., Angel P., Krammer P.H., Kirchhoff S. An unexpected role for FosB in activation-induced cell death of T cells. Oncogene. 2003;22(9):1333–1339. doi: 10.1038/sj.onc.1206126. [DOI] [PubMed] [Google Scholar]
- 55.Ebel M.E., Kansas G.S. Functions of smad transcription factors in TGF-β1-induced selectin ligand expression on murine CD4 Th cells. J. Immunol. 2016;197(7):2627–2634. doi: 10.4049/jimmunol.1600723. [DOI] [PubMed] [Google Scholar]
- 56.Lin Y., Zhang Q., Zhang H.M., Liu W., Liu C.J., Li Q., Guo A.Y. Transcription factor and miRNA co-regulatory network reveals shared and specific regulators in the development of B cell and T cell. Sci. Rep. 2015;5 doi: 10.1038/srep15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assmann T.S., Duarte G.C.K., Brondani L.A., de Freitas P.H.O., Martins É M., Canani L.H., Crispim D. Polymorphisms in genes encoding miR-155 and miR-146a are associated with protection to type 1 diabetes mellitus. Acta Diabetol. 2017;54(5):433–441. doi: 10.1007/s00592-016-0961-y. [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Wang X., Yang B., Wang H., Ma Z., Lu Z., Lu X., Gao B. 3β-Hydroxysteroid-Δ24 reductase (DHCR24) protects pancreatic β cells from endoplasmic reticulum stress-induced apoptosis by scavenging excessive intracellular reactive oxygen species. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/3426902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghiasi S.M., Krogh N., Tyrberg B., Mandrup-Poulsen T. The No-go and nonsense-mediated RNA decay pathways are regulated by inflammatory cytokines in insulin-producing cells and human islets and determine β-cell insulin biosynthesis and survival. Diabetes. 2018;67(10):2019–2037. doi: 10.2337/db18-0073. [DOI] [PubMed] [Google Scholar]
- 60.Weng Q., Zhao M., Zheng J., Yang L., Xu Z., Zhang Z., Wang J., Wang J., Yang B., Richard Lu Q., Ying M., He Q. STAT3 dictates β-cell apoptosis by modulating PTEN in streptozocin-induced hyperglycemia. Cell Death Differ. 2020;27(1):130–145. doi: 10.1038/s41418-019-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Golec E., Rosberg R., Zhang E., Renström E., Blom A.M., King B.C. A cryptic non-GPI-anchored cytosolic isoform of CD59 controls insulin exocytosis in pancreatic β-cells by interaction with SNARE proteins. FASEB J. 2019;33(11):12425–12434. doi: 10.1096/fj.201901007R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mollet I.G., Malm H.A., Wendt A., Orho-Melander M., Eliasson L. Integrator of stress responses calmodulin binding transcription activator 1 (Camta1) regulates miR-212/miR-132 expression and insulin secretion. J. Biol. Chem. 2016;291(35):18440–18452. doi: 10.1074/jbc.M116.716860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takei S., Nagashima S., Takei A., Yamamuro D., Wakabayashi T., Murakami A., Isoda M., Yamazaki H., Ebihara C., Takahashi M., Ebihara K., Dezaki K., Takayanagi Y., Onaka T., Fujiwara K., Yashiro T., Ishibashi S. β-Cell-Specific deletion of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase causes overt diabetes due to reduction of β-cell mass and impaired insulin secretion. Diabetes. 2020;69(11):2352–2363. doi: 10.2337/db19-0996. [DOI] [PubMed] [Google Scholar]
- 64.Santos L.R.B., Muller C., de Souza A.H., Takahashi H.K., Spégel P., Sweet I.R., Chae H., Mulder H., Jonas J.C. NNT reverse mode of operation mediates glucose control of mitochondrial NADPH and glutathione redox state in mouse pancreatic β-cells. Mol. Metabol. 2017;6(6):535–547. doi: 10.1016/j.molmet.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sebastiani G., Po A., Miele E., Ventriglia G., Ceccarelli E., Bugliani M., Marselli L., Marchetti P., Gulino A., Ferretti E., Dotta F. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetol. 2015;52(3):523–530. doi: 10.1007/s00592-014-0675-y. [DOI] [PubMed] [Google Scholar]
- 66.Martin B., Chadwick W., Janssens J., Premont R.T., Schmalzigaug R., Becker K.G., Lehrmann E., Wood W.H., Zhang Y., Siddiqui S., Park S.S., Cong W.N., Daimon C.M., Maudsley S. GIT2 acts as a systems-level coordinator of neurometabolic activity and pathophysiological aging. Front. Endocrinol. 2015;6:191. doi: 10.3389/fendo.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berchtold L.A., Størling Z.M., Ortis F., Lage K., Bang-Berthelsen C., Bergholdt R., Hald J., Brorsson C.A., Eizirik D.L., Pociot F., Brunak S., Størling J. Huntingtin-interacting protein 14 is a type 1 diabetes candidate protein regulating insulin secretion and beta-cell apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2011;108(37):E681–E688. doi: 10.1073/pnas.1104384108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilhelmi I., Grunwald S., Gimber N., Popp O., Dittmar G., Arumughan A., Wanker E.E., Laeger T., Schmoranzer J., Daumke O., Schürmann A. The ARFRP1-dependent Golgi scaffolding protein GOPC is required for insulin secretion from pancreatic β-cells. Mol. Metabol. 2021;45 doi: 10.1016/j.molmet.2020.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z., Wakabayashi N., Wakabayashi J., Tamura Y., Song W.J., Sereda S., Clerc P., Polster B.M., Aja S.M., Pletnikov M.V., Kensler T.W., Shirihai O.S., Iijima M., Hussain M.A., Sesaki H. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol. Biol. Cell. 2011;22(13):2235–2245. doi: 10.1091/mbc.E10-12-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He X.Q., Wang N., Zhao J.J., Wang D., Wang C.J., Xie L., Zheng H.Y., Shi S.Z., He J., Zhou J., Xin H.B., Deng K.Y. Specific deletion of CDC42 in pancreatic β cells attenuates glucose-induced insulin expression and secretion in mice. Mol. Cell. Endocrinol. 2020;518 doi: 10.1016/j.mce.2020.111004. [DOI] [PubMed] [Google Scholar]
- 71.Bacos K., Gillberg L., Volkov P., Olsson A.H., Hansen T., Pedersen O., Gjesing A.P., Eiberg H., Tuomi T., Almgren P., Groop L., Eliasson L., Vaag A., Dayeh T., Ling C. Blood-based biomarkers of age-associated epigenetic changes in human islets associate with insulin secretion and diabetes. Nat. Commun. 2016;7 doi: 10.1038/ncomms11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakamaki J., Fu A., Reeks C., Baird S., Depatie C., Al Azzabi M., Bardeesy N., Gingras A.C., Yee S.P., Screaton R.A. Role of the SIK2-p35-PJA2 complex in pancreatic β-cell functional compensation. Nat. Cell Biol. 2014;16(3):234–244. doi: 10.1038/ncb2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Axelsson A.S., Mahdi T., Nenonen H.A., Singh T., Hänzelmann S., Wendt A., Bagge A., Reinbothe T.M., Millstein J., Yang X., Zhang B., Gusmao E.G., Shu L., Szabat M., Tang Y., Wang J., Salö S., Eliasson L., Artner I., Fex M., Johnson J.D., Wollheim C.B., Derry J.M.J., Mecham B., Spégel P., Mulder H., Costa I.G., Zhang E., Rosengren A.H. Sox5 regulates beta-cell phenotype and is reduced in type 2 diabetes. Nat. Commun. 2017;8 doi: 10.1038/ncomms15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raum J.C., Soleimanpour S.A., Groff D.N., Coré N., Fasano L., Garratt A.N., Dai C., Powers A.C., Stoffers D.A. Tshz1 regulates pancreatic β-cell maturation. Diabetes. 2015;64(8):2905–2914. doi: 10.2337/db14-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piquer S., Casas S., Quesada I., Nadal A., Julià M., Novials A., Gomis R. Role of iduronate-2-sulfatase in glucose-stimulated insulin secretion by activation of exocytosis. Am. J. Physiol. Endocrinol. Metab. 2009;297(3):E793–801. doi: 10.1152/ajpendo.90878.2008. [DOI] [PubMed] [Google Scholar]
- 76.Brunham L.R., Kruit J.K., Pape T.D., Timmins J.M., Reuwer A.Q., Vasanji Z., Marsh B.J., Rodrigues B., Johnson J.D., Parks J.S., Verchere C.B., Hayden M.R. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13(3):340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 77.Fiaschi-Taesch N.M., Salim F., Kleinberger J., Troxell R., Cozar-Castellano I., Selk K., Cherok E., Takane K.K., Scott D.K., Stewart A.F. Induction of human beta-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes. 2010;59(8):1926–1936. doi: 10.2337/db09-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu Y., Lindner J., Kumar A., Yuan W., Magnuson M.A. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Backe M.B., Novotny G.W., Christensen D.P., Grunnet L.G., Mandrup-Poulsen T. Altering β-cell number through stable alteration of miR-21 and miR-34a expression. Islets. 2014;6(1) doi: 10.4161/isl.27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalis M., Bolmeson C., Esguerra J.L., Gupta S., Edlund A., Tormo-Badia N., Speidel D., Holmberg D., Mayans S., Khoo N.K., Wendt A., Eliasson L., Cilio C.M. Beta-cell specific deletion of Dicer1 leads to defective insulin secretion and diabetes mellitus. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorrepati K.D.D., He W., Lupse B., Yuan T., Maedler K., Ardestani A. An SCF(FBXO28) E3 ligase protects pancreatic β-cells from apoptosis. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19040975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang L., Yan M., Kirschke C.P. Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription. Exp. Cell Res. 2010;316(16):2630–2643. doi: 10.1016/j.yexcr.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 83.Ibrahim S., Johnson M., Stephens C.H., Xu J., Moore R., Mariani A., Contreras C., Syed F., Mirmira R.G., Anderson R.M., Sims E.K. β-Cell pre-mir-21 induces dysfunction and loss of cellular identity by targeting transforming growth factor beta 2 (Tgfb2) and Smad family member 2 (Smad2) mRNAs. Mol. Metabol. 2021;53 doi: 10.1016/j.molmet.2021.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.