Abstract
The role of invasive alien species in the transmission dynamics of zoonotic pathogens is often overlooked, despite the rapid escalation in biological invasions globally. Here we synthesise available information on the influence of invasive alien species on zoonotic pathogen dynamics in invaded ranges, focussing on Europe, and identify key associated knowledge gaps. We identified 272 documented interactions between alien species and zoonotic pathogens within invaded ranges. The majority of these involved invasive alien mammals followed by birds with only a few occurrences of other taxa documented. A wide range of potential interactions between invasive alien species and zoonotic pathogens were identified but few studies considered transmission to humans and so there was limited evidence of actual impacts on human health. However, there is an urgent need to raise awareness of the potential risks posed to human health by the transmission of zoonotic diseases by invasive alien species; the role of invasive alien species in zoonotic disease transmission may exceed that of native wildlife and occur in a relatively short period following the arrival of an invasive alien species within a new region. Ecological and social mechanisms govern the dynamics of zoonotic disease transmission but wildlife diseases are not consistently included within animal, plant and human policies. Rapid advances in the development of systems frameworks that integrate the ecological, economic and social processes promoting spillover in rapidly changing environments will increase understanding to inform decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10530-022-02978-1.
Keywords: Zoonotic pathogens, Invasive non-native species, Disease, Spillover, One health
Introduction
The number of alien species arriving in new regions is escalating globally (Seebens et al. 2017) and the interaction of invasive alien species with land use change could be of similar magnitude to the threat of climate change in shifting the distribution of hosts, vectors and reservoirs of pathogens (Hulme 2014). The adverse effects of invasive alien species 1 on biodiversity and ecosystems have been widely documented (Mazza and Tricarico 2018; Pyšek et al. 2020). However, the role of alien species in the transmission dynamics of emerging zoonotic diseases2 is often overlooked (Dunn and Hatcher 2015; Hulme, 2017; Roy et al. 2016).
Zoonotic diseases make up 60% of emerging infectious disease events worldwide (Jones et al. 2008) and disproportionately affect tropical communities (Halliday et al. 2012) accounting for around one-quarter of Disability Life Adjusted Years lost to infectious diseases in Lower Middle Income Countries (Grace et al. 2012). The impacts of these complex, multi-host pathogens are evolving in response to social-political and environmental change including agricultural intensification, deforestation and climate change (Jones et al. 2008; Plowright et al. 2021). An outcome of ongoing social-political and environmental change is the dramatic shifts and expansion of animal hosts (Johnson et al. 2020) which can increase the probability of contact amongst humans, animal hosts and disease vectors with knock-on consequences for exposure and transmission of zoonotic diseases. For example, abundant and widely distributed species, including those that have expanded their ranges by adapting to human-dominated landscapes, were found to harbour higher loads of zoonotic viruses and pose an increased risk of contributing to human spillover worldwide than those with limited distributions (Johnson et al. 2020). Another global analysis highlighted that disturbed habitats, under substantial human use, harbour a greater richness and total abundance of known wildlife hosts of zoonotic diseases than other habitats (Gibb et al. 2020).
Empirical and review studies have highlighted a wide range of mechanisms by which social-ecological and anthropogenic environmental change, including habitat loss, degradation and fragmentation, can alter the interactions between species within a disease transmission network and promote zoonotic disease spillover (Aguirre 2017; Plowright et al. 2021). These have included effects on behaviour, social strucuture and dispersal of species and composition and diversity of communities (Estrada-Peña et al. 2014). Alterations to the composition and diversity of local communities have been shown to increase or decrease zoonotic pathogen transmission. In some cases, new alien species within a community may be suitable hosts for endemic pathogens and so can increase pathogen transmission through “spillback” to native host species including humans. The connectedness between natural and anthropogenic systems highlights the importance of whole systems approaches to understanding the changing dynamics of pathogens in response to global environmental change (Wood et al. 2012).
|
Panel 1: SARS-CoV-2, mink and the potential for spillback of pathogens to humans and other animals Following an outbreak of SARS-CoV-2 on a farm of American mink, Neogale vison, there was strong evidence to suggest the mink had seemingly contracted the infection from spillover from the human pandemic, at least two farm workers have subsequently caught the virus from the mink (Enserink 2020). In December 2020, a wild American mink in Utah near a fur farm was found to be infected with SARS-CoV-2 representing the first case of a non-captive animal infected with this coronavirus. This is of particularly concern, considering that there are studies demonstrating a clear overlap in habitat use between free-ranging mink populations and farm animals (Hammershøj et al. 2005; Valnisty et al. 2020). A further case was reported in Spain with the capture of two American minks in the wild with SARS-CoV-2 infection, although in this case far from fur farms (Aguiló-Gisbert et al. 2021). Further study is required to examine the potential risk to other river-roaming species through indirect transmission routes. |
The extent to which invasive alien species are involved in zoonotic disease transmission in changing environments and through which mechanisms has not been well studied although a recent study demonstrated that the number of zoonosis events increase with the richness of alien zoonotic hosts (Zhang et al 2022).
It has been hypothesised that invasive alien species may have a disproportionate impact on the transmission of zoonotic pathogens for a number of reasons (Hulme 2014):
Alien species may be more effective hosts than other species or vectors in the transmission of endemic pathogens and amplify local pathogens (Chinchio et al. 2020)
Alien species may facilitate the establishment of new emerging diseases with which they have co-evolved with in their native range and which may be introduced with them (Dunn and Hatcher 2015)
Alien species often thrive in anthropogenic environments so may have high encounter rates with people and often exhibit high dispersal rates including through human-mediated dispersal, with the trade in many alien species being relatively unregulated.
The integration of a new host into an established zoonotic network can dramatically increase disease transmission.
Here we present a review of the literature on the role of alien species in the emergence and spread of zoonoses recognising the mounting evidence of links between zoonotic diseases and biodiversity change (Johnson et al. 2020; Nuñez et al. 2020). A recent analysis highlighted that the introduction of alien species is likely to have contributed to zoonosis emergences in recent history (Zhang et al 2022). Here we assess the extent to which the impacts of invasive alien species on zoonotic disease transmission have already been realised and assessed possible biases in the information available and opportunities for improving our scientific understanding of the role invasive alien species play in zoonotic disease transmission to inform interventions and policy.
Methods
We conducted a systematic review of published literature following PRISMA guidelines using the Web of Knowledge platform, which includes references from 1960 onwards. All databases within Web of Knowledge were searched, which included Web of Science Core Collection, BIOSIS Citation Index, BIOSIS Previews, KCI Korean Journal Database, MEDLINE, Russian Science Citation Index, SciELO Citation Index (search date: 12th July 2020). We utilised six sets of search strings to retrieve literature (Table 1), which yielded 603 unique references once duplicates were removed. No special operators were used which effectively performs an AND operation between the words in the set. Our search only included papers written in the English language although it should be noted that literature in other languages would undoubtedly have add to the information available.
Table 1.
Sets of search strings used to retrieve literature linking zoonotic diseases and invasive alien species and the number of references returned for each set
| Sets of search terms | Number of references returned |
|---|---|
| Alien species zoonoses | 48 |
| Alien species zoonotic diseases | 50 |
| Invasive species zoonoses | 262 |
| Invasive species zoonotic diseases | 272 |
| Non-native species zoonoses | 312 |
| Non-native species zoonotic diseases | 290 |
For extraction of data into summary tables, we agreed on inclusion and exclusion criteria which aligned with the scope of the review (Table 2). Full papers were identified following screening of all titles and abstracts, by one of three of the study authors (BVP, ET, HER), and further reviewed as necessary for eligibility and inclusion. Overall, 369 papers out of the 603 were excluded from the study.
Table 2.
Inclusion and exclusion criteria used to select studies for the review
| Inclusion Criteria |
|---|
| Contains primary data on populations of alien species established in the wild outside their native range and causing (or having potential to cause) zoonotic disease |
| Contains primary data on the role of alien species (or potential role) as a vector or reservoir species for a zoonotic disease in the wild outside their native range |
| Reviews the role of alien species in zoonotic disease transmission and spread in the wild outside their native range (either considering only the alien species or the alien species in comparison to native species) |
| Contains primary laboratory data on competence of alien species which are vectors or reservoirs for a zoonotic pathogen where the laboratory test populations arefrom outside the native range |
| Exclusion Criteria |
|---|
| Contains only ecological, taxonomic, genetic or physiological data on the alien species with no data on a zoonotic disease |
| Contains data on alien species involved as a vector or reservoir species for a zoonotic disease in the native range of the IAS only and with no information from outside the native range |
| Contains data on invasiveness of a pathogen inside host tissue as opposed to data on an alien species |
| Contains data on a non-invasive scientific method as opposed to data on alien species |
| Contains data on zoonotic disease in humans without linkage to ¹alien species |
| Contains data on zoonotic disease links to alien species hosts but the hosts are not identified to species |
| Contains data on alien species links to zoonotic disease where alien species populations are captive or kept as pets only with no free living alien species populations |
| Contains data on bites by an alien species as health problem rather than infectious zoonotic disease |
| Reviews that do not explicitly link alien species and zoonotic diseases (e.g. of invasion and biosecurity policy, zoonotic diseases and ecosystems, zoonotic diseases and biogeography, wildlife trade) |
Data synthesis and summary measures
Data on invasive alien species-zoonotic pathogen interactions from relevant papers was extracted into summary tables (Supplementary Information 1) including taxonomic information (order, family, species) of the invasive alien species and the species or genus name of the pathogen. The role of the invasive alien species in zoonotic disease transmission was classified within broad categories of direct and indirect roles. Direct roles in transmission included being an invasive alien species pathogen of humans, being a reservoir host for a zoonotic pathogen, being an arthropod vector for the zoonotic pathogen (biological or mechanical). Indirect roles included being a host for arthropod vectors of zoonotic pathogens, being a vector for a zoonotic pathogen, or altering vector-host–pathogen dynamics in ways that increase transmission to humans.
The type of study was also categorised for each paper. Some of the studies were laboratory-based such as those considering the competence of vectors or reservoirs within the context of zoonoses and others on molecular phylogenetics. Many of the studies were field-based including screening approaches to assess pathogen prevalence within an invasive alien species host through to ecological studies investigating the mechanisms behind zoonotic transmission. There were also a number of biogeographic studies and review papers identified. For each study and where available the following contextual factors were collected: the region and country in which the study took place, the timing of introduction of the alien species and current extent of establishment and spread in the country, information on the status of the zoonotic disease in the study region.
To clarify and quantify the extent of impact that invasive alien species are involved in transmission of the parasite or pathogen to humans, we extracted further information on the role of the invasive alien species in the pathways to zoonotic disease transmission (Plowright et al. 2017, 2021) from all the papers identified as relevant (conforming to the inclusion criteria outlined in Table 2).
The evidence for each individual invasive alien species and zoonotic pathogen interaction was revisited to evaluate the extent of impact that invasive alien species are having on transmission from a potential impact to an actual (= realised) impact along a continuum (identified by the authors) of available supporting evidence (Table 3). This provided an opportunity to give context to the interaction and specifically some measure of confidence in the extent and magnitude of the impact on humans. Pathogen type and transmission pathways were retrospectively retrieved for pathogens involved in the interactions using a wide range of literature sources (Supplementary Information 2).
Table 3.
Continuum of potential and actual impacts of IAS on zoonotic disease spillover with types of supporting evidence
| Type of impact | Certainty of impact | Type of supporting evidence |
|---|---|---|
| Potential | Very low | Detection of sporadic pathogen presence or low prevalence (< 5%) in populations of IAS outside their native range |
| Potential | Low | Detection of medium pathogen prevalence in populations of IAS (5–20%) outside their native range, especially where less than native species |
| Potential | Medium | High pathogen prevalence in populations of IAS outside their native range, especially where the same or higher than native species |
| Potential | High | High pathogen prevalence in populations of IAS outside their native range, especially where higher than native species, widespread and abundant in anthropogenic habitats, laboratory/dissection studies supporting role in transmission |
| Actual | Low | IAS shown to play a role in transmission to people but low case number or prevalence outside their native range |
| Actual | Medium | IAS shown to overlap spatially or temporally with the distribution of human outbreaks outside the native range of the IAS |
| Actual | High | IAS shown to have changed the distribution or spread of autochthonous transmission or human outbreaks outside the native range of the IAS |
| Actual | High | IAS shown to alter strain diversity and population structure of pathogens and strains shared with humans outside the native range of the IAS |
Additionally, further information is provided on type of transmission and broad host associations and human health impact specifically in Europe noting this study was funded by the European Commission (Supplementary Information 2). Transmission was coded as: A = aerosol transmission, C = contact (skin and mucosal) transmission, O = oral transmission through food (F) or water (W) or vector-borne transmission (V) by either flea (F), tick (T), mite (MI), lice (LI), biting flies (BF), Triatminae i.e. kissing bugs (Tri) or mosquitoes (MOS). The annual cases and case fatality rates are also reported within the tables and all information is taken from European Centre for Disease Prevention and Control (2012–2018) where data are available
Results
Overall 272 direct interactions between invasive alien species and zoonotic pathogens in the invaded range (Supplementary Information 1), conforming to our inclusion criteria, were identified from the literature review including other recent review papers (Hulme 2014; Zhu et al. 2019). Only five species (a reptile, three plants, and a plant pathogen) were identified as having an indirect impact on zoonotic pathogen transmission, by altering host-vector pathogen dynamics (Table 4; Supplementary Information 1). The vast majority of direct interactions identified involved invasive alien vertebrates as potential or actual reservoir species for zoonotic pathogens, with 213 (78.3%) invasive alien species-pathogen genus interactions identified for mammals, 26 (9.6%) for birds, 3 (1.1%) for non-avian reptiles and 4 (1.5%) for fish. For invertebrates, 14 (5.1%) invasive alien species-pathogen interactions involved invasive ticks (4) or insects (10) as biological vectors, whilst one (0.4%) involved an invasive alien crustacean and 11 (4.0%) involved invasive alien molluscs as intermediate reservoir hosts for zoonotic pathogens. Six nematodes and five platyhelminthes were identified as zoonotic endo-parasites that had been recently introduced to Europe, with some degree of impact on human health.
|
Panel 2. Examples of evidence of actual impacts on human health following transmission of a zoonotic disease by an invasive alien species There is unequivocal evidence that the spread and maintenance of serious human pathogens such as bacteria from the genera Leptospira, Bartonella and Yersinia followed the widespread human-mediated introduction of Norwegian rat, Rattus norvegicus, and the black rat, Rattus rattus to new areas, especially islands, through shipping during the sixteenth and seventeenth centuries. The evidence for the actual impacts on transmission was based on global biogeographical studies of pathogens detected from rats and humans, facilitated by advances in molecular methods (Kosoy and Bai 2019) alongside empirical studies on host-vector-pathogen interactions (Moseley et al. 2018). Other significant actual impacts come from more recent invaders to Europe, such as Aedes mosquito vectors including Ae. albopictus providing the conditions for autochthonous outbreaks of arboviruses such as Dengue and Chikungunya in Europe within a decade of initial invasion and establishment (Leta et al. 2018). |
Table 4.
Broad group of pathogen or parasite (V = Virus, B = Bacteria, F = Fungus, Pr = Protozoa, N = Nematode, C = Cestode, T = Trematode, Pl = Platyhelminth) associated with invasive alien species including the number of genera and species (where information was available) and the invaded countries in which the association has been observed. Further notes on taxonomic groups including detailed species-specific examples are provided within the Supplementary Information
| Invasive alien species | Common name | V | B | F | Pr | N | C | T | Pl | Number of parasite/pathogen genera | Number of parasite/pathogen species | Countries |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthropoda: Arachnida | ||||||||||||
| Rhipicephalus microplus | Asian blue tick | 1 | 1 | Guam | ||||||||
| Hyalomma marginatum | Mediterranean Hyalomma | ✓ | 1 | 1 | Austria | |||||||
| Rhipicephalus appendiculatus | Brown ear tick | ✓ | 1 | 1 | Comoros Islands | |||||||
| Rhipicephalus sanguineus | Kennel tick | ✓ | 1 | 1 | Switzerland | |||||||
| Arthropoda: Insecta | ||||||||||||
| Aedes aegypti | Yellowfever mosquito | ✓ | ✓ | 2 | 2 |
Argentina Switzerland USA |
||||||
| Culex pipiens | Common house mosquito | ✓ | 1 | 2 | Argentina | |||||||
| Aedes japonicus | Asian bush mosquito | ✓ | 1 | 2 | Switzerland | |||||||
| Aedes albopictus | Tiger mosquito | ✓ | 1 | 2 | Italy | |||||||
| Aedes japonicus japonicus | Asian bush mosquito | ✓ | 5 | 0 |
USA Switzerland |
|||||||
| Culex quinquefasciatus | Southern house mosquito | ✓ | 1 | 0 | USA | |||||||
| Maxillopoda | ||||||||||||
| Austrominius (Elminius) modestus | Acorn barnacle | ✓ | 1 | 0 | UK | |||||||
| Gastropoda | ||||||||||||
| Biomphalaria glabrata | Bloodfluke planorb | ✓ | 1 | 1 | Romania | |||||||
| Biomphaliaria tenagophila | ✓ | 1 | 1 | Romania | ||||||||
| Achatina fulica | Giant African land snail | ✓ | 1 | 2 | USA | |||||||
| Melanoides tuberculata | Red-rimmed melania | ✓ | 5 | 6 |
Costa Rica Peru Brazil Venezuela |
|||||||
| Alcadia striata | Straite drop | ✓ | 1 | 1 | USA | |||||||
| Bradybaena similaris | Asian trampsnail | ✓ | 1 | 1 | USA | |||||||
| Zachrysia provisoria | Cuban brown snail | ✓ | 1 | 1 | USA | |||||||
| Actinopterygii | ||||||||||||
| Oreochromis niloticus | Nile tilapia | ✓ | 2 | 0 | China | |||||||
| Cyprinus carpio | European carp | ✓ | 2 | 0 | China | |||||||
| Oncorhynchus keta | Chum salmon | ✓ | 1 | 1 | Europe | |||||||
| Aves | ||||||||||||
| Sturnus vulgaris | Common starling | ✓ | 4 | 2 |
USA New Zealand |
|||||||
| Branta canadensis | Canada goose | ✓ | 2 | 1 |
Belgium UK |
|||||||
| Alectoris chukar | Chukar partridge | ✓ | 1 | 1 | USA | |||||||
| Myiopsitta monachus | Monk parakeet | ✓ | 1 | 0 | Chile | |||||||
| Psittacula krameri | Rose-ringed parakeet | ✓ | ✓ | 1 | 1 |
France Japan |
||||||
| Columba livia | Common pigeon | ✓ | 2 | 0 | USA | |||||||
| Streptopelia chinensis | Spotted dove | ✓ | 2 | 0 | USA | |||||||
| Padda oryzivora | Java sparrow | ✓ | 2 | 0 | USA | |||||||
| Fringilla coelebs | Common chaffinch | ✓ | 1 | 0 | New Zealand | |||||||
| Turdus philomelos | Song thrush | ✓ | ✓ | 2 | 0 |
USA New Zealand |
||||||
| Turdus merula | Common blackbird | ✓ | 1 | 0 | New Zealand | |||||||
| Prunella modularis | Dunnock | ✓ | 1 | 0 | New Zealand | |||||||
| Passer domesticus | House sparrow | ✓ | ✓ | 3 | 1 |
USA New Zealand |
||||||
| Mammalia | ||||||||||||
| Sus scrofa | Wild boar | ✓ | ✓ | ✓ | 18 | 24 |
USA Australia Georgia Brazil Chile |
|||||
| Nyctereutes procyonoides | Raccoon dog | ✓ | ✓ | ✓ | ✓ | ✓ | 13 | 9 |
Germany Poland Estonia The Netherlands Denmark Lithuania Austria Latvia |
|||
| Canis lupus | Grey wolf | ✓ | 1 | 1 | Estonia | |||||||
| Canis lupus familiaris | Domestic dog | ✓ | 1 | 3 | Estonia | |||||||
| Canis lupus dingo | Dingo | ✓ | 1 | 1 | Australia | |||||||
| Vulpes vulpes | Fox | ✓ | ✓ | 3 | 4 | Australia | ||||||
| Felis catus | Domestic cat | ✓ | ✓ | ✓ | 10 | 9 |
Australia USA Denmark |
|||||
| Neogale vison | American mink | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | 3 |
Spain Chile Poland |
|||
| Procyon lotor | Northen raccoon | ✓ | ✓ | ✓ | 19 | 10 |
Japan Denmark Germany Poland Norway China Austria |
|||||
| Ondatra zibethicus | Muskrat | ✓ | ✓ | ✓ | ✓ | 7 | 6 |
Germany Poland France |
||||
| Myocastor coypus | Coypu | ✓ | ✓ | ✓ | ✓ | 5 | 7 |
Korea France Japan Italy |
||||
| Mus musculus | House mouse | ✓ | ✓ | ✓ | ✓ | 10 | 3 |
New Zealand Senegal Australia Argentina Chile Puerto Rico USA Nigeria Madagascar |
||||
| Tenrec ecaudatus | Common tenrec | ✓ | 1 | 1 | Mayotte | |||||||
| Herpestes javanicus | Javan mongoose | ✓ | 1 | 0 | Japan | |||||||
| Mustela putorius furo | Ferret | ✓ | 1 | 0 | New Zealand | |||||||
| Nasua nasua | Ring-tailed coati | ✓ | 1 | 0 | Norway | |||||||
| Paguma larvata | Masked palm civet | ✓ | 3 | 1 | Japan | |||||||
| Didelphis marsupialis | Common opossum | ✓ | 1 | 1 | USA | |||||||
| Trichosurus vulpecula | Common brushtail possum | ✓ | 2 | 0 | New Zealand | |||||||
| Erinaceus europaeus | European hedgehog | ✓ | 1 | 0 | New Zealand | |||||||
| Lepus europaeus | European hare | ✓ | 1 | 1 |
Chile Argentina |
|||||||
| Oryctolagus cuniculus | European rabbit | ✓ | 1 | 0 | New Zealand | |||||||
| Chlorocebus aethiops sabaeus | African green monkey | ✓ | 1 | 1 | St. Kitts | |||||||
| Macaca mulatta | Rhesus macaque | ✓ | 2 | 0 |
Puerto Rico USA |
|||||||
| Chinchilla lanigera | Long-tailed chinchilla | ✓ | 1 | 1 | Switzerland | |||||||
| Gerbillus nigeriae | Nigerian gerbil | ✓ | 1 | 1 | Senegal | |||||||
| Herpestes auropunctatus | Indian mongoose | ✓ | 1 | 0 |
Puerto Rico St. Kitts |
|||||||
| Suncus murinus | Asian house shrew | ✓ | 1 | 0 | Madagascar | |||||||
| Callosciurus finlaysonii | Finlayson's squirrel | ✓ | 1 | 0 | Germany | |||||||
| Sciurus carolinensis | Grey squirrel | ✓ | 1 | 0 |
UK Italy Germany |
|||||||
| Sciurus variagata | Variegated squirrel | ✓ | 1 | 0 | Germany | |||||||
| Tamias sibiricus barberi | Siberian chipmunk | ✓ | 1 | 1 | France | |||||||
| Tamiops swinhoei | Swinhoe's striped squirrel | ✓ | 1 | 0 | Germany | |||||||
| Rattus norvegicus | Brown rat | ✓ | ✓ | ✓ | 40 | 42 |
USA South Africa Grenada Australia Madagascar Puerto Rico Canada Argentina Chile Nigeria |
|||||
| Rattus rattus | Black rat | ✓ | ✓ | ✓ | ✓ | ✓ | 44 | 23 |
Australia USA Argentina South Africa Italy Chile Uganda Madagascar Senegal Diego Garcia New Zealand La Réunion Mauritius Seychelles Swaziland Mozambique Puerto Rico Malayasia Borneo Brazil Benin Nigeria |
|||
| Rattus tanezumi | Asian house rat | ✓ | 4 | 3 | South Africa |
Evidence of an actual rather than a potential impact of the invasive alien species on the zoonotic disease transmission
Evidence of an actual rather than a potential impact of the invasive alien species on the zoonotic disease transmission and human health was only demonstrated within a few studies despite a wide range of interactions between invasive alien species and zoonotic diseases being identified (Supplementary Information 1). The following groups of pathogens were implicated (actual or potential) with alien or invasive alien vectors or hosts in transmission: 52 bacteria, 23 viruses, 33 other endoparasites and these combined with the invasive alien species to give 204 zoonotic pathogen – alien or invasive alien species interactions. Of these 22 were aerosol-transmitted, 33 were contact-transmitted, 119 orally-transmitted, 22 were water-borne, 55 were food-borne and 55 were vector-borne (details in Supplementary Information 2).
Evidence for invasive alien species playing an equivalent or disproportionate role in zoonotic disease spillover
Many of the studies reviewed compared host–pathogen interactions between invasive alien and native host species, usually for a single pathogen at a time, but sometimes for multiple native hosts and multiple pathogen species. Of the 65 host–pathogen interactions identified from these comparative studies, 57 focused on mammalian hosts including 13 host species with the most numerous being raccoon dog, N. procyonoides (32%; n = 21), black rat, R. rattus (26%; n = 17), Norway rat, R. norvegicus (11%; n = 7), house mouse, M. musculus (5%; n = 3). The remaining eight invasive alien species were birds (8%; n = 5) and insects (5%; n = 3). Across all the comparative studies, the pathogen prevalence (derived from assessing pathogen presence using sero-prevalence or PCR-positive detections within host animals surveyed in a variety of ways) across sampled invasive alien species host individuals was lower than in the native host in 15 studies, equivalent to the native host in 14 studies, and higher than the native host in 33 studies.
Discussion
Invasive alien species are involved in the transmission of zoonotic pathogens in a range of endemic and epidemic disease situations and geographical contexts, although noting many studies identified potential rather than actual impacts on transmission, with a wide range of ecological, environmental and social processes modulating their role in transmission.
Concerning whether invasive alien species play a stronger role in transmission than native hosts, comparisons of infection prevalence between such sympatric hosts was only widely studied in mammal species. For this taxon, pathogen prevalence was higher in the invasive alien host than the native host in around half of the studies, indicating that invasive alien populations can be more widely infected than sympatric native host populations by zoonotic pathogens. Whether this translates into a stronger role of invasive alien species in transmission and spillover of zoonotic diseases (Hulme 2014) depends on a wide range of other physiological, behavioural factors and evolutionary (including habitat and resource use, overlap with people affecting rates of contact, rate and conditions favouring pathogen susceptibility and shedding by hosts; Murray and Daszak 2013; Hartemink et al. 2015; Plowright et al. 2017). These factors will need to be unravelled through detailed empirical ecological studies of interactions between sympatric native and invasive alien hosts, people and pathogens; such studies are currently rare.
Species ecological traits determine the role that invasive alien species play in transmission relative to native species. As an example, raccoon dogs, N. procyonoides, were assessed in a number of studies as a reservoir host for a range of intestinal pathogens, the bacterial pathogen Francisella tularensis and rabies. A number of the traits that have contributed to the invasion success of raccoon dogs were also considered to be important in altering the spatial and temporal dynamics of the tapeworm Echinococcus multilocularis; raccoon dogs can colonise wide areas over a short time and have a high reproductive rate. They are considered to be an important definitive host for the tapeworm. The disease, alveolar echinococcosis, caused by the larval form of this tapeworm, is a highly lethal helminthic disease in humans; the prevalence of the disease is increasing in Europe (Torgerson et al. 2015), particularly in western Europe, and has been attributed to the abundance of foxes and meadow voles. Studies from Poland, Germany and Estonia all demonstrated a lower prevalence of the tapeworm in raccoon dogs compared to the native red foxes sampled in the same period. It is thought that this might be a consequence of diet. Red foxes consumed more arvicoline rodents, the main intermediate hosts of the tapeworm, particularly during the coldest period of the year when raccoon dogs are in hibernation (Laurimaa et al. 2015). However, raccoon dogs can reach very high densities and so are potentially an important additional definitive host for E. multilocularis.
The roles of a wide diversity of native hosts and their pathogens and parasites, including livestock, should be considered when assessing the evidence for the importance of invasive alien species (and other alien species) in disease transmission (Mazza and Tricarico 2018). The majority of emerging human diseases are known to originate from mammals which may be attributed to their vast global distributions and diversity (Han et al. 2016). Moreover particular mammalian taxa, such as bats and rodents, with traits that foster adaptation to anthropogenic environments and to transmission roles, have been identified as being particularly important in spillover (Han et al. 2016; Johnson et al. 2020). A number of mammalian invasive alien species have been very well-studied and dominate amongst vertebrate invasive alien species in terms of numbers and diversity of known potential interactions with zoonoses. Such mammalian invasive alien species are often widely distributed beyond the native range and have close association with humans because they thrive in anthropogenic habitats (rats, Rattus spp., mice, Mus spp., raccoon dog, N. procyonoides, raccoon, P. lotor) or are managed by humans in one way or another (wild boar, Sus scrofa, feral American mink, N. vison). Amongst the invasive alien species that are commonly associated with human-dominated landscapes, mammals have been the focus of most of the studies with reptiles and birds being less well-studied.
Rodents are known to be the most species rich of zoonotic mammalian hosts and to harbour high diversities of zoonotic pathogens, alongside bats, compared to other mammalian taxa (Johnson et al. 2020); 17 invasive alien rodents were included in the interactions with zoonotic pathogens that were identified through this literature review. Rodent reservoir hosts are characterised by high reproductive potential (reproducing early in life and with high frequency) which favours pathogen transmission and maintenance within reservoir populations (Han et al. 2015). Furthermore, many of the rodents documented as invasive alien species favour urban habitats and as such live in close proximity with humans increasing the probability of human exposure to the zoonotic pathogens they carry. Norway rats (R. norvegicus) and black rats (R. rattus) are considered to be amongst the most damaging invasive alien species globally (Feng and Himsworth 2014) and inhabit every continent except for Antarctica. Their role in transmitting a number of zoonotic pathogens has been well documented (Pépin et al. 2016). Leptospira and hantaviruses, particularly Seoul virus, are the most important in terms of human morbidity and mortality (Pépin et al. 2016). It is intriguing to note the vast variability in prevalence of pathogens borne by rats, even within limited geographical distances with interacting host populations (Pépin et al. 2016) which has implications for surveillance and monitoring. Furthermore, molecular studies are expanding the known diversity of pathogens carried by rats, for many of which the human health implications are unknown, further demonstrating the dynamic nature of the role of invasive alien species in zoonoses transmission and epidemiology.
Consistent with studies of native species-zoonotic pathogen interactions, land use practices and changes were found to modulate a number of the invasive alien host–pathogen interactions. In Chile Leptospira species in rodent communities inhabiting agricultural areas were almost three times more infected than in wild areas (Correa et al. 2017) and the invasive Norwegian rats were the most infected species (38.1%). Similarly, the association between the black rat and sewers resulting in high population densities of this invasive alien species was seen to be a contributing factor to the increased prevalence of Leptospira in the western Indian Ocean islands and neighbouring Africa (Dietrich et al. 2018). Urbanisation has led to conditions that favour highly adaptable species such as mice and rats and in a study on the cities of Southern Benin the infection rates of black rats, R. rattus, and Norwegian rats, R. norvegicus, with Trypanosoma lewisi was higher than in native rodents (Dobigny et al. 2019).
Human management of livestock and wildlife populations can dramatically influence the extent to which invasive alien species play a role in zoonotic disease transmission. Wild boars, Sus scrofa, are reservoirs for many pathogens transmissible to humans such as foodborne zoonoses including bacterial diseases (brucellosis, salmonellosis, tuberculosis and yersiniosis), parasitic diseases (toxoplasmosis and trichinellosis) and the viral 19 hepatitis E. Supplemental feeding to increase the density of wild boar for hunting can increase transmission risk and hunters are at highest risk. Pathogen prevalence also varies with habitat; incidence of Brucella suis and Escherichia coli were highest in boar from forested and agricultural regions respectively (Lama and Bachoon 2018). Furthermore, hunting with dogs compared to other management practices can increase the risk of pathogen transmission from wild boar to humans (Carr et al. 2019). It is postulated that hunting with dogs may elevate stress and birth rates, leading to high rates of pathogen excretion, but may also alter animal movements and social structure in ways that increase contact rates and pathogen transmission (Carr et al. 2019). The complexity of disease transmission and the interplay of ecological and social factors has implications for the outcomes of invasive alien species management (eradication and population reduction).
Four of the studies we reviewed addressed indirect impacts of invasive alien species on disease transmission. Invasive alien species can alter the habitat use by native species and indirectly increase the human risk of exposure to a disease (Allan et al. 2010). In Missouri (U.S.) the white-tailed deer, Odocoileus virginianus, the dominant host for the tick Amblyomma americanum carrying the bacteria Ehrlichia spp. (agents of human ehrlichiosis), used areas invaded by the Amur honeysuckle, Lonicera maackii, more frequently. This led to considerably greater numbers of ticks infected with pathogens in honeysuckle-invaded areas than the adjacent honeysuckle-uninvaded areas. When honeysuckle was experimentally removed, a decrease in deer activity and infected tick numbers was observed. In contrast, invasive alien species can reduce the quality of habitat for vector-host contacts, thus decreasing the risk. Japanese stiltgrass, Microstegium vimineum, changes soil surface microclimate conditions, reducing habitat quality for ticks (Civitello et al. 2008). Invasive alien species can also alter vector-host–pathogen dynamics, increasing human spillover. In Florida, the invasive alien Burmese python, Python bivittatus, heavily predated the large mammals of the area (i.e. deer, raccoons and opossums), inducing the native mosquito, Culex cedecei, to feed more on hispid cotton rat Sigmodon hispidus (the primary reservoir host) and even on humans (Hoyer et al. 2017).
Conclusions and future directions
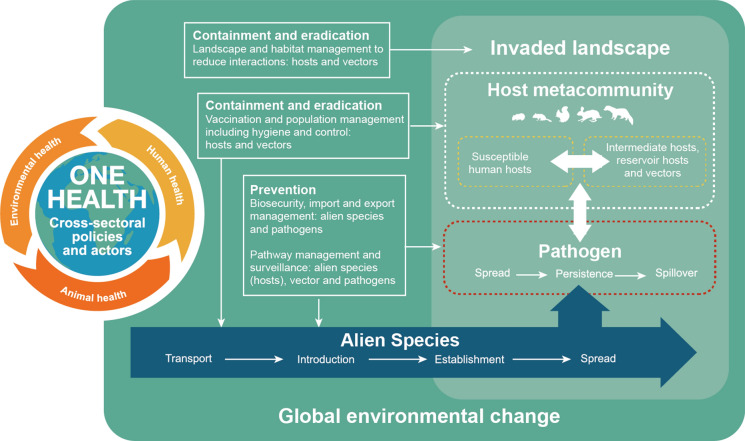
Given that invasive alien species can play a role in transmission of zoonotic diseases that exceeds or is equivalent to the role of native wildlife, sometimes in a relatively short period following their arrival, there is an urgent need to raise awareness of the potential risks posed to human health. Ecological and social mechanisms govern the dynamics of zoonotic disease transmission but wildlife diseases are not consistently included within animal, plant and human policies (Roy et al. 2016); however the World Animal Health Organisation and Convention on International Trade in Endangered Species of Wild Fauna and Flora have agreed to collaborate on animal health and welfare issues (https://cites.org/eng/node/18857). Consideration of ecological, evolutionary and social perspectives will enhance understanding of the threat of biological invasions which adversely affect biodiversity and ecosystems but also affect human health (Fig. 1).
Fig. 1.
The role of invasive alien species in zoonotic disease transmission within the host metacommunity is modulated by interacting social, environmental and ecological factors operating in the invaded landscape and can be interrupted by cross-sectoral actors and policies operating on invasion or spill-over pathways, particularly if these are linked within One Health frameworks
Most forecasts of the risk of emerging diseases neglect the potential role of alien species (Nuñez et al. 2020) and this consequently represents a gap in strategies underpinning responses for zoonoses. Modelling can inform prevention of zoonotic diseases and early warning of zoonotic pathogens carried or hosted by alien species (Cross et al. 2019). It is imperative to integrate biological invasion status and history into interpretation of host–pathogen networks, to better understand the role of invasive alien species in human spillover. Models that integrate heterogenous social and contact networks among wildlife, livestock and people (e.g. due to trade, social structure of animals and people, differential habitat use) are increasingly being used to understand infectious disease dynamics and spillover. Systems frameworks are being developed, that integrate the ecological, economic and social processes promoting spillover within ecosystems and policies and actors that interact with the disease systems in rapidly changing environments. It is critical to acknowledge and integrate the complex and diverse roles of invasive alien species into such model frameworks for zoonoses to better understand temporal and spatial trends in health risks to underpin public health reporting.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
With thanks to the European Commission for funding this review through the contract with IUCN No 07.0202/2019/812535/SER/ENV.D.2 “Technical and scientific support in relation to the implementation of Regulation 1143/2014 on IAS”. The EU keeps worldwide ownership of the results of this contract. HER is partly supported through Natural Environment Research Council award number NE/R016429/1 as part of the UK-SCAPE programme delivering National Capability. Thank you to the UK Centre for Ecology & Hydrology Graphic Designer Kate Randall for assisting with Figure 1. We are appreciative of the comments from the editor and reviewers.
Author contributions
HER, BVP, ET, RS, KS, RH contributed to the study conception and design. Data collection and analysis were performed by HER, BVP, ET, RH, CJ, KR. The first draft of the manuscript was written by HER and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The opinions given herein belong solely to the authors and do not represent the views or policies of IUCN or the European Commission.
Footnotes
Invasive alien species (IAS) are plants, animals and other organisms that are introduced directly or indirectly by people into places out of their natural range of distribution, and which may establish, spread and threaten or adversely impact upon biodiversity and nature’s contributions to people (including ecosystem services). Human health impacts are not always included in the definition of IAS but here we use a definition that extends to social and economic impacts including human health. There has been much debate over the inclusion of pathogens within the definition of IAS given the complexities of ascribing native or non-native status to microorganisms.
A zoonosis is a human disease occurring following the natural and reciprocal transmission of a pathogen or parasite between animals and humans.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguiló-Gisbert J, Padilla-Blanco M, Lizana V, Maiques E, Muñoz-Baquero M, Chillida-Martínez E, Cardells J, Rubio-Guerri C. First description of SARS-CoV-2 infection in two feral American mink (Neovison vison) caught in the wild. Animals. 2021;11:1422. doi: 10.3390/ani11051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA. Changing patterns of emerging zoonotic diseases in wildlife, domestic animals, and humans linked to biodiversity loss and globalization. ILAR J. 2017;58:315–318. doi: 10.1093/ilar/ilx035. [DOI] [PubMed] [Google Scholar]
- Allan BF, Dutra HP, Goessling LS, Barnett K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE, Orrock JL. Invasive honeysuckle eradication reduces tick-borne disease risk by altering host dynamics. Proc Natl Acad Sci. 2010;107:18523–18527. doi: 10.1073/pnas.1008362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AN, Milleson MP, Hernández FA, Merrill HR, Avery ML, Wisely SM. Wildlife management practices associated with pathogen exposure in non-native wild pigs in Florida, US. Viruses. 2019;11:14. doi: 10.3390/v11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchio E, Crotta M, Romeo C, Drewe JA, Guitian J, Ferrari N. Invasive alien species and disease risk: An open challenge in public and animal health. PLoS Pathog. 2020;16:e1008922. doi: 10.1371/journal.ppat.1008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello DJ, Flory SL, Clay K. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae) J Med Entomol. 2008;45:867–872. doi: 10.1093/jmedent/45.5.867. [DOI] [PubMed] [Google Scholar]
- Correa JP, Bucarey SA, Cattan PE, Landaeta-Aqueveque C, Ramírez-Estrada J. Renal carriage of Leptospira species in rodents from Mediterranean Chile: The Norway rat (Rattus norvegicus) as a relevant host in agricultural lands. Acta Trop. 2017;176:105–108. doi: 10.1016/j.actatropica.2017.07.032. [DOI] [PubMed] [Google Scholar]
- Cross PC, Prosser DJ, Ramey AM, Hanks EM, Pepin KM. Confronting models with data: the challenges of estimating disease spillover. Philos Trans R Soc B. 2019;374:20180435. doi: 10.1098/rstb.2018.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich M, Gomard Y, Lagadec E, Ramasindrazana B, Le Minter G, Guernier V, Benlali A, Rocamora G, Markotter W, Goodman SM. Biogeography of Leptospira in wild animal communities inhabiting the insular ecosystem of the western Indian Ocean islands and neighboring Africa. Emerg Microbes Infect. 2018;7:1–12. doi: 10.1038/s41426-018-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobigny G, Gauthier P, Houéménou G, Dossou H, Badou S, Etougbétché J, Tatard C, Truc P. Spatio-temporal survey of small mammal-borne Trypanosoma lewisi in Cotonou, Benin, and the potential risk of human infection. Infect Genet Evol. 2019;75:103967. doi: 10.1016/j.meegid.2019.103967. [DOI] [PubMed] [Google Scholar]
- Dunn AM, Hatcher MJ. Parasites and biological invasions: parallels, interactions, and control. Trends Parasitol. 2015;31:189–199. doi: 10.1016/j.pt.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Enserink M (2020) Coronavirus rips through Dutch mink farms, triggering culls. American Association for the Advancement of Science [DOI] [PubMed]
- Estrada-Peña A, Ostfeld RS, Peterson AT, Poulin R, de la Fuente J. Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol. 2014;30:205–214. doi: 10.1016/j.pt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Feng AY, Himsworth CG. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus) Urban Ecosyst. 2014;17:149–162. doi: 10.1007/s11252-013-0305-4. [DOI] [Google Scholar]
- Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, Jones KE. Zoonotic host diversity increases in human-dominated ecosystems. Nature. 2020;584:398–402. doi: 10.1038/s41586-020-2562-8. [DOI] [PubMed] [Google Scholar]
- Grace D, Gilbert J, Randolph T, Kang’ethe E. The multiple burdens of zoonotic disease and an ecohealth approach to their assessment. Tropical Anim Health Prod. 2012;44(1):67–73. doi: 10.1007/s11250-012-0209-y. [DOI] [PubMed] [Google Scholar]
- Halliday J, Daborn C, Auty H, Mtema Z, Lembo T, Bronsvoort BMD, Handel I, Knobel D, Hampson K, Cleaveland S. Bringing together emerging and endemic zoonoses surveillance: shared challenges and a common solution. Philos Trans Royal Soc B: Biol Sci. 2012;367(1604):2872–2880. doi: 10.1098/rstb.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammershøj M, Pertoldi C, Asferg T, Møller TB, Kristensen NB. Danish free-ranging mink populations consist mainly of farm animals: Evidence from microsatellite and stable isotope analyses. J Nat Conserv. 2005;13:267–274. doi: 10.1016/j.jnc.2005.03.001. [DOI] [Google Scholar]
- Han BA, Kramer AM, Drake JM. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016;32:565–577. doi: 10.1016/j.pt.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BA, Schmidt JP, Bowden SE, Drake JM. Rodent reservoirs of future zoonotic diseases. Proc Natl Acad Sci. 2015;112:7039–7044. doi: 10.1073/pnas.1501598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemink N, Vanwambeke SO, Purse BV, Gilbert M, Van Dyck H. Towards a resource-based habitat approach for spatial modelling of vector-borne disease risks. Biol Rev. 2015;90:1151–1162. doi: 10.1111/brv.12149. [DOI] [PubMed] [Google Scholar]
- Hoyer IJ, Blosser EM, Acevedo C, Thompson AC, Reeves LE, Burkett-Cadena ND. Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biol Let. 2017;13:20170353. doi: 10.1098/rsbl.2017.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme PE. Invasive species challenge the global response to emerging diseases. Trends Parasitol. 2014;30:267–270. doi: 10.1016/j.pt.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Hulme PE. Climate change and biological invasions: evidence, expectations, and response options. Biol Rev. 2017;92:1297–1313. doi: 10.1111/brv.12282. [DOI] [PubMed] [Google Scholar]
- Johnson CK, Hitchens PL, Pandit PS, Rushmore J, Evans TS, Young CC, Doyle MM. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc R Soc B. 2020;287:20192736. doi: 10.1098/rspb.2019.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M, Bai Y. Bartonella bacteria in urban rats: a movement from the jungles of Southeast Asia to metropoles around the globe. Front Ecol Evol. 2019;7:88. doi: 10.3389/fevo.2019.00088. [DOI] [Google Scholar]
- Lama JK, Bachoon DS. Detection of Brucella suis, Campylobacter jejuni, and Escherichia coli strains in feral pig (Sus scrofa) communities of Georgia. Vector-Borne Zoonotic Diseases. 2018;18:350–355. doi: 10.1089/vbz.2017.2187. [DOI] [PubMed] [Google Scholar]
- Laurimaa L, Süld K, Moks E, Valdmann H, Umhang G, Knapp J, Saarma U. First report of the zoonotic tapeworm Echinococcus multilocularis in raccoon dogs in Estonia, and comparisons with other countries in Europe. Vet Parasitol. 2015;212:200–205. doi: 10.1016/j.vetpar.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Leta S, Beyene TJ, De Clercq EM, Amenu K, Kraemer MU, Revie CW. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018;67:25–35. doi: 10.1016/j.ijid.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza G, Tricarico E. Invasive species and human health. Wallingford: CABI; 2018. [Google Scholar]
- Murray KA, Daszak P. Human ecology in pathogenic landscapes: two hypotheses on how land use change drives viral emergence. Curr Opin Virol. 2013;3:79–83. doi: 10.1016/j.coviro.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez MA, Pauchard A, Ricciardi A. Invasion science and the global spread of SARS-CoV-2. Trends Ecol Evol. 2020;35:642–645. doi: 10.1016/j.tree.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pépin M, Dupinay T, Zilber A-L, McElhinney LM. Of rats and pathogens: pathogens transmitted by urban rats with an emphasis on hantaviruses. CAB Rev. 2016;11:1–13. doi: 10.1079/PAVSNNR201611019. [DOI] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–510. doi: 10.1038/nrmicro.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Reaser JK, Locke H, Woodley SJ, Patz JA, Becker DJ, Oppler G, Hudson PJ, Tabor GM. Land use-induced spillover: a call to action to safeguard environmental, animal, and human health. Lancet Planet Health. 2021;5:e237–e245. doi: 10.1016/S2542-5196(21)00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P. Scientists' warning on invasive alien species. Biol Rev. 2020;95:1511–1534. doi: 10.1111/brv.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy HE, Hesketh H, Purse BV, Eilenberg J, Santini A, Scalera R, Stentiford GD, Adriaens T, Bacela-Spychalska K, Bass D, Beckmann KM. Alien pathogens on the Horizon: opportunities for predicting their threat to wildlife. Conserv Lett. 2017;10(4):477–484. doi: 10.1111/conl.12297. [DOI] [Google Scholar]
- Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pyšek P, Winter M, Arianoutsou M, Bacher S, Blasius B, Brundu G, Capinha C, Celesti-Grapow L, Dawson W, Dullinger S, Fuentes N, Jäger H, Kartesz J, Kenis M, Kreft H, Kühn I, Lenzner B, Liebhold A, Mosena A, Moser D, Nishino M, Pearman D, Pergl J, Rabitsch W, Rojas-Sandoval J, Roques A, Rorke S, Rossinelli S, Roy HE, Scalera R, Schindler S, Štajerová K, Tokarska-Guzik B, van Kleunen M, Walker K, Weigelt P, Yamanaka T, Essl F. No saturation in the accumulation of alien species worldwide. Nat Commun. 2017;8:14435. doi: 10.1038/ncomms14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, Rokni MB, Zhou X-N, Fèvre EM, Sripa B. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12:e1001920. doi: 10.1371/journal.pmed.1001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnisty AA, Homel KV, Kheidorova EE, Shpak AV, Nikiforov ME (2020) MOlecular genetic polymorphism of american mink populations (Neovison vison) in model fur farms and on the adjacent territories in belarus. Дoклaды Haциoнaльнoй aкaдeмии нayк Бeлapycи, pp. 685–693. Pecпyбликaнcкoe yнитapнoe пpeдпpиятиe Издaтeльcкий дoм Бeлopyccкaя нayкa.
- Wood JL, Leach M, Waldman L, MacGregor H, Fooks AR, Jones KE, Restif O, Dechmann D, Hayman DT, Baker KS. A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos Trans Royal Soc B: Biol Sci. 2012;367:2881–2892. doi: 10.1098/rstb.2012.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rohr J, Cui R, Xin Y, Han L, Yang X, Gu S, Du Y, Liang J, Wang X, Wu Z. Biological invasions facilitate zoonotic disease emergences. Nat Commun. 2022;13(1):1–11. doi: 10.1038/s41467-022-29378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G-L, Tang Y-Y, Limpanont Y, Wu Z-D, Li J, Lv Z-Y. Zoonotic parasites carried by invasive alien species in China. Infect Dis Poverty. 2019;8:1–17. doi: 10.1186/s40249-018-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.