Abstract
Background & Aims
Radiofrequency ablation (RFA) and ablative external beam radiotherapy (ablative RT) are commonly used to treat small intrahepatic malignancies. We meta-analysed oncologic outcomes and systematically reviewed the clinical consideration of tumour location and size.
Methods
PubMed, Medline, Embase, and Cochrane Library databases were searched on February 24, 2022. Studies comparing RFA and ablative RT, providing one of the endpoints (local control or survival), and encompassing ≥5 patients in each arm were included.
Results
Twenty-one studies involving 4,638 patients were included. Regarding survival, the odds ratio (OR) was 1.204 (p = 0.194, favouring RFA, not statistically significant) among all studies, 1.253 (p = 0.153) among hepatocellular carcinoma (HCC) studies, and 1.002 (p = 0.996) among colorectal cancer metastasis studies. Regarding local control, the OR was 0.458 (p <0.001, favouring ablative RT) among all studies, 0.452 (p <0.001) among HCC studies, favouring the ablative RT arm, and 0.649 (p = 0.484) among colorectal cancer metastasis studies. Pooled 1- and 2-year survival rates for HCC studies were 91.8% and 77.7% after RFA, and 89.0% and 76.0% after ablative RT, respectively; and for metastasis studies were 88.2% and 66.4% after RFA and 82.7% and 60.6% after RT, respectively. Literature analysis suggests that ablative RT can be more effective than RFA for tumours larger than 2–3 cm or for specific sublocations in the liver (e.g. subphrenic or perivascular sites), with moderate quality of evidence (reference to the grading system of the American Society for Radiation Oncology Primary Liver Cancer Clinical Guidelines). The pooled grade ≥3 complication rates were 2.9% and 2.8% in the RFA and ablative RT arms, respectively (p = 0.952).
Conclusions
Our study shows that ablative RT can yield oncologic outcomes similar to RFA, and suggests that it can be more effective for the treatment of tumours in locations where RFA is difficult to perform or for large-sized tumours.
Systematic Review Registration
This study was registered with PROSPERO (Protocol No: CRD42022332997).
Impact and implications
Radiofrequency ablation (RFA) and ablative radiotherapy (RT) are non-surgical modalities for the treatment of small intrahepatic malignancies. Ablative RT showed oncologic outcomes at least similar to those of RFA, and was more effective at specific locations (e.g. perivascular or subphrenic locations).
Keywords: Intrahepatic malignancy, Liver cancer, External beam radiation therapy, Radiofrequency ablation
Abbreviations: ASCO, American Society of Clinical Oncology; ASTRO, American Society for Radiation Oncology; CIRSE, cardiovascular and interventional radiological society of Europe; CRC, colorectal cancer; EBRT, external beam radiation therapy; EQD2, Equivalent dose, 2 Gy per Fraction; HCC, hepatocellular carcinoma; HFRT, hypofractionated radiotherapy; IPTW, inverse probability of treatment weighting; LC, local control; LT, liver transplantation; MWA, microwave ablation; NCDB, national cancer database; OS, overall survival; P, prospective; PBT, proton beam therapy; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PSM, propensity score matching; R, retrospective; RCT, randomised controlled trial; RFA, radiofrequency ablation; RT, radiotherapy; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolisation
Graphical abstract
Highlights
-
•
RFA is the most widely used non-surgical local modality for the treatment of small intrahepatic malignancies.
-
•
Ablative RT yields a curative effect by focusing high doses on small targets using computerised planning.
-
•
Ablative RT was associated with superior local control and overall survival compared to RFA.
-
•
RT could be more effective than RFA for tumours larger than 2–3 cm or for specific sublocations in the liver.
Introduction
Surgical resection is the most reliable curative treatment for small-sized, localised hepatic malignancies.1,2 Compared with surgery, radiofrequency ablation (RFA) is a simpler modality that inflicts less damage to the liver. Therefore, it has been used as a surrogate radical modality for localised liver malignancies.[1], [2], [3] However, external beam radiation therapy (EBRT) has gained popularity in the treatment of intrahepatic malignancies, especially with the advent of CT-based computerised planning, which enables precise targeting and normal liver sparing.4 Recent comparative studies have reported that the local control rate of stereotactic body radiotherapy (SBRT) for intrahepatic malignancies is comparable to that of RFA.5 To make clinical decisions pertaining to the most suitable treatment modality, consideration of the tumour location and size is necessary. RFA has been most efficient in treating small tumours (<2–3 cm),6,7 but has difficulty in treating specific sublocations (e.g. subphrenic or perivascular sites).8,9 EBRT is able to deliver a prescribed dose efficiently to relatively large tumours, and can be less affected by locational difficulties.10 Therefore we have systematically reviewed the literature on clinical considerations of tumour location and size to aid in clinical decision-making; we have also performed a comparative meta-analysis of the oncologic outcomes of RFA and ablative EBRT in the treatment of intrahepatic malignancies.
Materials and methods
Study design
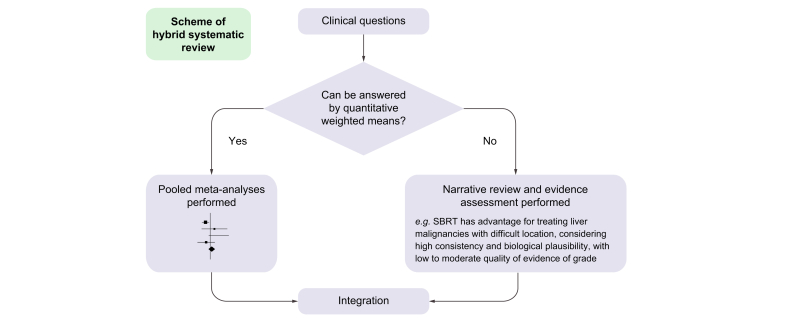
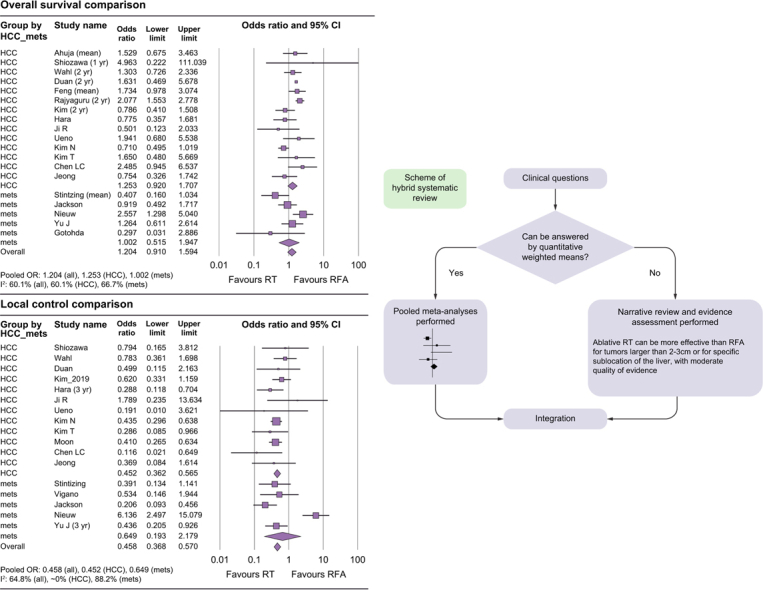
We conducted our systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,11 and also referred to the Cochrane Handbook for methodological aspects. We used patient, intervention, comparison, outcomes (PICO) to frame the main hypothetical question, ‘Does ablative radiotherapy (RT) have oncologic outcomes (e.g. survival and local control) and toxicity profiles comparable to RFA for the treatment of patients with localised liver malignancies?’ We then conducted a systematic review with formal meta-analyses. The auxiliary hypothetical question was ‘Is ablative RT more efficient than RFA for the treatment of large-sized (>2–3 cm) liver malignancies present in locations that are difficult to access (e.g. perivascular, liver dome)?’ A narrative review with evidence grading was performed on the auxiliary question because the criteria for tumour size and difficult locations varied in different studies, and it was necessary to subjectively evaluate the details of the treatment (i.e. a hybrid systematic review, Fig. 1). The primary endpoint was local control (LC) and the secondary endpoint was overall survival (OS). Grade ≥3 complications were investigated as the secondary endpoints. This study was registered with PROSPERO (Protocol No: CRD42022332997).
Fig. 1.
Flow diagram of a hybrid systematic review.
SBRT, stereotactic body radiotherapy.
Study inclusion and data collection
Four databases, including PubMed, MEDLINE, Embase, and Cochrane Library, were searched until February 24, 2022. The studies that fulfilled the following inclusion criteria were included: (1) clinical studies comparing RFA and ablative RT for treatment of liver malignancies; (2) data for at least 1 endpoint (LC or OS); and (3) each arm (RFA and ablative RT) should encompass 5 or more patients with hepatocellular carcinoma (HCC) or liver metastases. Studies regarding thermal ablation which include cases of both RFA and microwave ablation (MWA) were also included. The reference lists of the included studies were also checked to identify potentially missing studies. Language restrictions were not applied, and external consultation was performed when language translation was necessary. Conference abstracts were included if they met the inclusion criteria. Multiple studies from a single institution were included if they did not have overlapping patient data. Otherwise, the study was selected using the following criteria prioritised in numerical order: (1) larger sample size; (2) data on more endpoints; and (3) time elapsed since publication. Two independent reviewers searched the literature, and any disagreement was resolved through mutual discussion and re-investigation. Search terms and strategies according to the databases are shown in Supplementary Data 1. We used pre-designed sheets including (1) general information, including author name, publication source, patient recruitment period, affiliation, type of study, and study design and (2) clinical information including number of patients, target patients, follow-up periods, LC rates, overall survival rates, rate and detail of grade ≥3 complications, and differential clinical outcomes according to tumour size and location. OS and LC data were acquired from a descriptive graph in the absence of numerical data.
Quality assessment
According to a preliminary search, the majority of candidate studies were observational studies. We used the Newcastle–Ottawa scale,12 as recommended in the Cochrane Handbook for the Assessment of Observational Studies.13 A study with a score of 8–9 was evaluated as high quality, that with a score of 6–7 as medium quality, and a study with a score of 5 or less as low quality. As observational studies with a high risk of bias are not recommended for meta-analysis, as referenced by the Cochrane handbook,14 we excluded low-quality studies from the present systematic review, if the authors agreed.
Effect measures and data synthesis
The main effect measure to assess the primary and secondary endpoints was the pooled odds ratio (OR) of OS and LC rates, in comparison of RFA and SBRT. Considering the possible heterogeneity in response evaluation (e.g. complete or partial response), LC was set as an endpoint and acquired either reported LC rate or non-local failure rate. Pooled analyses of OS were weighted using the number of patients, and those of LC were based on the patient or tumour number as reported by individual studies. The pooled percentiles of LC and OS were also calculated for clinical reference. Regarding complications, the pooled percentile rate of grade ≥3 complications was calculated and subjectively reviewed. Because the vast majority of candidate studies were observational studies from different institutions, there would be a possible heterogeneity with regard to treatment detail and clinical characteristics; thus, a random-effects model was used for the pooled analysis of the endpoints, in accordance with the Cochrane handbook.14
Subgroup analyses were also performed for comparability. The studies were regarded as having reliable comparability if they were randomised studies, performed intentional statistical matching (e.g. propensity scoring matching, inverse-probability weighting), or reported no significant difference regarding known clinical factors (including but not limited to age, tumour size, Child–Pugh class, and tumour location). Studies without available comparative information or those with SBRT arms having inferior clinical profiles (e.g. p <0.05, or >20% difference) were regarded as not having reliable comparability. The stepwise-hierarchical pooled analysis by Shin et al.15 was referred to for the stepwise analysis methods and interpretation of the subject owing to scarce randomised literature. Subgroup analyses were also performed according to the disease, including HCC and colorectal cancer (CRC) with liver metastases.
We performed the Cochran Q test16 and I2 statistics17 to assess heterogeneity in the pooled analyses, and I2 values of 25%, 50%, and 75% were regarded as low, moderate, and high heterogeneity, respectively. Publication bias assessments for pooled analyses involving >10 studies were performed using a visual funnel plot assessment and quantitative Egger’s test.18 Possible publication bias was considered to exist if the funnel plots showed asymmetry and the 2-tailed p value was <0.1 in the Egger’s test. Duval and Tweedie’s trim-and-fill method19 was used for analyses with possible publication bias to yield the adjusted reference values. All statistical analyses were performed using the Comprehensive Meta-Analysis version 3 (Biostat Inc., Englewood, NJ, USA).
The advantages of a specific modality according to tumour location and size were assessed with reference to the grading system outlined in the liver cancer practice guidelines of the American Society of Radiation Oncology (ASTRO).20 The evidence grading system is summarised in Table S1.
Results
Study selection and characteristics
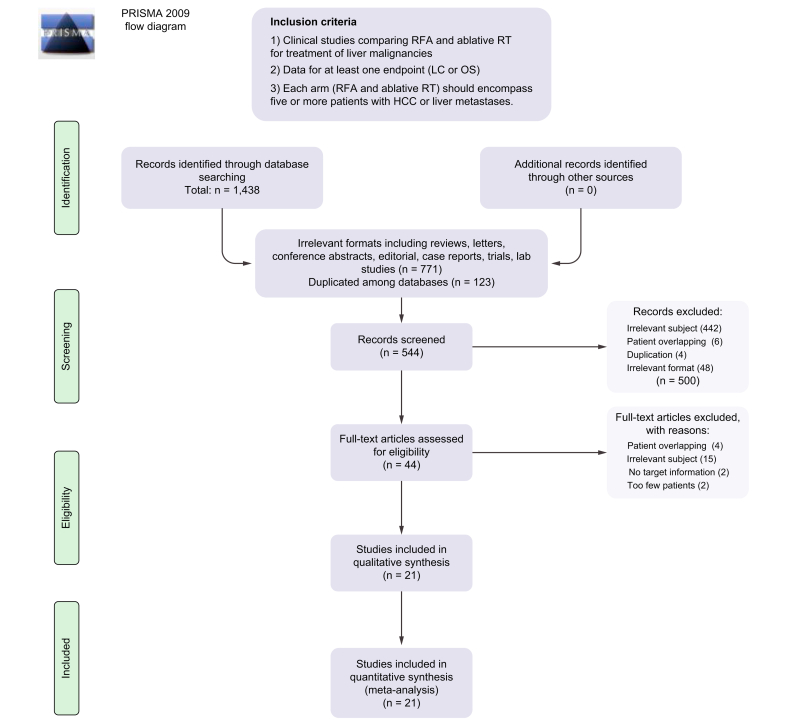
A total of 1,438 studies were initially searched. Those duplicated among databases and having irrelevant formats (e.g. reviews, letters, conference abstracts, editorials, case reports, trial protocols, and lab studies) were machine-filtered. Eventually, 544 studies were screened using the abstracts and citations. After excluding 500 articles for various reasons, 44 studies underwent full-text review, and 21 studies involving 4638 patients (RFA 2807, ablative RT 1831) that met all inclusion criteria were finally included.[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] The inclusion process is illustrated in Fig. 2.
Fig. 2.
Study inclusion plot.
HCC, hepatocellular carcinoma; LC, local control; OS, overall survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RFA, radiofrequency ablation; RT, radiotherapy.
Among the 21 studies, 16 had full text and 5 were conference abstracts. Fourteen studies involved patients with HCC, and 7 studies involved patients with CRC and liver metastases. With regard to radiation modalities, 18 studies investigated the results of SBRT, 2 of CyberKnife®, and 1 of proton therapy. The majority (17 of 21) were observational studies that were retrospectively designed, 2 were studies based on the US National Cancer Database, and there was 1 prospective observational study and 1 randomised study. General information regarding these studies is summarised in Table 1.
Table 1.
General information of included studies.
| Author | Year of publication | Years of patients recruit | Affiliation | Country | Study type | Source | No. of patients | Study design | Subject of study |
|---|---|---|---|---|---|---|---|---|---|
| Ahuja | 2014 | Louisiana State Univ. | US | Conference abstract | CIRSE | TACE and RFA 32 TACE and SBRT 32 |
R | HCC | |
| Shiozawa | 2015 | 2011–2014 | Toho Univ. | Japan | Full article | World J Gastroenterol | RFA 38, CyberKnife 35 (all solitary tumour) | R | HCC |
| Wahl | 2016 | 2004–2012 | Univ. of Michigan | US | Full article | J Clin Oncol | RFA 161, SBRT 63 (tumours: RFA 249, SBRT 83) | P | HCC |
| Duan | 2016 | 2011–2012 | Beijing 302 hospital | China | Conference abstract | Hepatol | RFA 40, SBRT 37 | R | HCC |
| Feng | 2016 | 2004–2011 | Univ. of Michigan | US | Conference abstract | ASCO | RFA 78, SBRT 78 (after PSM) | NCDB | HCC |
| Rajyaguru | 2018 | 2004–2013 | Gundersen Health System | US | Full article | J Clin Oncol | RFA 521, SBRT 296 (after PSM) | NCDB | HCC |
| Kim | 2019 | 2012–2016 | Yonsei Cancer Center | Korea | Full article | Radiother Oncol | RFA 95, SBRT 95 (after PSM) (tumour n = patient n) | R | HCC |
| Hara | 2019 | 2012–2016 | Yokohama City Univ., Ofuno Chao Univ. | Japan | Full article | Hepatology | RFA 106, SBRT-HFRT 106 (after PSM) | R | HCC |
| Ji R | 2022 | 2008–2021 | Univ. of Hong Kong | HK SAR, China | Full article | Medicine | RFA 38 SBRT 22 | R | HCC |
| Ueno | 2021 | 2014–2019 | Kurashiki Central Hospital | Japan | Full article | J Gastrointestinal Oncol | RFA 62 SBRT 31 (after PSM) | R | HCC |
| Kim N | 2020 | 2010–2016 | East Asian Multicentres | China, Japan, HK SAR, Taiwan, and Korea | Full article | J Hepatol | RFA 313 SBRT 313 (after PSM) | R | HCC |
| Kim T | 2021 | 2013–2017 | National Cancer Center | Korea | Full article | J Hepatol | RFA 56 PBT 80 | RCT | HCC |
| Moon | 2019 | 2006–2018 | Multicentres of US | US | Conference abstract | AASLD | RFA 529 (include 123 MWA) SBRT 387 lesions | R | HCC |
| Chen LC | 2019 | 2014–2017 | Dalin Tzu Chi Hosp. | Taiwan | Conference abstract | ASTRO | RFA 84 SBRT 24 | R | HCC |
| Stintzing | 2013 | 2005–2011 | Comprehensive Cancer Centre | Germany | Full article | Acta Oncol | RFA 30, CyberKnife 30 (tumours: RFA 35, CyberKnife 35) | R | CRC liver mets |
| Viganò | 2018 | 2004–2013 | Humanitas Univ. | Italy | Full article | World J Surg | RFA 19, SBRT 14 | R | CRC liver only mets |
| Jackson | 2018 | 2000–2015 | Univ. of Michigan | US | Full article | Int J Radiat Oncol Biol Phys | RFA 69, SBRT 92 | R | CRC and other liver mets |
| Nieuwenhuizen S | 2021 | from 2007 | Amsterdam registry | Netherland | Full article | Cancers | RFA 144 (include 81 MWA) SBRT 55 | R | CRC liver mets |
| Jeong | 2021 | 2013 | Asan hospital | Korea | Full article | J Gastroenterol Hepatol | RFA 172 SBRT 87 (after IPTW) | R | CRC liver mets |
| Yu J | 2021 | 2007–2014 | Asan hospital | Korea | Full article | Cancer Res Treat | RFA 178 SBRT 44 (after IPTW) | R | CRC liver mets |
| Gotohda | 2020 | 2010–2016 | Seven centres from Japan | Japan | Full article | JGH open | RFA 42 SBRT 5 | R | CRC liver mets |
ASCO, American Society of Clinical Oncology; ASTRO, American Society for Radiation Oncology; CIRSE, cardiovascular and interventional radiological society of Europe; HCC, hepatocellular carcinoma; LT, liver transplantation; NCDB, national cancer database; P, prospective; R, retrospective; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolisation.
Among HCC studies, the median 2-year OS rates were 78.5% (range: 52.9–92.9) in the RFA arm and 77.6% (range: 46.3–90.2) in the ablative RT arm; the median 2-year LC rates were 84.5% (63.8–94.7) in the RFA arm and 91.7% (74.9–100) in the ablative RT arm. Regarding CRC studies, the median 2-year OS rates were 64.3% (50.2–80) in the RFA arm and 65.4% (52.3–80) in the ablative RT arm; the median 2-year LC rates were 60.8% (56.4–93.3) in the RFA arm and 77.0% (71.5–88.2) in the ablative RT arm. Regarding comparability analysis methods, 9 studies used intentional patient-matching methods (e.g. propensity score matching; inverse probability of weighting). Eight studies performed statistical comparisons; 6 of them showed that the ablative RT arm had inferior clinical factors (e.g. p <0.05, or >20% numeral difference), and 2 of them reported no statistically significant difference between the RT and the RFA arms regarding clinical factors. Clinical factors included, but were not limited to, age, Child–Pugh score, tumour size, and difficult location to be treated. Three studies did not provide relevant information. One study performed a randomised allocation. Table S2 provides information regarding the clinical characteristics of the included studies.
Quality and bias assessments
According to the Newcastle–Ottawa scale, 10 of the 21 studies were regarded as having high quality (8–9 points) and 8 studies had medium quality (6 or 7 points). None of the studies were assessed as having low quality. Therefore, all studies that fulfilled the inclusion criteria were included in the present systematic review. Details of scoring and the reasons according to each scoring category are shown in Table S3.
Synthesis of clinical endpoints
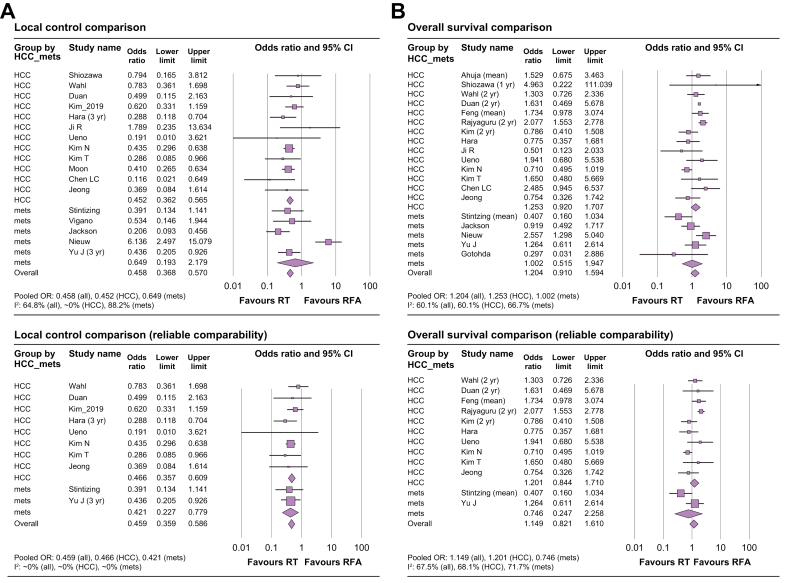
With regard to OS, the OR was 1.204 (95% CI: 0.910–1.594, p = 0.194) among all studies, 1.253 (95% CI: 0.920–1.707, p = 0.153) among HCC studies, and 1.002 (95% CI: 0.515–1.947, p = 0.996) among CRC metastases studies. Among studies with reliable comparability, the OR was 1.149 (95% CI: 0.821–1.610, p = 0.417) for all studies, 1.201 (95% CI: 0.844–1.710, p = 0.309) among HCC studies, and 0.746 (95% CI: 0.247–2.258, p = 0.64) among CRC metastases studies. These results are summarised in Table 2 and are shown in Fig. 3 as forest plots. With regard to LC, the OR was 0.458 (95% CI: 0.368–0.570, p <0.001) among all studies and 0.452 (95% CI: 0.362–0.565, p <0.001) among HCC studies, favouring the ablative RT arm, and 0.649 (95% CI: 0.193–2.179, p = 0.484) among CRC metastasis studies. Among studies with reliable comparability (e.g. randomised studies, studies performed with intentional statistical matching, no significant differences in known clinical factors), the OR was 0.466 (95% CI: 0.357–0.609, p <0.001) among all studies, 0.421 (95% CI: 0.227–0.779, p <0.001) among HCC studies, and 0.459 (95% CI: 0.359–0.586, p = 0.006) among CRC metastases studies, all favouring the ablative RT arm.
Table 2.
Pooled rate of odds ratio regarding local control and survival.
| Studies (subject) | No. of studies | No. of cases | Heterogeneity p | I2 (%) | Heterogeneity assessment | OR (95% CI) | RFA vs. SBRT (p value) |
|---|---|---|---|---|---|---|---|
| All studies (local control) | |||||||
| All | 17 | 3,670 | <0.001 | 64.8 | Moderate to high | 0.458 (0.368-0.570) | <0.001 |
| HCC | 11 | 2,974 | 0.555 | ∼0 | Very low | 0.452 (0.362-0.565) | <0.001 |
| CRC mets | 6 | 696 | <0.001 | 88.2 | High | 0.649 (0.193-2.179) | 0.484 |
| Studies with reliable comparability (local control) | |||||||
| All | 10 | 2,109 | 0.838 | ∼0 | Very low | 0.466 (0.357-0.609) | <0.001 |
| HCC | 8 | 1,817 | 0.68 | ∼0 | Very low | 0.421 (0.227-0.779) | <0.001 |
| CRC mets | 2 | 292 | 0.87 | ∼0 | Very low | 0.459 (0.359-0.586) | 0.006 |
| All studies (overall survival) | |||||||
| All | 19 | 3,504 | <0.001 | 60.10% | Moderate to high | 1.204 (0.910-1.594) | 0.194 |
| HCC | 14 | 2,875 | 0.002 | 60.1 | Moderate to high | 1.253 (0.920-1.707) | 0.153 |
| CRC mets | 5 | 629 | 0.017 | 66.7 | Moderate to high | 1.002 (0.515-1.947) | 0.996 |
| Studies with reliable comparability (overall survival) | |||||||
| All | 12 | 2,856 | <0.001 | 67.5 | Moderate to high | 1.149 (0.821-1.610) | 0.417 |
| HCC | 10 | 2,634 | 0.001 | 68.1 | Moderate to high | 1.201 (0.844-1.710) | 0.309 |
| CRC mets | 2 | 222 | 0.06 | 71.7 | Moderate to high | 0.746 (0.247-2.258) | 0.604 |
CRC, colorectal cancer; HCC, hepatocellular carcinoma; LC, local control; OR, odds ratio; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy.
Fig. 3.
Forest plots of local control and overall survival.
(A) local control comparison of all included studies (upper) and of studies with reliable comparability (lower); (B) overall survival comparison of all included studies (upper) and of studies with reliable comparability (lower). HCC, hepatocellular carcinoma; OR, odds ratio; RFA, radiofrequency ablation; RT, radiotherapy.
Pooled analyses of the LC percentile included all studies, and pooled analyses of the OS percentile included HCC and CRC metastasis studies separately. Among HCC studies, the pooled 1-, 2-, and 3-year OS rates were 91.8% (95% CI: 87.2–94.9), 77.7% (70.7–83.4), and 76.0% (64.4–84.7) in the RFA arm, respectively; the corresponding rates for the ablative RT arm were 89.0% (95% CI: 83.6–92.7), 76.0% (64.4–84.7), and 65.9% (53.7–76.3), respectively. Among CRC metastases studies, pooled 1-, 2-, and 3-year OS rates were 88.2% (95% CI: 77.9–94.0), 66.4% (50.9–79.0), and 52.1% (41.1–62.8) in RFA arm; the corresponding rates for the ablative RT arm were 82.7% (95% CI: 61.6–93.4), 60.6% (50.7–69.6), and 43.6% (26.6–62.1), respectively. Pooled 1-, 2-, and 3-year LC percentile rates were 82.3% (95% CI: 77.2–86.4), 80.1% (72.7–85.8), and 92.4% (89.2–94.7) in RFA arms, and 92.4% (95% CI: 89.2–94.7), 86.5% (81.7–90.2), 83.9% (77.7–88.7) in ablative RT arms, respectively.
Pooled results of OS and LC percentiles are summarised in Table 3.
Table 3.
Pooled percentile of clinical endpoints.
| Subject Modality | No. of cohorts | No. of cases | Effect size % (95% CI) | RFA vs. ablative RT (p value) | Heterogeneity p | I2(%) |
|---|---|---|---|---|---|---|
| One-year LC rate (HCC and CRC mets) | ||||||
| All | 32 | 3,687 | 87.2 (84.2–89.7) | <0.001 | 83.3 | |
| RFA | 16 | 2,172 | 82.3 (77.2–86.4) | <0.001 | 80.0 | |
| RT | 16 | 1,515 | 92.4 (89.2–94.7) | <0.001 | <0.001 | 63.9 |
| Two-year LC rate | ||||||
| All | 28 | 2,549 | 84 (80.0–87.3) | <0.001 | 84.8 | |
| RFA | 14 | 1,464 | 80.1 (72.7–85.8) | <0.001 | 88.2 | |
| RT | 14 | 1,084 | 86.5 (81.7–90.2) | 0.094 | <0.001 | 66.4 |
| Three-year LC rate | ||||||
| All | 26 | 2,809 | 79.6 (74.9–83.6) | <0.001 | 87.4 | |
| RFA | 13 | 1,649 | 75.7 (68.6–81.7) | <0.001 | 88.7 | |
| RT |
13 |
1,160 |
83.9 (77.7–88.7) |
0.062 |
<0.001 |
80.4 |
| One-year OS (HCC) | ||||||
| All | 24 | 2,875 | 90.3 (87.0–92.9) | <0.001 | 81.8 | |
| RFA | 12 | 1,686 | 91.8 (87.2–94.9) | <0.001 | 85.6 | |
| RT | 12 | 1,189 | 89.0 (83.6–92.7) | 0.333 | <0.001 | 79.7 |
| Two-year OS (HCC) | ||||||
| All | 22 | 2,802 | 77.2 (71.4–82.2) | <0.001 | 90.6 | |
| RFA | 11 | 1,648 | 77.7 (70.7–83.4) | <0.001 | 88.2 | |
| RT | 11 | 1,154 | 76.0 (64.4–84.7) | 0.775 | <0.001 | 92.7 |
| Three-year OS (HCC) | ||||||
| All | 18 | 2,518 | 67.5 (60.3–74.0) | <0.001 | 91.1 | |
| RFA | 9 | 1,449 | 68.5 (59.3–76.5) | <0.001 | 90.7 | |
| RT |
9 |
1,069 |
65.9 (53.7–76.3) |
0.718 |
<0.001 |
92.4 |
| One-year OS (CRC mets) | ||||||
| All | 8 | 629 | 86.6 (77.7–92.3) | <0.001 | 86.0 | |
| RFA | 4 | 433 | 88.2 (77.9–94.0) | 0.001 | 81.8 | |
| RT | 4 | 196 | 82.7 (61.6–93.4) | 0.507 | 0.001 | 81.1 |
| Two-year OS (CRC mets) | ||||||
| All | 8 | 629 | 62.2 (54.0–69.8) | <0.001 | 80.8 | |
| RFA | 4 | 433 | 66.4 (50.9–79.0) | <0.001 | 88.2 | |
| RT | 4 | 196 | 60.6 (50.7–69.6) | 0.517 | 0.195 | 36.3 |
| Three-year OS (CRC mets) | ||||||
| All | 8 | 629 | 49.9 (40.5–59.3) | <0.001 | 83.7 | |
| RFA | 4 | 433 | 52.1 (41.1–62.8) | 0.003 | 78.5 | |
| RT |
4 |
196 |
43.6 (26.6–62.1) |
0.444 |
0.002 |
79.5 |
| Grade ≥3 complication | ||||||
| All | 26 | 4,698 | 2.9 (1.9–4.4) | <0.001 | 72.5 | |
| RFA | 13 | 3,415 | 2.9 (1.4–6.1) | <0.001 | 73.0 | |
| RT | 13 | 1,283 | 2.8 (1.6–4.9) | 0.952 | <0.001 | 73.2 |
CRC, colorectal cancer; HCC, hepatocellular carcinoma; LC, local control; OS, overall survival; RFA, radiofrequency ablation; RT, radiotherapy.
Heterogeneity analyses and publication bias assessment
Heterogeneity in the pooled analyses of LC was moderate to high (I2=64.8%), very low (I2=∼0%), and high (I2=88.2%), including all studies, HCC studies, and CRC metastases studies. In the subgroup analyses, including studies with reliable comparability, heterogeneity was very low in pooled analyses of all HCC and CRC metastasis studies. With regard to OS, heterogeneity was moderate to high in all pooled analyses (Table 2). No publication bias was identified in the pooled analyses of local control (p = 0.824) and overall survival (p = 0.468). The funnel plots are shown in Fig. S1.
Complications
Thirteen studies provided comparative complication data.22,23,28,29,[31], [32], [33], [34], [35],[38], [39], [40],42 The Common Terminology Criteria for Adverse Events by the National Cancer Institute of the US was used in most studies except the study by Ji et al.32 of which used the Clavien–Dindo classification. Pooled grade ≥3 complication rates were 2.9% (95% CI: 1.4–6.1) and 2.8% (1.6–4.9) in the RFA and ablative RT arms, respectively (p = 0.952 for difference). The vast majority of complications in RFA arms were as a result of mechanical damage from the procedures (e.g. bleeding, perforation, and pneumothorax). Among 20 grade ≥3 complication events from the ablative RT arms of 13 studies, 55% were hepatic damage (e.g. ascites, biliary stricture, liver function worsening), whereas 45% were gastrointestinal damage (e.g. bleeding or ulcer). Table 4 summarises the reported complications.
Table 4.
Complications according to treatment modalities.
| Author | Source | Subject of study | No. of patients (no. of tumours) | Complications of grade ≥3 |
|---|---|---|---|---|
| Shiozawa, 2015 | World J Gastroenterol | HCC | RFA 38, CyberKnife 35 | No late adverse effect in RFA 11.4% (4 cases of ascites, 2 of them liver-related death) in SBRT 1-yr CP score in SBRT higher than RFA group (p = 0.003) |
| Wahl, 2016 | J Clin Oncol | HCC | RFA 161, SBRT 63 | ≥G3 complication: RFA 11% vs. SBRT 5%. (p = 0.31) 2 G5 bleeding in RFA arms |
| Kim, 2019 | Radiother Oncol | HCC | RFA 668, SBRT 105 (before PSM) | 3.7% in RFA group had grade 3 or 4 toxicities no G ≥3 toxicity in SBRT arm, however RILD in 7 cases (6.7%) |
| Hara, 2019 | Hepatology | HCC | RFA 231, SBRT-HFRT 143 (before PSM) | One G5 peritonitis and 1 G5 gastric haemorrhage in RFA |
| Ji R, 2022 | Medicine | HCC | RFA 38 SBRT 22 | No severe (Clavien–Dindo ≥III) complication in both arms |
| Ueno, 2021 | J Gastrointestinal Oncol | HCC | RFA 62 SBRT 31 (after PSM) | No serious complication noted in both arms |
| Kim N, 2020 | J Hepatol | HCC | RFA 1568 SBRT 496 (before PSM) | No difference in grade 3–4 toxicity (2.6% vs. 1.6%, p = 0.268) CP score change of >2 points was higher in SBRT arm at 3 months (4.7 vs. 11.2%, p <0.001) but restored at 6 months (8.1% vs. 6.3%, p = 0.278) |
| Kim T, 2021 | J Hepatol | HCC | RFA 56 PBT 80 | G3 LFT increase (14.3%) and G3 bleeding (1.8%) in RFA arm No grade 3/4 Cx in PBT arm |
| Jeong, 2021 | HCC | HCC | RFA 172 SBRT 87 (after IPTW) | G4 haemorrhage in RFA arm (0.6%); G3 biliary stricture in SBRT arm (1.1%) |
| Stintzing, 2013 | Acta Oncol | CRC liver mets | RFA 30, CyberKnife 30 | No ≥G3 complication in both arms |
| Jackson, 2018 | Int J Radiat Oncol Biol Phys | CRC and other liver mets | RFA 69, SBRT 92 | ≥G3 complication: RFA 4.3% vs. SBRT 4.3% (p = ns) |
| Nieuwenhuizen, 2021 | Cancers | CRC liver mets | RFA 144, SBRT 55 | 6.3% in RFA arm (all procedure related damage) vs. 0 cases |
| Yu J, 2021 | Cancer Res Treat | CRC liver mets | RFA 178, SBRT 44 (after IPTW) | No G3 or higher complication in both arms |
CP, Child-Pugh; CRC, colorectal cancer; HCC, hepatocellular carcinoma; HFRT, hypofractionated radiotherapy; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LFT, liver function test; LT, liver transplantation; PSM, propensity score matching; RFA, radiofrequency ablation; RILD, radiation-induced liver disease; SBRT, stereotactic body radiotherapy.
Evidence grading review considering tumour location
Seven studies reported treatment efficacy related to tumour location in comparison with the 2 arms. Among them, 2 studies28,39 reported that difficult location was a factor affecting inferior LC in RFA arms, whereas it was not a factor in ablative RT arms. Kim et al.34 reported that SBRT showed better LC in the treatment of subphrenic and segment 8 tumours. Two studies40,42 reported that LC was higher in the SBRT arm, although the SBRT arm included more tumours in difficult locations. Two studies32,33 reported that although the majority of the SBRT arm included tumours in difficult locations, as different from the RFA arm, LC was higher or non-inferior. To summarise, all the above studies28,[32], [33], [34],39,40,42 consistently reported that SBRT could be more effective in the treatment of tumours in difficult locations, and 4 studies33,34,40,42 reported better LC with SBRT (Table 5) than with RFA. This corresponds to a moderate quality of evidence based on the grading system proposed by ASTRO (Table S1).
Table 5.
Complications and size considerations in treatment efficacy.
| Author | Subject of study | No. of patients (no. of tumours) | Consideration of size in treatment efficacy |
|---|---|---|---|
| Wahl, 2016 | HCC | RFA 161, SBRT 63 (tumours: RFA 249, SBRT 83) | Favouring SBRT with tumours > 2 cm (HR 3.35, p = 0.025), no difference in LC with tumours <2 cm (HR 2.50, p = 0.15) |
| Kim, 2019 | HCC | RFA 668, SBRT 105 | Favouring SBRT with tumours > 2 cm (HR 2.18, p = 0.012), no difference in LC with tumours ≤2 cm (HR 2.25, p = 0.061) (before PSM) |
| Jackson, 2018 | CRC and other liver mets | RFA 69, SBRT 92 (tumours: RFA 122, SBRT: 170) | Favouring SBRT with tumours > 2 cm (HR 3.54, p <0.01), no difference in LC with tumours <2 cm (HR 2.18, p = 0.4) |
| Yu J, 2021 | CRC liver mets | RFA 178 SBRT 44 | Favouring SBRT with tumours > 2 cm (HR 0.153, p <0.001), no difference in LC with tumours <2 cm (HR 0.648, p = 0.1) (IPTW cohort) |
| Kim N, 2020 | HCC | RFA 1568 SBRT 496 | >3 cm size related to inferior LC with RFA (HR 1.26, p = 0.030) of which was not related with SBRT (HR 1.01, p = 0.960) (before PSM) |
| Nieuwenhuizen, 2021 | CRC liver mets | RFA 144 SBRT 55 | >3 cm size related to inferior LC with RFA (p <0.001) of which was not related with SBRT (p = 0.361) |
| Kim T, 2021 | HCC | RFA 56 PBT 80 | All ≤3 cm in size LC 83.9/77.6% vs. 94.8/88.3% (RFA vs. SBRT) at 2/3 years (p = 0.123) |
| Hara, 2019 | HCC | RFA 106 SBRT-HFRT 106 | All ≤3 cm in size LC: 79.8% vs. 93.2% (RFA vs. SBRT) at 3 years (p <0.01) (PSM cohort) |
| Ueno, 2021 | HCC | RFA 62 SBRT 31 | All ≤3 cm in size LC 93/87% vs. 100/100% (RFA vs. SBRT) at 2/3 years (p = 0.024) (PSM cohort) |
| Jeong, 2021 | HCC | RFA 172 SBRT 87 | All ≤3 cm in size LC 90.6% vs. 96.3% (RFA vs. SBRT) at 4 years (p = 0.167) (IPTW cohort) |
| Moon, 2019 | HCC | RFA 529 SBRT 387 lesions | For ≤2 cm tumours, 1-year LC was 87 vs. 93.4%; for >2 cm tumours, 1-year LC was 71.4 vs. 84.8% (RFA vs. SBRT) |
| Location consideration in treatment efficacy | |||
| Kim, 2019 | HCC | RFA 668, SBRT 105 | Subphrenic location related to inferior LC with RFA (HR 1.53, p = 0.003), which was not related with SBRT (HR 1.00, p = 0.996) (before PSM) |
| Jeong, 2021 | HCC | RFA 172 SBRT 87 | Perivascular location related to inferior LC with RFA (4-year LC: 72% vs. 97%, <0.001), which was not related with SBRT (4-year LC: 94.7% vs. 95.5%, p = 0.872) (IPTW cohort) |
| Kim N, 2020 | HCC | RFA 1568 SBRT 496 | Favouring SBRT with subphrenic tumours (2-year LC 77% vs. 84.7%, p = 0.005 Favouring SBRT with segment 8 tumours (2-year LC 77.4% vs. 85.5% p = 0.014) (before PSM) |
| Hara, 2019 | HCC | RFA 106 SBRT-HFRT 106 | More tumours in difficult location (attaching organs) in SBRT arm (42% vs. 100%) LC: 79.8% vs. 93.2% (RFA vs. SBRT) at 3 years (p <0.01) (PSM cohorts) |
| Yu J, 2021 | CRC liver mets | RFA 178 SBRT 44 | More tumours in difficult location in SBRT arm (60.7% vs. 90.9%, p = 0.001) LC 58% vs. 76% (RFA vs. SBRT) at 3 years (IPTW cohort) |
| Ueno, 2021 | HCC | RFA 62 SBRT 31 | Vast majority of SBRT arm had difficult location compared with few difficult locations in RFA arm (p <0.001) Cumulative LC 90.3 vs. 100% (RFA vs. SBRT) (p = 0.024) (PSM cohort) |
| Ji R, 2022 | HCC | RFA 38 SBRT 22 | Majority in SBRT arm had difficult locations whereas there were no difficult locations for patients in RFA arm LC (overall): 94.7 vs. 90.6 (p = 0.566) |
CRC, colorectal cancer; HCC, hepatocellular carcinoma; HFRT, hypofractionated radiotherapy; HR, hazard ratio; IPTW, inverse probability of treatment weighting; LC, local control; PBT, proton beam therapy; PSM, propensity score matching; RFA, radiofrequency ablation; SBRT, stereotactic body radiotherapy.
Evidence grading review considering tumour size
Eleven studies reported treatment efficacy related to size consideration in the 2 arms. In 4 studies, LC did not differ between the arms in the treatment of tumours <2 cm in size, but SBRT was preferred with regard to LC in the treatment of tumours >2 cm in size.23,28,31,40 Two studies reported that tumour size >3 cm was related to inferior LC in RFA arm but not in the SBRT arm.34,38 Four studies included only patients with tumours ≤3 cm in size, and 2 of them reported no difference between the arms, while the other 2 reported better LC rates in SBRT arms.33,35,39,42 Moon et al.36 showed that the 1-year LC rates were 87% vs. 93.4% for tumours ≤2 cm and 71.4% vs. 84.8% for tumours >2 cm (RFA vs. SBRT). In summary, 5 studies23,28,31,36,40 consistently suggested more efficient LC of SBRT compared with RFA for larger tumours (>2–3 cm), and 9 studies23,28,31,[34], [35], [36],38,40,42 consistently suggested at least non-inferior LC of SBRT compared with RFA for smaller tumours (<2–3 cm) (Table 5). This corresponds to a moderate quality of evidence in the grading system judging the literature on HCC by ASTRO (Table S1).
Discussion
Brief review of literature
According to a recent meta-analysis, the survival outcomes of RFA were comparable to that of surgical resection among HCC patients within the Milan criteria.43 For liver metastases, RFA has been used in the treatment of patients with adverse clinical conditions as a lesser invasive surrogate with fewer complications.3 Regarding EBRT, the ablative role by precisely targeting and delivering a high dose of external radiation to localised lesions has emerged. SBRT, which delivers a high-dose of X-ray beams (70–100 Gy in equivalent dose, 2 Gy per fraction scheme [EQD2]) within 1–2 weeks, and precise radiotherapy which delivers particle beams at doses within the ablative range, have both yielded local control comparable to that of other ablative modalities.5,35,44 As 2 modalities have overlapping roles in treating localised intrahepatic malignancies, several researchers reported comparative oncologic outcomes.5,10 Notably, Kim et al.35 reported non-inferior local control in the treatment of small HCCs by delivery of proton beam therapy in ablative doses (91.3 Gy in EQD2) in a phase III randomised study. Certain studies based on a national database reported that RFA yields favourable survival outcomes compared with those of SBRT26; whereas no significant difference was noted in studies with matched cohorts.23,34 RFA is most efficient in treating tumours <2 cm in size6,7; however, it is less efficient in the treatment of tumours larger than 2–3 cm or for specific sublocation of the liver (e.g. subphrenic or perivascular sites), and may even pose a risk of complications.8,9 EBRT is less affected by the location of the tumour, and it is possible to deliver a sufficient radiation dose covering tumours >2–3 cm with clinical margins.10 Several investigators reported that SBRT showed higher local control when treating tumours >2–3 cm in matched cohort studies, and can be advantageous for treating tumours in difficult locations.34,40,42
Local control and survival
Our study reported that ablative RT has a better efficacy with regard to LC than RFA. Comparative OR was significant in the pooled analyses of all studies (OR: 0.458, p <0.001) and HCC studies (OR: 0.452, p <0.001). Similarly, the comparative OR was significant in the pooled analyses of all studies (OR: 0.466, p <0.001), HCC studies (OR: 0.421, p <0.001), and CRC metastasis studies (OR: 0.459, p = 0.006) with very low heterogeneity (I2: ∼0% in all above analyses), among studies with reliable comparability. Although not as rigid as the pooled analysis of randomised controlled trials, the pooled results of studies with reliable comparability have very low heterogeneity and are consistently valid, increasing the reliability of hypothesis testing.15 Although both RFA and ablative RT confer a high LC probability for small intrahepatic malignancies, RFA might yield suboptimal LC for tumours near major vessels or the diaphragm, or those exceeding 2–3 cm in size.9,[45], [46], [47] However, ablative RT is less limited by tumour location because it does not cause mechanical damage and can provide a prescribed dose within a relatively wide range.10,48 In clinical practice, ablative RT is considered less preferred than RFA and is applied more often in recurrent settings.23,33,37,39 In some studies, patients who underwent ablative RT had less favourable clinical parameters than those who underwent RFA.22,31,32,[36], [37], [38] Considering the above, we assume that the difference in LC between modalities was a result of the characteristics of the modalities rather than clinical differences.
Controversy has existed on the effect on survival, of selection between RFA and ablative RT. A study based on a national database reported favourable OS in the RFA arm.26 However, it had a limitation – the data of liver fibrosis were missing in nearly 70% of patients; furthermore, many of the factors used for propensity matching were social but not clinical factors (e.g. race, location of treatment facility, etc.). In a randomised study by Kim et al.,35 no difference in OS was reported between ablative proton therapy and RFA. There was no difference in OS between the RFA and SBRT arms in a study that involved propensity matching in approximately 2,000 patients from 5 countries.34 In the present study, pooled analyses did not reveal a comparative difference in the overall and subgroup analyses according to the primary disease (Table 2). The survival of HCC patients is affected by several clinical factors including liver function, biological profile, previous treatment, and local control.26,34,40 In addition, both treatment methods were effective, with a 2-year LC rate >80% in the pooled analyses (Table 3). Therefore, investigating OS differences based only on the selection of local modalities might be difficult. Although the pooled analyses and the majority of individual studies reported no significant difference, future randomised studies are needed to define the effect of selection between the 2 modalities on OS.
Feasibility considering tumour location and size
Overall grade ≥3 complication rates in the pooled analyses were <3% in both modalities (RFA 2.9%; ablative RT 2.8%, p = 0.952), indicating their feasibility. The characteristics of the complications were different between the 2 methods: most of the toxicities caused by RFA were caused by mechanical damage, whereas ablative RT mainly caused hepatic or gastrointestinal damage.
Because RFA mainly causes mechanical complications, tumour location significantly affects treatment safety and efficacy. Cao et al.49 reported a major complication rate of 10.7% after RFA for periportal HCCs, which was higher than that of non-periportal controls (5.1%). Kang et al.9 reported that aggressive intrasegmental recurrence occurred in 15% of periportal tumours after RFA because of thermal damage to the intrahepatic vessels. Song et al.50 reported 9.5% of peritoneal seeding after RFA for subphrenic tumours and a local tumour progression rate of 37.8% at 3 years. Lee et al.45 reported that the local recurrence risk was significantly higher after RFA for HCCs in the periportal location (hazard ratio [HR]: 2.29) and subphrenic location (HR: 2.25). The higher recurrence risk related to difficult locations and could be owing to suboptimal ablation to avoid possible complications or the heat-sink effect for perivascular tumours (i.e. ineffective thermal ablation hindered by blood flow).46,49,51
Ablative RT uses X-ray beams from multiple directions, which penetrate the body and accumulate in the target tumour.52 Because cell death by X-rays is biological death caused by DNA damage, direct mechanical or thermal damage does not occur.52,53 Normal organs have various radiation tolerances; the major vessel can tolerate radiation doses as high as ≥90 Gy in EQD2 clinically, and partial fibrosis does not alter blood flow.[54], [55], [56] Application of ablative RT to subphrenic tumours rarely induces pulmonary plural toxicities because radiation can cause partial fibrosis or atrophy but not rupture34,40,57; therefore, organ functions can be maintained. Therefore, ablative RT is often administered to tumours in locations where it is difficult to perform RFA. Several studies have reported that SBRT arms showed higher33,34,40,42 or similar LC32 although they had more target tumours in difficult locations. Similarly, Kim et al.28 and Jeong et al.39 reported that difficult locations (e.g. subphrenic or perivascular) were related to inferior LC after RFA, but not after SBRT.
As tumour size increases, it might have a biologically aggressive profile, and the presence of microinvasion or subclinical satellite nodules is frequent.58,59 For large tumours, it is difficult to secure sufficient ablative margins because of the possible risk of damage to the heat-sink effects in adjacent organs.58,60 For HCCs ≥3 cm in size, the local recurrence rate of RFA has been reported to be 30–50%.47 When ablative RT is applied to liver tumours, the blood vessels or bile ducts can tolerate the radiation dose required for treatment.44 Therefore, ablative RT could be performed with clinical margins covering subclinical disease for patients with preserved liver function and tumours with a distance of 1–2 cm from the small bowel, which is less affected by tumour size. Accordingly, in our systematic review, SBRT showed better LC for treatment of intrahepatic tumours >2 cm in size23,28,31,40; however, tumour size >3 cm showed inferior LC with RFA but not with SBRT.34,38 LC being similar between modalities for treatment of small tumours (<2–3 cm) was shown as well.33,35,36,39,42
In summary, ablative RT could be more effective than RFA for treatment of tumours in difficult locations or with relatively large sizes. However, ablative RT should be cautiously applied to tumours near the small bowel or in patients with impaired liver function.
Limitations and future perspectives
Because most of the included studies were non-randomised, heterogeneity in clinical and methodological aspects could not be entirely overcome. For example, when analysing the effectiveness of treatment modalities according to tumour size, the reference size was not constant among studies, and the statistical methods and effect measures were also different. The definition of difficult location was subjective, and only 1 study34 provided values of oncologic outcome according to specific location. In addition, heterogeneity in treatment outcomes could exist between previously treated and treatment-naïve tumours. Although most studies did not report segregated results, future studies are expected to report separate results to enable subgroup analyses. Regarding RFA arms, we included the studies with subject of thermal ablation including both RFA and MWA. Although the 2 modalities are similar in applying thermal damage to the tissue using a needle, MWA is reported to be effective for relatively larger tumours and is less affected by the heat-sink effect.61 MWA cases must be separated into subgroups and evaluated if the relevant literature increases.
Many clinical decisions inevitably rely on information obtained from observational studies, particularly in the field of oncology. To the best possible extent, we performed an evidence-grading review for possible subjective outcomes, quantitative analyses for major oncologic outcomes, subgroup analyses, and formal heterogeneity assessments. As sufficient information from randomised studies is lacking, integration of clinical outcomes through quantitative and qualitative meta-analysis could be an alternative route to help in clinical decision-making.62,63
Our study suggests that ablative RT can yield oncologic outcomes similar to that with RFA and that ablative RT can be more effective for tumours in locations where it is difficult to perform RFA or in cases of large-sized tumours. However, no standardised guidance exists to clarify the indications for RFA and ablative RT. Therefore, it is necessary to establish decision criteria for selecting an optimum modality between the 2, considering the efficacy and feasibility according to specific location, tumour size, and other clinical circumstances. As ablative RT has been commonly applied as salvage therapy, further studies are needed to compare the efficacy of the 2 modalities in recurrent and primary treatment settings. As a randomised study has been limited to only 1 that compared proton therapy and RFA, future randomised trials involving SBRT and RFA arms are warranted to obtain more robust conclusions.
Financial support
This study was supported by the Research Supporting Program of the Korean Association for the Study of the Liver and the Korean Liver Foundation. This study was supported by National Research Fund of Korea (NRF-2021R1I1A2047475).
Authors’ contributions
Conception and study design: JS. Data collection: CHR, JSL, SYK. Analysis: CHR. Drafting: CHR. Editing and supervision: JS. Final approval: all authors. The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability statement
Data are available within the article or the Supplementary materials.
Conflicts of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100594.
Supplementary data
The following are the supplementary data to this article:
References
- 1.European Association for the Study of the Liver et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Korean Liver Cancer Association National cancer center. 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Amerongen M.J., Jenniskens S.F.M., van den Boezem P.B., Fütterer J.J., de Wilt J.H.W. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases – a meta-analysis. HPB (Oxford) 2017;19:749–756. doi: 10.1016/j.hpb.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Rim C.H., Seong J. Application of radiotherapy for hepatocellular carcinoma in current clinical practice guidelines. Radiat Oncol J. 2016;34:160–167. doi: 10.3857/roj.2016.01970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J., Shin I.-S., Yoon W.S., Koom W.S., Rim C.H. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother Oncol. 2020;145:63–70. doi: 10.1016/j.radonc.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee H., Heo J.S., Cho Y.B., Yun S.H., Kim H.C., Lee W.Y., et al. Hepatectomy vs radiofrequency ablation for colorectal liver metastasis: a propensity score analysis. World J Gastroenterol. 2015;21:3300. doi: 10.3748/wjg.v21.i11.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Q., Kobayashi S., Ye X., Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. 2014;4:1–9. doi: 10.1038/srep07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee D.H., Kim J.W., Lee J.M., Kim J.M., Lee M.W., Rhim H., et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer. 2021;10:25–37. doi: 10.1159/000510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang T.W., Lim H.K., Lee M.W., Kim Y-s, Rhim H., Lee W.J., et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology. 2015;276:274–285. doi: 10.1148/radiol.15141215. [DOI] [PubMed] [Google Scholar]
- 10.Rim C.H., Lee H.Y., Kim J.S., Kim H. Radiofrequency ablation and stereotactic body radiotherapy for hepatocellular carcinoma: should they clash or reconcile? Int J Radiat Biol. 2021;97:111–119. doi: 10.1080/09553002.2021.1857453. [DOI] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 12.Peterson J., Welch V., Losos M., Tugwell P. 2011. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Assessed May 1st, 2022] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. 2021. Tools for assessing methodological quality or risk of bias in non-randomized studies; Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021)www.training.cochrane.org/handbook Cochrane. Available from: [Google Scholar]
- 14.JPT H., J T., J C., M C., T L., MJ P., et al. 2021. Chapter 24: including non-randomized studies on intervention effects; Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021)www.training.cochrane.org/handbook Cochrane. Available from: [Google Scholar]
- 15.Shin I.S., Rim C.H. Stepwise-hierarchical pooled analysis for synergistic interpretation of meta-analyses involving randomized and observational studies: methodology development. J Med Internet Res. 2021;23 doi: 10.2196/29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 17.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 20.Apisarnthanarax S., Barry A., Cao M., Czito B., DeMatteo R., Drinane M., et al. External beam radiation therapy for primary liver cancers: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12:28–51. doi: 10.1016/j.prro.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja C., Kharoti Y., Chadha M., McLarty J., Kaufman J.A., Farsad K., et al. Comparison of transarterial chemoembolization with radiofrequency ablation versus transarterial chemoembolization followed by stereotactic body radiation therapy for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014;37(2 Suppl. 1):S234. [Abstract] [Google Scholar]
- 22.Shiozawa K., Watanabe M., Ikehara T., Matsukiyo Y., Kogame M., Kishimoto Y., et al. Comparison of percutaneous radiofrequency ablation and CyberKnife® for initial solitary hepatocellular carcinoma: a pilot study. World J Gastroenterol. 2015;21:13490–13499. doi: 10.3748/wjg.v21.i48.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahl D.R., Stenmark M.H., Tao Y., Pollom E.L., Caoili E.M., Lawrence T.S., et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan X., Zhang T., Xie H., Sun J., He W., Xu H. Stereotactic body radiotherapy vs. radiofrequency ablation in the treatment of small hepatocellular carcinoma. Hepatology. 2016;64(1 Suppl. 1):653A–654A. [Abstract] [Google Scholar]
- 25.Feng M.U.-S., Marshall V.D., Parikh N., MVD Use of radiofrequency ablation and stereotactic body radiotherapy for the treatment of hepatocellular carcinoma: an analysis of the SEER-Medicare database. J Clin Oncol. 2016;34(4 Suppl. 1) [Abstract] [Google Scholar]
- 26.Rajyaguru D.J., Borgert A.J., Smith A.L., Thomes R.M., Conway P.D., Halfdanarson T.R., et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the National Cancer Database. J Clin Oncol. 2018;36:600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 27.Hara K., Takeda A., Tsurugai Y., Sanuki N., Saigusa Y., Maeda S., et al. Clinical outcomes after treatment for hepatocellular carcinoma, stereotactic body radiotherapy vs radiofrequency ablation: a propensity score-matched analysis. Hepatology. 2018;68(Suppl. 1):848A. [Abstract] [Google Scholar]
- 28.Kim N., Kim H.J., Won J.Y., Kim D.Y., Han K.H., Jung I., et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. 2019;131:81–87. doi: 10.1016/j.radonc.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Stintzing S., Grothe A., Hendrich S., Hoffmann R.T., Heinemann V., Rentsch M., et al. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncol. 2013;52:971–977. doi: 10.3109/0284186X.2013.766362. [DOI] [PubMed] [Google Scholar]
- 30.Vigano L., Pedicini V., Comito T., Carnaghi C., Costa G., Poretti D., et al. Aggressive and multidisciplinary local approach to iterative recurrences of colorectal liver metastases. World J Surg. 2018;42:2651–2659. doi: 10.1007/s00268-018-4525-x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson W.C., Tao Y., Mendiratta-Lala M., Bazzi L., Wahl D.R., Schipper M.J., et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018;100:950–958. doi: 10.1016/j.ijrobp.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji R., Ng K.K., Chen W., Yang W., Zhu H., Cheung T.-T., et al. Comparison of clinical outcome between stereotactic body radiotherapy and radiofrequency ablation for unresectable hepatocellular carcinoma. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000028545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno M., Takabatake H., Itasaka S., Kayahara T., Morimoto Y., Yamamoto H., et al. Stereotactic body radiation therapy versus radiofrequency ablation for single small hepatocellular carcinoma: a propensity-score matching analysis of their impact on liver function and clinical outcomes. J Gastrointest Oncol. 2021;12:2334. doi: 10.21037/jgo-21-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim N., Cheng J., Jung I., Der Liang J., Shih Y.L., Huang W.-Y., et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73:121–129. doi: 10.1016/j.jhep.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Kim T.H., Koh Y.H., Kim B.H., Kim M.J., Lee J.H., Park B., et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol. 2021;74:603–612. doi: 10.1016/j.jhep.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Moon A., Owen D., Kim H., Sanders E., Beyer C., McGinty K.A., et al. Multicenter cohort study of thermal ablation versus stereotactic body radiation therapy for hepatocellular carcinoma. Hepatology. 2019;70(suppl.1) 2019 70 (215A) Supplement 1. [Google Scholar]
- 37.Chen L., Hung S., Lin H., Lee M., Chiou W., Huang L., et al. Comparing radiofrequency ablation with stereotactic body radiotherapy for localized hepatocellular carcinoma: analysis of 108 cases from a single academic radiotherapy center. Int J Radiat Oncol Biol Phys. 2019;105:E216. [Google Scholar]
- 38.Nieuwenhuizen S., Dijkstra M., Puijk R.S., Timmer F.E., Nota I.M., Opperman J., et al. Thermal ablation versus stereotactic ablative body radiotherapy to treat unresectable colorectal liver metastases: a comparative analysis from the prospective Amsterdam CORE registry. Cancers. 2021;13:4303. doi: 10.3390/cancers13174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong Y., Lee K.J., Lee S.J., Shin Y.M., Kim M.J., Lim Y.S., et al. Radiofrequency ablation versus stereotactic body radiation therapy for small (≤3 cm) hepatocellular carcinoma: a retrospective comparison analysis. J Gastroenterol Hepatol. 2021;36:1962–1970. doi: 10.1111/jgh.15442. [DOI] [PubMed] [Google Scholar]
- 40.Yu J., Kim D.H., Lee J., Shin Y.M., Kim J.H., Yoon S.M., et al. Radiofrequency ablation versus stereotactic body radiation therapy in the treatment of colorectal cancer liver metastases. Cancer Res Treat. 2022;54:850–859. doi: 10.4143/crt.2021.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotohda N., Nomura S., Doi M., Karasawa K., Ohki T., Shimizu Y., et al. Clinical impact of radiofrequency ablation and stereotactic body radiation therapy for colorectal liver metastasis as local therapies for elderly, vulnerable patients. JGH Open. 2020;4:722–728. doi: 10.1002/jgh3.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara K., Takeda A., Tsurugai Y., Saigusa Y., Sanuki N., Eriguchi T., et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. 2019;69:2533–2545. doi: 10.1002/hep.30591. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q., Tang M., Zhang S. Comparison of radiofrequency ablation and surgical resection for hepatocellular carcinoma conforming to the Milan criteria: a meta-analysis. ANZ J Surg. 2021;91:E432–E438. doi: 10.1111/ans.16560. [DOI] [PubMed] [Google Scholar]
- 44.Rim C.H., Kim H.J., Seong J. Clinical feasibility and efficacy of stereotactic body radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Radiother Oncol. 2019;131:135–144. doi: 10.1016/j.radonc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Lee M.W., Kang D., Lim H.K., Cho J., Sinn D.H., Kang T.W., et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma <3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30(4):2391–2400. doi: 10.1007/s00330-019-06575-0. [DOI] [PubMed] [Google Scholar]
- 46.Lee S., Kang T.W., Cha D.I., Song K.D., Lee M.W., Rhim H., et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70–78. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y.S., Lim H.K., Rhim H., Lee M.W., Choi D., Lee W.J., et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Miften M., Vinogradskiy Y., Moiseenko V., Grimm J., Yorke E., Jackson A., et al. Radiation dose-volume effects for liver SBRT. Int J Radiat Oncol Biol Phys. 2021;110:196–205. doi: 10.1016/j.ijrobp.2017.12.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao S., Lyu T., Fan Z., Guan H., Song L., Tong X., et al. Long-term outcome of percutaneous radiofrequency ablation for periportal hepatocellular carcinoma: tumor recurrence or progression, survival and clinical significance. Cancer Imaging. 2022;22:2. doi: 10.1186/s40644-021-00442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song K.D., Lim H.K., Rhim H., Lee M.W., Kang T.W., Paik Y.H., et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol. 2019;11:227–237. doi: 10.4251/wjgo.v11.i3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Z.-Y., Li G.-L., Chen J., Chen Z.-W., Chen Y.-P., Lin S.-Z. Effect of heat sink on the recurrence of small malignant hepatic tumors after radiofrequency ablation. J Cancer Res Therapeut. 2016;12:153. doi: 10.4103/jcrt.JCRT_959_16. [DOI] [PubMed] [Google Scholar]
- 52.Rubio C., Morera R., Hernando O., Leroy T., Lartigau S.E. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. 2013;18:387–396. doi: 10.1016/j.rpor.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan F.M., Gibbons J.P. Lippincott Williams & Wilkins; Philadelphia, PA: 2014. Khan's the physics of radiation therapy. [Google Scholar]
- 54.Xue J., Kubicek G., Patel A., Goldsmith B., Asbell S.O., LaCouture T.A. Validity of current stereotactic body radiation therapy dose constraints for aorta and major vessels. Semin Radiat Oncol. 2016;26:135–139. doi: 10.1016/j.semradonc.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Rim C.H., Yoon W.S. Leaflet manual of external beam radiation therapy for hepatocellular carcinoma: a review of the indications, evidences, and clinical trials. OncoTargets Ther. 2018;11:2865. doi: 10.2147/OTT.S164651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans J.D., Gomez D.R., Amini A., Rebueno N., Allen P.K., Martel M.K., et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol. 2013;106:327–332. doi: 10.1016/j.radonc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sioshansi S., Rava P., Karam A., Ding L., FitzGerald T. Tolerance dose of the diaphragm with liver SBRT. Int J Radiat Oncol Biol Phys. 2014;90:S371. [Google Scholar]
- 58.Ke S., Ding X.M., Qian X.J., Zhou Y.M., Cao B.X., Gao K., et al. Radiofrequency ablation of hepatocellular carcinoma sized >3 and ≤5 cm: is ablative margin of more than 1 cm justified? World J Gastroenterol. 2013;19:7389–7398. doi: 10.3748/wjg.v19.i42.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeda K., Seki T., Umehara H., Inokuchi R., Tamai T., Sakaida N., et al. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol. 2007;31:485–491. [PubMed] [Google Scholar]
- 60.Dodd G.D., 3rd, Frank M.S., Aribandi M., Chopra S., Chintapalli K.N. Radiofrequency thermal ablation: computer analysis of the size of the thermal injury created by overlapping ablations. Am J Roentgenol. 2001;177:777–782. doi: 10.2214/ajr.177.4.1770777. [DOI] [PubMed] [Google Scholar]
- 61.Wang Z., Liu M., Zhang D.Z., Wu S.S., Hong Z.X., He G.B., et al. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3–5-cm HCC. Hepatology. 2022;76:66–77. doi: 10.1002/hep.32323. [DOI] [PubMed] [Google Scholar]
- 62.Frieden T.R. Evidence for health decision making—beyond randomized, controlled trials. New Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394. [DOI] [PubMed] [Google Scholar]
- 63.Shin I.-S., Rim C.H. Updating perspectives on meta-analyses in the field of radiation oncology. Medicina. 2021;57:117. doi: 10.3390/medicina57020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available within the article or the Supplementary materials.