Abstract
Inflammatory bowel disease (IBD) is characterized by the chronic inflammation of the gastrointestinal tract and impacts almost 7 million people across the globe. Current therapeutics are effective in treating the symptoms, but they often do not address the root cause or selectively target areas of inflammation. Notably, self-assembled nanoparticles show great promise as drug delivery systems for the treatment of IBD. Nanoparticles can be designed to survive the harsh gastric conditions and reach inflamed areas of the gastrointestinal tract. Oral drug delivery with nanoparticles can localize drugs to the impacted inflamed region using active and/or passive targeting and promote a high rate of drug dispersion in local tissues, thus reducing potential off-target toxicities. Since a dysregulated gut microbiome is implicated in the development and progression of IBD, it is also important to develop nanoparticles and biomaterials that can restore symbiotic microbes while reducing the proliferation of harmful microbes. In this review, we highlight recent advances in self-assembled nanosystems designed for addressing inflammation and dysregulated gut microbiomes as potential treatments for IBD. Nanoparticles have a promising future in improving the delivery of current therapeutics, increasing patient compliance by providing an oral method of medication, and reducing side effects. However, remaining challenges include scale-up synthesis of nanoparticles, potential side effects, and financial obstacles of clinical trials. It would be in the patients’ best interest to continue research on nanoparticles in the pursuit of more effective therapeutics for the treatment of IBD.
Keywords: Inflammatory bowel disease, Drug delivery, Self-assembly, Nanoparticles, Gut microbiome, Reactive oxygen species
Graphical Abstract
INTRODUCTION
Interest in finding treatments for inflammatory bowel disease (IBD) has been increasing in recent years, with the rise of big data helping to elucidate the complex interactions associated with the illness. Crohn’s disease (CD) and ulcerative colitis (UC), the two forms of IBD, are both disorders described by chronic inflammation of the gastrointestinal (GI) tract. While they often have similar symptoms, they have different clinical and pathological criteria. The shared symptoms include fatigue, loss of appetite, abdominal pain, cramping, diarrhea, blood in stool, and unintended weight loss.1 While the exact pathogenesis of IBD is unknown, potential causes of IBD include a dysregulated immune system, genetic predisposition, diet, and other environmental factors.2 Genome-wide association studies found that 163 genes are involved in IBD, 110 of which were shared by both CD and UC.3 Despite this, genetics are not the singular cause as a small study reported concordance of monozygotic twins to be 95.4% and 49.5% for CD and UC, respectively, while for dizygotic twins the percentages were 42.4% and 0% for CD and UC, respectively.4 Interestingly, environmental factors are also nonuniform.5 With this multifactorial etiology in mind, it is clear why the leading views like the hygiene hypothesis focus on the premise of how increasing worldwide standard of living decreases childhood exposure to pathogens, possibly preventing the typical development of the immune system.6,7
Patients with IBD often have periods of illness and remission, and their symptoms can vary in severity. There are many possible complications from IBD, including primary sclerosing cholangitis, eye/skin/joint inflammation, blood clots, and an increased risk of colorectal cancer. The manifestations of these diseases and their subsequent recognition are quite different. CD can present with small bowel disease, antisaccharomyces cerevisiae antibodies, and granulomas, which are specific findings that help distinguish the disease. UC is typically associated with rectal inflammation, antineutrophil cytoplasmic antibodies, and crypt abscesses.2 While specific complications from CD include malnutrition, bowel obstructions, fistulas, and anal fissure, complications from UC include severe dehydration, a perforated colon, and a toxic megacolon.8
IBD has been increasing in prevalence in the industrialized world. Some 1.6 million Americans are diagnosed with IBD, with 80 000 of those cases being children and 70 000 new diagnoses every year.9,10 People living in industrialized countries have an increased risk of developing IBD, and this is thought to be related to key factors such as a Western diet with increased consumption of fat and refined food.2 Globally, the number of cases of IBD has increased dramatically from 3.7 million to over 6.8 million between 1990 and 2017, an increase of 85.1%.11 Therefore, there is an urgent need for new therapeutic approaches against IBD. While developed nations have the greatest incidence of IBD, emerging countries have seen an increase in the incidence of IBD as they have become more industrialized.12–14 Interestingly, studies have shown that IBD can lead to a relative lack of diversity in the microbiome, possibly leading to unchecked bacterial proliferation of pathobionts and even barrier infiltration, prompting tissue destruction from the resulting immune-mediated inflammatory response.15–20 With the pathogenesis of IBD still unclear, it remains to be seen whether specific microbes are responsible for the disease or if a dysregulated microbiome is a downstream effect. Regardless, the symbiotic relationship that exists between the gut flora and the host clearly has repercussions on the wellbeing of people and could be a cornerstone to the future of IBD treatment.
The current standard of care for IBD includes anti-inflammatory drugs such as corticosteroids and aminosalicylates that help suppress symptoms caused by the immune response. Systemic corticosteroids are highly efficient at treating inflammation but carry a heavy side effect profile. This class of drugs often targets inflammation by neutralizing inflammatory mediators and stimulating the production of anti-inflammatory molecules.21 Aminosalicylates work through the scavenging of reactive oxygen species (ROS) and antioxidant effects via immobilizing leukocytes. They also upregulate endogenous antioxidant effects, helping lessen downstream effects.22,23 Immune suppressors are used in conjunction with anti-inflammatory drugs to help and prevent the damage that occurs from the malfunctioning immune response. For patients with moderate to severe IBD, monoclonal antibody therapies targeted against inflammatory cytokines are given through intravenous (IV) infusions or injections. These biologics for IBD have grown as a more prominent form of treatment ever since Remicade was approved by the Food and Drug Administration (FDA) in 1998.7 However, these biologics aiming to block cytokines can make patients more susceptible to infection, so this class of drugs is clinically recommended as a therapeutic for people who are unresponsive to traditional medications.8 While there is a significant array of treatments available for IBD patients, they may no longer be effective after an extended amount of time because of system tolerance, requiring patients to use other medications.9 In severe cases, some patients have little to no remission from any of the therapeutics available on the market, significantly decreasing their quality of life and increasing the chances of serious complications. Some patients must resort to costly, life-altering surgeries, and this adds to the medical expenses. In the US alone, it was estimated that the annual financial burden of IBD patients was $14.6–31.6 billion in 2014.10 On a per-patient basis, it costs about $10,364 per year for someone with CD, which is more than the cost for people with chronic obstructive pulmonary disease, coronary artery disease, multiple sclerosis, stroke, or diabetes.11 Thus, more research is needed to develop more cost-effective treatments along with better end results.
In this regard, nanoparticles (NPs) show great promise for potential treatment against IBD. NPs are a class of nanomedicine that can undergo dynamic changes in structural properties, which affect how they interact with their surroundings. They often use bonding and chemical reactions to facilitate changes to their structure at a desired site for drug release. In addition, self-assembled NPs often mimic cellular behaviors. For peptide self-assemblies, the ingredients are simple amino acids formed by widely available chemicals, making their scale-up process economical24 and applicable for oral delivery.25 With advancements in technology, targeted delivery systems can become even easier to make. There are abundant opportunities within this field to improve delivery systems, tissue healing, and even modulation of organ behavior.26,27 Additionally, the bottom-up production approach is easily modifiable, similar to the delivery system of COVID-19 mRNA vaccines. Making specific changes for individualized care or specifying targets can be simple and cost-effective.28 There are numerous applications in medicine and nanobiotechnology, providing a positive outlook for many chronic illnesses.
Nanoparticles can be produced in a short step synthesis process and often use polyethylene glycol (PEG) and polycaprolactone (PCL), which are polymers used in FDA approved products.29 These molecules have high drug loading capacities. The inner fold can be filled with minimal effects on the delivery of the drug or the behavior of the molecule, facilitating the development of both an efficacious and safe delivery system.30–32 Their long polymeric chains can protect the cargo against immune cells or antibodies prior to reaching their activation point.
An important aspect of this research is the development of mouse models to evaluate the therapeutic effects of drugs against IBD. Dextran sulfate sodium (DSS) induced colitis in mice is currently the most common model for research on this chronic disease.33 DSS is a sulfated polysaccharide that is toxic to the intestinal epithelial cells. DSS added in drinking water impedes the functions of the small intestines and colonic barrier and induces an immune reaction similar to IBD. Clinical correlations often observed in these mice are reduced colon lengths, absorption difficulties, malnutrition, and blood in the stool. DSS-inflamed tissue also triggers immune responses typically seen in IBD,34 including local infiltration of neutrophils, macrophages,35 and lymphocytes.36 It is notable that while DSS-induced colitis is an acute model of IBD and phenocopies some of its pathogenic features, it does not mimic the chronic inflammation present in IBD.
Oral drug delivery is attractive because of the many benefits this route provides. Drugs taken in pill form are generally cheaper for patients as they require no additional equipment or help to be administered. It comes with the added benefit of not needing to set aside time to receive IV infusions or subcutaneous injections in their treatment course, resulting in higher patient compliance because of the convenience and painless application.37 One of the greatest advantage of the oral delivery route comes from not having to use a needle. A recent study estimated that an astonishing 6.7 billion injections are given per year using reused equipment, mostly in resource-poor countries.38 That puts individuals at risk for chronic bloodborne infections, such as Hepatitis B, Hepatitis C, and HIV. In fact, the World Health Organization estimated that in the year 2000, the usage of dirty needles contributed to 33%, 40%, and 5% of new infections in those respective diseases worldwide.39 Giving the option for patients in third world countries to receive cheaper and safer healthcare for their IBD can be lifesaving.
Oral drug delivery systems have to circumvent the harsh conditions of the stomach in order to reach the target tissue in the intestines. The best way to overcome these difficulties is often with a pH self-assembly that can help adapt to the surrounding systems when it reaches target conditions.27 A nanoparticle system that is responsive to a certain physiological environment can properly colocalize to the necessary area. The configuration of self-assembled NPs is reversible, so it is important that the structure and/or payload remains intact before reaching the desired target area. There are noticeable differences between pH-dependent delivery systems and ROS-targeting nanoparticles, and it is important to understand how artificial self-assemblies behave.27,40 Moreover, nanoparticles can be delivered to specific tissues guided by external stimulations, such as ultrasound. The multitude of ways of delivering nanoparticles to specific tissues will help produce better results not only for IBD treatment but for other drug delivery applications as well.40
While oral drug delivery is generally the preferred route, the effectiveness of the treatment is the most important factor for drug development. Injections and enemas provide alternatives for delivery that circumvent the need to design nanoparticles that can survive the harsh conditions of the GI tract to reach the site of inflammation. However, this comes with the risk of blood borne pathogens and reliance on appropriate clinics to distribute care with additional cost. Again, physicians must weigh not only the effectiveness of the treatment but also the lifestyle of the patient to make better decisions for the treatment process. Table 1 summarizes the articles covered in this review, including the different delivery routes used.
Table 1.
Overview of Key Features of the Nanoparticles and Therapies Covered in the Review
| Name | Mechanism of Action | Type of Targeting | In Vivo Model | Delivery Route | Therapeutic Components | Ref |
|---|---|---|---|---|---|---|
| Budesonide-aromatized thioketal-tempol (B-ATK-T) | ROS | Active, passive | DSS mice | Oral | Budesonide (anti-inflammatory) | 41 |
| Tempol (antioxidant) | ||||||
| Polymers paired polyphenol nanoparticle (PPNP) | ROS | Active, passive | DSS mice | Oral | Corticosteroid dexamethasone DEX (anti-inflammatory) | 42 |
| Tannic acid (mucoadhesive, antioxidant) | ||||||
| Tempol-loaded β-cyclodextrin-derived material (OxbCD) | ROS | Active, passive | DSS mice TNBS mice | Oral | OxbCD (eliminates hydrogen peroxide) | 43 |
| Tempol (antioxidant) | ||||||
| anti-TNF-α antibody carrier | ROS | Active, passive | DSS mice | Oral | Tannic acid as therapeutic carrier (mucoadhesive, antioxidant) | 44 |
| anti-TNF-α antibody (anti-inflammatory) | ||||||
| Rosmarinic acid nanoparticles (RANPs) | ROS | Passive | DSS mice | IV injection | Rosmarinic acid (antioxidant, anti-inflammatory, antibacterial, anticancer) | 45 |
| Can be loaded with DEX (corticosteroid, anti-inflammatory) | ||||||
| Vasoactive intestinal peptide-sterically stabilized micelle (VIP-SSM) | Carrier | Passive | DSS mice | Intraperitoneal injection | VIP (anti-inflammatory hormone) | 46 |
| Inflammation-targeting hydrogel (IT-hydrogel) | Carrier | Passive | DSS mice TRUC mice | Enema | Can be loaded with DEX (corticosteroid, anti-inflammatory) | 47 |
| Carboxymethyl inulin (CMI) NPs | Carrier | Active | DSS mice | Oral | No therapeutics (testing uptake in active macrophages for future targeted drug delivery or imaging applications) | 48 |
| Poly(lactic-co-glycolic acid)-folic acid-resveratrol (PLGA-FA-RSV) | ROS | Passive | TNBS rats | Oral | Resveratrol (antioxidant, anti-inflammatory) | 49 |
| Sulfasalazine polyethylene glycol (S-PEG) | Carrier | Active, passive | In vitro only (HEK 293) | Designed for oral | Sulfasalazine (common IBD drug, anti-inflammatory) Ornidazole (antibiotic) | 50 |
| Hyaluronic acid-bilirubin (HABN) | ROS, microbiome | Active, passive | DSS mice | Oral | Hyaluronic acid (immunomodulatory)Bilirubin (ROS-scavenging, antioxidant, cytoprotective) | 51 |
| Ac2-26-loaded OxbCD (AON) | ROS, microbiome | Active, passive | DSS mice | Oral | OxbCD (eliminates hydrogen peroxide) | 52 |
| Ac2-26 (annexin peptide that inhibits inflammatory responses) | ||||||
| Ginger exosome-like nanoparticles (GELNs) | Microbiome | Passive | DSS mice | Oral | miR7267–3p (promotes IL-22 expression, gut barrier function) | 53 |
| Amyloid-templated epigallocatechin gallate (EGCG) hydrogel | Microbiome | Passive | DSS mice | Oral | EGCG (antioxidant, anti-inflammatory) | 54 |
| Lipid membrane coated bacteria (LCB) | Microbiome | Passive | DSS mice STm mice | Oral | Probiotic Escherichia coli Nissle 1917 (EcN) | 55 |
NANOPARTICLES ENGINEERED FOR ROS TARGETING
As previously discussed, the pathogenesis of IBD is not yet fully understood. However, a few factors have been identified that may reduce inflammation and lead patients to remission. One of the targets for treatment is ROS, which is mainly composed of peroxide, superoxide, and hydroxyl radicals. ROS are thought to regulate oxygen homeostasis and cellular signaling, making them necessary for cells to function normally.56 However, overproduction of ROS has been shown to be involved in the development and pathogenesis of inflammatory diseases.57 Higher concentrations of ROS in IBD leads to increased mucosal membrane permeability, inflammatory response, and ulceration.58,59 Patients with IBD have been shown to have 10–100 times higher concentrations of mucosal ROS, thus making ROS an attractive target.60 While there have been a few research studies showing the promise of targeting ROS in IBD,61 treatments have not yet reached the market because of their nonspecificity toward inflamed sites, instability within the GI tract, and inability to neutralize ROS in local tissues.43 Recently, however, some nanoparticle drug designs have shown the potential to be applicable clinically and will be covered in this section of the review.
One potential therapy, a B-ATK-T nanoparticle prodrug, aims to treat IBD through the self-assembly of a nanoparticle that incorporates both budesonide (B), an anti-inflammatory drug, and tempol (T), an antioxidant (Figure 1a).41 This nanoparticle was synthesized by reacting aromatized thioketal (ATK) with budesonide to form B-ATK, which was subsequently reacted with tempol to form B-ATK-T. The nanoparticle had a 41% and 16% loading capacity for budesonide and tempol, respectively. The designed thioketal bonds between the drugs were effective in releasing the drugs with ~55–100% rates of hydrolysis, depending on the level of ROS, whereas BT/poly(lactic-co-glycolic acid) (PLGA) NPs showed no hydrolysis. The nanoparticle was tested for its responsiveness to ROS, including hydrogen peroxide, hypo-chlorite, hydroxyl radical, superoxide anion, and peroxynitrite. The prodrug only reacted upon the exposure to overproduced ROS species, leading to the disassembly of the nanoparticle and release of budesonide and tempol at the site of inflammation in the colon. This effectively increased the drug concentrations at the desired sites and decreased undesired systemic effects. These nanoparticles remained stable in simulated intestinal and gastric fluid. When evaluated in vivo on mice with DSS induced colitis, daily oral administration of B-ATK-T NP exerted robust therapeutic efficacy, as shown by significant improvement in the histological scores of colonic tissues. B-ATK-T NP also reduced weight loss while improving disease activity index (DAI) and colon length of the DSS-induced mice, as shown in Figure 1b. Mice treated with B-ATK-T NP did not exhibit any major toxicity as shown by normal major organs and blood chemistry.
Figure 1.
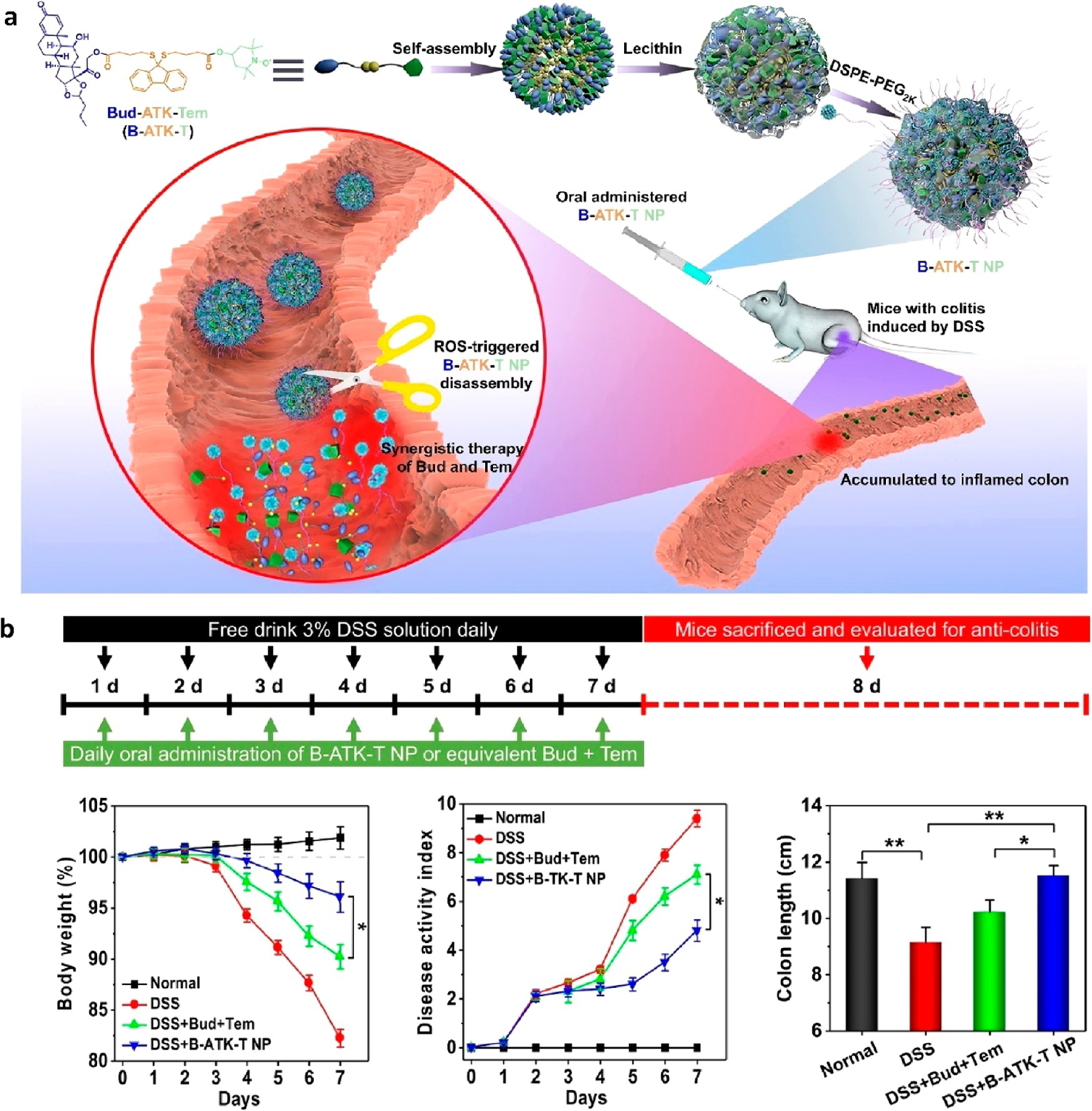
(a) Overview of B-ATK-T nanoparticle assembly and mechanism of release at site of inflamed colon. (b) DSS mouse treatment regimen and efficacy data. All groups were given 3 wt % DSS except for the normal control, which was given purified water. B-TK-T NP indicates the prodrug, while Bud + Tem is the unconjugated control. *p < 0.05, **p < 0.01. Reprinted with permission from Li et al.41 Copyright 2019 Elsevier.
Another research group engineered a self-assembled supramolecular nanoparticle using natural polyphenols (tannic acid, catechin, epigallocatechin gallate), which are considered Generally Recognized as Safe (GRAS) compounds by the US FDA.42 The benefit of using these polyphenols is that they have strong antioxidant and radical-scavenging activity through their inhibition of ROS-generating enzymes and reducing ROS, and have been applied to IBD treatment in the past. Tannic acid (TA) is a degradable mucoadhesive polyphenol, which allows for targeted drug delivery to the colon. Additionally, upregulation of esterases occurs when there is inflammation, and because polyphenols can be hydrolyzed by these enzymes, it provides a targeted approach to the colon. The nanoparticles were prepared using PEG, poloxamer 407 (F-127), and poloxamer 188 (F-68), which are considered safe by the US FDA. The polymers were each paired with a polyphenol and assembled into nanoparticles, termed PPNP. The polydispersity index (PDI) of TA with F-68 had a PDI lower than 0.05, which is the lowest of all pairings, indicating that it can provide the PPNP with the highest degree of uniformity and smallest diameter. The PPNP was then loaded with dexamethasone (DEX), which is commonly used for colitis treatment. The resulting PPNP-DEX had a drug encapsulation efficiency of ~23% and weight ratio of ~35%. Testing of PPNP-DEX in simulated gastric and intestinal fluid suggested that only 10% of DEX would be released before reaching the intestines, and 30% released thereafter. However, when the colitis environment was simulated by adding esterase, the amount of DEX released rose to 62%, indicating responsiveness of PPNP-DEX to models of inflamed tissues. Esterase enzymes are not typically localized but are indicative of a generalized inflammatory response. This increase in effectiveness was demonstrated in DSS-induced colitis mice. A dye encapsulated in PPNP was given to healthy and colitis mice, and the fluorescence intensity in colitis mice was about 3, 2, and 2 times greater than healthy mice at hours 6, 12, and 24 h, respectively. After PPNP-DEX treatment, mice with DSS-induced colitis showed an increase in body weight, a decrease in TNF-α levels, and reduced colonic myeloperoxidase activity.
Another approach for decreasing ROS was taken by mimicking the body’s natural enzymes. Superoxide dismutase (SOD) turns the superoxide into hydrogen peroxide, which can become a hydroxyl radical through reduction or water through decomposition by catalase.43 They utilized the free radical scavenger tempol as the payload and β-cyclodextrin-derived material (OxbCD) for drug loading, serving as SOD and catalase mimics, respectively. They achieved a loading capacity of approximately 5.9% for tempol. Using 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-PEG, these materials were then self-assembled into nanoparticles which are stable through the GI tract. When there are high levels of ROS, the nanoparticles can release tempol at the inflammation site. The resulting nanoparticles were delivered orally and preferentially targeted the inflamed colon because of the increased binding interaction and epithelial permeability. The nanoparticles showed efficacy in both TNBS and DSS-induced mice and decreased inflammatory cytokines and disease index with good toxicological results. Notably, the nanoparticle treatment was more effective compared with tempol/PLGA NPs or free tempol. While this system is designed for tempol, it may also be applicable for delivering nucleic acids, peptides, and proteins, making it a promising alternative for current treatments.
Monoclonal antibodies have been used as treatment of IBD for over two decades, and the market has been continually growing. However, these treatments require injections, leading to lower levels of patient compliance, and they are typically more expensive than oral therapeutics. Moreover, delivery through injection leads to higher levels of systemic exposure and cytotoxicity, thus increasing side effects. Researchers designed a self-assembled nanoparticle that can encapsulate infliximab (INF) antibody using tannic acid and PEG for successful oral delivery.44 Tannic acid (TA), a natural polyphenol, was mixed with a PEG-containing polymer (DSPE-PEG2K, PEG10K, or Pluronic F68) to form nanoparticles. TA was chosen because of its ability to form hydrogen bonds with macromolecules from its catechol and galloyl groups, making it ideal for the encapsulation of the antibody. This strategy is significant because it uses aqueous self-assembly as opposed to organic solvents. Thus, potential toxicity of organic solvent can be avoided and scale-up becomes simpler and more practical. These polyphenol-PEG-containing polymers self-assembled into nanoparticles (termed PPCNPs) with drug loading efficiency greater than 90%, making them an effective encapsulation strategy. The nanoparticles decreased in size once exposed to the ROS, allowing for the release of the antibodies. Upon oral administration of INF-loaded PPCNPs, the concentration of INF was much higher in inflamed regions of the colon of DSS mice compared with that in healthy controls, demonstrating the dynamic responsiveness of the nanotherapy. Notably, IV administration of INF resulted in 4.4, 5.5, 14.6, 29.4, and 30-times higher levels of INF in the lung, heart, kidney, spleen, and liver, respectively, compared with those after oral administration of INF-loaded PPCNPs. This demonstrates the potential of nanoparticle-mediated oral delivery to reduce off-target toxicity of drugs. In addition, oral treatment with INF-loaded PPCNPs produced significant better results than free INF treatment in terms of colon length, disease activity, myeloperoxidase activity, and proinflammatory cytokines (i.e., TNF-α, IL-1β. and IL-6). Overall, this self-assembled nanoparticle carrier has shown promising results in reducing inflammatory response with reduced systemic effects and has the potential to transition the biologics market to adopt oral delivery of antibodies.
Rosmarinic acid (RA), a polyphenol-based antioxidant, has shown potential as a promising therapeutic because of its anticancer, antibacterial, and anti-inflammatory properties. However, RA is difficult to administer because of its low natural availability and water solubility. To address these issues, researchers have used one-step amide coupling to react RA with mPEG2X·NH2 and form PEGylated RA, which then self-assembled into spherical nanoparticles, termed RANP (Figure 2a).45 RANP nanotherapy demonstrated similar ROS-scavenging properties as RA for hydrogen peroxide, and RANP was effective at protecting CHO-K1 cells from H2O2-induced cytotoxicity. RANP exhibited high colloidal stability, and after intravenous administration, RANPs showed at least a 4 times larger elimination half-life, mean residence time, and maximal concentration compared with free RA. In a murine DSS-induced colitis model, RANP administered intravenously was localized to the inflamed colon and outperformed their parent RA, leading to lowered colon inflammation, disease activity scores, body weight loss, and pathological damage (Figure 2b). RANP also addressed the pathophysiology of the disease by limiting the secretion of pro-inflammatory cytokines within the digestive system. Finally, DEX-loaded RANP was shown to be more effective at decreasing the DAI than RANP alone. This proof-of-concept study shows that RANP has potential to work as a treatment against IBD and carrier for other nanotherapies.
Figure 2.
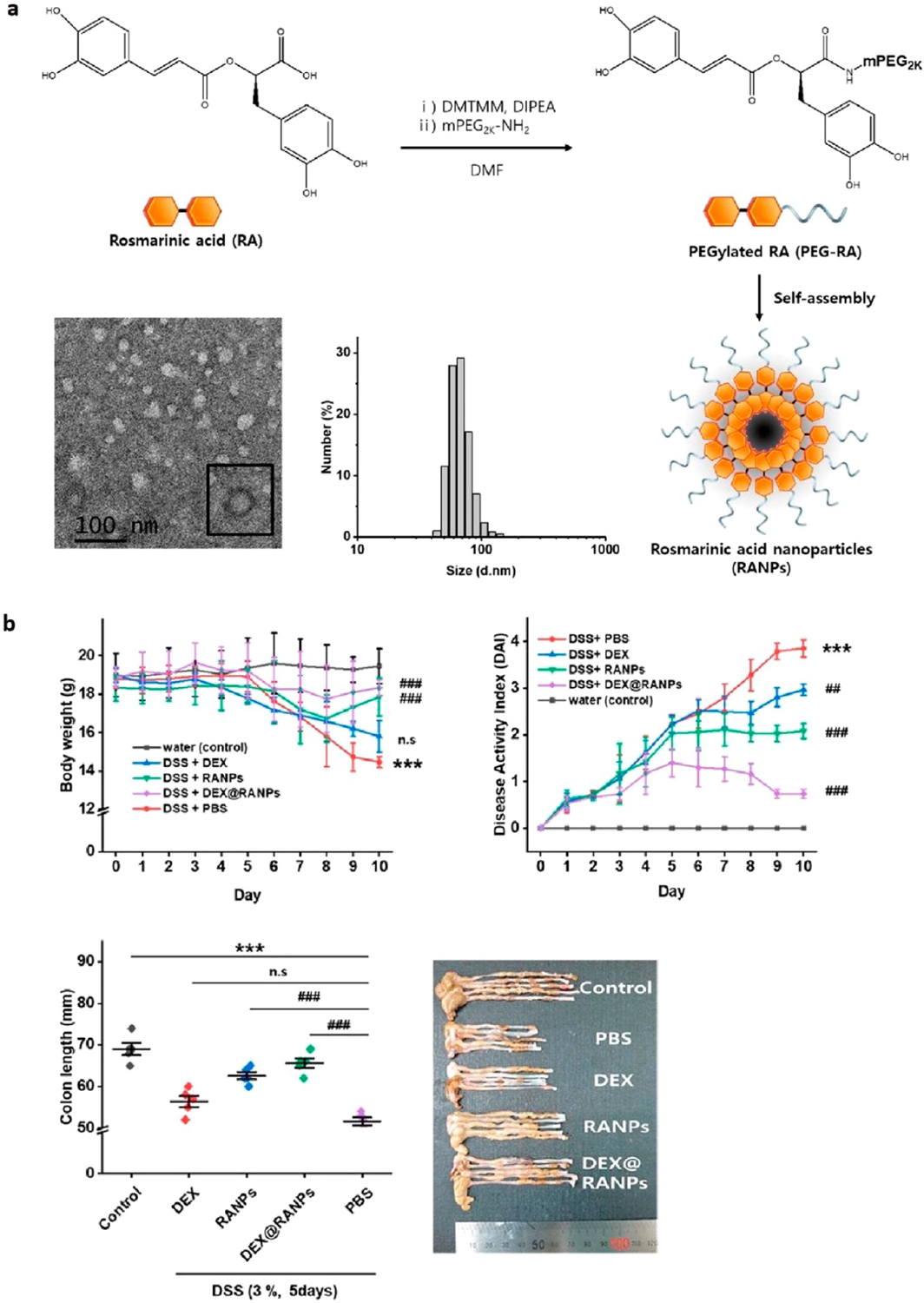
Overview of (a) RANP synthesis and structure. A transmission electron microscopy (TEM) image of the nanoparticle with an average diameter of 67.5 nm is shown along with the dynamic light scattering size distribution. (b) Efficacy on DSS-induced colitis mice for different experimental groups: control (water), phosphate buffered saline (PBS) control (DSS + PBS), free DEX control (DSS + DEX), RANPs as carrier control (DSS + RANPs), and DEX-loaded RANPs (DSS + DEX@RANPs). All mice (except the water control) were given 3 wt % DSS for 5 days before being sacrificed on day 10. All mice (except the water control) were treated with the indicated formulations on odd days. Modified and reprinted with permission from Chung et al.45 Copyright 2020 American Chemical Society.
NANOPARTICLES ENGINEERED AS DRUG CARRIERS
While ROS-targeting can be an effective strategy for IBD treatment, the field can also benefit from innovative drug carriers that are capable of utilizing drugs that have already been FDA approved. Existing treatments for IBD lack the localization needed to prevent their systemic side effects, and the use of nanoparticles as drug carriers for the existing drugs and new novel treatments is the imminent future of nanotechnology. For example, studies have shown that in IBD, the mucosal layer is thinned, exposing mucus-secreting goblet cells.62 This makes mucoadhesive nanoparticles an attractive option because of the increased penetrability. Delivery of nanoparticles to the other layers of the mucosa like the lamina propria as well as microbiota are being explored as well.63 The localized delivery may reduce the dosage and dosing frequency64 and may lead to a lessened toxicokinetic profile,65 thus improving patients’ quality of life.
Vasoactive intestinal peptide (VIP) has been shown to be effective because of its anti-inflammatory activity in animal models for CD,66,67 multiple sclerosis,68,69 rheumatoid arthritis,70–72 and septic shock.73,74 It has also been shown to be immunomodulatory to the adaptive and innate immune systems. However, in UC, there has been varied responses reported, so researchers designed a sterically stabilized micelle (SSM), which is a lipid-based nanoparticle that can incorporate VIP, an amphiphilic peptide, into its palisade layer, termed VIP-SSM.46 With a mean particle size of 15 nm, this nanoparticle can protect VIP from degradation, reducing hypotensive toxicity and increasing targeting. When tested on DSS-induced colitis mice, both free VIP and VIP-SSM increased the expression of occludin, a key tight junction protein, suggesting restoration of intestinal epithelial integrity against colitis. While both VIP and VIP-SSM improved histology, proinflammatory cytokine, and antidiarrhea in preventative studies, only VIP-SSM showed those results in a therapeutic study. In addition, VIP-SSM reduced protein and mRNA expression of down-regulated in adenoma (DRA), a key chloride bicarbonate exchanger in the colon responsible for colitis-induced diarrhea. Notably, free VIP given frequently increased toxicity whereas SSM removed any unfavorable side effects. While injections were used in this study, the research group is currently working to develop an oral formulation for VIP-SSM which has shown promise in in vitro release experiments.75
An alternative delivery approach for treatment is through enemas, which researchers have utilized to deliver therapeutics using amphiphile ascorbyl palmitate (AP), designated as a GRAS material by the US FDA, to self-assemble into a hydrogel carrier for therapeutics.47 Their inflammation-targeting hydrogel, IT-hydrogel, was effective at encapsulating hydrophobic drugs with a maximum drug loading and encapsulation efficiency of approximately 14% and 75%, respectively. Because of the negative surface charge of the hydrogel, IT-hydrogel targeted and adhered to inflamed mucosa. IT-hydrogel releases its payload after degradation by hydrolytic enzymes prominent in inflamed regions. The hydrogel was also stable for 16 days at 37 °C in PBS and did not release loaded DEX, demonstrating its stability. Compared with free DEX, the hydrogel with DEX significantly reduced cumulative systemic drug absorption while improving histopathology score, colon weight, and TNF expression. Using human biopsies, IT-hydrogel was found to adhere better on inflamed sites of UC patients. IT-hydrogel loaded with DEX and administered through enema has been shown to decrease the systemic exposure to DEX and increase its efficacy in DSS and TRUC-induced colitis mice models. As IT-hydrogel is composed of a GRAS material and requires a simple synthesis process that can easily be scaled up, it is an ideal candidate as a delivery system for other IBD treatments.
One study utilized inulin, a polysaccharide that can remain intact through the stomach’s acidic environment while also being digested by inulinase and is found in the colon.48 To prepare this, mannosylated nanoparticles were self-assembled and attached to carboxymethyl inulin (CMI), with side chains containing carboxyl groups. The mannose backbone serves as a ligand that is recognized by mannose receptors found on the surface of macrophages. This exclusivity ensures uptake by the inflammatory cells rather than nontargeted cells within the colon. Using apremilast, a TNF-α inhibitor, the researchers achieved a drug loading efficiency of 16% with encapsulation efficiency of 44%. In vivo analysis showed that in DSS-induced colitis mice, 60% of the nanocomposite material accumulated in the inflamed colon. With a size of 240 nm and a drug loading of 26% weight percent, this nanoparticle system is suitable for macrophage-targeted drug delivery.
Another study reported a drug delivery system using PLGA NPs as the carrier and resveratrol (RSV) for treatment.49 PLGA nanoparticles have been shown to improve diffusion on inflamed intestinal areas because of the size and charge. PLGA nanoparticles can enhance the ability of insoluble drugs to survive oral administration, and PLGA is used in FDA-approved products. When treated with folic acid (FA), PLGA nanoparticles formed the outer shell. Resveratrol is an antioxidant and anti-inflammatory agent, which has a high efficiency of loading within the PLGA-FA complex of 59% and 91% for PLGA-RSV alone. PLGA nanoparticles treated with FA were shown to maintain more resveratrol through gastric conditions. The in vitro and in vivo experiments were successful with the former showing the best permeability with the aforementioned PLGA-FA-RSV compound. The in vivo experiments were done on TNBS-treated rats and also compared to Asacol, a drug typically used for moderate UC. The colons of the rats treated with PLGA-RSV, PLGA-FA-RSV, and FDA-approved Asacol were comparable to those of healthy rats. Unlike Asacol and free resveratrol, which caused 5% and 17% body weight loss, the other two treatments had no observable body weight loss or issues with colon length. PLGA-RSV and PLGA-FA-RSV were effective against colitis progression while lowering the immune system reactions and maintaining normal villi, submucosal, and lamina layers as in healthy rat intestines. On the other hand, Asacol therapy resulted in moderate immune cell accumulation. Overall, this showed the promise of the PLGA-FA system for delivering RSV as well as other compounds.
Sulfasalazine is a common drug used to treat IBD,76 and ornidazole is an antibiotic often used after ileocolonic resection to prevent CD relapse.77 Researchers have developed a more efficient delivery method by conjugating sulfasalazine with low molecular weight PEG to make S-PEG, an amphiphilic conjugate.50 This resulted in the self-assembly of a micellar nanostructure with a hydrophobic cavity that allowed for the uptake of hydrophobic drugs and a hydrophilic outer surface for increased solubility (Figure 3). The maximum drug entrapment and loading efficiency for S-PEG loaded with sulfasalazine was 60.3% and 6.4%, while those for ornidazole were 29.9% and 3.1%, respectively. These nanoparticles contained an azo-moiety that can be cleaved by azoreductase, an enzyme naturally produced by the gut microbiome. The sulfasalazine conjugate also allowed for a targeting mechanism from the chemical reductant, sodium dithionite. This enzyme mimic was used as an in vitro replacement for colonic lumen behavior, where there is azoreductase activity. S-PEG showed the potential to survive the GI tract while also releasing at the necessary pH over a significant amount of time. Providing the necessary treatment in small doses reduces the side effects, and over 90% of encapsulated sulfasalazine was released in the presence of sodium dithionite within 4 h in the colonic pH of 7.2. This self-assembled nanostructure demonstrated its ability to release the drug in colon-mimicking conditions and to be nontoxic to HEK293 cells (Figure 3c).
Figure 3.
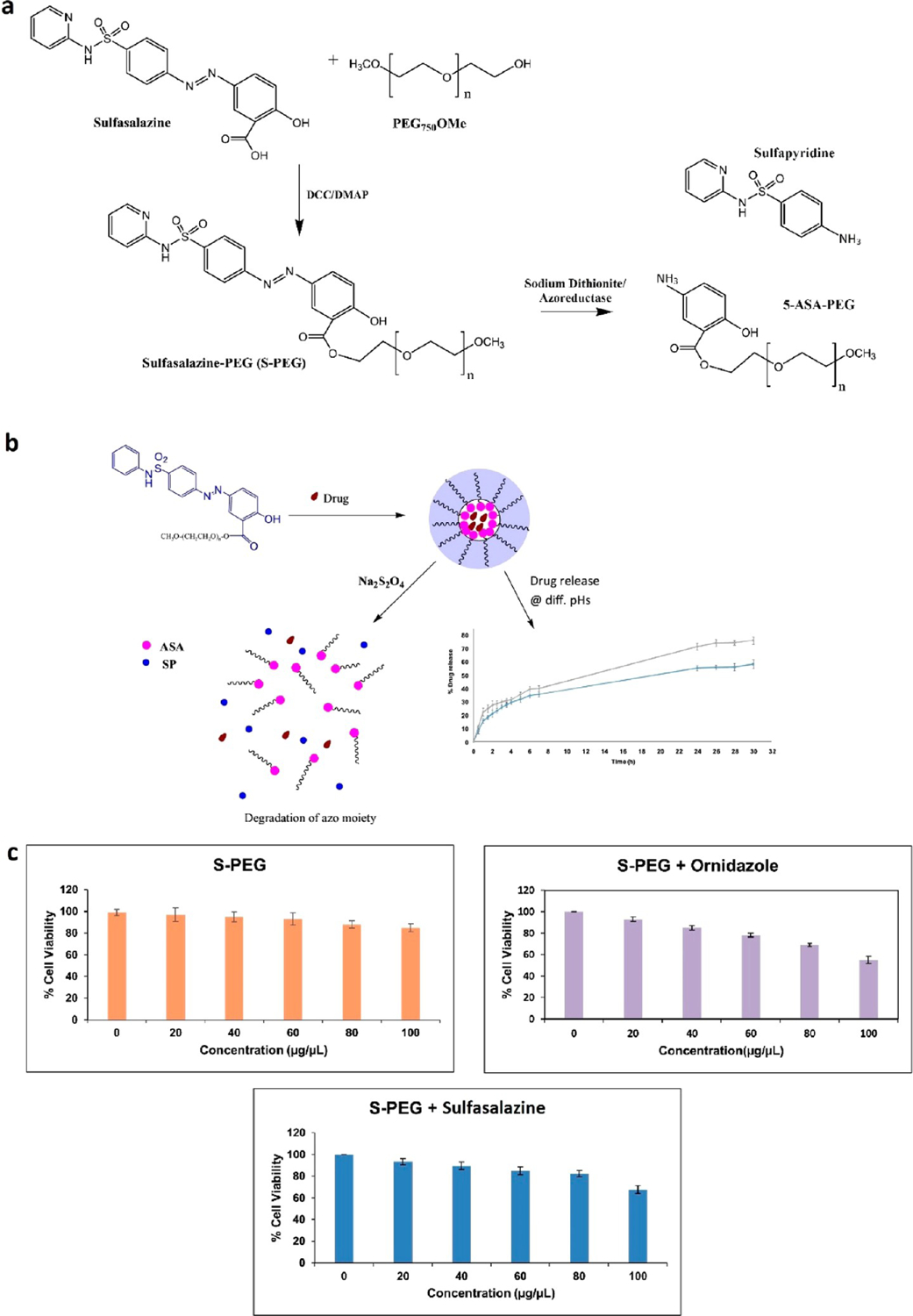
Overview of (a) chemical synthesis of S-PEG and degradation by sodium dithionite or azoreductase into sulfapyridine (SP) and 5-ASA-PEG. (b) Diagram of S-PEG self-assembly and encapsulation of a drug and subsequent release. The drug release profile of ornidazole from S-PEG is shown over 30 h at pH 4.5 (blue) and 7.2 (gray). (c) Cytotoxicity assay on HEK 293 cells evaluating the S-PEG carrier alone (orange) and loaded drugs ornidazole (purple) and sulfasalazine (blue). Modified and reprinted with permission from Priyam et al.50 Copyright 2019 Elsevier.
NANOPARTICLES ENGINEERED FOR MICROBIOME MODULATION
Emerging evidence has shown that the development and progression of IBD is associated with a dysregulated gut microbiome. IBD patients show a different gut microbial community structure as well as decreased overall diversity and relative abundance of anti-inflammatory taxa compared tohealthy subjects.78 Manipulation of the dysregulated gut microbiome via fecal microbiota transplantation (FMT) and probiotics has been studied in some diseases, such as in a Clostridium difficile infection. However, FMT has major limitations, including biosafety concerns especially during the current COVID-19 pandemic, frequency of oral administration, restricted storage conditions for the anaerobic bacteria, and the potential high cost. Although it is still at its infancy stage, engineered nanoparticles or biomaterials have shown potential to modulate the gut microbiota for therapy against IBD as well as inflammation associated colorectal cancer.79
We have recently developed hyaluronic acid-bilirubin treatment, coined HABN, as a potential therapy to modulate the gut microbiome and reduce inflammation in IBD.51 The current IBD treatments based on immunosuppressants aim to suppress immune responses and limit inflammation without addressing the root issues. HABN instead focuses on repairing damage to the mucus layer and restoring microbial diversity in the gut microbiome. Hyaluronic acid (HA) is an aqueous compatible molecule found in the human body that has the ability to modulate the immune system. Bilirubin (BR) is a hydrophobic byproduct from the breakdown of red blood cells and is typically secreted from the liver or gallbladder to the digestive tract. Chemical conjugation between HA and BR allowed the formation of self-assembled nanoparticles that are robust for oral administration (Figure 4). The BR core exhibited potent ROS-scavenging capability while the HA shell promoted immune modulation. HABN administered orally was localized to inflamed colon tissues better than separate HA or BR molecules, and HABN exerted robust efficacy against DSS-induced colitis. When compared with conventional IBD therapeutics, such as DEX and methylprednisolone, HABN significantly outperformed them. Mice regained full body weight while reducing the colonic damage in pathology examinations and restoring the normal gut microbiome without any overt sign of serious side effects. With the pathogenesis of IBD being closely correlated to intestinal barrier disruptions and the imbalance of the gut microbiome, this work presents HABN as an initial foray into addressing the underlying pathophysiology of IBD. In essence, this ROS-targeting approach shows promising results to serve as a potent immunomodulator and a therapeutic agent against IBD.
Figure 4.
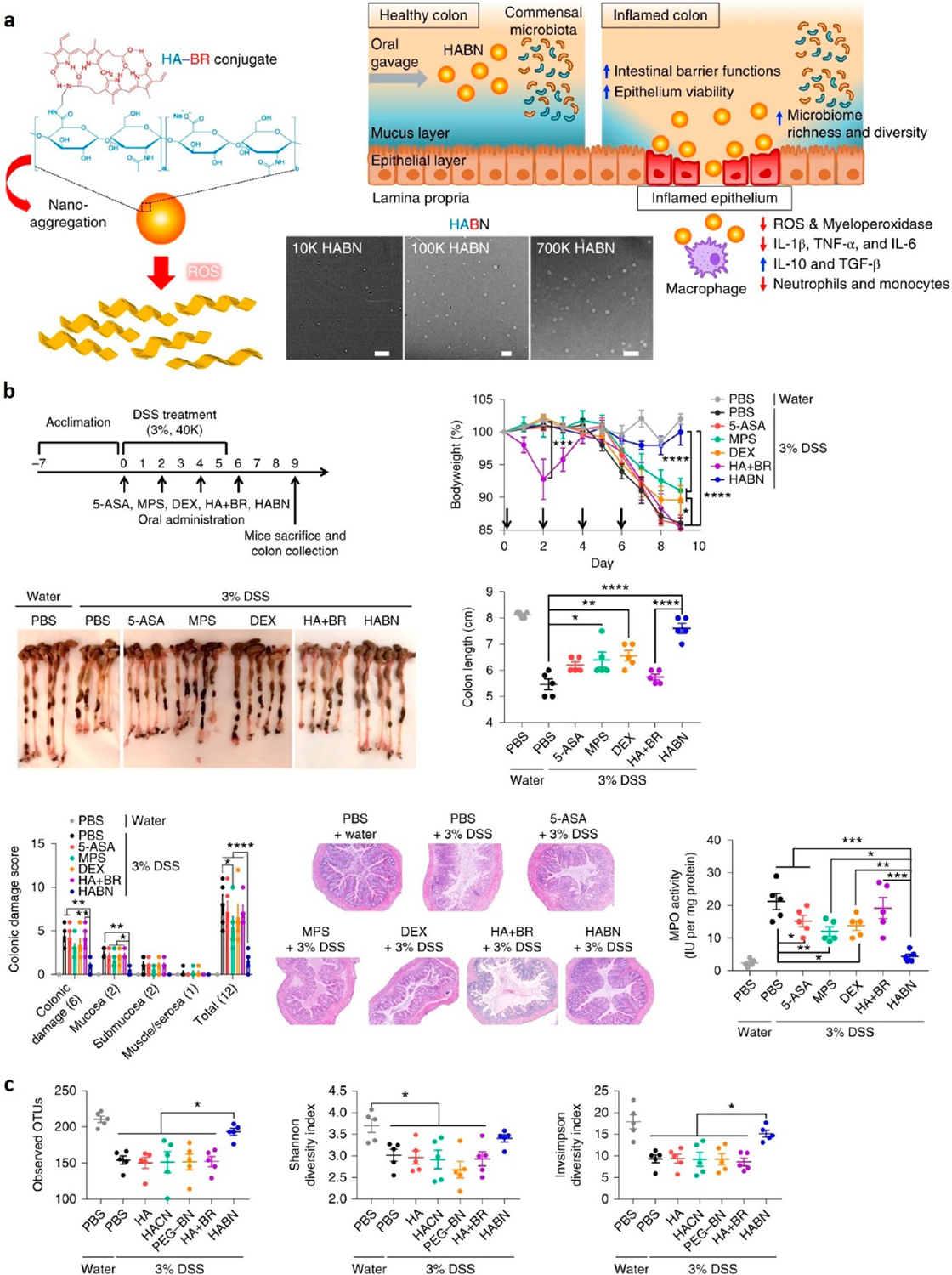
Overview of (a) HABN structure and mechanism of action. TEM images are shown for HA at molecular weights of 10, 100, and 700 kDa. Scale bars are 500, 300, and 400 nm, respectively. (b) Effects on DSS-induced colitis mice for different experimental groups: control (PBS–water), PBS control (PBS–3% DSS), 5-aminosalicyclic acid (5-ASA), methylprednisolone (MPS), free DEX (DEX), free HA and BN (HA + BN), and HABN nanoparticle (HABN). All mice were given 3 wt % DSS for 6 days except the water control. All treatments were administered orally. (c) Effects on microbiome diversity using metrics such as OTUs, Shannon diversity index, and Invsimpson diversity index. HACN is the control HA–cholesterol conjugate. Modified and reprinted with permission from Lee et al.51 Copyright 2019 Nature Publishing Group.
HABN has a ROS-scavenging property, which led to a protective effect from ROS-cytotoxicity on human HT-29 colonic epithelial cells. We showed the rapid disaggregation and response of the nanoparticle in response to ROS. Interestingly, when dosed orally in the DSS-induced colitis model, HABN was detected in DSS-inflamed colonic macrophages, especially inflammatory M1 macrophages, but not in the colon of healthy mice. In addition, HABN was shown to modulate the gut microbiota. 16S rRNA gene sequencing analyses showed an increase in bacterial richness (observed operational taxonomic unit, OTU) and α-diversity (Shannon and inverse-Simpson indices) from fecal samples of HABN treated DSS-colitis mice (Figure 4c). Compared with other treatments, HABN-treated mice demonstrated distinct gut microbiome composition, harboring higher relative abundance of Akkermansia muciniphila, Clostridium XIVα, and Lactobacillus. Notably, these microbes have been reported to have beneficial effects against IBD in both animal models and patients. Importantly, when the microbiota was disrupted by oral antibiotics, the therapeutic efficacy of HABN was markedly abrogated, highlighting the importance of microbiome modulation for the treatment of IBD.
The previously covered research group that engineered OxbCD NPs43 extended their study by replacing tempol with Ac2-26.52 Ac2-26 is a peptide that was identified to inhibit the inflammatory responses and reduce the penetration of monocytes, macrophages, and neutrophils into the region.80 The peptide also showed promising results when it was delivered via nanoparticles using intramucosal and intraperitoneal injection.81 Similar to their previous work, OxbCD was used as the carrier to encapsulate Ac2-26, resulting in a nanostructure termed AON. The design allowed for the hydrolysis of OxbCD at high ROS regions, releasing the peptide therapy at the inflamed regions. These nanoparticles were then exposed to lecithin and DSPE-PEG to coat the hydrophobic core with an amphiphilic top layer for colloidal stability (Figure 5a). AON exhibited a loading capacity of 0.086% for Ac2-26 with an entrapment efficiency of 43%. When orally administered on DSS-induced mice, AON nanotherapy reduced proinflammatory cytokine production by altering macrophages from a proinflammatory to an anti-inflammatory phenotype while also increasing gut microbiota diversity from a state of dysbiosis. It also reduced the weight loss and disease activity of the DSS-induced mice while restoring colon length (Figure 5b). AON nanotherapy was safe and effective at decreasing the severity of oxidative stress, inflammation, and fecal bleeding and increasing stool consistency and mucosal healing.
Figure 5.
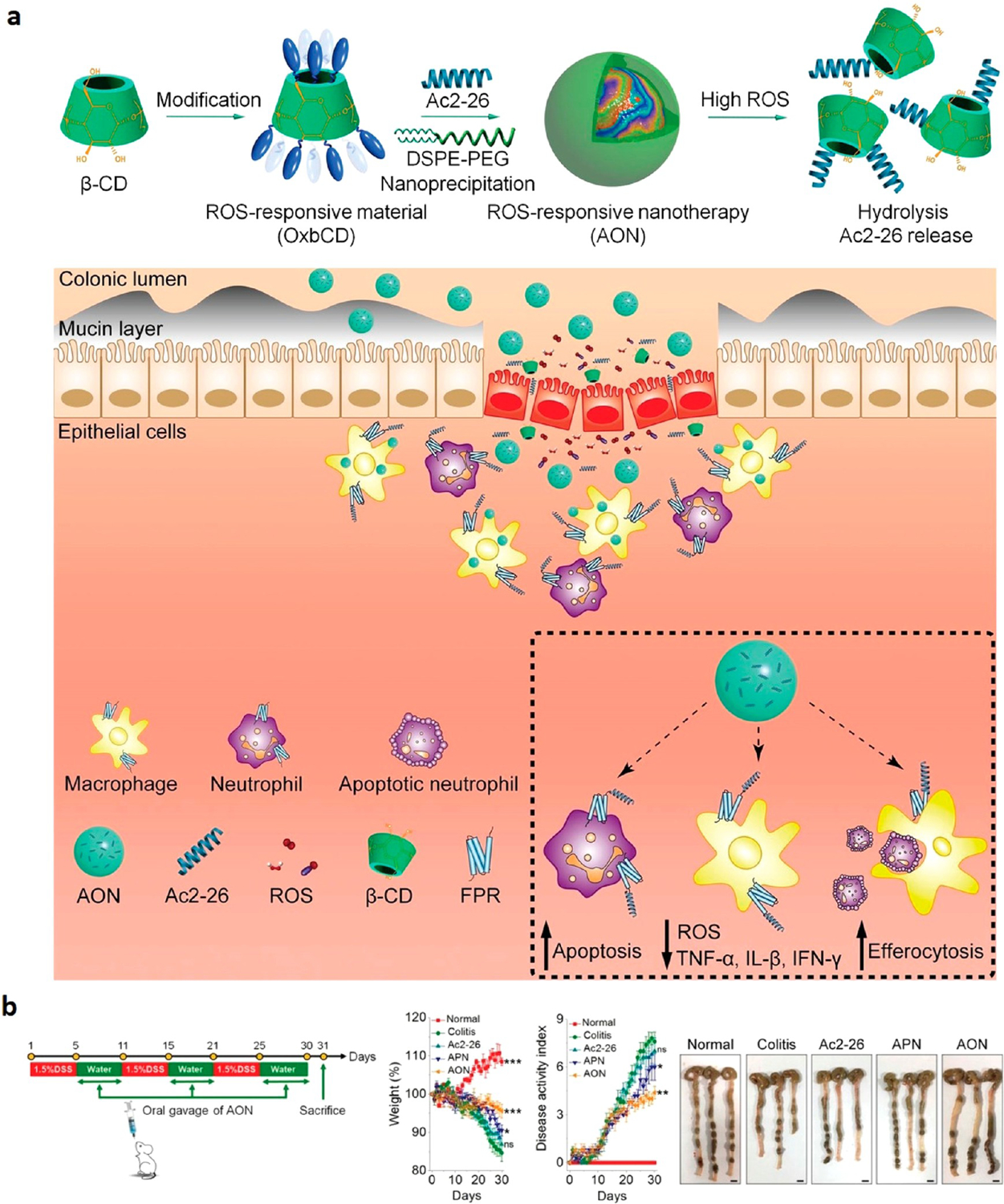
Overview of (a) assembly of AON and responsiveness to ROS species in inflamed colon lumen. (b) DSS treatment regimens of mice and efficacy for different experimental groups: non-DSS control (Normal), DSS-induced mouse control (Colitis), free Ac2-26 peptide (Ac2-26), Ac2-26-loaded PLGA NP (APN), and Ac2-loaded OxbCD NP (AON). APN was used as a non-ROS-responsive control. “A Proresolving Peptide Nanotherapy for Site-Specific Treatment of Inflammatory Bowel Disease by Regulating Proinflammatory Microenvironment and Gut Microbiota” by John Wiley and Sons, used under Creative Commons CC BY license (https://creativecommons.org/licenses). Panels A and B cropped and simplified from the original (ref 52).
This Ac2-26-loaded compound responded to inflammatory conditions by tapering the effect of the immune cells and normalizing the proinflammatory microenvironments, helping reduce the impact of the disease. OxbCD reduced the expansion ofEscherichia–Shigellaand increasedPrevotellaceaelevels, which is reported to be a short-chain fatty acids (SCFAs) producer.82 Consequently, OxbCD significantly restored the level of gut microbial-derived SCFAs, which were associated with a reduced risk of IBD.82 We have recently reported similar results and shown that oral administration of inulin gel reshaped the gut microbiome, promoting the SCFAs production.79 Using the colon-retentive hydrogel with anti-PD-1 resulted in changes in the gut microbiome characterized by increased frequency of Roseburia, Lactobacillus, and Akkermansia, the former two of which are major SCFA producers, and a reduction in Oscillibacter that produces lipopolysaccharides. Importantly, when combined with anti-PD-1 treatment, inulin gel consumption inhibited the DSS-accelerated tumor growth in the CDX2–cre NLS–APCfl/fl transgenic mice, which is a model of IBD-associated colorectal cancer.
As opposed to engineering nanoparticles that release drugs and peptides to inflamed colonic tissue and restore healthy gut microbes, Teng et al.53 developed ginger exosome-like nanoparticles (GELNs) that delivered their microRNAs to Lactobacillus rhamnosus (LGG), altering the bacteria’s gene expression (Figure 6a). This preferential uptake behavior by Lactobacillus rhamnosus was lipid-dependent, and GELN-derived lipids were enriched with 1,2-dilinoleoyl-sn-glycero-3-phosphate, C18:1/C18:3 (36:4), and 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphate, C16:0/C18:2 (34:2). GELN-derived mdo-miR7267–3p has a potential binding site for mRNA encoding LGG monooxygenase ycnE. As a result, GELN-RNAs inhibited the ycnE gene expression, increased indole-3-carboxaldehyde (I3A, a known ligand for the aryl hydrocarbon receptor), and induced the production of I3A and IL-22, thereby ameliorating colitis.53 Important metrics such as body weight, survival, and colon length were all improved when using GELN-RNA treatment (Figure 6b). Moreover, there have been attempts to use NP-mediated gene therapy for the treatment of IBD, focusing on the delivery of nucleic acids using biomaterials such as chitosan, polyamino acid, lipids, and polyethylenimine, as outlined in a recent review paper.83
Figure 6.
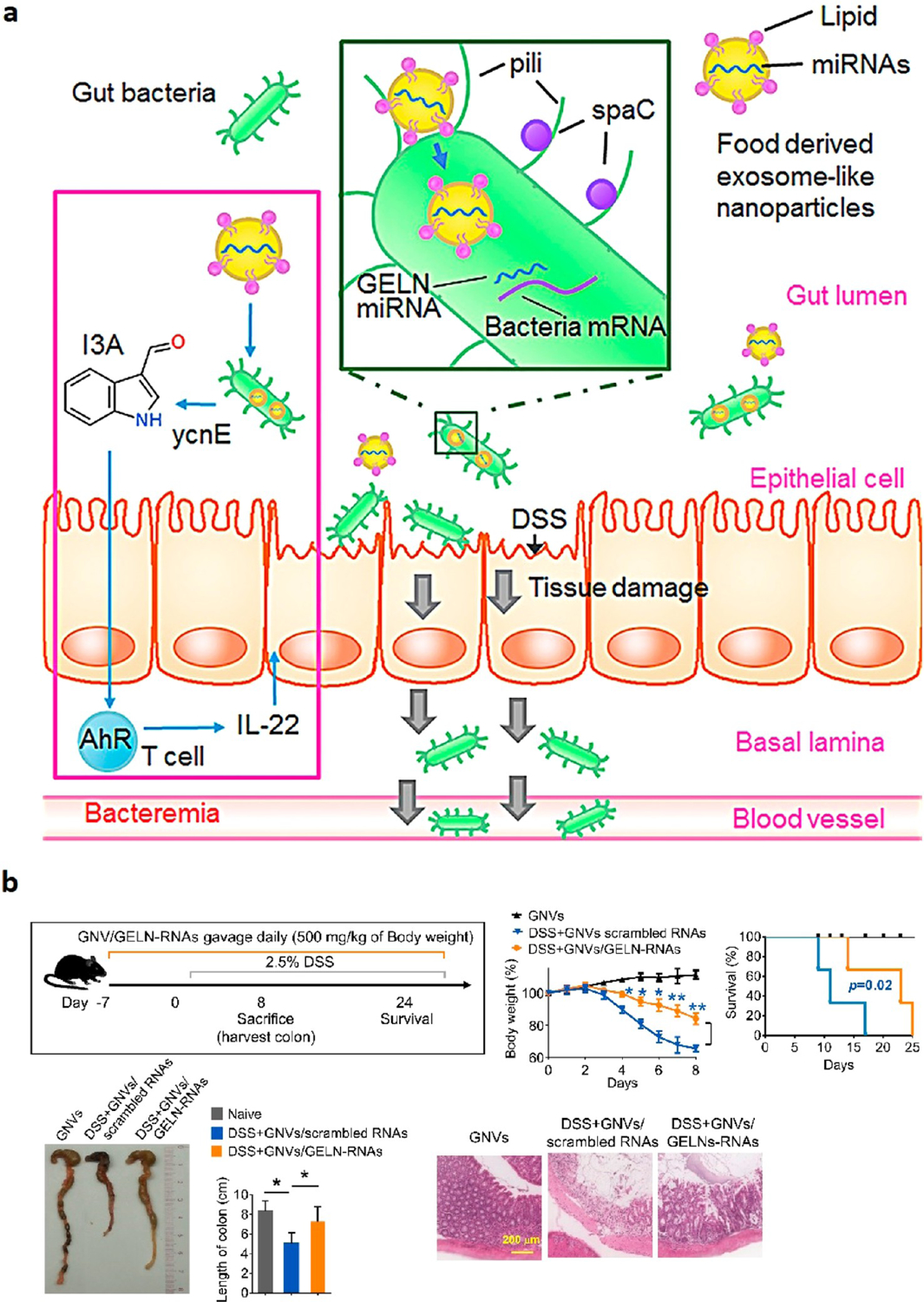
Overview of (a) proposed mechanism for how plant-derived exosome-like microRNA delivery system shapes the gut microbiota for the treatment of colitis. (b) DSS treatment regimen and NP efficacy. GNVs are GELN nanovectors, and scrambled RNAs encapsulated in GNV was used as the control. H&E-stained colon samples are used to show tissue effects. Reprinted with permission from Teng et al.53 Copyright 2018 Cell Press.
Polyphenols demonstrate anti-inflammatory properties, and the ability to treat the diseased tract without any noticeable side effects, and have even been recognized for their ability to modulate the microbiome. Figure 7a shows the hybrid filament hydrogel developed with polyphenols as the attachment between side groups of the amyloid fibrils.54 This hydrogel system increased the loading capacity of polyphenols and improved stability. For example, when epigallocatechin gallate (EGCG) was tested as the polyphenol, it was shown to have a dissociation factor of 3.4 × 10−7 with the amyloid fibers. This allowed for greater retention in the colon, allowing for the attachment of these hybrid filaments to help reduce inflammation associated with colitis. It was shown to improve body weight, DAI, and colon length of DSS-induced mice (Figure 7b). Additionally, the hydrogel system promoted the symbiosis for the gut microbiota, addressing mRNA expression for inflammatory immune cells and stabilizing the intestinal barrier functions. The hydrogel is hypothesized to have two modes of action to reduce antigen presentation on immune cells and inflammation: reduction of colitis-associated bacteria because of the antioxidant effects of EGCG and the physical barrier formed by the hydrogel, which protects epithelial cells. This represents a multifactorial drug delivery system that can stabilize the colitis microenvironment.
Figure 7.
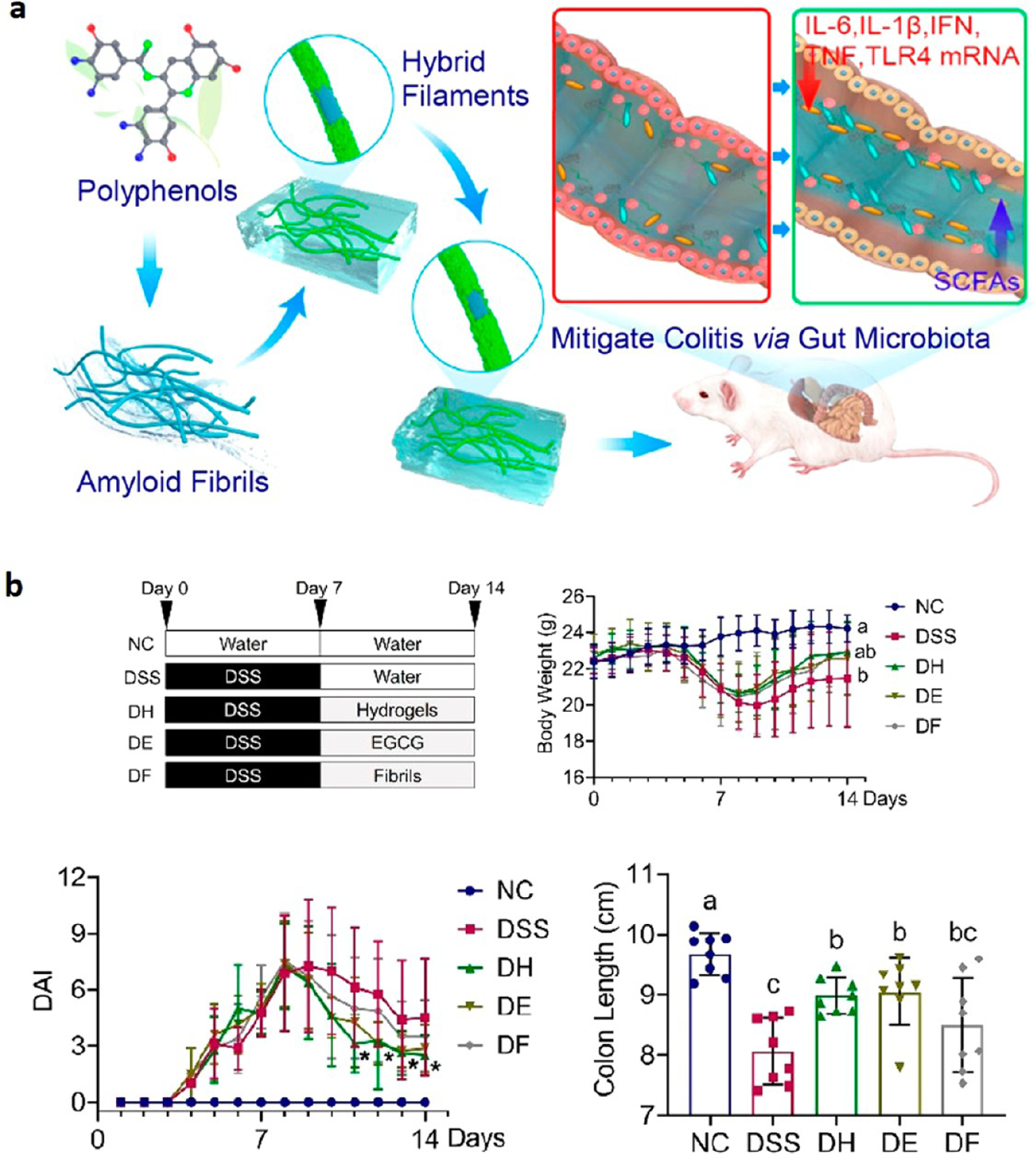
Overview of (a) formation of hybrid nanofilaments as a delivery system for the treatment of colitis. (b) Treatment regimens and efficacy for different experimental groups: normal control (NC), DSS-induced mouse control (DSS), amyloid-EGCG hydrogel treatment (DH), EGCG treatment (DE), and amyloid fibrils treatment (DF). All were given 2 wt % DSS except for NC, which was given purified water. Different letters between data groups indicate statistically significant differences. Reprinted with permission from Hu et al.54 Copyright 2020 American Chemical Society.
Another potential therapeutic for addressing a gut microbiota imbalance is the development of bacterial solutions that present a unique approach to the transplantation of microbiomes.55 A biointerfacial supramolecular self-assembly was made by vortexing cholesterol, dioleoylphosphatydic acid (DOPA), calcium phosphate buffer, and Escherichia coli Nissle 1917 together in as quickly as 15 min to develop lipid-coated high-retention probiotic bacteria (LCB). The purpose was to circumvent and improve upon the current bacterial therapeutics, which suffer from low retention and survivability through the GI tract. The encapsulated bacteria could still grow both in vitro and in vivo. LCB were shown to remain viable up to 4 days postadministration and prevented histological damage while increasing the beneficial proliferation of the microbiome. They also prevented Salmonella enterica serovar Typhimurium infection in mouse models. The lengthened retention time and the influx of probiotic bacteria resulted in increased treatment efficacy for induced colitis. These results show great promise for microbiota transplantation or even potentially live biotherapeutic products as treatment for IBD.
FUTURE OUTLOOK
With all these promising results both in vitro and in vivo, what are the remaining challenges to overcome for translating nanoparticles for treating IBD? First, the field of nanoparticle drug delivery, especially within IBD, is still in its infancy and has not yet been adopted by large pharmaceutical companies. Nanoparticle-based therapy against IBD has to be validated in clinical trials, and this clinical translation is not easy to achieve, especially for transitioning from an academic to scale-up process and GMP manufacturing. In the age of COVID-19, the scientific advancement in nanomedicine is clear. However, as multiple treatments are available for IBD with large market shares, pharmaceutical companies are not as incentivized to create these new, innovative medicines against IBD, unlike in the case of SARS-COV-2.27
Beyond that, these artificial self-assemblies are still not as efficient as their natural counterparts. The consistency of nanoparticles’ size is critical when manufacturing as it affects the pharmacokinetics and pharmacodynamics of the drug. There are a multitude of barriers in using nanoparticles reliably for treating IBD. Without significant monetary incentives from pharmaceutical companies, it will be challenging to overcome the current technological or scientific barriers. At the same time, it is important to continue developing research, especially from the academic side, so treatments can become more readily accessible and efficacious for this chronic life-altering pathology.26
The future of nanoparticle-based therapeutics for IBD is promising. Researchers should first focus on improving nanoparticles as a carrier for traditional treatments. This effort would go hand in hand with the development of a bottom-up individualized approach for the treatment of patients. IBD represents a field of medicine that is well-suited for this methodology to be tested. The mix-and-match approach clinicians use to improve patients’ quality of life can be transitioned to biomarker genomics that allow for the optimization of nanoparticle selection. Moreover, additional research on the microbiome should also be pursued to better understand how nanoparticles can influence the gut microbiome and improve patient outcomes. Recent studies have shed new light on how the gut microbiotas impact the pathobiology of IBD. Yet, more research is warranted to understand how the specific microbes interact with one another and host cells and how new therapeutics can be designed to target and modulate the pathogenic microbes as a new therapeutic approach for IBD patients.
CONCLUSIONS
Our review presents recent advancements in the field of nanoparticle engineering that have been applied toward IBD to potentially serve as drug carriers, treatment, or a combination of both. The researchers are adapting historically proven drugs for IBD, like DEX or anti-TNF antibodies, or testing new therapeutics as alternatives to the established standard. ROS have become a major focus in recent years as a potential mechanism for bringing patients to remission. Additionally, the microbiome has been growing in importance as a crucial indicator of disease and can be a target for therapy. Many of the nanotherapeutics utilized targeted drug delivery by attaching and/or releasing the encapsulated load at the site of inflammation. The predominant focus has been on oral formulations as it is more likely to increase patient compliance and reduce systemic exposure. Adapting monoclonal antibodies for oral delivery through self-assembled structures is especially promising as it can improve biologics already FDA-approved and proven to work on patients by reducing possible side effects and making it easier to administer. However, injections and enemas are still a valid way to deliver therapeutics and have shown improvements over the years. Although these treatments have not yet been tested in human clinical trials, most have the potential to provide benefits without the immunosuppressant side effects that accompany typical treatments. Additionally, improving delivery to the inflammation sites can increase effectiveness, allowing for decreased dosage and the off-target toxicity of the drug. This review presents the most cutting-edge advancements in the field for treating the increasing number of IBD-diagnosed patients at anticipated lower costs.
ACKNOWLEDGMENTS
This work was supported in part by the US National Institutes of Health (NIH) (R01DE030691, R01DK125087, R01CA271799, R01NS122536, and U01CA210152). O.A.A. is supported by the National Science Foundation Graduate Research Fellowship. We thank Ms. April Kim for critical review of the paper. The graphical abstract was created by the authors using BioRender.com.
The authors declare the following competing financial interest(s): J.J.M. declares financial interests as board membership, a paid consultant, research funding, and/or equity holder in EVOQ Therapeutics, Saros Therapeutics, and Intrinsic Medicine.
ABBREVIATIONS
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- IV
intravenous
- FDA
Food and Drug Administration
- NPs
nanoparticles
- PEG
polyethylene glycol
- PCL
polycaprolactone
- DSS
dextran sulfate sodium
- ROS
reactive oxygen species
- B
budesonide
- T
tempol
- ATK
aromatized thioketal
- DAI
disease activity index
- GRAS
Generally Recognized as Safe
- TA
tannic acid
- PPNP
polyphenols and polymers self-assembled nanoparticles
- PDI
polydispersity index
- DEX
dexamethasone
- SOD
superoxide dismutase
- OxbCD
β-cyclodextrin-derived material
- CMI
carboxymethyl inulin
- PLGA
poly(lactic-co-glycolic acid)
- FA
folic acid
- INF
infliximab
- PPCNPs
polyphenol-PEG-containing polymers self-assembled nanoparticles
- RA
rosmarinic acid
- VIP
vasoactive intestinal peptide
- SSM
sterically stabilized micelle
- DRA
down-regulated in adenoma
- AP
ascorbyl palmitate
- FMT
fecal microbiota transplantation
- HA
hyaluronic acid
- BR
bilirubin
- HACN
control HA-cholesterol conjugate
- OTU
observed operational taxonomic unit
- AON
Ac2-loaded OxbCD NP
- APN
Ac2-26-loaded PLGA NP
- SCFAs
short-chain fatty acids
- GELNs
ginger exosome-like nanoparticles
- LGG
Lactobacillus rhamnosus
- I3A
indole-3-carboxaldehyde
- ECGC
epigallocatechin gallate
- DOPA
dioleoylphosphatydic acid
- LCB
lipid coated bacteria
- LBPs
live biotherapeutic products
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.molpharmaceut.2c00222
Contributor Information
Omar A. Abed, Department of Chemical Engineering, University of Michigan, Ann Arbor, Michigan 48109, United States; Biointerfaces Institute, University of Michigan, Ann Arbor, Michigan 48109, United States.
Younes Attlassy, Department of Medicine, New York University School of Medicine, New York, New York 10012, United States.
Jin Xu, Biointerfaces Institute and Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, Michigan 48109, United States.
Kai Han, Biointerfaces Institute and Department of Pharmaceutical Sciences, University of Michigan, Ann Arbor, Michigan 48109, United States.
James J. Moon, Department of Chemical Engineering, University of Michigan, Ann Arbor, Michigan 48109, United States; Biointerfaces Institute, Department of Pharmaceutical Sciences, and Department of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan 48109, United States
REFERENCES
- (1).Singh S; Blanchard A; Walker JR; Graff LA; Miller N; Bernstein CN Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2011, 9 (9), 769–75. [DOI] [PubMed] [Google Scholar]
- (2).Baumgart DC; Sandborn WJ Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007, 369 (9573), 1641–57. [DOI] [PubMed] [Google Scholar]
- (3).Jostins L; Ripke S; Weersma RK; Duerr RH; McGovern DP; Hui KY; Lee JC; Schumm LP; Sharma Y; Anderson CA; Essers J; Mitrovic M; Ning K; Cleynen I; Theatre E; Spain SL; Raychaudhuri S; Goyette P; Wei Z; Abraham C; Achkar JP; Ahmad T; Amininejad L; Ananthakrishnan AN; Andersen V; Andrews JM; Baidoo L; Balschun T; Bampton PA; Bitton A; Boucher G; Brand S; Buning C; Cohain A; Cichon S; D’Amato M; De Jong D; Devaney KL; Dubinsky M; Edwards C; Ellinghaus D; Ferguson LR; Franchimont D; Fransen K; Gearry R; Georges M; Gieger C; Glas J; Haritunians T; Hart A; Hawkey C; Hedl M; Hu XL; Karlsen TH; Kupcinskas L; Kugathasan S; Latiano A; Laukens D; Lawrance IC; Lees CW; Louis E; Mahy G; Mansfield J; Morgan AR; Mowat C; Newman W; Palmieri O; Ponsioen CY; Potocnik U; Prescott NJ; Regueiro M; Rotter JI; Russell RK; Sanderson JD; Sans M; Satsangi J; Schreiber S; Simms LA; Sventoraityte J; Targan SR; Taylor KD; Tremelling M; Verspaget HW; De Vos M; Wijmenga C; Wilson DC; Winkelmann J; Xavier RJ; Zeissig S; Zhang B; Zhang CK; Zhao HY; Silverberg MS; Annese V; Hakonarson H; Brant SR; Radford-Smith G; Mathew CG; Rioux JD; Schadt EE; Daly MJ; Franke A; Parkes M; Vermeire S; Barrett JC; Cho JH; IIBDGC, I. I. G. C. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491 (7422), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bengtson MB; Aamodt G; Vatn MH; Harris JR Concordance for IBD among twins compared to ordinary siblings - A Norwegian population-based study. Journal of Crohns & Colitis 2010, 4 (3), 312–318. [DOI] [PubMed] [Google Scholar]
- (5).Cosnes J Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Practice & Research Clinical Gastroenterology 2004, 18 (3), 481–496. [DOI] [PubMed] [Google Scholar]
- (6).Garn H; Renz H Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology 2007, 212 (6), 441–452. [DOI] [PubMed] [Google Scholar]
- (7).Okada H; Kuhn C; Feillet H; Bach JF The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin. Exp. Immunol 2010, 160 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Thoreson R; Cullen JJ Pathophysiology of inflammatory bowel disease: an overview. Surg Clin North Am. 2007, 87 (3), 575–85. [DOI] [PubMed] [Google Scholar]
- (9).Shivashankar R; Tremaine WJ; Harmsen WS; Loftus EV Jr. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clin Gastroenterol Hepatol 2017, 15 (6), 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kappelman MD; Moore KR; Allen JK; Cook SF Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig. Dis. Sci 2013, 58 (2), 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Jairath V; Feagan BG Global burden of inflammatory bowel disease. Lancet Gastroenterology & Hepatology 2020, 5 (1), 2–3. [DOI] [PubMed] [Google Scholar]
- (12).Desai HG; Gupte PA Increasing incidence of Crohn’s disease in India: is it related to improved sanitation? Indian J. Gastroenterol 2005, 24 (1), 23–24. [PubMed] [Google Scholar]
- (13).Zheng JJ; Zhu XS; Huangfu Z; Gao ZX; Guo ZR; Wang Z Crohn’s disease in mainland China: a systematic analysis of 50 years of research. Chin J. Dig Dis 2005, 6 (4), 175–81. [DOI] [PubMed] [Google Scholar]
- (14).Manichanh C; Borruel N; Casellas F; Guarner F The gut microbiota in IBD. Nature Reviews Gastroenterology & Hepatology 2012, 9 (10), 599–608. [DOI] [PubMed] [Google Scholar]
- (15).Martinez C; Antolin M; Santos J; Torrejon A; Casellas F; Borruel N; Guarner F; Malagelada JR Unstable Composition of the Fecal Microbiota in Ulcerative Colitis During Clinical Remission. American Journal of Gastroenterology 2008, 103 (3), 643–648. [DOI] [PubMed] [Google Scholar]
- (16).Swidsinski A Mucosal flora in inflammatory bowel disease: Intraepithelial bacteria or endocrine epithelial cell secretory granules? - Reply. Gastroenterology 2002, 123 (3), 956–956. [PubMed] [Google Scholar]
- (17).Willing BP; Dicksved J; Halfvarson J; Andersson AF; Lucio M; Zheng Z; Jarnerot G; Tysk C; Jansson JK; Engstrand L A Pyrosequencing Study in Twins Shows That Gastrointestinal Microbial Profiles Vary With Inflammatory Bowel Disease Phenotypes. Gastroenterology 2010, 139 (6), 1844–U105. [DOI] [PubMed] [Google Scholar]
- (18).Chassaing B; Rolhion N; de Vallee A; Salim SY; Prorok-Hamon M; Neut C; Campbell BJ; Soderholm JD; Hugot JP; Colombel JF; Darfeuille-Michaud A Crohn disease-associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J. Clin. Invest 2011, 121 (3), 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sokol H; Pigneur B; Watterlot L; Lakhdari O; Bermudez-Humaran LG; Gratadoux JJ; Blugeon S; Bridonneau C; Furet JP; Corthier G; Grangette C; Vasquez N; Pochart P; Trugnan G; Thomas G; Blottiere HM; Dore J; Marteau P; Seksik P; Langella P Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U.S.A 2008, 105 (43), 16731–16736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sokol H; Seksik P; Furet JP; Firmesse O; Nion-Larmurier L; Beaugerie L; Cosnes J; Corthier G; Marteau P; Dore J Low Counts of Faecalibacterium prausnitzii in Colitis Microbiota. Inflammatory Bowel Diseases 2009, 15 (8), 1183–1189. [DOI] [PubMed] [Google Scholar]
- (21).Ramamoorthy S; Cidlowski JA Corticosteroids Mechanisms of Action in Health and Disease. Rheumatic Disease Clinics of North America 2016, 42 (1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Campregher C; Gasche C Aminosalicylates. Best Practice & Research Clinical Gastroenterology 2011, 25 (4–5), 535–546. [DOI] [PubMed] [Google Scholar]
- (23).Greenfield SM; Punchard NA; Teare JP; Thompson RPH The Mode of Action of the Aminosalicylates in Inflammatory Bowel-Disease. Alimentary Pharmacology & Therapeutics 1993, 7 (4), 369–383. [DOI] [PubMed] [Google Scholar]
- (24).Andersson L; Blomberg L; Flegel M; Lepsa L; Nilsson B; Verlander M Large-scale synthesis of peptides. Biopolymers 2000, 55 (3), 227–250. [DOI] [PubMed] [Google Scholar]
- (25).Petit N; Dyer JM; Clerens S; Gerrard JA; Domigan LJ Oral delivery of self-assembling bioactive peptides to target gastrointestinal tract disease. Food & Function 2020, 11 (11), 9468–9488. [DOI] [PubMed] [Google Scholar]
- (26).Giron F; Pasto A; Tasciotti E; Abraham BP Nanotechnology in the Treatment of Inflammatory Bowel Disease. Inflamm Bowel Dis 2019, 25 (12), 1871–1880. [DOI] [PubMed] [Google Scholar]
- (27).Ferreira NN; Ferreira LMB; Cardoso VMO; Boni FI; Souza ALR; Gremiao MPD Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J 2018, 99, 117–133. [Google Scholar]
- (28).Chen FQ; Liu Q; Xiong Y; Xu L Current Strategies and Potential Prospects of Nanomedicine-Mediated Therapy in Inflammatory Bowel Disease. Int. J. Nanomed 2021, 16, 4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gou PF; Zhu WP; Shen ZQ Synthesis, self-assembly, and drug-loading capacity of well-defined cyclodextrin-centered drug-conjugated amphiphilic A(14)B(7) Miktoarm star copolymers based on poly(epsilon-caprolactone) and poly(ethylene glycol). Biomacro-molecules 2010, 11 (4), 934–43. [DOI] [PubMed] [Google Scholar]
- (30).Uhrich KE; Cannizzaro SM; Langer RS; Shakesheff KM Polymeric systems for controlled drug release. Chem. Rev 1999, 99 (11), 3181–3198. [DOI] [PubMed] [Google Scholar]
- (31).Drumheller PD; Hubbell JA Densely Cross-Linked Polymer Networks of Poly(Ethylene Glycol) in Trimethylolpropane Triacrylate for Cell-Adhesion-Resistant Surfaces. J. Biomed. Mater. Res 1995, 29 (2), 207–215. [DOI] [PubMed] [Google Scholar]
- (32).Desai NP; Hubbell JA Biological Responses to Polyethylene Oxide Modified Polyethylene Terephthalate Surfaces. J. Biomed. Mater. Res 1991, 25 (7), 829–843. [DOI] [PubMed] [Google Scholar]
- (33).Chassaing B; Aitken JD; Malleshappa M; Vijay-Kumar M Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc Immunol 2014, 104, 15 25 1–15 25 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Okayasu I; Hatakeyama S; Yamada M; Ohkusa T; Inagaki Y; Nakaya R A Novel Method in the Induction of Reliable Experimental Acute and Chronic Ulcerative-Colitis in Mice. Gastroenterology 1990, 98 (3), 694–702. [DOI] [PubMed] [Google Scholar]
- (35).Elson CO; Beagley KW; Sharmanov AT; Fujihashi K; Kiyono H; Tennyson GS; Cong YZ; Black CA; Ridwan BW; McGhee JR Hapten-induced model of murine inflammatory bowel disease - Mucosal immune responses and protection by tolerance. J. Immunol 1996, 157 (5), 2174–2185. [PubMed] [Google Scholar]
- (36).Krawisz JE; Sharon P; Stenson WF Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity - Assessment of Inflammation in Rat and Hamster Models. Gastroenterology 1984, 87 (6), 1344–1350. [PubMed] [Google Scholar]
- (37).Pridgen EM; Alexis F; Farokhzad OC Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin Drug Deliv 2015, 12 (9), 1459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hutin YJF; Hauri AM; Armstrong GL Use of injections in healthcare settings worldwide, 2000: literature review and regional estimates. Bmj-British Medical Journal 2003, 327 (7423), 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hauri AM; Armstrong GL; Hutin YJF The global burden of disease attributable to contaminated injections given in health care settings. International Journal of Std & Aids 2004, 15 (1), 7–16. [DOI] [PubMed] [Google Scholar]
- (40).Cong Y; Qiao ZY; Wang H Molecular Self-Assembly Constructed in Physiological Conditions for Cancer Diagnosis and Therapy. Adv. Therapeutics 2018, 1 (6), 1800067. [Google Scholar]
- (41).Li SS; Xie AQ; Li H; Zou X; Zhang QX A self-assembled, ROS-responsive Janus-prodrug for targeted therapy of inflammatory bowel disease. J. Controlled Release 2019, 316, 66–78. [DOI] [PubMed] [Google Scholar]
- (42).Wang XY; Yan JJ; Wang LZ; Pan DH; Yang RL; Xu YP; Sheng J; Huang QH; Zhao HM; Yang M Rational Design of Polyphenol-Poloxamer Nanovesicles for Targeting Inflammatory Bowel Disease Therapy. Chem. Mater 2018, 30 (12), 4073–4080. [Google Scholar]
- (43).Zhang QX; Tao H; Lin YY; Hu Y; An HJ; Zhang DL; Feng SB; Hu HY; Wang RB; Li XH; Zhang JX A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 2016, 105, 206–221. [DOI] [PubMed] [Google Scholar]
- (44).Wang XY; Yan JJ; Wang LZ; Pan DH; Xu YP; Wang F; Sheng J; Li XN; Yang M Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy. Theranostics 2020, 10 (23), 10808–10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Chung CH; Jung W; Keum H; Kim TW; Jon S Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14 (6), 6887–6896. [DOI] [PubMed] [Google Scholar]
- (46).Jayawardena D; Anbazhagan AN; Guzman G; Dudeja PK; Onyuksel H Vasoactive Intestinal Peptide Nanomedicine for the Management of Inflammatory Bowel Disease. Mol. Pharmaceutics 2017, 14 (11), 3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zhang SF; Ermann J; Succi MD; Zhou A; Hamilton MJ; Cao BN; Korzenik JR; Glickman JN; Vemula PK; Glimcher LH; Traverso G; Langer R; Karp JM An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Translational Med 2015, 7 (300), 300ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Sun QJ; Arif M; Chi Z; Li GT; Liu CG Macrophages-targeting mannosylated nanoparticles based on inulin for the treatment of inflammatory bowel disease (IBD). Int. J. Biol. Macromol 2021, 169, 206–215. [DOI] [PubMed] [Google Scholar]
- (49).Naserifar M; Hosseinzadeh H; Abnous K; Mohammadi M; Taghdisi SM; Ramezani M; Alibolandi M Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci. 2020, 262, 118555. [DOI] [PubMed] [Google Scholar]
- (50).Priyam A; Shivhare K; Yadav S; Sharma AK; Kumar P Enhanced solubility and self-assembly of amphiphilic sulfasalazine-PEG-OMe (S-PEG) conjugate into core-shell nanostructures useful for colonic drug delivery. Colloid. Surf. A: Physicochem. Eng. Aspects 2018, 547, 157–167. [Google Scholar]
- (51).Lee Y; Sugihara K; Gillilland MG 3rd; Jon S; Kamada N; Moon JJ Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater 2020, 19 (1), 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Li CW; Zhao Y; Cheng J; Guo JW; Zhang QX; Zhang XJ; Ren J; Wang FC; Huang J; Hu HY; Wang RB; Zhang JX A Proresolving Peptide Nanotherapy for Site-Specific Treatment of Inflammatory Bowel Disease by Regulating Proinflammatory Microenvironment and Gut Microbiota. Adv. Sci 2019, 6 (18), 1900610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Teng Y; Ren Y; Sayed M; Hu X; Lei C; Kumar A; Hutchins E; Mu JY; Deng ZB; Luo C; Sundaram K; Sriwastva MK; Zhang LF; Hsieh M; Reiman R; Haribabu B; Yan J; Jala VR; Miller DM; Van Keuren-Jensen K; Merchant ML; McClain CJ; Park JW; Egilmez NK; Zhang HG Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host & Microbe 2018, 24 (5), 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Hu B; Yu SJ; Shi C; Gu J; Shao Y; Chen Q; Li YQ; Mezzenga R Amyloid-Polyphenol Hybrid Nanofilaments Mitigate Colitis and Regulate Gut Microbial Dysbiosis. ACS Nano 2020, 14 (3), 2760–2776. [DOI] [PubMed] [Google Scholar]
- (55).Cao ZP; Wang XY; Pang Y; Cheng SS; Liu JY Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun 2019, 10, 5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Winterbourn CC Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol 2008, 4 (5), 278–286. [DOI] [PubMed] [Google Scholar]
- (57).Mittal M; Siddiqui MR; Tran K; Reddy SP; Malik AB Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxidants & Redox Signaling 2014, 20 (7), 1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Dagli U; Balk M; Yucel D; Ulker A; Over H; Saydam G; Sahin B The role of reactive oxygen metabolites in ulcerative colitis. Inflammatory Bowel Diseases 1997, 3 (4), 260–264. [PubMed] [Google Scholar]
- (59).Grisham MB Oxidants and Free-Radicals in Inflammatory Bowel-Disease. Lancet 1994, 344 (8926), 859–861. [DOI] [PubMed] [Google Scholar]
- (60).Sedghi S; Fields JZ; Klamut M; Urban G; Durkin M; Winship D; Fretland D; Olyaee M; Keshavarzian A Increased Production of Luminol Enhanced Chemiluminescence by the Inflamed Colonic Mucosa in Patients with Ulcerative-Colitis. Gut 1993, 34 (9), 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Segui J; Gironella M; Sans M; Granell S; Gil F; Gimeno M; Coronel P; Pique JM; Panes J Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. Journal of Leukocyte Biology 2004, 76 (3), 537–544. [DOI] [PubMed] [Google Scholar]
- (62).Swidsinski A; Loening-Baucke V; Theissig F; Engelhardt H; Bengmark S; Koch S; Lochs H; Dorffel Y Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 2007, 56 (3), 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Yang CH; Merlin D Nanoparticle-Mediated Drug Delivery Systems For The Treatment Of IBD: Current Perspectives. Int. J. Nanomed 2019, 14, 8875–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Melero A; Draheim C; Hansen S; Giner E; Carreras JJ; Talens-Visconti R; Garrigues TM; Peris JE; Recio MC; Giner R; Lehr CM Targeted delivery of Cyclosporine A by polymeric nanocarriers improves the therapy of inflammatory bowel disease in a relevant mouse model. Eur. J. Pharm. Biopharm 2017, 119, 361–371. [DOI] [PubMed] [Google Scholar]
- (65).Dahan A; Amidon GL; Zimmermann EM Drug targeting strategies for the treatment of inflammatory bowel disease: a mechanistic update. Expert Review of Clinical Immunology 2010, 6 (4), 543–550. [DOI] [PubMed] [Google Scholar]
- (66).Abad C; Martinez C; Juarranz MG; Arranz A; Leceta J; Delgado M; Gomariz RP Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology 2003, 124 (4), 961–971. [DOI] [PubMed] [Google Scholar]
- (67).Abad C; Gomariz R; Waschek J; Leceta J; Martinez C; Juarranz Y; Arranz A VIP in inflammatory bowel disease: state of the art. Endocr Metab Immune Disord Drug Targets 2012, 12 (4), 316–22. [DOI] [PubMed] [Google Scholar]
- (68).Fernandez-Martin A; Gonzalez-Rey E; Chorny A; Martin J; Pozo D; Ganea D; Delgado M VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Vip, Pacap, and Related Peptides: From Gene to Therapy 2006, 1070, 276. [DOI] [PubMed] [Google Scholar]
- (69).Cobo M; Anderson P; Benabdellah K; Toscano MG; Munoz P; Garcia-Perez A; Gutierrez I; Delgado M; Martin F Mesenchymal Stem Cells Expressing Vasoactive Intestinal Peptide Ameliorate Symptoms in a Model of Chronic Multiple Sclerosis. Cell Transplantation 2013, 22 (5), 839–854. [DOI] [PubMed] [Google Scholar]
- (70).Juarranz Y; Abad C; Martinez C; Arranz A; Gutierrez-Canas I; Rosignoli F; Gomariz RP; Leceta J Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Research & Therapy 2005, 7 (5), R1034–R1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Foey AD; Field S; Ahmed S; Jain A; Feldmann M; Brennan FM; Williams R Impact of VIP and cAMP on the regulation of TNF-alpha and IL-10 production: implications for rheumatoid arthritis. Arthritis Research & Therapy 2003, 5 (6), R317–R328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Arranz A; Gutierrez-Canas I; Carrion M; Juarranz Y; Pablos JL; Martinez C; Gomariz RP VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. Molecular Immunology 2008, 45 (11), 3065–3073. [DOI] [PubMed] [Google Scholar]
- (73).Tuncel N; Tore F; Sahinturk V; Ak D; Tuncel M Vasoactive intestinal peptide inhibits degranulation and changes granular content of mast cells: a potential therapeutic strategy in controlling septic shock. Peptides 2000, 21 (1), 81–89. [DOI] [PubMed] [Google Scholar]
- (74).Delgado M; Martinez C; Pozo D; Calvo JR; Leceta J; Ganea D; Gomariz RP Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activation polypeptide (PACAP) protect mice from lethal endotoxemia through the inhibition of TNF-α and IL-6. J. Immunol 1999, 162 (2), 1200–1205. [PubMed] [Google Scholar]
- (75).Jayawardena D; Anbazhagan AN; Priyamvada S; Kumar A; Saksena S; Onyuksel H; Dudeja PK Colonic delivery of vasoactive intestinal peptide nanomedicine alleviates colitis and shows promise as an oral capsule. Nanomedicine 2020, 15 (25), 2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Peppercorn MA Sulfasalazine - Pharmacology, Clinical Use, Toxicity, and Related New Drug Development. Ann. Int. Med 1984, 101 (3), 377–386. [DOI] [PubMed] [Google Scholar]
- (77).Rutgeerts P; Van Assche G; Vermeire S; D’Haens G; Baert F; Noman M; Aerden I; De Hertogh G; Geboes K; Hiele M; D’Hoore A; Penninckx F Ornidazole for prophylaxis of post-operative Crohn’s disease recurrence: A randomized, double-blind, placebo-controlled trial. Gastroenterology 2005, 128 (4), 856–861. [DOI] [PubMed] [Google Scholar]
- (78).Knox NC; Forbes JD; Peterson CL; Van Domselaar G; Bernstein CN The Gut Microbiome in Inflammatory Bowel Disease: Lessons Learned From Other Immune-Mediated Inflammatory Diseases. American Journal of Gastroenterology 2019, 114 (7), 1051–1070. [DOI] [PubMed] [Google Scholar]
- (79).Han K; Nam J; Xu J; Sun XQ; Huang XH; Animasahun O; Achreja A; Jeon JH; Pursley B; Kamada N; Chen GY; Nagrath D; Moon JJ Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng 2021, 5 (11), 1377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Perretti M; D’Acquisto F Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nature Reviews Immunology 2009, 9 (1), 62–70. [DOI] [PubMed] [Google Scholar]
- (81).Leoni G; Neumann PA; Kamaly N; Quiros M; Nishio H; Jones HR; Sumagin R; Hilgarth RS; Alam A; Fredman G; Argyris I; Rijcken E; Kusters D; Reutelingsperger C; Perretti M; Parkos CA; Farokhzad OC; Neish AS; Nusrat A Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest 2015, 125 (3), 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Vinolo MAR; Rodrigues HG; Nachbar RT; Curi R Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3 (10), 858–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Verma P; Srivastava A; Srikanth CV; Bajaj A Nanoparticle-mediated gene therapy strategies for mitigating inflammatory bowel disease. Biomaterials Science 2021, 9 (5), 1481–1502. [DOI] [PubMed] [Google Scholar]


