Abstract
Over the past two decades, China has introduced significant changes to drug regulations through regulatory innovations to accelerate drug review and approvals, keeping in line with the rapidly growing scientific innovation in drug research and development (R&D). In this study, we outlined the revolution of drug regulation in China since the establishment of the State Drug Administration in 1998. More particularly, we performed a comprehensive analysis of newly approved anticancer drugs in China from the year 2005 to May 2021, as a powerful illustration of how the revolution has changed the drug R&D landscape. Innovative drug development in China has boomed, benefiting in particular from pro-innovation policies as well as expedited program designations by the authority. We found a significant increase in the number of both imported and domestic new anticancer drugs from 2005 to 2021, with the emergence of drugs with novel mechanisms of action, including immune checkpoint inhibitors and cell therapy products. Drug lag has also been dramatically shortened by more than 70% for imported drugs in years 2016–2020 compared to years 2006–2010. Furthermore, we provide an insight into the potential approaches to further optimize the science-based and clinical value-based regulatory and R&D drug ecosystem in China. This review provides evidence of significant impacts of regulations and policies on drug R&D and suggests that the constantly adapting regulatory ecosystem will speed up drug development in China and worldwide.
Key words: Regulatory innovations, Drug R&D, Anticancer drugs, Innovative drugs, Expedited program designations, Drug lag, Globalization, Unmet medical need
Graphical abstract
This review is an overview of newly approved cancer drugs in China between 2005 and 2021, as a powerful illustration of how revolution in drug regulation has influenced the drug R&D in China.
1. Introduction
Since its foundation, China’s pharmaceutical regulatory authority has evolved over the past two decades. In 1998, the State Drug Administration (SDA) was established to tackle the inconsistency of standards for drug approval across provinces, which marked the commencement of a legal system1 (Fig. 1). The Chinese Good Clinical Practice guidance first issued by the SDA in 1999 and amended as needed set the standard of quality and integrity of clinical research. In 2003, the functions of food administration were incorporated into the SDA, and the Agency turned into the State Food and Drug Administration. The subsequent China Food and Drug Administration (CFDA) was founded in 2013 and elevated to a ministerial-level agency under the State Council. In 2018, as part of China’s government administration overhaul, the CFDA became the present “National Medical Products Administration” (NMPA) under the newly-established State Administration for Market Regulation2. Affiliated with the NMPA and its predecessors, the Center for Drug Evaluation (CDE) has been responsible for scientific evaluation of clinical trial applications, marketing authorization applications, and subsequent amendments and renewals3 for drugs (including chemical, biological products, and traditional Chinese medicine). With the commitment to modernizing its structure, the CDE implemented several rounds of agency reorganization to streamline the review procedures. The quadrupled number of employers at the CDE by the end of 20164 and further expansion also made great contributions to the more efficient review processes. By adopting a series of regulatory innovations to accelerate drug development and drug review, the regulatory authority is becoming a science-based and clinical value-based regulatory agency intended to ensure drug efficacy, safety, and quality.
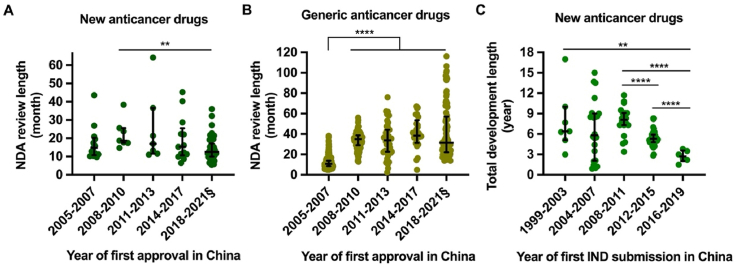
Figure 1.
Iteration of China’s key pharmaceutical laws and policies, 1998–2021. Major policies and announcements were grouped according to their main objectives and effects: reform and deepening, drug registration regulation (DRR), drug administration law (DAL), expedited programs, imported drug policies, and generic drug policies. SDA, State Drug Administration; SFDA, State Food and Drug Administration; CFDA, China Food and Drug Administration; NMPA, National Medical Products Administration; CDE, Center for Drug Evaluation; ICH, International Council for Harmonization; IND, investigational new drug; NDA, new drug application; MRCT, multi-regional clinical trials; MAH, Marketing Authorization Holder.
Given the rising unmet medical needs associated with various malignancies, anticancer drugs have been the priority for drug research and development (R&D)5, which represent the majority of expedited program-designated drugs and dominate the newly approved medicines in China6,7. Although the effects of certain designations in drug review have been recognized8, the full implications of drug regulations are not fully understood. Here we investigated the trends and characteristics of anticancer drug approvals in China from 2005 to 2021 as an illustration of the impacts of regulatory innovation on drug R&D.
2. Iterations of key laws and policies
China’s drug regulatory framework consists of multiple tiers. The primary statute regulating drugs (including biologics) is the Drug Administration Law of the People’s Republic of China (DAL), supplemented by one set of general implementing rules, referred to as the DAL Implementing Regulations. Under the DAL, the Drug Registration Regulation (DRR) is the core document governing clinical trials and drug registration. The CDE also participates in drafting guidance documents related to drug development and registration. During the past decades, significant iterations and adaptations of legislation and policies have been made in response to historical events. The regulatory body was originally based on the first version of the DAL (promulgated in 1984 and revised in 2001) and the DRR (enacted in 2002 and effective in 2005)9. In 2007, in the wake of several drug misadventures, such as the serious neurological adverse reactions caused by intrathecal injection of methotrexate contaminated with vincristine10,11, the authority made substantial amendments to the DRR to strengthen supervision of drug quality. No further comprehensive revisions to the DAL and the DRR were adopted in the following decade until 2015.
In 2015, as the conflict between the public’s need for health and the slow pace of review and approval of therapeutics continued to intensify, the trumpet for regulatory reform was blown. Under the guidance of landmark documents “Opinions on Reforming the Review and Approval System of Drugs and Medical Devices” (State Council [2015] No.44) released in August 201512 and “Opinions on Deepening the Reform of Review and Approval Processes to Encourage Innovation of Drugs and Medical Devices” (State Council [2017] No.42) released in October 201713, an array of unprecedented initiatives were introduced to enhance the efficiency of the drug review process, stimulate clinical value-based innovation, and improve drug quality (Fig. 1)2,14. In conjunction with the implementation of regulatory innovations, the CFDA joined the International Council for Harmonisation (ICH) as a regulatory member in 2017 and pledged to implement international standards in China15,16. In parallel to innovative drugs, generic drugs were also encouraged, accompanied by emphasizing drug quality through improving the technical standards stipulated by the Generic Quality Consistency Evaluation (GQCE) requirements17.
To keep in line with the rapidly growing technological and scientific innovation in drug R&D, the NMPA has progressively adopted a clinical value-based drug regulatory system. The upgraded system is now leading the industry to value innovation and focus on patients’ needs. The latest revisions of DAL and DRR (effective on 1 December 2019 and 1 July 2020, respectively) have incorporated and optimized the time-tested and effective regulatory processes including expedited designation programs, communication mechanisms with the regulators, and 60-working-day silent approval procedures for investigational new drug (IND) applications (Fig. 1)18. A growing number of scientific and technical guidelines have been released to address the newly revised DAL and DRR. In 2021, the CDE issued a record 87 guidelines. Notably, a recently enacted guideline stressed the driving force of “clinical value” in anticancer drug R&D19, which lays the foundation for patient-focused drug development. Furthermore, China’s National Health Commission published a pilot guidance of a comprehensive and scientific framework of drug evaluation on drug safety, efficacy, economic value, innovation, appropriateness, and accessibility20.
3. Approval of anticancer drugs in China
Between January 2005 and May 2021, China’s authority approved 660 anticancer drugs, including 94 new drugs (Table 1). During the period of 2005–2007, the vast majority (241/258) were generic drugs, while the other 13 were new drugs. Triggered by several cases of drug misadventures and corruption matters, the authority determined to strengthen drug management and regulation by mandating collective review and approval for generic drugs in 2008. Collective review and the following GQCE requirements in 2016 are the two most important milestones that significantly raised the standards for generic drugs and ruled out redundant generics. As a result, multiple clinical applications were voluntarily withdrawn by sponsors. Furthermore, the registration backlog compromised the approval of both new drugs and generics21. The number of annually approved anticancer drugs declined remarkably. In particular, only 13 new and 31 generic drugs were approved between 2014 and 2017 (Table 1). As a positive impact of the regulatory reform and improved R&D capacity, China has seen an explosion in drug development since 2018 (Fig. 2A and B, Table 1). Notably, the number of approved new drugs reached 54 (33.5%) between 2018 and May 2021 (Table 1). During this same period, 99 generic drugs, 6 biosimilars, and 2 modified new drugs were approved. Consistently, the number of investigational drug candidates has expanded considerably in recent years22,23.
Table 1.
Summary of anticancer drugs approved by the year of first approval in China.
| Year of first approval in China | 2005–2007 | 2008–2010 | 2011–2013 | 2014–2017 | 2018–2021∗ | Overall |
|---|---|---|---|---|---|---|
| Total number | 258 | 140 | 57 | 44 | 161 | 660 |
| Registration type, No. (%) | ||||||
| New drug | 13 (5.0%) | 7 (5.0%) | 7 (12.3%) | 13 (29.5%) | 54 (33.5%) | 94 |
| Generic | 241 (93.4%) | 130 (92.9%) | 50 (87.7%) | 31 (70.5%) | 99 (61.5%) | 551 |
| Modified new drug | 1 (0.4%) | 2 (1.4%) | 0 | 0 | 2 (1.2%) | 5 |
| Biosimilar | 3 (1.2%) | 1 (0.7%) | 0 | 0 | 6 (3.7%) | 10 |
| Drug type, No. (%) | ||||||
| Chemical | 250 (96.9%) | 137 (97.9%) | 57 (100.0%) | 44 (100.0%) | 140 (87.0%) | 628 |
| Biologicals |
8 (3.1%) |
3 (2.1%) |
0 |
0 |
21 (13.0%) |
32 |
| New drugs | ||||||
| MOA type, No. (%) | ||||||
| Cytotoxic | 5 (38.5%) | 3 (42.9%) | 2 (28.6%) | 2 (15.4%) | 5 (9.3%) | 17 |
| Targeted | 5 (38.5%) | 4 (57.1%) | 5 (71.4%) | 11 (84.6%) | 39 (72.2%) | 64 |
| Immuno-oncology | 1 (7.7%) | 0 | 0 | 0 | 8 (14.8%) | 9 |
| Miscellaneous | 2 (15.4%) | 0 | 0 | 0 | 2 (3.7%) | 4 |
| Drug origin, No. (%) | ||||||
| Domestic | 4 (30.8%) | 1 (14.3%) | 2 (28.6%) | 2 (15.4%) | 18 (33.3%) | 27 |
| Imported | 9 (69.2%) | 6 (85.7%) | 5 (71.4%) | 11 (84.6%) | 36 (66.7%) | 67 |
| NDA review length (months) |
14.8 (10.8–20.3) |
18.7 (17.5–25.6) |
17 (11.6–36.6) |
15.3 (10.6–25.6) |
12.5 (10.0–17.2) |
13.5 (11.2–20.0) |
| Generics | ||||||
| MOA type, No. (%) | ||||||
| Cytotoxic | 166 (68.9%) | 82 (63.1%) | 38 (76.0%) | 25 (80.6%) | 54 (54.5%) | 365 |
| Targeted | 11 (4.5%) | 4 (3.1%) | 7 (14.0%) | 2 (6.5%) | 40 (40.4%) | 64 |
| Immuno-oncology | 0 | 0 | 0 | 0 | 0 | 0 |
| Miscellaneous | 64 (26.6%) | 44 (33.8%) | 5 (10.0%) | 4 (12.9%) | 5 (5.1%) | 122 |
| Drug origin, No. (%) | ||||||
| Domestic | 237 (98.3%) | 127 (97.7%) | 49 (98.0%) | 29 (93.5%) | 94 (94.9%) | 536 |
| Imported | 4 (1.7%) | 3 (2.3%) | 1 (2.0%) | 2 (6.5%) | 5 (5.1%) | 15 |
| NDA review length (months) | 10.8 (8.5–13.9) | 35.0 (29.0–38.7) | 33.8 (22.7–44.2) | 38.4 (31.3–53.4) | 31.5 (22.1–57.0) | 22.2 (11.5–36.0) |
Note: MOA, mechanism of action; %, calculated by each period; NDA, new drug application. NDA review length and total development length are presented with median (IQR).
Data until 31 May 2021 was included.
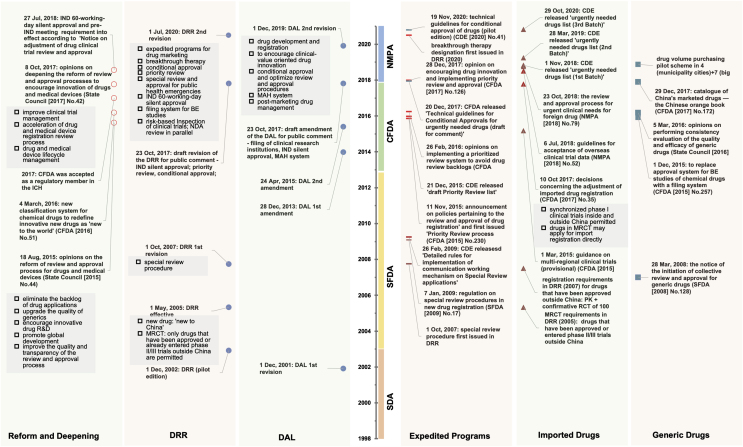
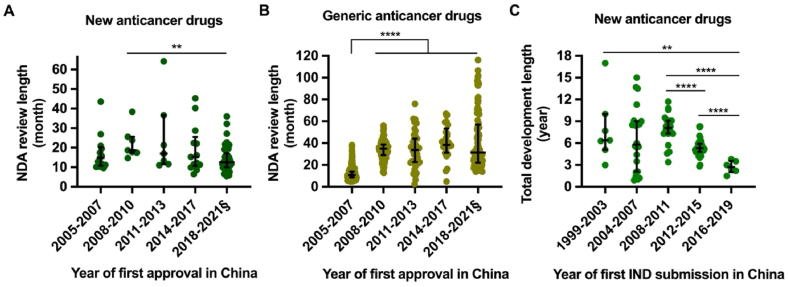
Figure 2.
Trend of NDA review length and total development length of new anticancer drugs approved in China, 2005 to 2021. The new drug application (NDA) review length is shown by the time between NDA submission and approval among new anticancer drugs (A) and generic drugs (B). Drugs were grouped into quintiles of the first approval year in China spanning from January 2005 to May 2021. Only four generic drugs (no new anticancer drugs) were approved in 2016; therefore, drugs approved between 2014 and 2017 were presented as a group. The total development length of new anticancer drugs (C) is shown by the time between the submission of investigational new drug (IND) application to first approval in China. Drugs are grouped according to the year of first IND submission in China. Horizontal lines indicate medians, and error bars depict interquartile ranges. §Data until 31 May 2021. ∗∗P < 0.01, ∗∗∗∗P < 0.0001.
As a regulatory speed-limit step in drug R&D, IND and new drug application (NDA) review timelines were significantly reduced. According to the 2013 CDE annual report24, there was an average 6-month and 13-month waiting period for IND applications for chemicals and biologic therapeutics, respectively. Since the practice of silent approval limit of 60 working days, it was reported that 99.86% of IND applications were approved within the time limit in 20217. In terms of NDA review length, we found that the median length of new anticancer drugs decreased from 18.7 (17.5–25.6) months in 2008–2010 to 12.5 (10.0–17.2, P = 0.0028) between January 2018 and May 2021 (Fig. 2A). The total median time to marketing licensure for new drugs that submitted IND applications during 2008–2011 was 8.1 (7.3–9.1) years, and this time decreased to 5.3 (4.8–5.9, P < 0.0001) years for those that submitted IND during 2012–2015 (Fig. 2C).
The growing scientific and technological innovation has facilitated the development of drugs with various mechanisms of action (MOA) (Table 1). Prior to 2010, most anticancer drugs approved in China were cytotoxic chemotherapies, and the majority of them were generics. In the following decade, the number of cytotoxic drugs declined while drugs of other MOAs expanded. Specifically, the number of new targeted drugs has been growing, from 5 products (38.5%) in 2005–2007 to 11 products (84.6%) in 2014–2017, and reaching 39 products (72.2%) between 2018 and May 2021. Subsequently, targeted generics and biosimilars have recently shown a rapid increase, with 40 generics and six biosimilars approved in 2018–May 2021. Immunotherapy started to thrive in 2018, with 8 products approved as of May 2021. Furthermore, two chimeric antigens receptor (CAR)-T cell therapies were approved in China in June and September 2021.
4. Promoting domestic drug development
Since 2007, several expedited programs have been developed in China25, constantly catalyzing domestic innovation. The first action was to create a communication channel between the industry and the regulatory authority. The Special Review (SR) program was initially introduced in the 2007 revised DRR for new drugs treating severe conditions and demonstrating substantial clinical benefits (Fig. 1 and Table 2). Drugs with SR designation would receive more intense guidance from the CDE for development and registration. Based on our findings, 39 (41.5%) approved new anticancer drugs were granted SR designations (Fig. 3). The SR designation focused on communication but had little impact on NDA review length and total development length (Fig. 4). The benefits of this program became less tangible after a formal avenue of communication between sponsors and regulators was established in 2016. The SR designation was therefore terminated in 2020.
Table 2.
Expedited programs developed in China.
| Review type | Description |
|---|---|
| Special Review (SR) | A program issued in the 2007 DRR and applicable for new drugs that not yet launched in any countries. With SR designation, the applicants would receive more intensive guidance from the regulatory authority. The SR program was removed from the 2020 DRR. |
| Priority Review (PR) | A program introduced by the former CFDA in November 2015 and issued in the 2020 DRR. PR applies to primarily new drugs that showing substantial clinical benefits, for urgently needed conditions and diseases such as major infectious diseases and rare diseases, and new or modified drugs for pediatrics. PR designation shortens the NDA review length to 130 working days as stipulated in the 2020 DRR. |
| Conditional Approval (CA) | A program introduced by the CFDA in December 2017 and issued in the 2020 DRR. CA applies to drugs and medical devices specified for serious life-threatening conditions, significant unmet medical requirements or rare diseases where early or mid-stage clinical data can be used to anticipate clinical benefits. CA designation allowed NMPA to approve drugs based on outstanding early trial results or surrogate endpoints. |
| Breakthrough Therapy (BT) | A designation issued in the 2020 DRR and applicable to new drugs that are used for the prevention and the treatment of diseases that seriously endanger life or affect the quality of life, for which there is no effective prevention and treatment, or, compared with existing measures of treatment, there is sufficient evidence proving the obvious clinical advantages. |
DRR, drug registration regulation; CFDA, China Food and Drug Administration; NMPA, National Medical Product Administration.
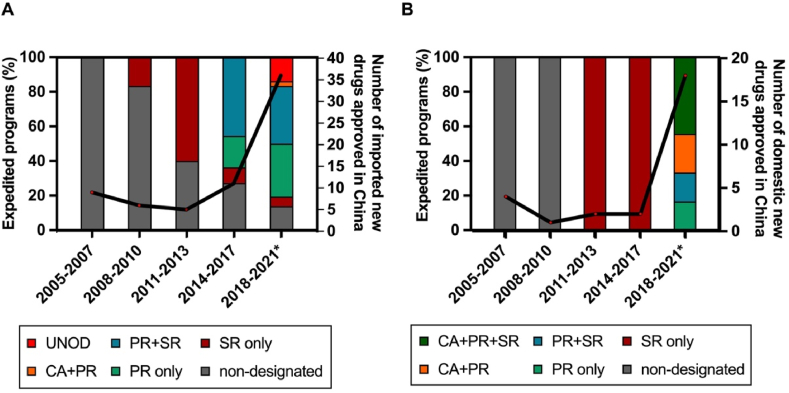
Figure 3.
Uptake of expedited programs (EP). The proportion of EP designations and overall number of imported (A) and domestic (B) new anticancer drugs are shown in three-year intervals from January 2005 to May 2021. SR, Special Review designation, effective between October 2007 and June 2020; PR, Priority Review, effective from November 2015; CA, Conditional Approval, effective since December 2017; UNOD, Urgently Needed Overseas Drugs, three batches of UNOD released since November 2018. ∗Data until 31 May 2021.
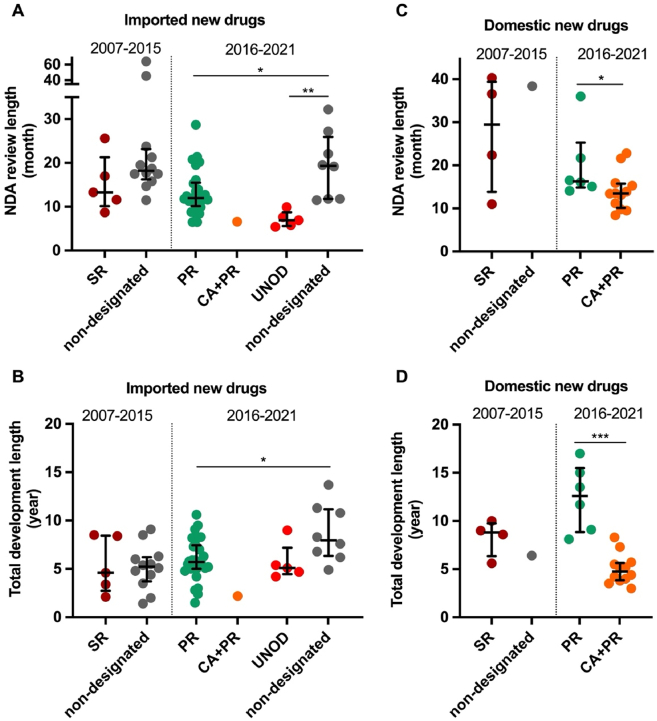
Figure 4.
NDA review length and total development length of approved new anticancer drugs in China by expedited programs, 2007 to May 2021. The new drug application (NDA) review length (month) is shown by the time between NDA submission and approval for imported (A) and domestic new drugs (C), and the total development length (year) is shown by the time between the submission of investigational new drug (IND) application to first approval in China for imported (B) and domestic (D) new drugs, respectively. Dark red circles denote anticancer drugs granted Special Review (SR) designation from October 2007 to December 2015. Green, bright red, and orange circles denote anticancer drugs with Priority Review (PR), Urgently Needed Overseas Drugs (UNOD), and PR plus conditional approval (CA) designations, respectively, since Dec 2015. Comparators (non-SR or non-PR/UNOD) are approval year-matched anticancer drugs without expedited programs designation. Horizontal lines indicate medians, and error bars depict interquartile ranges. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
In December 2015, as one of the pioneer measures of the comprehensive regulatory reform, the Priority Review (PR) program was launched to shorten the drug review length. The objective of the PR program was initially intended to reduce the registration backlog26 and subsequently shifted to improving clinical value in December 201725. Drugs that address unmet medical need and show a large magnitude of clinical benefits are eligible for this designation. Eighteen (100%) domestic anti-cancer new drugs approved after 2017 were granted PR designation (Fig. 3), with a median drug review time of 14.6 months (Fig. 4).
Two additional programs were developed to introduce regulatory flexibilities, including Conditional Approval (CA) and Breakthrough Therapy (BT) (Table 2). In December 2017, the CA pathway was established for drugs to be approved on the basis of surrogate endpoints that are reasonably predictive of clinical benefits or less comprehensive clinical data27. CA applies to drugs with high clinical value over existing therapeutics in the setting of life-threatening or rare conditions. For instance, two home-made next-generation Bruton tyrosine kinase (BTK) inhibitors, zanubrutinib and orelabrutinib, possibly conferring efficacy and safety advantages compared with the first-generation inhibitor ibrutinib, received conditional approval by the NMPA for patients with relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma28, 29, 30. Similar to the Accelerated Approval program in the US, post-marketing commitment to confirm actual clinical benefits is required for drugs receiving CA in China9. According to our findings, CA designation significantly correlated with reduced total development length (Fig. 4D). Twelve domestic PR-designated drugs were approved under the CA pathway with a median of 4.8 (3.9–5.7) years after IND submission, compared with 12.6 (8.9–15.5) years for domestic PR-designated counterparts not receiving CA (difference, 7.8 years; P = 0.0002). BT was first introduced in the revised 2020 DRR for drugs with preliminary clinical data showing a large magnitude of clinical benefits over existing therapies. The development of BT-designated drugs can be expedited through intensive communication with the authority, and these drugs are potentially eligible for the CA and PR pathways. As of May 2022, 46 new anticancer drugs have been granted BT designation in China. Five of them have been approved by the NMPA for their BT-designated indications.
5. Reducing drug lag
Promoting accessibility to imported new drugs is another way to address unmet medical needs. Historically, before 2000, drugs were approved without requiring additional clinical trials conducted in China when enduring medical demands were identified. With the accumulation of R&D and supervisory experience, the authority began requiring Chinese data prior to marketing approval31. Before 2017, for novel drugs, initiation of phase 2 trials overseas was a prerequisite for Chinese sites to participate in multi-regional clinical trials (MRCT), and holding licensure issued by other countries was a must prior to Chinese marketing approval32. Multi-national pharmaceutical companies thus introduced globally approved drugs to China by bridging studies, which dominated the registration strategies in China between 2008 and 2013. Moreover, the inefficient drug review process was another barrier to the entry of imported drugs. According to our findings, the NDA review length for imported new drugs between 2008 and 2013 was the longest at 17.7 (15.9–22.6) months. As reported in the 2013 CDE annual report, there was an increasing backlog of NDA review (up to 14 months waiting period), and the waiting period of IND review for chemical drugs conducting bridging studies was even longer (up to 19 months)24. Hence, drug development and approval in China have lagged behind the West significantly21, with 5.8 (3.4–7.8) years lags of entering the clinical phase for anticancer drugs that started clinical trials in the US in 2001–2005, and 6.6 (2.8–9.5) years gap in drug approval for anticancer drugs that approved in the US in 2006–2010 (Fig. 5). This drug lag (defined as the time between a new drug being approved in the US and the subsequent approval in China) limited drug access and hampered drug innovation8,21.
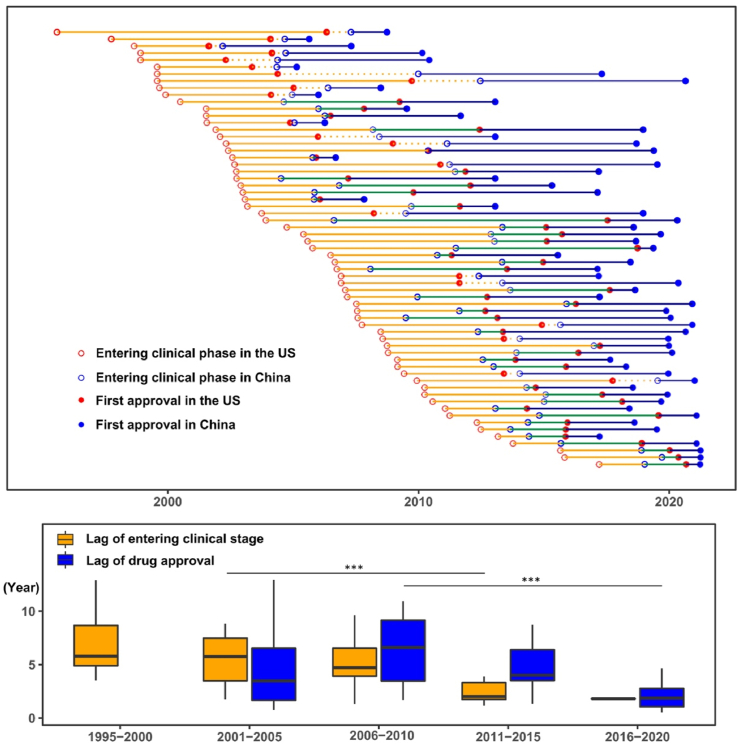
Figure 5.
Clinical development and first approval of imported new anticancer drugs in the US and China. Clinical development and approval dates are shown by clinical development and registration strategies and development timelines (five-year interval from 1995 to 2021). Each line represents one drug approved in China between Jan 2005 to May 2021 that was approved in the US after 2000. The red and blue hollow circles represent the start date of first clinical trial in the US and dates of investigational new drug (IND) submission in China. Red and blue solid circles are dates of first approval in the US and China, respectively. Orange line segments (from red hollow circle to blue hollow circle) indicate the time difference of entering clinical stage between the US and China, which is plotted as orange boxes on the bottom by five-year intervals. Similarly, blue line segments (from red solid circle to blue solid circle) indicate the lag of drug approval between the US and China, which is further plotted as blue boxes. ∗∗∗P < 0.001.
In response, China’s regulatory authorities have taken multiple ground-breaking actions to improve China’s regulatory and R&D environments33 since 2015 (Fig. 1). Effective in October 2017, the authority allowed synchronized first-in-human studies tested in Chinese participants for new agents developed outside China34 (Fig. 1). In addition, joining the ICH in 2017 paved the way for harmonizing the technical requirements for registration in China with global standards. These measures are supportive of China joining multi-regional clinical trials at an early stage and promoting patient access. Subsequently, the proportion of drugs adopting MRCT pathway began to expand, reaching at 52% between 2015 and May 202135. A trend of narrowed drug lags was observed between the US and China for drugs adopting MRCT in China in comparison to those adopting bridging strategies in China (3.8 vs. 4.7 years, P = 0.225). Moreover, China has been streamlining the review and approval processes. The review timeline for PR-designated new drugs was significantly reduced. For 31/44 imported drugs with PR designation, the median NDA review lengths were 12.0 (10.2–15.5) months, compared with 19.4 (11.8–25.9, P = 0.040) months for non-designated drugs (Fig. 4A). This was also accompanied by relatively shorter total development lengths (PR, 5.7 [5.0–7.5] years vs. non-designated, 8.0 [6.4–11.2] years; Fig. 4B).
The acceptance of clinical data from trials conducted outside China was formally adopted in the guideline issued by the NMPA in 201836. Accelerated approval of foreign-approved drugs on the Urgently Needed Overseas Drugs (UNOD) lists released between 2018 and 2020 was a canonical illustration of accepting overseas data when recognizing the unmet needs and drug lag in treating serious or life-threatening diseases (Table 2). UNOD-designated drugs, usually with substantial clinical benefits, were allowed to apply for marketing authorization with limited or even no Chinese clinical data if no ethnic differences were predicted and were entitled to have a shortened NDA/Biologic License Application (BLA) review length of three to six months37. Five anticancer drugs on the UNOD lists were approved with a median of 5.1 (4.5–7.2) years after IND submission (Fig. 4B) with a median review length of 6.9 (5.6–8.8) months (Fig. 4A). Among them, the first-in-class (FiC) FLT3 inhibitor gilteritinib, approved by the US Food and Drug Administration (FDA) in 2018, significantly improved the survival of patients with relapsed or refractory (r/r) acute myeloid leukemia with FLT3 mutations, compared to salvage chemotherapy38. In light of the limited treatment options for these patients in China, gilteritinib was included in the UNOD list in November 202039 and was approved by the NMPA in February 2021 with an ongoing confirmatory study40.
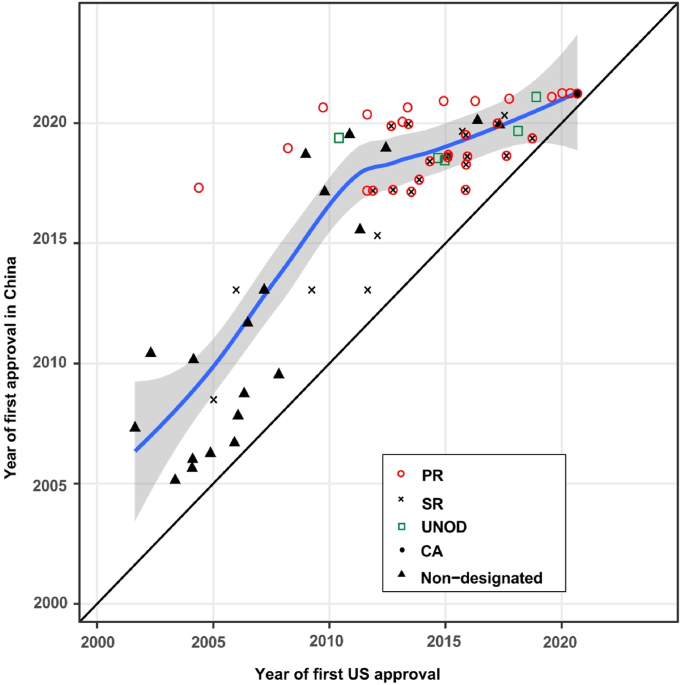
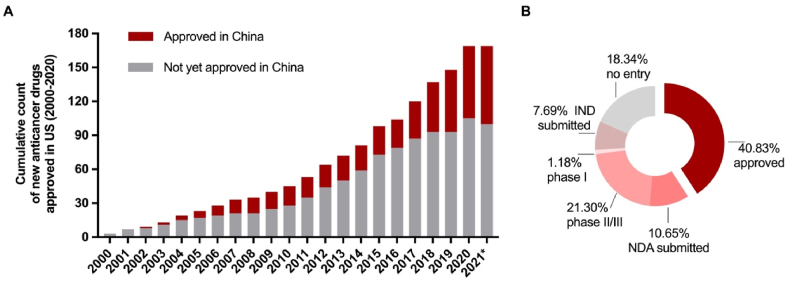
Thanks to the collective efforts of the pharmaceutical industry, clinical sites, and the regulatory authority, a remarkably shorter drug lag has been witnessed in recent years. Lags of entering the clinical phase have decreased dramatically to 2.0 (1.4–3.5) years for anticancer drugs that entered the clinical phase in the US in 2011–2015 (compared to 5.8 [3.4–7.8] years in 2001–2005, P = 0.0009), accompanied by a shortened gap in drug approval (1.9 [1.0–2.9] years, 2016–2020 vs. 6.6 [2.8–9.5] years, 2006–2010; P = 0.0006, Fig. 5). The fitted LOWESS curve demonstrated the trend of reduction in drug lag, approaching 1.4 years in 2021 (Fig. 6). Accordingly, the proportion of new anticancer drugs licensed in China among those approved in the US has been rising over the past two decades (Fig. 7A). As of May 2021, 40.8% (69/169) of anticancer drugs licensed in the US between 2000 and 2020 have achieved marketing licensures in China, with another 10.7% awaiting review of the submitted NDA/BLA by the authority (Fig. 7B).
Figure 6.
First approval dates of imported new anticancer drugs in the US and China. Red circles, black solid circles, and black X marks denote anticancer drugs granted Priority Review (PR), Conditional Approval (CA), and Special Review (SR) designations, respectively. Green squares indicate new drugs that were on the lists of Urgently Needed Overseas Drugs (UNOD) released since Nov 2018. Black triangles denote the drug approvals undergoing non-designated procedures. The diagonal solid line represents the same day of approval in the U.S. and China. The fitted line in blue with 95% confidence interval based on the locally weighted scatterplot smoothing (LOWESS) approach indicates the tendency of drug lag. The line approaching the diagonal line means the shortened drug lag.
Figure 7.
Current status in China of new anticancer drugs approved in the US (A) A cumulative number of new anticancer drugs approved in the U.S. from 2000 to 2020 and of which the number of drugs approved in China as of May 2021 (B) Proportion of drugs in various stages in China as of May 2021 among new anticancer drugs approved in the US between 2000 and 2020. IND, investigational new drug application; NDA, new drug application.
6. Discussion
Following over two decades of reforms, China’s pharmaceutical regulatory authority has been upgraded to be a more efficient, scientific, and clinical value-based system to keep pace with the rapidly growing technological and scientific innovations. We have witnessed a growing number of imported and domestic new anti-cancer drugs approved in China, which largely benefitted from the recent regulatory revolutions, R&D capabilities, and technical innovation.
Improving drug accessibility to address unmet medical needs has always been the priority for Chinese regulators and drug developers. Mitigating the lag for imported new drugs is one critical step. As shown here, the drug lag compared with the US has been significantly reduced, although a two-year lag of imported drugs still exists in China compared with the US. To close the gap, in-parallel drug review and risk-based on-site inspections, as stipulated in the latest revision of DRR in 2020, are expected to further shorten the review length. Pralsetinib was an example of in-parallel review, with a review length of 6.6 months under the PR and CA designations41. In addition, in-licensing drugs to domestic companies has become an efficient approach for overseas biotech companies to gain early access to the Chinese market22. For instance, the first in-licensed agent niraparib, granted to Zai Lab from Tesaro in 2016, was first approved by the NMPA 2.8 years after the FDA’s first approval. Axicabtagene ciloleucel, a CD19-directed CAR-T cell therapy, was the first CAR-T cell therapy approved in China in June 2021, with the technology of YESCARTA® (axicabtagene ciloleucel) transferred from Kite Pharma.
Home-grown innovation is booming in recent years, but mainly from drugs in established drug classes with similar efficacy and safety profiles, i.e., so-called “me-too” drugs. Indeed, moderate competition is necessary for the health system to control costs and increase drug affordability42, and also critical to managing the emergence of drug shortages43. In the end, patients can benefit from a cascade of available anticancer drugs with reasonable prices, including me-too drugs, generic drugs, and biosimilars. However, redundant development of similar drugs would result in an improper allocation of resources and inadequate investments in therapeutic areas that lack sufficient drugs22,44. For instance, over ten anti-PD-1 or PD-L1 monoclonal antibodies have been approved in China till today, however, there are still a substantial number of similar candidates in clinical phase pursuing efficacy-proven indications45. Resemblances have been observed in generics, as illustrated by over 60 generic versions of azithromycin tablets in the Chinese market, while there are no generic atazanavir sulfate available even after the patent of the reference drug has expired46. Imbalance of drug development has long been a global issue44. To confront such obstacles, the FDA has offered a package of regulatory incentives such as the orphan drug designation47, and has established the drug shortage database to monitor drug accessibility48. These collective efforts brought increasing drugs approved in the US in areas with urgent need, including hundreds of orphan drug approvals49 and generic drug approvals involving drugs previously in shortage50. Likewise, China’s authority issued regulatory guidelines emphasizing unmet medical needs and published several lists of drugs that are off-patent or close to patent expiration without generic counterparts51. In the future, a comprehensive value and quality evaluation framework are warranted with respect to procurement, hospital use, and pricing, which will encourage the development of innovative drugs with actual clinical values and high-quality generics.
The Chinese pharmaceutical industry is currently in the middle of a transition from largely “me-too” to “me-better” drugs in established therapeutic classes22,23. It is anticipated that new drugs with optimized structures or based on advanced techniques and platforms will demonstrate better efficacy or safety profiles, or provide alternative benefits to patients. For instance, the world’s first subcutaneously administered PD-L1 antibody, envafolimab may show compliance and safety advantages over intravenously administered PD-(L)1 antibodies52.
Indeed, first-in-class innovation would inevitably pose greater unforeseen risks to both sponsors and regulators. Proportionate risk management and reliable benefit-risk assessments are crucial to proactively address such challenges. It is encouraging that the Chinese regulatory system constantly adapts to external changes to make science-based regulatory decisions. For example, the “global first” bispecific PD-1/CTLA-4 antibody, cadonilimab, domestically developed by Akesobio, received BT designation by China’s CDE based on its favorable preliminary data53 showing higher objective response rate in advanced cervical cancer, compared with data from other trials evaluating combination therapies of anti-PD-1 plus anti-CTLA-4 antibodies54,55. Based on these superior results, the drug has been approved under expedited pathways in June 202256. This reflects the strides made by Chinese companies in developing FiC drugs and the professionals of reviewers in benefit-risk evaluation for drugs with novel mechanisms of action.
Globalization is becoming a norm for drug development in China57. In addition to overseas pharmaceutical companies incorporating China in their global registrational strategy33,58, local companies are beginning to step up to the international arena. In 2020, around one-fifth of investigational anticancer products discovered in China were also being developed outside China22. Successful global development requires a rigorous and prospective strategic plan. Using China-only data to support drug marketing in other countries is inevitably challenging. For instance, the FDA has issued complete response letters for sintilimab and surufatinib. When making regulatory decisions, the FDA has indicated that the degree of unmet medical need and the magnitude of clinical benefits of investigational drugs will determine the degree of regulatory flexibility, which is similar to China’s authority35,59. Notably, zanubrutinib received double approvals from the FDA and NMPA using pivotal data mainly from patients in China, becoming the first domestic innovative drug achieving simultaneous development across countries14. China's first locally developed antibody–drug conjugate disitamab vedotin received BT designation by the FDA for the second-line treatment of HER2 positive locally advanced or metastatic urothelial cancer60. As a novel and promising therapeutic method for this indication61, disitamab vedotin fulfilled the unmet medical need in the US, underscored by the high prevalence of metastatic urothelial cancer and the limited effective treatments. China’s NMPA has been promoting clinical value-based drug development, requiring the use of the best standard of care available as control, and encouraging timely communication about pivotal trial designs and major decisions. The NMPA has also committed to improving the Good Manufacturing Practice and has been accepted as a pre-accession member of the Pharmaceutical Inspection Cooperation Scheme last year. In the future, simultaneous drug development and joint regulatory review will further accelerate the globalization of drug R&D.
Multiple regulatory and health policy communities around the globe have been actively engaging with patients throughout the entire drug development process62. In the US, FDA’s Patient-Focused Drug Development (PFDD) initiative was incorporated into the Prescription Drug User Fee Act (PDUFA V) (reauthorized in 2012) and the 21st Century Cures Act (enacted into law in 2016)63,64. The FDA has made significant progress in implementing the fundamental goals regarding patient engagement, including the inclusion of patient experience data in the review process and providing PFDD guidance64,65. Novel web-based platforms such as “Project Patient Voice” were established to voluntarily report patient-reported symptoms and side effects, supplementing the safety information of clinical trial data66. In China, patient centeredness is evolving into the core philosophy of R&D, as underscored by the recently released guidance series on Patient-Focused Clinical Trials: Design, Implementation and Benefit-Risk Assessment67, 68, 69. The Patient-Focused Guidance series encouraged proper adoptions of decentralized clinical trials (DCTs) using digital solutions to alleviate the burden of trial participation and enable trial continuity.
7. Conclusions
Regulations and policies have significant impacts on pharmaceutical R&D. During the past decades, China’s authority has transformed into a science-based and clinical value-based regulatory system and witnessed an expansion of innovative anticancer drugs and high-quality generics. Expedited programs further facilitated innovation for drugs with high clinical value, accompanied by a remarkably shortened review length and R&D total development length. Drug launch delays have been significantly mitigated to tackle drug availability. In the future, the constantly adapting Chinese regulatory ecosystem will further speed up drug development and provide earlier accessibility for patients in China and worldwide.
8. Methods
8.1. Pharmaceutical laws and regulations
We reviewed relevant laws, regulations, and technical guidelines and summarized key regulatory milestones (Fig. 1) including major modifications of the DAL and the DRR, landmark documents and significant measures along the history of drug regulations in China.
8.2. Data extraction
Publicly available drug information of all cancer drugs that received marketing authorization in China between 1 January 2005 and 31 May 2021, were extracted from the commercial database Pharmcube, one of China’s most authoritative drug information platforms. All data were manually verified based on the official databases of China’s NMPA (https://www.nmpa.gov.cn) and CDE (https://www.cde.org.cn). Drug data from the US were cross-checked on the Drugs@FDA and Orange Book database from the US FDA website (https://www.fda.gov/drugs). Basic characteristics consisted of drug types (new drugs [new to China]-including pharmacologics and biologics-regardless of approval status in other countries, modified new drugs, biosimilars, or generics), mechanisms of action (cytotoxic, targeted, immune-oncology therapy, or miscellaneous), and origins (locally developed [domestic] or foreign-developed [imported]). Regulatory characteristics comprise the submission dates of IND application and NDA/BLA, the first approval dates in China, and expedited programs (Special Review [SR], Priority Review [PR], and Conditional Approval [CA]) received.
For imported drugs, we also collected the launch dates of the first clinical trials in the US documented on ClinicalTrials.gov (https://www.clinicaltrials.gov/, a publicly available clinical study registry database developed by the US National Library of Medicine) and the dates of first approval in the US. Drugs on the lists of Urgently Needed Overseas Drugs (UNOD) were marked. Based on the clinical development strategies in China, imported drugs were further categorized into 1) waiving trials (not conducted clinical trials in Chinese population before drug registration in China due to no predicted ethnic differences), 2) bridging studies in China according to the guideline ICH-E5, and 3) China joining multi-regional clinical trials (MRCT) groups.
We also collected anticancer drugs in clinical development granted with Breakthrough Therapy (BT) designation in China (effective as of July 2020).
8.3. Analysis of drug review length, total development length, and drug lag
To assess the metrics of drug development and approval, we calculated the drug review length (NDA review length), total development length, and drug lag compared with the US25. Briefly, the NDA review length was measured as the time difference between NDA/BLA approval and submission. The total development length of innovative drugs was calculated as the time from the date of IND application (which marks the commencement of the clinical development phase) to the first approval. Drug lags of entering the clinical phase and drug approvals between the US and China were defined as the gaps between starting clinical trials in the US and dates of IND submission in China, and the gaps between the US’s first approval dates and that of China, respectively.
8.4. Statistical analysis
Numerical data are presented with median and interquartile ranges. A non-parametric Mann–Whitney–Wilcoxon test was performed to examine the differences of total development length and drug review length between periods and EP-designated versus year-matched non-EP-designated drugs. To analyze the drug lag, we fit a locally weighted scatterplot smoothing model to assess the trend of drug lags over time and conducted an Mann–Whitney–Wilcoxon test to assess lag time of clinical phase and lag time of drug approval between periods. Statistical analyses were performed using R version 4.0.2 and GraphPad Prism version 9.3.1. A two-tailed P-value <0.05 was considered statistically significant.
9. Limitations
This study has some limitations. First, the dates of the first IND and first marketing approval were extracted to estimate total development length in China regardless of indications. Second, we evaluated the trend of the total development length of new drugs by periods grouped by the dates of first IND submission in China. As a result, the total development length of recent periods might be underestimated, given that some drugs are not yet approved as of the cut-off date of this study (May 2021) and are therefore not included. Similarly, the drug lag of recent years might be underestimated in this study. Third, we used the registered start dates of the first US clinical trials as the commencement of trials in the US, due to the inaccessibility of IND data on the FDA website. Therefore, drug lag times to clinical phase between the US and China were underestimated. Fourth, because different expedited programs were created at different times, there are inherent challenges in assessing the regulatory outcomes between various programs. To simplify, we evaluated the total development time and review time between the designated (including PR, CA and UNOD) and non-designated drug counterparts approved between 2016 and 2021. Fifth, the impact of the IND silent approval policy enacted in July 2020 was fully illustrated by our data since drugs included were approved before May 2021, with all IND applications submitted before 2020. Finally, drug R&D can be affected by factors other than new regulations and programs. Therefore, causal inferences may not be made between specific regulatory policies and faster drug development or review time length.
Acknowledgment
We thank Dr. Ningjun Jiang from CStone Pharmaceuticals for his helpful input in the preparation of this manuscript.
Author contributions
Yang Liu, Cuicui Xie, and Guanqiao Li performed the centrality analysis, and drafted and revised the manuscript. Yale Jiang, Yunhe Qin, and Wei Xie participated in the data cleansing and statistical analysis. Liyun Zhou and Yi Fan verified the analytical methods. Lianjie Ren, Chen Yin, and Huan Yang participated in the design of figures and manuscript writing. Qing Zhai contributed to the revision of the manuscript. Ning Zhang, Hongzhuan Chen and Xiaoyuan Chen conceived and supervised this study. All authors have approved the final article.
Conflicts of interest
Cuicui Xie, Yunhe Qin, Liyun Zhou, and Wei Xie are staff at Pharmcube. The other authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Guanqiao Li, Email: guanqiaoli@tsinghua.edu.cn.
Hongzhuan Chen, Email: yaoli@shsmu.edu.cn.
Xiaoyuan Chen, Email: cxya02648@mail.tsinghua.edu.cn.
References
- 1.Tang W., Huang Y., Zhou D., Huang Y., Chen Y., Ren S., et al. Evolving drug regulatory landscape in China: a clinical pharmacology perspective. Clin Transl Sci. 2021;14:1222–1230. doi: 10.1111/cts.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L., Gao H., Kaitin K.I., Shao L. Reforming China’s drug regulatory system. Nat Rev Drug Discov. 2018;17:858–859. doi: 10.1038/nrd.2018.150. [DOI] [PubMed] [Google Scholar]
- 3.Center for Drug Evaluation of NMPA. National Medical Product Administration. Available from: http://english.nmpa.gov.cn/2019-07/19/c_389169.htm [accessed July 26, 2021].
- 4.Cyranoski D. China cracks down on fake data in drug trials. Nature. 2017;545:275. doi: 10.1038/nature.2017.21977. [DOI] [PubMed] [Google Scholar]
- 5.Brown D.G., Wobst H.J. A decade of FDA-approved drugs (2010–2019): trends and future directions. J Med Chem. 2021;64:2312–2338. doi: 10.1021/acs.jmedchem.0c01516. [DOI] [PubMed] [Google Scholar]
- 6.Su X., Wang H., Zhao N., Wang T., Cui Y. Trends in innovative drug development in China. Nat Rev Drug Discov. 2022 doi: 10.1038/d41573-022-00077-3. Available from: https://doi.org/10.1038/d41573-022-00077-3. [DOI] [PubMed] [Google Scholar]
- 7.Center for Drug Evaluation, NMPA . 2021. Drug review annual report.https://www.cde.org.cn/main/news/viewInfoCommon/f92b7bdf775bbf4c4dc3a762f343cdc8 Cde.Org.Cn. Available from: [Google Scholar]
- 8.Zhu X., Liu B. Launch delay of new drugs in China and effect on patients’ health. Clin Therapeut. 2020;42:1750–1761. doi: 10.1016/j.clinthera.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 9.China Promulgates Revised Drug Registration Regulation. Available from: https://www.cov.com/-/media/files/corporate/publications/2020/04/china-promulgates-revised-drug-registration-regulation.pdf [accessed January 12, 2021].
- 10.Zhao N. Review of Adverse Drug Events in China: Methotrexate Event Causing Serious Neurological Adverse Reactions. sina.cn. Available from: https://k.sina.cn/article_2144896680_7fd886a802000w4l8.html?from=health [accessed June 16, 2021].
- 11.Cancer drug company slammed after probe. China.Org.Cn. Available from: http://www.china.org.cn/english/health/235508.htm [accessed June 30, 2021].
- 12.Shao L., Xu L., Li Q., Chakravarthy R., Yang Z., Kaitin K.I. Innovative drug availability in China. Nat Rev Drug Discov. 2016;15:739–740. doi: 10.1038/nrd.2016.200. [DOI] [PubMed] [Google Scholar]
- 13.China’s State Council . 2017. Official opinions on deepening the review and approval policies reform and encouraging drug and medical device innovations. China med device.https://chinameddevice.com/chinas-state-council-official-opinions-deepening-review-approval-policies-reform-encouraging-drug-medical-device-innovations/ Available from: [Google Scholar]
- 14.Li G., Liu X., Chen X. Simultaneous development of zanubrutinib in the USA and China. Nat Rev Clin Oncol. 2020;17:589–590. doi: 10.1038/s41571-020-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ICH Current Members and Observers. ICH Official Web Site. Available from: https://www.ich.org/page/members-observers [accessed July 26, 2021].
- 16.China Joins ICH as Full Regulatory Member, Pledges to Implement Guidelines. Available from: https://www.fdanews.com/articles/182330-china-joins-ich-as-full-regulatory-member-pledges-to-implement-guidelines [accessed July 26, 2021].
- 17.Chinese patients to see more affordable, high-quality generic drugs. National Medical Product Administration. Available from: http://english.www.gov.cn/state_council/ministries/2018/08/03/content_281476247616990.htm [accessed July 26, 2021].
- 18.Newly Revised Drug Administration Law Adopted After Deliberation—To Comprehensively Implement the Four Strictest Requirements and Effectively Protect Drug Safety for the Public. Available from: http://subsites.chinadaily.com.cn/nmpa/2019-08/26/c_409529.htm [accessed July 26, 2021].
- 19.Center for Drug Evaluation, NMPA. Guidelines of Clinical Value-Oriented Clinical Development for Anti-tumor Drugs. Cde.Org.Cn. Available from: https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=67c30813bd94792b5b2a9f9bd7121763 [accessed June 21, 2022].
- 20.China’s National Health Commission. Guidance of Comprehensive and Scientific Framework of Drug Evaluation (Pilot Edition). Available from: https://www.med66.com/upload/resources/file/2020/11/09/163534.pdf [accessed August 27, 2021].
- 21.Zhou Q., Chen X.Y., Yang Z.M., Wu Y.L. The changing landscape of clinical trial and approval processes in China. Nat Rev Clin Oncol. 2017;14:577–583. doi: 10.1038/nrclinonc.2017.10. [DOI] [PubMed] [Google Scholar]
- 22.Li G., Qin Y., Xie C., Wu Y.L., Chen X. Trends in oncology drug innovation in China. Nat Rev Drug Discov. 2021;20:15–16. doi: 10.1038/d41573-020-00195-w. [DOI] [PubMed] [Google Scholar]
- 23.Li G., Liu Y., Hu H., Yuan S., Zhou L., Chen X. Evolution of innovative drug R&D in China. Nat Rev Drug Discov. 2022;21:553–554. doi: 10.1038/d41573-022-00058-6. [DOI] [PubMed] [Google Scholar]
- 24.Center for Drug Evaluation, NMPA Drug review annual report. 2013. https://www.cde.org.cn/main/news/viewInfoCommon/821e227c1c5a6b40d2e5b841d05e8b69 Available from:
- 25.Li G., Liu Y., Xie C., Zhou Q., Chen X. Characteristics of expedited programmes for cancer drug approval in China. Nat Rev Drug Discov. 2021;20:416. doi: 10.1038/d41573-021-00080-0. [DOI] [PubMed] [Google Scholar]
- 26.Announcement on Policies Pertaining to the Review and Approval of Drug Registration. Available from: http://www.gov.cn/xinwen/2015-11/12/content_2964983.htm [accessed July 27, 2021].
- 27.CFDA released Technical Guidelines for Conditional Approvals for Urgently Needed Drugs (draft for comment). Available from: https://med.sina.com/article_detail_103_2_38632.html [accessed July 27, 2021].
- 28.Hillmen P., Brown J.R., Eichhorst B.F., Lamanna N., O’Brien S.M., Qiu L., et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16:517–523. doi: 10.2217/fon-2019-0844. [DOI] [PubMed] [Google Scholar]
- 29.BRUKINSA® (Zanubrutinib) Demonstrates Superior Objective Response Rate by Investigator Assessment and Reduced Rates of Atrial Fibrillation or Flutter at Interim Analysis in Head-to-Head Trial Against Ibrutinib in Chronic Lymphocytic Leukemia. BeiGene LTD. Available from: https://ir.beigene.com/news-releases/news-release-details/brukinsar-zanubrutinib-demonstrates-superior-objective-response/[accessed July 5, 2021].
- 30.Hillmen P., Eichhorst B., Brown J.R., Lamanna N., O’Brien S., Tam C.S., et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibruitinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. European Hematology Association EHA. 2021 https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/330170/peter.hillmen.first.interim.analysis.of.alpine.study.results.of.a.phase.3.html Available from: [Google Scholar]
- 31.Kang C., Gao C. Clinical research strategies for imported drugs registration. Chin J Clin Pharmacol. 2009;25:375–379. [Google Scholar]
- 32.Bajaj G., Gupta M., Wang H.G.H., Barrett J.S., Tan M., Rupalla K., et al. Challenges and opportunities with oncology drug development in China. Clin Pharmacol Ther. 2019;105:363–375. doi: 10.1002/cpt.1017. [DOI] [PubMed] [Google Scholar]
- 33.Wang M. Clinical trials and drug approvals continue to accelerate in China. Lancet Oncol. 2017;18:855. doi: 10.1016/S1470-2045(17)30406-0. [DOI] [PubMed] [Google Scholar]
- 34.CFDA. Decisions Concerning the Adjustment of Imported Drug Registration. The Central People’s Government of the People’s Republic of China. Available from: http://www.gov.cn/xinwen/2017-10/10/content_5230906.htm [accessed July 27, 2021].
- 35.Li G., Liu Y., He R., Su L., Chen X. FDA decisions on new oncological drugs. Lancet Oncol. 2022;23:583–585. doi: 10.1016/S1470-2045(22)00136-X. [DOI] [PubMed] [Google Scholar]
- 36.Guidelines for Acceptance of Overseas Clinical Trial Data (NMPA [2018] No.52). National Medical Product Administration. Available from: https://www.nmpa.gov.cn/zhuanti/ypqxgg/ggzhcfg/20180710151401465.html [accessed July 1, 2021].
- 37.The Review and Approval Process for Urgent Clinical Needs for Foreign Drug (NMPA [2018] No.79). National Medical Product Administration. Available from: https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20181030171201646.html [accessed July 27, 2021].
- 38.Perl A.E., Martinelli G., Cortes J.E., Neubauer A., Berman E., Paolini S., et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-Mutated AML. N Engl J Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 39.Center for Drug Evaluation, NMPA. Notice on the release of the third batch of clinically urgently needed overseas new drugs. Available from: http://www.cde.org.cn/news.do?method=viewInfoCommon&id=5ed6430be031fc66 [accessed September 3, 2021].
- 40.Astellas’ XOSPATA® (gilteritinib) Receives Conditional Approval by China’s National Medical Products Administration for Relapsed or Refractory Acute Myeloid Leukemia with a FLT3 Mutation | Astellas Pharma Inc. GLOBAL WEBSITE. Available from: https://www.astellas.com/en/news/16411 [accessed September 9, 2021].
- 41.CStone Pharmaceuticals. CStone Pharmaceuticals Voluntary Announcement. Available from:: http://spider.pharmcube.com/spider_298d3f11fde7cbd6af914fe9b4a7edd0.pdf [accessed September 3, 2021].
- 42.Daniel H., Serchen J., Cooney T.G. Health and public policy committee of the American college of physicians policy recommendations to promote prescription drug competition: a position paper from the American college of physicians. Ann Intern Med. 2020;173:1002–1003. doi: 10.7326/M19-3773. [DOI] [PubMed] [Google Scholar]
- 43.FDA Drug Shortages Task Force. Drug Shortages: Root Causes and Potential Solutions. Fda.Gov. Available from:: https://www.fda.gov/media/132058/download [accessed August 27, 2021].
- 44.Gagne J.J., Choudhry N.K. How many “me-too” drugs is too many?. JAMA. 2011;305:711–712. doi: 10.1001/jama.2011.152. [DOI] [PubMed] [Google Scholar]
- 45.Li G., Jiang Y., Qin Y., Yuan S., Chen X. Comparing development strategies for PD1/PDL1-based immunotherapies. Nat Rev Drug Discov. 2022;21:484. doi: 10.1038/d41573-022-00003-7. [DOI] [PubMed] [Google Scholar]
- 46.lynn.huang The Second Batch Of Offpatent Drugs Published By China CDE. Accestra Consulting n.d. Available from: https://www.accestra.com/the-second-batch-of-offpatent-drugs-published-by-china-cde/[accessed September 9, 2021].
- 47.Coté T.R., Xu K., Pariser A.R. Accelerating orphan drug development. Nat Rev Drug Discov. 2010;9:901–902. doi: 10.1038/nrd3340. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Food and Drug Administration. Drug Shortages. Fda.Gov. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/drug-shortages [accessed August 27, 2021].
- 49.Tambuyzer E. Rare diseases, orphan drugs and their regulation: questions and misconceptions. Nat Rev Drug Discov. 2010;9:921–929. doi: 10.1038/nrd3275. [DOI] [PubMed] [Google Scholar]
- 50.Jiao K., Gupta R., Fox E., Kesselheim A., Ross J.S. Characteristics of recent generic drug approvals by the US Food and Drug Administration. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.China announces generic drug list to reduce pharmaceutical costs. European Pharmaceutical Review n.d. Available from: https://www.europeanpharmaceuticalreview.com/news/90765/china-announces-generic-drug-list-to-reduce-pharmaceutical-costs/[accessed July 6, 2021].
- 52.TRACON Pharmaceuticals Inc. TRACON Pharmaceuticals Announces Acceptance of the Envafolimab (KN035) NDA by the NMPA in China for Priority Review. GlobeNewswire News Room. Available from: https://www.globenewswire.com/news-release/2021/01/19/2160596/10391/en/TRACON-Pharmaceuticals-Announces-Acceptance-of-the-Envafolimab-KN035-NDA-by-the-NMPA-in-China-for-Priority-Review.html [accessed September 3, 2021].
- 53.Akeso Inc. Akeso Voluntary Announcement: Clinical Study Progress of Cadonilimab. Available from: https://pdf.dfcfw.com/pdf/H2_AN202011031425973314_1.pdf?1604383897000.pdf [accessed September 3, 2021].
- 54.Naumann R.W., Oaknin A., Meyer T., Lopez-Picazo J.M., Lao C., Bang Y.J., et al. LBA62 - efficacy and safety of nivolumab (Nivo) + ipilimumab (Ipi) in patients (pts) with recurrent/metastatic (R/M) cervical cancer: results from CheckMate 358. Ann Oncol. 2019;30:v898–v899. [Google Scholar]
- 55.O’Malley D.M., Oaknin A., Monk B.J., Leary A., Selle F., et al. LBA34 - single-agent anti-PD-1 balstilimab or in combination with anti-CTLA-4 zalifrelimab for recurrent/metastatic (R/M) cervical cancer (CC): preliminary results of two independent phase II trials. Ann Oncol. 2020;31(Suppl 4):S1142–S1215. [Google Scholar]
- 56.Akeso’s Cadonilimab (PD-1/CTLA-4), the First Dual Immune Checkpoint Inhibitor to Treat Cancer, Approved for Marketing in China. Akeso, Inc. Available from: https://akesobio.com/en/media/akeso-news/20220629/[accessed July 26, 2022].
- 57.Mullard A. Chinese biopharma starts feeding the global pipeline. Nat Rev Drug Discov. 2017;16:443–446. doi: 10.1038/nrd.2017.94. [DOI] [PubMed] [Google Scholar]
- 58.Aburto J.M., Villavicencio F., Basellini U., Kjærgaard S., Vaupel J.W. Dynamics of life expectancy and life span equality. Proc Natl Acad Sci U S A. 2020;117:5250–5259. doi: 10.1073/pnas.1915884117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh H., Pazdur R. Importing oncology trials from China: a bridge over troubled waters?. Lancet Oncol. 2022;23:323–325. doi: 10.1016/S1470-2045(22)00071-7. [DOI] [PubMed] [Google Scholar]
- 60.RemeGen Announces US FDA Has Granted Breakthrough Therapy Designation for Disitamab Vedotin (RC48) in Urothelial Cancer-RemeGen Co., Ltd. Available from: http://www.remegen.com/Invest2.aspx?id=138 [accessed May 19, 2022].
- 61.Patelli G., Zeppellini A., Spina F., Righetti E., Stabile S., Amatu A., et al. The evolving panorama of HER2-targeted treatments in metastatic urothelial cancer: a systematic review and future perspectives. Cancer Treat Rev. 2022;104 doi: 10.1016/j.ctrv.2022.102351. [DOI] [PubMed] [Google Scholar]
- 62.Kluetz P.G., O’Connor D.J., Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19:e267–e274. doi: 10.1016/S1470-2045(18)30097-4. [DOI] [PubMed] [Google Scholar]
- 63.Mullard A. Patient-focused drug development programme takes first steps. Nat Rev Drug Discov. 2013;12:651–652. doi: 10.1038/nrd4104. [DOI] [PubMed] [Google Scholar]
- 64.Focusing on the patient: implementation of key 21st Century Cures provisions and recommendations for the future. Food and Drug Law Institute (FDLI) 2020. Available from: https://www.fdli.org/2020/12/focusing-on-the-patient-implementation-of-key-21st-century-cures-provisions-and-recommendations-for-the-future/[accessed May 19, 2022].
- 65.Fiero M.H., Roydhouse J.K., Vallejo J., King-Kallimanis B.L., Kluetz P.G., Sridhara R. US Food and Drug Administration review of statistical analysis of patient-reported outcomes in lung cancer clinical trials approved between January, 2008, and December, 2017. Lancet Oncol. 2019;20:e582–e589. doi: 10.1016/S1470-2045(19)30335-3. [DOI] [PubMed] [Google Scholar]
- 66.Kluetz P.G., Bhatnagar V. The FDA’s patient-focused drug development initiative. Clin Adv Hematol Oncol. 2021;19:70–72. [PubMed] [Google Scholar]
- 67.Center for Drug Evaluation, NMPA. Notice of Public Consultation on “Technical Guidance on Patient-Focused Clinical Trial Design (Draft for Comments).” Cde.Org.Cn. Available from: https://www.cde.org.cn/main/news/viewInfoCommon/0cccaa1f5aeb73dcebf7d4d3e3e88b1c [accessed August 24, 2022].
- 68.Center for Drug Evaluation, NMPA. Notice of Public Consultation on “Technical Guidance on Patient-Focused Clinical Trial Implementation (Draft for Comments).” Cde.Org.Cn. Available from: https://www.cde.org.cn/main/news/viewInfoCommon/47f15561b3121091d100e6146fc5249a [accessed August 24, 2022].
- 69.Center for Drug Evaluation, NMPA. Notice of Public Consultation on “Technical Guidance on Patient-Focused Clinical Trial Benefit-Risk Assessment (Draft for Comments).” Cde.Org.Cn. Available from: https://www.cde.org.cn/main/news/viewInfoCommon/fc162e0cda62ebf42754ee90a98035dd [accessed August 24, 2022].