Abstract
Drug-induced liver injury (DILI) is a type of bizarre adverse drug reaction (ADR) damaging liver (L-ADR) which may lead to substantial hospitalizations and mortality. Due to the general low incidence, detection of L-ADR remains an unsolved public health challenge. Therefore, we used the data of 6.673 million of ADR reports from January 1st, 2012 to December 31st, 2016 in China National ADR Monitoring System to establish a new database of L-ADR reports for future investigation. Results showed that totally 114,357 ADR reports were retrieved by keywords searching of liver-related injuries from the original heterogeneous system. By cleaning and standardizing the data fields by the dictionary of synonyms and English translation, we resulted 94,593 ADR records reported to liver injury and then created a new database ready for computer mining. The reporting status of L-ADR showed a persistent 1.62-fold change over the past five years. The national population-adjusted reporting numbers of L-ADR manifested an upward trend with age increasing and more evident in men. The annual reporting rate of L-ADR in age group over 80 years old strikingly exceeded the annual DILI incidence rate in general population, despite known underreporting situation in spontaneous ADR reporting system. The percentage of herbal and traditional medicines (H/TM) L-ADR reports in the whole number was 4.5%, while 80.60% of the H/TM reports were new findings. There was great geographical disparity of reported agents, i.e. more cardiovascular and antineoplastic agents were reported in higher socio-demographic index (SDI) regions and more antimicrobials, especially antitubercular agents, were reported in lower SDI regions. In conclusion, this study presented a large-scale, unbiased, unified, and computer-minable L-ADR database for further investigation. Age-, sex- and SDI-related risks of L-ADR incidence warrant to emphasize the precise pharmacovigilance policies within China or other regions in the world.
Key words: Adverse drug reactions, Drug-induced liver injury, Spontaneous reporting system, Database, Socio-demographic index, Pharmacoepidemiology, Pharmacovigilance, Geographical disparity
Graphical abstract
This study provides a whole epidemiological picture of L-ADR in mainland China, and concluded that L-ADR has a persistent increase. This work provides a powerful tool to mine the potential hepatotoxic drugs in the future.
1. Introduction
Adverse drug reaction (ADR) is unwanted “secondary injury” to patients, which is one of the leading causes of hospitalizations and morbidity resulting nonnegligible public heath burdens1. Prevention of ADR is a particularly challenging work since over 50% of them belong to bizarre type and are unpredictable in nature2. Liver-related ADR, usually called as drug-induced liver injury (DILI) in clinical diagnosis, is a clinically-important ADR damaging liver (L-ADR), which may cause substantial hospitalizations, acute liver failure and even death3,4. L-ADR is either the leading causes of acute liver failure in the United States or the most frequent reason for the postmarketing drug withdrawal5,6. However, since the very low incidence (19–23.8/100,000)7,8, detection of L-ADR is great challenging9. This situation leads to little knowledges of the epidemiological trends and characteristics of L-ADRs and in turn limits our ability to initiate precise policies to prevent and control this critical drug-induced disease.
L-ADR studies have been conducted mainly in developed countries10,11, where the spectra of diseases and drugs may be significantly different from those countries in low- and middle-income levels8. Besides, in many low-income developing countries where the availability of modern drugs is limited, a greater amount of herbal and traditional medicines (H/TM) are used in place of conventional pharmaceuticals (CP). Unfortunately, the knowledge of H/TM-related L-ADR is largely limited12. Therefore, there is an urgent need for studies on epidemiological trends of L-ADR covering wide drug spectrum in large populations with different income levels.
The national spontaneous reporting systems, covering the entire population and a wide range of drugs, is of advantage to discover this kind of rare but serious ADRs cost-effectively. In the recent two decades, especially by the 2009 Healthcare reform plan13, China has established a complete ADR spontaneous reporting and monitoring system in all provinces of the country, covering all levels in the medical system ranging from tertiary hospitals to primary healthcare units14,15. Owing to the largest population, great multiformity of drug spectrum and heterogeneity of socio-demographic index (SDI)16 in China, this ADRs big data has opened a new window for understanding the real-world epidemiological patterns of L-ADRs and can guide the precise initiation of prevention and control policies for different SDI regions in China and the world.
2. Methods
2.1. Data source
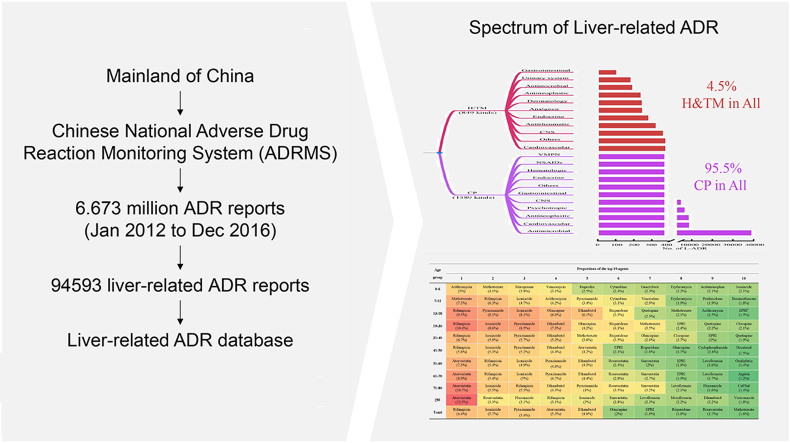
Epidemiological trends of L-ADR in mainland China were investigated. The ADR data set (6.673 million) from January 1st, 2012 to December 31st, 2016 were obtained from the Chinese National Adverse Drug Reaction Monitoring System (ADRMS) database, China Food and Drug Administration. The research data covered all 31 provinces, autonomous regions, and direct-controlled municipalities in mainland China.
Firstly, the L-ADR data were retrieved by computer key word searching from the above ADRMS dataset (all in Chinese). The ADR reports recorded as liver injury-related ADR, such as “drug-induced liver injury”, “drug-induced liver damage” and “abnormal liver function caused by drug”, were included. Meanwhile, reports with key words of non-drug etiology, such as “viral liver disease”, “alcoholic liver disease”, and “autoimmune liver disease”, were excluded. It has been also reported to use keywords or the standardized codes to search adverse drug events from electronic medical records recently17. The complete searching key words and their translations used in this study were listed in Supporting Information Table S1.
Secondly, the drug names and subclasses, outcomes, biochemical indices, adverse reaction names, and clinical symptoms were manually standardized to create a dictionary of synonyms for the data fields to ensure accuracy of subsequent analyses. Then the dataset was standardized based on the dictionary.
Thirdly, we exclude incomplete records and those reports with the causality judgement of “possibly not” or “not”. The causality assessment of ADRMS was obligatorily required to use the WHO-Uppsala Monitoring Centre (UMC) causality judgment method for adverse reactions18 and each report was reviewed by the provincial drug administration and at last by the CFDA. Although the Roussel-Uclaf Causality Assessment Method (RUCAM) is much specific for drug-induced liver injury in clinical diagnosis, spontaneous ADR reporting systems are not designed for only L-ADR and the reporting data are sometimes insufficient for use in the RUCAM scoring scale19,20. In our study, the reports judged to be “possibly not” or “not” by the provincial or national drug administration were excluded, that means those reports with “very likely”, “probably” and “possible” causality judgements were included (Supporting Information Fig. S1).
2.2. Stratified analysis
A total of 94,593 L-ADR reports were used for stratified analysis. The demographic distribution of L-ADR reports was analyzed by sex and age. The age standardized demographic distribution of patients with L-ADR was analyzed with adjustment for age based on the population of the whole country. The developing trend of L-ADR reports was also analyzed by year groups of reporting.
The proportion of L-ADR in all types of ADRs was calculated as annual number of L-ADR reports divided by annual number of all types of ADRs recorded in the ADRMS database.
The spectrum of implicated agents of L-ADRs were analyzed, including rankings and proportions of the drug classes, subclasses, and certain agent. The implicated agents were classified into two major classes: conventional pharmaceuticals (CP, including either chemical or biological drugs) and herbal and traditional medicines (H/TM). The drug subclasses were classified by the major therapeutic purposes of drugs (e.g., antimicrobial, cardiovascular, and antineoplastic). The drug spectrum of L-ADR was also analyzed by age and sex. We also separate the L-ADR reports into two groups, one is composed of those 338 kinds of agents with pre-known hepatotoxicity knowledge (namely pre-known group, referred by the LiverTox and the critical reviewed literature20); and the other consisted to new-finding group (without pre-known hepatotoxicity knowledge).
Furthermore, the spectrum of implicated agents of L-ADR was investigated by the region development levels, according to the five-level stratification of socio-demographic index (SDI) proposed by the World Health Organization (WHO) and the Global Burden of Disease (GBD) Study16. Notably, there are only three SDI levels of provinces in China, e.g., low-middle, middle and high-middle levels, without presence of the low and high SDI levels.
2.3. Statistics
Data were processed using SPSS software and were expressed as mean ± standard deviation, or median (M) with 25th and 75th percentiles (P25, P75), and rate. Data with significant differences were tested by regression analysis. The trends analysis was analyzed by curve regression model (APC), which were performed by the Joinpoint regression program (National Cancer Institute of the United States). P < 0.05 is considered statistically significant.
2.4. Role of the funding source
The funding source of this study did not participate in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding and lead authors had full access to all data in the study, and all authors had final responsibility for the decision to submit for publication.
3. Results
3.1. Establishment of the L-ADR database
The national ADR monitoring system is designed for unbiased reporting of all types of ADRs and thus limited our ability to assess ADRs focusing on a specific organ. In this regard, we created a new L-ADR database based on the national ADR monitoring system. To overcome the heterogenicity of terms reported in the spontaneous system, we therefore performed computer searching and manual unifying for all the terms (in Chinese, see Table S1) related to drug-induced liver injury. The searching works resulted 114,357 putative L-ADR reports from the original 6.673 million ADR records. The various terms retrieved from the dataset were manually standardized to create a synonyms dictionary. All the synonyms were replaced as the key terminology and translated to the English ones respectively. Then we excluded the incomplete records and those ones judged to be “possibly not” or “not” related to L-ADR. At last, a total of 94,593 L-ADR reports were obtained to create the new L-ADR database.
The new database are computer-searchable, including identification number, drug generic names, drug categories, drug registration number, pharmaceutical dosage forms, route of administration, manufacturer, product lot number, dosage information, time of administration, time of drug cessation, purpose of drug treatment, ethnics, age, sex, weight, comorbidities, history of ADRs, familial ADR history, name of ADR, onset time of ADR, description of ADR, outcome of ADR, improving status after drug cessation, rechallenge, influence to underlying diseases, causality assessment results (by reporter, institutional supervisor, provincial regulator, and national regulator), reporting time, reporting institution, region of reporting institution, SDI level of the region of reporting institution, and career of reporter, etc.
3.2. Temporal trends
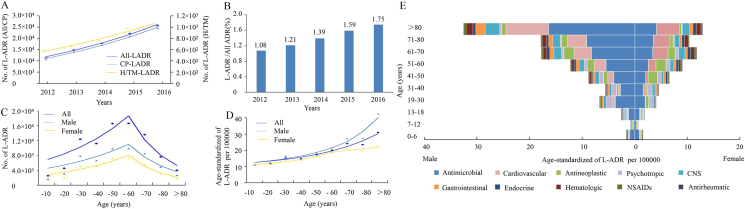
In this study, a total of over 6.673 million ADR records covering all the administrative regions throughout mainland China were obtained and a total of 94,593 L-ADR reports were retrieved. L-ADR reports in mainland China had increased continuously in recent five years (Fig. 1A). Among this, CP showed a trend of continuously steady increase of reports numbers, while H/TM showed a slower rate of increase over the past two years.
Figure 1.
The overall trend and epidemiological characteristics of L-ADR reports in Mainland China. (A) Temporal changes of L-ADR reports by implicated agent classes. The fitting models for All-LADR, CP-LADR and H/TM-LADR were regressed with APC values as 17.54, 17.75 and 12.74, respectively. (B) Proportion of L-ADR reports in all types of ADRs over the year. (C) Distribution trends of L-ADR reports in different sex and age groups showed two-phase pattern with increasing trend below 60 years old and decreasing trend beyond 60 years old. APC values for all sex are 22.89 (< 60 years) and −35.55 (> 60 years); for male ones are 20.81 (< 60 years) and −33.05 (> 60 years); and for female ones are 25.79 (< 60 years) and −39.31 (> 60 years), respectively. (D) Distribution trends of L-ADR reports in different sex and age groups after adjustment by the national age distribution showed increasing patterns. APC values for all sex is 27.50; for male ones are 31.55 (< 70 years) and 39.38 (> 70 years); for female ones are 40.31 (< 60 years) and 9.87 (> 60 years). (E) Proportions of implicated CP drug subclasses in different sex and age groups according to the age-distribution adjusted reporting numbers. X-axis indicates the proportion of L-ADR in general population (per 100,000) and Y-axis indicates age groups.
Despite the overall increase of ADR reports in mainland China, the proportion of L-ADR reports in the whole ADRs had increased 62% from 1.08% to 1.75% (Fig. 1B). The average value during the five years was 1.42%.
3.3. Epidemiological characteristics
The relationship curve between the number of L-ADR reports and age was parabolic (Fig. 1C). The highest number of L-ADR reports existed in the age group of 41–60 years, followed by 19–40 years, and 61–80 years. These age groups accounted for 90.2% of the total number of reports (Supporting Information Table S2). Of note, the number of L-ADR reports from males was 1.50-fold greater than that from females in the total dataset; but in H/TM-L-ADRs, the proportion of male was 1.11-fold higher than that of females (Table S2). Most of the reports indicated good outcome (Table S2), without relationship to age, sex or drug classifications (Supporting Information Table S3).
Intriguingly, the national age distribution-adjusted number of L-ADR reports showed a generally upward trend with age, especially in those over the age of 81 years (more evident in men) (Fig. 1D). Besides, the nation population-adjusted number of L-ADR reports was significantly higher in males than in females over the age of 19 years. To understand this phenomenon, we further analyzed the proportions of drug subclasses within different sex and age groups (Fig. 1E). The results showed that there were higher proportions of antibiotics (predominantly antitubercular agents) in men over the age of 19 years compared to the corresponding age groups of women. In the age group over 81 years, cardiovascular, CNS and gastrointestinal subclasses also contributed to the rapid increase of age distribution-adjusted number in men compared to women.
3.4. Classification of implicated drugs
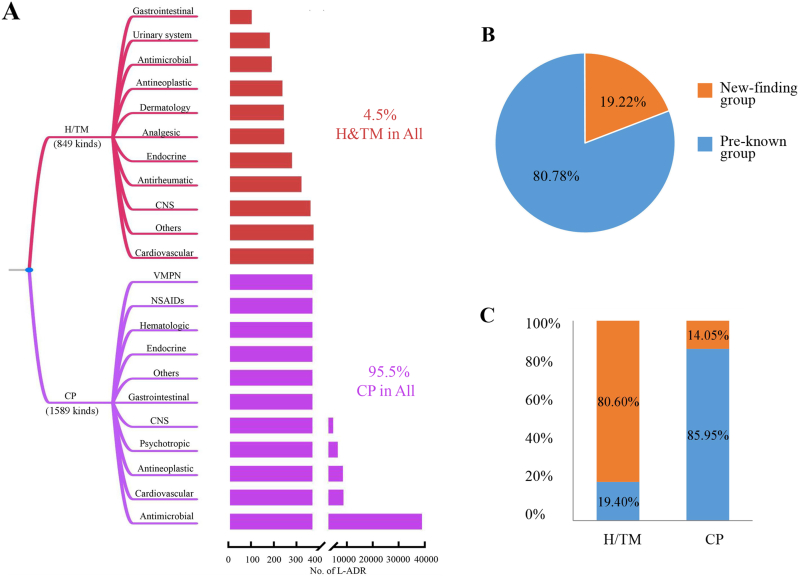
There were totally 2438 kinds of drugs reported to liver injury in the database, including 1589 kinds of conventional pharmaceuticals (CPs) and 849 kinds of H/TM agents (Fig. 2A). Although the number of H/TM drugs was over a half of the number of CPs, the L-ADR reports of H/TM was less than 1/20 of the report number of CPs (Table S2).
Figure 2.
The overall spectrum of drugs reported to liver injury. (A) The drug category spectrum of L-ADR. (B) The proportion of L-ADR reports of pre-known hepatotoxicity group and new-finding group. (C) The proportions of pre-known and new-finding groups categorized by H/TM and CP agents.
Furthermore, since the researches on the hepatotoxicity of H/TM are very limited before, we argue the reported L-ADR cases of H/TM are predominantly new findings. We then separate the L-ADR reports into two groups, pre-known group (with pre-known hepatotoxicity knowledges referred by the LiverTox and the critical reviewed literature19) and new-finding group (without pre-known hepatotoxicity knowledge). Firstly, we found that the pre-known group accounted for 80.8% of total L-ADR reports; and the new-finding group accounted for 19.2% of total L-ADR reports (Fig. 2B). Then, we examined the proportion of H/TM and CP agents in each group. The results showed that 85.95% of L-ADR reports in CP agents are pre-known hepatotoxic agents; while in H/TM category, only 19.40% are pre-known hepatotoxic H/TM (Fig. 3B). That is to say, 80.60% of L-ADR reports of H/TM are new findings.
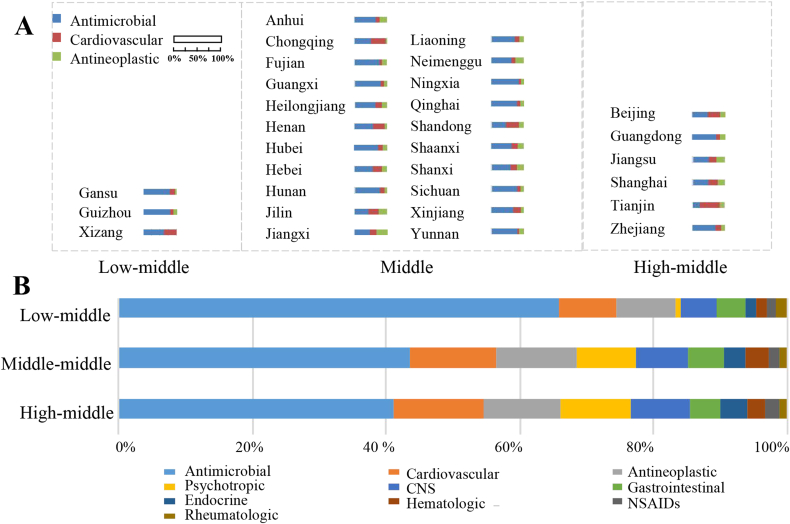
Figure 3.
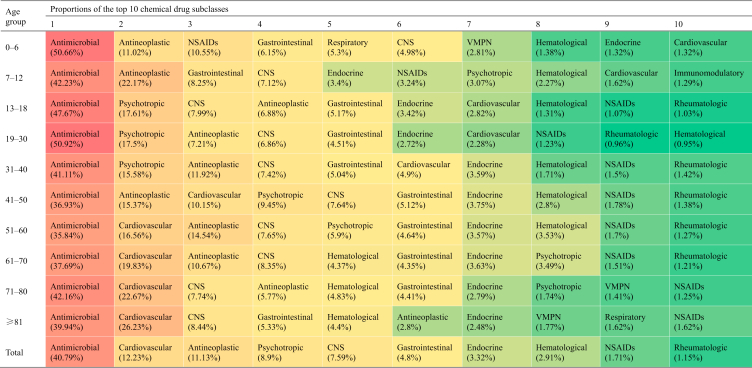
SDI-related differences of L-ADR in Mainland China. (A) The proportions of the national top three drug subclasses (antimicrobial, cardiovascular, and antineoplastic agents) in each province were indicated by the column width. The provinces were clustered into three groups (e.g., Low-middle, Middle and High-middle levels) according to WHO SDI quintile. There are no Low and High SDI levels of provinces in China. (B) Top 10 subclasses of chemical drugs-associated to DILI reports by SDI levels in Mainland China. CNS, central nervous system; NSAIDs, non-steroidal anti-inflammatory drugs.
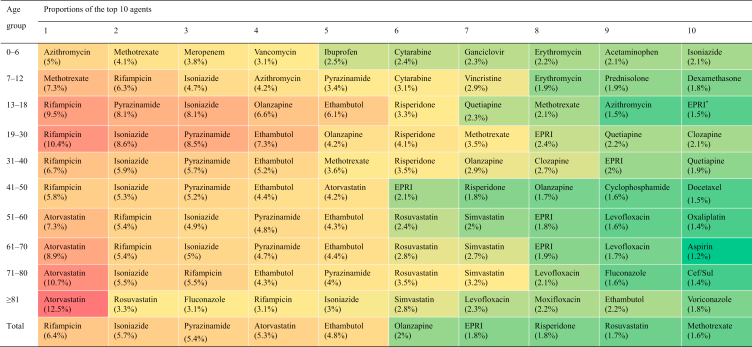
Among CP agents (chemical and biological drugs), the top five subclasses with the highest proportions were antimicrobial (including antitubercular agents), cardiovascular, antineoplastic, psychotropic, and central nervous system agents (Table 1). The top 10 agents-implicated with liver injury by age groups were listed in Table 2.
Table 1.
Top 10 subclasses associated with DILI reports by age groups in Mainland China.
CNS, central nervous system; NSAIDs, non-steroidal anti-inflammatory drugs; VMPN, vitamins, minerals, and parenteral nutrition.
Table 2.
Top 10 agents associated to L-ADR reports by age groups in Mainland China.
EPRI, ethambutol, pyrazinamide, rifampicin and isoniazid compound preparation; PRI, pyrazinamide, rifampicin and isoniazid compound preparation; Cef/Sul, cefoperazone and sulbactam compound preparation.
The proportions of antimicrobial drug-associated L-ADR reports exceeded 35% in each age group (Table 1) and the values were lower in females than in males in all age groups (Fig. 1E). Compared to tertiary hospitals, there was a higher proportion of antimicrobial drug DILI reports in primary healthcare units (Supporting Information Table S4) and such increase was contributed by higher proportion of anti-tubercular (anti-TB) agents (Supporting Information Table S5). The top 10 implicated CP or H/TM agents were listed in Supporting Information Table S6.
The number of L-ADR reports related to cardiovascular agents tended to increase with age, with the 0–6 years age group ranked tenth (accounting for 1.32% of numbers) and the age group over 51 years ranked second (accounting for more than 16% of numbers) (Table 1).
The proportions of L-ADR reports related to antineoplastic agents to that of all this subclass could be roughly divided into three ranges: 1) the age group with a proportion less than 5% (over 81 years); 2) the age groups with a percentage of 5%–10% (13–30 and 71–80 years); and 3) the age groups with a proportion more than 10% (0–12 and 31–70 years). The peak percentage groups were 7–12 years (22.17%) and 41–50 years (15.37%) (Table 1).
3.5. Effect of regional developing level
We found that the spectrum of implicated agents with L-ADR was significantly different among provinces. To show this difference, the proportions of the top three drug subclasses (ranked by the national data) associated to L-ADR in different provinces by SDI levels were represented in Fig. 3A, which revealed great regional disparities. We also combined the provinces data by SDI levels. It could be found that the proportion of reports related to antimicrobial agents was higher with decreasing SDI levels (Fig. 3B). Similarly, cardiovascular and antineoplastic agents also had different proportions among different SDI regions. Further analysis of the ranking of specific varieties of antimicrobial agents showed that the proportion of antitubercular agents increased as SDI levels declined (Supporting Information Table S7).
4. Discussion
The tremendous population and wide geo-sociological coverage of ADRMS in mainland China have qualified this spontaneous ADR reporting data source as the best resource to study the reporting landscape of L-ADR, especially to discover those L-ADR with very low incidence, the geo-sociological difference, and the demographical predisposing variance. The wellness of data coverage in different regions illustrated the reporting robustness throughout mainland China, which is recorded by the unified system regulated under the national administration. Another property of this data source is the unbiased design of the ADRMS for all types of ADR. This makes the data reflect the real-world status of L-ADR. We then created a L-ADR database by computer searching and manual standardization based on the ADRMS. The new database makes the study for L-ADR in a computer-minable way since all the terminologies have been unified. To our knowledge, this study provides the first resource and a powerful tool to mine the real-world epidemiological patterns of L-ADR.
This nation-wide census obtained the first landscape on the pharmacovigilance status of L-ADR throughout mainland China. From the temporal trends, we observed a persistently increasing curve of L-ADR reports during the period between 2012 and 2016, with a 1.71-fold change. This result suggests that the incidence of L-ADR in China has been persistently expanding, deserving greater attention.
We also observed significant age- and sex-related differences in L-ADR reports. Without adjustment for age distribution, the absolute number of L-ADR was greater in the middle-aged group. This was similar to the previous surveys based mainly on hospitalization medical records7,10,11,21. However, after adjustment for age distribution of the population in mainland China, the result showed that the number of L-ADR reports increased generally with age, suggesting that advanced age is an important risk factor for drug-induced liver injury7. And the L-ADR in male people were more frequently reported than female. Nevertheless, the age- or sex-related risk was not observed in the clinical outcome, which manifested as generally good outcomes. Elderly individuals tend to have more diseases, take more drugs, and have reduced liver detoxification and elimination capacity4, so more liver injury may occur. Notably, in this study, the annual reporting rate of L-ADR reached 25/100,000 in the 81 years and older age group, which exceeds the annual DILI incidence rate in either Iceland population7 or Chinese population8. Given that the rate of underreporting in the ADR spontaneous reporting system is generally high (over 90%)21, the actual incidence of DILI in the elderly population may be severely underestimated. As the global population ages, the number of cases and social burden of L-ADRs will increase and will bring more challenges to global public health management.
Moreover, we found obvious region-related differences in L-ADR reports. For instance, the proportion of L-ADR implicated to antimicrobial agents was higher in low-SDI regions, which was consistent with higher prevalence of infectious diseases in low-SDI regions, as reported by the WHO GBD study20. Specifically, the high contribution of antimicrobial agents in low-SDI regions was mainly due to a higher proportion of antitubercular agents, consistent with higher incidence of tuberculosis in low-SDI regions19. Also, compared to tertiary hospitals (involved much more patients in high-income population), the antitubercular agents contributed much more proportion of L-ADR reports in primary healthcare units (involved higher proportion of low-income population). These data indicated important needs to strengthen monitoring of liver injury and rational use of antitubercular agents to aid in prevention and control of DILI. Similarly, a significantly higher proportion of cardiovascular drug was observed in high-SDI regions compared with low-SDI regions, which was related to a higher cardiovascular burden in developed regions22. In general, it is of great policy interest for different regions all over the world with different SDI levels to develop precise and targeted prevention and control policies.
As the data shown, anti-tuberculosis (anti-TB) drugs were the most frequently reported agents in the L-ADR database. The hepatotoxicity of anti-TB drugs has been well recorded. As the multidrug-resistance of tuberculosis rises persistently in recent years globally, the increase of duration and dosage of anti-TB drugs as well combinational uses become more common in the management of tuberculosis. In our study, two, three or four compound anti-TB drugs were observed in the top 10 list, especially in the low-income regions. Together, these situation causes much more risk of liver injury to fight tuberculosis. Liver injury poses a huge challenge to the task of tuberculosis fight globally.
Due to the untargeted nature of reporting, the present data reveals the real-world drug spectrum in mainland China. Intriguingly, the percentage of H/TM-related L-ADR reports in the whole number was only 4.5%, which is much lower than the data 26.8% reported in the previous literature in mainland China8. The literature was a sampling study involved 308 medical centers rather than whole coverage of all population in mainland China. Thus, it suggests the necessity to reconsider the real proportion of H/TM and western medicines regarding to L-ADR. On the other hand, we also noted that most (80.60%) of the H/TM-related L-ADR reports were new findings, while the new findings in CP agents were only 14.05%. These results indicate that, compared to CP agents, the knowledge of hepatotoxicity of H/TM are significantly limited, which warrants urgent needs to investigate the L-ADR of H/TM agents. The information will help to improve the pharmacovigilance of H/TM and decrease the underreporting rate or reporting bias.
There were limitations in this study. We did not involve the data before 2009 in the first development stage of the ADRMS, since the data had not covered all the regions and levels in China. In addition, the data could be mined to discover new hepatotoxic drugs which have not been identified in current clinical observation. But the new L-ADR database offers possibility to do this discovery in the future. And the modified liver-specific causality assessment such as RUCAM for spontaneous ADR reporting systems is needed and will be considered in the future.
5. Conclusions
In the largest national monitoring of its kind, we have provided a whole epidemiological picture of L-ADR in mainland China. We concluded that L-ADR has a persistent increase. Our work provides a powerful tool to mine the potential hepatotoxic drugs in the future. The outline of reporting status provides a real-world window for developing novel management strategies of drug-related injuries in China and other regions of the world.
Acknowledgments
We are very grateful to the China National Adverse Drug Reaction Monitoring Center for providing data. This work was financially supported by the National Natural Science Foundation of China (grant numbers Nos. 82074112, 81630100 and 81721002), the National Science and Technology Directorate Major Project (2015ZX09501-004-001-008, China), the National Industry Program of China (201507004–04), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202005, China), the Beijing Talent Youth Program (JQ21026, China) and the Project of China PLA General Hospital (2019-JQPY-003 and 2019MBD-023).
Author contributions
Xiaohe Xiao, Jiabo Wang and Chuanyong Shen initiated and designed the study. Jiabo Wang, Haibo Song, Feilin Ge, Yuming Guo, and Guangbin Luo drafted the manuscript. Jiabo Wang, Haibo Song, Feilin Ge, Peng Xiong, Jing Jing, Tingting He, Zhuo Shi, Chao Zhou, Zixin Han, Yanzhong Han, and Ming Niu analyzed the data. Zhaofang Bai commented and revised the text. Jiabo Wang and Ming Niu established the database.
Conflicts of interest
The authors declare that they have no conflict of interests.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.04.019.
Contributor Information
Chuanyong Shen, Email: shenchuanyong@cdr-adr.org.cn.
Xiaohe Xiao, Email: pharmacy302xxh@126.com.
Appendix A. Supporting information
The following is the Supporting information to this article:
References
- 1.Vecino-Ortiz A.I., Jafri A., Hyder A.A. Effective interventions for unintentional injuries: a systematic review and mortality impact assessment among the poorest billion. Lancet Global Health. 2018;6:e523–e534. doi: 10.1016/S2214-109X(18)30107-4. [DOI] [PubMed] [Google Scholar]
- 2.Hakkarainen K.M., Hedna K., Petzold M., Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions—a meta-analysis. PLoS One. 2012;7:e33236. doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X.H., Tang J.Y., Mao Y.M., Li X.H., Wang J.B., Liu C.H., et al. Guidance for the clinical evaluation of traditional Chinese medicine-induced liver injury Issued by China Food and Drug Administration. Acta Pharm Sin B. 2019;9:648–658. doi: 10.1016/j.apsb.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z.L., Xu G., Wang H.B., Zhan X.Y., Gao Y., Chen N., et al. Icariside II, a main compound in Epimedii Folium, induces idiosyncratic hepatotoxicity by enhancing NLRP3 inflammasome activation. Acta Pharm Sin B. 2020;10:1619–1633. doi: 10.1016/j.apsb.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuben A., Koch D.G., Lee W.M. Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a US multicenter, prospective study. Hepatology. 2010;52:2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro V.J., Senior J.R. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 7.Björnsson E.S., Bergmann O.M., Björnsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Shen T., Liu Y., Shang J., Xie Q., Li J., Yan M., et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology. 2019;156:2230–2241. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Hazell L., Shakir S.A. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 10.Stephens C., Robles-Diaz M., Medina-Caliz I., Garcia-Cortes M., Ortega-Alonso A., Sanabria-Cabrera J., et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol. 2021;75:86–97. doi: 10.1016/j.jhep.2021.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N., Bonkovsky H.L., Fontana R., Lee W., Stolz A., Talwalkar J., et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148:1340–1352. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplowitz N. Herb-induced liver injury: a global concern. Chin J Integr Med. 2018;24:643–644. doi: 10.1007/s11655-018-3004-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z. Launch of the health-care reform plan in China. Lancet. 2009;373:1322–1324. doi: 10.1016/S0140-6736(09)60753-4. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y., Li X., Wu G., Ye X. National ADR monitoring system in China. Drug Saf. 2016;39:1043–1051. doi: 10.1007/s40264-016-0446-5. [DOI] [PubMed] [Google Scholar]
- 15.China National Medical Products Administration. Report of the China National Center for ADR monitoring. Available from: https://www.cdr-adr.org.cn/drug_1/aqjs_1/drug_aqjs_sjbg/202103/t20210326_48418.html.
- 16.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeboah-Korang A., Louissaint J., Tsung I., Prabhu S., Fontana R.J. Utility of a computerized ICD-10 algorithm to identify idiosyncratic drug-induced liver injury cases in the electronic medical record. Drug Saf. 2020;43:371–377. doi: 10.1007/s40264-019-00903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO), Uppsala Monitoring Centre. The use of the WHO-UMC system for standardized case causality assessment. WHO [EB/OL]. [2012-6-11]. Available from: http://www.who-umc.org/graphics/26649.pdf.
- 19.Danan G., Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions ofinternational consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 20.Björnsson E., Hoofnagle J. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology. 2016;63:590–603. doi: 10.1002/hep.28323. [DOI] [PubMed] [Google Scholar]
- 21.Xu J.M. A multicenter survey on hospital inpatients with drug-induced acute liver injury in China. Chin J Digestion. 2007;27:439–442. [Google Scholar]
- 22.Alatawi Y.M., Hansen R.A. Empirical estimation of under-reporting in the U.S. Food and drug administration adverse event reporting system (FAERS) Expet Opin Drug Saf. 2017;16:761–767. doi: 10.1080/14740338.2017.1323867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.