Abstract
Amyloidosis concomitant to aortic stenosis usually occurs with myocardial infiltration by the transthyretin protein. To our knowledge, this is the first report of localized amyloidosis of indeterminate type in a severely calcified and functionally unicuspid aortic valve. Isolated dystrophic valvular amyloidosis is believed to be related to fibrocalcific valve disease. In light of the literature on this topic, the present case raises new hypotheses on pathophysiology and further supports the contributory role of unusual non-tricuspid valve morphology in the development of dystrophic amyloid, likely secondary to altered hemodynamic stress.
Résumé
Une amyloïdose associée à une sténose aortique survient généralement avec infiltration du myocarde par la protéine transthyrétine. Le cas que nous décrivons est, à notre connaissance, le premier cas rapporté d’amyloïdose localisée de type indéterminé dans une valve aortique sévèrement calcifiée et fonctionnellement unicuspide. L’amyloïdose valvulaire dystrophique isolée serait liée à l'atteinte fibrocalcique de la valve. À la lumière de la littérature à ce sujet, le cas décrit ici permet de soulever de nouvelles hypothèses physiopathologiques et appuie le lien entre une morphologie valvulaire inhabituelle (non tricuspide) et l’apparition de substances amyloïdes dystrophiques, probablement secondaire à une altération des contraintes hémodynamiques.
Most of the patients with aortic stenosis (AS) and concomitant amyloidosis present with myocardial infiltration, mainly resulting from the misfolding of the transthyretin protein. Isolated valvular amyloidosis has been reported much less often, and when reports have been made, it has been referred to as dystrophic valvular amyloidosis, most frequently involving the transthyretin protein or apolipoprotein A11,2 and believed to be related to fibrocalcific valve disease.
To our knowledge, this is the first report of localized amyloidosis of indeterminate type in a severely calcified and functionally unicuspid valve. This case raises new hypotheses on the pathophysiology of valvular amyloidosis and supports the contributory role of unusual non-tricuspid valve morphology in the development of a dystrophic amyloid substance.
Case Presentation
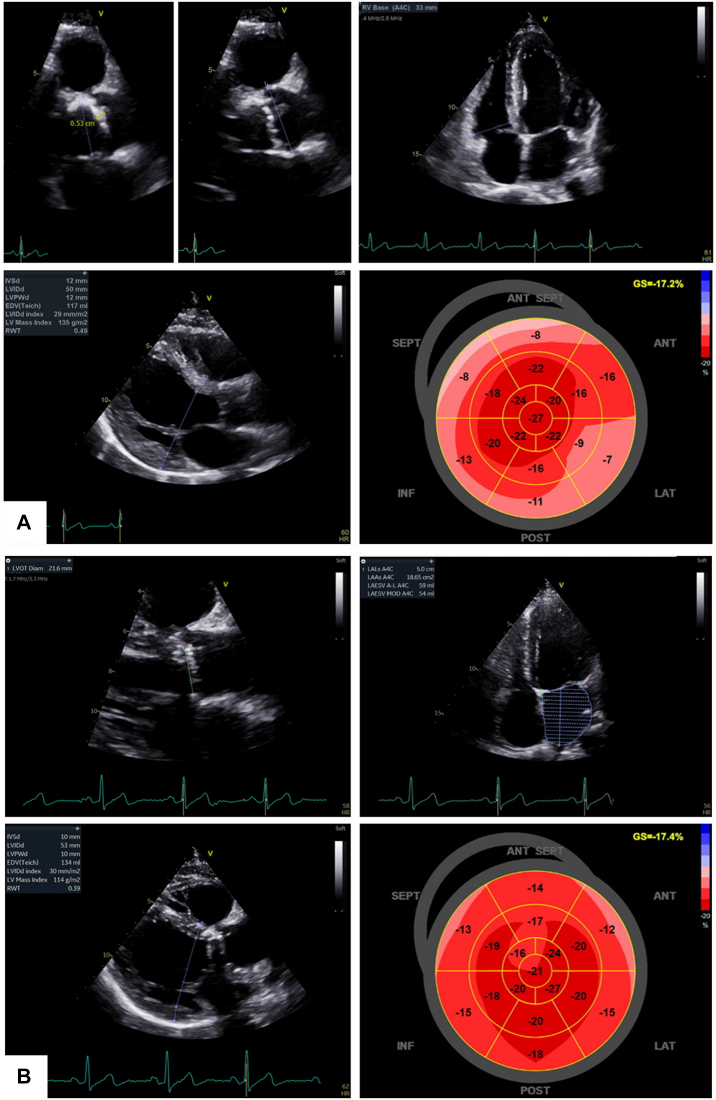
In June 2019, a 53-year-old French Canadian Caucasian man, working as a mechanic, complained of progressive dyspnea and fatigue to the cardiac surgeon at our institution. On cardiac auscultation, a loud systolic murmur was appreciated. Further evaluation revealed exertional angina, dyspnea (New York Heart Association functional class III/IV), and a systolic murmur with soft S2. An asymptomatic cardiac murmur had been known of since his childhood but never investigated. The patient had no other cardiovascular risk factor apart from smoking. An electrocardiogram revealed sinus bradycardia (52 beats per minute) without electrical hypertrophy. Transthoracic echocardiography showed a heavily calcified aortic valve, most likely bicuspid, with severe AS and severe concentric left ventricle (LV) hypertrophy with an indexed mass of 150 g/m2, but an only slightly increased wall thickness with interventricular septum and posterior wall thicknesses of 12 mm (Fig. 1A; Supplemental Table S1). The ejection fraction was preserved, strain was mildly to moderately reduced with apical sparing (Fig. 1A), and the left atrium was mildly dilated. Coronary angiography revealed a 70% stenosis of the first diagonal artery. Surgical aortic valve replacement with a 23 mm On-X mechanical prosthesis (CryoLife, Inc, Kennesaw, GA) and concomitant single coronary artery bypass were successfully performed in July 2019.
Figure 1.
Transthoracic echocardiographic (TTE) data (A) before and (B) 9 months after aortic valve replacement. (A) Before intervention, TTE revealed a thickened aortic valve with one leaflet > 5 mm thick and increased echogenicity, left ventricle hypertrophy, and apical sparing of strain. (B) After intervention, TTE revealed the well-placed prosthesis and normalization of left ventricle hypertrophy and basal strain.
Clinical Findings and Differential Diagnosis
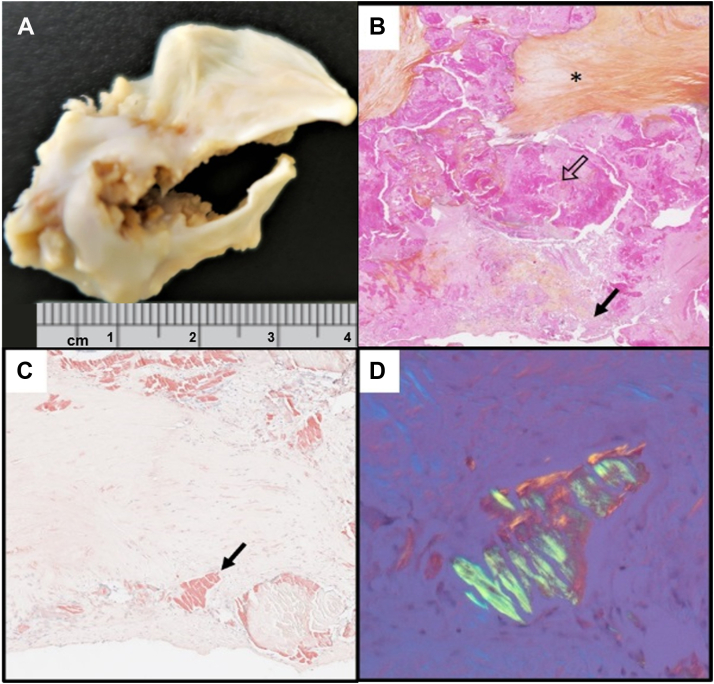
The explanted specimen was severely calcified, with only one functional cusp. Despite a definitive phenotype described as unicuspid, the presence of a probable raphe, heavy fibrocalcific changes, and acute-angle fusion of the other fused commissure suggested acquired fusion of an initially congenital bicuspid valve (Fig. 2A). As this morphology and texture were somewhat unusual in the setting of a congenitally bicuspid valve, suspicion was raised of other etiologies, including rheumatic disease and valvular amyloidosis. Histology excluded rheumatic disease, due to the absence of postinflammatory changes, but it revealed Congo-red positive amorphous extracellular material, birefringent to polarized light, consistent with amyloid (Fig. 2, B-D). Amyloid deposits like the one depicted in these images were seen throughout the valve tissue in a patchy but diffuse distribution. In accordance with guidelines, the patient was investigated for cardiac amyloidosis. Serum and urine protein electrophoreses and free light-chains were normal. 99m technetium- pyrophosphate scintigraphy was negative for transthyretin cardiac amyloidosis, the most common form in AS (visual uptake grade 0/3; Supplemental Fig. S1). Amyloid subtyping by mass spectrometry on the excised valve tissue confirmed the presence of amyloid, but neither light-chain nor transthyretin were identified. Rather, the profile consisted of numerous proteins, including serum amyloid P component and apolipoprotein A4 and E. Additionally, genetic testing did not reveal any variant in genes commonly associated with hereditary amyloidosis. At subsequent 9-month and 2-year follow-ups, physical exam and cardiac biomarkers were normal, and echocardiography showed satisfactory prosthesis hemodynamics, LV mass regression, normal LV function, and homogenous strain profile (Fig. 1B; Supplemental Table S1). Final diagnosis was AS on a functionally unicuspid valve with concomitant valvular amyloidosis, most likely isolated and likely secondary to a local degenerative process.
Figure 2.
Representative macroscopic and histologic images of the explanted valve. (A) Excised specimen showing marked fibrocalcific remodeling and a single functional commissure, resulting in a unicuspid-like valve morphology. (B) Hematoxylin-phloxine-safron staining (original magnification ×15) showing marked chronic tissue remodeling including fibrosis (asterisk), calcification (open arrow), and amorphous pale pink material (solid arrow), (C) Congo Red positive (original magnification ×100), (D) with green birefringence under polarized light (original magnification ×200), consistent with amyloid.
Discussion
Most of the patients with concomitant AS and amyloidosis present with myocardial infiltration, most often resulting from the misfolding of the transthyretin protein (transthyretin amyloidosis type), or rarely, of the immunoglobulin light chains (amyloid light-chain type). Isolated valvular amyloidosis, thought to be uncommon, has likely been underreported, as diagnosis requires histologic analysis of the excised valve. Although we did not have an absolute pathologic proof of the absence of amyloidosis deposits within the myocardium, we believe that, in addition to the pathologic findings within the explanted valve, the clinical and imaging presentation and course of this patient were both strongly in favour of this diagnosis. Indeed, postintervention evaluation revealed complete recovery of the patient, with no remaining heart failure sign or symptom, and with normalization of global longitudinal strain. This observation further reminds us that despite common beliefs in the cardiology community, apical strain sparing is not specific to cardiac amyloidosis, especially in patients with severe AS. What is currently unclear is whether the persistence of apical sparing after aortic valve replacement could be a better marker of cardiac amyloidosis. To determine this, longitudinal studies with repeated strain imaging are required in patients with dual AS and cardiac amyloidosis.
Previous studies have observed colocalization of amyloid fibrils, fibrosis, and calcification in explanted valves, without generalized amyloidosis, a condition referred to as dystrophic amyloidosis.1,2 Apolipoprotein A1, serum amyloid P component, and transthyretin have been identified as constitutive proteins.1,3 Our patient had a severely calcified valve, with amyloid deposits containing P component and other proteins, resembling the previously reported cases. The possibility has been suggested that amyloid results from protein accumulation following apoptosis of native valve cells in response to tissue repair and fibrosis following valve trauma.2 Also, in vitro studies have shown that the amyloid itself promotes apoptosis and subsequent mineralization of valvular cells, potentially setting up a repetitive loop of apoptosis and amyloid accumulation.1 In addition, lipid infiltration (low-density lipoprotein; apolipoproteins A, B, E, A1) and their oxidation are known protagonists in fibrocalcific remodeling.2,4 Chronic inflammation and oxidative stress also have been identified as important players in the amylogenic process.5 Lipid infiltration occurs in areas of valve tissue injury, which can result from local inflammation, age-related degeneration, and congenital malformation. A recently published multiomic study explored the altered pathways in calcific valve disease.6 The majority of highlighted genes and proteins involved—among which are apolipoproteins, amyloid precursor protein (resulting from platelets activation), and transthyretin—are known to be related to the formation of amyloid plaques. The novel multiomic network that was highlighted revealed that coagulation/complement and platelet activation/degranulation pathways play a major role. These findings suggest that molecular mechanisms of calcification in calcific AS may be linked to the formation of amyloid-like deposits as found in Alzheimer’s disease and cardiac amyloidosis. In our relatively young patient, we hypothesize that the amyloid-fibrocalcific remodeling of the valve was secondary to the underlying congenitally bicuspid morphology, resulting in altered hemodynamics, increased mechanical stress, and subsequent tissue injury, potentially triggering proinflammatory mechanisms, which are known to be involved, at least in part, in both fibrocalcific valve disease and amyloid formation. Interesting to note is that 2 cases have been reported previously of localized valvular amyloidosis without significant fibrocalcific disease in bicuspid aortic valves. The first bicuspid valve was congenital with amyloid mass containing AL7; in the second bicuspid valve (type not provided in original publication), the amyloid was of unknown type.8 Considering that localized valvular amyloidosis can occur with or without fibrocalcific deposits, regardless of the involved proteins, the underlying pathophysiology remains unclear. However, available reports of non-tricuspid valves strongly suggest that such malformations play a major role in amyloidogenesis, by inducing high mechanical stress related to both leaflet deformation and nonphysiological flow patterns.
Novel Teaching Points.
-
•
Valvular amyloidosis is not always a systemic manifestation of transthyretin amyloidosis, amyloid light-chain, amyloid A (AA), or apolipoprotein A1 types amyloidoses. This fact calls into question the pathophysiology of amyloid formation and its role in the development of AS. The physiopathologies of aortic valve stenosis, valvular amyloidosis, and cardiac amyloidosis are not well understood and should be explored further to better identify patients at risk of developing one of these conditions.
-
•
Mechanical factors—resulting from unusual valve morphology and altered hemodynamics—might play a major role in localized valvular amyloidosis, either independently or in synergy with fibrocalcific mechanisms.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The patient gave informed consent for this publication.
See page 1072 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.09.002.
Supplementary Material
References
- 1.Audet A., Côté N., Couture C., et al. Amyloid substance within stenotic aortic valves promotes mineralization. Histopathology. 2012;61:610–619. doi: 10.1111/j.1365-2559.2012.04265.x. [DOI] [PubMed] [Google Scholar]
- 2.Ladefoged C., Rohr N. Amyloid deposits in aortic and mitral valves. A clinicopathological investigation of material from 100 consecutive heart valve operations. Virchows Arch A Pathol Anat Histopathol. 1984;404:301–312. doi: 10.1007/BF00694895. [DOI] [PubMed] [Google Scholar]
- 3.Singal A.K., Bansal R., Singh A., et al. Concomitant transthyretin amyloidosis and severe aortic stenosis in elderly Indian population: a pilot study. JACC CardioOncol. 2021;3:565–576. doi: 10.1016/j.jaccao.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindman B.R., Clavel M.A., Mathieu P., et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlini G., Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 6.Heuschkel M.A., Skenteris N.T., Hutcheson J.D., et al. Integrative multi-omics analysis in calcific aortic valve disease reveals a link to the formation of amyloid-like deposits. Cells. 2020;9:2164. doi: 10.3390/cells9102164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groves P.H., Douglas-Jones A.G., Hall R.J.C. Amyloid, thrombosis and acute myocardial infarction in association with a bicuspid aortic valve. Br Heart J. 1993;70:560–562. doi: 10.1136/hrt.70.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal S., Reehana S., Lawrence D. Unique type of isolated cardiac valvular amyloidosis. J Cardiothorac Surg. 2006;1:38. doi: 10.1186/1749-8090-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.