Abstract
The Afa/Dr family of diffusely adhering Escherichia coli (Afa/Dr DAEC) includes bacteria expressing afimbrial adhesins (AFA), Dr hemagglutinin, and fimbrial F1845 adhesin. We show that infection of human intestinal Caco-2/TC7 cells by the Afa/Dr DAEC strains C1845 and IH11128 is followed by clustering of CD55 around adhering bacteria. Mapping of CD55 epitopes involved in CD55 clustering by Afa/Dr DAEC was conducted using CD55 deletion mutants expressed by stable transfection in CHO cells. Deletion in the short consensus repeat 1 (SCR1) domain abolished Afa/Dr DAEC-induced CD55 clustering. In contrast, deletion in the SCR4 domain does not modify Afa/Dr DAEC-induced CD55 clustering. We show that the brush border-associated glycosylphosphatidylinositol (GPI)-anchored protein CD66e (carcinoembryonic antigen) is recruited by the Afa/Dr DAEC strains C1845 and IH11128. This conclusion is based on the observations that (i) infection of Caco-2/TC7 cells by Afa/Dr DAEC strains is followed by clustering of CD66e around adhering bacteria and (ii) Afa/Dr DAEC strains bound efficiently to stably transfected HeLa cells expressing CD66e, accompanied by CD66e clustering around adhering bacteria. Inhibition assay using monoclonal antibodies directed against CD55 SCR domains, and polyclonal anti-CD55 and anti-CD66e antibodies demonstrate that CD55 and CD66e function as a receptors for the C1845 and IH11128 bacteria. Moreover, using structural draE gene mutants, we found that a mutant in which cysteine replaced aspartic acid at position 54 displayed conserved binding capacity but failed to induce CD55 and CD66e clustering. Taken together, these data give new insights into the mechanisms by which Afa/Dr DAEC induces adhesin-dependent cross talk in the human polarized intestinal epithelial cells by mobilizing brush border-associated GPI-anchored proteins known to function as transducing molecules.
Diffusely adhering Escherichia coli (DAEC) strains are considered a heterogeneous group of E. coli. It has been well established that some DAEC strains expressing related adhesins (Afa/Dr DAEC) cause symptomatic urinary tract or intestinal infections (6, 22, 23, 33, 35). Members of this family of virulent E. coli express a family of gene operons including the afa (13, 14, 19, 22, 23), dra (44), and daa (5, 25) genes. For example, in these operons the genes A to D encode mostly accessory functions and are very similar in terms of amino acid sequence and functional homology. The last gene, E, encodes the major structural proteins which function as adhesins. The gene afaE encodes the afimbrial adhesins Afa-I and Afa-III, draE encodes the Dr hemagglutinin, and daaE encodes the fimbrial F1845 adhesin. Despite the similarities among AfaE-I, AfaE-III, Dr hemagglutinin, and F1845, several differences have been observed. AfaE-I and Afa-III are afimbrial, while Dr hemagglutinin and F1845 have fimbrial structures. There is evidence that the afimbrial Afa-I and Afa-III adhesins, the Dr hemagglutinin, and the fimbrial F1845 adhesin mediate the recognition of the membrane glycosylphosphatidylinositol (GPI)-anchored protein decay-accelerating factor (DAF; CD55) as a receptor (32, 33). CD55 is a 70- to 75-kDa protein that acts primarily to protect cells against lysis by autologous complement (for reviews, see references 26 30). Based on these similarities among adhesins and receptor recognition, it was proposed that these bacterial strains belong to a group of E. coli named the Afa/Dr DAEC family. We have previously reported that the Afa/Dr DAEC bacteria adhere to cultured human epithelial intestinal cells (20, 21) by recognition of the brush border-associated CD55 (4), inducing signaling (34) and brush border injuries (3). Activation of GPI-anchored molecules, such as Thy-1 and CD55, after cross-linking by antibodies leads to recruitment of GPI proteins characterized by the appearance of punctate foci indicating clustering (41, 43). Mobilization of GPI-anchored proteins into a complex of signaling molecules localized in particular cell invaginations named caveolae has been recently described (for reviews, see references 2 and 24). It is known that several GPI-anchored molecules are brush border associated in human polarized intestinal cells (12). To gain further insight in the mechanism of pathogenicity of Afa/Dr DAEC, we decided to examine whether strains C1845, expressing the fimbrial F1845 adhesin, and IH11128, expressing the Dr hemagglutinin, recruit brush border-associated GPI-anchored proteins in human polarized intestinal cells.
MATERIALS AND METHODS
Cell lines and culture.
The Caco-2/TC7 clone (9), established from the cultured human colonic adenocarcinoma parental Caco-2 cell line which spontaneously differentiates in culture (37), was used. Cells were routinely grown in Dulbecco modified Eagle's minimal essential medium (25 mM glucose) (Life Technologies, Cergy, France) supplemented with 20% heat-inactivated (30 min, 56°C) fetal calf serum (FCS; Boehringer, Mannheim, Germany) and 1% nonessential amino acids (Life Technologies) as previously described (3, 4). For maintenance purposes, cells were passaged weekly using 0.02% trypsin in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Experiments and maintenance of the cells were carried out at 37°C in a 10% CO2–90% air atmosphere. The culture medium was changed daily. Cells were used at postconfluence after 15 days of culture (fully differentiated cells) for infection assay.
The Chinese hamster ovary (CHO) cell transfectant clones that stably express human CD55 cDNA (DAF/A9), cDNA deletion constructs (DAFΔSCR1/029-6B, DAFΔSCR2/043-7A, DAFΔSCR3/044-2D, DAFΔSCR4/054-5×4), deletion in the serine/threonine-rich (S/T) region (DAFΔS/T/021-C7), a construct for a transmembrane (TM) version of CD55 using the TM domain of the membrane cofactor protein (DAF-TM/2H), or the vector alone were used (11, 27). Cells were cultured in Ham's F-12 medium supplemented with 10% FCS and grown to confluence. Cells were cultured at 37°C in a 5% CO2–95% air atmosphere.
Stably transfected HeLa cells expressing CD66e (HeLa-CD66e) or containing the expression vector alone (HeLa-SFFV.neo) were obtained from F. Grunert (Immunbiologisches Institut, Universität Freiburg, Freiburg, Germany) (7, 10, 17). Cells were cultured at 37°C in a 5% CO2–95% air atmosphere in RPMI 1640 with l-glutamine (Life Technologies) supplemented with 10% FCS and 500 μg of Geneticin per ml.
Bacterial strains.
The bacterial strains used were the clinical isolate E. coli C1845 harboring the fimbrial F1845 adhesin (6), the clinical isolate E. coli IH11128 harboring the Dr hemagglutinin (33), the laboratory E. coli strain HB101 transformed with plasmid pSSS1 expressing F1845 adhesin (6), and the laboratory strain E. coli K-12 EC901 carrying the recombinant plasmid pBJN406 expressing Dr hemagglutinin (32). E. coli K-12 or HB101 was used as a control.
C1845 was grown on Mueller-Hinton agar containing 1% Casamino Acids (Difco Laboratories, Detroit, Mich.), 0.15% yeast extract, 0.005% magnesium sulfate, and 0.0005% manganese chloride in 2% agar for 18 h at 37°C. IH11128 was grown at 37°C for 18 h on Luria broth. K-12 EC901(pBN406) was grown on Luria broth supplemented with chloramphenicol. HB101(pSSS1) was grown at 37°C for 18 h on Luria agar.
Mutant strains carrying plasmid pCC90 in which point mutations in draE were made by site-directed mutagenesis were used (8). E. coli DH5α(pCC90) carries the plasmid encoding the Dr hemagglutinin. The mutant strains carried pCC90-D54 stop, or a plasmid in which threonine 90 is replaced by methionine (pCC90T90M), isoleucine 113 is replaced by threonine (pCC90-I113T), or aspartic acid 54 (Asp-54) is replaced by valine (pCC90-D54V), tyrosine (pCC90-D54Y), glycine (pCC90-D54G), or cysteine (pCC90-D54C).
Cell infection.
The method used for Afa/Dr DAEC infection of cultured cells has been described previously (3, 4). Briefly, the cells were washed twice with PBS. Infecting E. coli bacteria were suspended in the culture medium, and a total of 0.5 ml (108 CFU/well) of this suspension was added to each well of the tissue culture plate. The plates were incubated at 37°C in 10% CO2–90% air for 3 h. The monolayers were then washed three times with sterile PBS.
Quantification of E. coli binding.
Quantitative binding assays of E. coli onto cultured cells were conducted with metabolically labeled bacteria. E. coli was radiolabeled by the addition of 14C-acetic acid (94 mCi/mmol; 100 μCi/10-ml tube; Amersham) in Luria broth as previously reported (3, 4). The cell monolayers were infected with radiolabeled bacteria (108 CFU/well; 50,000 to 70,000 cpm) in the presence of 1% mannose to prevent type 1 fimbria-mediated binding and incubated at 37°C in 10% CO2–90% air for 3 h. The monolayers were then washed three times with sterile PBS. Adhering bacteria and intestinal cells were dissolved in a 1 N NaOH solution. The level of bacterial adhesion was evaluated by liquid scintillation counting. Each adherence assay was conducted in triplicate with three successive cell passages. Inhibition of E. coli adhesion was conducted using anti-CD55 or anti-CD66e polyclonal antibodies (all diluted 1:20 in PBS). Before bacterial adhesion assays, the cell monolayers were preincubated 1 h at 37°C each antibody and then incubated 3 h at 37°C with radiolabeled E. coli.
Antibodies.
The rabbit immunoglobulin G (IgG), anti-Dr adhesin antibody was a generous gift from B. Nowicki (Texas University, Galveston). The mouse monoclonal antibody (MAb) CY-CD55 raised against human CD55 was obtained from Valbiotech (Paris, France). The polyclonal anti-CD55 antibody and mAbs 1H4 and 8D11 directed against CD55 short consensus repeat 3 (SCR3) and SCR4 domains, respectively, were from D. M. Lublin (Washington University, St. Louis, Mo.). Ascites fluid containing the IF7 antibody against the CD55 SCR2 domain was a generous gift from J. M. Bergelson (Dana-Farber Cancer Institute, Harvard Medical School, Boston, Mass.). MAb IA10 against the CD55 SCR1 domain was generously provided by V. Nussenzweig (New York University Medical Center). MAb D14HD1 recognizing CD66a, CD66c, CD66d, and CD66e was a generous gift from F. Grunert. The polyclonal anti-carcinoembryonic antigen (CEA) rabbit antibody was from Dako (Tebu, France).
Immunofluorescence.
Monolayers of cells were prepared on glass coverslips which were placed in 24-well tissue culture plates (Corning Glass Works, Corning, N.Y.). CD55 and CD66e were detected on unpermeabilized cell layers by indirect immunofluorescence labeling with anti-CD55 MAb and monoclonal or polyclonal anti-CEA antibodies, respectively. Preparations were fixed for 10 min at room temperature in 3.5% paraformaldehyde in PBS. Cell monolayers were incubated with specific primary antibody for 45 min at room temperature, washed, and then incubated with a secondary fluorescein isothiocyanate (FITC)- or tetramethyl rhodamine isothiocyanate (TRITC)-conjugated antibody. Primary antibodies were diluted 1:20 to 1:100 in PBS in 2% gelatin–PBS (anti-Dr, 1/100; CY-CD55, 1/20; D14HD1, 1/100). Secondary antibodies were either FITC- or TRITC-conjugated goat anti-mouse IgG from Immunotech (Luminy, France), or FITC-conjugated goat anti-rabbit IgG from Institut Pasteur Productions (Paris, France), used at a dilution of 1:20 in 2% gelatin-PBS.
When indirect immunofluorescence labeling was used, no fluorescent staining was observed when nonimmune serum was used and when the primary antibody was omitted. Specimens were mounted in DABCO antifade mounting medium (Citifluor Laboratories, Birmingham, United Kingdom). Specimens were examined by epifluorescence using a Leitz Aristoplan microscope with epifluorescence. All photographs were taken on Kodak T-MAX 400 black-and-white or color film (Eastman Kodak Co., Rochester, N.Y.).
Statistics.
Data are expressed as mean ± standard error of the mean of experiments. A typical experiment was conducted at least in three successive passages of cells. For each cell passage, examination was conducted at least with three cell monolayers. The statistical significance was assessed by a Student t test.
RESULTS
Clustering of CD55 around Afa/Dr DAEC adhering onto human polarized intestinal Caco-2/TC7 cells.
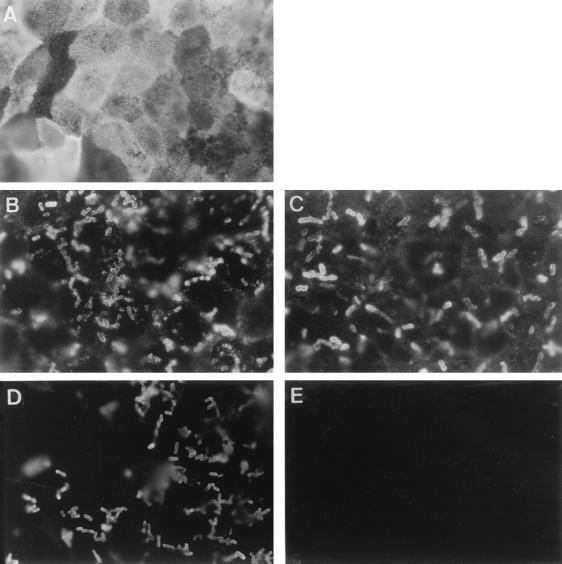
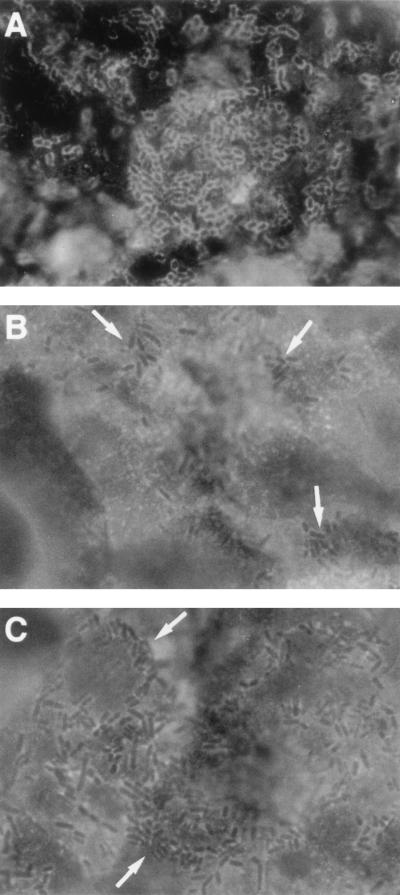
We have previously observed that the CD55 molecule is strikingly localized at the apical domain of the polarized fully differentiated Caco-2 cells (3). We examined by indirect immunofluorescence labeling at high magnification the distribution of CD55 in Afa/Dr DAEC-infected, fully differentiated Caco-2/TC7 cells. As seen in Fig. 1A, immunolabeling of the CD55 molecule in control cells is characterized by a fine punctate cell labeling and expression showing a mosaic pattern. This result is consistent with the typical distribution of the brush border-associated molecules in fully differentiated intestinal cells. We observed disorganization of CD55 distribution in infected cells characterized by the disappearance of the cell diffuse punctate labeling and by intensive CD55 clustering around adhering C1845 (Fig. 1B) and HB101(pSSS1) bacteria harboring the F1845 adhesin (Fig. 1C). As a control, C1845 bacteria plated on a glass slide showed no cross-reaction with the anti-CD55 MAb (Fig. 1E). Identical results were obtained with the uropathogenic strain IH11128 and with the recombinant E. coli EC901(pBN406) harboring Dr hemagglutinin (not shown).
FIG. 1.
Change in CD55 distribution in Afa/Dr DAEC C1845-infected human fully differentiated intestinal Caco-2/TC7 cells. Cells were infected with E. coli C1845 or recombinant strain HB101(pSSS1) (F1845+) for 3 h at 37°C before washing and fixing. The paraformaldehyde-fixed cells were stained with MAb CY-CD55 and examined at the apical domain. (A) CD55 in control cells showing punctate labeling and mosaic pattern distribution. (B) Immunolabeling of CD55 in C1845-infected cells showing disappearance of CD55 mosaic pattern distribution and appearance of positive CD55 immunolabeling around adhering bacteria indicating CD55 clustering. (C) CD55 in E. coli recombinant strain HB101(pSSS1) (F1845+)-infected cells showing disappearance of CD55 mosaic pattern distribution and appearance of positive CD55 immunolabeling around adhering bacteria indicating CD55 clustering. (D and E) As a control, C1845 plated on glass slide and immunolabeled with anti-Dr or anti-CD55 antibodies, respectively, shows positive Dr labeling (D) and negative CD55 labeling (E). Magnifications, ×100.
Mapping of CD55 epitopes involved in CD55 clustering.
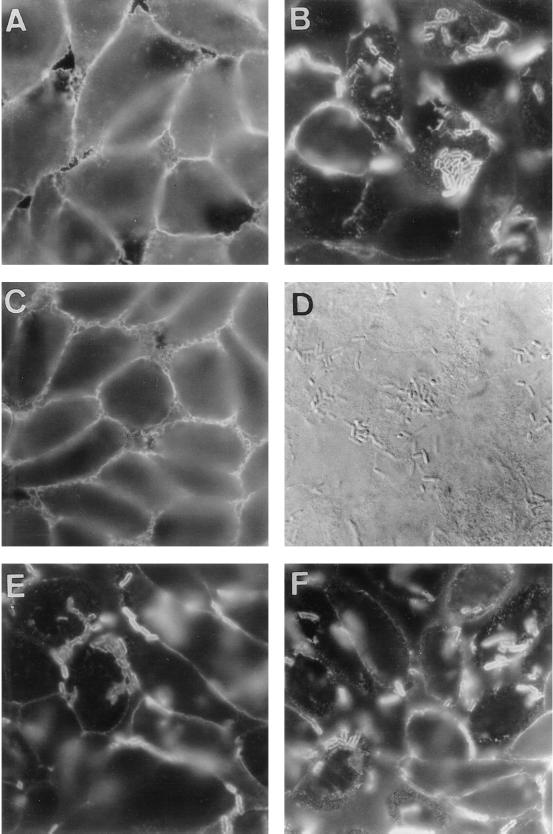
The CD55 molecule has five extracellular domains: four contiguous SCR domains, followed by an S/T, heavily O-glycosylated C-terminal domain. Moreover, a GPI anchor attaches the molecule to the outer leaflet of the cell membrane (for reviews, see references 26 and 30). Mapping of CD55 epitopes involved in Afa/Dr DAEC-induced CD55 clustering was further conducted using the CHO cell transfectant clones that stably express human CD55 cDNA (DAF/A9), cDNA deletion constructs (DAFΔSCR1/029-6B, DAFΔSCR2/043-7A, DAFΔSCR3/044-2D, DAFΔSCR4/054-5×4, and DAFΔS/T/021-C7), a construct for a TM version of CD55 (DAF-TM/2H), or the vector alone (11, 26). Analyzed by flow cytometry and CD55 immunoprecipitation with rabbit polyclonal anti-CD55, these clones demonstrated a high expression of CD55 (11, 27). As shown in Fig. 2, the CD55 clustering around adhering Afa/Dr DAEC C1845 bacteria occurred in CHO cell transfectant clones DAF/A9, DAFΔSCR4/054-5×4, and DAF-TM/2H. In contrast, no CD55 clustering around adhering C1845 bacteria was found to clone DAFΔSCR1/029-6B. Although the binding of C1845 bacteria in clones DAFΔSCR2/043-7A, DAFΔSCR3/044-2D, and DAFΔS/T/021-C7 was dramatically decreased, in agreement with a previous report (31), no CD55 clustering was found around the sparse, randomly adhering C1845 bacteria observed (not shown).
FIG. 2.
CD55 clustering around adhering Afa/Dr DAEC C1845 infecting CHO cell transfectant clones that stably express human CD55 cDNA, cDNA deletion constructs, or a construct for a TM version of CD55. Cells were infected with strain C1845 for 3 h at 37°C before washing and fixing. The paraformaldehyde-fixed cells were stained with MAb CY-CD55. (A and B) Expression of CD55 in uninfected DAF/A9 clone (A) and CD55 clustering around adhering bacteria (B) in infected DAF/A9 clone. (C and D) No CD55 clustering (C) around adhering bacteria observed by phase-contrast microscopy (D) in infected DAFΔSCR1/029-6B clone. (E and F) CD55 clustering around adhering bacteria in infected DAFΔSCR4/054-5×4 and DAF-TM/2H clones, respectively. magnifications, ×100.
We have previously reported that recognition of CD55 in cultured human embryonic intestinal INT407 cells is followed by reorganization of F-actin stress fibers into F-actin ruffles through activation of signaling molecules associated with the CD55 molecule (34). Here, we examine whether CD55 clustering around adhering bacteria into CHO cell transfectant clone DAF/A9 is followed by F-actin reorganization. F-actin labeled with fluorescein-phalloidin show similar organization in stress fibers both in noninfected and Afa/Dr DAEC-infected DAF/A9 cells (not shown).
Clustering of the brush border-associated CD66e (CEA) around Afa/Dr DAEC adhering to human polarized intestinal Caco-2/TC7 cells.
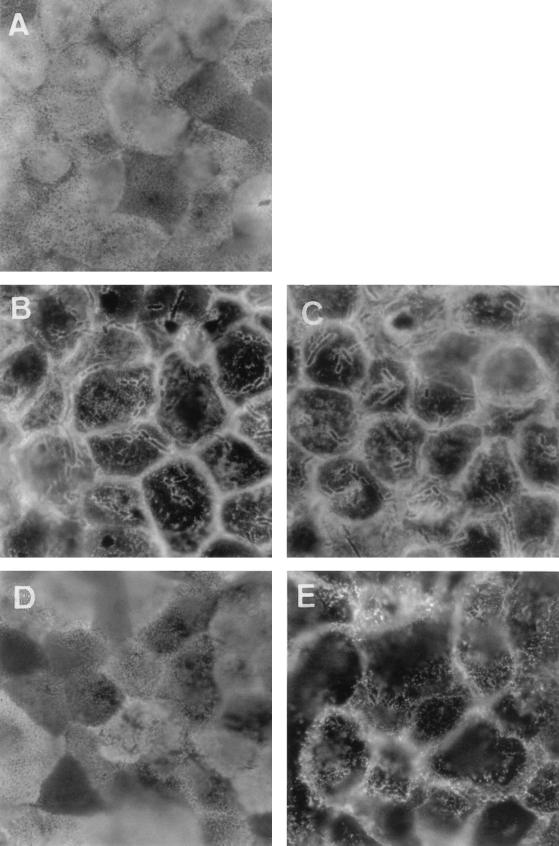
We examined the apical distribution of other known brush border-associated GPI proteins upon Afa/Dr DAEC infection (Fig. 3). We found disorganization of CD66e distribution in the Afa/Dr DAEC C1845-infected Caco-2/TC7 cells, characterized by the disappearance of punctate diffuse labeling of CD66e and the appearance of CD66e clustering around adhering bacteria (Fig. 3B). The same was found in E. coli HB101(pSSS1) (F1845+)-infected cells (Fig. 3C). As for CD55, C1845 bacteria plated on glass slides showed no cross-reaction with the MAb or polyclonal anti-CD66e (not shown). In contrast, when examining the distribution of brush border-associated alkaline phosphatase (AP) in Afa/Dr DAEC C1845-infected cells, we observed disorganization of the punctate diffuse labeling of AP at the apical cell surface without AP clustering around adhering bacteria (Fig. 3E).
FIG. 3.
Change in apical distribution of the GPI-anchored CD66e and AP in Afa/Dr DAEC C1845-infected human fully differentiated intestinal Caco-2/TC7 cells. Cells infected with E. coli C1845 or recombinant strains for 3 h at 37°C before washing and fixing. Paraformaldehyde-fixed cells were stained with MAb anti-CD66E or anti-AP and examined at the apical domain. (A) CD66e in control cells showing punctate labeling and mosaic pattern distribution. (B) Disappearance of CD66e mosaic pattern distribution in C1845-infected cells and appearance of positive CD66e immunolabeling around adhering bacteria. (C) Disappearance of CD66e mosaic pattern distribution in E. coli recombinant strain HB101(pSSS1) (F1845+)-infected cells and appearance of positive CD66e immunolabeling around adhering bacteria. (D) AP in control cells showing punctate labeling and mosaic pattern distribution. (E) Disorganization of the AP mosaic pattern distribution in C1845-infected cells and absence of AP labeling around infecting bacteria. We noticed that as for CD55 (Fig. 1), the C1845 bacteria plated on glass slide and immunolabeled with appropriate antibodies presented no immunolabeling of CD66e or AP around adhering bacteria (not shown). Magnifications, ×100.
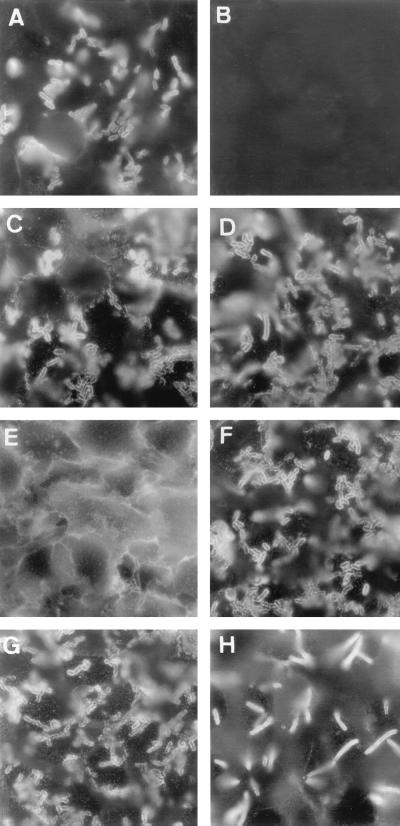
To confirm that CD66e was mobilized upon Afa/Dr DAEC infection, we used stably transfected HeLa cells expressing recombinant CD66e protein (HeLa-CD66e) or containing the expression vector alone (HeLa-SFFV.neo) (Fig. 4). It has been previously observed that the Afa/Dr DAEC adhered onto HeLa cells (15, 16, 19), since these human nonpolarized epithelial cells constitutively express CD55. In agreement with this, we found efficient binding of C1845 bacteria onto HeLa-SFFV.neo (Fig. 4A). Immunolabeled with an anti-CD66e antibody, we found no CD66e clustering around the adhering bacteria in HeLa-SFFV.neo (Fig. 4B). In contrast, we observed intense CD55 clustering around adhering C1845 bacteria in both the infected HeLa-SFFV.neo and HeLa-CD66e cells (Fig. 4C and D, respectively), which constitutively expressed CD55. In the infected HeLa-CD66e cells, we observed intense CD66e clustering around adhering C1845, HB101(pSSS1) (F1845+), and EC901(pBN406) (Dr+) bacteria (Fig. 4F to H, respectively).
FIG. 4.
CD66e clustering around adhering Afa/Dr DAEC bacteria infecting stably transfected HeLa cells expressing CD66e. HeLa cells were infected with strains C1845 (B, F, and H), HB101(pSSS1) (F1845+) (C), and EC901(pBN406) (Dr+) (D). The paraformaldehyde-fixed cells were stained with MAb anti-CEA D14HD11 recognizing CD66e (A, B, E to H), anti-CD55 (C and D) or with polyclonal anti-Dr antibody (A). (A to C) HeLa cells containing the expression vector (HeLa-SFFV.neo) alone. (D to H) HeLa cells stably transfected with CD66e cDNA (HeLa-CD66e). (A) Immunolabeling with anti-Dr antibody in C1845-infected HeLa cells-SFFV.neo reveals adhering bacteria. (B) No clustering of CD66e around C1845 bacteria infecting the HeLa-SFFV.neo cells. (C) Clustering of CD55 around C1845 bacteria infecting HeLa-SFFV.neo cells. (D) Clustering of CD55 around C1845 bacteria infecting HeLa-CD66e cells. (E) Positive CD66e immunofluorescence in HeLa-CD66e cells. (F to H) Clustering of CD66e around adhering C1845 (F), HB101(pSSS1) (F1845+) (G), and EC901(pBN406) (Dr+) (H) bacteria infecting the HeLa-CD66e cells. Magnifications, ×100.
To ascertain whether the brush border-associated CD66e acts as a coreceptor with the CD55 for Afa/Dr DAEC in human intestinal cells, we conducted an adhesion inhibition assay with antibodies directed against CD55 or CD66e (Table 1). In agreement with results obtained by Nowicki et al. (31) for transfected CHO cells, MAbs directed against the SCR2 and SCR3 domains of CD55 resulted in a high level of inhibition of Afa/Dr DAEC binding, whereas MAbs directed against the SCR1 and SCR4 domains did not. Significantly high inhibition of bacterial attachment was obtained with the polyclonal anti-CD66e antibody. Moreover, a significant increase in inhibition of bacterial binding was obtained when the polyclonal anti-CD55 and anti-CD66e antibodies were used in conjunction, compared with the inhibition obtained with each antibody used separately.
TABLE 1.
Inhibition of adhesion of the metabolically radiolabeled 14C-Afa/Dr DAEC strains C1845 and IH11128 by antibodies directed against CD55 and CD66e in fully differentiated human intestinal Caco-2/TC7 cells.
| Condition | % of 14C labeled bacteria bound ± SEMa
|
|
|---|---|---|
| E. coli C1845 | E. coli IH11128 | |
| Control infected cells | 5.60 ± 0.25 | 5.50 ± 0.24 |
| MAb IA10 directed against CD55 SCR1 domain | 4.90 ± 0.27 | 4.99 ± 0.16 |
| MAb IF7 directed against CD55 SCR2 domain | 2.87 ± 0.24∗ | 2.76 ± 0.14∗ |
| CY-CD55 directed against CD55 SCR3 domain | 2.78 ± 0.11∗ | 2.64 ± 0.11∗ |
| MAb 8D11 directed against CD55 SCR4 domain | 5.60 ± 0.24 | ND |
| Anti-CD55 polyclonal antibody | 2.85 ± 0.14∗ | 2.80 ± 0.11∗ |
| Anti-CD66e polyclonal antibody | 3.74 ± 0.14∗ | 4.29 ± 0.14∗ |
| Anti-CD66e + anti-CD55 polyclonal antibodies | 1.26 ± 0.07∗ | 1.42 ± 0.11∗ |
Inhibition appeared to be specific since preimmune rabbit sera did not affect binding. Statistical analysis between antibody-treated infected cells versus control infected cells was performed with a Student t test. ∗, significant difference at P < 0.01. Statistical analysis between anti-CD66e and anti-CD55 polyclonal antibody-treated cells versus anti-CD55 polyclonal antibody or anti-CD66e polyclonal antibody-treated cells shows in both cases a significant difference at P < 0.01. Statistical analysis between anti-CD55 polyclonal antibody versus anti-CD66e polyclonal antibody treated cells shows no significant difference. ND, not determined.
Effects of point mutations in Dr hemagglutinin on CD55 and CD66e clustering in Caco-2/TC7 cells.
Carnoy and Moseley (8) recently constructed mutants at positions 32, 40, 54, 90, and 113 in Dr hemagglutinin. We further investigated whether several point mutations could affect CD55 and CD66e recruitment around Afa/Dr DAEC bacteria adhering to Caco-2/TC7 cells (Table 2).
TABLE 2.
Effects of point mutations within Dr hemagglutinin on binding to CHO CD55+ cells and on CD55 and CD66e clustering in fully differentiated human intestinal Caco-2/TC7 cells.
| Plasmid | % of 14C-labeled bacteria bound ± SEMa
|
CD55 and CD66e clusteringb | |
|---|---|---|---|
| CHO CD55+ cells | TC7 cells | ||
| pCC90 | 6.60 ± 0.3 | 5.5 ± 0.4 | Positive |
| pCC90-D54stop | 0.40 ± 0.04∗ | 0.7 ± 0.2∗ | Negativec |
| BN17 (draE) | 0.25 ± 0.03∗ | 0.5 ± 0.2∗ | Negativec |
| pCC90-D54V | 5.70 ± 0.3 | 5.1 ± 0.5 | Positive |
| pCC90-D54Y | 6.60 ± 0.2 | 5.5 ± 0.2 | Positive |
| pCC90-T90M | 6.30 ± 0.3 | 5.8 ± 0.4 | Positive |
| pCC90-I113T | 6.30 ± 0.3 | 5.8 ± 0.5 | Positive |
| pCC90-D54G | 4.00 ± 0.2∗ | 2.0 ± 0.3∗ | Weakly positive |
| pCC90-D54C | 4.70 ± 0.3∗ | 3.6 ± 0.5∗ | Negative |
Statistical analysis between pCC90 versus mutants was performed with a Student t test. ∗, significant difference at P < 0.01.
CD55 and CD66e clustering around bacteria adhering onto fully differentiated human intestinal Caco-2/TC7 cells observed after indirect immunolabeling of CD55 and CD66e with anti-CD55 MAb IF7 and polyclonal rabbit anti-CEA antibody, respectively.
Although E. coli(pCC90-D54stop) and E. coli BN17 (draE) were not adherent to Caco-2/TC7 cells, isolated adhering E. coli could be observed in randomly distributed cells for which no CD55 and CD66e clustering was observed.
Dr+ E. coli DH5α(pCC90) carrying the plasmid encoding the Dr hemagglutinin and all of the mutants showed a low level of adhesion to the CHO CD55− cells (not shown). Dr+ E. coli DH5α(pCC90) showed a high level of adhesion to the CHO CD55+ cells. This recombinant E. coli bound efficiently to Caco-2/TC7 cells expressing CD55 and promoted pronounced CD55 (Fig. 5A) and CD66e (not shown) clustering. E. coli DH5α, used as a host for the mutated plasmids, showed no binding to CHO CD55+ and Caco-2/TC7 cells. The insertion mutant E. coli BN17 (EC901[pBJ17:Tn3]) (draE) and E. coli (pCC90-D54stop) lost adhesion to the CHO CD55+ and Caco-2/TC7 cells. Interestingly, when examining the randomly distributed Caco-2/TC7 cells to which a small number of pCC90-D54stop-carrying and BN17 E. coli mutants adhered, we found no CD55 and CD66e clustering. The mutants carrying pCC90-T90M, pCC90-I113T, pCC90-D54V, and pCC90-D54Y retained the capacity to bind to CHO CD55+ and Caco-2/TC7 cells as well CD55 and CD66E clustering activity. The mutant carrying pCC90-D54G retained CHO CD55+ binding but lost 64% of binding to Caco-2/TC7 cells. This mutant promoted weak CD55 and CD66e clustering activity. The mutant carrying pCC90-D54C lost only 29 and 34% of binding to the CHO CD55+ and Caco-2/TC7 cells, respectively. Interestingly, this mutant lost entirely CD55 (Fig. 5B) and CD66e (Fig. 5C) clustering activity.
FIG. 5.
CD55 and CD66e immunolabeling in human fully differentiated Caco-2/TC7 cells infected by recombinant E. coli(pCC90) and a Dr mutant carrying pCC90-D54C. Experimental conditions were as for Fig. 1. (A) CD55 clustering around adhering pCC90 (Dr+-carrying bacteria. (B and C) No CD55 (B) and CD66e (C) clustering around pCC90-D54C-carrying adhering bacteria. Arrows indicate adhering bacteria. Magnifications, ×100.
DISCUSSION
The signaling GPI-anchored proteins are now recognized as playing a role in microbial pathogenicity. For example, coxsackievirus recognizes the functional SCR3 domain of CD55, cross-links CD55, and subsequently is internalized within the cells (39, 40). In this study, we were attempting to gain additional insights into Afa/Dr DAEC pathogenicity by examining how these pathogenic bacteria mobilize membrane-associated GPI proteins upon cell infection. Golusko et al. (16) recently reported that an intense accumulation of CD55 outlined the adhering Afa/Dr DAEC in infected HeLa cells. We found here that the same phenomenon occurs in fully differentiated Caco-2/TC7 cells expressing a brush border, since intense clustering of the brush border-associated CD55 was observed around the adhering Afa/Dr DAEC bacteria.
We observed that the cell surface expression of CD55 in Caco-2/TC7 and CHO DAF+ cells is very different. This is due to the fact that the Caco-2/TC7 cells are fully differentiated, expressing a well-organized brush border with well-ordered microvilli endowed by functional proteins such as CD55. Moreover, the observed mosaic pattern of CD55 in Caco-2/TC7 cells is characteristic of brush border-associated proteins since this pattern results from the fact that the level of expression of brush border-associated functional proteins could vary from one cell to another (4, 37). In contrast, the fine and more homogenous CD55 expression observed in CHO DAF+ cells results from the presence of a smooth cell surface characteristic of undifferentiated cells. Interestingly, we observed that the binding of recombinant E. coli pCC90 onto these cell lines is not significantly different. This result demonstrates that the binding capacity of the CD55 receptor is similar despite the pattern of CD55 distribution.
It has been previously established by mapping of complement regulatory domains on the human CD55 molecule that different parts of the molecule control the CD55 function (11, 27). For example, the complement regulatory function of CD55 is highly dependent of the SCR2, SCR3, and SCR4 domains, while the SCR1 domain and GPI anchor do not play a role. Afa/Dr DAEC binding (31), and in consequence bacterial internalization (38), are highly dependent on the SCR2 and SCR3 domains, while the SCR1 and SCR4 domains do not play a role. We examined using the stably transfected CHO cells carrying individual deletion in the CD55 molecule (11, 27) whether or not a single deletion in CD55 affected CD55 clustering around adhering bacteria. We found that deletion in SCR2 and SCR3 domains, which dramatically decreased Afa/Dr DAEC binding (31), abolished CD55 clustering around the sparsely remaining adhering bacteria observed. Again, deletion in the S/T-rich region abolished CD55 clustering around the sparsely remaining adhering bacteria observed. Interestingly, the S/T region serves as a nonspecific spacer projecting CD55 at the membrane surface (for a review, see reference 26). Deletion in S/T region abrogates the CD55 function (11, 27), abolishes Afa/Dr DAEC binding (31), and in a close consequence terminates bacterial internalization (38). The deletion in the SCR4 domain, which abolished the CD55 function (11, 27), did not affect Afa/Dr DAEC binding and CD55 clustering around the adhering bacteria. Interestingly, we found that deletion of the SCR1 domain, which did not influence the CD55 complement regulatory function (11, 27), abolished CD55 clustering around the adhering bacteria without affecting the level of bacterial binding. This result now suggests that the SCR1 domain plays a role in the Afa/Dr DAEC mechanism of pathogenicity. We hypothesize that the disappearance of the CD55 clustering around adhering bacteria could reflect a failure in the CD55-associated functions such as signaling.
Results obtained with Dr mutants are in agreement with the hypothesis that Afa/Dr DAEC binding and Afa/Dr DAEC-induced GPI clustering can be traced back to different sites in the CD55 molecule. Indeed, when examining whether site-directed mutagenesis in Dr hemagglutinin affected the Afa/Dr DAEC cellular response in human intestinal cells, we found that when the aspartic acid residue at position 54 was replaced by cysteine, the mutant retained a high capacity to bind but lost entirely CD55 and CD66e clustering activity. This result suggests that this point mutation in the Dr adhesin could promote a failure in the GPI-associated signaling without affecting binding. It is known that the N-terminal 54-amino-acid region in Afa/Dr adhesins is involved in expression of phenotypes (8, 23, 32, 48). Mannose-resistant hemagglutination (MRHA) exhibited by the Afa/Dr family of adhesins is sensitive to chloramphenicol for Dr hemagglutinin, while MRHA for Afa-I, Afa-III, and F1845 adhesins is not sensitive (23, 32). Moreover, Dr hemagglutinin, but not AfaE-I, AfaE-III, and F1845 adhesins, expresses a chloramphenicol-sensitive (Cms) adhesion to type IV collagen (8, 48). Interestingly, Carnoy and Moseley (8) using site-directed mutagenesis have demonstrated that mutations at positions 32, 40, 54, 90, and 113 affected differently type IV collagen binding and chloramphenicol sensitivity of binding, while they had no effect on MRHA. These authors concluded that Asp-54 appeared involved in a conformational domain for the Cms hemagglutination (CSHA) and type IV collagen binding. Le Bouguenec et al. (23) demonstrated that in strain A30 expressing the Afa-III adhesin, aspartic acid 52 was associated with the CSHA phenotype. A comparison of the deduced amino acid sequences in Afa-III adhesin of strain A30 and Dr hemagglutinin showed that position 52 corresponds to an asparagine residue in AFA-III and to an asparatic residue in Dr hemagglutinin (23). Like Afa-III of strain A30, the other Cmr adhesins (Afa-I and F1845) do not contain an aspartic acid residue at position 52. Interestingly, Le Bouguenec et al. (23) reported that Afa-III adhesins of strains AL845 and AL847 differed from that of the strain A30 by the presence of an aspartic acid residue instead of an asparagine at position 52, and that these adhesins conferred Cms MRHA properties to the strains.
When examining the distribution of known brush border-associated GPI-anchored proteins, we found here that Afa/Dr DAEC infection promotes the clustering of the brush border-associated GPI-anchored protein CD66e, whereas another brush border-associated GPI-anchored protein, AP, was not affected. CD66e is a member of the CEA gene family, which belongs to the immunoglobulin superfamily (for a review, see reference 45). The CEA family consists of highly homologous glycoproteins subdivided into the CEA and pregnancy-specific glycoproteins subgroups (for a review, see reference 28). The CEA subgroup includes biliary glycoprotein (CD66a), CEA gene family member 6 (CGM6; CD66b), nonspecific cross-reacting antigen (NCA; CD66c), CGM1 (CD66d), and CEA (CD66e). CD66e is a 180- to 200-kDa GPI-anchored glycoprotein found in epithelia of the gastrointestinal tract, lungs, and testes and in high levels in a variety of carcinomas and cultured human colonic cells (4). The function of these molecules in vivo is not known (for a review, see reference 30). However, some subgroup members act as homotypic and heterotypic cell adhesion molecules, and CD66e plays a role as an accessory molecule in binding tumor cells to collagen type I. Recently, reports have demonstrated that several protein (Opa)-expressing pathogenic Neisseria gonorrhoeae strains interact with glycoproteins belonging to the CD66 family including GPI-anchored glycoproteins (7, 46). In HeLa cells that were stably transfected with five different members of the CEA family, immunofluorescence experiments show that clustering of CEA-likes molecules occurred around Opa+ bacteria, indicating Opa-specific recruitment of CEA-likes molecules (7). Several CEA subgroup members, in addition of their surface-exposed regions, are membrane bound through a GPI anchor (CEA, NCA, and CGM6). Recent studies show that host signaling via CD66-Opa interactions results in stimulation of the opsonin-independent phagocytic uptake in polymorphonuclear cells (17), internalization in transfected HeLa cells expressing CEA family proteins (10, 17), and actin polymerization and transcellular passage in colonic polarized T84 cells (47). Interestingly, we observed that in Afa/Dr DAEC-infected CHO DAF/A9 cells infection is not followed by F-actin rearrangements unlike in infected INT407 (34) and Caco-2/TC7 cells (3). Two hypotheses could explain this discrepancy between the cell lines. The CHO cell transfectant clone DAF/A9 could lack expression of one or several GPI-associated signaling molecules that function downstream of CD55 and which are essential for the Afa/Dr DAEC-induced Ca2+-dependent signaling promoting cytoskeleton rearrangements (34). The second explanation could come from the fact that the CHO DAF/A9 cells lacked expression of CD66e. Indeed, it is tempting to speculate that the costimulation of CD55 and CD66e could be required to induce the F-actin reorganization as in INT407 (34) and Caco-2/TC7 (3) cells, which both expressed constitutively CD55 and CD66e (4).
In conclusion, the results presented here and previously (3, 4, 34) demonstrate that strains C1845 and IH11128 of the Afa/Dr DAEC family develop in human intestinal cells a common mechanism including the recognition of two brush border-associated GPI-anchored proteins: CD55 and CD66e. This new result is important in terms of Afa/Dr DAEC pathogenicity, in particular to explain how these bacteria induce adhesin-mediated host-pathogen cross talk (1). Indeed, our results are consistent with the current mechanism for GPI-associated signal transduction in that the GPI-anchored glycoproteins seem to be laterally mobile in the membrane through their GPI anchor. They could associate with some other membrane-associated signal-transducing protein(s) into a complex of signaling molecules localized in caveolae or caveola-like structures (for reviews, see references 2 and 2, 24). It has been established that CD55 and CD66e are capable of triggering different cellular responses by signaling. For example, CD55 coimmunoprecipitates with the Src family member tyrosine kinases p56lck and p59fyn1 and other phosphorylated proteins (41, 43). CD66 associates with tyrosine kinases of the Src family in neutrophils for CD66-mediated cell signaling (42). Furthermore, it was recently demonstrated that the CD66-mediated opsonin-independent phagocytosis of Opa52 N. gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signaling pathway (18).
ACKNOWLEDGMENTS
J. Guignot and I. Peiffer contributed equally to this work.
We are grateful to B. J. Nowicki for the generous gift of the recombinant K-12 EC901(pBN406), insertion mutant E. coli BN17, and anti-Dr antibody. We thank F. Gruner for the generous gift of CD66e-transfected HeLa cells.
J. Guignot is supported by a doctoral fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT). C. Carnoy is supported by a grant from the Délégation à la Recherche (CHRU Lille). A.L. Servin is supported for this work by a grant from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires (PRFMMIP-MENRT). S.L. Moseley is supported for this work by grant DK49862 from the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Abraham S N, Jonsson A-B, Normark S. Fimbriae-mediated host-pathogen cross-talk. Curr Opin Microbiol. 1998;1:75–81. doi: 10.1016/s1369-5274(98)80145-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson R G W. The caveola membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 3.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1818–1828. doi: 10.1128/iai.64.6.1918-1928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut. 1996;38:248–253. doi: 10.1136/gut.38.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 6.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos M P, Grunert F, Belland R J. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnoy C, Moseley S L. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol Microbiol. 1997;23:365–379. doi: 10.1046/j.1365-2958.1997.2231590.x. [DOI] [PubMed] [Google Scholar]
- 9.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Zweibaum A, Rousset M. Differential expression of sucrase isomaltase in clones isolated from early and late passages of the cell line Caco-2 evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Grunert F, Medina-Marino A, Gotschlich E C. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coyne K E, Hall S E, Thompson E S, Acre M A, Kinoshita T, Fujita T, Anstee D J, Rosse W, Lublin D M. Mapping epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992;149:2906–2913. [PubMed] [Google Scholar]
- 12.Garcia M, Mirre C, Quaroni A, Reggio H, Le Bivic A L. GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J Cell Sci. 1993;104:1281–1290. doi: 10.1242/jcs.104.4.1281. [DOI] [PubMed] [Google Scholar]
- 13.Garcia M I, Gounon P, Courcoux P, Labigne A, Le Bouguenec C. The afimbrial adhesive sheat ancoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol Microbiol. 1996;19:683–693. doi: 10.1046/j.1365-2958.1996.394935.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia M I, Labigne A, Le Bouguenec C. Nucleotide sequence of the afimbrial-adhesin-encoding afa-3 gene cluster and its translocation via flanking IS1 insertion sequences. J Bacteriol. 1994;76:7601–7613. doi: 10.1128/jb.176.24.7601-7613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golusko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki B J. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 16.Golusko P, Selvarangan R, Popov V, Pham T, Wen J W, Singhal J. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect Immun. 1999;67:3989–3997. doi: 10.1128/iai.67.8.3989-3997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray-Owen S D, Dehio C, Haude A, Grunert F, Meyer T F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauck C R, Meyer T F, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–54. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouve M, Garcia M I, Courcoux P, Labigne A, Gounon P, Le Bouguenec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfAD proteins, respectively. Infect Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernéis S, Bernet M F, Gabastout J M, Coconnier M H, Nowicki B J, Servin A L. Human cultured intestinal cells express attachment sites for uropathogenic Escherichia coli bearing adhesins of the Dr adhesin family. FEMS Microbiol Lett. 1994;119:27–32. doi: 10.1111/j.1574-6968.1994.tb06862.x. [DOI] [PubMed] [Google Scholar]
- 21.Kernéis S, Bilge S S, Fourel V, Chauvière G, Coconnier M H, Servin A L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli (DAEC) during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991;59:4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labigne-Roussel A, Schmidt M A, Waltz W, Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985;162:1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bouguenec C, Garcia M I, Ouin V, Desperrier J M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisanti M, Tang Z, Scherer P, Kubler E, Koleske A, Sargiacomo M. Caveolae, transmembrane signaling and cellular transformation. Mol Membr Biol. 1995;12:121–124. doi: 10.3109/09687689509038506. [DOI] [PubMed] [Google Scholar]
- 25.Loomis W P, Moseley S L. Translational control of mRNA processing in the F1845 fimbrial operon of Escherichia coli. Mol Microbiol. 1998;30:843–853. doi: 10.1046/j.1365-2958.1998.01117.x. [DOI] [PubMed] [Google Scholar]
- 26.Lublin D M. Glycosyl-phosphatidylinositol anchoring of membrane proteins. Curr Top Microbiol Immunol. 1992;178:141–162. doi: 10.1007/978-3-642-77014-2_9. [DOI] [PubMed] [Google Scholar]
- 27.Lublin D M, Coyne K E. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J Exp Med. 1991;174:35–44. doi: 10.1084/jem.174.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagel G, Grunert F. From genes to proteins: the non-specific cross-reacting antigens. Tumour Biol. 1995;16:17–22. doi: 10.1159/000217924. [DOI] [PubMed] [Google Scholar]
- 29.Nagel G, Grunert F, Kuijpers T W, Watt S M, Thompson J, Zimmerman W. Genomic organization, splice variants and expression of CGM2, a CD66-related member of the carcinoembryonic antigene gene family. Eur J Biochem. 1993;214:27–35. doi: 10.1111/j.1432-1033.1993.tb17892.x. [DOI] [PubMed] [Google Scholar]
- 30.Nosgean O, Briolay A, Roux B. Mammalian GPI proteins: sorting, membrane residence and functions. Biochim Biophys Acta. 1997;1331:153–186. doi: 10.1016/s0304-4157(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 31.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I, AFA-II, and AFA-II, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with the Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki B, Moulds J, Hull R, Hull S. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect Immun. 1988;56:1057–1060. doi: 10.1128/iai.56.5.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peiffer I, Servin A L, Bernet-Camard M-F. Piracy of decay-accelerating factor (DAF-CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect Immun. 1998;66:4036–4042. doi: 10.1128/iai.66.9.4036-4042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham T, Kaul A, Hart A, Goluszko P, Moulds J, Nowicki S, Lublin D M, Nowicki B J. Dra-related X adhesins of gestational pyelonephritis-associated Escherichia coli recognize SCR-3 and SCR-4 domains of recombinant decay-accelerating factor. Infect Immun. 1995;63:1663–1668. doi: 10.1128/iai.63.5.1663-1668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlandi P A, Fishman P H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveola-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 38.Selvarangan R, Golusko P, Popov V, Singhal J, Pham T, Lublin D M, Nowicki S, Nowicki B. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect Immun. 2000;68:1391–1399. doi: 10.1128/iai.68.3.1391-1399.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafren D R. Viral entry induced by cross-linked decay-accelerating factor. J Virol. 1998;72:9407–9412. doi: 10.1128/jvi.72.11.9407-9412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafren D R, Bates R C, Agrez M V, Herd R L, Burns G F, Barry R D. Coxsakieviruses B1, B3, and B5 use decay accelerating factor as a receptor for cell attachment. J Virol. 1995;69:3873–3877. doi: 10.1128/jvi.69.6.3873-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenoy-Scaria A M, Kwong J, Fijita T, Olszowy M W, Shaw A S, Lublin D M. Signal transduction through decay-accelerating factor. Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn1. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 42.Skubitz K M, Campbell K D, Ahmed K, Skubitz A P N. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol. 1995;155:5382–5390. [PubMed] [Google Scholar]
- 43.Stefanova I, Horejsi V, Ansotegui I J, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 44.Swanson T N, Bilge S S, Nowicki B, Moseley S L. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect Immun. 1991;59:261–268. doi: 10.1128/iai.59.1.261-268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson J A, Grunert F, Zimmerman W. Carcinoembryonic antigen family: molecular biology and clinical perspective. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 46.Virj M, Makepeace K, Fergusson D J-P, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseria. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Gray-Owen S D, Knorre A, Meyer T F, Dehio C. Opa binding to cellular CD66 receptors mediates the transcellular transversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol Microbiol. 1998;30:657–671. doi: 10.1046/j.1365-2958.1998.01102.x. [DOI] [PubMed] [Google Scholar]
- 48.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]