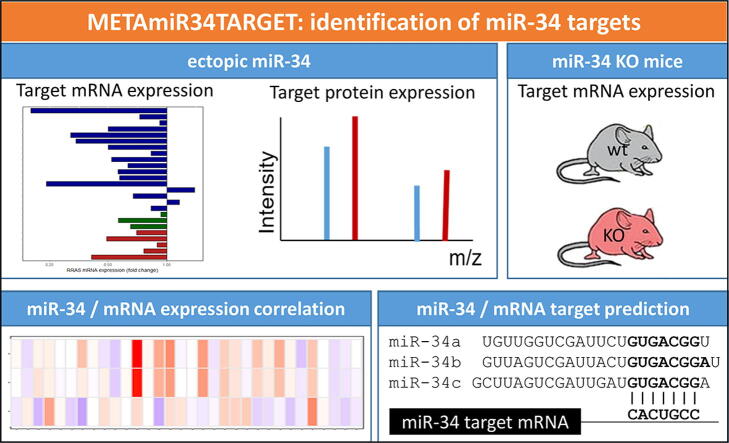
Graphical abstract
Keywords: microRNA, miR-34, Meta-analysis, Target
Abstract
Members of the microRNA-34/miR-34 family are induced by the p53 tumor suppressor and themselves possess tumor suppressive properties, as they inhibit the translation of mRNAs that encode proteins involved in processes, such as proliferation, migration, invasion, and metastasis. Here we performed a comprehensive integrative meta-analysis of multiple computational and experimental miR-34 related datasets and developed tools to identify and characterize novel miR-34 targets. A miR-34 target probability score was generated for every mRNA to estimate the likelihood of representing a miR-34 target. Experimentally validated miR-34 targets were strongly enriched among mRNAs with the highest scores providing a proof of principle for our analysis. We integrated the results from the meta-analysis in a user-friendly METAmiR34TARGET website (www.metamir34target.com/) that allows to graphically represent the meta-analysis results for every mRNA. Moreover, the website harbors a screen function, which allows to select multiple miR-34-related criteria/analyses and cut-off values to facilitate the stringent and comprehensive prediction of relevant miR-34 targets in expression data obtained from cell lines and tumors/tissues. Furthermore, information on more than 200 miR-34 target mRNAs, that have been experimentally validated so far, has been integrated in the web-tool. The website and datasets provided here should facilitate further investigation into the mechanisms of tumor suppression by the p53/miR-34 connection and identification of potential cancer drug targets.
1. Introduction
MicroRNAs (miRNAs) are short non-coding RNAs that bind to partially complementary sequences in 3′-untranslated regions (3′-UTR) of their target mRNAs via a 7 nucleotide seed sequence located at their 5′-ends [1]. Via this interaction, miRNAs suppress the translation of target mRNAs by recruiting the RISC complex. As a secondary consequence, target mRNAs are degraded. Both effects lead to down-regulation of target expression by miRNAs. Since miRNAs exert their function by the regulation of their target mRNAs, the identification of miRNA targets is important to understand the biological function of miRNAs. Certain miRNAs play important roles in cancer, because they regulate the expression of cancer-related genes [2], [3]. For example, the miR-34a targets c-Met and Axl represent important oncogenes and inhibitors of these proteins have been translated into clinical trials or are approved for the treatment of certain types of tumors [3], [4], [5], [6]. So far, putative microRNA target RNAs were identified by using miRNA prediction algorithms that identify potential miRNA targets based on a match between a miRNA and its target mRNA in the seed sequence [reviewed in [7]. Since such an approach is entirely computational it may generate false positive assignments. In the last years comprehensive genome-wide miRNA and mRNA expression profiling studies of tumors have been performed within The Cancer Genome Atlas (TCGA) program, which allow correlation analyzes between miRNA and mRNA expression [8]. These datasets have been integrated with computational algorithms in miRNA portals, such as miRGator to improve miRNA target prediction [9]. However, correlational analyses are very indirect and provide only the information as to whether the expression of miRNA and mRNA correlate positively or negatively. Besides mRNA expression profiling studies in tumors, many studies applying genome-wide mRNA profiling in cell lines and mouse models after ectopic miRNA expression or miRNA knockout have been performed in last years. These datasets allow a more direct and straightforward identification of putative miRNA targets. However, to our knowledge such datasets have not yet been integrated into webtools for miRNA target identification. Here we integrated experimental datasets based on ectopic miRNA expression in cell lines or miRNA knockout in mouse models, miRNA/mRNA correlation datasets and computational prediction algorithms to develop a user friendly web-based tool that allows a comprehensive identification of miRNA targets (Fig. 1). Exemplarily, we focused on miR-34a, miR-34b, and miR-34c, which are members of the miR-34 family and represent some of the most prevalent p53-induced miRNAs (Fig. 2A) [4], [10], [11], [12], [13], [14]. miR-34a is ubiquitously expressed in most organs, whereas miR-34b and miR-34c are expressed mainly in the brain, lungs, and uterus [15]. miR-34 host genes are often inactivated/silenced in cancer [16]. miR-34 family members possess tumor suppressor properties, which are mediated by the repression of their target mRNAs [17]. Published miR-34 targets include mRNAs encoded by prominent oncogenes that promote proliferation, migration, invasion, metastasis, such as AXL [18], [19], MET [10], KIT [20], CSF1R [21], and NTN1 [22], as well as central regulators of cancer-related processes, such as SNAIL [23], [24], IL6R [25], SERPINE1 [26], PPP1R11 [27], WASF1 [28] and LDHA [18]. However, many mediators of miR-34 functions still have to be identified and experimentally validated. Since the p53/miR-34 axis is often inactivated in tumors, the targets of miR-34 are up-regulated during tumor initiation and progression and mediate oncogenic effects [22]. Therefore, certain miR-34 targets may represent attractive targets for therapeutic inhibition, as shown for PAI1 and IL6R in a mouse model of CRC [26]. However, miR-34 and their targets also play important roles in normal tissue development and homeostasis. For example, while miR-34 is protective in cancer, it is detrimental in cardiovascular environment (e.g. hypertrophy) [29]. The miR-34a target PNUTS reduces telomere shortening, DNA damage responses and cardiomyocyte apoptosis, and improves functional recovery after acute myocardial infarction [29]. Therefore, the suppression of certain miR-34 targets may also have toxic side effects.
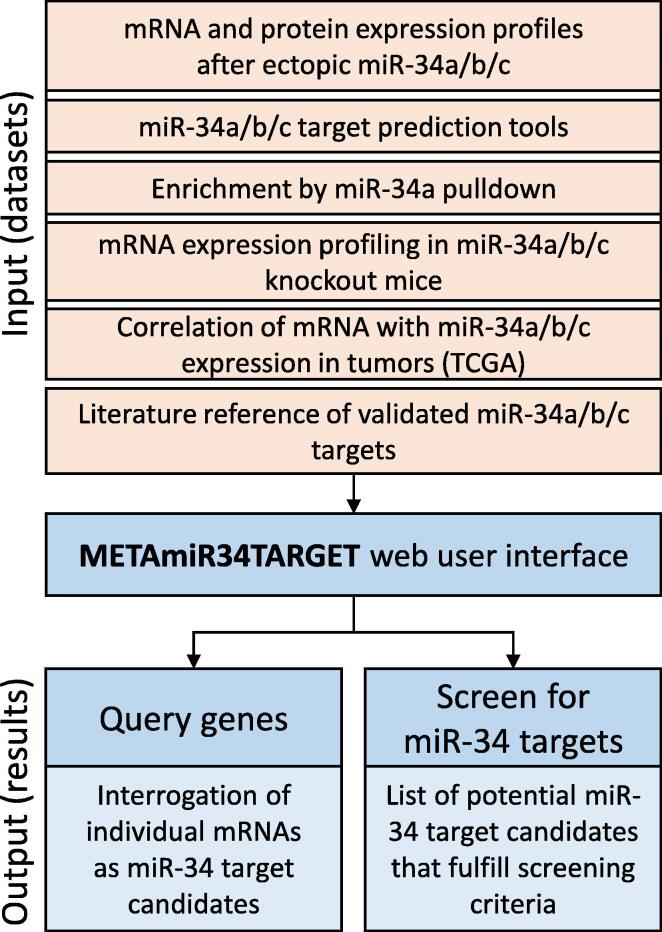
Fig. 1.
Flowchart of METAmiR34TARGET development. Indicated input datasets were analyzed and integrated into the METAmiR34TARGET website, which allows a comprehensive identification of miR-34 targets.
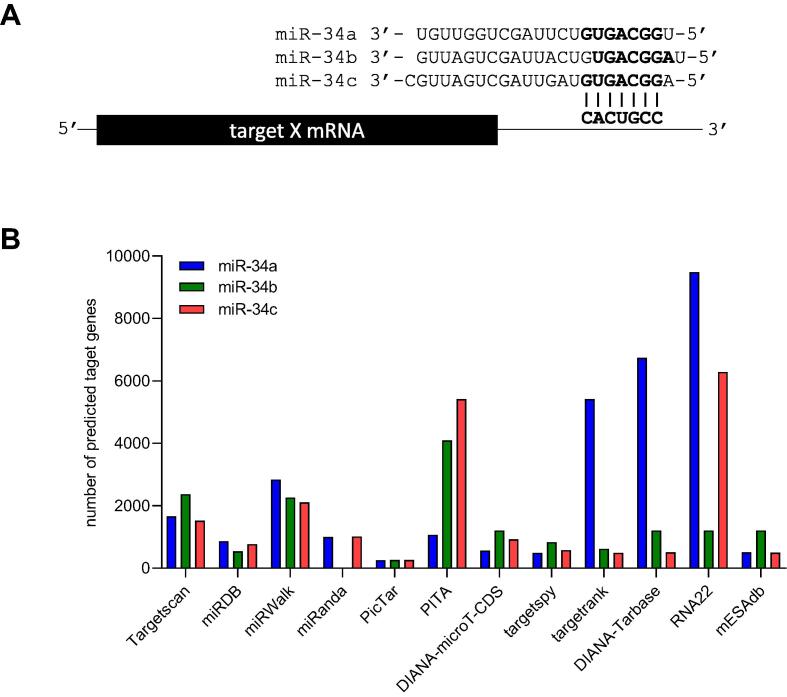
Fig. 2.
Sequence alignment of miR-34 family members and miR-34 target identification rates obtained by public prediction tools. (A) Sequence alignment of the mature miR-34a, miR-34b, and miR-34c. The seed-sequences are high-lighted in bold. A target mRNA with ideal seed-match binding site is shown in the bottom. (B) Overview of frequencies of predicted miR-34a, miR-34b, and miR-34c target mRNAs identified by the respective miRNA target prediction tools/algorithms.
2. Materials and methods
2.1. Datasets and data processing
18 datasets containing genome-wide mRNA expression profiling data obtained from cell lines after transfection with miR-34a/b/c mimics or expression vectors (Table S1) and 6 mRNA expression profiling datasets derived from miR-34a/b/c knockout mice (Table S2) were downloaded from NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/). For datasets obtained by RNA-seq (GSE99401, GSE123628, GSE133775, GSE69484, GSE84138, and GSE99452), RPKM values were used and for datasets obtained by microarrays (all other datasets), normalized signal intensity values were used. The fold change was calculated by dividing mRNA expression levels after ectopic miR-34 or miR-34 knockout by the control expression levels for every mRNA. Two protein profiling datasets generated from cell lines after ectopic miR-34a and miR-34c were obtained from literature [18], [30]. Normalized SILAC H/M ratio values represent fold changes in protein expression for ectopic miR-34a/c versus control. Furthermore, two miR-34a pulldown datasets that identified mRNAs that are bound by miR-34a were obtained from the literature [31], [32]. Average fold enrichment ratio values of miR-34a vs miR-scrambled pulldown were used. TCGA datasets containing mRNA and miRNA expression profiling of tumors from 32 cancer entities were down-loaded from the NIH GDC data portal (https://portal.gdc.cancer.gov/). The correlation between the expression of mature miR-34a/b/c and every mRNA was calculated by Pearson correlation coefficient (RSEM expression values were used). Data from 12 miRNA target prediction tools/algorithms was downloaded and analyzed from webpages listed in Table S3. Validated/published miR-34 targets were identified by manual analysis of the literature available in Pubmed until August 2022 with keywords: miR-34a, miR-34b, miR-34c.
2.2. Calculation of miR-34a/b/c target probability scores
The datasets described above were integrated to calculate a score for every mRNA in order to estimate the likelihood of representing a potential miR-34a/b/c target. First, partial scores were calculated for each dataset type. For datasets generated after ectopic miR-34a/b/c expression the score was calculated as the number of datasets in which the mRNA or protein expression was repressed by more than 1.5-fold divided by the total number of datasets. For miR-34a/b/c knockout mice datasets the score was calculated as the number of datasets in which the mRNA expression was induced more than 1.5-fold in knockout mice, divided by the total number of datasets. The TCGA mRNA/miR34a/b/c correlation score was calculated as the number of datasets/cancer types in which the mRNA/miR-34a/b/c/ Pearson correlation coefficient is lower than −0.1 divided by the total number of datasets/cancer types. For miR-34 pulldown datasets the score was calculated as the number of datasets in which the mRNA enrichment in the pull-down with miR-34 probes was more than 1.5-fold than with control probes, divided by the total number of datasets. For miRNA target prediction tools/algorithms dataset types, the score was calculated by dividing the number of prediction tools predicting a mRNA as a miR-34a/b/c target by the total number of prediction tools. All partial scores were added together with all dataset types weigh equally to obtain the miR34a/b/c probability score.
miR-34 target probability score = + + + + +
Finally, the score was rescaled between 0 and 100 with the highest score set to 100 to obtain the final miR34a/b/c probability score.
2.3. Gene set enrichment analysis
The association between the probability score and experimentally validated miR-34 targets was calculated using ranked GSEA analyses with genes ranked according to the miR-34a/b/c target probability score. The enrichment of top predicted miR-34a/b/c targets in hallmark MSigDB gene sets was analyzed using GSEA [33].
2.4. METAmiR34TARGET webpage
The METAmiR34TARGET webpage was designed using the R package shiny (https://shiny.rstudio.com/).
3. Results and discussion
3.1. Meta-analysis of publicly available miR-34 related datasets
Here we developed a bioinformatics approach to comprehensively identify potential targets of the miR-34 family (flow-chart provided in Fig. 1). To identify mRNAs that are regulated by miR-34, we first performed a query of the NCBI GEO database for datasets that were obtained by genome-wide mRNA expression profiling of cell lines after transfection with miR-34a/b/c mimics or expression vectors. Thereby, we obtained 18 GEO-datasets (Table S1) that were subsequently analyzed as described in Materials and methods. In addition, two studies that comprehensively characterized changes in protein expression after ectopic miR-34a and miR-34c expression [18], [30], were included. For each mRNA or protein, the fold change caused by ectopic miR-34a/b/c was calculated. Since miRNAs generally repress their targets, mRNAs/proteins that are consistently suppressed by ectopic miR-34 in multiple datasets represent potential miR-34a/b/c targets. Next, we analyzed 6 mRNA expression profiling datasets derived from multiple tissues and tumors from miR-34a/b/c knockout mice (Table S2) to determine differential expression of miR-34 target mRNAs between miR-34a/b/c knockout vs wild-type samples. mRNAs consistently induced in samples from knockout mice were regarded as potential miR-34a/b/c targets. Although these studies provide information as to whether the mRNA abundance is regulated by miR-34a/b/c, it remained unknown whether the regulation is mediated directly via binding of the miRNA to the 3′-UTR or by an indirect mechanism, e.g. an effect on factors regulating the activity of the host gene of the target mRNA. To investigate whether an mRNA is potentially a direct miR-34a/b/c target, 12 different miRNA target prediction tools/algorithms were included (Table S3). The number of predicted miR-34a/b/c targets varied from 254 to 9482, depending on the miRNA target prediction tool used (Fig. 2B). We also included the results from two studies, which used miR-34a pulldown assays to identify mRNAs directly bound to miR-34a. Finally, correlations between the expression of mRNAs and miR-34a/b/c in TCGA datasets of 32 different tumor entities were analyzed. Since miRNAs repress their targets, mRNAs that display a negative correlation with miR-34a/b/c represent potential targets. In addition, we performed a literature search to identify miR-34a, miR-34b, and miR-34c target mRNAs that had already been validated experimentally. For this we examined all miR-34-related publications available in PubMed until August 2022 and identified 210 miR-34a, 28 miR-34b, and 53 miR-34c targets that had previously been validated by 3′-UTR luciferase reporter assays (Table S4).
3.2. Calculation of miR-34a/b/c target probability scores
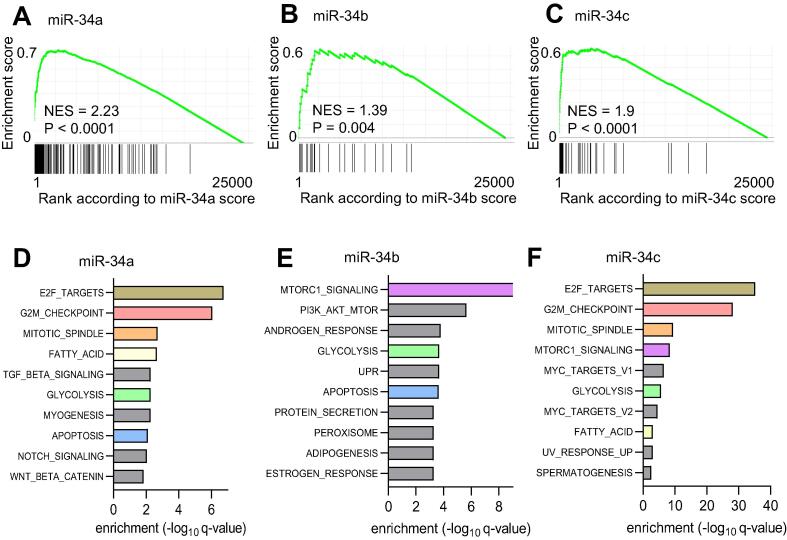
Next, we integrated all mRNA expression, binding, and correlation analyses described above and calculated a score for every mRNA (described in Materials and methods) to estimate the likelihood that an mRNA represents a potential miR-34a/b/c target (Table S5). Target mRNAs were ranked according to their scores. Ranked GSEA analysis showed that previously published and therefore validated miR-34a targets listed in Table S4 are strongly enriched among mRNAs with highest miR-34a target probability scores (Fig. 3A). Similar results were obtained for miR-34b and miR-34c (Fig. 3B and 3C). These results indicated that the algorithm developed here is suitable for the identification of mRNAs targeted by miR-34a, miR-34b, and miR-34c. The forty most highly ranked potential miR-34a, miR-34b, and miR-34c target mRNAs are depicted in Table 1. GSEA analyses of the 300 top-ranked potential miR-34a target mRNAs revealed that they were enriched in Hallmark MSigDB gene sets associated with E2F targets and cell cycle related signatures, such as those representing mitotic spindle and G2/M checkpoint components (Fig. 3D). The 300 top-ranked, potential miR-34b targets showed the strongest enrichment for components of MTORC1 signaling (Fig. 3E), whereas the 300 top-ranked potential miR-34c targets displayed enrichment in E2F targets and cell cycle related gene sets (Fig. 3F). Indeed, members of the miR-34 family were shown to inhibit cell cycle progression by repressing their targets E2F3, Cyclin D1, CDC25A, CDK4 and CDK6, which regulate the transition from G1- to S-phase or G2- to M−phase, in a variety of cancer cell lines [17]. Furthermore, PDK1, which is an activator of PI3K/MTOR signaling has been identified as a miR-34 target that regulates glucose metabolism and glycolysis [34].
Fig. 3.
Gene set enrichment analysis of predicted miR-34a, miR-34b, and miR-34c targets. (A) Enrichment of published miR-34a targets (2 1 0) in genes with highest miR-34a target probability scores. (B) Enrichment of published miR-34b targets (28) in genes with highest miR-34b target probability scores. (C) Enrichment of published miR-34c targets (53) in genes with highest miR-34c target probability scores. (D-F) GSEA analyses of 300 top ranked potential miR-34a (D), miR-34b (E), and miR-34c (F) targets in Hallmark MSigDB gene sets. Common gene sets enriched in potential targets of multiple miR-34 miRNAs are displayed in same colors.
Table 1.
Top 40 predicted miR-34a, miR-34b, and miR-34c target mRNAs.
| miR-34a targets |
miR-34b targets |
miR-34c targets |
||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Score | Validation | Gene | Score | Validation | Gene | Score | Validation |
| ACSL4 | 100.00 | [18], [37] | ZZZ3 | 100.00 | – | VCL | 100.00 | [38] |
| RDH11 | 81.89 | – | HMGN4 | 98.24 | – | ACSL4 | 98.51 | – |
| AXL | 89.71 | [18], [19], [39] | C6orf89 | 97.06 | – | ZDHHC16 | 98.51 | – |
| ARHGAP1 | 81.48 | [40] | TUFT1 | 96.47 | – | PRR11 | 97.01 | – |
| RRAS | 81.89 | [18] | API5 | 95.29 | – | E2F5 | 97.01 | – |
| TPD52 | 81.48 | [18] | DDX21 | 93.53 | – | FLOT2 | 94.03 | – |
| ERLIN1 | 77.78 | – | REEP5 | 91.76 | – | MTA2 | 88.81 | – |
| LRRC40 | 77.78 | – | ZC3H15 | 90.00 | – | FUT8 | 88.81 | – |
| VCL | 81.48 | [38] | RHOU | 84.71 | – | TUFT1 | 88.81 | – |
| GINS3 | 80.66 | – | SH3BGRL2 | 84.71 | – | UHRF2 | 88.06 | – |
| TAF5 | 68.31 | – | ARNT2 | 84.12 | – | PREB | 87.31 | – |
| MET | 76.13 | [10], [41] | AP3M1 | 82.35 | – | AXL | 86.57 | [18], [19], [39] |
| CDC23 | 72.02 | – | ETS1 | 80.59 | – | PACS1 | 86.57 | – |
| CDK6 | 74.90 | [42], [43] | GLCE | 80.59 | – | CDK6 | 86.57 | – |
| LMAN2L | 64.61 | [44] | DHTKD1 | 79.41 | – | PPARGC1B | 86.57 | – |
| EFNB1 | 69.55 | – | E2F5 | 79.41 | – | LGR4 | 85.82 | [45] |
| SURF4 | 65.84 | – | KIF1A | 79.41 | – | ARHGAP1 | 85.82 | – |
| TBC1D13 | 71.19 | – | PDK1 | 79.41 | – | ERGIC1 | 85.07 | – |
| NUP210 | 71.60 | – | SLC39A9 | 79.41 | – | SH3GL1 | 84.33 | – |
| MAP2K1 | 68.31 | [46] | SNX1 | 79.41 | – | MAP2K1 | 84.33 | – |
| POU2F1 | 63.37 | – | TMED4 | 79.41 | – | CDC25A | 83.58 | – |
| TSEN15 | 69.55 | – | ZNRF3 | 79.41 | – | GNPDA1 | 82.84 | – |
| SMAD4 | 64.61 | [47] | PURB | 78.82 | – | LMAN2L | 82.09 | – |
| MPV17L2 | 69.55 | – | UHRF2 | 78.82 | – | TGIF2 | 82.09 | [48] |
| PACS1 | 66.26 | [49] | YWHAZ | 78.82 | – | LDHA | 81.34 | – |
| QDPR | 69.55 | – | ANKS1A | 78.24 | – | FAM167A | 80.60 | – |
| GORASP2 | 68.31 | – | DEPDC1 | 78.24 | – | METAP1 | 80.60 | – |
| E2F5 | 61.32 | [50] | SLC16A14 | 77.65 | – | SGPP1 | 80.60 | – |
| MAGT1 | 61.32 | – | PLEKHA1 | 77.65 | – | ERLIN1 | 79.85 | – |
| IQGAP3 | 67.90 | – | SYT4 | 77.65 | – | TSPAN14 | 79.85 | – |
| SLC29A1 | 68.72 | – | CHRAC1 | 76.47 | – | MKI67 | 79.10 | – |
| SH3GL1 | 63.79 | – | INCENP | 76.47 | – | PIP5K1A | 78.36 | – |
| TMEM109 | 60.91 | – | PPM1K | 76.47 | – | E2F3 | 77.61 | – |
| FOXJ2 | 58.02 | [51] | BRIP1 | 76.47 | – | XBP1 | 77.61 | [52], [53] |
| HMMR | 61.32 | – | RAD54L2 | 76.47 | – | MCM2 | 76.87 | – |
| RRP1B | 62.96 | – | RCC2 | 76.47 | – | ARSB | 76.87 | – |
| SIRT1 | 57.20 | [54], [55] | TTC33 | 76.47 | – | SMPD1 | 76.87 | – |
| SGPP1 | 63.37 | [56] | WNK3 | 76.47 | – | BAZ2A | 76.12 | – |
| TGIF2 | 58.85 | – | AP1S2 | 75.88 | – | SPEN | 76.12 | – |
| CCNE2 | 63.37 | [10], [43] | AMMECR1 | 75.88 | – | SYT1 | 76.12 | [57], [58] |
3.3. Exemplary applications of the METAmiR34TARGET website
Individual gene queries. Next we generated an intuitive user interface, which allows to query individual genes (Fig. 4). The analysis of specific mRNAs of interest in all datasets described above can be graphically presented in the “query genes” tab (Fig. 4). The user can query mRNAs by entering a gene symbol in the search box (Fig. 4, panel I). If the entered candidate encodes a published/validated miR-34a/b/c target mRNA, a green box is displayed in the upper right corner with the reference of the publication (Fig. 4, panel II). As an example, ACSL4 was entered. Acyl-CoA Synthetase Long Chain Family Member 4 plays an important role in long chain fatty acids metabolism, immune signaling transduction, and ferroptosis [35]. The expression of ACSL4 is significantly upregulated in multiple types of cancer and increased expression of ACSL4 typically indicates an unfavorable prognosis [36]. The results for this known miR-34 target are shown in Fig. 4. Multiple charts are displayed for every entered mRNA (Fig. 4): The fold change in mRNA expression caused by ectopic miR-34a (blue), miR-34b (green), or miR-34c (red) expression is displayed in chart III. Since miRNAs repress their target mRNAs, mRNAs consistently suppressed by ectopic miR-34 in multiple datasets represent potential miR-34a/b/c targets. Pearson correlation coefficients between the candidate’s mRNA expression and the expression of miR-34a, miR-34b, and miR-34c in TCGA datasets of 32 cancer types are shown in chart IV. Since miRNAs repress their targets, mRNAs with a negative correlation to miR-34 expression represent potential miR-34a/b/c targets. Information as to whether the candidate is a predicted miR-34a (blue), miR-34b (green), miR-34c (red) target based on 12 miRNA target prediction tools/algorithms is displayed in chart V. The fold change of the candidate's protein expression by ectopic miR-34a is shown in chart VI. The enrichment of the queried mRNA in miR-34a pulldown studies is displayed in chart VII. Chart VIII shows the fold change in mRNA expression in tumors or normal tissues from miR-34 knockout mice when compared to wild-type mice. Finally, the miR-34 target probability scores and ranks are displayed in chart IX. By clicking on bars representing individual studies displayed in charts III and VIII additional information about the study (panel X) and the expression data of the queried gene (chart XI) can be obtained. Many of published miR-34 targets represent oncogenes or pro-tumorigenic factors. To analyze whether the candidates possess oncogenic properties and may represent potential drug targets, a link to the DepMap portal is provided (panel XII), which allows the user to analyze whether the queried gene represents a cancer vulnerability and therefore may serve as therapeutic targets. As an example, the expression of ACSL4 mRNA was consistently suppressed by ectopic miR-34a, miR-34b, and miR-34c in 23 from 25 studies. miR-34a and ACSL4 mRNA show a negative correlation in most cancer types. ACSL4 was consistently predicted as a miR-34a and miR-34c target. ACSL4 protein expression was suppressed more that 1.5-times by ectopic miR-34a and miR-34c, and ACSL4 mRNA was enriched more than 30-times in miR-34a pulldown assays. According to our miR-34 target probability score, ACSL4 ranked number 1 and 2 as a potential miR-34a and miR-34c target, respectively. Therefore, based on our meta-analysis, ACSL4 is a very good miR-34 target candidate, which was previously experimentally confirmed [18], [37].
Fig. 4.
Layout of the METAmiR34TARGET website. The “query genes” tab showing the graphical representation of analysis of the queried gene in indicated datasets. Panel I: Search box for entering a gene symbol. Panel II: Message box indicating whether the queried gene encodes a published/validated miR-34a/b/c target mRNA. Panel III: Fold-change of the queried gene’s mRNA expression by ectopic miR-34a (blue), miR-34b (green), or miR-34c (red). Panel IV: Heat-map showing correlation coefficients between the mRNA expression of the queried gene and the expression of miR-34a, miR-34b, and miR-34c in TCGA datasets. Panel V: Chart displaying whether the queried gene is a predicted miR-34a (blue), miR-34b (green), miR-34c (red) target based on 12 miRNA target prediction tools/algorithms. Panel VI: Fold-change of the queried gene’s protein expression by ectopic miR-34a. Panel VII: Enrichment of queried gene’s mRNA in miR-34a pulldown studies. Panel VIII: Fold-change of the queried gene’s mRNA expression in miR-34 knockout mice versus wt mice. Panel IX: miR-34 target probability scores and ranks of the queried gene. Panel X and XI: Additional information about the selected study (panel X) and the expression data of the queried gene (chart XI). A link to the DepMap portal results for the queried gene is provided in panel XII. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
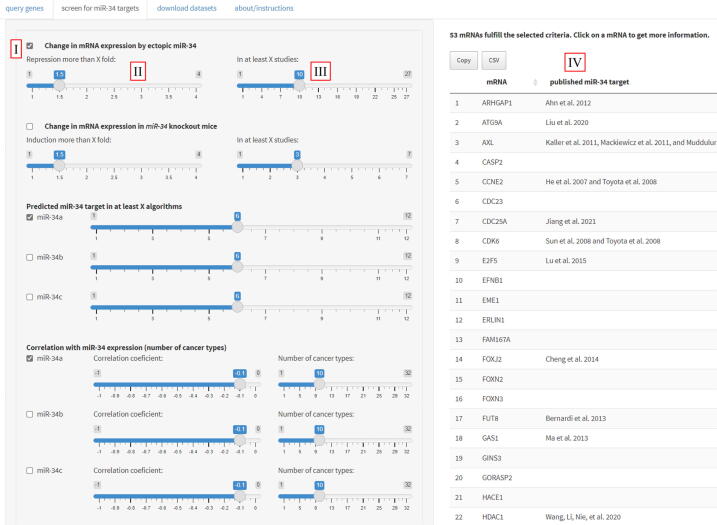
Screening for miR-34a, miR-34b, or miR-34c targets. Comprehensive screens for multiple novel miR-34a/b/c targets can be performed using the tools in the “screen for miR-34 targets” tab (Fig. 5). The user can select multiple screening criteria to obtain a list of mRNAs that fulfill them. The type of datasets that should be employed in the screen is selected within the field that describes the dataset (Fig. 5, panel I). For the datasets describing the effect of ectopic miR-34, the user can set a cut-off value for the minimum fold of mRNA repression (Fig. 5, panel II) and the number of studies/datasets that have to fulfill that cut-off (Fig. 5, panel III). For example, by selecting the minimum fold repression of 1.5 and the number of studies/datasets of 10, the tool will provide a list of mRNAs that are repressed by ectopic miR-34a/b/c by more than 1.5 fold in at least 10 studies. Similarly, for other types of datasets the user may select a minimal value of fold-change in expression and the number of studies/datasets that have to fulfill that criterion. The resulting list of identified candidate miR-34 targets is displayed in Fig. 5, panel IV. Individual genes in the list can be clicked to obtain detailed information in the “query genes” tab.
Fig. 5.
Screening for multiple miR-34 targets. The “screen for miR-34 targets” tab showing the selection of multiple criteria/datasets for the identification of potential miR-34 targets. Panel I: Checkboxes to select the type of datasets that should be included in the screen. Panel II: Slide-bars to select the cutoff for fold change in expression. Panel III: Slide-bars to select the number of studies/datasets that have to fulfill that cutoff. Panel IV: List of identified genes that fulfill the selected criteria.
The advantage of the meta-analysis described here is that it combines a large amount of computational datasets generated by prediction tools, such as TARGETSCAN, with experimental datasets derived from cell lines and tissues. The result from our integrative meta-analysis can be useful for experimental design of future miR-34 related cell culture and animal studies. Moreover, the meta-analysis includes correlation analyses of miR-34 with mRNA expression in primary tumors, which together with the cancer dependency map may facilitate the identification of potential drug targets for cancer treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2022.12.003.
Contributor Information
Matjaz Rokavec, Email: matjaz.rokavec@med.uni-muenchen.de.
Heiko Hermeking, Email: heiko.hermeking@med.uni-muenchen.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pasquinelli A.E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 2.Bracken C.P., Scott H.S., Goodall G.J. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17(12):719–732. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 3.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nature Reviews Cancer. 2012;12(6):613–626. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 4.Hermeking H. p53 enters the microRNA world. Molecular Cell. 2007;12(5):414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Malvankar C., Kumar D. AXL kinase inhibitors- A prospective model for medicinal chemistry strategies in anticancer drug discovery. Biochim Biophys Acta Rev Cancer. 2022;1877(5) doi: 10.1016/j.bbcan.2022.188786. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y., Xu J., Sun B., Wang J., Wang Z. MET-Targeted Therapies and Clinical Outcomes: A Systematic Literature Review. Mol Diagn Ther. 2022;26(2):203–227. doi: 10.1007/s40291-021-00568-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatun M.S., Alam M.A., Shoombuatong W., Mollah M.N.H., Kurata H., et al. Recent Development of Bioinformatics Tools for microRNA Target Prediction. Curr Med Chem. 2022;29(5):865–880. doi: 10.2174/0929867328666210804090224. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen A., Silber J., Harinath G., Huse J.T., Schultz N., et al. Analysis of microRNA-target interactions across diverse cancer types. Nat Struct Mol Biol. 2013;20(11):1325–1332. doi: 10.1038/nsmb.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho S., Jang I., Jun Y., Yoon S., Ko M., et al. MiRGator v3.0: a microRNA portal for deep sequencing, expression profiling and mRNA targeting. Nucleic Acids Res. 2013;41(Database issue):D252–D257. doi: 10.1093/nar/gks1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L., He X., Lim L.P., de Stanchina E., Xuan Z., et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarasov V., Jung P., Verdoodt B., Lodygin D., Epanchintsev A., et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6(13):1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 12.Bommer G.T., Gerin I., Feng Y., Kaczorowski A.J., Kuick R., et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17(15):1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 13.Chang T.C., Wentzel E.A., Kent O.A., Ramachandran K., Mullendore M., et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raver-Shapira N., Marciano E., Meiri E., Spector Y., Rosenfeld N., et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26(5):731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Wei X., He J., Cao Q., Du D., et al. The comprehensive landscape of miR-34a in cancer research. Cancer Metastasis Rev. 2021;40(3):925–948. doi: 10.1007/s10555-021-09973-3. [DOI] [PubMed] [Google Scholar]
- 16.Lodygin D., Tarasov V., Epanchintsev A., Berking C., Knyazeva T., et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 17.Rokavec M., Li H., Jiang L., Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6(3):214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 18.Kaller M., Liffers S.T., Oeljeklaus S., Kuhlmann K., Roh S., et al. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10(8):M111. doi: 10.1074/mcp.M111.010462. 010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackiewicz M., Huppi K., Pitt J.J., Dorsey T.H., Ambs S., et al. Identification of the receptor tyrosine kinase AXL in breast cancer as a target for the human miR-34a microRNA. Breast Cancer Res Treat. 2011;130(2):663–679. doi: 10.1007/s10549-011-1690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemens H., Jackstadt R., Kaller M., Hermeking H. Repression of c-Kit by p53 is mediated by miR-34 and is associated with reduced chemoresistance, migration and stemness. Oncotarget. 2013;4(9):1399–1415. doi: 10.18632/oncotarget.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi X., Kaller M., Rokavec M., Kirchner T., Horst D., et al. Characterization of a p53/miR-34a/CSF1R/STAT3 Feedback Loop in Colorectal Cancer. Cell Mol Gastroenterol Hepatol. 2020;10(2):391–418. doi: 10.1016/j.jcmgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F., Bouznad N., Kaller M., Shi X., Konig J., et al. Csf1r mediates enhancement of intestinal tumorigenesis caused by inactivation of Mir34a. Int J Biol Sci. 2022;18(14):5415–5437. doi: 10.7150/ijbs.75503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemens H., Jackstadt R., Hunten S., Kaller M., Menssen A., et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle. 2011;10(24):4256–4271. doi: 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- 24.Kim N.H., Kim H.S., Li X.Y., Lee I., Choi H.S., et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195(3):417–433. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rokavec M., Oner M.G., Li H., Jackstadt R., Jiang L., et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124(4):1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oner M.G., Rokavec M., Kaller M., Bouznad N., Horst D., et al. Combined Inactivation of TP53 and MIR34A Promotes Colorectal Cancer Development and Progression in Mice Via Increasing Levels of IL6R and PAI1. Gastroenterology. 2018;155(6):1868–1882. doi: 10.1053/j.gastro.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Li H., Rokavec M., Jiang L., Horst D., Hermeking H. Antagonistic Effects of p53 and HIF1A on microRNA-34a Regulation of PPP1R11 and STAT3 and Hypoxia-induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells. Gastroenterology. 2017;153(2):505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Jiang L., Hermeking H. miR-34a and miR-34b/c Suppress Intestinal Tumorigenesis. Cancer Res. 2017;77(10):2746–2758. doi: 10.1158/0008-5472.CAN-16-2183. [DOI] [PubMed] [Google Scholar]
- 29.Boon R.A., Iekushi K., Lechner S., Seeger T., Fischer A., et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495(7439):107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 30.Ebner O.A., Selbach M. Quantitative proteomic analysis of gene regulation by miR-34a and miR-34c. PLoS One. 2014;9(3):e92166. doi: 10.1371/journal.pone.0092166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awan H.M., Shah A., Rashid F., Wei S., Chen L., et al. Comparing two approaches of miR-34a target identification, biotinylated-miRNA pulldown vs miRNA overexpression. RNA Biol. 2018;15(1):55–61. doi: 10.1080/15476286.2017.1391441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lal A., Thomas M.P., Altschuler G., Navarro F., O'Day E., et al. Capture of microRNA-bound mRNAs identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 2011;7(11):e1002363. doi: 10.1371/journal.pgen.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H.R., Roe J.S., Lee J.E., Cho E.J., Youn H.D. p53 regulates glucose metabolism by miR-34a. Biochem Biophys Res Commun. 2013;437(2):225–231. doi: 10.1016/j.bbrc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Quan J., Bode A.M., Luo X. ACSL family: The regulatory mechanisms and therapeutic implications in cancer. Eur J Pharmacol. 2021;909 doi: 10.1016/j.ejphar.2021.174397. [DOI] [PubMed] [Google Scholar]
- 36.Hou J., Jiang C., Wen X., Li C., Xiong S., et al. ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.949863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W., Li X., Ding N., Teng J., Zhang S., et al. miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. 2020;21(1):33. doi: 10.1186/s12863-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardo B.C., Gao X.M., Winbanks C.E., Boey E.J., Tham Y.K., et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA. 2012;109(43):17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mudduluru G., Ceppi P., Kumarswamy R., Scagliotti G.V., Papotti M., et al. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene. 2011;30(25):2888–2899. doi: 10.1038/onc.2011.13. [DOI] [PubMed] [Google Scholar]
- 40.Ahn Y.H., Gibbons D.L., Chakravarti D., Creighton C.J., Rizvi Z.H., et al. ZEB1 drives prometastatic actin cytoskeletal remodeling by downregulating miR-34a expression. J Clin Invest. 2012;122(9):3170–3183. doi: 10.1172/JCI63608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N., Fu H., Tie Y., Hu Z., Kong W., et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275(1):44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Sun F., Fu H., Liu Q., Tie Y., Zhu J., et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582(10):1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 43.Toyota M., Suzuki H., Sasaki Y., Maruyama R., Imai K., et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68(11):4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 44.Geng Y., Wu Y., Xu C., Li T., Zhang L. Long Non-Coding RNA LINC00662 Regulated Proliferation and Migration by Targeting miR-34a-5p/LMAN2L Axis in Glioma. Onco Targets Ther. 2020;13:10161–10172. doi: 10.2147/OTT.S272616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J., Li X., Li D., Ren X., Li Y., et al. MicroRNA-34 Family Enhances Wound Inflammation by Targeting LGR4. J Invest Dermatol. 2020;140(2):465–476 e11. doi: 10.1016/j.jid.2019.07.694. [DOI] [PubMed] [Google Scholar]
- 46.Ichimura A., Ruike Y., Terasawa K., Shimizu K., Tsujimoto G. MicroRNA-34a inhibits cell proliferation by repressing mitogen-activated protein kinase kinase 1 during megakaryocytic differentiation of K562 cells. Mol Pharmacol. 2010;77(6):1016–1024. doi: 10.1124/mol.109.063321. [DOI] [PubMed] [Google Scholar]
- 47.Werner T.V., Hart M., Nickels R., Kim Y.J., Menger M.D., et al. MiR-34a-3p alters proliferation and apoptosis of meningioma cells in vitro and is directly targeting SMAD4, FRAT1 and BCL2. Aging (Albany NY) 2017;9(3):932–954. doi: 10.18632/aging.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Wang C.M., Jiang Z.Z., Yu X.J., Fan C.G., et al. MicroRNA-34c targets TGFB-induced factor homeobox 2, represses cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma. Oncol Lett. 2015;10(5):3095–3102. doi: 10.3892/ol.2015.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veena M.S., Raychaudhuri S., Basak S.K., Venkatesan N., Kumar P., et al. Dysregulation of hsa-miR-34a and hsa-miR-449a leads to overexpression of PACS-1 and loss of DNA damage response (DDR) in cervical cancer. J Biol Chem. 2020;295(50):17169–17186. doi: 10.1074/jbc.RA120.014048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu G., Sun Y., An S., Xin S., Ren X., et al. MicroRNA-34a targets FMNL2 and E2F5 and suppresses the progression of colorectal cancer. Exp Mol Pathol. 2015;99(1):173–179. doi: 10.1016/j.yexmp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Cheng B.B., Qu M.J., Wu L.L., Shen Y., Yan Z.Q., et al. MicroRNA-34a targets Forkhead box j2 to modulate differentiation of endothelial progenitor cells in response to shear stress. J Mol Cell Cardiol. 2014;74:4–12. doi: 10.1016/j.yjmcc.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 52.Krammes L., Hart M., Rheinheimer S., Diener C., Menegatti J., et al. Induction of the Endoplasmic-Reticulum-Stress Response: MicroRNA-34a Targeting of the IRE1alpha-Branch. Cells. 2020;9(6) doi: 10.3390/cells9061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartoszewska S., Cabaj A., Dabrowski M., Collawn J.F., Bartoszewski R. miR-34c-5p modulates X-box-binding protein 1 (XBP1) expression during the adaptive phase of the unfolded protein response. FASEB J. 2019;33(10):11541–11554. doi: 10.1096/fj.201900600RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamakuchi M., Ferlito M., Lowenstein C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito T., Yagi S., Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398(4):735–740. doi: 10.1016/j.bbrc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 56.He J., Zhao H., Liu X., Wang D., Wang Y., et al. Sevoflurane suppresses cell viability and invasion and promotes cell apoptosis in colon cancer by modulating exosomemediated circHMGCS1 via the miR34a5p/SGPP1 axis. Oncol Rep. 2020;44(6):2429–2442. doi: 10.3892/or.2020.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agostini M., Tucci P., Steinert J.R., Shalom-Feuerstein R., Rouleau M., et al. microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A. 2011;108(52):21099–21104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Z., Zhang K., Zhou H., Jiang L., Xie B., et al. Increased miR-34c mediates synaptic deficits by targeting synaptotagmin 1 through ROS-JNK-p53 pathway in Alzheimer's Disease. Aging Cell. 2020;19(3):e13125. doi: 10.1111/acel.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.