Abstract
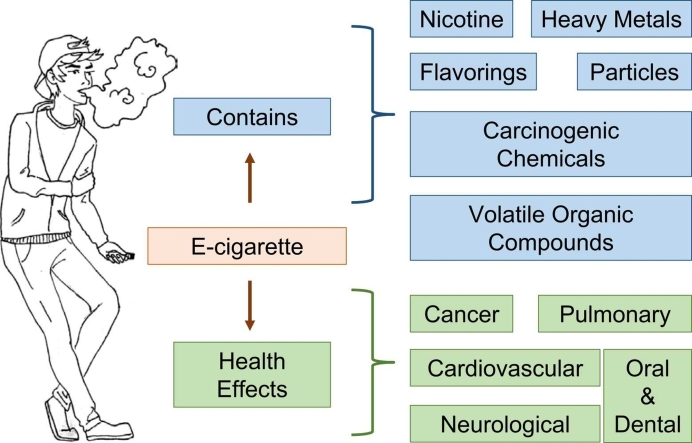
In recent years, new nicotine delivery methods have emerged, and many users are choosing electronic cigarettes (e-cigarettes) over traditional tobacco cigarettes. E-cigarette use is very popular among adolescents, with more than 3.5 million currently using these products in the US. Despite the increased prevalence of e-cigarette use, there is limited knowledge regarding the health impact of e-cigarettes on the general population. Based on published findings by others, E-cigarette is associated with lung injury outbreak, which increased health and safety concerns related to consuming this product. Different components of e-cigarettes, including food-safe liquid solvents and flavorings, can cause health issues related to pneumonia, pulmonary injury, and bronchiolitis. In addition, e-cigarettes contain alarmingly high levels of carcinogens and toxicants that may have long-lasting effects on other organ systems, including the development of neurological manifestations, lung cancer, cardiovascular disorders, and tooth decay. Despite the well- documented potential for harm, e-cigarettes do not appear to increase susceptibility to SARS-CoV- 2 infection. Furthermore, some studies have found that e-cigarette users experience improvements in lung health and minimal adverse effects. Therefore, more studies are needed to provide a definitive conclusion on the long-term safety of e-cigarettes. The purpose of this review is to inform the readers about the possible health-risks associated with the use of e-cigarettes, especially among the group of young and young-adults, from a molecular biology point of view.
Abbreviations: ENDS, electronic nicotine delivery system; e-cigarettes, electronic cigarettes; e-liquid, e-cigarette liquid; e-vapor, e-cigarette vapor; VG, vegetable glycerin; PG, propylene glycol; FDA, Food and Drug Administration; THC, Tetrahydrocannabinol; HNSCC, head and neck squamous cancer cells; XPC, xeroderma pigmentosum complementation group C; OGG1/2, 8-oxoguanine glycosylase; CYP, cytochrome P450; PAHs, polycyclic aromatic hydrocarbons; ROS, reactive oxygen species; EMT, epithelial-to-mesenchymal transition; CSC, Cancer Stem Cell; Sox2,, SRY (sex determining region Y)-box 2; Yap1, Yes associated protein 1; E2F1, E2F transcription factor 1; Oct4,, Octamer-binding transcription factor 4; ZEB, zinc finger E-box binding homeobox; ZO-1, zonula occludens-1; EVALI, e-cigarette or vaping product use-associated lung injury; VAPI, vaping-associated pulmonary injury; AM, alveolar macrophages; MPP, Mycoplasma pneumoniae pneumonia; BAL, bronchial alveolar lavage; PAFR, platelet-activating factor receptor; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; CNS, central nervous system; CS, cigarette smoke; OS, oxidative stress; iPSC-EC, induced pluripotent stem cell-derived endothelial cells; NOX, NADPH oxidase; FOXO3, forkhead box O3; TNF‐α, tumor necrosis factor alpha; AEC, airway epithelial cells; NET, neutrophil extracellular traps; NK, natural killer; MMP9, matrix metallopeptidase 9; CC16, Clara cell protein 16; CM, cardiomyocyte; COPD, chronic obstructive pulmonary disease; pAMPK, phospho-AMP-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; NQO-1, NAD(P)H quinone dehydrogenase 1; LDL, low-density lipoprotein; HUVEC, human umbilical vein endothelial cells
Keywords: E-cigarettes, Vaping, Nicotine, EVALI, Health risks
Graphical Abstract
Highlights
-
•
There is an increased prevalence of e-cigarette use among young adolescents.
-
•
E-cigarette use has potential side-effects on numerous organ systems.
-
•
There are not sufficient data supporting the safety of e-cigarettes.
-
•
More studies are needed to understand the long-term effects of e-cigarette use.
1. Introduction
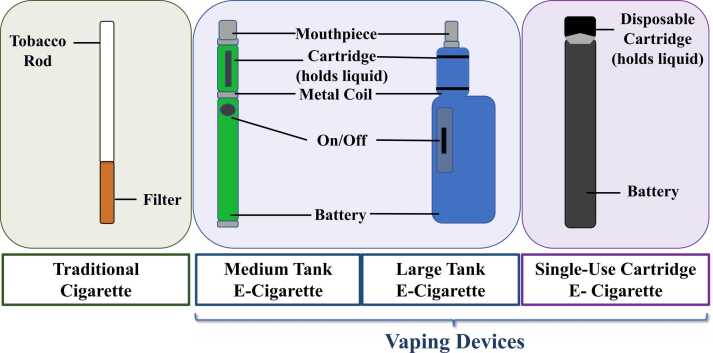
An E-cigarette is a type of electronic nicotine delivery system (ENDS) that uses battery-operated non-combustible tobacco products and is considered to be the latest technology available on the market [1]. Colloquially known as vaping devices, e-cigarettes generate a near odorless vapor, typically by heating and aerosolizing nicotine, vegetable glycerin (VG), and propylene glycol (PG), which is subsequently inhaled [1], [2]. E-cigarettes were introduced as a smoking cessation aid for adults with an unfounded claim of a superior safety profile when compared to traditional tobacco cigarettes [3]. In contrast to the relatively simple-shaped traditional cigarettes, composed of a filtration zone and a tobacco rod, vape products have a more complex design and continue to evolve [1]. The 3rd generation of traditional vapes, commonly known as tanks, were most commonly used among smokers in 2015–2017 [4], [5]. Tanks contain larger batteries with refillable e-cigarette liquid (e-liquid) cartridges [1], [2] and have adjustable voltage/wattage delivery [5]. As illustrated in Fig. 1, the e-liquid is heated using replaceable metallic coils, generating a vapor inhaled by the user [1]. Newer devices consist of a single use, hybrid small-battery with a non-refillable cartridge [2], [6] that usually contains acidified nicotine salts, which are easily absorbed by the endothelial airway system [7]. Despite the high levels of nicotine and other additives in e-cigarettes, these devices have gained huge popularity among the general population, particularly among young adults, with an estimated 35 million users worldwide [8]. The United States (US) is by far the largest market for the consumption and utilization of e-cigarettes [9]. In fact, an estimated 8.1 million adults were using e-cigarettes in 2018 [10]. Alarmingly, the use of e-cigarettes among middle-and high-school students has seen a sharp increase since 2014 [11]. Funding for marketing campaigns on e-cigarettes also increased from $6.4 million spent in 2011, to $115.3 million in 2014 [12], [13], suggesting that as with traditional cigarettes, marketing strategies for e-cigarettes was predominately targeted toward adolescents. During 2014, e-cigarette advertisements reached approximately 18.3 million adolescents in the US [14]. Adolescents exposed to advertisements via the internet, in grocery stores, in print, and on TV were 1.52 times more susceptible to use e-cigarettes for the first time, and 2.22 times more likely to repeat use, if they were current users [15]. Vaping among middle-and high-school students has seen an exponential increase between 2017 and 2019, leading to the highest number of 5 million users in the year 2019 [16], [17]. Compounding the potential harm of e-cigarette use, the combined consumption of both nicotine and Tetrahydrocannabinol (THC), the main psychoactive agent in cannabis, has substantially risen in recent years [18]. E-liquids include a variety of flavors such as mint, fruit, or candy, which have been attributed as one of the main driving forces that attract adolescents to use tobacco products [16], [19], [20], [21]. During the year of 2019, a lung injury outbreak associated with e-cigarette use, known as e-cigarette or vaping product use-associated lung injury (EVALI), raised the alarm about the harmful health effects associated with these products, as many patients died from the condition [22], [23], [24]. However, the negative health effects of e-cigarettes use are not limited to EVALI. Cancer, neurological, cardiovascular, and oro-dental diseases have all been linked to e-cigarettes use [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]. On the contrary, e-cigarettes use does not appear to increase susceptibility to SARS-CoV-2 infection [35]. Furthermore, some studies have found that e-cigarette users experience improvements in lung health [36], [37], [38], [39]. While others, specifically funded by the tobacco industry, have consistently reported few adverse respiratory health outcomes in smokers transitioning to e-cigarette use [33], [40], [41]. It is worth mentioning that the mass marketing of e-cigarettes has been available for only 15 years, and not enough time has been given to provide long-term studies on the safety of e-cigarettes [42]. Furthermore, several studies on e-cigarettes were performed in vitro, and may not be directly comparable to clinical and or in vivo studies. Financial conflict of interest and potential bias from industry-funded articles on e-cigarettes [43], [44], should also be taken into consideration. Therefore, this review will focus more on studies from independent researchers who investigated the pathology of e-cigarettes and have identified molecular and cellular mechanisms that could lead to disease onset and progression associated with vaping.
Fig. 1.
Comparison of nicotine delivery systems. Schematic representation of commercially available vaping devices and their different components. The delivery systems of nicotine in vaping devices are very different from traditional cigarettes that generate smoke by combustion of tobacco and are inhaled through a filter [1]. Tank e-cigarettes are battery-powered devices that deliver a charge to the metal coil when the activation “on/off button” is pressed, which heats the e-liquid in the cartridge, generating vapor that is inhaled through the mouthpiece [1], [2]. On the other hand, single-use e-cigarettes do not utilize a mechanical ‘on’ trigger but activate vapor production through the user’s suction on a disposable, combined cartridge, and mouthpiece [2], [6].
2. The health impacts of vaping e-cigarettes
2.1. E-cigarettes and pulmonary diseases
Based on a retrospective cohort study of 98 patients, e-cigarettes were directly implicated in the development of a clinical finding referred to as EVALI [22]. Another term for EVALI is vaping-associated pulmonary injury (VAPI). Currently, as the clinical data on the effects of e-cigarette products is limited, case reports serve a critical role, providing insights on e-cigarette-related pathology. Clinical presentations have been reported as (i) lipoid pneumonias, (ii) acute eosinophilic pneumonias, (iii) pneumonias with pleural effusion, (iv) acute pneumonitis, (v) respiratory bronchiolitis, (vi) interstitial lung disease, (vii) bronchiolitis obliterans organizing pneumonia and (viii) diffuse alveolar hemorrhages [45] (Fig. 2). However, further research is necessary as a multitude of patients with acute lung presentations have been noted to have pre-existing conditions. In addition, a large proportion of individuals presenting with e-cigarette-related acute conditions have reported using traditional tobacco cigarettes in addition to e-cigarettes [45]. More studies are required to approximate whether concomitant e-cigarette use was the cause of these conditions or whether additional confounding factors played a role. Since the effects of e-cigarette use are still to a great extent uncharacterized, traditional cigarette smoke (CS) exposure is used as a reference point in many studies and may provide the basis for initial insight into e-cigarette effects. Several recent meta-analyses have drawn strong associations between e-cigarette use with the prevalence of asthma or COPD [46], [47]. Moreover, in conditions such as asthma, where the respiratory immune system is already dysregulated, e-cigarette use may exacerbate the pathology [48]. The overlap of asthmatic symptoms and underlying mechanism that drive pathophysiology may resemble that of e-cigarette use, but more concrete and targeted studies need to be performed in human subjects to determine if and how e-cigarette may cause asthma and/or worsen symptoms in patients with existing respiratory diseases.
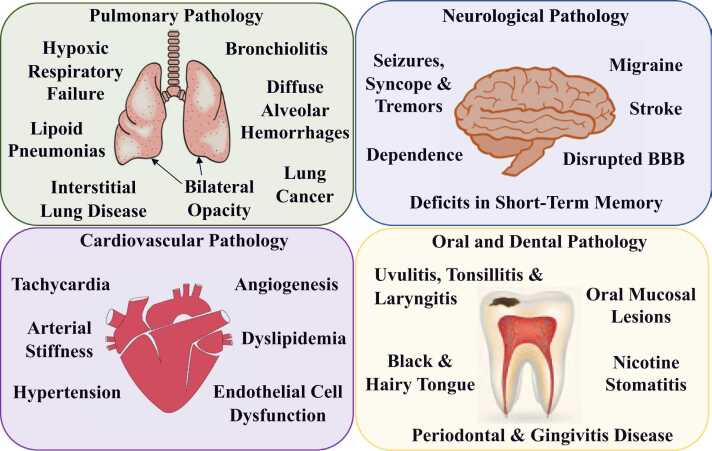
Fig. 2.
Summary of the clinical manifestations related to e-cigarette use. In the lungs vaping causes hypoxic respiratory failure, bilateral opacity, lipoid pneumonias, interstitial lung disease, bronchiolitis and diffuse alveolar hemorrhages [45]. Furthermore, increased production of CYP that metabolically activate PAHs from the e-vapor, has been linked to lung cancer by promoting ROS production and enhancing genotoxicity [25]. Vaping causes neurological disorders like seizures, syncope and tremors, disrupts the integrity of the BBB in the brain and increases the risk of developing a stroke [27], [28], [29], [93], [94], [95]. E-vapor could also induce changes in the embryonic brain that may contribute to future cognitive and behavioral abnormalities, like deficits in short-term memory [98]. Many e-cigarette brands contain acidified nicotine salts which result in delivery of high nicotine levels and potentially create dependence [7]. Impact of vaping on the cardiovascular system includes transient elevation of blood pressure, arterial stiffness, tachycardia, angiogenesis and dyslipidemia [30], [106], [107], [108], [112], [113], [114]. Additionally, vascular endothelial cells exposed to e-liquid showed decreased viability and higher production of ROS, leading to overall dysfunction [31]. Vaping also has detrimental effects in the oral cavity including black and hairy tongue, oral mucosal lesions, nicotine stomatitis, uvulitis, tonsillitis, laryngitis, periodontal and gingivitis disease [29], [30], [31], [32], [33], [124], [125], [126], [127], [130], [131], [132], [133], [134].
2.1.1. Lipid-laden alveolar macrophages and pneumonia in EVALI
The most common symptoms of EVALI include fever, shortness of breath, cough, and chest pain [22], [23]. Approximately two thirds of patients exhibit gastrointestinal symptoms such as nausea, emesis, and diarrhea [22], [23]. Patients classically exhibit the radiological finding of bilateral pulmonary opacity [22], [23]. As of February 18th, 2020, a total of 2807 EVALI cases and 68 related deaths have been reported in the US [24]. In September 2019, there was a peak in the number of e-cigarette-related emergency room visits, but the number of patients has since declined [24]. The observed decline could be related to more stringent law enforcement and increased awareness regarding e-cigarette use [24]. Several studies have identified Vitamin E Acetate (VEA) as the main driver of EVALI. In fact, VEA was identified as one of the major contaminates in bronchial alveolar lavage (BAL) fluid recovered from EVALI patients consuming THC products [49], [50]. As mentioned above, besides nicotine, e-cigarettes also contain the solvents, PG and VG, that are widely used in the food industry and considered safe for human consumption [51]. PG and VG are highly hygroscopic and water-soluble, which has led to their extensive use as humectants in e-liquids [52]. However, literature is sparse for the health effects of PG and VG when inhaled in vapor form. In a study on chronic e-cigarette-exposed murine model, the effects of PG, VG, and nicotine in pulmonary pathology were analyzed [53]. The data showed no inflammatory response or emphysematous changes in the lungs from mice exposed to e-cigarette vapor (e-vapor) compared to CS exposed mice, but there were significant alterations in the lipid profiles of both alveolar type II cells and alveolar macrophages (AM) after e-vapor exposure [53]. This study was the first to establish an experimental model of lipid-laden AM. The authors found morphologically disrupted lamellar bodies in type II alveolar cells with a shift towards phospholipid secretion that impeded surfactant function and opsonization capability [53]. Together with lipid-laden AM, a significantly delayed and reduced immune response occurred. Moreover, mice infected with the influenza A virus, exhibited higher morbidity and mortality when exposed to chronic e-cigarette as compared to air-exposed controls [53]. Similar clinical presentation of lipid-laden AM was detected in BAL fluid recovered from vaping patients, in clinical studies [54], [55]. E-vapor has been shown to enhance pneumococcal adherence to pulmonary epithelial cells due to the increased response of platelet-activating factor receptor (PAFR) [56]. A case reported in a young female vaper with acute hypoxic respiratory failure coupled with bilateral radiological opacities demonstrated that the patient was also positive for Mycoplasma pneumoniae pneumonia (MPP) [57], which generally has a mild clinical presentation, and is referred to as atypical or “walking pneumonia” [58]. BAL fluid recovered from the patient contained lipid-laden AM and was the first report that correlated long-term e-cigarette use with an active infection [57]. However, since this report was based on an isolated case, a larger cohort or case control study is needed to further verify a correlation between e-cigarette use and MPP. (Fig. 3). In addition to PG and VG solvents used in e-cigarettes [53], [55], flavorings may contribute to the development of lipoid pneumonias and other pulmonary related pathologies [59]. Lastly, in a systematic review published by others, they reported that the amount of variability in the levels of toxic components such as tobacco-specific nitrosamines, volatile organic chemicals, metals, flavoring chemicals, and others across different brands of e-cigarettes made it difficult to draw definitive conclusions [52].
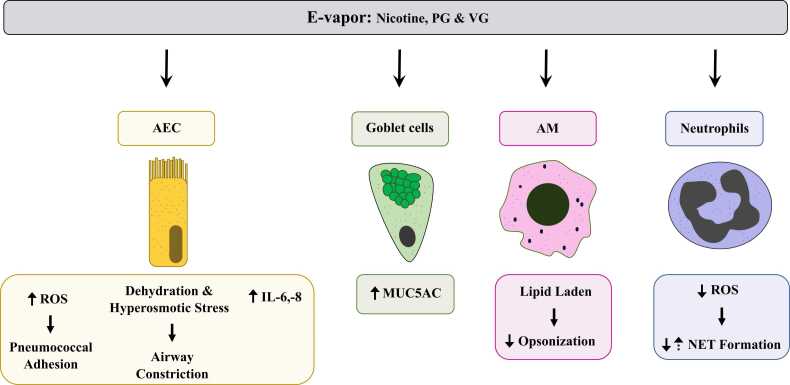
Fig. 3.
Main molecular mechanisms mediating e-cigarette pulmonary pathology. E-cigarette use has been demonstrated to elicit molecular responses in various cell types of the respiratory system. The effects of e-vapor exposure in AEC are 1) elevated ROS production which can lead to decreased antimicrobial efficiency, enhancing pneumococcal adhesion [56], 2) dehydration and osmotic stress leading to airway constriction [64], [65], and 3) increased cytokine secretion such as IL-6 and IL-8 [69]. E-vapor exposure also leads to increased secretion of the mucin MUC5AC by mucus secretory goblet cells [78]. Lipid profiles in AM are altered in response to e-vapor exposure [53], [55], [57]. Morphological changes in these cells showed a shift toward phospholipid secretion, leading to opsonization impairment [53]. E-vapor exposure also leads to reduction of neutrophilic ROS production which decreases NET formation [73], [76]. However, some studies have shown an increase of NET formation after e-vapor exposure [76], [78], highlighting the need to further study the effect of e-cigarettes on neutrophils.
2.1.2. E-cigarettes and mechanisms of airway epithelial disfunction
AEC are the first to be exposed to pathogens and toxicants. AEC coordinate responses to both endogenous and exogenous stimuli, orchestrating pulmonary inflammatory and mucus secretory responses [60]. AEC are comprised of basal cells which can regenerate and differentiate in response to injury and produce bioactive molecules [60]. Mucus secretory, or goblet, cells secrete the mucins which are a vital innate defense mechanism and subsequently ciliated cells move the secreted mucins via directional ciliary beating to remove pathogen or pollutant [61]. Clara cells are also secretory cells that are primarily located at the terminal airways [61]. The rates of differentiation and interplay between all these cells is critical for normal lung function and response to injury [60]. PG and VG may potentially act in a dehydrating manner on airway epithelium [62] and can induce epithelial stress and potentially induce inflammatory responses [63]. Furthermore, PG and VG do not cross the airway membranes and, when inhaled in large amounts, can induce hyperosmotic stress which may further induce both proinflammatory responses as well as cell stress responses [64], [65]. In addition, airway constriction may be strongly induced by PG/VG containing nicotine, which independently acts as an airway constrictor [65]. These stress responses may impact both epithelial and smooth muscle cells, leading to highly complex airway responses that need to be further investigated. A clinical study demonstrated that e-vapor containing PG/VG with or without nicotine could cause inflammation in the lungs and vasoconstriction [65]. The authors used 25 healthy, occasional smokers in their study and found that regardless of nicotine, the presence of PG/VG in the e-vapor led to the observed results and pathology [65]. They showed that PG/VG inhalation alone led to increased serum levels of Clara cell protein 16 (CC16), which is a well-known anti-inflammatory epithelial lung injury marker [65]. They further conducted physiological testing on their participants, including pulse oximetry, gas tension, pulmonary function tests and blood analyses [65]. The data showed an increased and consistent vasoconstriction upon PG/VG exposure coupled with disturbed gas exchange [65] (Fig. 3).
Traditional smoke in COPD patients has been shown to exacerbate mucus secretion, airway constriction and inflammation of the pulmonary epithelium, which are also overlapping pathways seen with e-cigarette-induced airway epithelial dysfunction and toxicity [66]. Several independent research groups showed an increase in ROS production after exposure to e-vapor extract in human umbilical vein endothelial cells (HUVEC) [67] and in small airway epithelial cells [68]. Increased ROS production and increased secretion of the pro-inflammatory cytokines, interleukin (IL)− 6 and IL-8 occurred in pulmonary epithelium exposed to e-vapor [69]. In a murine model, acute (3-day) e-vapor exposure increased levels of IL-1α, IL-13, IL-6 and the chemokine CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), in BAL fluids [69]. Inhalation of e-vapor by study participants that were non-smokers showed significantly increased serum markers of inflammatory responses and ROS production 1–2-hours post-exposure, with a return to baseline after 6-hours [70]. Toxicants present in CS and e-vapor increase secretion of ROS that can further induce cell stress responses, such as proinflammatory pathways, unfolded protein response pathways, and alterations in cell differentiation, including goblet cell hyperplasia [71]. Short term exposure to e-vapor in an in vitro airway epithelial cells (AEC) model led to increased mitochondrial ROS generation as well as increased secretion of IL-8 and IL-6 release [69] (Fig. 3). In conjunction with mitochondrial changes, the authors indicate that there is a significant increase in DNA damage and DNA fragmentation, suggesting there may be long-term effects associated with e-cigarette use [69].
2.1.3. E-cigarettes and neutrophilic-driven pulmonary inflammation
Airway epithelial secretion of the interleukin (IL)17 is what primarily drives neutrophilic inflammation [72]. Neutrophil recruitment is aided by the epithelial intercellular adhesion molecule (ICAM)− 1, to which the neutrophilic cell surface molecules CD11b and CD18 bind [72]. Therefore, in addition to general airway constriction, the most common response to AEC exposure to PG/VG containing nicotine is neutrophilic-driven inflammation.
A recent study has evaluated in detail the neutrophilic response to PG/VG exposure [73]. The study found a highly significant decrease of neutrophilic chemotaxis towards bacterial cell wall components upon exposure to e-vapor [73]. In addition, neutrophil membrane fluidity was affected and most notably, ROS were reduced by almost 50% [73]. Neutrophils generate extensive extracellular fibers which have been termed neutrophil extracellular traps (NET) and have been extensively characterized [74], [75]. NET contain DNA, histones, various proteolytic enzymes, proteins and ROS that kill pathogens [74], [75]. As such, in neutrophils with reduced ROS due to e-vapor exposure, NET production was reduced by 3.5-fold, suggesting an extensive reduction in neutrophilic efficiency [73] (Fig. 3). Using an in vivo murine model, the authors found that the Pseudomonas burden was significantly increased upon e-vapor exposure and that neutrophil migration to infection sites was reduced [73]. E-vapor may cause a reduction in neutrophil recruitment and a reduction in antimicrobial efficiency [73]. ROS signaling is distinct and integral in its downstream effects on neutrophil function as compared to other cell types where ROS is often detrimental. In fact, it has been shown that in AEC, e-vapor induces the generation of ROS and increases oxidative stress (OS), which is highly detrimental for cellular function and epithelial innate immune responses [69]. Another study further recapitulated these findings, however in the context of very specific flavoring compounds such as cinnamaldehyde [76]. They found a dose-dependent suppression of general innate immune responses spanning across macrophages, neutrophils, and natural killer (NK) cells [76]. They further suggest thiolation as the mechanism of action regarding immune suppression [76]. In addition, some flavoring agents were found to reduce NET formation, while others were shown to increase NET formation [76]. In another clinical study that included e-cigarette users as well as traditional tobacco cigarette users, the authors examined the nasal immune architecture and further corroborated the immunosuppressive effect of e-cigarette use [77].
In addition to the inflammatory response, a critical innate immune response is the mucus secretion [60], [61]. Mucins are large glycoproteins capable of binding various pathogens [60], [61]. Mucins, MUC5AC and MUC5B, are the primary secreted mucins in the context of CS or toxicant exposure and are highly upregulated upon insult [60], [61]. To better understand the neutrophilic activation and mucin secretion profiles of e-cigarette users, a clinical study that included 15 e-cigarette users, 15 nonsmokers, and 14 traditional tobacco users was conducted [78]. The study performed proteomic analyses, mucin quantification, and NET formation quantification. The data showed numerous well-known markers of CS exposure, including the matrix metallopeptidase 9 (MMP9) and thioredoxin were also upregulated with e-cigarette use [78]. An increase of mucin MUC5AC concentrations in e-cigarette users was also observed, which is the principal marker of mucus hypersecretion in the CS-related pathology [78] (Fig. 3). This is one of the first reports that may have indicated that e-cigarette use has detrimental effects similar to those of traditional tobacco cigarettes. Findings further implicate e-cigarette use as a risk factor for development of chronic pulmonary diseases such as chronic obstructive pulmonary disease (COPD) [78]. Similar to other studies, effects of e-cigarette were primarily focused on the neutrophilic response [78]. The discrepancy lies in that the latter study reported an increased level of NET-related proteins in e-cigarette users as compared to both nonsmokers and traditional tobacco users [78]. The results are further complicated as the authors were unable to find increased levels of NET-inducing proinflammatory elements such as IL-8 and/or tumor necrosis factor (TNF)-α [78]. Overall, this study provided strong evidence that the immuno-mucosal effects of e-cigarette use are more complicated than what was previously reported and that e-cigarettes may in fact have similarly detrimental effects as compared to traditional tobacco cigarettes [78].
The molecular effects of e-cigarettes are highly debated. There are those who showed increased neutrophilic inflammation, NET generation, and inflammatory response, while others showed the exact opposite (Table 1). The discrepancy in findings can create a significant problem when addressing regulatory or clinical questions posed by the expansion of e-cigarette use. As such, there is a dire need for standardization of studies, particularly in terms of e-liquid constituents. This discrepancy may also imply variability amongst different e-cigarette products in terms of what components are included. This may be a reason for the scattered incidence of EVALI and suggests that, in addition to standardization of research approaches, there may be a need for regulatory practices to standardize the contents of e-liquids.
Table 1.
Health impacts of vaping.
| Clinical studies | Animal studies | Cell line studies | |
|---|---|---|---|
| Cancer | N/A |
|
|
| Pulmonary |
|
||
| Neurological & Neurovascular | |||
| Cardiovascular | |||
| Oral & Dental | N/A | N/A |
Summary of relevant clinical, animal and cell studies on the adverse health effects of vaping which include cancer, pulmonary injury, neurological symptoms, cardiovascular disease and oro-dental symptoms. N/A, not applicable.
2.2. E-cigarettes and cancer
As mentioned above, e-cigarettes are often advertised as a “less-harmful” alternative to smoking traditional tobacco cigarettes. As a consequence, e-cigarette use has gained popularity among cancer survivors in the US [79], [80]. According to the National Health Interview Survey, the prevalence of e-cigarette use has increased in cancer patients from 8.5 % in 2014 to 10.7 % in 2017 [81]. A study on head and neck squamous cancer (HNSCC) and normal epithelial cell lines exposed to e-vapor from different brands, with or without nicotine, showed reduced cell viability and significant evidence of apoptosis and necrosis when compared to unexposed controls [82]. In addition, the exposed cell lines showed a higher expression of H2A histone family member X (γ-H2AX), a well-defined marker for double-strand DNA breaks. Regardless of nicotine, e-vapor was considered cytotoxic and a DNA strand break-inducing agent [82]. Tobacco-specific nitrosamines, such as N‐nitrosonornicotine, 4–(methylnitrosamino)‐1–(3–pyridyl)‐1–butanone, N‐nitrosoanabasine, and N‐nitrosoanatabine, are potent carcinogens found in e-cigarettes [83]. Inadequate storage and the manufacturing process of e-liquid and tobacco flavoring led to nitrosamines formation and subsequently increased their concentration [84]. Studies on human bronchial epithelial cells, human urothelial cells, and lung and bladder tissues recovered from postmortem mice exposed to e-vapor, showed high tumorigenic transformation due to the nicotine metabolites N-nitrosonornicotine and nicotine-derived nitrosamine ketone [85]. Protein expression levels of xeroderma pigmentosum complementation group C (XPC) and 8-oxoguanine glycosylase (OGG1/2) involved in DNA-repair mechanism, were decreased in both the human bronchial epithelial cells and mice lungs following e-vapor exposure [85]. Another study using a rat model of lung cancer showed multiple effects of e-vapor on cancer initiation [25]. Specifically, e-vapor increased carcinogen-metabolizing enzymes, such as cytochrome P450 (CYP) family genes and activators of polycyclic aromatic hydrocarbons (PAHs) [25]. These enzymes were associated with overproduction of ROS and DNA oxidation to 8-hydroxy2′-deoxyguanosine. Furthermore, e-vapor induced DNA damage in peripheral blood, exhibited by DNA fragmentation and strand breaks in leukocytes, as well as micronuclei formation in reticulocytes [25].
Metastasis is one of the leading causes of cancer deaths, and the epithelial-to-mesenchymal transition (EMT) is one of the markers of metastasis [86]. Exposure to e-liquid and e-vapor significantly increased the expression of EMT markers in adenocarcinoma alveolar basal epithelial cells, along with fibroblast-like morphology, loss of cell-to-cell junctions, internalization of E-cadherin, increased motility, and nuclear translocation of active β-catenin [87]. Nicotine induced Cancer Stem Cell (CSC) properties of non-small cell lung cancer, referred to as stemness [88]. The induction of stemness in lung cancer, associated with e-cigarette use, was mediated through upregulation of a stemness marker SRY (sex determining region Y)-box 2 (Sox2), a gene that is highly expressed in stem cells and responsible for self-renewal along with the Yes associated protein 1/ E2F transcription factor 1/Octamer-binding transcription factor 4 (Yap1/E2F1/Oct4) signaling axis [26]. In vitro studies using lung cell lines, A549 and H1650, exposed to e-liquid extracts showed high sphere formation and self-renewal activity that were diminished with the knock-out of Sox2, as increased expression of Sox2 increases sphere formation or self-renewal ability [26]. Exposure to e-liquid increased CSC properties by interfering with Sox2, as validated in Sox2 knocked-out cells. Exposure to e-liquid also altered the expression of EMT markers [26]. Specifically, in A549 and H1650 cells exposed to e-liquid, expression of mesenchymal markers such as vimentin, fibronectin, zinc finger E-box binding homeobox (ZEB)1, and ZEB2 were induced [26]. Moreover, a decreased expression of the epithelial marker E-cadherin and the tight junction protein zonula occludens-1 (ZO-1) was observed in A549 and H1650 cells exposed to e-liquid [26]. In a case report of a 45-year-old female patient, she had extensive inflammation in lungs and liver due to e-cigarette use, which mimic metastatic cancer [89]. Findings confirmed the association of e-cigarette use with cancer progression by inducing EMT transition and impairing DNA repair mechanisms.
Despite available insights from in vitro and in vivo data (Table 1), as yet no clinical study has been performed to analyze the effect of e-cigarette on carcinogenesis in human subjects. This would require a longitudinal effort to establish a direct link or causality with the latency period of cancer formation.
2.3. E-cigarettes and neurological disorders
One of the most frightening consequences of CS is the potential neurological manifestations. Multiple studies have explored the effect of nicotine, the main neuroactive ingredient in CS, on the central nervous system (CNS). In addition to nicotine’s ability to induce an antidepressant effect in mice [90], nicotine is also highly addictive. The limbic-dopaminergic system, tasked with regulating emotional and reward-driven behaviors, undergoes expansion in adolescence and is particularly vulnerable to nicotine induced damage during this developmental period [91]. Other components of CS have been shown to regulate neurotransmitter release, activate receptors, and inhibit monoamine oxidase, ultimately leading to an antidepressant effect [92].
As more time has passed since the production of e-cigarettes, evidence has accumulated, further linking e-cigarettes use and its associated detrimental health effects (Table 1). For example, data collected between December 2010 to June 2019 on 123 e-cigarette users reported seizures, syncope, or tremors [28]. Among the patients that experienced seizure, 85 % reported occurrence within 24-hours after e-cigarette use, while 62 % reported occurrence within 30-minutes of e-cigarette use. The study concluded a link between e-cigarette use and seizures [28]. In an attempt to find e-cigarette side effects that were not reported to physicians, an internet forum and data mining study on e-cigarettes was performed [29]. This study found an alarmingly high number of side effects, with the most reported health effects affecting neurological and respiratory systems [29]. The most common neurological symptoms reported were headaches, malaise, nausea, tiredness, dizziness, fatigue, and lightheadedness, with dehydration, migraine, and stroke reported to be the most common neurological disorders [29]. Taken together, these two studies highlighted valid concerns since e-vapor does lead to neurological dysfunction, resulting in a wide range of neurological symptoms and/or disorders. Additionally, e-cigarette use has been implicated in new onset and exacerbation of pre-existing seizures. In fact, in 2019, at the height of the EVALI outbreak (explained in detail above) the Food and Drug Administration (FDA) released a statement warning of seizures induced from e-cigarette use [93]. A case study reported vaping induced seizures with a tonic-clonic seizure beginning while the patient was driving, and after hospital admission and stabilization, re-initiation of a tonic-clonic seizure began minutes after vaping in the emergency department [94]. Moreover, empirical data was collected from a responsive neurostimulator implanted in patients using e-cigarettes, further corroborating the association between e-cigarette use and seizure episodes [95].
Of the utmost concern is the consumption of e-cigarettes by pregnant women and the effect of e-vapor on the development of the fetus. Around 8.5 % of all births delivered are by women under the age of 25 [96]. This age cohort is also the group that has the highest prevalence of smoking [96]. A common belief among this population is that pregnant women who use e-cigarettes have less adverse health effects than those who smoke traditional tobacco cigarettes. However, there is not enough evidence to suggest that e-cigarettes cannot harm an unborn fetus [97]. In fact, pregnant mice exposed to e-vapor with and without nicotine throughout gestation delivered offspring with deficits in short-term memory, as well as reduced anxiety and hyperactivity, when compared to offspring born to dams exposed to ambient air [98]. Further, epigenetic testing in brains recovered postmortem from offspring showed increased DNA methylation, and alteration of 13-genes involved in neurological activity [98]. The same research group showed that switching pregnant dams from CS to e-vapor during gestation and lactation had decreased neurological deficits in their offspring, when compared to offspring born to dams exclusively exposed to CS [99]. Interestingly, despite a reduction in neurological deficits, genetic alterations were still detected in offspring, suggesting that e-cigarette use should not be recommended during pregnancy [99].
Accumulating evidence suggesting that e-cigarette use can lead to neurological deficits are largely attributed to nicotine, as its effects are well studied in traditional tobacco cigarettes. However, other constituents of e-liquid and e-vapor have not been thoroughly studied. Prospective studies are needed to establish a direct causality between e-cigarettes and neurological disorders (Fig. 2).
2.4. E-cigarettes and cerebrovascular disorders
Nicotine has been linked to blood brain barrier (BBB) dysfunction and an increased risk of ischemic manifestations [100]. Although marketed as a smoking cessation tool, nicotine delivery through e-cigarettes can result in higher levels of nicotine in serum when compared to traditional tobacco cigarettes. For example, a recent study in rats compared the serum levels of nicotine after exposure to e-vapor (JUUL and previous generation e-cigarettes) versus CS (Marlboro Red cigarettes, produced by Philip Morris USA Inc.) [7]. JUUL e-vapor delivered 136.4 ng/ml of nicotine, which is substantially higher than the 17.1 ng/ml delivered by other e-cigarettes and 26.1 ng/ml delivered by Marlboro cigarettes [7]. This study concluded that e-cigarettes are not safer than traditional cigarettes. In addition, traditional tobacco cigarettes are associated with the production of ROS and with vascular endothelial dysfunction, both of which can affect the integrity of the BBB [101]. Furthermore, nicotine has been shown to exacerbate cerebral ischemia and post-ischemic inflammation [102], [103]. Although e-cigarettes may not contain the multitude of carcinogens and toxic compounds known to be contained within traditional tobacco cigarettes, their potential to induce OS and negative impact on the BBB was considered not be significantly different as compared to traditional tobacco products [27]. Increased expression and nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) and NAD(P)H quinone dehydrogenase 1 (NQO-1) are typical mechanisms activated during OS and were detected in mouse primary brain microvascular endothelial cells after exposure to e-vapor extract [27], [101]. In addition to OS, increased cellular inflammation together with a significant decrease in BBB integrity were detected after e-vapor exposure [27]. However, the impairment of the cerebrovascular system may be the result of the other various compounds contained within the e-liquid, and not necessarily a direct result of the nicotine [27]. Therefore, further studies dissecting the specific constituents within e-cigarette smoke that may contribute to the negative effects on cerebrovascular system are needed to form specific causal relationship. In terms of thrombosis, e-cigarette use may be a contributing factor for stroke, as chronic exposure of mice to e-vapor for 2 weeks decreased the concentration of thrombomodulin and increased TNF‐α expression [27]. These results were similar to those obtained from mice chronically exposed to traditional cigarette smoke [27]. Additionally, e-cigarette use was found to exacerbate stroke injury and its outcome, confirming that e-cigarettes induced OS resulting from dysregulation of the cellular antioxidant response system is a strong candidate in the mechanism for elevated stroke risk and cerebrovascular toxicity [27].
2.5. E-cigarettes and cardiovascular diseases
Cardiovascular disease is one of the main causes of CS-related deaths. According to the American Heart Association, smoking causes more than 20% of deaths due to heart disease in the US [104]. The most common examples of CS-related cardiovascular diseases are myocardial infarction, stroke, and aortic aneurysm [105]. Smoking is also correlated with peripheral vascular disease, hypertension, and dyslipidemia [105]. The underlying causes of cardiovascular disease in traditional tobacco and e-cigarette users are endothelial cell dysfunction, OS, and angiogenesis [106]. Recent studies on the impact of e-cigarettes on cardiovascular diseases showed that while e-cigarettes have milder effects than traditional tobacco cigarettes, increased heart rate and blood pressure still occurred [106], [107], [108]. A randomized, double-blinded study found that using e-cigarettes containing nicotine may impact peripheral and central hemodynamics and arterial stiffness, with negative effects on the cardiovascular system at levels comparable to traditional tobacco cigarettes [30]. Peripheral and central blood pressure, and parameters of arterial stiffness were measured in subjects for 2-hours after vaping e-cigarettes with nicotine [30]. The peripheral systolic blood pressure increased significantly 45-minutes after the use of nicotine-containing e-cigarettes, which was not observed in the group vaping e-cigarettes without nicotine [30]. In addition, the heart rate of nicotine-containing e-cigarette users remained elevated for approximately 45-minutes after vaping [30].
A recent study investigated the effect of nicotine and different flavors of e-cigarettes on endothelial cells and a potential crosstalk with macrophage activation [31]. Serum samples were collected from e-cigarette users and human-induced pluripotent stem cell-derived endothelial cells (iPSC-EC) were collected from healthy individuals and subsequently exposed to e-liquids [31]. In the serum of e-cigarette users, the levels of inflammatory cytokines were elevated. In iPSC-EC exposed to e-liquids, evidence of endothelial dysfunction were observed including lower cell viability, higher production of IL-1β and − 6, ROS production, activation of OS-related pathway, caspase 3/7 activity, low-density lipoprotein uptake (LDL), and disrupted tube formation and migration [31]. Furthermore, multiple studies on commercial and isolated HUVEC revealed the negative impact of e-cigarette use [67], [109], [110]. Exposure to high levels of e-vapor caused DNA damage, cell death (both apoptotic and necrotic), and significantly elevated ROS levels in HUVECs [67], [110]. The treatment of e-vapor exposed HUVEC cells with antioxidants decreased necrotic cell death, which suggests that increased ROS production is the underlying reason for necrotic cell death due to e-cigarette use. Exposure to e-vapor reduced the cell viability and metabolic activity in HUVECs [67], [110]. E-vapor exposure also induced an inflammatory response through the deposition of the complement cascade components, C1q, gC1qR, cC1qR, C5b-9, and C3b, involved in the innate immune response [109]. The expression of complement inhibitors/regulators, CD35, CD55 and CD59 were also increased after exposure to e-vapor [109]. Complement cascade activity also increased in platelet cells obtained from healthy donors when exposed to e-vapor extracts [111]. Platelet activity and aggregation were enhanced, as well as increased levels of platelet adhesion markers CD41, CD42b, and CD62p were measured after e-vapor exposure [111]. Interestingly, platelet functions were inhibited by nicotine alone, which suggests that other components in e-cigarettes are responsible for enhancing platelets aggregation that may negatively impact cardiovascular disease outcomes [111].
In addition to cell culture studies, the negative health effects of e-cigarette use have been demonstrated in different mouse models. In a cardiovascular mouse model, increased collagen content was found in tissues exposed to e-cigarettes, along with high angiogenesis activity and high levels of the endothelial cell markers CD31 and CD34 [112]. Exposure of wild type mice to e-vapor, particularly the ingredient acrolein aldehyde, led to increased vascular OS, inflammation, and lipid peroxidation by a phagocytic NADPH oxidase (NOX-2) mechanism [113]. The risk of cardiovascular disorder in offspring exposed to combination nicotine-containing e-vapor in utero along with maternal obesity was observed [114]. The gestational exposure to e-vapor in combination with a high fat diet led to increased cardiomyocyte (CM) apoptosis and abnormal ventricular structure by postnatal day 14 [114]. The CMs showed chromatin fragmentation, convoluted nuclear membrane, enlarged mitochondria, and decreased phospho-AMP-activated protein kinase (pAMPK) immunoreactivity as the signs of apoptosis [114]. Cell death signaling including cleaved caspase-3 was enhanced, the anti-apoptotic BAX was reduced, and the pro-apoptotic BCL-2 was elevated [114]. The expression levels of the lipid peroxidation product, 4-HNE, were also increased as evidenced by OS detection in the ventricles of 14-day-old mice [114].
Studies provided summarized the detrimental cardiovascular health effects associated with e-cigarette use (Table 1), which have been shown in many cases to be equally as harmful as traditional cigarette use. Moreover, certain chemicals found in e-cigarettes such as acrolein aldehyde are particularly harmful to the vascular system and further highlight the need for prospective cohort studies.
2.6. E-cigarettes and oro-dental hygiene
The inhalation of e-vapor has the potential to cause harm to the oral cavity. Toxicology studies have shown many potentially toxic components in e-cigarettes which include volatile organic compounds like benzene, lead, ultrafine particulate matter, diacetyl, nicotine, nickel, and tin [115]. As a result of e-cigarette use, there have been reports of symptoms affecting the tongue, soft tissue, lips, and the hard palate [116]. E-cigarette users commonly reported symptoms including burning [117], poor taste [118], dryness [118], irritation [119], [120], [121], bad breath [122] and pain [123]. These studies reported decreased oral symptomology relative to those reported in traditional tobacco cigarette users, with the above symptoms occurring with a greater frequency than non-smokers [123]. In addition to these mild symptoms, more noticeable symptoms have also been reported as a result of e-cigarette use, including burns, black tongue, and oral mucosal lesions [32], [124], [125], [126]. Of note, hairy tongue and nicotine stomatitis were significantly more prevalent in consumers of e-cigarettes relative to former smokers [127].
Symptoms associated with e-cigarette use are not limited to the mouth but expand to affect the throat as well. Evidence of mild symptoms including irritation [119], dryness of the throat, cough, and soreness were also reported [32], [33], [41]. It is also possible that some of these symptoms negatively impacting the throat are related to e-cigarette flavoring, such as cola, cinnamon, sour, citrus, or custard [128], [129]. Additionally, para-tracheal edema, uvulitis, tonsilloliths, tonsillitis, and laryngitis were observed in e-cigarette users [29], [33], [130]. Smoking of traditional tobacco cigarettes is known to negatively impact dental implant treatments, resulting in an increased rate of implantation failure and peri-implant bone loss [131]. However, in comparison to traditional tobacco cigarettes, e-cigarettes were not found to affect peri-implant health [131]. A significant decrease in marginal bone loss was observed in patients who smoked traditional tobacco cigarettes [131]. Although the use of e-cigarettes was not found to cause bone loss, they were found to significantly increase the release of pro-inflammatory cytokines, such as TNF‐α and IL‐1β, similar to users of traditional tobacco cigarettes [131]. Interestingly e-cigarette use also resulted in significantly lower levels of gingival bleeding or bleeding on probing (BOP) relative to the non-smoking group [131], [132]. However, when established users of traditional tobacco cigarettes switched to use e-cigarettes, they exhibited a significant rise in gingival inflammation or BOP [133]. Although only a pilot study, it demonstrated the negative impact that switching to use e-cigarettes may have on former tobacco smokers, either for its social aspects or to facilitate their termination of traditional tobacco use [133].
Studies have drawn associations between periodontal disease and e-cigarette use, presenting with increased concentrations of localized inflammatory markers, an expansion in bone loss, increased levels of plaque, sulcular fluid volume increases, and deeper probing depths [132]. Further evidence demonstrates that when compared to non-smokers, e-cigarette consumers had a 2-fold increase in the probability of developing periodontal disease and a 3-fold increase in the likelihood to develop gingival disease [34], [134]. Currently, although there are some data examining the effects of e-cigarettes on the oral cavity (Table 1), more research in this area is necessary to understand the relationship between e-cigarettes and oral health.
3. Conclusions
In this review we provided a summary on the possible health-risks associated with e- cigarette use among adolescents and young-adults from a molecular biology perspective, focusing on basic science such as DNA repair, signaling pathways, and immunological responses. The negative findings of e-cigarette use broadly affects numerous organ systems. Some adverse effects are unique to e-cigarettes use, while others are similar to those of traditional tobacco cigarettes. Although majority of the literature cited have shown evidence of a possible pathology with e- cigarettes, others have found an improvement in lung health with limited adverse effects and/or comorbidities. Potential flaws in scientific methods and data analysis may have provided some biased perspectives. Nonetheless, the discrepancies in findings addressed the need for longer studies that are more clinically relevant. It is also important to highlight that currently there are major limitations when investigating the effects of e-cigarettes, as most data gathered on e-cigarette use and its effects on human subjects are from cross-sectional studies. To address the direct causality on how e-cigarettes consumption may negatively impact human health, further prospective studies are necessary. A better understanding in the potential risks and benefits of long-term use of e-cigarette can provide an unbiased opinion on what this product has to offer.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
ME-L: Assisted in writing the introduction and conclusions, provided assistance with Table 1, Fig. 2, Fig. 3, editing and formatting of the manuscript. MP: Assisted in writing the section on neurological/cerebrovascular disorders and provided assistance with Fig. 1, Fig. 2, and editing of the manuscript. MA: Assisted in writing the section on cancer and provided assistance with Fig. 3. LG, JR, PP, and MM: Assisted in writing the section on pneumonia/EVALI. YC: Assisted in writing the sections on cancer and cardiovascular diseases. LSL: Assisted in writing the section on neurological/cerebrovascular disorders. FO, JS, and EA: Assisted in writing the section on cardiovascular diseases. AC: Assisted in writing the section on oro-dental hygiene. NE-H: Course director, assisted in writing the abstract and conclusions as well as editing and formatting of the manuscript, table and figures. All authors have read and approved the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the Biomedical Science Program in the College of Medicine at Florida International University for providing funding towards the publication of this manuscript, which was written as a collaborative effort among Ph.D. graduate students as part of the curriculum for the seminar course GMS 6939.
Handling Editor:
References
- 1.Brown C.J., Cheng J.M. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23(Suppl 2) doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. E-cigarette, or vaping, products visual dictionary(accessed 2 Feb 2021). Available from: 〈https://www.cdc.gov/tobacco/basic_information/e-cigarettes/pdfs/ecigarette-or-vaping-products-visual-dictionary-508.pdf〉.
- 3.Centers for Disease Control and Prevention. Adult smoking cessation – The use of E-cigarettes. A summary of smoking cessation: a report of the surgeon general2020 (accessed 2 Feb 2021). Available from: 〈https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/fact-sheets/pdfs/adult-smoking-cessation-e-cigarettes-use-h.pdf〉.
- 4.Walley S.C., Jenssen B.P. Electronic nicotine delivery systems. Pediatrics. 2015;136(5):1018–1026. doi: 10.1542/peds.2015-3222. [DOI] [PubMed] [Google Scholar]
- 5.Aherrera A., Aravindakshan A., Jarmul S., Olmedo P., Chen R., Cohen J.E., et al. E-cigarette use behaviors and device characteristics of daily exclusive e-cigarette users in Maryland: implications for product toxicity. Tob Induc Dis. 2020;18:93. doi: 10.18332/tid/128319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delnevo C., Giovenco D.P., Hrywna M. Rapid proliferation of illegal pod-mod disposable e-cigarettes. Tob. Control. 2020;29(e1):e150–e151. doi: 10.1136/tobaccocontrol-2019-055485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao P., Liu J., Springer M.L. JUUL and combusted cigarettes comparably impair endothelial function. Tob. Regul. Sci. 2020;6(1):30–37. doi: 10.18001/trs.6.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besaratinia A., Tommasi S. Vaping: a growing global health concern. EClinicalMedicine. 2019;17 doi: 10.1016/j.eclinm.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euromonitor International. Smokless tobacco and vapour products2020 (accessed 2 Feb 2021). Available from: 〈https://www.euromonitor.com/smokeless-tobacco-and-vapour-products〉.
- 10.Villarroel M.A., Cha A.E., Vahratian A. Electronic cigarette use among U.S. adults, 2018. NCHS Data Brief. 2020;(365):1–8. [PubMed] [Google Scholar]
- 11.Gentzke A.S., Creamer M., Cullen K.A., Ambrose B.K., Willis G., Jamal A., et al. Vital signs: tobacco product use among middle and high school students - United States, 2011-2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68(6) doi: 10.15585/mmwr.mm6806e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim A.E., Arnold K.Y., Makarenko O. E-cigarette advertising expenditures in the U.S., 2011-2012. Am. J. Prev. Med. 2014;46(4):409–412. doi: 10.1016/j.amepre.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Truth Initiative. Vaporized:youth and young adult exposure to e-cigarette marketing.2015 (accessed 2 Feb 2021). Available from: 〈https://truthinitiative.org/sites/default/files/media/files/2019/03/Vaporized-Youth-Exposure-To-E-Cigarette-Marketing.pdf〉.
- 14.Singh T., Marynak K., Arrazola R.A., Cox S., Rolle I.V., King B.A. Vital signs: exposure to electronic cigarette advertising among middle school and high school students - United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;64(52):1403–1408. doi: 10.15585/mmwr.mm6452a3. [DOI] [PubMed] [Google Scholar]
- 15.Mantey D.S., Cooper M.R., Clendennen S.L., Pasch K.E., Perry C.L. E-cigarette marketing exposure is associated with e-cigarette use among US youth. J. Adolesc. Health. 2016;58(6):686–690. doi: 10.1016/j.jadohealth.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen K.A., Gentzke A.S., Sawdey M.D., Chang J.T., Anic G.M., Wang T.W., et al. e-cigarette use among youth in the United States, 2019. JAMA. 2019;322(21):2095–2103. doi: 10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miech R., Johnston L., O’Malley P.M., Bachman J.G., Patrick M.E. Trends in adolescent vaping, 2017-2019. N Engl J Med. 2019;381(15):1490–1491. doi: 10.1056/NEJMc1910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miech R.A., Patrick M.E., O’Malley P.M., Johnston L.D., Bachman J.G. Trends in reported Marijuana vaping among US adolescents, 2017-2019. Jama. 2019;323(5):475–476. doi: 10.1001/jama.2019.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrose B.K., Day H.R., Rostron B., Conway K.P., Borek N., Hyland A., et al. Flavored tobacco product use among US youth aged 12-17 Years, 2013-2014. JAMA. 2015;314(17):1871–1873. doi: 10.1001/jama.2015.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan-Sarin S., Morean M.E., Camenga D.R., Cavallo D.A., Kong G. E-cigarette use among high school and middle school adolescents in Connecticut. Nicotine Tob. Res. 2015;17(7):810–818. doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald E.A., Ling P.M. One of several ‘toys’ for smoking: young adult experiences with electronic cigarettes in New York City. Tob. Control. 2015;24(6):588–593. doi: 10.1136/tobaccocontrol-2014-051743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Layden J.E., Ghinai I., Pray I., Kimball A., Layer M., Tenforde M.W., et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin - Final Report. N. Engl. J. Med. 2020;382(10) doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 23.Kalininskiy A., Bach C.T., Nacca N.E., Ginsberg G., Marraffa J., Navarette K.A., et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir. Med. 2019;7(12):1017–1026. doi: 10.1016/s2213-2600(19)30415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for disease control and prevention. Outbreak of lung injury associated with E-cigarette use, or vaping2020 (accessed 3 Feb 2021). Available from: 〈https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html〉.
- 25.Canistro D., Vivarelli F., Cirillo S., Babot Marquillas C., Buschini A., Lazzaretti M., et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017;7(1):2028. doi: 10.1038/s41598-017-02317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaal C.M., Bora-Singhal N., Kumar D.M., Chellappan S.P. Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer. Mol. Cancer. 2018;17(1):149. doi: 10.1186/s12943-018-0901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaisar M.A., Villalba H., Prasad S., Liles T., Sifat A.E., Sajja R.K., et al. Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: is Metformin a viable countermeasure. Redox Biol. 2017;13:353–362. doi: 10.1016/j.redox.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faulcon L.M., Rudy S., Limpert J., Wang B., Murphy I. Adverse experience reports of seizures in youth and young adult electronic nicotine delivery systems users. J. Adolesc. Health. 2020;66:15–17. doi: 10.1016/j.jadohealth.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Hua M., Sadah S., Hristidis V., Talbot P. Health effects associated with electronic cigarette use: automated mining of online forums. J. Med. Internet Res. 2020;22(1) doi: 10.2196/15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franzen K.F., Willig J., Cayo Talavera S., Meusel M., Sayk F., Reppel M., et al. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc. Med. 2018;23(5):419–425. doi: 10.1177/1358863x18779694. [DOI] [PubMed] [Google Scholar]
- 31.Lee W.H., Ong S.G., Zhou Y., Tian L., Bae H.R., Baker N., et al. Modeling cardiovascular risks of E-cigarettes with human-induced pluripotent stem cell-derived endothelial cells. J. Am. Coll. Cardiol. 2019;73(21):2722–2737. doi: 10.1016/j.jacc.2019.03.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farsalinos K.E., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int. J. Environ. Res. Public Health. 2014;11(4):4356–4373. doi: 10.3390/ijerph110404356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cravo A.S., Bush J., Sharma G., Savioz R., Martin C., Craige S., et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regul. Toxicol. Pharmacol. 2016;81(Suppl 1) doi: 10.1016/j.yrtph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Jeong W., Choi D.W., Kim Y.K., Lee H.J., Lee S.A., Park E.C., et al. Vol. 91. 2020. Associations of electronic and conventional cigarette use with periodontal disease in South Korean adults; pp. 55–64. (J. Periodontol.). [DOI] [PubMed] [Google Scholar]
- 35.Jose T., Croghan I.T., Hays J.T., Schroeder D.R., Warner D.O. Electronic cigarette use is not associated with COVID-19 diagnosis. J Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211024391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cibella F., Campagna D., Caponnetto P., Amaradio M.D., Caruso M., Russo C., et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin. Sci. 2016;130(21):1929–1937. doi: 10.1042/cs20160268. [DOI] [PubMed] [Google Scholar]
- 37.Hajek P., Phillips-Waller A., Przulj D., Pesola F., Myers Smith K., Bisal N., et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N. Engl. J. Med. 2019;380(7):629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 38.Polosa R., Morjaria J.B., Caponnetto P., Caruso M., Campagna D., Amaradio M.D., et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov. Med. 2016;21(114):99–108. [PubMed] [Google Scholar]
- 39.Veldheer S., Yingst J., Midya V., Hummer B., Lester C., Krebs N., et al. Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial. Addict. Behav. 2019;91:95–101. doi: 10.1016/j.addbeh.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Ruiz C.D., O’Connell G., Graff D.W., Yan X.S. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regul. Toxicol. Pharmacol. 2017;87:36–53. doi: 10.1016/j.yrtph.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Walele T., Bush J., Koch A., Savioz R., Martin C., O’Connell G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regul. Toxicol. Pharmacol. 2018;92:226–238. doi: 10.1016/j.yrtph.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Lanspa M.J., Blagev D.P., Callahan S.J. Use of e-cigarettes for smoking cessation. JAMA. 2021;325(10):1006. doi: 10.1001/jama.2020.27207. [DOI] [PubMed] [Google Scholar]
- 43.Hendlin Y.H., Vora M., Elias J., Ling P.M. Financial conflicts of interest and stance on tobacco harm reduction: a systematic review. Am. J. Public Health. 2019;109(7):e1–e8. doi: 10.2105/ajph.2019.305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pisinger C., Godtfredsen N., Bender A.M. A conflict of interest is strongly associated with tobacco industry-favourable results, indicating no harm of e-cigarettes. Prev. Med. 2019;119:124–131. doi: 10.1016/j.ypmed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Khan M.S., Khateeb F., Akhtar J., Khan Z., Lal A., Kholodovych V., et al. Organizing pneumonia related to electronic cigarette use: a case report and review of literature. Clin. Respir. J. 2018;12(3):1295–1299. doi: 10.1111/crj.12775. [DOI] [PubMed] [Google Scholar]
- 46.Xian S., Chen Y. E-cigarette users are associated with asthma disease: a meta-analysis. Clin. Respir. J. 2021;15(5):457–466. doi: 10.1111/crj.13346. [DOI] [PubMed] [Google Scholar]
- 47.Wills T.A., Soneji S.S., Choi K., Jaspers I., Tam E.K. E-cigarette use and respiratory disorders: an integrative review of converging evidence from epidemiological and laboratory studies. Eur. Respir. J. 2021;57(1) doi: 10.1183/13993003.01815-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickman E., Jaspers I. Current E-cigarette research in the context of asthma. Curr. Allergy Asthma Rep. 2020;20(10):62. doi: 10.1007/s11882-020-00952-2. [DOI] [PubMed] [Google Scholar]
- 49.Blount B.C., Karwowski M.P., Shields P.G., Morel-Espinosa M., Valentin-Blasini L., Gardner M., et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N. Engl. J. Med. 2020;382(8):697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strongin R.M. Toxic ketene gas forms on vaping vitamin E acetate prompting interest in its possible role in the EVALI outbreak. Proc. Natl. Acad. Sci. 2020;117(14):7553–7554. doi: 10.1073/pnas.2003384117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Food and Drug Administration. CFR - Code of Federal Regulations Title 21, Volume 3 - Part 172. Revised as of May 28, 2020(accessed 3 Feb 2021). Available from: 〈https://www.ecfr.gov/cgi-bin/text-idx?SID=11d1b7724a191200c67a85132474aaed&mc=true&tpl=/ecfrbrowse/Title21/21tab_02.tpl〉.
- 52.Cheng T. Chemical evaluation of electronic cigarettes. Tob. Control. 2014;23(Suppl 2) doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madison M.C., Landers C.T., Gu B.H., Chang C.Y., Tung H.Y., You R., et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019;129(10):4290–4304. doi: 10.1172/jci128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maddock S.D., Cirulis M.M., Callahan S.J., Keenan L.M., Pirozzi C.S., Raman S.M., et al. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 55.Viswam D., Trotter S., Burge P.S., Walters G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyashita L., Suri R., Dearing E., Mudway I., Dove R.E., Neill D.R., et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018;51(2) doi: 10.1183/13993003.01592-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kooragayalu S., El-Zarif S., Jariwala S. Vaping associated pulmonary injury (VAPI) with superimposed mycoplasma pneumoniae infection. Respir. Med. Case. Rep. 2020;29 doi: 10.1016/j.rmcr.2020.100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajantri B., Venkatram S., Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection. J. Clin. Med. Res. 2018;10(7):535–544. doi: 10.14740/jocmr3421w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrington-Trimis J.L., Samet J.M., McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA. 2014;312(23):2493–2494. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hiemstra P.S., McCray P.B., Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015;45(4):1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitsett J.A. Airway epithelial differentiation and mucociliary clearance. Ann. Am. Thorac. Soc. 2018;15(Suppl 3) doi: 10.1513/AnnalsATS.201802-128AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin V.Y., Fain M.D., Jackson P.L., Berryhill T.F., Wilson L.S., Mazur M., et al. Vaporized E-cigarette liquids induce ion transport dysfunction in airway epithelia. Am. J. Respir. Cell Mol. Biol. 2019;61(2):162–173. doi: 10.1165/rcmb.2017-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Arcos I., Geraghty P., Baumlin N., Campos M., Dabo A.J., Jundi B., et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 2016;71(12):1119–1129. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.In vitro systems toxicology assessment of nonflavored e-cigarette liquids in primary lung epithelial cells. Appl. In Vitro Toxicol. 2017;3(1):41–55. doi: 10.1089/aivt.2016.0040. [DOI] [Google Scholar]
- 65.Chaumont M., van de Borne P., Bernard A., Van Muylem A., Deprez G., Ullmo J., et al. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316(5) doi: 10.1152/ajplung.00492.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Farrell H.E., Brown R., Brown Z., Milijevic B., Ristovski Z.D., Bowman R.V., et al. E-cigarettes induce toxicity comparable to tobacco cigarettes in airway epithelium from patients with COPD. Toxicol. In Vitro. 2021;75 doi: 10.1016/j.tiv.2021.105204. [DOI] [PubMed] [Google Scholar]
- 67.Anderson C., Majeste A., Hanus J., Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. 2016;154(2):332–340. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 68.Zhao J., Zhang Y., Sisler J.D., Shaffer J., Leonard S.S., Morris A.M., et al. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018;344:549–557. doi: 10.1016/j.jhazmat.2017.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLOS One. 2015;10(2) doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatterjee S., Tao J.Q., Johncola A., Guo W., Caporale A., Langham M.C., et al. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317(2) doi: 10.1152/ajplung.00110.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manevski M., Muthumalage T., Devadoss D., Sundar I.K., Wang Q., Singh K.P., et al. Cellular stress responses and dysfunctional mitochondrial-cellular senescence, and therapeutics in chronic respiratory diseases. Redox Biol. 2020;33 doi: 10.1016/j.redox.2020.101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo-Parke H., Linden D., Weldon S., Kidney J.C., Taggart C.C. Mechanisms of virus-induced airway immunity dysfunction in the pathogenesis of COPD disease, progression, and exacerbation. Front. Immunol. 2020;11:1205. doi: 10.3389/fimmu.2020.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corriden R., Moshensky A., Bojanowski C.M., Meier A., Chien J., Nelson R.K., et al. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am. J. Physiol. Cell Physiol. 2020;318(1) doi: 10.1152/ajpcell.00045.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 75.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18(2):134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 76.Clapp P.W., Pawlak E.A., Lackey J.T., Keating J.E., Reeber S.L., Glish G.L., et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313(2) doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin E.M., Clapp P.W., Rebuli M.E., Pawlak E.A., Glista-Baker E., Benowitz N.L., et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016;311(1):L135–L144. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reidel B., Radicioni G., Clapp P.W., Ford A.A., Abdelwahab S., Rebuli M.E., et al. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am. J. Respir. Crit. Care Med. 2018;197(4):492–501. doi: 10.1164/rccm.201708-1590OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mravec B., Tibensky M., Horvathova L., Babal P. E-cigarettes and cancer risk. Cancer Prev. Res. 2020;13(2):137–144. doi: 10.1158/1940-6207.Capr-19-0346. [DOI] [PubMed] [Google Scholar]
- 80.Symes Y.R., Ribisl K.M., Boynton M.H., Westmaas J.L., Mayer D.K., Golden S.D. Dual cigarette and e-cigarette use in cancer survivors: an analysis using population assessment of tobacco health (PATH) data. J. Cancer Surviv. 2019;13(2):161–170. doi: 10.1007/s11764-019-0735-y. [DOI] [PubMed] [Google Scholar]
- 81.Sanford N.N., Sher D.J., Xu X., Aizer A.A., Mahal B.A. Trends in smoking and e-cigarette use among US patients with cancer, 2014-2017. JAMA Oncol. 2019;5(3):426–428. doi: 10.1001/jamaoncol.2018.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu V., Rahimy M., Korrapati A., Xuan Y., Zou A.E., Krishnan A.R., et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hecht S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999;91(14):1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 84.Kim H.J., Shin H.S. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2013;1291:48–55. doi: 10.1016/j.chroma.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 85.Lee H.W., Park S.H., Weng M.W., Wang H.T., Huang W.C., Lepor H., et al. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA. 2018;115(7):E1560–e9. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18(1–2):43–73. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zahedi A., Phandthong R., Chaili A., Remark G., Talbot P. Epithelial-to-mesenchymal transition of A549 lung cancer cells exposed to electronic cigarettes. Lung Cancer. 2018;122:224–233. doi: 10.1016/j.lungcan.2018.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perumal D., Pillai S., Nguyen J., Schaal C., Coppola D., Chellappan S.P. Nicotinic acetylcholine receptors induce c-Kit ligand/stem cell factor and promote stemness in an ARRB1/ β-arrestin-1 dependent manner in NSCLC. Oncotarget. 2014;5(21):10486–10502. doi: 10.18632/oncotarget.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ring Madsen L., Vinther Krarup N.H., Bergmann T.K., Bærentzen S., Neghabat S., Duval L., et al. A cancer that went up in smoke: pulmonary reaction to e-cigarettes imitating metastatic cancer. Chest. 2016;149(3):e65–e67. doi: 10.1016/j.chest.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 90.Andreasen J.T., Redrobe J.P. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav. Pharmacol. 2009;20(3):286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- 91.Yuan M., Cross S.J., Loughlin S.E., Leslie F.M. Nicotine and the adolescent brain. J. Physiol. 2015;593(16) doi: 10.1113/jp270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xia W., Veljkovic E., Koshibu K., Peitsch M.C., Hoeng J. Neurobehavioral effects of selected tobacco constituents in rodents following subchronic administration. Eur. J. Pharmacol. 2019;865 doi: 10.1016/j.ejphar.2019.172809. [DOI] [PubMed] [Google Scholar]
- 93.Michael Felberbaum. FDA In Brief: FDA encourages continued submission of reports related to seizures following e-cigarette use as part of agency’s ongoing scientific investigation of potential safety issue2019 (accessed 4 Feb 2021). Available from: 〈https://www.fda.gov/news-events/fda-brief/fda-brief-fda-encourages-continued-submission-reports-related-seizures-following-e-cigarette-use〉.
- 94.Quezada T., Sianati B., Valeriano J. Vaping and seizure risk: a case report. Neurology. 2020;94(15 Supplement):1814. [Google Scholar]
- 95.Oster J., Tatum P., Monigan C., Kryzanski J. E-cigarette use (Vaping) causes seizures on EEG detected by responsive neurstimulation: evidence from electrocorticography. Neurology. 2020;94(15 Supplement):1809. [Google Scholar]
- 96.Drake P., Driscoll A.K., Mathews T.J. Cigarette smoking during pregnancy: United States, 2016. NHCS Data Brief. 2018;305:1–8. [PubMed] [Google Scholar]
- 97.Wagner N.J., Camerota M., Propper C. Prevalence and perceptions of electronic cigarette use during pregnancy. Matern. Child Health J. 2017;21(8):1655–1661. doi: 10.1007/s10995-016-2257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen T., Li G.E., Chen H., Cranfield C.G., McGrath K.C., Gorrie C.A. Maternal E-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol. 2018;31(7):601–611. doi: 10.1021/acs.chemrestox.8b00084. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen T., Li G.E., Chen H., Cranfield C.G., McGrath K.C., Gorrie C.A. Neurological effects in the offspring after switching from tobacco cigarettes to e-cigarettes during pregnancy in a mouse model. Toxicol. Sci. 2019:191–200. doi: 10.1093/toxsci/kfz194. [DOI] [PubMed] [Google Scholar]
- 100.Mazzone P., Tierney W., Hossain M., Puvenna V., Janigro D., Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int. J. Environ. Res. Public Health. 2010;7(12):4111–4126. doi: 10.3390/ijerph7124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prasad S., Sajja R.K., Kaisar M.A., Park J.H., Villalba H., Liles T., et al. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradford S.T., Stamatovic S.M., Dondeti R.S., Keep R.F., Andjelkovic A.V. Nicotine aggravates the brain postischemic inflammatory response. Am. J. Physiol. Heart Circ. Physiol. 2011;300(4):H1518–H1529. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paulson J.R., Yang T., Selvaraj P.K., Mdzinarishvili A., Van der Schyf C.J., Klein J., et al. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J. Pharmacol. Exp. Ther. 2010;332(2):371–379. doi: 10.1124/jpet.109.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/cir.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 105.Darville A., Hahn E.J. E-cigarettes and atherosclerotic cardiovascular disease: what clinicians and researchers need to know. Curr. Atheroscler. Rep. 2019;21(5):15. doi: 10.1007/s11883-019-0777-7. [DOI] [PubMed] [Google Scholar]
- 106.Qasim H., Karim Z.A., Rivera J.O., Khasawneh F.T., Alshbool F.Z. Impact of electronic cigarettes on the cardiovascular system. J. Am. Heart Assoc. 2017;6(9) doi: 10.1161/jaha.117.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vansickel A.R., Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob. Res. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vlachopoulos C., Ioakeimidis N., Abdelrasoul M., Terentes-Printzios D., Georgakopoulos C., Pietri P., et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J. Am. Coll. Cardiol. 2016;67(23):2802–2803. doi: 10.1016/j.jacc.2016.03.569. [DOI] [PubMed] [Google Scholar]
- 109.Barber K.E., Ghebrehiwet B., Yin W., Rubenstein D.A. Vol. 10. 2017. Endothelial cell inflammatory reactions are altered in the presence of e-cigarette extracts of variable nicotine; pp. 124–133. (Cell. Mol. Bioeng.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Putzhammer R., Doppler C., Jakschitz T., Heinz K., Förste J., Danzl K., et al. Vapours of US and EU market leader electronic cigarette brands and liquids are cytotoxic for human vascular endothelial cells. PLOS One. 2016;11(6) doi: 10.1371/journal.pone.0157337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hom S., Chen L., Wang T., Ghebrehiwet B., Yin W., Rubenstein D.A. Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets. 2016;27(7):694–702. doi: 10.3109/09537104.2016.1158403. [DOI] [PubMed] [Google Scholar]
- 112.Shi H., Fan X., Horton A., Haller S.T., Kennedy D.J., Schiefer I.T., et al. The effect of electronic-cigarette vaping on cardiac function and angiogenesis in mice. Sci. Rep. 2019;9(1):4085. doi: 10.1038/s41598-019-40847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuntic M., Oelze M., Steven S., Kröller-Schön S., Stamm P., Kalinovic S., et al. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2) Eur. Heart J. 2019:2472–2483. doi: 10.1093/eurheartj/ehz772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hasan K.M., Munoz A., Tumoyan H., Parveen M., Espinoza-Derout J., Shao X.M., et al. Adverse effects of fetal exposure of electronic-cigarettes and high-fat diet on male neonatal hearts. Exp. Mol. Pathol. 2021;118 doi: 10.1016/j.yexmp.2020.104573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2016.
- 116.Yang I., Sandeep S., Rodriguez J. The oral health impact of electronic cigarette use: a systematic review. Crit. Rev. Toxicol. 2020;50(2):97–127. doi: 10.1080/10408444.2020.1713726. [DOI] [PubMed] [Google Scholar]
- 117.Nides M.A., Leischow S.J., Bhatter M., Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav. 2014;38(2):265–274. doi: 10.5993/ajhb.38.2.12. [DOI] [PubMed] [Google Scholar]
- 118.Etter J.F. Electronic cigarettes: a survey of users. BMC Public Health. 2010;10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control. 2010;19(2):98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 120.King J.L., Reboussin B.A., Wiseman K.D., Ribisl K.M., Seidenberg A.B., Wagoner K.G., et al. Adverse symptoms users attribute to e-cigarettes: results from a national survey of US adults. Drug Alcohol Depend. 2019;196:9–13. doi: 10.1016/j.drugalcdep.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hughes A., Hendrickson R.G. An epidemiologic and clinical description of e-cigarette toxicity. Clin. Toxicol. 2019;57(4):287–293. doi: 10.1080/15563650.2018.1510503. [DOI] [PubMed] [Google Scholar]
- 122.Baweja R., Curci K.M., Yingst J., Veldheer S., Hrabovsky S., Wilson S.J., et al. Views of experienced electronic cigarette users. Addict. Res. Theory. 2016;24(1):80–88. doi: 10.3109/16066359.2015.1077947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho J.H. The association between electronic-cigarette use and self-reported oral symptoms including cracked or broken teeth and tongue and/or inside-cheek pain among adolescents: a cross-sectional study. PLOS One. 2017;12(7) doi: 10.1371/journal.pone.0180506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yao T., Max W., Sung H.Y., Glantz S.A., Goldberg R.L., Wang J.B., et al. Relationship between spending on electronic cigarettes, 30-day use, and disease symptoms among current adult cigarette smokers in the U.S. PLOS One. 2017;12(11) doi: 10.1371/journal.pone.0187399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bartram A., Jones N., Endersby S. Lichenoid eruption associated with use of an e-cigarette. Br. J. Oral Maxillofac. Surg. 2016;54(4):475. doi: 10.1016/j.bjoms.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Cant A., Collard B., Cunliffe D. Electronic cigarettes: necrotic ulcer. Br. Dent. J. 2017;222(4):226. doi: 10.1038/sj.bdj.2017.150. [DOI] [PubMed] [Google Scholar]
- 127.Bardellini E., Amadori F., Conti G., Majorana A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol. Scand. 2018;76(3):226–228. doi: 10.1080/00016357.2017.1406613. [DOI] [PubMed] [Google Scholar]
- 128.Li Q., Zhan Y., Wang L., Leischow S.J., Zeng D.D. Analysis of symptoms and their potential associations with e-liquids’ components: a social media study. BMC Public Health. 2016;16(1):674. doi: 10.1186/s12889-016-3326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tongyao F, Mondal P, Foulds J, Du P. (2018). Flavor use in a large cohort of long-term electronic cigarette users: implications for future pulmonary research. D22 Cutting edge research in smoking cessation and e-cigarettes. p. A6244-A6244.
- 130.Volansky, P.M., Endara-Bravo, A.S., (2015). Acute uvulitis secondary to electronic cigarette use. A40 a hard day's night: drug induced lung disease. p. A1556-A1556.
- 131.ArRejaie A.S., Al-Aali K.A., Alrabiah M., Vohra F., Mokeem S.A., Basunbul G., et al. Proinflammatory cytokine levels and peri-implant parameters among cigarette smokers, individuals vaping electronic cigarettes, and non-smokers. J. Periodontol. 2019;90(4):367–374. doi: 10.1002/jper.18-0045. [DOI] [PubMed] [Google Scholar]
- 132.Al-Aali K.A., Alrabiah M., ArRejaie A.S., Abduljabbar T., Vohra F., Akram Z. Peri-implant parameters, tumor necrosis factor-alpha, and interleukin-1 beta levels in vaping individuals. Clin. Implant Dent. Relat. Res. 2018;20(3):410–415. doi: 10.1111/cid.12597. [DOI] [PubMed] [Google Scholar]
- 133.Wadia R., Booth V., Yap H.F., Moyes D.L. A pilot study of the gingival response when smokers switch from smoking to vaping. Br. Dent. J. 2016;221(11):722–726. doi: 10.1038/sj.bdj.2016.914. [DOI] [PubMed] [Google Scholar]
- 134.Vora M.V., Chaffee B.W. Tobacco-use patterns and self-reported oral health outcomes: a cross-sectional assessment of the population assessment of tobacco and health study, 2013-2014. J. Am. Dent. Assoc. 2019;150(5) doi: 10.1016/j.adaj.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]