Abstract
Background & Aims:
Patients admitted to the hospital for alcoholic hepatitis (AH) are at increased risk of readmission and death. We aimed to identify factors associated with readmission, alcohol relapse, and mortality.
Methods:
We performed a retrospective analysis of consecutive patients admitted with AH to a tertiary care hospital from 1999 through 2016 (test cohort, n=135). We validated our findings in a prospective analysis of patients in a multi-center AH research consortium from 2013 through 2017 (validation cohort, n=159). Alcohol relapse was defined as any amount of alcohol consumption within 30 days after hospital discharge. Early alcohol rehabilitation was defined as residential or outpatient addiction treatment or mutual support group participation within 30 days after hospital discharge.
Results:
Thirty-day readmission rates were 30% in both cohorts. Alcohol relapse rates were 37% in the test and 34% in the validation cohort. Following hospital discharge, 27 patients (20%) in the test cohort and 19 patients (16%) in the validation cohort attended early alcohol rehabilitation. There were 53 deaths (39%) in a median follow-up time of 2.8 years and 42 deaths (26%) in a median follow-up time of 1.3 years, respectively. In the test cohort, early alcohol rehabilitation reduced odds for 30-day readmission (adjusted odds ratios [AOR] 0.16; 95% CI, 0.04–0.65; P=.01), 30-day alcohol relapse (AOR, 0.11; 95% CI, 0.02–0.53; P<.001), and death (adjusted hazard ratio [AHR], 0.20; 95% CI, 0.05–0.56; P=.001). In the validation cohort early alcohol rehabilitation reduced odds for 30-day readmission (AOR, 0.30; 95% CI, 0.09–0.98; P=.04), 30-day alcohol relapse (AOR 0.09; 95% CI, 0.01–0.73; P=.02), and death (AHR, 0.20; 95% CI, 0.01–0.94; P=.04). A model combining alcohol rehabilitation and bilirubin identified patients with readmission to the hospital within 30 days with an area under the receiver operating characteristic curve of 0.73.
Conclusions:
In an analysis from two cohorts of patients admitted with AH, early alcohol rehabilitation can reduce risk of hospital readmission, alcohol relapse, and death and should be considered as a quality indicator in AH hospitalization treatment.
Keywords: chemical dependency, addiction treatment, alcohol use disorder, alcohol abstinence
Introduction
Alcoholic hepatitis or alcohol-associated hepatitis (AH) is the most severe form of alcohol related liver disease (ALD) and accounts for 0.71% of all admissions in the United States with in-hospital mortality rate up to 46%.1, 2 Patients surviving an admission for AH are at increased risk of readmission and subsequent death. Previous studies have mainly evaluated short-term mortality outcomes,3, 4 while other clinically important outcomes i.e. alcohol relapse and hospital readmission after surviving an index AH admission have not been well studied.
In patients surviving an AH admission, preventing alcohol relapse is crucial because long-term survival is dependent on abstinence rather than severity of liver disease.5 Medical therapy with corticosteroids improve short-term survival and no pharmacologic intervention has been shown to improve long-term survival.4 Thus, it is important to prevent alcohol relapse after hospital discharge to ensure long-term alcohol abstinence. Previous studies reported alcohol relapse rates in this population as high as 25% at 1-year5 and 60% at 5-years follow up.6 Multidisciplinary teams including addiction specialists are recommended in treating patients with ALD.2 However, this integrated approach is prevalent only in liver transplant settings.7 A gap in knowledge is whether participation in alcohol rehabilitation programs after hospital discharge can improve clinical outcomes in patients with AH.
In addition to early alcohol relapse, early hospital readmission is another important clinical outcome of AH that has not been well studied. Hospital readmissions are associated with increased costs to the healthcare system, patient mortality, and may be preventable.8 Therefore, 30-day readmission rate has become a key quality indicator used by Center for Medicare and Medicaid Services (CMS) to assess reimbursement penalties.9 For these reasons, it is pertinent to evaluate the rate of 30-day hospital readmission in patients with AH.
The aims of the study were to determine the rates and predictors of 30-day readmission, 30-day alcohol relapse, and mortality after surviving an index AH admission.
Patients and Methods
Patient cohorts
The test cohort (N=135) was derived retrospectively from consecutive patients admitted with AH at Mayo Clinic (Rochester, MN) between 1999 and 2016. A validation cohort (n=159) was derived prospectively from the ongoing NIAAA Translational Research and Evolving Alcoholic Hepatitis Treatment (TREAT) consortium 001 study including patients admitted with AH at three tertiary care hospitals: Mayo Clinic (Rochester, MN), Indiana University (Indianapolis, IN), and Virginia Commonwealth University (Richmond, VA), between 2013 and 2017. The consortium design has been reported previously.10, 11 Overlapping patients were removed from the test cohort (n=19). Exclusion criteria include 1) death during index admission (n=15 and n=5 in test and validation cohort) and 2) Patients who died or were lost to follow within 1 month after hospital discharge (n=29 and n=50 in test and validation cohort) and 3) Patients who had previously undergone liver transplantation (n=3 in test cohort). The study was approved by the institutional review boards (IRB) for all study sites. Some patients included in the validation cohort have been included in other papers published previously by the TREAT consortium.10–14
Clinical criteria for alcoholic hepatitis diagnosis
As per NIAAA clinical criteria,15 the AH diagnosis was based on the average daily ethanol consumption of more than 40 g/day for women and more than 60 g/day for men for a minimum of 6 months and within the 6 weeks before study enrollment; and on clinical evaluation and appropriate laboratory testing as defined by a total bilirubin concentration greater than 3 mg/dL and an aspartate aminotransferase (AST) concentration greater than 50 U/L, and AST to alanine aminotransferase (ALT) ratio of > 1.5. When the diagnosis of AH remained in question, a liver biopsy was obtained for confirmation of AH. Patients with suspected or proven cirrhosis were eligible for enrollment if they met inclusion criteria for AH. Individuals with positive hepatitis B or hepatitis C virus markers were eligible for enrollment to include the full spectrum of AH patients seen in clinical practice. However, cases with current or previous diagnosis of autoimmune hepatitis, drug induced liver disease, hemochromatosis, or Wilson disease were excluded.
Data collection at the time of admission
Baseline data were collected on (1) demographic factors, body mass index (BMI) and average number of alcohol drinks per day (1 standard drink equals 12–14 grams of pure alcohol) (2) hepatic decompensation (ascites, variceal bleeding, hepatic encephalopathy); (3) laboratory values (4) AH severity scores: Maddrey discriminant (mDF); Model for End-Stage Liver Disease (MELD); Age, serum bilirubin, international normalized ratio (INR), and serum Creatinine (ABIC); and Child-Turcotte-Pugh (CTP) scores16, 17 and (5) the use of alcohol relapse prevention medications during admission.
Follow up
In test cohort, subjects were followed until death or last follow up date or August 5, 2017. Alcohol relapse was based on patient’s report and/or detection of ethanol or its metabolites in urine or blood. In the validation cohort, subjects were followed for 1 year after enrollment. Follow up included telephone call at 1 month, clinic visit at 6 months, and clinic visit at 1 year, in addition to regular clinical follow up. At each follow up visit, trained interviewers asked subjects about attendance to alcohol rehabilitation, and hospital readmission. The latter was confirmed by review of the medical records. At each follow up visit, subjects self-reported alcohol consumption in the past 30 days using a validated timeline followback questionnaire.18 Alcohol biomarkers (i.e. blood ethanol level or urine ethyl glucuronide) were not routinely collected and were collected at the discretion of the medical providers.
Outcomes of interest
The 30-day hospital readmission was defined as any hospital admission within 30 days after discharge. Alcohol relapse was defined as any alcohol consumption within 30 days after hospital discharge. Early alcohol rehabilitation was defined as attending either residential or outpatient addiction treatment or mutual support group (i.e. alcoholics anonymous) within 30 days after hospital discharge. Mortality status was collected at the time of last follow up or August 5, 2017 for test cohort, and at last follow up or at one year for the validation cohort.
Statistical analysis
The rates of 30-day hospital readmission, 30-day alcohol relapse, and death at last follow up were evaluated. Multivariable Logistic regression analysis was performed to determine independent predictors for 30-day hospital readmission and 30-day alcohol relapse. The prognostic model for 30-day hospital readmission (AH readmission score) was developed using independent predictors from the test cohort and validated in the validation cohort. The performance of the AH readmission score was evaluated using the area under the receiver operating characteristics (AUC). Multivariable Cox regression analysis was performed to determine independent predictors of mortality. Landmark analysis was performed, i.e. all patients were alive at 30 days after hospital discharge, thus limiting the immortal time bias associated with alcohol rehabilitation attendance. Variables with P<.05 in the univariate analysis were included in the multivariable model to identify independent predictors for each outcome. All statistical analyses were performed using JMP statistical software, version 9.0 (SAS Institute Inc, Cary, NC). All comparisons were 2-sided. P<0.05 was considered statistically significant.
Results
Baseline characteristics
After excluding patients who were lost to follow up, there were 135 and 159 patients in the test and validation cohorts respectively. Baseline clinical characteristics of both cohorts are shown in Table 1. In the test cohort, mean age was 48.0±10.2 years and mean MELD score was 20.5±7.2. In the validation cohort, mean age was 50.0±11.0 years old and mean MELD score was 21.8±7.2. Alcohol relapse prevention medications (all were gabapentin) were prescribed to 10(7.4%), and 7(4.4%) patients in test and validation cohorts respectively. Of 10 patients who received gabapentin in test cohort, five were for alcohol use disorder and the others were for combination of alcohol use disorder and neuropathic pain.
Table 1.
Baseline characteristics of retrospective and prospective cohort
| Test cohort | Validation cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| All patients (n=135) | Readmission in 30 days (n=41) | No readmission (n=94) | P* | All patients (n=159) | Readmission in 30 days (n=49) | No readmission (n=110) | P** | |
| Age, years (mean±SD) | 48.0±10.2 | 47.2±8.7 | 48.4±10.8 | 0.50 | 50.0±11.0 | 48.4±12.0 | 50.8±10.5 | 0.24 |
| Male (n, %) | 90 (66.7%) | 29 (70.7%) | 61 (64.9%) | 0.51 | 92 (57.9%) | 29 (59.2%) | 63 (57.3%) | 0.82 |
| BMI, kg/m2 (mean±SD) | 28.6±7.4 | 28.4±7.7 | 28.7±7.3 | 0.86 | 28.9±7.0 | 28.2±6.0 | 29.2±7.3 | 0.33 |
| White (n, %) | 130 (96.3%) | 39 (95.1%) | 91 (96.8%) | 0.64 | 137 (86.2%) | 41 (83.7%) | 96 (87.3%) | 0.54 |
| Married or living with significant other (n, %) | 42 (31.1%) | 14 (33.3%) | 28 (29.8%) | 0.61 | 59 (40.1%) | 21 (44.7%) | 38 (38.0%) | 0.44 |
| College degree or above (n, %) | n/a | n/a | n/a | 73 (49.7%) | 25 (53.2%) | 48 (48.0%) | 0.56 | |
| Average number of drinks/day (median, IQR) | n/a | n/a | n/a | 8 (5, 13) | 7 (5, 11) | 9 (5, 15) | 0.12 | |
| Hepatic decompensation (n, %) | 49 (36.3%) | 22 (53.7%) | 27 (28.7%) | 0.006 | 106 (66.7%) | 35 (71.4%) | 71 (64.6%) | 0.40 |
| - Hepatic encephalopathy (n, %) | 17 (12.6%) | 7 (17.1%) | 10 (10.6%) | 0.30 | 40 (25.2%) | 10 (20.4%) | 30 (27.3%) | 0.35 |
| - Variceal bleeding (n, %) | 3 (2.2%) | 2 (4.9%) | 1 (1.7%) | 0.22 | 24 (15.1%) | 8 (16.3%) | 16 (14.6%) | 0.77 |
| - Ascites (n, %) | 53 (39.3%) | 20 (48.8%) | 33 (35.1%) | 0.13 | 90 (56.6%) | 29 (59.2%) | 61 (55.5%) | 0.66 |
| Use of alcohol relapse prevention medication | 10 (7.4%) | 5 (12.2%) | 5 (5.3%) | 0.17 | 7 (4.4%) | 0 (0%) | 7 (6.4%) | 0.10 |
| Labs | ||||||||
| Platelet, 109/L (median, IQR) | 147 (86, 196) | 159 (122, 205) | 129 (78, 190) | 0.02 | 127 (87, 184) | 119 (74, 184) | 130 (87, 187) | 0.76 |
| Albumin, g/dL (median, IQR) | 3.0 (2.7, 3.4) | 3.1 (2.5, 3.3) | 3.0 (2.7, 3.5) | 0.17 | 2.8 (2.4, 3.2) | 2.8 (2.3, 3.2) | 2.8 (2.4, 3.3) | 0.51 |
| Creatinine, mg/dL (median, IQR) | 0.7 (0.6, 1.1) | 0.6 (0.5, 1.0) | 0.7 (0.6, 1.1) | 0.10 | 0.7 (0.6, 1.1) | 0.82 (0.63, 1.2) |
0.7 (0.6, 1.0) | 0.45 |
| Total bilirubin, mg/dL (median, IQR) | 8.7 (4.0, 19.6) | 14.4 (6.3, 23.5) | 6.9 (3.7, 14.3) | 0.004 | 9.6 (3.9, 19.1) | 13.3 (5.5, 19.1) | 8.3 (3.5, 19.4) | 0.25 |
| INR (median, IQR) | 1.4 (1.2, 1.7) | 1.5 (1.3, 1.8) | 1.3 (1.2, 1.7) | 0.21 | 1.7 (1.4, 2.1) | 1.8 (1.5, 2.1) | 1.6 (1.3, 2.1) | 0.19 |
| PT, seconds (median, IQR) | 15 (13, 19) | 15 (13, 18) | 15 (12, 19) | 0.52 | 20 (16, 23) | 20 (18, 23) | 19 (15, 27) | 0.07 |
| Severity scores | ||||||||
| MELD score (mean±SD) | 20.5±7.2 | 21.6±6.8 | 20.0±7.4 | 0.22 | 21.8±7.2 | 23.3±6.7 | 21.2±7.3 | 0.04 |
| CTP score (mean±SD) | 8.9±1.5 | 9.3±1.3 | 8.8±1.6 | 0.03 | 9.5±1.8 | 9.9±1.5 | 9.4±1.9 | 0.04 |
| mDF score (median, IQR) | 30 (16, 50) | 37 (19, 56) | 25 (14, 50) | 0.11 | 37 (15, 61) | 45 (26, 63) | 34 (13, 57) | 0.15 |
| mDF score≥32 (n, %) | 64 (49.2%) | 25 (62.5%) | 39 (43.3%) | 0.044 | 89 (56.3%) | 31 (64.6%) | 58 (52.7%) | 0.17 |
| ABIC score (mean±SD) | 7.4±1.5 | 7.6±1.4 | 7.3±1.6 | 0.39 | 7.8±1.3 | 7.7±1.3 | 7.8±1.3 | 0.82 |
| Outcomes | ||||||||
| Hospital stay, days (median, IQR) | 7 (4, 12) | 7 (4, 12) | 6 (4,12) | 0.74 | 6 (3, 10) | 6 (3, 10) | 7 (3, 10) | 0.75 |
| Attendance to alcohol rehabilitation within 30 days after discharge (n, %) | 27 (20%) | 3 (7.3%) | 24 (25.5%) | 0.02 | 19 (16.0%) (n=119) | 4 (8.2%) (n=49) | 15 (21.4%) (n=70) | 0.04 |
| Alcohol relapse within 30 days after discharge (n, %) | 50 (37%) | 23 (56.1%) | 27 (28.7%) | 0.003 | 48 (34.0%) (n=141) | 27 (57.5%) (n=47) | 21 (22.3%) (n=94) | <0.001 |
P-value comparing those who readmitted vs. who did not readmit in test cohort
P-value comparing those who readmitted vs. who did not readmit in validation cohort n/a = data not available in test cohort
Abbreviations: ABIC = Age, serum Bilirubin, international normalized ratio, and serum Creatinine, BMI = body mass index, CTP= Child-Turcotte-Pugh Score, INR = international normalized ratio, mDF = Maddrey’s Discriminant Function, MELD = Model for End-Stage Liver Disease, PT = prothrombin time
Alcohol rehabilitation within 30 days after hospital discharge
In the test cohort, 96 out of 135(71.1%) patients were referred for evaluation by alcohol addiction specialists (either board certified addiction psychiatrists or licensed alcohol and drug counselor) during index admission, and complete evaluation was performed in 87 out of 135(64.4%) patients. The main reason for incomplete evaluation was patient’s refusal. This information was not available in the validation cohort.
Alcohol rehabilitation data was available in all patients in the test cohort and in 74.8% (119 out of 159 patients) in the validation cohort. The analyses evaluating the effect of alcohol rehabilitation were performed using only cases with available data. There were 27(20.0%) and 19(16.0%) patients attended alcohol rehabilitation in test and validation cohort, respectively (Table 1). The types of alcohol rehabilitation were residential rehabilitation (n=12, n=11), outpatient rehabilitation (n=12, n=2), and mutual support group, i.e. alcoholics anonymous (n=3, n=6) in test and validation cohort respectively.
Next, we evaluate predictors of alcohol rehabilitation attendance. Patients who attended alcohol rehabilitation were more likely to be evaluated by alcohol addiction specialists and have college degrees (Supplemental Table 1). Interestingly, the liver severity scores were not different between the two groups. For test cohort, evaluation by alcohol addiction specialists was a predictor of attending alcohol rehabilitation (OR 2.91 [95%CI 1.02–8.27], P=0.03) in univariate analysis (Supplemental Table 2) but not in multivariable analysis (Supplemental Table 3). For validation cohort, having college degree or above (AOR 4.62 [95%CI 1.19–17.9], P=0.03) was an independent predictor of attending alcohol rehabilitation in multivariable analysis (Supplemental Table 3).
30-day readmission after hospital discharge
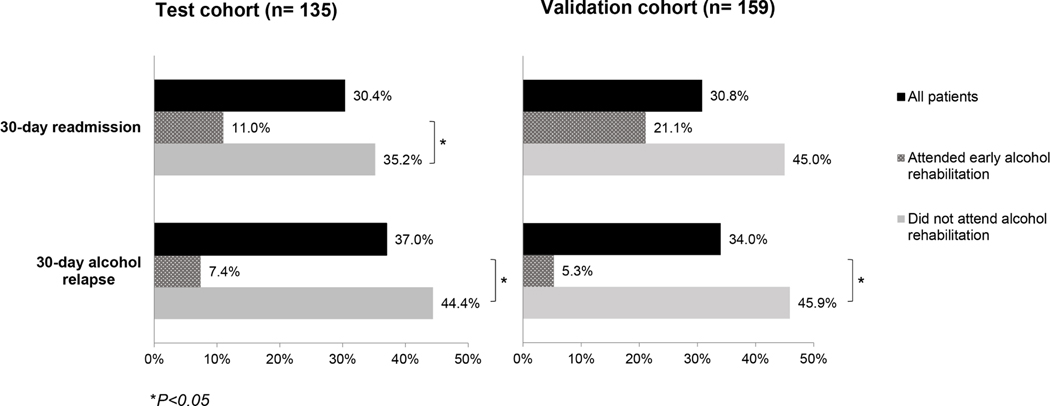
The 30-day readmission rate was 30.4% (n=41) and 30.8% (n=49) in test and validation cohorts, respectively (Figure 1). The 30-day readmission rate was lower in those who attended early alcohol rehabilitation compared to those who did not attend alcohol rehabilitation in both test (11.0% vs. 35.2%, P=0.02) and validation cohorts (21.1% vs. 45.0%, P=0.07) (Figure 1).
Figure 1.
Rates of 30-day readmission and 30-day alcohol relapse
In test cohort, patients readmitted within 30 days were less likely to attend alcohol rehabilitation (7.3% vs. 25.5%, P=0.02) and were more likely to experience alcohol relapse within 1 month after hospital discharge (56.1% vs 28.7%, P=0.003) (Table 1). Patients readmitted within 30 days had higher rates of hepatic decompensation, higher total bilirubin level and higher CTP score compared to those without readmission. Similarly, in validation cohort, patients readmitted within 30 days were less likely to attend alcohol rehabilitation (8.2% vs. 21.4%, P=0.04) and were more likely to experience alcohol relapse (57.5% vs 22.3%, P=0.003). Additionally, patients with readmission were more likely to have higher MELD and CTP scores compared to those without readmission.
In test cohort, causes of readmission included hepatic decompensation (39%), recurrent AH (19%), infection (15%), other medical conditions (19%) and alcohol intoxication or withdrawal (8%). In validation cohort, causes of readmission included hepatic decompensation (51%), other medical conditions (29%), infection (12%), alcohol intoxication or withdrawal (6%) and recurrent AH (2%).
For test cohort, early alcohol rehabilitation was a protective factor (AOR 0.16 [95%CI 0.04–0.65], P=0.01) and increase in total bilirubin (AOR 1.04 [95%CI 1.09–0.96, P=0.04) was a risk factor for 30-day readmission in multivariable analysis after adjusting for hepatic decompensation (Table 2). For validation cohort, early alcohol rehabilitation was a protective factor against 30-day readmission (AOR 0.30 [95%CI 0.09–0.98], P=0.04) after adjusting for hepatic decompensation and total bilirubin level. Univariate analysis is shown in Supplemental Table 4.
Table 2.
Multivariable analyses of variables associated with 30-day readmission, 30-day alcohol 2 relapse and mortality
| Test cohort | Validation cohort | |||
|---|---|---|---|---|
| Multivariable logistic regression analysis | ||||
| 30-day readmission | Adjusted Odds Ratio | P | Adjusted Odds Ratio | P |
| Hepatic decompensation | 2.14 (0.90–5.06) | 0.08 | 1.95 (0.87–4.38) | 0.10 |
| Total bilirubin | 1.04 (1.01–1.09) | 0.04 | 1.02 (0.99–1.06) | 0.23 |
| Attendance to alcohol rehabilitation | 0.16 (0.04–0.65) | 0.01 | 0.30 (0.09–0.98) | 0.04 |
| Multivariable logistic regression analysis | ||||
| 30-day alcohol relapse | Adjusted Odds Ratio | P | Adjusted Odds Ratio | P |
| College degree or above | n/a | 0.74 (0.31–1.77) | 0.50 | |
| MELD score | 0.98 (0.89–1.07) | 0.60 | 0.97 (0.90–1.04) | 0.36 |
| Child-Turcotte-Pugh Score | 0.79 (0.53–1.17) | 0.23 | 0.82 (0.60–1.12) | 0.20 |
| Attendance to alcohol rehabilitation | 0.11 (0.02–0.53) | <0.001 | 0.09 (0.01–0.73) | 0.02 |
| Multivariable Cox regression analysis | ||||
| Mortality | Adjusted Hazard Ratio | P | Adjusted Hazard Ratio | P |
| Age | 1.01 (0.98–1.03) | 0.79 | 1.04 (1.01–1.08) | 0.04 |
| Male | 0.74 (0.41–1.32) | 0.30 | 0.79 (0.41–1.58) | 0.49 |
| Hepatic decompensation | n/a | 2.11 (1.01–4.99) | 0.04 | |
| Variceal bleeding | 11.1 (2.52–34.4) | 0.004 | n/a | |
| ABIC score | n/a | 1.12 (0.81–1.55) | 0.49 | |
| Attendance to alcohol rehabilitation | 0.20 (0.05–0.56) | 0.001 | 0.20 (0.01–0.94) | 0.04 |
n/a = variables that were not statistically significant in univariate analysis
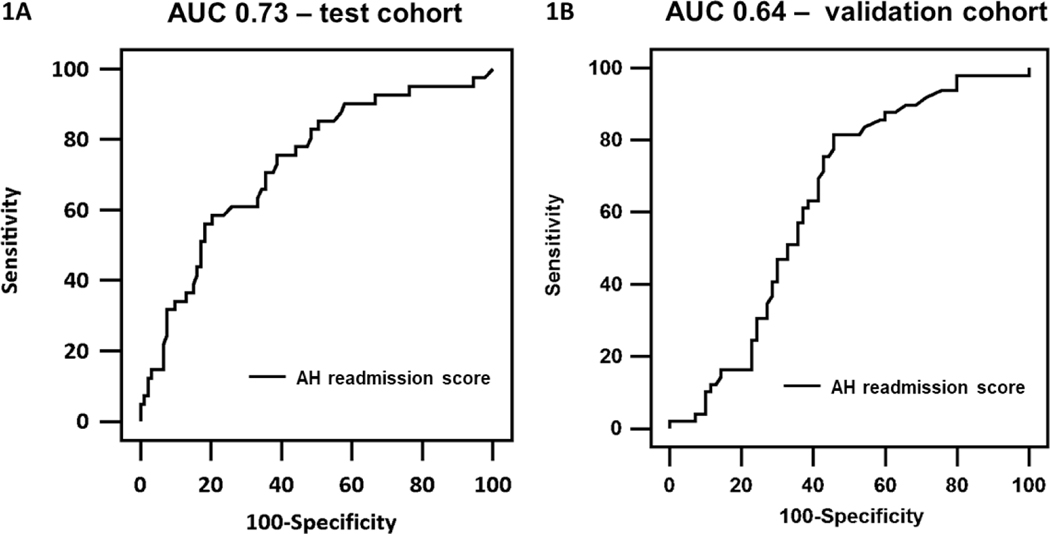
To generate an AH readmission score for 30-day readmission, independent predictors of readmission in the test cohort were used. The AH readmission score = −1.32 + 0.06*(total bilirubin) – 1.80*(alcohol rehabilitation attendance: yes=1, no=0). The area under the receiver operating characteristic curve (AUC) for the AH readmission score were 0.73 (95%CI 0.64–0.80) in test cohort and 0.64 (95%CI 0.54–0.72) in validation cohort (Figure 2).
Figure 2.
Area under the receiver operating characteristic curve of AH readmission score for predicting 30-day readmission in A) test and B) validation cohort.
Alcohol relapse within 30 days after hospital discharge
The 30-day alcohol relapse data were available in all patients in test cohort and in 88.7% (141 out of 159) in validation cohort. The 30-day alcohol relapse rate was 37.0% (n=50) and 34.0% (n=48) in test and validation cohort, respectively (Figure 1). The 30-day alcohol relapse was lower in those who attended early alcohol rehabilitation compared to those who did not attend alcohol rehabilitation in both test (7.4% vs. 44.4%, P<0.001) and validation cohorts (5.3% vs. 45.9%, P<0.001) (Figure 1). Among those who relapsed, only 2 out of 50 in test cohort and only 1 out of 48 in the validation cohort attended alcohol rehabilitation after hospital discharge.
In univariate analysis (Supplemental Table 5), early alcohol rehabilitation was a protective factor for 30-day alcohol relapse in both test and validation cohorts. Additionally, higher MELD score, CTP score, mDF scores, INR and having college degree were protective factors for alcohol relapse. In multivariable analysis (Table 2), participation in alcohol rehabilitation within 30-days after hospital discharge was the only independent protective factor against 30-day alcohol relapse (AOR 0.11, [95%CI 0.02–0.53], P<0.001 and AOR 0.09, [95%CI 0.01–0.73], P=0.02) in test and validation cohort, respectively.
Mortality
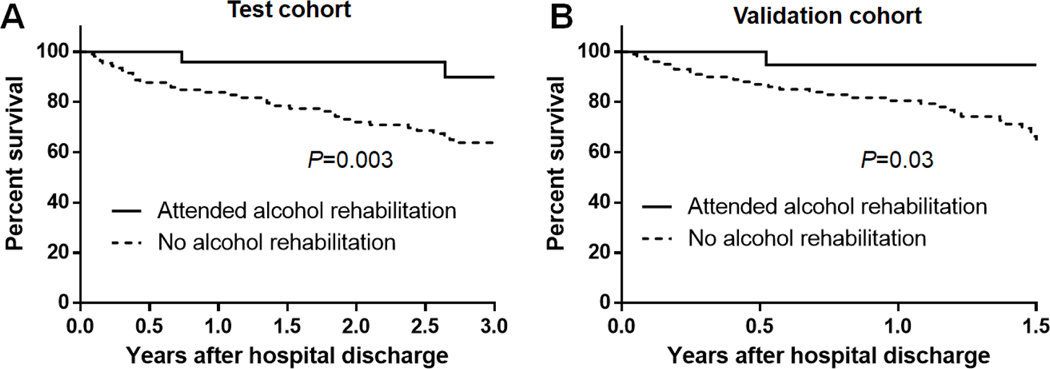
There were 53 (39.3%) and 42 (26.4%) deaths during a median follow up time of 2.8 and 1.3 years in test and validation cohorts, respectively. The mortality rate at one year was 14% in the test and 16% in the validation cohort. As shown in Figure 3, patients who attended early alcohol rehabilitation had significantly improved survival compared to patients who did not attend alcohol rehabilitation in both test and validation cohorts.
Figure 3.
Kaplan–Meier curves showing in patients attending vs. not attending alcohol rehabilitation in A) test and B) validation cohort
Univariate analysis is shown in Supplemental Table 6. In multivariable analysis (Table 2), attendance to early alcohol rehabilitation was an independent protective factor (adjusted hazard ratio [AHR] 0.20 [95%CI 0.05–0.56], P=0.001), while variceal bleeding was an independent predictor of mortality (AHR 11.1 [95%CI 2.52–34.4], P=0.004) after adjusting for age and sex. In validation cohort, attendance to alcohol rehabilitation was an independent protective factor (AHR 0.20 [95%CI 0.01–0.94], P=0.04), whereas age (AHR 1.04 [95%CI 1.01–1.08], P=0.04) and hepatic decompensation (AHR 2.11 [95%CI 1.01–4.99], P=0.04) were significant predictors of mortality, after adjusting for sex and ABIC score.
Discussion
Recent studies on AH reported that alcohol relapse is the most important predictor of long-term mortality.5, 6 However, hospital readmission rates, alcohol relapse along with alcohol rehabilitation attendance have not been well characterized in the AH population. We utilized a test (single-center retrospective) and a validation (multi-center prospective) cohort of hospitalized AH patients to evaluate 30-day readmission, 30-day alcohol relapse, and long-term mortality. At 30-days after surviving AH admission, readmission rate was 30%, alcohol relapse rate was 34–37%, and only 16–20% of patients participated in alcohol rehabilitation. The current study showed that alcohol rehabilitation after hospital discharge was associated with decreased 30-day readmission, decreased 30-day alcohol relapse and importantly, decreased mortality. While alcohol rehabilitation is routinely recommended in this clinical scenario, this is one of the first studies that documents the benefits of this intervention on clinically significant patient endpoints including mortality.
In multivariable analysis, participating in alcohol rehabilitation was associated with 70–84% lower risk of 30-day readmission, 89–91% lower risk of 30-day alcohol relapse, and 80% lower risk of long-term mortality. This is an important finding because alcohol relapse is the most important predictor for long-term mortality and there is currently no proven pharmacological intervention to prevent alcohol relapse in patients with AH.5, 6, 19, 20 Evidence suggests that psychosocial interventions including cognitive behavioral therapy and motivational interviewing may be effective in supporting alcohol abstinence in patients with alcoholic cirrhosis.21–23 It is also important to emphasize that the most common type of alcohol rehabilitation was at a residential (44% in test cohort and 53% in validation cohort) level of care. Future studies are necessary to test whether the intensity of treatment along with timelines (within 30 days of discharge) are important prerequisites of success.
After AH admission, only 16–20% of patients with AH attended alcohol rehabilitation. The results challenge us to do better as a specialty in connecting patients who survive AH episodes to alcohol rehabilitation and to build stronger bridges with our addiction specialist colleagues. Our study results strongly suggest that alcohol rehabilitation should be arranged for AH patients during their index hospital admission and early alcohol rehabilitation referral should be documented as a quality metric in the management of all hospitalized AH patients. Patients evaluated by alcohol addiction specialists during their hospitalization demonstrated higher attendance rates of alcohol rehabilitation compared to patients who were not assessed (25.3% vs. 10.4%). Therefore, an addiction specialist should meet with the patient during hospital admission. Unfortunately, less than 8% of patients received alcohol relapse prevention medication, which were all gabapentin. It is important to note that the use of gabapentin was an off-label use.24 The integration of addiction medicine in hepatology practice is becoming even more important with the development of effective treatments for hepatitis C viral(HCV) infection because alcohol abstinence is necessary for optimal treatment of HCV.20
Our results emphasize the high disease burden of AH. The 30-day readmission rate was 30%, which is comparable to patients with decompensated cirrhosis (32%).25 Recurrent AH and alcohol intoxication or withdrawal were causes of readmission in 19% and 8% in the test cohort, respectively. A previous study suggested that subsequent episodes of AH in alcohol relapsers are more severe compared to the index episode with close to 60% mortality.26 Only total bilirubin at the time of admission and participation in alcohol rehabilitation after hospital discharge were independent predictors of readmission. Interestingly, the liver severity scores, e.g. MELD score or CTP score, were not predictors for readmission. In contrast, MELD score has been previously found to be an independent predictor of readmission in cirrhotic patients.27 Although we attempted to create a model to predict 30-day readmission using alcohol rehabilitation attendance and total bilirubin, the AUC were only 0.73 and 0.63 in test and validation cohorts, respectively. Unfortunately, the risk of readmission in this patient population is likely difficult to predict due to many socioeconomic factors involved. Similar findings have been reported in patients with decompensated cirrhosis.28
The major strengths of our study were the utilization of two separate cohorts. Importantly, the validation group was a multicenter prospective cohort with well-defined AH patients. The findings from both cohorts were consistent. However, our study has few limitations worth mentioning. First, the diagnosis of AH was made without a liver biopsy, as is common in real practice, which may occasionally lead to misdiagnosis. However, using the NIAAA clinical criteria, less than 10% of patients would have a diagnosis other than AH on liver biopsy.15 Second, alcohol relapse was based on self-report and the data may not be fully accurate. However, the well validated timeline followback questionnaire was used and we considered any alcohol consumption after discharge as relapse. Future studies should include blood or urine markers to screen for alcohol relapse. Third, we were not able to evaluate the effect of central obesity, factor shown to have negative effect on outcomes in patients with alcoholic hepatitis.29 This is because waist and hip circumference data were not available. However, BMI data was analyzed and did not affect the outcomes in our cohort. Lastly, the reasons for patients not attending rehabilitation are difficult to ascertain and the benefit of rehabilitation may be reflective of the high motivation of those patients to discontinue alcohol rather than the rehabilitation process itself.
In conclusion, the 30-day readmission and 30-day alcohol relapse rates are 30% and 37% respectively after an AH admission. Early alcohol rehabilitation is associated with reduction in hospital readmission, alcohol relapse, and death and should be pursued in all AH patients during and/or immediately following their hospitalization.
Supplementary Material
What You Need to Know.
Background:
Patients admitted to the hospital for alcoholic hepatitis (AH) are at increased risk of hospital readmission and death.
Findings:
In two cohort of patients admitted with AH, early alcohol rehabilitation reduced risk of hospital readmission, alcohol relapse, and death.
Implications for patient care:
All patients with AH should be formally evaluated by addiction specialists during hospital stay and referred for treatment within 30 days of hospital discharge.
Abbreviations List:
- ABIC
Age, serum Bilirubin, international normalized ratio, and serum Creatinine
- AH
Alcoholic hepatitis
- BMI
Body mass index
- CTP
Child-Turcotte-Pugh scores
- mDF
Maddrey discriminant
- MELD
Model for End-Stage Liver Disease
- PT
Prothrombin time
- INR
International normalized ratio
Footnotes
Conflict of interest:
The authors who have taken part in this study declared that they do not have any conflict of interest with respect to this manuscript. The work was supported by the NIAAA U01AA 021788 grant to PSK and VHS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. Journal of clinical gastroenterology. 2011;45(8):714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singal AK, Bataller R, Ahn J, et al. ACG Clinical Guideline: Alcoholic Liver Disease. The American journal of gastroenterology. 2018;113(2):175–94. Epub 2018/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology (Baltimore, Md). 2005;41(2):353–8. Epub 2005/01/22. [DOI] [PubMed] [Google Scholar]
- 4.Thursz MR, Richardson P, Allison M, et al. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. New England Journal of Medicine. 2015;372(17):1619–28. [DOI] [PubMed] [Google Scholar]
- 5.Alexandre L, Julien L, Florent A, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatology (Baltimore, Md). 2017;66(5):1464–73. [DOI] [PubMed] [Google Scholar]
- 6.Altamirano J, Lopez-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: Prediction and impact on long-term survival. Hepatology (Baltimore, Md). 2017;66(6):1842–53. Epub 2017/06/25. [DOI] [PubMed] [Google Scholar]
- 7.Dom G, Peuskens H. Addiction specialist’s role in liver transplantation procedures for alcoholic liver disease. World J Hepatol. 2015;7(17):2091–9. Epub 2015/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. The American journal of gastroenterology. 2012;107(2):247–52. Epub 2011/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirapongsathorn S, Talwalkar JA, Kamath PS. Strategies to Reduce Hospital Readmissions. Seminars in liver disease. 2016;36(2):161–6. Epub 2016/05/14. [DOI] [PubMed] [Google Scholar]
- 10.Lourens S, Sunjaya DB, Singal A, et al. Acute Alcoholic Hepatitis: Natural History and Predictors of Mortality Using a Multicenter Prospective Study. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2017;1(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liangpunsakul S, Puri P, Shah VH, et al. Effects of Age, Sex, Body Weight, and Quantity of Alcohol Consumption on Occurrence and Severity of Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2016;14(12):1831–8 e3. Epub 2016/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Amet T, Xing Y, et al. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: A prospective observational study. Hepatology (Baltimore, Md). 2017;66(2):575–90. Epub 2017/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puri P, Liangpunsakul S, Christensen JE, et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology (Baltimore, Md). 2018;67(4):1284–302. Epub 2017/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samala N, Lourens SG, Shah VH, et al. Posttraumatic Stress Disorder in Patients with Heavy Alcohol Consumption and Alcoholic Hepatitis. Alcohol Clin Exp Res. 2018;42(10):1933–8. Epub 2018/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabb DW, Bataller R, Chalasani NP, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150(4):785–90. Epub 2016/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. The American journal of gastroenterology. 2008;103(11):2747–56. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 17.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with endstage liver disease. Hepatology (Baltimore, Md). 2001;33(2):464–70. Epub 2001/02/15. [DOI] [PubMed] [Google Scholar]
- 18.Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. p. 41–72. [Google Scholar]
- 19.Lee MR, Leggio L. Management of Alcohol Use Disorder in Patients Requiring Liver Transplant. Am J Psychiatry. 2015;172(12):1182–9. Epub 2015/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leggio L, Lee MR. Treatment of Alcohol Use Disorder in Patients with Alcoholic Liver Disease. The American Journal of Medicine. 2017;130(2):124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addolorato G, Mirijello A, Barrio P, et al. Treatment of alcohol use disorders in patients with alcoholic liver disease. Journal of Hepatology. 2016;65(3):618–30. [DOI] [PubMed] [Google Scholar]
- 22.Khan A, Tansel A, White DL, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients With Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol. 2016;14(2):191–202.e1–4; quiz e20. Epub 2015/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinrieb RM, Van Horn DH, Lynch KG, et al. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(5):539–47. Epub 2011/04/21. [DOI] [PubMed] [Google Scholar]
- 24.Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert opinion on investigational drugs. 2018;27(1):113–24. Epub 2017/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirapongsathorn S, Krittanawong C, Enders FT, et al. Incidence and cost analysis of hospital admission and 30-day readmission among patients with cirrhosis. Hepatology communications. 2018;2(2):188–98. Epub 2018/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potts JR, Howard MR, Verma S. Recurrent severe alcoholic hepatitis: clinical characteristics and outcomes. Eur J Gastroenterol Hepatol. 2013;25(6):659–64. Epub 2013/05/01. [DOI] [PubMed] [Google Scholar]
- 27.Berman K, Tandra S, Forssell K, et al. Incidence and Predictors of 30-Day Readmission Among Patients Hospitalized for Advanced Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9(3):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakkarin C, Chayakrit K, EF T, et al. Incidence and cost analysis of hospital admission and 30-day readmission among patients with cirrhosis. Hepatology Communications. 2018;2(2):188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aberg F, Helenius-Hietala J, Puukka P, et al. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology (Baltimore, Md). 2018;67(6):2141–9. Epub 2017/11/23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.