Abstract
Objectives:
We sought to determine whether hyperinflammatory and hypoinflammatory ARDS, which have been associated with differences in plasma biomarkers and mortality risk, also display differences in bronchoalveolar lavage (BALF) biomarker profiles. We then described the relationship between hyperinflammatory and hypoinflammatory ARDS to novel subphenotypes derived using BALF biomarkers.
Design:
Secondary analysis of a randomized control trial testing omega-3 fatty acids for the treatment of ARDS
Setting:
Five North American intensive care units
Patients:
Adults (n=88) on invasive mechanical ventilation within 48 hours of ARDS onset
Interventions:
None
Measurements and main results:
We classified 57 patients as hypoinflammatory and 31 patients as hyperinflammatory using a previously validated logistic regression model. Of 14 BALF biomarkers analyzed, interleukin-6 and granulocyte colony stimulating factor were higher among patients with hyperinflammatory ARDS compared to hypoinflammatory ARDS, though the differences were not robust to multiple hypothesis testing. We then performed a de novo latent class analysis of the 14 BALF biomarkers to identify 2 classes well-separated by alveolar profiles. Class 2 (n=63) displayed significantly higher interleukin-6, von Willebrand factor, soluble programmed cell death receptor-1, % neutrophils, and other biomarkers of inflammation compared to Class 1 (n=25). These BALF-derived classes had minimal overlap with the plasma-derived hyperinflammatory and hypoinflammatory classes, and the majority of both plasma-derived classes were in BALF-derived Class 2 and characterized by high BALF biomarkers. Additionally, the BALF-derived classes were associated with clinical severity of pulmonary disease, with Class 2 exhibiting lower PaO2 to FIO2 and distinct ventilatory parameters, unlike the plasma-derived classes which were only related to non-pulmonary organ dysfunction.
Conclusions:
Hyperinflammatory and hypoinflammatory ARDS subphenotypes did not display significant differences in alveolar biologic profiles. Identifying ARDS subgroups using BALF measurements is a unique approach that complements information obtained from plasma, with potential to inform enrichment strategies in trials of lung-targeted therapies.
Keywords: phenotype, precision medicine, acute respiratory distress syndrome, bronchoalveolar lavage fluid
Introduction
Heterogeneity across patients with the acute respiratory distress syndrome (ARDS) can hinder the identification of effective, targeted therapeutics (1). To address this challenge, multiple investigators have identified two ARDS subphenotypes by applying a type of finite mixture modelling called latent class analysis (LCA), to a panel of clinical and biologic variables. These subphenotypes, often termed “hyperinflammatory” and “hypoinflammatory” based on differences in inflammatory plasma biomarkers, are associated with differences in overall mortality and response to therapies (2). Although they have potential to inform therapeutic trials, their relationship to alveolar inflammation and respiratory physiology—the hallmarks of ARDS pathobiology—remains unclear.
Our aims were to (1) describe the bronchoalveolar lavage fluid (BALF) biomarker profile in subjects with hyperinflammatory or hypoinflammatory ARDS, (2) determine whether a de novo LCA restricted to BALF biomarkers aligns with existing plasma-derived classes, and (3) compare how each subphenotype classification relates to severity of respiratory failure and non-pulmonary organ failures.
Materials and Methods
We performed a secondary analysis of a phase-II trial of omega-3 fatty acids that enrolled patients within 48 hours of ARDS onset at 5 North American centers (n=88), and measured biomarkers of lung injury and inflammation in paired plasma and BALF (3). These data were collected with approval of local human subjects committees and a Data Safety Monitoring Board (DSMB) under trial number NCT00351533 (Appendix 1). The trial collected samples on enrollment, and employed a standardized bronchoscopy procedure to avoid systematic differences in BALF dilution (3).
To classify patients into plasma-derived hyperinflammatory or hypoinflammatory subphenotypes, we used a validated regression-based model with plasma soluble tumor necrosis factor receptor-1 (sTNFR-1), interleukin-8 (IL-8), and serum bicarbonate concentrations (4).
We then derived novel subphenotypes by applying LCA to 14 biomarkers measured in BALF, fitting models for one to four classes. Biomarkers with skewed distributions were log2 transformed, then all biomarkers were z-score normalized. Biomarkers highly correlated (Pearson’s correlation >0.80) or with >25% of values below the lower detection limit were excluded, as these could impede model fit and convergence (Supplemental Table 1) (5). We compared BALF biomarkers, plasma biomarkers, and clinical features between plasma-derived and BALF-derived subphenotypes using Mann-Whitney U and Fisher’s exact tests.
Results
When examining plasma-derived subphenotypes, we classified 57 patients as hypoinflammatory and 31 patients as hyperinflammatory ARDS. Hyperinflammatory ARDS displayed higher BALF interleukin-6 (IL-6) and granulocyte colony stimulating factor (G-CSF) levels compared with hypoinflammatory ARDS, although these were no longer significant after a Bonferroni correction for multiple hypothesis tests (Figure 1A; Supplemental Figure 1; Supplemental Table 2). Biomarkers such as soluble programmed cell death-ligand 1, interleukin-17A (IL-17A), and MCP-1 were not different in BALF, although they displayed stark differences when measured in plasma (Supplemental Table 3).
Figure 1: Mean alveolar biomarkers by plasma-derived and BALF-derived classes.
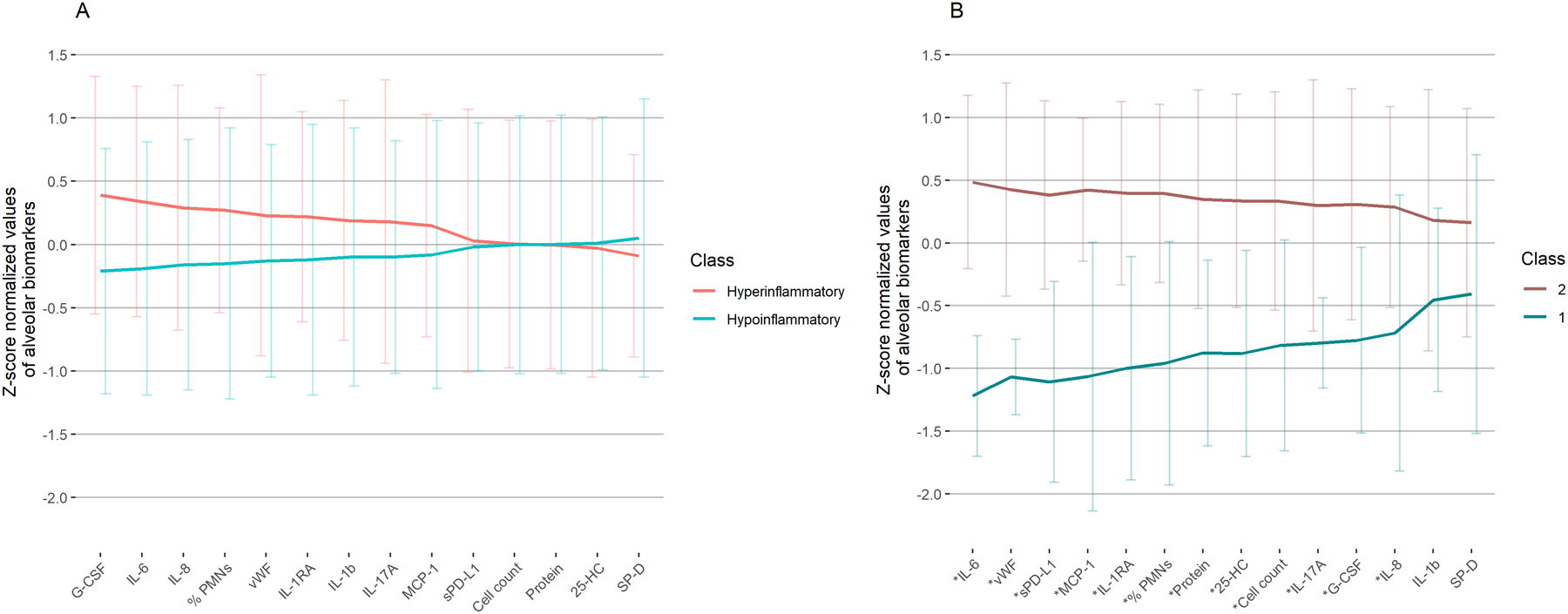
(A) Alveolar biomarkers by plasma-derived classes. (B) Alveolar biomarkers by BALF-derived classes. (Both panels) Lines indicate mean and error bars indicate standard deviation of these z-score normalized alveolar biomarkers, sorted by magnitude of difference between classes. *Bonferroni corrected P < 0.05 for difference between classes using Mann Whitney U tests. Abbreviations: BALF = bronchoalveolar lavage fluid, G-CSF = granulocyte colony stimulating factor, IL-6 = interleukin-6, IL-8 = interleukin-8, % PMNs = % neutrophils of total leukocyte count, vWF = von Willebrand factor, IL-1RA = interleukin-1 receptor antagonist, IL-1b = interleukin-1 beta, IL-17A = interleukin-17A, MCP-1 = monocyte chemoattractant protein-1, sPD-L1 = soluble programmed cell death ligand 1, cell count = total leukocyte count, protein = total protein, 25-HC = 25-hydroxycholesterol, SP-D = surfactant protein D.
Next, we classified patients into BALF-derived subphenotypes using LCA. We selected a two-class model because the Vuong-Lo-Mendell-Rubin test indicated this was a significant improvement over a one-class model (p=0.004), and three or four-class models did not significantly increase explanatory power of class identification. A two-class model also had the highest entropy index (0.97). In the two-class model, 25 (28%) patients were in BALF Class 1 and 63 (72%) were in BALF Class 2. Class 2 had higher concentrations of total protein, % neutrophils, and all other BALF biomarkers of inflammation and lung injury compared to Class 1 (Figure 1B; Supplemental Figure 1; Supplemental Table 2). Except for interleukin-1 beta (IL-1b) and surfactant protein D (SP-D), these differences were significant even after Bonferroni correction.
Overlap between plasma-derived and BALF-derived ARDS subphenotypes was minimal (Cohen’s kappa =0.07). Notably, most hypoinflammatory ARDS patients were in BALF Class 2 (39/57, 68%), and were characterized by high alveolar inflammation. Additionally, 24/31 (77%) hyperinflammatory ARDS patients also were in Class 2.
Finally, we compared clinical features, severity of respiratory failure, and severity of non-pulmonary organ failures between plasma-derived and BALF-derived subphenotypes (Table 1). Hyperinflammatory patients were older and had higher APACHE-II scores compared to hypoinflammatory patients, but respiratory parameters such as lung injury score (LIS) (6), PaO2 to FIO2 ratio (PaO2:FIO2), and ventilatory parameters were not different. In contrast, BALF Class 2 patients displayed lower PaO2:FIO2 (152 vs. 202, p = 0.008), higher LIS (54% with severe LIS vs. 20%, p=0.004), and received higher PEEP on enrollment compared to BALF Class 1. Consistent with this, the median duration of mechanical ventilation for survivors was numerically higher in BALF Class 2, although the difference was not statistically significant. In terms of severity of non-pulmonary organ dysfunction, we observed no significant differences in APACHE-II score or mortality between BALF-derived subphenotypes, although numerically we observed lower mortality among Class 2 (13% vs. 28%).
Table 1:
Clinical features by plasma-derived and BALF-derived classes
| Age, years | 47 (38–59) | 60 (46–68) | 0.025 | 58 (44–65) | 48 (41–63) | 0.14 |
| Female | 21 (37%) | 11 (35%) | >0.99 | 9 (36%) | 23 (37%) | >0.99 |
| Pneumonia | 25 (44%) | 12 (39%) | 0.65 | 10 (40%) | 27 (43%) | >0.99 |
| Direct Lung Injury | 30 (53%) | 18 (58%) | 0.82 | 12 (48%) | 36 (57%) | 0.63 |
| PaO2 to FIO2 ratio | 163 (126–204) | 152 (116–187) | 0.30 | 202 (146–221) | 152 (116–185) | 0.008 |
| Severe LIS | 23 (40%) | 16 (52%) | 0.37 | 5 (20%) | 34 (54%) | 0.004 |
| PEEP, cm H2O | 8 (5–10) | 8 (5–10) | 0.46 | 5 (5–10) | 10 (5–10) | 0.018 |
| Plateau pressure, cm H2O | 24 (20–30) | 24 (20–29) | 0.71 | 22 (19–29) | 24 (21–29) | 0.21 |
| Duration of mechanical ventilation in survivors | 10 (7–28) | 10 (9–28) | 0.43 | 8 (5–28) | 10 (7–28) | 0.48 |
| Severity of non-pulmonary organ failure | ||||||
| Vasopressors | 18 (32%) | 16 (52%) | 0.072 | 9 (36%) | 25 (40%) | 0.81 |
| AKI | 5 (9%) | 8 (26%) | 0.055 | 3 (12%) | 10 (16%) | 0.75 |
| APACHE-II | 20 (16–24) | 25 (21–29) | <0.001 | 24 (17–25) | 22 (18–27) | 0.82 |
| 28-day mortality | 9 (16%) | 6 (19%) | 0.77 | 7 (28%) | 8 (13%) | 0.12 |
Median (interquartile range) listed for continuous variables, N (%) for categorical variables. Abbreviations: BALF = bronchoalveolar lavage fluid, severe LIS = Lung injury score > 2.5, PEEP = positive end-expiratory pressure, AKI = Acute kidney injury, APACHE = acute physiology and chronic health evaluation.
In order to investigate whether these BALF-derived classes were simply reflecting ARDS severity as captured by routine clinical measurements, we compared BALF biomarker concentrations among patients with PaO2:FIO2 ≤ 150 to those with PaO2:FIO2 >150 (a common threshold for ARDS trial enrollment) (7). Only BALF total protein and 25-hydroxycholesterol were significantly higher among patients with PaO2:FIO2 ≤ 150 (Supplemental Table 4). Finally, as a step towards integrating data from circulating and lung compartments, we calculated ratios of BALF to plasma concentrations for biomarkers measured in both spaces (Supplemental Table 5). We compared these ratios by direct (e.g. pneumonia, aspiration) and indirect (e.g. trauma, non-pulmonary sepsis) etiologies of ARDS, to further explore how integrating BALF with plasma can offer new insights into lung pathobiology. We found that BALF-to-plasma ratios of IL-1RA and IL-17a were higher in direct than indirect ARDS, driven largely by differences in the BALF measurements rather than plasma.
Discussion
In summary, this study expands our understanding of hypoinflammatory and hyperinflammatory ARDS subphenotypes by analyzing the lung compartment, and is the first to compare their molecular profiles in BALF. Although hyperinflammatory ARDS exhibited nominally higher BALF concentrations of IL-6 and G-CSF compared to hypoinflammatory ARDS, these differences were not robust to multiple hypothesis testing. Instead, biomarkers that were clearly different between hypoinflammatory and hyperinflammatory ARDS in plasma had minimal differences in BALF. Additionally, our exploratory LCA of BALF biomarkers revealed 2 novel classes of patients, with Class 2 displaying markedly higher neutrophilic inflammation, protein, and biomarkers of inflammation and endothelial injury in the lung. This BALF molecular profile was prevalent among both hypoinflammatory and hyperinflammatory ARDS. These findings build upon a smaller study that showed similar alveolar biomarker profiles by plasma-derived subphenotypes using non-invasive mini-bronchoalveolar lavage, as well as our previous work in this population showing divergent peripheral and alveolar transcriptomic profiles (8, 9). Collectively, these support the concept that plasma-derived ARDS classes do not consistently reflect differences in early alveolar inflammatory profiles.
Our LCA of BALF biomarkers is proof-of-concept that BALF is a unique approach to selecting ARDS subgroups for future study. Prior ARDS trials focused enrollment on moderate-severe ARDS by PaO2:FIO2 to improve prognostic enrichment, however this strategy did not delineate molecular differences in the lung as effectively as the BALF-derived classification in this population (1). The BALF-derived classes identified not only strong distinctions in multiple lung injury biomarkers, which we expected given the clustering approach, but also revealed differences in hypoxemia, LIS, and ventilator parameters even though these were not considered in the LCA. In contrast, the plasma-based subphenotypes did not discriminate subjects based on severity of respiratory failure, but were related to overall severity of illness as measured through APACHE-II scores. Although we ostensibly might expect BALF-derived Class 2 to display higher mortality than Class 1 from their higher degree of lung injury, prior research suggests a minority of ARDS patients actually die of hypoxemic respiratory failure (10). To advance precision medicine for ARDS, it is important to instead identify subgroups with high risk for disease-related outcomes and/or shared biologic features that can targeted for therapy. We propose that utilizing BALF or other organ-specific measurements, instead of plasma alone, can support trials that target lung-specific biology and outcomes.
Although we identified important differences in BALF biomarkers by ARDS subphenotypes, the sample size limited our power to accurately compare clinical outcomes or treatment response. LCA in this small population is also susceptible to missing low-prevalence classes. As such, our findings, particularly the LCA, need external validation. Accounting for dilution is another common challenge in studies of BALF. Consistent with other recent analyses of BALF, we minimized systematic differences in BALF dilution factor by employing a rigorous bronchoscopy protocol, and chose not to perform other corrections for dilution, as the validity of existing methods are limited (11–13). There may be some random differences in dilution factor across patients that we expect would bias towards the null; despite this, we found significant differences between BALF-derived classes. Finally, mortality was relatively low compared to early ARDS trials, which could affect generalizability of our findings. Notwithstanding these limitations, our study demonstrates that BALF may have potential to deepen our understanding of ARDS subphenotypes, and guide the design of future ARDS trials. Future studies should examine ways to further integrate BALF with plasma and clinical data.
Conclusions
Alveolar biomarker profiles were not significantly different between hyperinflammatory and hypoinflammatory ARDS, although the groups displayed differences in plasma biomarkers of inflammation and non-pulmonary organ dysfunction. Deriving novel subphenotypes with BALF helped identify a subgroup of patients with an alveolar biomarker profile consistent with lung injury as well as high clinical severity of respiratory failure, and offers new opportunities for designing trials of lung-targeted therapeutics.
Supplementary Material
KEY POINTS.
Question:
How do previously described plasma-derived hyperinflammatory and hypoinflammatory ARDS subphenotypes compare to novel subphenotypes derived from BALF biomarkers?
Findings:
In a secondary analysis of a phase-II ARDS trial (n=88), hyperinflammatory and hypoinflammatory ARDS did not have significant differences in BALF biomarkers or severity of respiratory failure. Latent class analysis of BALF biomarkers identified two novel ARDS subphenotypes characterized by significant differences in alveolar inflammation, as well as lung injury severity, hypoxemia, and ventilator parameters.
Meaning:
BALF offers distinct insights from plasma into lung-specific biology and disease severity, which may help support precision medicine ARDS trials.
Funding
This research was supported by an American Thoracic Society/Acute Respiratory Distress Syndrome Foundation Award, an American Society for Parenteral and Enteral Nutrition Rhoads Research Foundation Award, and National Institutes of Health Grants P50HL073996, P20RR015557, K12RR023265. Authors received support from F32HL158088 (Sathe), K23HL144916 (Morrell), K23HL105654 (Stapleton), R01HL149676 (Mikacenic) from the National Heart, Lung, and Blood Institute; K23DK116967 (Bhatraju) from the National Institute of Diabetes, Digestive and Kidney Disease; Z01ES102005 (Fessler) from the National Institute of Environmental Health Sciences.
The funding sources had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Copyright Form Disclosure:
Drs. Sathe, Morrell, Bhatraju, Fessler, Stapleton, and Mikacenic received support for article research from the National Institutes of Health (NIH). Dr. Fessler disclosed government work. Dr. Stapleton’s institution received funding from the NIH, the ATS/ARDS Foundation, and ASPEN; she received funding from Altimmune and CSL-Behring. Dr. Mikacenic’s institution received funding from the National Heart, Lung, and Blood Institute. Dr. Wurfel has disclosed that he does not have any potential conflicts of interest.
Footnotes
Work performed at the University of Washington
References
- 1.Prescott HC, Calfee CS, Thompson BT, et al. : Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med 2016; 194:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calfee CS, Delucchi K, Parsons PE, et al. : Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2:611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton RD, Martin TR, Weiss NS, et al. : A Phase II Randomized Placebo-Controlled Trial of Omega-3 Fatty Acids for the Treatment of Acute Lung Injury. Crit Care Med 2011; 39:1655–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Famous KR, Delucchi K, Ware LB, et al. : Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017; 195:331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P, Calfee CS, Delucchi KL: Practitioner’s Guide to Latent Class Analysis: Methodological Considerations and Common Pitfalls. Crit Care Med 2021; 49:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray JF, Matthay MA, Luce JM, et al. : An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988; 138:720–723 [DOI] [PubMed] [Google Scholar]
- 7.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, et al. : Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heijnen NFL, Hagens LA, Smit MR, et al. : Biological Subphenotypes of ARDS Show Prognostic Enrichment in Mechanically Ventilated Patients Without ARDS. Am J Respir Crit Care Med 2021; [DOI] [PubMed] [Google Scholar]
- 9.Morrell ED, Radella F, Manicone AM, et al. : Peripheral and Alveolar Cell Transcriptional Programs Are Distinct in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2018; 197:528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ketcham SW, Sedhai YR, Miller HC, et al. : Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: a retrospective cohort study. Crit Care 2020; 24:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendib I, Beldi-Ferchiou A, Schlemmer F, et al. : Alveolar compartmentalization of inflammatory and immune cell biomarkers in pneumonia-related ARDS [Internet]. Crit Care 2021; 25[cited 2021 Mar 31] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7794625/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargaville PA, South M, Vervaart P, et al. : Validity of markers of dilution in small volume lung lavage. Am J Respir Crit Care Med 1999; 160:778–784 [DOI] [PubMed] [Google Scholar]
- 13.Marcy TW, Merrill WW, Rankin JA, et al. : Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am Rev Respir Dis 1987; 135:1276–1280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


