Abstract
Sex (whether one is ‘male’ or ‘female’, based on biological characteristics) and gender (defined by socially constructed roles and behaviors) influence asthma diagnosis and management. For example, women generally report more severe asthma symptoms than men; men and women are exposed to different asthma-causing triggers; men tend to be more physically active than women. Furthermore, implicit, often unintended gender bias by healthcare professionals (HCPs) is widespread, and may result in delayed asthma diagnosis, which can be greater in women than men. The sex and gender of the HCP can also impact asthma management. Pregnancy, menstruation, and menopause can all affect asthma in several ways and may be associated with poor asthma control. This review provides guidance for considering sex- and gender-associated impacts on asthma diagnosis and management and offers possible approaches to support HCPs in providing personalized asthma care for all patients, regardless of their sex or gender.
Subject terms: Asthma, Respiratory signs and symptoms
Introduction
Globally, ~300 million people live with asthma1. There are well-established sex and gender differences in the prevalence of asthma: more boys than girls suffer from asthma pre-puberty, while post-puberty, the prevalence of asthma is higher in women than men2. Furthermore, women are more likely to have severe asthma, comorbidities, worse quality of life, and a higher rate of exacerbations, hospitalizations, and mortality compared with men1,3. These differences have been attributed to sex-specific physiological differences (e.g., sex hormones)2, but may also be driven by gender-specific sociocultural and behavioral differences (e.g., gender roles/occupations, symptom perception)4,5.
Importantly, ‘sex’ and ‘gender’ are not always clearly defined in scientific literature and are often used interchangeably and/or incorrectly6. Sex refers to the biological and physiological characteristics of females and males (e.g., chromosomes, hormones, and reproductive organs), while gender is a sociocultural construct that refers to the identities, characteristics, roles, and behaviors of men, women, boys, and girls and gender-diverse people6. Therefore, gender characteristics can vary between societies and may change over time.
In a previous review7, we outlined current evidence for sex- and gender-related differences that influence asthma pathogenesis, clinical course, severity, symptoms, and management. The aim of this narrative review is to provide guidance for healthcare professionals (HCPs) to consider sex- and gender-associated differences in asthma diagnosis and management when deciding the best course of action for their patients. These suggestions are based on the authors’ assessment of current evidence and are outlined in each section as “author guidance”.
There are limited studies on gender-diverse people with asthma, and many studies have been carried out using ‘traditional’ sex and gender definitions. As such, we have used the terminology of ‘men’ and ‘women’ throughout this review.
Gender differences in patient health behaviors
Patient reporting of symptoms
Patient reporting of symptoms may influence the way symptoms are interpreted by HCPs, and, therefore, how the patient’s asthma is managed. Evidence suggests that women perceive their asthma as more symptomatic than men and report more frequent, severe, and bothersome symptoms, even if the severity and level of asthma control are similar4. Compared with men, women also report poorer quality of life and greater symptom impact, including more limitations on sports, social activities, sleep, and day-to-day activities8. It may be for these reasons that women are more likely than men to report their symptoms to a HCP8. Men may also understate their symptoms and be reluctant to seek HCP support due to societal expectations that men should not complain about their health9.
Author guidance:
Recognize that men and women may present with different symptom profiles and that gender can affect how and when patients report their symptoms.
Confirm diagnosis with spirometry10, and use validated measurements of asthma control e.g., asthma control questionnaire or asthma control test, which help to objectively assess the severity of patients’ symptoms and response to treatment10.
Discussion guides that help patients understand their asthma may prompt conversations to gain insight into their symptoms and daily life limitations so that appropriate support can be offered by HCPs and asthma educators11,12.
Triggers and long-term exposures
While certain triggers, such as air pollution, are likely to be similar for both genders, women and men may be exposed to different triggers and asthma-causing substances due to their gender roles and occupations (Fig. 1)5. Globally, women are more likely to be exposed to cleaning chemicals and biomass fuels, while men are more likely to be exposed to pyrolysis products, plant-based materials, isocyanates, metals, and metalloids (all of which are associated with an increased risk of asthma and respiratory disease [Fig. 1])5,13–17. However, traditional gender roles are evolving, and it is important to explore the potential exposures faced by men and women in occupations that were once strongly aligned with a particular gender. Changing roles at work and home may result in the reduction of gender differences in exposure to asthma-exacerbating triggers.
Fig. 1. Major categories for reported exposures associated with work-related asthma by gender.
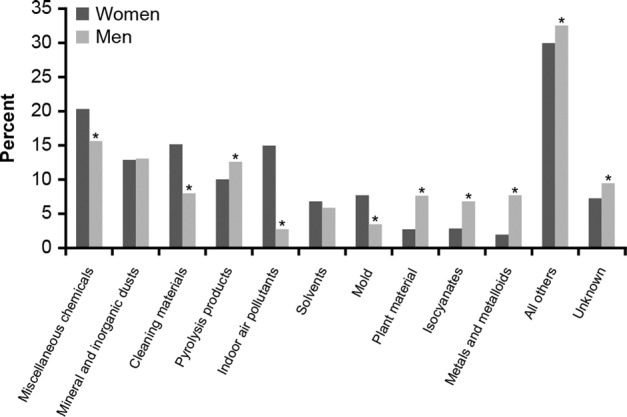
Categories defined by the Association of Occupational and Environmental Clinics (AOEC), September 20125; *p-value for gender differences <0.05. Percentages are based on the number of females (n = 4973) and males (n = 3264). Adapted from White et al.5. Reproduced with permission from Taylor & Francis © 2014. www.tandfonline.com.
On average, more men than women smoke18; however, it appears that women are more susceptible than men to smoking-related asthma symptoms19. Women who smoke are less likely than men to quit successfully as they are more susceptible to tobacco addiction: nicotine is metabolized faster in women than in men, resulting in a need for a higher level of nicotine to produce pleasurable feelings20. There may also be concerns for women surrounding cessation-related weight gain21.
Author guidance:
Be aware that, generally, men and women are exposed to different occupational and domestic triggers that may affect their asthma.
Explore patient’s occupation, lifestyle and history to identify possible exposure to triggers. Discuss personal behaviors, workplace strategies, and explore protective measures to minimize exposure. If asthma symptoms persist or worsen, explore possible lifestyle and occupation changes.
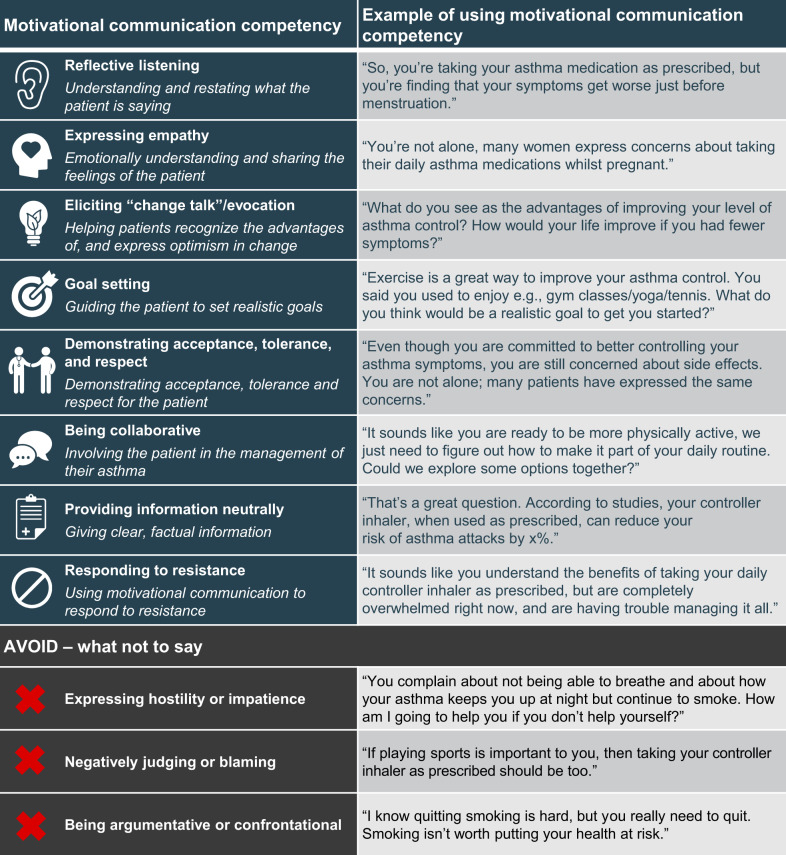
Although smoking cessation may be more difficult in women than men, due to factors such as susceptibility to tobacco addiction and weight-gain concerns, it should be encouraged regardless of patient sex/gender. Men and women often have different reasons for smoking or not quitting, and motivational communication (Fig. 212) could be used to understand these reasons and tailor cessation advice.
Fig. 2. Motivational communication competencies, definitions, and examples.
Adapted from Gosselin Boucher et al.12 under Creative Commons Attribution license CC-BY.
Physical activity and diet
Obesity and low levels of physical activity are associated with an increased risk of asthma symptoms22. Compared with women and girls, men and boys are more likely to participate in regular physical activity23,24 and are less likely to be obese25. However, men may have poorer diets than women; a large study that examined the relationship between gender, sexuality, and diet, reported that “very gender conforming males” (males with more ‘male’ personality traits) had unhealthier diets than the other groups examined26. It is known that a high intake of fruits and vegetables has anti-inflammatory properties that may reduce asthma risk and improve asthma control27, although more studies are needed on the relationship between diet and asthma outcomes.
Interventions that promote physical activity and healthy eating have been shown to improve asthma outcomes in both men and women28. It is important to note that men and women may have different goals and motivations for physical activity. Men have been shown to be motivated by competition, maintaining health, and enhancing body shape, whereas women are more motivated by emotional support and social aspects, as well as attaining well-being and a positive body image29.
Author guidance:
Promote initiatives to educate all patients on the benefits of weight control, physical activity, and healthy eating on asthma control. Guide patients towards physical activities that they find enjoyable and beneficial.
Adherence to asthma medication regimen
Taking medications correctly is important for asthma control. A Swedish study reported that, overall, men and women displayed similar levels of intentional nonadherence, but men with certain self-reported personality traits (e.g., agreeableness and conscientiousness) were more likely to adhere to their medication than men with more self-reported neurotic-type personality traits (e.g., vulnerability and self-consciousness)30; there was no association between personality traits and medication adherence in women31.
Women may be more likely to use their inhalation device incorrectly, which impacts asthma control32,33. Moreover, many patients rely on short-acting beta-agonists (SABAs) to treat their immediate asthma symptoms rather than taking daily maintenance medications that decrease the risk of exacerbations10,34. A Canadian study in patients ≥66 years old reported that women filled fewer prescriptions for maintenance inhalers and more prescriptions for reliever inhalers than men, suggesting that the women may also have had poorer asthma control31. As some people may not take their maintenance inhalers as prescribed and instead depend on reliever medication, the Global Initiative for Asthma (GINA) 2022 report recommends (in Track 1) low-dose inhaled corticosteroid (ICS)-formoterol as needed for mild asthma, or low-to-high dose ICS-formoterol as maintenance for more severe asthma (with low-dose ICS-formoterol as needed as a reliever). A second treatment track (Track 2) is possible, but not preferred10. It is important to consider that communities with limited resources can be found across low-, middle- and high-income countries; for these patients, Track 1 may not be affordable, and so Track 2 may be chosen while checking for adherence to treatment10.
Author guidance:
Help patients to understand that asthma is a chronic condition that can be controlled by taking asthma medication as prescribed and using inhalers correctly, in combination with self-management (e.g., reducing allergen exposure, engaging in a healthy lifestyle).
Educate patients on self-management to help them identify symptom worsening. Self-management education that includes a written action plan, regular review and symptom monitoring, reduces unscheduled visits, hospitalization and time lost from school or work10. A plan to explain when and how to increase medication(s) and when to seek HCP input may help patients gain greater control of their asthma, increase confidence about getting active and reduce asthma exacerbations35.
Assess the frequency of SABA use. In patients who are dependent on SABAs and/or are avoiding regular maintenance medication, ICS-formoterol can be used “on demand” in mild asthma (anti-inflammatory reliever therapy), or regularly twice-daily plus on demand if asthma is more severe10. For severe asthma, treatment regimens may be simplified (if considered appropriate and the patient is adherent) with a single once-daily inhaler with a long-acting (24 h) ICS/beta-agonist association, plus long-acting muscarinic antagonist if required10,36. Alternatively, add-on biologic therapy can be considered if these therapies are not sufficient to treat persistent symptoms, following a specialized consultation and review of potential care gaps10.
Assess asthma phenotype to tailor treatment and optimize asthma control.
Check inhaler technique regularly. Poor inhaler technique is common, and contributes to poor asthma control, along with overuse of SABAs and short-course oral corticosteroids (OCS)10,37.
Gender bias in HCP behavior
Implicit gender bias by HCPs is widespread and may affect the diagnosis, management, and health outcomes of diseases/conditions38. A 2019 analysis in Denmark found that in 72% of cases (of various diseases, including respiratory diseases), the median time span from symptom onset to diagnosis was longer in women than in men39. Moreover, in patients with chronic pain, men are often viewed as ‘brave’ while women are viewed as ‘emotional’ and ‘complaining’40. In a recent survey of women in England, 84% said they felt they were not listened to by HCPs41. This implicit bias may prevent women from receiving adequate asthma care40.
Asthma with comorbid anxiety is more prevalent in women than men42,43. As anxiety and stress can lead to HCPs taking patients’ symptoms less seriously40, it is possible that people with comorbid anxiety (women in particular) may be more likely to be misdiagnosed and/or receive sub-optimal asthma management44. In a 2006 study of patients with chronic obstructive pulmonary disease (COPD), a hypothetical case study was given to primary care physicians (PCPs), with half told the patient was a woman, and half told the same patient was a man. Results showed that COPD was more likely to be diagnosed in men than women, although this gender bias no longer appeared once the physicians were shown the patients’ spirometry results45. It is possible that assumptions that women are less likely to smoke and more likely to manifest anxiety as respiratory complaints may have played a role.
Spirometry is a vital tool to help confirm an asthma diagnosis but is under-utilized in diagnosing asthma in primary care10,46; this has been exacerbated by infection-control precautions during the COVID-19 pandemic. Under-utilization of spirometry may be more predominant in women: a recent study in patients ≥66 years old with asthma, reported that women experienced significantly lower rates of spirometry than men31. The reasons for this are unclear, but the authors suggest it could be related to either provider and/or patient behaviors. Nevertheless, the study highlights the importance of basing asthma diagnoses on objective measures like spirometry, peak flow, and fractional exhaled nitric oxide (FeNO) tests, as women in the study also had higher rates of Emergency Department (ED) visits than men. Recent studies of spirometry data from transgender and gender non-binary patients have also demonstrated that, although spirometry reference values should be based on birth sex and not gender47, until very recently, there has been a lack of guidance and hence inconsistent use of male and female reference ranges48. HCPs are often unsure whether reference ranges for birth sex or gender should be used, especially if patients feel discriminated against if birth sex is used49. This uncertainty may result in systemic or unconscious provider biases48 and may lead to misdiagnosis and inappropriate treatment50.
The gender of the HCP may also influence disease management. In a study of women general practice nurses, the nurses provided significantly more comprehensive information to women but discussed disease management more with men51. Furthermore, a 2020 study showed that female HCPs consulting with male patients discussed preventative interventions and lifestyle modification more often than any other patient–HCP gender combination52. This could be significant as it is probable that asthma educators are more likely to be women than men (as frontline HCPs are more likely to be women).
Author guidance:
Be aware of implicit gender bias, and the impact this has on asthma diagnosis, time to diagnosis, choice and interpretation of tests, and management. Gender bias training and self-assessment tools are recommended to gain these insights. HCPs can assess their gender bias using the Harvard University self-assessment tool53.
Eliminating gender bias should help in treating patients as individuals. Health behavior concerns should be addressed equally in men and women; for example, although more women than men may be physically inactive, it is important to still ask men about their physical activity as well as women.
Be aware of the importance of spirometry and its utilization to avoid both under- and over-treatment of asthma in men and women. Bronchial provocation (e.g., with methacholine), may also be used if a diagnosis cannot be reached10.
As sex is one of the predictive criteria for spirometry, consider what reference value ranges should be used for transgender patients. The 2019 American Thoracic Society and European Respiratory Society guidelines specify that patients should be informed that “birth sex and not gender is the determinant of predicted lung size” and using non-birth sex to calculate predicted spirometry values may lead to misdiagnosis and inappropriate treatment47.
HCP‒patient relationship
Good HCP–patient communication is vital, and time spent with patients exploring their concerns, encouraging health behavior changes, and supporting self-management can improve medication adherence and asthma control11. Motivational communication is a form of patient-centered behavior-change counseling that focuses on enhancing internal motivation to engage in appropriate self-management behaviors11,12,54. There is evidence that the impact of motivational communication can depend on the gender of the patient and of the HCP51.
The GINA 2022 report recommends that patients’ own healthcare goals and treatment preferences are incorporated into their asthma management plan10. However, it is important to recognize that women and men may have different healthcare goals. A recent study reported that men were more likely than women to focus on disease-specific goals (e.g., asthma control and medication reduction) rather than function-related (e.g., social, emotional) or knowledge-related (e.g., asthma education) goals. Better asthma control was achieved when patients (regardless of sex/gender) focused on disease-specific goals55.
Author guidance:
Consider undertaking evidence-based training on motivational communication (Fig. 2)12. As the impact of motivational communication can depend on the gender of the patient and the HCP51, training undertaken should try to address this discrepancy.
Prioritize spending time with patients at the start of the relationship, as this can save time spent in the long run11. Specialist asthma educators, pharmacists, school nurses and other HCPs can also support PCPs in educating/supporting patients with asthma56,57.
Perform regular asthma reviews with all patients; discuss the patient’s own healthcare goals and encourage them to focus on disease-specific goals.
Implement the GINA cycle of care for all patients10.
Management of asthma during pregnancy
Pregnancy affects asthma control in many women; approximately one-third of women report symptom worsening, one-third report symptom improvement, and one-third report no noticeable difference10. Poor maternal asthma control is associated with adverse outcomes, including increased risk of preterm birth, low birthweight, congenital malformations, perinatal death, and risk of childhood asthma58–60. In addition, a small reduction in the mother’s oxygen levels (e.g., during an asthma exacerbation) can result in severe, life-threatening fetal hypoxia61. It is, therefore, vital that pregnant women (and women who are thinking of becoming pregnant) are educated on taking their asthma medications as prescribed and have a plan for managing exacerbations62.
However, understandably, many women report being apprehensive about using asthma medication during pregnancy over concerns of teratogenicity, meaning that adherence to medications may decrease63. While the safety of most asthma medications (e.g., ICS, SABAs, long-acting beta-agonists, leukotriene receptor antagonists, OCS, and biologics) has not been unequivocally proven in pregnancy, they have now been used successfully for decades. Overall, evidence indicates asthma medications are safe in pregnancy, and their use is justified, as the benefits of good symptom control markedly outweigh the potential risks to mother and baby10,64,65.
Managing asthma symptoms during labor and delivery is also important, and guidelines advise women to continue with their usual asthma medications during this time10. Asthma symptoms occur in ~10% of deliveries61, and a cesarean section may be required if an acute exacerbation occurs66. Neonatal hypoglycemia is also a risk, especially if the woman takes high doses of beta-agonists in the 48 h before birth or if the baby is premature10. Oxytocin is the preferred drug to induce labor where necessary67. However, although this is a rare event, there is evidence from a small number of case studies that oxytocin can cause anaphylaxis in women with asthma68.
Author guidance:
Inform pregnant patients of the detrimental effects of poorly controlled maternal asthma for their baby, both during pregnancy and after.
Validate concerns using motivational communication and encourage pregnant patients to keep taking their asthma medication(s) as usual. The benefits of good symptom control outweigh the risks.
As asthma symptoms may worsen or improve during pregnancy, instruct pregnant women on how to adjust their medication(s) appropriately and have an action plan for the management of exacerbations during pregnancy and labor.
Inform pregnant women that although exacerbations during labor are rare, a cesarean section may be required in some instances if an exacerbation occurs66.
Aspects of pregnancy can mimic asthma symptoms (e.g., breathlessness). Understanding differential diagnosis and how to assess it (e.g. using spirometry67), could be useful when treating pregnant women with asthma.
Differential diagnoses
There may be difficulties in differentiating between symptoms caused by asthma and symptoms caused by other respiratory conditions (Table 120,42,43,69–79). For example, asthma and COPD share common features which make differentiating them complicated, especially in older adults and smokers80. In developed countries, the prevalence of COPD in men and women is similar81. However, potentially due to HCP implicit bias and lack of spirometry use, women are more likely to receive a diagnosis of asthma rather than COPD45,82. Additionally, because anxiety disorders (e.g., panic disorders, which may be associated with hyperventilation and dysfunctional breathing) and obesity are more commonly diagnosed in women than men42,43,69, women who report respiratory symptoms may have their symptoms attributed to anxiety or obesity, rather than taken seriously and investigated further.
Table 1.
Differential diagnoses of asthma in men and women.
| More common in women | More common in men |
|---|---|
| Obesity42,43 | COPD43 a |
| Dysfunctional breathing, including vocal cord dysfunction and exercise-induced laryngeal obstruction (EILO)69,70 | Lung cancer71 a |
| Anxiety43 | Idiopathic pulmonary fibrosis72 |
| Bronchiectasis73 | Heart failure74 |
| Gastro-esophageal reflux42,43 | Tuberculosis75 |
| Upper airway cough syndrome76 | |
| Pulmonary embolism77 | |
| Systemic sclerosis-associated interstitial lung disease78 |
Author guidance:
Determine whether symptoms are due to asthma or another condition and assess the severity of symptoms in all patients by using objective tests such as spirometry.
Be cautious about inferring anxiety disorders or obesity as a cause of symptoms based on the patient’s sex or gender.
Comorbidities
Comorbidities are associated with poor asthma outcomes43. Compared with men, women with asthma are more likely to have comorbidities, including obesity, osteoporosis, anxiety, and depression43. In addition, regular or frequent intake of OCS (and possibly high doses of ICS) to manage asthma symptoms increases the risk of developing side effects such as osteoporosis and cataracts83. As women are more likely than men to be prescribed OCS to manage their asthma symptoms84 (possibly because women report more severe symptoms than men4), they may be more at risk of these side effects. However, it is worth noting that corticosteroid use increases the risk of these comorbidities (including osteoporosis) in men as well as women85. Therefore, identifying patients who overuse corticosteroids is crucial for minimizing steroid-related comorbidities86.
Author guidance:
Be aware that not all symptoms that occur in someone with asthma are due to their asthma. Careful discussions with the patient and objective tests to explore the distinguishing characteristics may be needed to avoid over-treatment with SABAs.
Monitor both men and women for adverse events and comorbidities, including osteoporosis, particularly when using OCS as part of their asthma management regimen.
Sex hormones
Some women with asthma find that their symptoms worsen during certain phases in their menstrual cycle, during pregnancy, and at menopause10,87,88. Menopause is also associated with an increased risk of new-onset asthma89. Certain hormonal contraceptives have been shown to improve asthma symptoms and decrease the risk of asthma in pre-menopausal women90. It is interesting to note that, while estrogen and progesterone are involved in asthma pathogenesis, testosterone may protect against inflammatory processes that cause asthma7. However, there is a paucity of research into whether testosterone replacement therapy has beneficial effects on asthma, or if low testosterone (e.g., during andropause) affects asthma in men91.
Author guidance:
Recognize that some women with asthma may experience worse symptoms around menstruation, pregnancy and menopause.
Hormonal contraceptives have the potential to improve asthma control in women whose symptoms fluctuate with their menstrual cycle.
Fully assess women during pregnancy and at menopause who develop chest tightness or dyspnea to determine whether these symptoms are due to new-onset asthma, poor adherence to current asthma medication or other factors.
Sex/gender-specific phenotypes
Neutrophilic, obese asthma is a distinct phenotype that is more common in women than men and is often difficult to manage92,93. Women with neutrophilic, obese asthma tend to have lower lung function and a poor response to corticosteroids compared with non-obese women; this is not the case with men who have this phenotype93,94. Women may be more likely to have neutrophilia than men due to body composition. Women tend to have more subcutaneous than abdominal adipose fat, which secretes more leptin (a pro-inflammatory mediator that recruits neutrophils to the airways95), leading to increased neutrophilic inflammation96. Identifying a patient’s phenotype is useful when ascertaining the most effective medication to prescribe. More recently, ‘treatable traits’ have been proposed for the management of complex airway diseases. These are phenotypic or endotypic characteristics that are clinically relevant, measurable, and treatable97.
Author guidance:
Be wary of putting patients into ‘boxes’, and view asthma in terms of ‘treatable traits’ for which there are evidence-based interventions. With personalized medicine in mind, be adaptable to each patient’s individual characteristics.
Discussion and conclusion
Sex and gender can affect patient health behaviors, and implicit gender bias by HCPs is widespread and may affect diagnosis, management, and health outcomes38. Our suggestions should support HCPs to provide personalized asthma care for all patients, regardless of sex or gender. Figure 3 is a simple algorithm for navigating the considerations surrounding sex and gender differences when diagnosing and managing asthma. Figure 4 provides suggestions for minimizing the impact of sex and gender differences in asthma diagnosis and management.
Fig. 3. Considerations of sex and gender differences in patient asthma management.
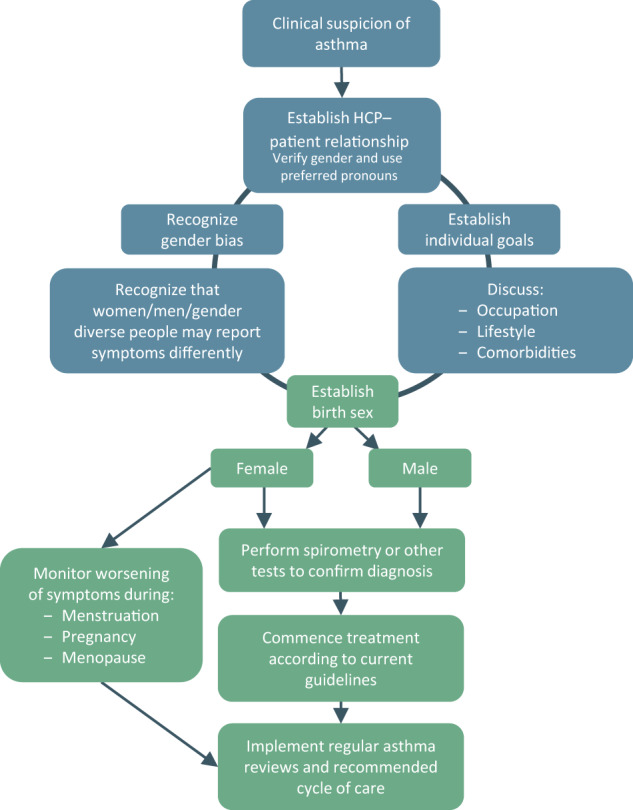
HCP healthcare professional.
Fig. 4. Summary: Suggested actions to minimize the impact of sex and gender differences in asthma diagnosis and management.
GINA Global Initiative for Asthma, HCP healthcare professional.
There are now many gender terms that people can use for self-identification. In everyday practice, HCPs will increasingly start to see patients with complex gender identities—so, although it is important to keep sex/gender differences in mind while diagnosing/managing asthma, it is crucial that HCPs treat patients as individuals and strive to provide personalized asthma care for all patients, regardless of sex or gender.
In addition, good collaboration must exist between all HCPs involved in the management of each patient (PCP, pneumologist, gynecologist etc.). Further research would allow HCPs to better account for sex/gender in diagnosing and treating their patients with asthma. Urgent research is needed to investigate the links between hormone changes and asthma in women and men, and the effects of testosterone. To this end, there is a need for greater consideration of sex and gender in the design and analysis of clinical trials. Clarity regarding the use and definition of ‘male/female’ and ‘women/men’ is also necessary and would be a good starting point. Ultimately, knowledge of the causes of sex and gender disparities in asthma diagnosis and management should be a high priority for new research on how to increase gender equity and improve quality in clinical practice. In view of the evidence that sex- and gender-related differences and biases can significantly and adversely impact diagnosis and management if not recognized, it is concerning that these differences are largely not taught during HCP training, including in curricula, or discussed in guidance followed by HCPs10,98. It is, therefore, our opinion, that updated guidance and resources are urgently needed to help HCPs minimize the impact that sex and gender have on asthma diagnosis and management. For individualized asthma management to become part of normal HCP practice, it is essential that a new approach to asthma research, diagnosis, and management is taken, one that considers sex and gender, while treating the patient as an individual.
Acknowledgements
This publication was funded by Novartis Pharma, Basel, Switzerland. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Ellen Maxwell, PhD and Anna King, PhD. This publication was written in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). D.S. is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Author contributions
L.P.B.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published; K.L.L.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published; C.R.S.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published; A.K.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published; D.S.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published; C.R.J.: made a substantial contribution to the initial concept of this review article, critically reviewed the intellectual content during the development process, and approved the final version to be published.
Data availability
This is a narrative review manuscript and does not report original research/data; thus, data sharing is not applicable.
Competing interests
L.P.B. reports research grants, consulting fees and honoraria from AstraZeneca, GlaxoSmithKline, Merck, Novartis, and Sanofi-Regeneron; research grants from Amgen and Biohaven; and honoraria from Covis, Cipla and Novartis all outside the submitted work. K.L. reports consulting fees and honoraria from AstraZeneca, Novartis, GlaxoSmithKline, AbbVie, Boehringer Ingelheim; consulting fees from Baucsh and Sojecci Inc; and honoraria from Astellas, Janssen, xFacto, Respiplus, outside the submitted work. C.R.S. reports grants and personal fees from Novartis, Chiesi, and AstraZeneca; and personal fees from GlaxoSmithKline, outside the submitted work. A.K. reports personal fees from AstraZeneca, Behring, Boehringer Ingelheim, Cipla, Covis, GlaxoSmithKline, Merck Frosst, Novartis, NovoNordisk, Pfizer, Sanofi-Genzyme, Teva, Trudel, and Valeo. D.S. reports personal fees from Aerogen, AstraZeneca, Boehringer Ingleheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Synairgen, Teva, Theravance, and Verona, outside the submitted work. C.J. reports personal fees for advisory board participation and educational content from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Sanofi-Genzyme; writing support from Novartis, and grant funding from GlaxoSmithKline, outside the submitted work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mattiuzzi C, Lippi G. Worldwide asthma epidemiology: insights from the Global Health Data Exchange Database. Int. Forum Allergy Rhinol. 2020;10:75–80. doi: 10.1002/alr.22464. [DOI] [PubMed] [Google Scholar]
- 2.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma Rep. 2017;17:19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senna G, et al. Sex differences in severe asthma: results from severe asthma network in Italy-SANI. Allergy Asthma Immunol. Res. 2021;13:219–228. doi: 10.4168/aair.2021.13.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo D, Zagni E, Ferri F, Canonica GW. Gender differences in asthma perception and its impact on quality of life: a post hoc analysis of the PROXIMA (Patient Reported Outcomes and Xolair(®) In the Management of Asthma) study. Allergy Asthma Clin. Immunol. 2019;15:65. doi: 10.1186/s13223-019-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White GE, et al. Gender differences in work-related asthma: surveillance data from California, Massachusetts, Michigan, and New Jersey, 1993-2008. J. Asthma. 2014;51:691–702. doi: 10.3109/02770903.2014.903968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarstrom A, Annandale E. A conceptual muddle: an empirical analysis of the use of ‘sex’ and ‘gender’ in ‘gender-specific medicine’ journals. PLoS ONE. 2012;7:e34193. doi: 10.1371/journal.pone.0034193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins CR, Boulet LP, Lavoie KL, Raherison-Semjen C, Singh D. Personalized treatment of asthma: the importance of sex and gender differences. J. Allergy Clin. Immunol. Pract. 2022;10:963–971.e3. doi: 10.1016/j.jaip.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Borges, R. C., Alith, M. B., Nascimento, O. A. & Jardim, J. R. Gender differences in the perception of asthma respiratory symptoms in five Latin American countries. J. Asthma10.1080/02770903.2021.1922914 (2021). [DOI] [PubMed]
- 9.Vitulano LA. Psychosocial issues for children and adolescents with chronic illness: self-esteem, school functioning and sports participation. Child Adolesc. Psychiatr. Clin. N. Am. 2003;12:585–592. doi: 10.1016/S1056-4993(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Asthma (GINA). 2022 GINA Report, Global Strategy for Asthma Management and Prevention. https://ginasthma.org/gina-reports/ (2022).
- 11.Lavoie KL, et al. Efficacy of brief motivational interviewing to improve adherence to inhaled corticosteroids among adult asthmatics: results from a randomized controlled pilot feasibility trial. Patient Prefer. Adherence. 2014;8:1555–1569. doi: 10.2147/PPA.S66966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosselin Boucher V, et al. Assessing Physician’s Motivational Communication Skills: The development of the Motivational Communication Competency Assessment Test (MC-CAT) JMIR Med. Educ. 2022;8:e31489. doi: 10.2196/31489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okello G, Devereux G, Semple S. Women and girls in resource poor countries experience much greater exposure to household air pollutants than men: Results from Uganda and Ethiopia. Environ. Int. 2018;119:429–437. doi: 10.1016/j.envint.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oluwole O, Arinola GO, Huo D, Olopade CO. Household biomass fuel use, asthma symptoms severity, and asthma underdiagnosis in rural schoolchildren in Nigeria: a cross-sectional observational study. BMC Pulm. Med. 2017;17:3. doi: 10.1186/s12890-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscato G, et al. Gender and occupational allergy: Report from the task force of the EAACI Environmental and Occupational Allergy Interest Group. Allergy. 2020;75:2753–2763. doi: 10.1111/all.14317. [DOI] [PubMed] [Google Scholar]
- 16.Global Burden of Disease (GBD) Occupational Chronic Respiratory Risk Factors Collaborators. Global and regional burden of chronic respiratory disease in 2016 arising from non-infectious airborne occupational exposures: a systematic analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020;77:142. doi: 10.1136/oemed-2019-106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svanes O, et al. Cleaning at home and at work in relation to lung function decline and airway obstruction. Am. J. Respir. Crit. Care Med. 2018;197:1157–1163. doi: 10.1164/rccm.201706-1311OC. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie, H. Who smokes more, men or women?https://ourworldindata.org/who-smokes-more-men-or-women (2022).
- 19.Langhammer A, Johnsen R, Holmen J, Gulsvik A, Bjermer L. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT) J. Epidemiol. Community Health. 2000;54:917–922. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SJ, et al. To quit or not: vulnerability of women to smoking tobacco. J. Environ. Sci. Health C. Enviro. n Carcinog. Ecotoxicol. Rev. 2016;34:33–56. doi: 10.1080/10590501.2015.1131539. [DOI] [PubMed] [Google Scholar]
- 21.Allen AM, Oncken C, Hatsukami D. Women and smoking: the effect of gender on the epidemiology, health effects, and cessation of smoking. Curr. Addict. Rep. 2014;1:53–60. doi: 10.1007/s40429-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters U, Dixon AE, Forno E. Obesity and asthma. J. Allergy Clin. Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, H., Millward, P. & Buraimo, B. Adult participation in sport: Analysis of the Taking Part Survey, Department for Culture, Media and Sport (2011). https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/137986/tp-adult-participation-sport-analysis.pdf (2022).
- 24.Trost SG, et al. Age and gender differences in objectively measured physical activity in youth. Med. Sci. Sports Exerc. 2002;34:350–355. doi: 10.1097/00005768-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Cooper AJ, Gupta SR, Moustafa AF, Chao AM. Sex/gender differences in obesity prevalence, comorbidities, and treatment. Curr. Obes. Rep. 2021;10:458–466. doi: 10.1007/s13679-021-00453-x. [DOI] [PubMed] [Google Scholar]
- 26.VanKim NA, et al. Gender expression and sexual orientation differences in diet quality and eating habits from adolescence to young adulthood. J. Acad. Nutr. Diet. 2019;119:2028–2040. doi: 10.1016/j.jand.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilleminault, L. et al. Diet and asthma: is it time to adapt our message? Nutrients10.3390/nu9111227 (2017). [DOI] [PMC free article] [PubMed]
- 28.Nyenhuis SM, Dixon AE, Ma J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: what is the evidence? J. Allergy Clin. Immunol. Pract. 2018;6:751–763. doi: 10.1016/j.jaip.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld CS. Sex-dependent differences in voluntary physical activity. J. Neurosci. Res. 2017;95:279–290. doi: 10.1002/jnr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, et al. The relationship between big five personality traits and psychotic experience in a large non-clinical youth sample: the mediating role of emotion regulation. Front. Psychiatry. 2018;9:648. doi: 10.3389/fpsyt.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To, T. et al. Sex differences in health services and medication use among older adults with asthma. ERJ Open Res. 10.1183/23120541.00242-2019 (2019). [DOI] [PMC free article] [PubMed]
- 32.Chrystyn H, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. npj Prim. Care Respir. Med. 2017;27:22. doi: 10.1038/s41533-017-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Self TH, et al. Gender differences in the use of peak flow meters and their effect on peak expiratory flow. Pharmacotherapy. 2005;25:526–530. doi: 10.1592/phco.25.4.526.61026. [DOI] [PubMed] [Google Scholar]
- 34.Nwaru BI, et al. Overuse of short-acting beta2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur. Respir. J. 2020;55:1901872. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royal College of Physicians UK. National Review of Asthma Deaths NRAD. Why asthma still kills. https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills (2015).
- 36.Zhang S, King D, Rosen VM, Ismaila AS. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int. J. Chron. Obstruct. Pulmon. Dis. 2020;15:417–438. doi: 10.2147/COPD.S234823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melani AS, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir. Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 38.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med. Ethics. 2017;18:19. doi: 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westergaard D, Moseley P, Sorup FKH, Baldi P, Brunak S. Population-wide analysis of differences in disease progression patterns in men and women. Nat. Commun. 2019;10:666. doi: 10.1038/s41467-019-08475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samulowitz A, Gremyr I, Eriksson E, Hensing G. “Brave Men” and “Emotional Women”: a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain. Res. Manag. 2018;2018:6358624. doi: 10.1155/2018/6358624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Department of Health and Social Care. Our vision for the Women’s Health Strategy for England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042631/dhsc-our-vision-for-the-women_s-health-strategy-for-england.pdf (2021).
- 42.Hekking PP, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in difficult-to-control asthma. J. Allergy Clin. Immunol. Pract. 2018;6:108–113. doi: 10.1016/j.jaip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Veenendaal M, et al. Age- and sex-specific prevalence of chronic comorbidity in adult patients with asthma: a real-life study. npj Prim. Care Respir. Med. 2019;29:14. doi: 10.1038/s41533-019-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavoie KL, et al. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients? Respir. Med. 2005;99:1249–1257. doi: 10.1016/j.rmed.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Miravitlles M, et al. [Attitudes toward the diagnosis of chronic obstructive pulmonary disease in primary care] Arch. Bronconeumol. 2006;42:3–8. doi: 10.1157/13083272. [DOI] [PubMed] [Google Scholar]
- 46.Sokol KC, Sharma G, Lin YL, Goldblum RM. Choosing wisely: adherence by physicians to recommended use of spirometry in the diagnosis and management of adult asthma. Am. J. Med. 2015;128:502–508. doi: 10.1016/j.amjmed.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Graham BL, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019;200:e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foer D, et al. Gender reference use in spirometry for transgender patients. Ann. Am. Thorac. Soc. 2021;18:537–540. doi: 10.1513/AnnalsATS.202002-103RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez A, Agardh A, Asamoah BO. Self-reported discrimination in health-care settings based on recognizability as transgender: a cross-sectional study among transgender U.S. citizens. Arch. Sex. Behav. 2018;47:973–985. doi: 10.1007/s10508-017-1028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haynes JM, Stumbo RW. The impact of using non-birth sex on the interpretation of spirometry data in subjects with air-flow obstruction. Respir. Care. 2018;63:215–218. doi: 10.4187/respcare.05586. [DOI] [PubMed] [Google Scholar]
- 51.Noordman J, van Dulmen S. The consequences of task delegation for the process of care: female patients seem to benefit more. Women Health. 2016;56:194–207. doi: 10.1080/03630242.2015.1086467. [DOI] [PubMed] [Google Scholar]
- 52.Delpech R, et al. Physicians’ preventive practices: more frequently performed for male patients and by female physicians. BMC Health Serv. Res. 2020;20:331. doi: 10.1186/s12913-020-05136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harvard University. Project Implicit. https://implicit.harvard.edu/implicit/ethics.html (2022).
- 54.Dragomir AI, et al. An international Delphi consensus study to define motivational communication in the context of developing a training program for physicians. Transl. Behav. Med. 2021;11:642–652. doi: 10.1093/tbm/ibaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulvey C, et al. Patient-selected treatment goals in severe asthma. J. Allergy Clin. Immunol. Pract. 2021;9:2732–2741.e2731. doi: 10.1016/j.jaip.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 56.Kovacevic M, et al. Impact of community pharmacists’ interventions on asthma self-management care. Res. Soc. Adm. Pharm. 2018;14:603–611. doi: 10.1016/j.sapharm.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Francisco B, Rood T, Nevel R, Foreman P, Homan S. Teaming up for asthma control: EPR-3 compliant school program in missouri is effective and cost-efficient. Prev. Chronic Dis. 2017;14:E40. doi: 10.5888/pcd14.170003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdullah, K., Zhu, J., Gershon, A., Dell, S. & To, T. Effect of asthma exacerbation during pregnancy in women with asthma: a population-based cohort study. Eur. Respir. J. 10.1183/13993003.01335-2019 (2020). [DOI] [PubMed]
- 60.Liu X, et al. Maternal asthma severity and control during pregnancy and risk of offspring asthma. J. Allergy Clin. Immunol. 2018;141:886–892.e883. doi: 10.1016/j.jaci.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Vieira, A. C., Pité, H. & Morais-Almeida, M. Asthma and pregnancy in the 2020 decade: still a matter of concern. J. Matern. Fetal Neonatal Med. 10.1080/14767058.2021.1916462 (2021). [DOI] [PubMed]
- 62.UpToDate. Patient education: Asthma and pregnancy (Beyond the Basics). https://www.uptodate.com/contents/asthma-and-pregnancy-beyond-the-basics. (2022).
- 63.Ibrahim WH, et al. Asthma knowledge, care, and outcome during pregnancy: the QAKCOP study. Chron. Respir. Dis. 2019;16:1479972318767719. doi: 10.1177/1479972318767719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.British Thoracic Society & Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax69(Suppl 1), 1–192 (2014). [PubMed]
- 65.Murphy VE, Schatz M. Asthma in pregnancy: a hit for two. Eur. Respir. Rev. 2014;23:64–68. doi: 10.1183/09059180.00008313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robijn, A. L. et al. Effect of maternal asthma exacerbations on perinatal outcomes: a population-based study. ERJ Open Res. 10.1183/23120541.00295-2020 (2020). [DOI] [PMC free article] [PubMed]
- 67.Weinberger, S. E. Patient education: Asthma and pregnancy (Beyond the Basics). https://www.uptodate.com/contents/asthma-and-pregnancy-beyond-the-basics/print#:~:text=For%20women%20with%20asthma%2C%20the,sleep)%20for%20women%20with%20asthma (2021).
- 68.Liccardi G, et al. Oxytocin: an unexpected risk for cardiologic and broncho-obstructive effects, and allergic reactions in susceptible delivering women. Multidiscip. Respir. Med. 2013;8:67. doi: 10.1186/2049-6958-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vidotto LS, Carvalho CRF, Harvey A, Jones M. Dysfunctional breathing: what do we know? J. Bras. Pneumol. 2019;45:e20170347. doi: 10.1590/1806-3713/e20170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walsted ES, et al. Characteristics and impact of exercise-induced laryngeal obstruction: an international perspective. ERJ Open Res. 2021;7:00195–02021. doi: 10.1183/23120541.00195-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci. Rep. 2017;7:14300. doi: 10.1038/s41598-017-14513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin. Epidemiol. 2013;5:483–492. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quint JK, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur. Respir. J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Savarese G, Lund LH. Global public health burden of heart failure. Card. Fail. Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horton, K. C., MacPherson, P., Houben, R. M., White, R. G. & Corbett, E. L. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 13, e1002119 (2016). [DOI] [PMC free article] [PubMed]
- 76.Morice AH, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur. Respir. J. 2014;44:1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal S, et al. Gender disparities in outcomes and resource utilization for acute pulmonary embolism hospitalizations in the United States. Am. J. Cardiol. 2015;116:1270–1276. doi: 10.1016/j.amjcard.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 78.Bergamasco A, Hartmann N, Wallace L, Verpillat P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin. Epidemiol. 2019;11:257–273. doi: 10.2147/CLEP.S191418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson J, et al. Differential diagnosis of asthma. Allergy Asthma. 2019;23:383–400. doi: 10.1007/978-3-030-05147-1_17. [DOI] [Google Scholar]
- 80.American Academy of Family Physicians. COPD and Asthma. https://www.aafp.org/dam/AAFP/documents/journals/fpm/COPD-Asthma.pdf (2016).
- 81.Ntritsos G, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018;13:1507–1514. doi: 10.2147/COPD.S146390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raherison-Semjen C, Mezzi K, Kostikas K, Mackay AJ, Singh D. The perception of physicians on gender-specific differences in the diagnosis of COPD: results from a Questionnaire-based survey. Int. J. Chron. Obstr. Pulmon. Dis. 2021;16:901–907. doi: 10.2147/COPD.S271505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bloechliger M, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir. Res. 2018;19:75. doi: 10.1186/s12931-018-0742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tran, T. N. et al. Oral corticosteroid prescription patterns for asthma in France, Germany, Italy and the UK. Eur. Respir. J. 10.1183/13993003.02363-2019 (2020). [DOI] [PMC free article] [PubMed]
- 85.Barry LE, et al. Age and sex associations with systemic corticosteroid-induced morbidity in asthma. J. Allergy Clin. Immunol. Pract. 2018;6:2014–2023 e2012. doi: 10.1016/j.jaip.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Hew M, et al. Cumulative dispensing of high oral corticosteroid doses for treating asthma in Australia. Med. J. Aust. 2020;213:316–320. doi: 10.5694/mja2.50758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sánchez-Ramos JL, et al. Risk factors for premenstrual asthma: a systematic review and meta-analysis. Expert Rev. Respir. Med. 2017;11:57–72. doi: 10.1080/17476348.2017.1270762. [DOI] [PubMed] [Google Scholar]
- 88.McCleary N, Nwaru BI, Nurmatov UB, Critchley H, Sheikh A. Endogenous and exogenous sex steroid hormones in asthma and allergy in females: a systematic review and meta-analysis. J. Allergy Clin. Immunol. 2018;141:1510–1513.e1518. doi: 10.1016/j.jaci.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Triebner K, et al. Menopause as a predictor of new-onset asthma: a longitudinal Northern European population study. J. Allergy Clin. Immunol. 2016;137:50–57.e56. doi: 10.1016/j.jaci.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 90.Nwaru BI, Sheikh A. Hormonal contraceptives and asthma in women of reproductive age: analysis of data from serial national Scottish Health Surveys. J. R. Soc. Med. 2015;108:358–371. doi: 10.1177/0141076815588320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med. Hypotheses. 2011;76:585–588. doi: 10.1016/j.mehy.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsiao HP, Lin MC, Wu CC, Wang CC, Wang TN. Sex-specific asthma phenotypes, inflammatory patterns, and asthma control in a cluster analysis. J. Allergy Clin. Immunol. Pract. 2019;7:556–567.e515. doi: 10.1016/j.jaip.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Hancox RJ, et al. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am. J. Respir. Crit. Care Med. 2005;171:440–445. doi: 10.1164/rccm.200405-623OC. [DOI] [PubMed] [Google Scholar]
- 95.Souza-Almeida G, et al. Leptin mediates in vivo neutrophil migration: involvement of tumor necrosis factor-alpha and CXCL1. Front. Immunol. 2018;9:111. doi: 10.3389/fimmu.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Telenga ED, et al. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 97.McDonald, V. M. et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur. Respir. J. 10.1183/13993003.02058-2018 (2019). [DOI] [PubMed]
- 98.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis And Management of Chronic Obstructive Pulmonary Disease.https://goldcopd.org/2021-gold-reports/ (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a narrative review manuscript and does not report original research/data; thus, data sharing is not applicable.