Abstract
Background
The current coronavirus disease (COVID-19) pandemic makes it difficult to obtain physical therapy in rehabilitation centres, particularly for persons with multiple sclerosis (pwMS), who are a population at high risk, since viral infections may contribute to MS exacerbations and relapses. Active video games could be a way to maintain physical therapy at home as part of the rehabilitation. The aim of this review is to summarise the current best evidence for the effectiveness of home-based active video games on gait and balance, user compliance, feasibility and safety for pwMS.
Methods
We searched for studies in five databases (PubMed, Scopus, Cochrane, CINAHL and Science direct) up to October 2020. Selection of studies, extraction of data and methodological quality assessment through the PEDro scale were made independently by two authors and discussed with a third author.
Results
Nine studies were included in this systematic review. We found significant improvements in balance; results were mixed concerning mobility, physical activity and gait. Home-based active video games are feasible and safe, with good compliance and adherence. The methodological quality of the studies was moderate (PEDro scale: 5.3 ± 2).
Conclusion
Overall, home-based active video games were found safe and effective improving static and dynamic balance in pwMS. Compliance was good, probably because it is a motivating and enjoyable training. Active video games can be a relevant alternative for physical rehabilitation at home in pwMS. Future studies should follow more rigorous methodological standards (larger sample sizes, more randomised controlled trials) to improve the quality of evidence and include cost-effectiveness in the analysis.
Keywords: Multiple sclerosis, Rehabilitation, Home, Active video game, Balance
1. Introduction
Multiple sclerosis (MS) is a chronic neurological demyelinating disease affecting the central nervous system. This disease is the leading cause of nontraumatic neurological disability in young adults in Europe and North America (Browne et al., 2014). Its symptoms are various, depending on the severity and spatial distribution of the lesions (Milo and Miller, 2014), but the current clinical manifestations of MS are: deterioration of motor, sensory, visual, and genitosphincterian functions (Compston and Coles, 2008). Regarding locomotor aspects, MS decreases strength, coordination, gait (Comber et al., 2017), balance and increases the fear of falling (FoF) (Perrochon et al., 2017) and risk of falls in persons with MS (pwMS) (Nilsagård et al., 2015). The literature reports that physical capabilities (i.e., mobility, aerobic capacity and muscle strength) (Amatya et al., 2019) and balance (Paltamaa et al., 2012) can be improved by physical therapy for pwMS. In the current coronavirus disease (COVID-19) context, it is particularly difficult for pwMS to obtain their usual health care, such as rehabilitation. Indeed, pwMS are a population with an increased risk of infection or serious complications due to COVID-19 (Sadeghmousavi and Rezaei, 2020). Some studies have demonstrated the benefit of telemedicine and active video games (AVG) during the pandemic period for continuity of health care delivery at home (Ambrosino et al., 2020; Hollander and Carr, 2020).
AVG are defined as the integration of physical activity into a video game environment requiring active body movements to control the game (Mat Rosly et al., 2017). AVG use a wide range of interfaces (Baranowski et al., 2008) and a tow-dimensional virtual environment projected on a standard screen, less immersive than virtual reality (Tieri et al., 2018). AVG enhance adherence and motivation in rehabilitation programs (Bonnechère et al., 2016; Maggio et al., 2019; Massetti et al., 2016; Taylor and Griffin, 2015). Several studies have shown the relevance of AVG in aging (S. Gallou-Guyot et al., 2020a, 2020b) and in neurological diseases (Bonnechère et al., 2016; Mat Rosly et al., 2017; Prosperini et al., 2020) such as stroke (Laver et al., 2017), Parkinson's disease (Triegaardt et al., 2019) and MS (Casuso-Holgado et al., 2018; Maggio et al., 2019; Massetti et al., 2016; Taylor and Griffin, 2015). AVG offer numerous advantages, such as the ability to practice moderate intensity physical activity (Mat Rosly et al., 2017) and dual-task training while playing, which increases motivation for the patient (Bonnechère et al., 2016; Perrochon et al., 2019), and prevents of monotony and boredom while providing direct feedback (Bonnechère et al., 2016). AVG are reported to be enjoyable and may enhance adherence to rehabilitation (Maggio et al., 2019; Massetti et al., 2016; Taylor and Griffin, 2015). Systematic reviews have reported that AVG have positive effects on gait and balance in pwMS (Casuso-Holgado et al., 2018; Maggio et al., 2019; Massetti et al., 2016; Taylor and Griffin, 2015). They seem at least as effective as conventional rehabilitation in improving balance and gait (Casuso-Holgado et al., 2018) and can therefore be an alternative therapy. While a major interest of AVG is their possible use at home (Miller et al., 2014), all reviews in pwMS have focused on centre or laboratory-based interventions.
A recent systematic review demonstrated an overall effectiveness of AVG at home at least equivalent to conventional therapy or usual care in people with neurological disease (i.e., stroke, Parkinson's disease, MS) (Perrochon et al., 2019). To date, no systematic review has been conducted to specifically assess the effectiveness and user compliance of home-based AVG in MS. Some studies seem to show positive effects of home-based AVG on motor function in pwMS (Chanpimol et al., 2020; Hoang et al., 2016; Prosperini et al., 2013) and report that patients felt more independent and confident and reported having fun with friends and family members (Palacios-Cena et al., 2016; Plow and Finlayson, 2014).
The aim of this review is to summarise the current best evidence for the effectiveness of home-based AVG on gait and balance in pwMS, as well as user compliance, feasibility and safety for this type of rehabilitation.
2. Methods
2.1. Search strategy
In order to perform this review, we used a protocol established prior to conducting the review that was registered on PROSPERO (registration ID: CRD42020200328). This systematic review was structured according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009), and by addition of some information from the AMSTAR-2 tool (Shea et al., 2017). We searched studies published since 2010 on PubMed, Scopus, Cochrane, CINAHL and Science Direct databases. The research was conducted up to 26 October 2020. We used the same search strategy, adapted for all databases. It combined the following terms: [("multiple sclerosis") AND ("virtual reality" OR exergam* OR "active video gam*" OR "interactive video gam*" OR "video gam*" OR "computer gam*" OR Kinect OR Nintendo Wii OR Wii OR Xbox) AND (rehabilitation OR intervention OR training OR program*)], where * designates a wildcard to allow other suffixes. To avoid missing relevant articles we also searched the grey literature.
2.2. Selection of studies
Only research articles in English were considered, excluding review articles, conference abstracts and case reports. The inclusion criteria were persons with a diagnosis of MS and home-based AVG as intervention. The exclusion criteria were qualitative studies, clinical trials, not home-based interventions and no literature access.
Two authors (MD and MGG) independently performed the database research and removed duplicates using Zotero software. The same authors removed studies which did not match the criteria, based on their titles and abstracts. The remaining articles were screened full-text for eligibility, and in case of uncertainty or disagreement the decision was resolved by a third author (AP).
2.3. Data extraction
Two authors (MD and AP) extracted the relevant information and another author (MGG) verified the extracted data. We extracted author names, year of the study, country, objectives, study design, follow-up, population (i.e., the number, age and EDSS score), modalities of intervention, comparator, outcome, and conclusion on effectiveness. We also extracted data concerning compliance (i.e., satisfaction, drop-outs and discontinued), feasability (supervision and follow-up, material used, installation and setting) and safety (appearance of adverse events).
2.4. Quality assessment
Two authors (MD and MGG) independently assessed the methodological quality of the selected studies using the Physiotherapy Evidence Database (PEDro) scale (Maher et al., 2003; Verhagen et al., 1998) which gaves a score of 10 for each study. Disagreements between authors or ambiguities during the quality assessment were resolved by a third author (AP).
3. Results
3.1. Study selection
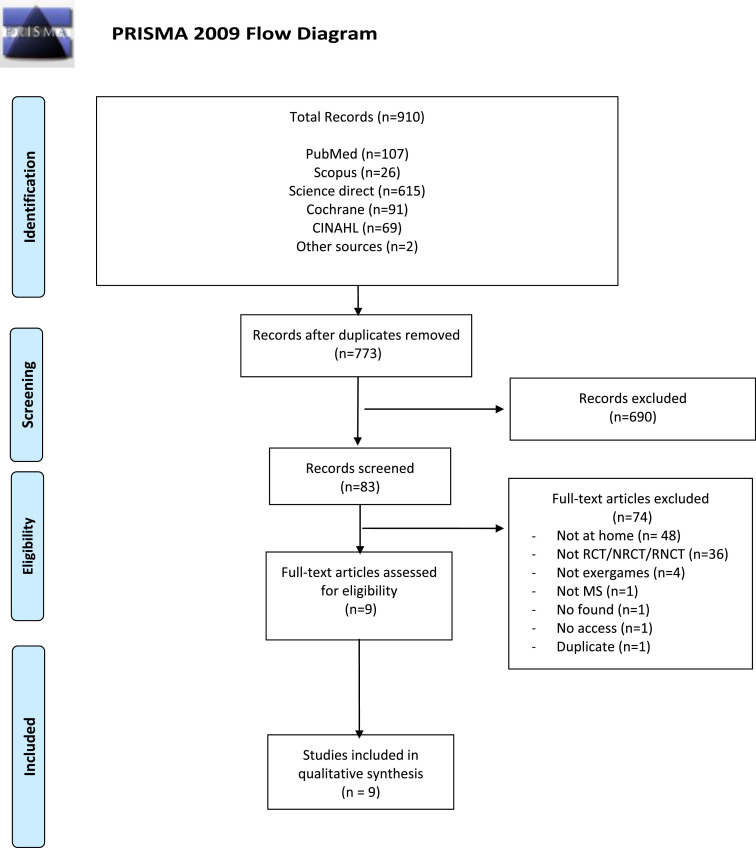
The initial database search revealed 910 potentially relevant articles. After removing duplicates, 772 papers were screened by title and abstract. Eighty-three (83) articles were analysed by full text, and 9 were included (Fig. 1 ). The reasons for exclusion, conflicts of interest and study funding are detailed respectively in Appendices A and B.
Fig. 1.
PRISMA flowchart for study selection.
3.2. Study design and sample characteristics
The main characteristics of the studies are summarised in Table 1 . The nine studies were published between 2011 and 2020. Four studies were randomised controlled trials (RCT) (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013; Thomas et al., 2017), two were non-randomised studies (NRCT) (Gutierrez et al., 2013; Kramer et al., 2014) and three were non-controlled (NCT) (Chanpimol et al., 2020; Pau et al., 2015; Plow and Finlayson, 2011). Six studies compared EG (i.e., experimental group) with a control group (CG), including a passive CG (i.e., usual care/no intervention) (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013; Thomas et al., 2017) or active CG (i.e., conventional therapy) (Gutierrez et al., 2013; Kramer et al., 2014). One study used a 12 week crossover design (Prosperini et al., 2013) and another required the CG to use the AVG after an observational period (usual care) of 6 months (Thomas et al., 2017). Finally, in one study the EG began with 3 weeks of rehabilitation in the rehabilitation centre, followed by 6 months at home (Kramer et al., 2014).
Table 1.
Main characteristics of included studies (intervention, outcome and main findings) (n = 9).
| First Author, Year Country | Objectives | Type of studies Design Follow (w) | Population Nb EG/CG Age (mean±SD) EG/CG EDSS (mean±SD) or median [range] Disease duration (y) | Interventions EG HardwareEG SoftwareCG | Modalities Duration, Frequency, Length Total time (calculate in h) Supervision | Outcomes | Conclusion Effectiveness (EG)Comparator (EG/CG)Nb of dropouts or discontinued Total (EG/CG) Training time achievedCompliance h (%) achievement of totalSafety (nb AE) Total (EG/CG) | PEDro Score |
|---|---|---|---|---|---|---|---|---|
| Chanpimol, 2020 USA | Effectiveness of exergame on physical function Acceptability |
NCT - |
10 49.6 ± 9.0 5 [3.5–6.0] 8.0 ± 6.3 |
Xbox 360 ® with Kinect Tablet Jintronix® rehabilitation software (VITAL Rehab) - |
30 min, 3x/w, 12w 18h Teleconferencing (1x/w) | Gait: MSWS-12, 2MWT Mobility: SPPB, 25FW Other: MFIS |
↗ mobility (SPPB, 25FW), gait (2MWT) - No dropout 14.9 h (83%) Higher satisfaction No AE |
3 |
| Gutierrez, 2013 Spain | Effectiveness of exergame on balance and postural control | NRCT - |
47 (24/23) 39.7 ± 8.1/42.8 ± 7.4 4 [3–5]/4 [3–5] 9.7 ± 6.8/10.9 ± 5.4 |
Xbox 360 ® with Kinect 3 games: Kinect Sports®, Joy Ride® and Adventures® Conventional therapy | 20 min, 4x/w, 10w 13.3h Teleconferencing (all sessions) | Balance: CES, MCT, BBS, POMA | ↗ balance (all tests) EG>CG for balance (all tests) 3 (1/2) >10.6 h, >80% Satisfactory compliance for EG and CG n.r. |
6 |
| Hoang, 2015 Australia |
Effectiveness of exergame on balance, stepping, functional performance, and cognition compared to usual care | RCT 24w |
50 (28/22) 53.4 ± 10.7/51.4 ± 12.8 4.1 ± 1.4/4.2 ± 1.2 11.6 ± 9.1/13.4 ± 6.9 |
Step training (modified DDR) Stepmania open-source including rhythm video game and CSRT Usual care |
30 min, 2x/w, 12 w 12 h Home visit: to install system Phone call: 1x, in the first 2 w |
Balance: COP Falls: nb of falls Gait: 10MWT, 6MWT Mobility: CSRT, SST, TUG (ST and DT), 9-HPT, MSFC Other: SDMT, TMT |
- EG>CG for mobility (CSRT, STT, 9-HPT, TUG DT, MSFC), balance (COP), gait (10MWT) 6 (5/1) 14.2 h No AE |
8 |
| Kramer, 2014 Germany |
Effectiveness of exergame on balance and adherence compared to two balance training programs | NRCT - |
61 (21/20/20) 47±9 3 ± 1 n.r. |
Nintendo Wii Wii Sports/Sports Resort/Fit games 10 games with mainly table tennis, tennis and tilt city Posturomed training group: 5 ST balance exercises rehabilitation program of the clinic Conventional training group |
30 min, 3x/w, 3 w in center for all groups + 6 months at home n.r. | Balance: tests on a forceplate and on Posturomed Gait: 10MWT | ↗ balance (Forceplate, Posturomed), gait (10MWT) No effects 9 before randomization 25.5 h EG > other groups in adherence n.r. |
5 |
| Novotna, 2019 Czech Republic |
Effectiveness and feasibility of exergame on balance and gait | RCT 4w |
39 (23/16) 39.4 ± 9.7/42.6 ± 10.6 3.9 ± 1.9/3.6 ± 1.9 14.9 ± 8.6/14.5 ± 9.9 |
Homebalance system® (Clevertech, CZ) including Wii balance platform and a tablet computer 2 games: chessboard, planets No intervention |
At least 15 min, 7x/w, 4 w 7 h Home visit: for the first session |
Balance: BBS, miniBESTest, ABC scale Falls: FESI Gait: walking task, MSWS-12 Mobility: TUG |
↗ balance (BBS, Mini-BESTest), gait parameters - No dropouts 5.6 h Good compliance No AE |
4 |
| Pau, 2015 Italy |
Effectiveness of exergame on balance | NCT - |
20 44.6 ± 10.6 3.4 ± 1.3 n.r. |
Nintendo Wii Fit 3 games: Penguin Slide, Table Tilt and balance bubble - | At least 30 min or 2 × 15 min, 5x/w, 5 w 12.5h Unsupervised |
Balance: COP (EO/EC) | ↗ balance (only COPD in ML) - 7 24.2 h n.r n.r. |
3 |
| Plow, 2011 USA |
Effectiveness of exergame on PA behaviour, QoL and fatigue | NCT - |
26 43.2 ± 9.3 n.r. 12.2 ± 7.9 |
Nintendo Wii Wii-Fit with balance board Game: Basic run - |
Total 14 w 3x/w, 10–15–30 min according to an RPE, 7 w 3–5x/w for 20–30 min/sessions, 7 w Minimum 10.5 h Phone call: every other w for the first 7 w (4 times) |
Balance: BESTest Mobility: TUG Other: PADS, YMCA fitness test, SF-36, MFIS, SES, strength |
↗ balance, strength - 14 6.7 h n.r 1 |
4 |
| Prosperini, 2013 Italy | Effectiveness of exergame on postural and balance control | RCT- crossover 12w |
34 (17/17) 35.3 ± 8.6/37.1 ± 8.8 3 [1.5–5.0]/3.5 [1.5–5] 12.2 ± 6.0/9.3 ± 5.3 |
Nintendo Wii Fit Plus with balance board 7 games (zazen, Table tilt, Ski slalom, Penguin slide, Tightrope walk, soccer heading, balance bubble) | 30 min, 4–5x/w, 12w [24.0–31.3]h Home visit: for the first session and every 4 w during intervention period Phone contacts every w | Balance: COP, FSST Mobility: 25FWT Falls: nb of falls Other: MSIS-29 |
↗ balance (COP, FSST), mobility (25FWT), other (MSIS-29) ↗ nonfallers - 2 (1/1) 27.4 h n.r. 24 – no falls |
8 |
| Thomas, 2017 United Kingdom |
Effectiveness, acceptability and suitability of exergame | RCT - |
30 (15/15) 50.9 ± 8.1/47.6 ± 9.3 n.r. - |
Mii-vitaliSe = Nintendo Wii (Wii Fit Plus, Wii Sports and Wii Sports Resort), and a balance board + usual care Mii-vitaliSe program after 6-months of usual care | EG: 12 months of intervention CG:6 months of intervention 2 supervised Exergame sessions in center Home visit: to install Exergame Phone call or visit home: Regular one-to-one support |
Gait: 2MWT, Gait-Stride Rhythmic, MSSE Mobility: iTUG, 9HPT, SST, step test Balance: static posturography, Other: GLTEG, ActivPAL3, EQ-5D-5 L, SF-36, MSIS-29, FSI, HADS, SCI-ESES |
only descriptive data 2 (2/0) 28% of day first 6months high satisfaction No AE |
7 |
2MWT: 2 min walking test; 6MWT: 6 min walking test; 9HPT: nine hole plug test; 10MW: 10 meter walk; 25FW: 25 foot walking test; ABC: activities specific balance confidence scale; AE: adverse events; BBS: berg balance scale; CG: control group; CES: Composite Equilibrium Score; COP: centre of pressure; DDR: dance revolution; CSRT: choice stepping reaction time; DT: dual task; EC: eyes closed; EDSS: expended disability status scale; EG: exergames; EO: eyes open; EQ5D5L: euroqol five dimensions five levels; FESI: falls efficacy scale international; FSI; fatigue symptom inventory; FSST: 4 step square test; GLTEG: Godin Leisure time exercise questionnaire; HADS: hospital anxiety and depression scale; iTUG: instrumented TUG; MCT: motor control test; min: minutes; MFIS: modified fatigue impact scale; ML: mediolateral; MSIS-29: multiple sclerosis impact scale; MSSE: multiple sclerosis self-efficacy scale; MSW-12: multiple sclerosis walking scale 12; nb: number; NCT: non controlled trial; n.r.: not reported; NRCT: non randomised controlled trial; PA: physical activity; PADS: physical activity and disability survey; POMA: performance oriented mobility assessment; QOL: quality of life; RCT: randomized controlled trial; RPE: rate of perceived exertion; SCI-ESES: Spinal Cord Injury Exercise Self-Efficacy Scale; SDMT: symbol digit modalities test; SES: self-efficiency scale; SF36: 36 item short form health status survey; SPPB: short physical performance battery; SST: steady stand test; ST: single task; TMT: trail making test; TUG: timed up-and-go; USA: United State America; y: years; w: week.
↗: improvement of function.
Sample sizes varied across the studies between 10 (Chanpimol et al., 2020) and 70 participants (Kramer et al., 2014). Participants were on average aged 44.9 ± 9.4 years old, ranged from 36.2 (Prosperini et al., 2013) to 52.4 (Hoang et al., 2016) and diagnosed for 8.0 (Chanpimol et al., 2020) to 14.9 (Novotna et al., 2019) years. Studies used the Expanded Disability Status Scale (EDSS) to classify the level of disability; the lowest mean EDSS was 3 (Kramer et al., 2014) and the highest was 4.2 (Hoang et al., 2016), and two studies presented a median EDSS: 3.3 (Prosperini et al., 2013) and 5 (Chanpimol et al., 2020).
3.3. Intervention characteristics
There were considerable variations in study intervention modalities. The intervention duration ranged from 4 weeks (Novotna et al., 2019) to 12 months (Thomas et al., 2017), the frequency ranged from 2 (Hoang et al., 2016) to 7 (Novotna et al., 2019) sessions per week and the duration of each session ranged from 10 min (Plow and Finlayson, 2011) to 30 min (Chanpimol et al., 2020; Hoang et al., 2016; Kramer et al., 2014; Pau et al., 2015; Prosperini et al., 2013). Total intervention time ranged from 7 h (Novotna et al., 2019) to 24 h (Prosperini et al., 2013). It was not possible to calculate the total time for two interventions because there were no specific modalities (i.e., intervention frequency) (Kramer et al., 2014) or because of lack of information (Thomas et al., 2017).
All studies used AVG to rehabilitate static and dynamic balance. The majority of the interventions used commercially available technologies, including the Nintendo® Wii (Kramer et al., 2014; Pau et al., 2015; Plow and Finlayson, 2011; Prosperini et al., 2013; Thomas et al., 2017) and Xbox 360 console with Microsoft® Kinect (Gutierrez et al., 2013). Three studies used a customised system designed for rehabilitation: a step training system (modified Dance Dance Revolution) (Hoang et al., 2016), an interactive system for home-based rehabilitation of balance disorders (Homebalance®) (Novotna et al., 2019), and the Jintronix Rehabilitation system for rehabilitation and senior care (Chanpimol et al., 2020). Most interventions required postural control, with tasks such as throwing, hitting, dodging objects with different body parts and managing virtual elements (Chanpimol et al., 2020; Gutierrez et al., 2013; Kramer et al., 2014; Novotna et al., 2019; Pau et al., 2015; Plow and Finlayson, 2011; Prosperini et al., 2013; Thomas et al., 2017), whereas another study required accurate steps in terms of direction and timing in synchronisation with stimuli presented on a screen (Hoang et al., 2016).
3.4. Effectiveness of EG
3.4.1. Balance & gait
Studies assessed intervention effects on static or dynamic balance through many outcomes (Table 1). Most studies demonstrated significant improvement for EG in postural sway (Kramer et al., 2014; Pau et al., 2015; Prosperini et al., 2013), FSST (Prosperini et al., 2013), CES, MCT and POMA (Gutierrez et al., 2013), BBS (Gutierrez et al., 2013; Novotna et al., 2019) and mini-BESTest (Novotna et al., 2019; Plow and Finlayson, 2011). There was no significant improvement in the EG only for the ABC scale (Novotna et al., 2019). Compared with the CG, there were significant improvements in the EG on balance through postural sway (Hoang et al., 2016), CES, MCT, BBS and POMA (Gutierrez et al., 2013).
Concerning gait, the studies showed an improvement in some parameters of gait (Novotna et al., 2019; Kramer et al., 2014) and in 2MWT (Chanpimol et al., 2020). The MSWS-12 was used in two studies to measure limitation of walking (Chanpimol et al., 2020; Novotna et al., 2019) and there were no significant improvements for the MSWS-12. Finally, one study showed significant improvement in the EG compared to the CG in 10MWT (Hoang et al., 2016).
3.4.2. Mobility & falls
Four studies assessed mobility using the TUG (Hoang et al., 2016; Novotna et al., 2019; Plow and Finlayson, 2011; Thomas et al., 2017), and they reported no significant improvement (Novotna et al., 2019; Plow and Finlayson, 2011; Thomas et al., 2017) and no between-group difference (Hoang et al., 2016). In contrast, many authors reported significant improvement on the 25FWT and the SPPB in the EG (Chanpimol et al., 2020; Prosperini et al., 2013) and in SPPB (Chanpimol et al., 2020). One study showed a significant improvement in the EG compared to the CG in stepping reaction time and ability (CRST, SST) (Hoang et al., 2016). Studies also assessed physical activity (Hoang et al., 2016; Plow and Finlayson, 2011; Thomas et al., 2017). Plow et al. indicated significant improvement in physical activity (PADS) and strength increased significantly (Plow and Finlayson, 2011). Another study evaluated patient functional performance by the MSFC and indicated less disability in the EG compared to the CG (Hoang et al., 2016). In contrast, Thomas et al. reported no significant improvement of GLTEQ (Thomas et al., 2017).
Concerning falls, Prosperini et al. reported a decrease in the number of falls after AVG (Prosperini et al., 2013). Other studies showed no significant difference in both groups concerning the number of falls (Hoang et al., 2016; Kramer et al., 2014) and FoF (FES-I) (Novotna et al., 2019).
3.4.3. Other findings
One study showed significant improvement in quality of life (QoL) by MSIS-29 (Prosperini et al., 2013), whereas other authors found no improvement in the SF-36 (Plow and Finlayson, 2011; Thomas et al., 2017) and MSIS-29 (Thomas et al., 2017). Moreover, two studies assessed self-efficacy using the barrier self-efficacy scale (Plow and Finlayson, 2011), the SCI-ESES (Thomas et al., 2017) and the MSSE Scale (Thomas et al., 2017). Plow et al. showed no improvement compared to baseline (Plow and Finlayson, 2011), and Thomas et al. reported no significant improvement for MSSE and SCI-ESES (Thomas et al., 2017). Finally, the studies reported no AVG effects on fatigue (MFIS) (Chanpimol et al., 2020; Plow and Finlayson, 2011) and on cognition (TMT, SDMT) (Hoang et al., 2016).
3.4.4. Follow-up
Only three studies assessed retention of benefits through follow-up (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013). The number of falls during the six-month period following intervention did not differ between the two groups (Hoang et al., 2016). Balance improvement was conserved after 4 weeks (Novotna et al., 2019) and 12 weeks (Prosperini et al., 2013).
3.5. User compliance, feasibility and safety
The details of user compliance, feasibility and safety are presented in Table 1. Most studies reported the intervention time achieved by the participants, and it varied between 5.6 h (Novotna et al., 2019) to 27.4 h (Prosperini et al., 2013), when reported. The percentage of prescribed sessions completed was 83% (Chanpimol et al., 2020) or more than 80% (Gutierrez et al., 2013; Thomas et al., 2017; Novotna et al., 2019). Two studies reported that the time of activity exceeded the total sessions scheduled (Hoang et al., 2016; Pau et al., 2015). In one study, adherence to balance training was better in the EG than CG (Kramer et al., 2014).
The number of dropouts and discontinued ranged from 2/36 (Prosperini et al., 2013) or 2/30 (Thomas et al., 2017) to 14/30 (Plow and Finlayson, 2011). There were no dropouts in two studies (Chanpimol et al., 2020; Novotna et al., 2019). Most of the dropouts and discontinued interventions were due to relapses (Gutierrez et al., 2013; Hoang et al., 2016; Plow and Finlayson, 2011; Prosperini et al., 2013), medical reasons (Thomas et al., 2017), family reasons (Hoang et al., 2016), and uncompleted tests or interventions (Pau et al., 2015; Plow and Finlayson, 2011). Chanpimol et al. assessed satisfaction through a survey, and all participants were “satisfied” (10%) or “very satisfied” (90%) (Chanpimol et al., 2020). Most study participants were positive about AVG, which was seen to be acceptable, fun and convenient (Thomas et al., 2017).
Concerning feasibility, 7 studies were supervised by online meetings via videoconference (Chanpimol et al., 2020; Gutierrez et al., 2013), telephone calls (Hoang et al., 2016; Plow and Finlayson, 2011; Prosperini et al., 2013; Thomas et al., 2017) and home visits (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013; Thomas et al., 2017). Phone calls ranged from once in the first two weeks (Hoang et al., 2016) to once a week during the intervention (Prosperini et al., 2013). There was a home visit for the first session in four studies (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013; Thomas et al., 2017). Two studies organised home visits during the intervention: three during the first 16 weeks (Thomas et al., 2017) and one every 4 weeks (Prosperini et al., 2013). Two studies were unsupervised (Kramer et al., 2014; Pau et al., 2015), including Kramer et al., whose pwMS performed a 3 week training period in centre before the home sessions (Kramer et al., 2014). A learning phase was reported in four studies: a physiotherapist taught participants how to use the AVG during a home visit and supervised the first session (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013), or two sessions were conducted in hospital before the intervention (Thomas et al., 2017).
Regarding safety, two studies conducted a risk assessment in the patient's home before setting up the AVG (Hoang et al., 2016; Thomas et al., 2017). Only two studies reported the occurrence of adverse events such as knee and back pain (Plow and Finlayson, 2011; Prosperini et al., 2013). One study indicated that 24 (70%) persons reported at least one adverse event, of which 5 were considered attributable to the AVG (Prosperini et al., 2013), whereas another study reported a repetitive knee injury from stepping (Plow and Finlayson, 2011).
3.6. Methodological quality
The methodological quality of the 9 studies according to the PEDro scale is presented in Appendix C. The PEDro score ranged from 3 to 8, with a mean of 5.3 ± 2. The criteria 9: “all subjects received treatment or control condition” and 11: “point measured and variability” was fulfilled for all studies. Only three studies used randomisation (Hoang et al., 2016; Prosperini et al., 2013; Thomas et al., 2017) and blinded assessors (Gutierrez et al., 2013; Hoang et al., 2016; Prosperini et al., 2013). For one study, criteria 2, 3 and 7 were not reported (Novotna et al., 2019). The blinding of participants and therapists (criteria 5 and 6) was impossible for this type of intervention, resulting in no studies with a 10/10 score.
4. Discussion
This is the first systematic review assessing the effects of home-based AVG on gait and balance in pwMS. Active video gaming seems a relevant alternative to rehabilitation in the home setting for pwMS, effectively improving balance and gait. However, there was a lack of evidence for mobility and falls and a lack of information for other functions (cognition, fatigue, QoL). Compliance was satisfactory, and AVG appear feasible and safe for pwMS.
4.1. Effectiveness of AVG
The greater benefits of AVG seem to be on balance (Kramer et al., 2014; Pau et al., 2015; Prosperini et al., 2013; Gutierrez et al., 2013; Novotna et al., 2019; Plow and Finlayson, 2011). AVG induced equivalent or superior improvement on balance compared to usual care and conventional training (Gutierrez et al., 2013; Hoang et al., 2016). This may be explained by the fact that balance training requires maintaining a stance in challenging static or dynamic balance activities such as lateral weight shifting, single-leg stance, side stepping and stepping in all directions. One study included even showed that the improvement in postural control was limited to the medio-lateral direction, which was precisely the movement performed during the game (Pau et al., 2015). In the literature, reviews reported that active video gaming could be as effective as conventional training and more effective than no intervention for improving balance in pwMS (Casuso-Holgado et al., 2018; Massetti et al., 2016). Recent studies (Maggio et al., 2019; Prosperini et al., 2020) have related this improvement of balance control to specific mechanisms of active video gaming, including i) muscle reinforcement (high-intensity repetition of task-oriented exercises); ii) specific retraining of sensory strategies by the coupling perception action (audio-visual biofeedback); iii) engagement of a mirror neuron system mediated by embodiment (the sense of presence in the game associated with a virtual avatar). Finally, the characteristics of AVG, such as high-intensity repetition of task-oriented exercises, incremental increase in task difficulty, real-time feedback and motivation, can lead to an enhancement of both the function and structure of neural mechanisms (Kleim and Jones, 2008). In order to enhance effectiveness, some studies reported the possibility of adapting the intervention to the participant's fitness level (Chanpimol et al., 2020; Novotna et al., 2019; Plow and Finlayson, 2011).
As in the literature (Casuso-Holgado et al., 2018; Massetti et al., 2016), the pooled evidence suggests that AVG also improve gait (Chanpimol et al., 2020; Novotna et al., 2019; Kramer et al., 2014). One study showed effects on gait superior to usual care (Hoang et al., 2016). Peruzzi et al. reported a larger improvement of gait parameters in AVG interventions for pwMS (Peruzzi et al., 2017).
The results are more debatable for mobility and falls. Many studies reported no improvement for the TUG (Novotna et al., 2019; Plow and Finlayson, 2011), whereas authors showed positive effects of AVG on functional tests (Chanpimol et al., 2020; Prosperini et al., 2013) and the stepping test (Hoang et al., 2016). Moreover, one study reported that the proportion of non-fallers was greater with the AVG (Prosperini et al., 2013), whereas the other results were not significant compared to the CG (Hoang et al., 2016; Kramer et al., 2014). Another study of AVG-based balance training, made in clinic, showed a significant improvement in fall risk for pwMS (Eftekharsadat et al., 2015).
Finally, the impact of AVG on other variables such as the QoL and fatigue were poorly studied. Only one study reported the effects of AVG on cognition through attention and executive functions (Hoang et al., 2016). A meta-analysis reported that AVG may have an effect on executive functions, not on global cognition or attention; but this analysis gather together several neurological disabilities (Mura et al., 2018). In pwMS, it is possible that AVG have a positive impact on attention and processing speed through far transfer effect from balance to cognition (Prosperini et al., 2015).
4.2. Characteristics of intervention
The intervention modalities varied in terms of total intervention time, length and frequency. A recent meta-analysis showed that the weekly frequency of sessions, rather than the duration of a single session and the overall duration of the intervention, significantly modified AVG effectiveness in neurological disorders (Prosperini et al., 2020). All interventions had non-immersive approaches (i.e., AVG), and a VR system enabling a full immersive experience was never found. In rehabilitation, the term VR has often been inappropriately used (Tieri et al., 2018) and seems confused with AVG in the literature. VR immersion provides a feeling of presence in the virtual environment (Holden, 2005) and may improve understanding and the perception of movement (mirror neuron system). In future interventions, VR with a head-mounted display could be used in pwMS to assess the impact on motor function. Commercial and custom AVG technologies were used in the interventions at home. Six studies included commercial devices (Gutierrez et al., 2013; Kramer et al., 2014; Pau et al., 2015; Plow and Finlayson, 2011; Prosperini et al., 2013; Thomas et al., 2017) often used in rehabilitation (mainly Wii, Kinect) (Bonnechère et al., 2016), while three studies used a customised games system (Chanpimol et al., 2020; Hoang et al., 2016; Novotna et al., 2019). These customised systems allow adjusting the challenge of the AVG according to balance impairment and patient feedback (Chanpimol et al., 2020; Novotna et al., 2019). A scoping review reported that customised and commercially available systems seemed to have the same beneficial effects on balance and gait in stroke patients (Darekar et al., 2015).
4.3. User compliance and feasibility at home
User compliance was satisfactory for all studies because participants completed more than 80% of the total sessions scheduled (Chanpimol et al., 2020; Gutierrez et al., 2013; Novotna et al., 2019; Thomas et al., 2017) or exceeded the total sessions scheduled (Hoang et al., 2016; Pau et al., 2015). This adherence seems superior than most home-exercise program interventions in chronic disease (average adherence) (Peek et al., 2016) and in pwMS (40 to 63%) (Paul et al., 2019). These results were nuanced by one study in which 9 persons were unable to use Wii-Fit for at least one week due to an increase in symptoms or illness (Plow and Finlayson, 2011). Good compliance may be explained by the fact that AVG are enjoyable and motivating (Maggio et al., 2019; Massetti et al., 2016; Taylor and Griffin, 2015). Kramer et al. reported that adherence to balance training was higher for AVG than other training (Kramer et al., 2014), while pwMS would use AVG repetitively, and recommend them to others (Chanpimol et al., 2020).
Despite this strong adherence, Perrochon et al. reported more dropouts for AVG than the CG in their review of individuals with neurological disease (Perrochon et al., 2019). In our review, dropouts and discontinued were mainly associated with relapse (Gutierrez et al., 2013; Hoang et al., 2016; Plow and Finlayson, 2011; Prosperini et al., 2013) or personal circumstances (e.g., lack of time, scheduling problems, etc.) (Hoang et al., 2016; Pau et al., 2015). Moreover, a home-based intervention needs adequate space (Plow and Finlayson, 2011), a suitable television (Plow and Finlayson, 2011; Thomas et al., 2017) and internet connection (Gutierrez et al., 2013). In the literature, other reasons are given, such as technical issues, lack of space at home and discouragement due to technological devices (Perrochon et al., 2019). This overall result tends to confirm the fact that opinions are divided among persons (Palacios-Cena et al., 2016; Plow and Finlayson, 2014). Some participants did not appreciate the feedback that they felt was inaccurate or that reinforced their limitations, and some reported a lack of time or motivation or boredom (Palacios-Cena et al., 2016; Plow and Finlayson, 2014). In practise, home-based interventions require an organisation which considers the social and family environment (Chen et al., 2019). The home-based setting of AVG required participants with the ability to use the system without technical barriers. This is why some studies supervised the first session (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013), or conducted the first two sessions (Thomas et al., 2017) or the first three weeks (Kramer et al., 2014) in hospital.
Most of the included studies were supervised by an online meeting via videoconference (Chanpimol et al., 2020; Gutierrez et al., 2013), telephone calls (Hoang et al., 2016; Plow and Finlayson, 2011; Prosperini et al., 2013; Thomas et al., 2017) and home visits (Hoang et al., 2016; Novotna et al., 2019; Prosperini et al., 2013; Thomas et al., 2017). Supervision allowed following a participant's development and compensated for the potential loss/reduction in the social contact associated with training in clinic. Improvement was observed in Plow's study during the initial supervised 7 weeks; after this period, the intervention was unsupervised, and participant levels returned to baseline (Plow and Finlayson, 2011). The literature reported greater improvements for supervised compared to unsupervised programs (Feger et al., 2015; Olney et al., 2006).
4.4. Safety
Mild to moderate adverse events were reported in a minority of studies (Plow and Finlayson, 2011; Prosperini et al., 2013), not specifically related to AVG. Jalink et al. reported that the use of Nintendo Wii can cause musculoskeletal problems, but the prevalence is low in a healthy population (Jalink et al., 2014). AVG can be used safely at home for pwMS (Hoang et al., 2016; Plow and Finlayson, 2011), but attention must be paid to the risk of falls in pwMS with moderate disability (Nilsagård et al., 2015). In order to practice safely at home, many authors recommended setting up a support around the balance board (Novotna et al., 2019; Pau et al., 2015) and to perform the AVG when other people are at home (Gutierrez et al., 2013).
4.5. Limitations
A limitation of this review is the heterogeneity of in the EG (duration and frequency of intervention, AVG systems (commercial or customised), outcomes), which make the interpretation of results difficult and does not permit a meta-analysis. The current results must be interpreted with caution because of the risk of bias and the heterogenous design (i.e., RCT, non-RCT and uncontrolled). Most of the studies were pilot studies without sample size calculation for clinical effectiveness. Finally, one study was not totally home-based, with 3 weeks of rehabilitation in clinic (Kramer et al., 2014).
Our sample comprised pwMS with mild disability, so findings cannot be generalised to people with more disabling MS. The inclusion criteria were sufficiently close to ensure that the study samples were similar, with a low EDSS scores, an absence of cognitive impairment (Chanpimol et al., 2020; Gutierrez et al., 2013; Hoang et al., 2016; Novotna et al., 2019) and of visual deficit.
4.6. Future studies
We limited our review to gait and balance, but AVG intervention can also have an impact on other disease symptoms, such as fatigue and cognitive function. First, fatigue is one of the most common symptoms, affecting 80% of pwMS (Rottoli et al., 2017), and one study showed that fatigue can have a negative impact on motor performance (Al-Sharman et al., 2019). An overview reported that rehabilitation could reduce patient-reported fatigue (Amatya et al., 2019). In our systematic review, three studies assessed the impact of fatigue in pwMS (Chanpimol et al., 2020; Plow and Finlayson, 2011; Thomas et al., 2017), but there was no significant improvement. However, Khalil et al. reported significant improvement in the EG over the CG on fatigue in clinic (Khalil et al., 2018). Second, a systematic review reported that AVG are effective in improving specific cognitive domains, such as executive functions (with dual-task performance) and perceptual or visuo-spatial abilities in neurological disabilities (Maggio et al., 2019; Mura et al., 2018). In our systematic review, only one study assessed cognitive function (Hoang et al., 2016), and significant cognitive improvement was demonstrated, but no significant difference between groups, which may be explained by an intervention duration too short to detect changes. Future studies should focus on these symptoms to confirm the impact of AVG on fatigue and cognitive function, facing previous positive results (Prosperini et al., 2015).
High quality studies with larger sample sizes and systematic RCT are necessary to improve the strength of the evidence. Future studies should evaluate the long-term effects with follow-up, since the main objective of AVG is to continue them over time.
4.7. EG in a pandemic context
A recent review suggested that technology-based (i.e., internet, telephone, active video gaming and pedometers) physical rehabilitation interventions could have greater effects on physical activity than usual care and no treatment for pwMS (Rintala et al., 2018). Our review confirms the notion that AVG may be a particularly interesting solution in situations in which conventional therapy is not readily available. AVG were effective in balance function and offer many advantages, such as their relative low-cost, high portability, off-the-shelf software and available and provide the opportunity to deliver an engaging, high-repetitive, standardised rehabilitation.
In addition to the current context of COVID-19, home-based AVG allow access to rehabilitation for pwMS who lack time flexibility (Kamm et al., 2015), frequent in this still young and active population. PwMS perceive barriers to physical activity in the environment (e.g., lack of physical activity options, lack of access to facilities for the disabled, and transportation inflexibilities) and personal barriers (Learmonth and Motl, 2016), which can limit access and adherence to rehabilitation in clinic. This may also explain a total duration of intervention greater than the prescribed dose.
5. Conclusion
This systematic review showed that home-based AVG can be effective in improving balance and gait functions, while the results were more contrasting for mobility and falls. AVG seem feasible, with good compliance and safe use for pwMS with low EDSS. AVG can be considered at least as an alternative for rehabilitation in the home for pwMS, especially in the current context of COVID-19 for pwMS. Nevertheless, future studies are necessary to confirm these results in the general MS population.
Funding sources
This research did not receive any funding from agencies in the public, commercial or not-for-profit sectors.
Declaration of Competing Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.102928.
Appendix. Supplementary materials
References
- Al-Sharman A., Khalil H., El-Salem K., Alghwiri A.A., Khazaaleh S., Khraim M. Motor performance improvement through virtual reality task is related to fatigue and cognition in people with multiple sclerosis. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2019;24:e1782. doi: 10.1002/pri.1782. [DOI] [PubMed] [Google Scholar]
- Amatya B., Khan F., Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD012732.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino P., Fuschillo S., Papa A., Di Minno M.N.D., Maniscalco M. Exergaming as a Supportive Tool for Home-Based Rehabilitation in the COVID-19 Pandemic Era. Games Health J. 2020 doi: 10.1089/g4h.2020.0095. [DOI] [PubMed] [Google Scholar]
- Baranowski T., Buday R., Thompson D.I., Baranowski J. Playing for Real. Am. J. Prev. Med. 2008;34:74–82. doi: 10.1016/j.amepre.2007.09.027. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnechère B., Jansen B., Omelina L., Van Sint Jan S. The use of commercial video games in rehabilitation: a systematic review. Int. J. Rehabil. Res. Int. Z. Rehabil. Rev. Int. Rech. Readaptation. 2016;39:277–290. doi: 10.1097/MRR.0000000000000190. [DOI] [PubMed] [Google Scholar]
- Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B.V., Thompson A.J. Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83:1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casuso-Holgado M.J., Martín-Valero R., Carazo A.F., Medrano-Sánchez E.M., Cortés-Vega M.D., Montero-Bancalero F.J. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: a systematic review and meta-analysis. Clin. Rehabil. 2018;32:1220–1234. doi: 10.1177/0269215518768084. [DOI] [PubMed] [Google Scholar]
- Chanpimol S., Benson K., Maloni H., Conroy S., Wallin M. Acceptability and outcomes of an individualized exergaming telePT program for veterans with multiple sclerosis: a pilot study. Arch. Physiother. 2020;10:18. doi: 10.1186/s40945-020-00089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yu, Abel K.T., Janecek J.T., Chen Yunan, Zheng K., Cramer S.C. Home-based technologies for stroke rehabilitation: a systematic review. Int. J. Med. Inf. 2019;123:11–22. doi: 10.1016/j.ijmedinf.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comber L., Galvin R., Coote S. Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. 2017;51:25–35. doi: 10.1016/j.gaitpost.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. Multiple sclerosis. Lancet Lond. Engl. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Darekar A., McFadyen B.J., Lamontagne A., Fung J. Efficacy of virtual reality-based intervention on balance and mobility disorders post-stroke: a scoping review. J. NeuroEng. Rehabil. 2015:12. doi: 10.1186/s12984-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharsadat B., Babaei-Ghazani A., Mohammadzadeh M., Talebi M., Eslamian F., Azari E. Effect of virtual reality-based balance training in multiple sclerosis. Neurol. Res. 2015;37:539–544. doi: 10.1179/1743132815Y.0000000013. [DOI] [PubMed] [Google Scholar]
- Feger M.A., Herb C.C., Fraser J.J., Glaviano N., Hertel J. Supervised rehabilitation versus home exercise in the treatment of acute ankle sprains: a systematic review. Clin. Sports Med. 2015;34:329–346. doi: 10.1016/j.csm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Gallou-Guyot, Mandigout S., Bherer L., Perrochon A. Effects of exergames and cognitive-motor dual-task training on cognitive, physical and dual-task functions in cognitively healthy older adults: an overview. Ageing Res. Rev. 2020;63 doi: 10.1016/j.arr.2020.101135. [DOI] [PubMed] [Google Scholar]
- Gallou-Guyot, Mandigout S., Combourieu-Donnezan L., Bherer L., Perrochon A. Cognitive and physical impact of cognitive-motor dual-task training in cognitively impaired older adults: an overview. Neurophysiol. Clin. 2020;50:441–453. doi: 10.1016/j.neucli.2020.10.010. [DOI] [PubMed] [Google Scholar]
- Gutierrez R.O., Galan Del Rio F., Cano de la Cuerda R., Alguacil Diego I.M., Gonzalez R.A., Page J.C.M. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation. 2013;33:545–554. doi: 10.3233/NRE-130995. [DOI] [PubMed] [Google Scholar]
- Hoang P., Schoene D., Gandevia S., Smith S., Lord S.R. Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis – a randomized controlled trial. Mult. Scler. J. 2016;22:94–103. doi: 10.1177/1352458515579442. [DOI] [PubMed] [Google Scholar]
- Holden M.K. Virtual environments for motor rehabilitation: review. Cyberpsychol. Behav. Impact Internet Multimed. Virtual Real. Behav. Soc. 2005;8:187–211. doi: 10.1089/cpb.2005.8.187. discussion 212-219. [DOI] [PubMed] [Google Scholar]
- Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- Jalink M.B., Heineman E., Pierie J.-P.E.N., ten Cate Hoedemaker H.O. The BMJ 349; 2014. Nintendo Related Injuries and Other problems: Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm C.P., Mattle H.P., Müri R.M., Heldner M.R., Blatter V., Bartlome S., Lüthy J., Imboden D., Pedrazzini G., Bohlhalter S., Hilfiker R., Vanbellingen T. Home-based training to improve manual dexterity in patients with multiple sclerosis: a randomized controlled trial. Mult. Scler. Houndmills Basingstoke Engl. 2015;21:1546–1556. doi: 10.1177/1352458514565959. [DOI] [PubMed] [Google Scholar]
- Khalil H., Al-Sharman A., El-Salem K., Alghwiri A.A., Al-Shorafat D., Khazaaleh S., Abu Foul L. The development and pilot evaluation of virtual reality balance scenarios in people with multiple sclerosis (MS): a feasibility study. NeuroRehabilitation. 2018;43:473–482. doi: 10.3233/NRE-182471. [DOI] [PubMed] [Google Scholar]
- Kleim J.A., Jones T.A. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008:51. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kramer A., Dettmers C., Gruber M. Exergaming with additional postural demands improves balance and gait in patients with multiple sclerosis as much as conventional balance training and leads to high adherence to home-based balance training. Arch. Phys. Med. Rehabil. 2014;95:1803–1809. doi: 10.1016/j.apmr.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Laver K.E., Lange B., George S., Deutsch J.E., Saposnik G., Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017;11 doi: 10.1002/14651858.CD008349.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth Y.C., Motl R.W. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil. Rehabil. 2016;38:1227–1242. doi: 10.3109/09638288.2015.1077397. [DOI] [PubMed] [Google Scholar]
- Maggio M.G., Russo M., Cuzzola M.F., Destro M., La Rosa G., Molonia F., Bramanti P., Lombardo G., De Luca R., Calabrò R.S. Virtual reality in multiple sclerosis rehabilitation: a review on cognitive and motor outcomes. J. Clin. Neurosci. 2019;65:106–111. doi: 10.1016/j.jocn.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003;83:713–721. doi: 10.1093/ptj/83.8.713. [DOI] [PubMed] [Google Scholar]
- Massetti T., Trevizan I.L., Arab C., Favero F.M., Ribeiro-Papa D.C., de Mello Monteiro C.B. Virtual reality in multiple sclerosis - A systematic review. Mult. Scler. Relat. Disord. 2016;8:107–112. doi: 10.1016/j.msard.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Mat Rosly M., Mat Rosly H., Davis Oam G.M., Husain R., Hasnan N. Exergaming for individuals with neurological disability: a systematic review. Disabil. Rehabil. 2017;39:727–735. doi: 10.3109/09638288.2016.1161086. [DOI] [PubMed] [Google Scholar]
- Miller K.J., Adair B.S., Pearce A.J., Said C.M., Ozanne E., Morris M.M. Effectiveness and feasibility of virtual reality and gaming system use at home by older adults for enabling physical activity to improve health-related domains: a systematic review. Age Ageing. 2014;43:188–195. doi: 10.1093/ageing/aft194. [DOI] [PubMed] [Google Scholar]
- Milo R., Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014;13:518–524. doi: 10.1016/j.autrev.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura G., Carta M.G., Sancassiani F., Machado S., Prosperini L. Active exergames to improve cognitive functioning in neurological disabilities: a systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018;54:450–462. doi: 10.23736/S1973-9087.17.04680-9. [DOI] [PubMed] [Google Scholar]
- Nilsagård Y., Gunn H., Freeman J., Hoang P., Lord S., Mazumder R., Cameron M. Falls in people with MS—An individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult. Scler. J. 2015;21:92–100. doi: 10.1177/1352458514538884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotna K., Janatova M., Hana K., Svestkova O., Preiningerova Lizrova J., Kubala Havrdova E. Biofeedback based home balance training can improve balance but not gait in people with multiple sclerosis. Mult. Scler. Int. 2019;2019 doi: 10.1155/2019/2854130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney S.J., Nymark J., Brouwer B., Culham E., Day A., Heard J., Henderson M., Parvataneni K. A randomized controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke. 2006;37:476–481. doi: 10.1161/01.STR.0000199061.85897.b7. [DOI] [PubMed] [Google Scholar]
- Palacios-Cena D., Ortiz-Gutierrez R.M., Buesa-Estellez A., Galan-Del-Rio F., Cachon Perez J.M., Martinez-Piedrola R., Velarde-Garcia J.F., Cano-DE-LA-Cuerda R. Multiple sclerosis patients’ experiences in relation to the impact of the kinect virtual home-exercise programme: a qualitative study. Eur. J. Phys. Rehabil. Med. 2016;52:347–355. [PubMed] [Google Scholar]
- Paltamaa J., Sjögren T., Peurala S., Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2012;44:811–823. doi: 10.2340/16501977-1047. [DOI] [PubMed] [Google Scholar]
- Pau M., Coghe G., Corona F., Leban B., Marrosu M.G., Cocco E. Effectiveness and limitations of unsupervised home-based balance rehabilitation with Nintendo Wii in people with multiple sclerosis. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/916478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L., Renfrew L., Freeman J., Murray H., Weller B., Mattison P., McConnachie A., Heggie R., Wu O., Coulter E.H. Web-based physiotherapy for people affected by multiple sclerosis: a single blind, randomized controlled feasibility study. Clin. Rehabil. 2019;33:473–484. doi: 10.1177/0269215518817080. [DOI] [PubMed] [Google Scholar]
- Peek K., Sanson-Fisher R., Mackenzie L., Carey M. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: a systematic review. Physiotherapy. 2016;102:127–135. doi: 10.1016/j.physio.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Perrochon A., Borel B., Istrate D., Compagnat M., Daviet J.-.C. Exercise-based games interventions at home in individuals with a neurological disease: a systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019;62:366–378. doi: 10.1016/j.rehab.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Perrochon A., Holtzer R., Laidet M., Armand S., Assal F., Lalive P.H., Allali G. Postural control is associated with cognition and fear of falling in patients with multiple sclerosis. J. Neural Transm. Vienna Austria 1996. 2017;124:495–500. doi: 10.1007/s00702-016-1668-5. [DOI] [PubMed] [Google Scholar]
- Peruzzi A., Zarbo I.R., Cereatti A., Della Croce U., Mirelman A. An innovative training program based on virtual reality and treadmill: effects on gait of persons with multiple sclerosis. Disabil. Rehabil. 2017;39:1557–1563. doi: 10.1080/09638288.2016.1224935. [DOI] [PubMed] [Google Scholar]
- Plow M., Finlayson M. A qualitative study exploring the usability of Nintendo Wii Fit among persons with multiple sclerosis. Occup. Ther. Int. 2014;21:21–32. doi: 10.1002/oti.1345. [DOI] [PubMed] [Google Scholar]
- Plow M., Finlayson M. Potential benefits of nintendo wii fit among people with multiple sclerosis: a longitudinal pilot study. Int. J. MS Care. 2011;13:21–30. doi: 10.7224/1537-2073-13.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosperini L., Fortuna D., Gianni C., Leonardi L., Marchetti M.R., Pozzilli C. Home-based balance training using the Wii balance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabil. Neural Repair. 2013;27:516–525. doi: 10.1177/1545968313478484. [DOI] [PubMed] [Google Scholar]
- Prosperini L., Petsas N., Sbardella E., Pozzilli C., Pantano P. Far transfer effect associated with video game balance training in multiple sclerosis: from balance to cognition? J. Neurol. 2015;262:774–776. doi: 10.1007/s00415-015-7640-8. [DOI] [PubMed] [Google Scholar]
- Prosperini L., Tomassini V., Castelli L., Tacchino A., Brichetto G., Cattaneo D., Solaro C.M. Exergames for balance dysfunction in neurological disability: a meta-analysis with meta-regression. J. Neurol. 2020 doi: 10.1007/s00415-020-09918-w. [DOI] [PubMed] [Google Scholar]
- Rintala A., Hakala S., Paltamaa J., Heinonen A., Karvanen J., Sjogren T. Effectiveness of technology-based distance physical rehabilitation interventions on physical activity and walking in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Disabil. Rehabil. 2018;40:373–387. doi: 10.1080/09638288.2016.1260649. [DOI] [PubMed] [Google Scholar]
- Rottoli M., La Gioia S., Frigeni B., Barcella V. Pathophysiology, assessment and management of multiple sclerosis fatigue: an update. Expert Rev. Neurother. 2017;17:373–379. doi: 10.1080/14737175.2017.1247695. [DOI] [PubMed] [Google Scholar]
- Sadeghmousavi S., Rezaei N. COVID-19 and Multiple Sclerosis: predisposition and Precautions in Treatment. Sn Compr. Clin. Med. 2020:1–6. doi: 10.1007/s42399-020-00504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. BMJ j4008; 2017. AMSTAR 2: a Critical Appraisal Tool For Systematic Reviews That Include Randomised Or Non-Randomised Studies of Healthcare interventions, Or Both. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M.J.D., Griffin M. The use of gaming technology for rehabilitation in people with multiple sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2015;21:355–371. doi: 10.1177/1352458514563593. [DOI] [PubMed] [Google Scholar]
- Thomas S., Fazakarley L., Thomas P.W., Collyer S., Brenton S., Perring S., Scott R., Thomas F., Thomas C., Jones K., Hickson J., Hillier C. Mii-vitaliSe: a pilot randomised controlled trial of a home gaming system (Nintendo Wii) to increase activity levels, vitality and well-being in people with multiple sclerosis. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieri G., Morone G., Paolucci S., Iosa M. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev. Med. Devices. 2018;15:107–117. doi: 10.1080/17434440.2018.1425613. [DOI] [PubMed] [Google Scholar]
- Triegaardt J., Han T.S., Sada C., Sharma S., Sharma P. The role of virtual reality on outcomes in rehabilitation of Parkinson's disease: meta-analysis and systematic review in 1031 participants. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019 doi: 10.1007/s10072-019-04144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen A.P., de Vet H.C., de Bie R.A., Kessels A.G., Boers M., Bouter L.M., Knipschild P.G. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998;51:1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.