Abstract
BACKGROUND
Schwannomas of the peripheral nerves are benign tumors that can very rarely undergo malignant transformation. These lesions are particularly challenging to diagnose via noninvasive techniques but can have significant implications for treatment.
OBSERVATIONS
This is a case of a 70-year-old female with a prior history of a right sciatic notch tumor that was diagnosed as a conventional schwannoma via histology from an initial biopsy and subsequent surgical debulking. Unfortunately, she experienced significant worsening of her motor deficit, whereby her postoperative foot weakness progressed to complete foot drop in less than 2 years. In addition, she demonstrated significant radiological progression, with more than 1 to 2 cm of growth in each dimension at her subsequent evaluation, along with intractable right leg pain. An additional operation was performed to completely remove the 7 × 8 cm tumor, and histology demonstrated angiosarcoma within a schwannoma. There was no evidence of recurrence at 15 months, and the patient had significant improvement in her pain.
LESSONS
Rapidly worsening function and radiological progression are not typically seen with conventional benign nerve sheath tumors and should prompt consideration of other lesions. Angiosarcoma within schwannoma is a rare pathology and optimal therapies for these tumors in terms of surgical timing and adjuvant therapy are still unknown.
Keywords: hybrid tumor, hybrid schwannoma, nerve tumor
ABBREVIATIONS: MPNST = malignant peripheral nerve sheath tumor, MRI = magnetic resonance imaging, NF1 = neurofibromatosis type 1
Schwannomas of the peripheral nervous system are benign tumors with a generally favorable prognosis after operative intervention. Very rarely, these lesions undergo malignant transformation, usually in the form of epithelioid malignant peripheral nerve sheath tumor (MPNST) or angiosarcoma.1 Schwannomas with angiosarcomatous changes are particularly rare, with only 22 cases previously reported in the literature.1–15 These hybrid tumors are challenging to diagnose and have a significant impact on patient treatment as well as subsequent outcomes. We present a 70-year-old female patient with an angiosarcoma arising within a schwannoma found in the right sciatic notch, highlighting the difficulties of establishing a definitive diagnosis.
Illustrative Case
History of Present Illness
A 70-year-old female with a past medical history of breast adenocarcinoma treated with mastectomy presented for evaluation of a suspected schwannoma of the right sciatic nerve. During salpingo-oophorectomy 3 years earlier, a large pelvic mass was incidentally found. Biopsy demonstrated a schwannoma, and the tumor was debulked 1 year later through a transabdominal approach. Pathology confirmed a schwannoma. After the surgery, she noted new foot weakness. Over the next 2 years, she developed progressive numbness and weakness of her right foot, which resulted in a complete foot drop, requiring an ankle foot orthosis, as well as paroxysmal pain on the dorsal aspect of the right foot and buttock. She re-presented for neurosurgical evaluation due to persistent, debilitating pain in the setting of radiological evidence of tumor progression on follow-up magnetic resonance imaging (MRI). Pathological review at our institution of the previous debulking confirmed a conventional schwannoma.
Imaging
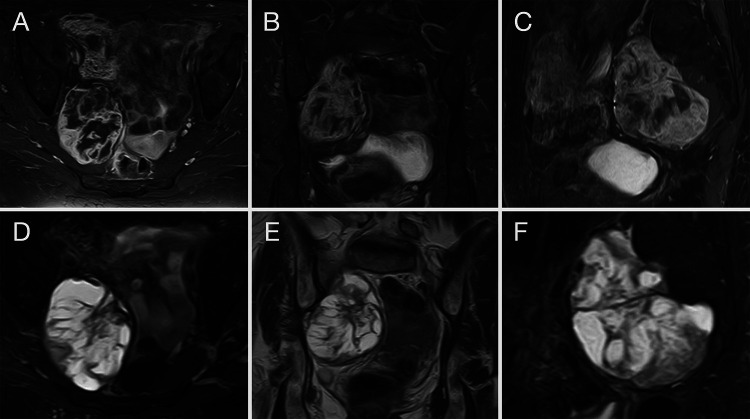
MRI of the pelvis with and without contrast demonstrated a large, heterogeneously contrast-enhancing mass extending from the presacral space out through the right sciatic notch along the course of the sciatic nerve. Her initial tumor several years earlier had measured 49.8 × 54.3 × 58.6 mm in the axial, coronal, and sagittal planes, respectively (Fig. 1A–C). Six months after her limited debulking, the tumor was measured at 47.7 × 58.0 × 60.1 mm. This subsequently increased in size after an additional 8 months (55.3 × 64.4 × 69.8 mm). On her final preoperative evaluation prior to her second resection, her tumor had grown to 66.2 × 69.6 × 79.0 mm (Fig. 2A–C).
FIG. 1.
MRI of the pelvis with and without contrast depicting the original tumor from several years earlier, measuring 49.8 × 54.3 × 58.6 mm in the axial (A), coronal (B), and sagittal (C) planes. Corresponding T2-weighted images are presented in panels D, E, and F.
FIG. 2.
MRI of the pelvis with and without contrast obtained just prior to the second resection, demonstrating tumor progression with a size of 66.2 × 69.6 × 79.0 mm in the axial (A), coronal (B), and sagittal (C) planes. Corresponding T2-weighted images are presented in panels D, E, and F.
Neurological Examination
The patient exhibited dense weakness in the right foot, with no active dorsiflexion and only trace toe extension. She demonstrated trace inversion and no eversion as well as grade 2/5 plantarflexion strength. She also exhibited severe weakness in abduction and hamstring strength as well as decreased sensation below the knee, with the exception of the saphenous distribution. Due to the patient’s tumor progression as well as intractable pain despite medications, she was offered an additional resection, which she elected to pursue. The principal goal of surgery was to improve the patient’s persistent pain and obtain a definitive histological diagnosis given the atypical behavior of this tumor for a conventional schwannoma.
Operative Intervention
The patient was treated by an interdisciplinary team. Rigid cystourethroscopy was performed by a urologist with placement of whistle-tip stents into each ureter. The colorectal team then performed a transabdominal approach to the lesion, with identification and mobilization of the right internal iliac artery and vein. Bleeding from peritumoral venous branches was controlled with clips and suture ligation. An intracapsular dissection was performed and the tumor was grossly removed. Residual presacral bleeding was controlled by the colorectal team and the wound was closed in anatomical layers. Nerve monitoring using electromyography was used during the operation and no abnormal firing was noted.
Postoperative Course
The patient did well after surgery. On postoperative day 2, she reported moderate improvement in the sensation throughout her right foot. By the date of discharge, her ambulation had improved significantly. Her motor examination remained stable. Postoperative MRI did not demonstrate any evidence of residual tumor.
Subsequent Treatment
The patient was evaluated by medical and radiation oncology. She opted to pursue postoperative observation, with interval 3-month computed tomography scans of the chest, abdomen, and pelvis. Her most recent imaging was obtained at her 15-month follow-up, and there was no evidence of local tumor recurrence or metastatic disease. Her pain was significantly improved compared to before surgery.
Pathological Findings
Gross examination revealed an 8.5 × 6.1 × 4.2 cm well-circumscribed mass with a lobulated tan to dark brown cut surface demonstrating multiple foci of hemorrhage (Fig. 3).
FIG. 3.

An 8.5 × 6.1 × 4.2 cm well-circumscribed mass with a lobulated, tan to dark brown cut surface exhibiting multiple foci of hemorrhage.
Microscopic examination revealed the features of a conventional schwannoma, composed of fascicles of benign Schwann cells intermixed with dilated and hyalinized blood vessels (Fig. 4A) and highlighted by the S100 protein (not depicted). In addition, multiple scattered foci of angiosarcoma were identified throughout the tumor, characterized by atypical and hyperchromatic, plump spindle to epithelioid cells with vesicular chromatin, inconspicuous nucleoli and amphophilic cytoplasm, arranged in sheets and slit-like spaces as well as poorly formed vascular channels with abundant extravasation of red blood cells, frequent mitotic figures, and rare foci of necrosis (Fig. 4B). By immunohistochemistry, the Schwannoma cells were highlighted by the S100 protein (not depicted) and the SOX10 antibody (Fig. 4C), while the angiosarcoma cells were highlighted by the ERG immunostain (Fig. 4D), further supporting their endothelial nature. The final diagnosis was determined to be angiosarcoma arising in schwannoma and the surgical margins were free of tumor.
FIG. 4.

A: Area of conventional schwannoma characterized by poorly formed fascicles of cytologically bland spindle cells with variable cellularity (so-called Antoni A and Antoni B areas), numerous vessels with hyalinized walls, scattered lymphocytes and a focus of hemosiderin deposition. B: Angiosarcoma, composed of plump spindle to epithelioid cells with vesicular chromatin, conspicuous nucleoli, and amphophilic cytoplasm, arranged in sheets and slit-like and poorly formed vascular spaces. Notice the frequent mitotic figures and the focus of necrosis on the left side of the image. C: SOX10 antibody, a Schwann cell marker, highlights the schwannoma cells (right), but it is negative in the area of angiosarcoma (left). D: ERG antibody, an endothelial marker, highlights the area of angiosarcoma (left) but is negative in the focus of conventional schwannoma (right).
Discussion
Observations
To the best of our knowledge, this is the first report of a sciatic notch tumor that was presumed to be a conventional schwannoma on two previous biopsy samples and was ultimately discovered to be an angiosarcoma within a schwannoma. Two key observations were noted in this patient that are not typical for patients with classic peripheral nerve schwannomas. First, this woman was developing progressive motor weakness. While she experienced a motor deficit after her initial resection, this progressed to complete right foot drop in less than 2 years after her first surgery. Rapid progression of sensorimotor deficits can often be seen in more aggressive tumors, such as MPNST,16 but significantly less commonly in schwannomas. Second, this tumor exhibited rapid radiological growth, with enlargement noted on MRI after just 6 months after the initial debulking. There was more than 1–2 cm of growth in each dimension noted at her subsequent evaluation. These features were particularly aggressive and atypical for conventional benign nerve sheath tumors.
Overview of the Literature
Malignant transformation in neurofibromas is a well-recognized phenomenon, most commonly in the form of MPNST in patients with neurofibromatosis type 1 (NF1), who carry an 8%–13% lifetime risk of malignant transformation.17 Conversely, malignant transformation in schwannomas is an exceptionally rare event, having no association with NF1 or NF2 and occurring in the form of epithelioid MPNST or angiosarcoma. The first documented case of angiosarcoma arising in schwannoma was reported by Trassard et al.14 in 1996, and to the best of our knowledge, only 21 additional cases have been reported since, mostly as isolated case reports, with the largest series including only four cases.1 A recent literature review of the published cases reveals that the single most commonly affected structure is the vagus nerve, followed by the sciatic nerve.15 Males are affected more than females (ratio 2:1) and although the age of presentation is wide (17–73), these lesions have been shown to commonly present in the sixth and seventh decades of life. By contrast, pure schwannomas have a wider anatomical distribution, are equally prevalent among males/females, and typically present in younger patients between the ages of 20 and 50 years.1 The presenting symptoms of angiosarcomas within schwannomas have generally been reported to be similar to those of conventional schwannomas; however, numerous studies depicted aggressive features, including rapidly growing masses, sometimes accompanied by pain and paresthesias, or dyspnea in the case of mediastinal lesions. No prior study described a tumor that was thought to be a conventional schwannoma, with rapidly progressing sensorimotor deficits. The size of the tumors varies and has been reported to be as small as 2.5 cm and as large as 30 cm.15 In the review by Xiang et al.,15 19 of 21 patients had clinical follow-up, with a mean length of 15.7 months (range, 10 days to 36 months) and seven patients died of disease.
Angiosarcoma within schwannomas has major implications for perioperative treatment. Conventional schwannomas of the peripheral nerves are almost always benign and can thus be successfully managed via complete resection. These tumors can frequently be separated from the nerve of origin, avoiding the need for adjuvant radiotherapy. By contrast, angiosarcomas within schwannomas have been shown to exhibit a more aggressive clinical course. Lee et al.6 reported a case of an epithelioid angiosarcoma within a 30-cm left sciatic plexiform schwannoma in a 73-year-old male. Despite complete resection of the tumor, the patient experienced local recurrence as well as pulmonary metastases 3 months after surgery. No adjuvant radiotherapy or chemotherapy was provided to this patient.6 Similarly, Li et al.7 reported a case of an 18-year-old male with a right sciatic nerve mass that was completely resected. No adjuvant therapy was administered, and the patient presented 9 months later with bloody sputum secondary to pulmonary metastasis. This was subsequently resected, and he patient received chemotherapy, without signs of further recurrence or metastatic disease 28 months after surgery.7 At present, there are very few reports of documented, long-term survival after gross-total resection and upfront adjuvant therapy of these tumors. Trassard et al.14 reported a case of a 65-year-old male with a 7-cm right tibial nerve tumor that was completely resected and treated with 2 rounds of chemotherapy and 50.4 Gy of radiotherapy. There was no evidence of local recurrence or metastatic disease at 36 months postoperatively. Given the numerous reports of early local or distant disease recurrence after incomplete resection, it would appear that complete resection at the time of surgery may play a role in achieving good outcomes.15 However, further studies are needed to better delineate the optimal adjuvant chemotherapy and radiotherapy regimen for patients with these higher-risk tumors.
Lessons
Angiosarcoma within schwannoma is a rare pathology that may have significant implications for treatment. However, optimal therapies for these tumors in terms of surgical timing and adjuvant therapy are still unknown. Rapidly worsening sensorimotor function and aggressive radiological progression are not typically seen with conventional nerve sheath tumors and should prompt consideration of other lesions in the differential. Traditional biopsy and sampling techniques may still be unreliable in establishing a definitive diagnosis.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Lakomkin, Torres-Mora, Spinner. Acquisition of data: Lakomkin, Torres-Mora, Spinner. Analysis and interpretation of data: all authors. Drafting the article: Lakomkin, Torres-Mora, Spinner. Critically revising the article: Lakomkin, Dozois, Spinner. Reviewed submitted version of manuscript: Lakomkin, Dozois, Spinner. Administrative/technical/material support: Spinner. Study supervision: Spinner.
References
- 1. McMenamin ME, Fletcher CD. Expanding the spectrum of malignant change in schwannomas: epithelioid malignant change, epithelioid malignant peripheral nerve sheath tumor, and epithelioid angiosarcoma: a study of 17 cases. Am J Surg Pathol. 2001;25(1):13–25. doi: 10.1097/00000478-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal SK, Munjal M, Rai D, Rao S. Malignant transformation of vagal nerve schwannoma in to angiosarcoma: a rare event. J Surg Tech Case Rep. 2015;7(1):17–19. doi: 10.4103/2006-8808.184941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demiröz ŞM, Fındık G, Aydoğdu K, et al. Mediastinal epithelioid angiosarcoma arising in schwannoma: The first case in the literature. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26(2):305–308. doi: 10.5606/tgkdc.dergisi.2018.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iannaci G, Crispino M, Cifarelli P, et al. Epithelioid angiosarcoma arising in schwannoma of the kidney: report of the first case and review of the literature. World J Surg Oncol. 2016;14(1):29. doi: 10.1186/s12957-016-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ito T, Tsutsumi T, Ohno K, Takizawa T, Kitamura K. Intracranial angiosarcoma arising from a schwannoma. J Laryngol Otol. 2007;121(1):68–71. doi: 10.1017/S0022215106003070. [DOI] [PubMed] [Google Scholar]
- 6. Lee FY, Wen MC, Wang J. Epithelioid angiosarcoma arising in a deep-seated plexiform schwannoma: a case report and literature review. Hum Pathol. 2007;38(7):1096–1101. doi: 10.1016/j.humpath.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 7. Li C, Chen Y, Zhang H, Zheng X, Wang J. Epithelioid angiosarcoma arising in schwannoma: report of three Chinese cases with review of the literature. Pathol Int. 2012;62(7):500–505. doi: 10.1111/j.1440-1827.2012.02827.x. [DOI] [PubMed] [Google Scholar]
- 8. Mahajan V, Rao S, Gupta P, Munjal M, Agrawal S. angiosarcoma developing in a vagal schwannoma: a rare case report. Head Neck Pathol. 2015;9(3):405–411. doi: 10.1007/s12105-014-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mentzel T, Katenkamp D. Intraneural angiosarcoma and angiosarcoma arising in benign and malignant peripheral nerve sheath tumours: clinicopathological and immunohistochemical analysis of four cases. Histopathology. 1999;35(2):114–120. doi: 10.1046/j.1365-2559.1999.00714.x. [DOI] [PubMed] [Google Scholar]
- 10. Ogawa T, Kato T, Ikeda A, et al. Case of malignant transformation of vagus nerve schwannoma to angiosarcoma. Head Neck. 2014;36(2):E17–E20. doi: 10.1002/hed.23390. [DOI] [PubMed] [Google Scholar]
- 11. Rückert RI, Fleige B, Rogalla P, Woodruff JM. Schwannoma with angiosarcoma. Report of a case and comparison with other types of nerve tumors with angiosarcoma. Cancer. 2000;89(7):1577–1585. [PubMed] [Google Scholar]
- 12. Sakai Y, Hirose T, Tomono A, et al. Angiosarcoma arising in schwannoma of cerebellopontine angle and later associating with meningioma in a patient with neurofibromatosis type 2. Brain Tumor Pathol. 2014;31(4):293–298. doi: 10.1007/s10014-014-0180-6. [DOI] [PubMed] [Google Scholar]
- 13. Shundo Y, Ota S, Inaba H, et al. [Angiosarcoma arised from a solitary schwannoma of the chest wall] Kyobu Geka. 2002;55(10):847–851. [PubMed] [Google Scholar]
- 14. Trassard M, Le Doussal V, Bui BN, Coindre JM. Angiosarcoma arising in a solitary schwannoma (neurilemoma) of the sciatic nerve. Am J Surg Pathol. 1996;20(11):1412–1417. doi: 10.1097/00000478-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 15. Xiang Y, Yan L, Lin X, Zhang X, Zhang F, Wu Z. Posterior mediastinal epithelioid angiosarcoma arising in schwannoma: a case report and review of the literature. Front Surg. 2021;8:666389. doi: 10.3389/fsurg.2021.666389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19(2):193–201. doi: 10.1634/theoncologist.2013-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]