Abstract
Previous work suggested that a metalloprotease, Vvp, may be a virulence factor of Vibrio vulnificus, which causes severe wound infection and septicemia in humans. To determine the role of Vvp in pathogenesis, we isolated an isogenic protease-deficient (PD) mutant of Vibrio vulnificus by in vivo allelic exchange. This PD mutant was as virulent as its parental strain in mice infected intraperitoneally and was 10-fold more virulent in mice infected via the oral route. Furthermore, the PD mutant was indistinguishable from its parental strain in invasion from peritoneal cavity into blood stream, enhancement of vascular permeability, growth in murine blood, and utilization of hemoglobin and transferrin. These data suggest that Vvp is not essential for virulence in the mouse. However, the cytolysin activity in the culture supernatant of the PD mutant was found to be twofold higher than that of the wild-type strain and remained for a much longer period. The higher cytolysin activity of the PD mutant may be associated with the enhanced virulence in mice infected via the oral route.
Vibrio vulnificus, a halophilic gram-negative marine bacterium, causes infectious diseases in humans, especially in people who are immunocompromised or have underlying conditions such as hemochromatosis, liver cirrhosis, or alcoholism (3, 4, 5, 29). It mainly produces two types of disease: primary sepsis via gastrointestinal infection, and wound infection. In either case, skin lesions with ulcer and edema are observed (13, 15, 36, 37).
This organism produces a few extracellular products implicated in bacterial virulence and pathogenesis, including cytolysin, protease, phospholipases, and siderophores (17, 18, 24, 38). Only a single extracellular protease (designated Vvp) has been identified (16, 24). This protease has been shown to increase vascular permeability and edema through activating the Hageman factor-plasma kallikrein-kinin cascade (25, 26, 27). It can also facilitate iron acquisition by the organism by digesting heme proteins, transferrin, and lactoferrin (31). Recently, Miyoshi et al. reported that the purified protease is able to cause hypodermic hemorrhage in guinea pigs (28). Based on these observations, Vvp was thought to be important in bacterial growth and disease developments.
We have previously cloned the vvp gene (6), which encodes a protease with its amino acid composition and the N-terminal sequence derived from the deduced amino acid sequence identical to those of the purified Vvp (16). To further determine the role of Vvp in pathogenesis by genetic approaches, we isolated a protease-deficient (PD) mutant by introducing an in-frame deletion to the vvp gene of a clinical V. vulnificus strain using an allelic exchange technique. By comparing the pathogenicity of this PD mutant with that of its parental strain, we would be able to determine whether the protease is an important virulence factor. Here we report the characterization of this PD mutant with respect to (i) virulence in mice, (ii) ability to invade the blood stream from the peritoneal cavity, (iii) ability to increase vascular permeability, (iv) growth in murine blood, and (v) ability to acquire iron from various iron-containing proteins.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The V. vulnificus and Escherichia coli strains and the plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| V. vulnificus strains | ||
| CS9133 | Clinical isolate | Gift from J. H. Rhee |
| CP103 | CS9133 vvp::pCP3 | This study |
| CP104 | CS9133, Δvvp | This study |
| CP110 | CP104/pIF3 | This study |
| E. coli strains | ||
| DH5α | supE44 lacU169(φ80lacZM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 12 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir Kmr nalidixic acid resistant | 22 |
| Plasmids | ||
| pUC19 | Cloning vector; Apr | Laboratory collection |
| pJC8 | pUC19 derivative with a 3.1-kb HindIII-HindIII fragment from V. vulnificus chromosome containing the vvp gene inserted in the multiple cloning site | 6 |
| pSI005 | pJC8 with a 426-bp BsrGI-BsrGI deletion in the vvp gene | This study |
| pCVD442 | Suicide vector; pGP704 with sacB gene inserted in the multiple cloning site; Apr | 9 |
| pCP3 | pCVD442 inserted in the SmaI site with a 1.8-kb SacI-HindIII fragment containing the deletion in vvp cloned from pSI005 | This study |
| pJRD215 | Shuttle vector; Tcr | 8 |
| pIF3 | pJRD215 derivative with a 3.1-kb HindIII-HindIII fragment from V. vulnificus chromosome containing the vvp gene inserted in the multiple cloning site | This study |
Abbreviations: Kmr, Apr, and Tcr, resistance to kanamycin, ampicillin, and tetracycline, respectively.
Animals.
Six- to eight-week-old C3H/HeNcrj and C3H/HeJcrj mice and 6- to 8-week-old Wistar rats were purchased from the animal center of the College of Medicine at National Cheng-Kung University.
Media.
All liquid cultures were grown in Luria broth (LB) at 37°C with vigorous aeration. All strains were maintained at −70°C in LB medium containing 17% glycerol. The bacteria were grown at 37°C on LB agar plates containing 1% skim milk for detecting protease activity. LB agar plates were depleted of iron by adding 75 μg of ethylenediamide-di(o-hydroxyphenyl) acetic acid (EDDHA; Sigma Chemical Co.) per ml of medium. Ampicillin (100 μg/ml), polymyxin B (50 U/ml), and tetracycline (15 μg/ml) were added as appropriate.
DNA preparation, manipulation, and analyses.
Plasmid DNA was extracted from the bacterial cells by the alkaline lysis method of Birnboim and Doly (2). Standard techniques were used to construct recombinant plasmids (33). DNA restriction endonucleases and T4 DNA ligase were purchased mainly from New England Biolabs. DNA fragments were purified from the agarose gels by using the GeneClean Glassmilk kit (Bio 101, Inc.).
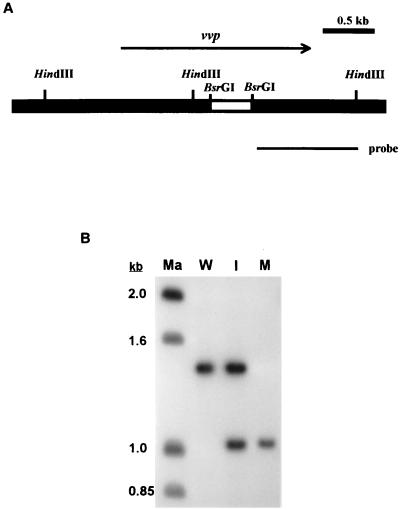
PCR was performed in a thermocycler (GeneAmp PCR system 9600; Perkin-Elmer Cetus) with 30 cycles of the following conditions: denaturing temperature of 94°C for 40 s; annealing temperature of 55°C for 90 s; and extension temperature of 72°C for 2 min. An additional 10 min at 72°C completed the reaction. Before Taq DNA polymerase (Amersham Pharmacia Biotech Inc.) was added, each reaction was preceded by a 2-min denaturing step at 94°C. The PCR primers used for amplifying a sequence in vvp in which the deletion was introduced (Fig. 1A) were 5′-GTCGCGGAAGAAGAGCC and 5′-GGCCGTGAGAGCACTCCGG. For Southern hybridization, chromosomal DNA of V. vulnificus was prepared by a method described by Ausubel et al. (1). Ten-microgram aliquots of the chromosomal DNA were completely digested with HindIII, fractionated by electrophoresis on a 1.2% agarose gel, and transferred to a nylon membrane (Hybond N+; Amersham Pharmacia Biotech). The probe was prepared and labeled with [α-32P]dCTP by random priming with a kit (Megaprime DNA labeling system; Amersham Pharmacia Biotech) using a BsrGI-HindIII fragment from pJC8 as the template (Fig. 1A). The membrane was prehybridized for 30 min at 68°C, hybridized for 1.5 h at the same temperature, washed, and visualized by autoradiography.
FIG. 1.
Detection of the deletion in vvp in the chromosome of V. vulnificus. (A) Restriction map of the insert in pCP3, which was used to construct the V. vulnificus vvp mutant. The deletion (blank bar) and the probe used in Southern blot analysis are indicated. (B) Southern blot analysis. Ten-microgram aliquots of genomic DNA were digested by HindIII, and the samples were electrophoresed on a 1.2% agarose gel. Ma, molecular weight standards; W, V. vulnificus CS9133 (wild type); I, CP103 (CS9133 with pCP3 integrated in the chromosome); M, CP104 (Δvvp).
Construction of the vvp mutant.
A vvp deletion was introduced into the chromosome of V. vulnificus by in vivo allelic exchange (9). Plasmid pSI005 was first constructed by removing a sequence between two BsrGI sites, within which the catalytically important zinc-binding region is located, in the vvp gene carried by pJC8. This resulted in a 426-bp in-frame deletion in the vvp gene which was confirmed by DNA sequence determination. The SacI-HindIII fragment from pSI005 was then made blunt ended using Klenow fragment and cloned into the SmaI site in pCVD442 to create pCP3. Plasmid pCVD442 is a suicide vector containing the sacB gene, which allows negative selection with sucrose for loss of the vector. Plasmid pCP3 was transferred from E. coli SM10λpir to V. vulnificus by conjugation. The transconjugants, which had pCP3 integrated in the chromosome via homologous recombination, were selected by ampicillin and polymyxin B and tested for sensitivity to 10% sucrose. Such transconjugants were obtained at a rate of 1 per 108 recipients. One of the sucrose-sensitive transconjugants was grown in LB broth containing 10% sucrose at 37°C overnight and then spread onto a 10% sucrose-containing LB plate for selecting the sucrose-resistant clones. The resultant strains were further tested for ampicillin sensitivity. Of the 600 sucrose-resistant colonies tested, 590 were ampicillin sensitive. Some of these sucrose-resistant, ampicillin-sensitive strains were then plated on the skim milk plate to screen for those that had lost protease activity.
Virulence in mice.
C3H/HeN mice were infected either by intraperitoneal (i.p.) injection or by force feeding of a bacterial suspension. For infection by i.p. injection, the mice were given 10-fold serially diluted bacterial suspension in phosphate-buffered saline (PBS) and mortality was recorded 48 h postinfection. For infection by the oral route, mice were pretreated with cyclophosphamide and iron dextran to render them neutropenic and iron overloaded, or they were not susceptible (our unpublished data). Each mouse was injected intraperitoneally with 3.75 mg of cyclophosphamide (Sigma) 72 h prior to challenge and intramuscularly with 25 mg of iron dextran (Sigma) 2 h prior to challenge. Pretreated mice were fasted for 12 h and then fed, by gastric intubation, with 500 μl of 10-fold serially diluted bacterial suspension in PBS containing 3% sodium bicarbonate. Mortality was recorded 72 h after challenge. The LD50 (the dose lethal to 50% of the mice) was calculated by the method of Reed and Muench (32).
Assay for bacterial invasion from peritoneal cavity into the bloodstream.
Bacteria (106 CFU) were injected into the peritoneal cavity of C3H/HeN and C3H/HeJ mice. C3H/HeJ is a lipopolysaccharide (LPS)-nonresponsive strain derived from C3H/HeN and was used to exclude the effect of LPS. The bacterial concentrations in the blood were estimated by plate counts at 1, 2, and 3 h postadministration.
Assay for enhancement of vascular permeability.
Rats that had been injected intravenously with 5% Evans' blue (1 ml/kg of body weight; Boehringer Mannheim GmbH, Mannheim, Germany) were injected intradermally into the dorsal skin with various concentrations of a bacterial suspension (100 μl per rat). At 6 h postinjection, the rats were sacrificed and the skin with the blue spot was stripped off. Increment of vascular permeability was determined by measuring the amount of Evans' blue extracted from the excised skin, as described by Katayama et al. (14).
Assay for bacterial growth in murine blood.
Seventy microliters of bacterial suspension in PBS was mixed with 130 μl of heparinized C3H/HeN murine whole blood at an initial concentration of 5 × 103 CFU per ml, and the mixture was incubated at 37°C with end-over-end rotation. The concentration of bacteria in the blood was determined at intervals by plate counts.
Assay for utilization of iron-containing compounds.
Bacterial culture (about 5 × 107 CFU) were seeded in iron-depleted top agar, which was then poured onto an iron-depleted LB agar plate (19). Five microliters of hemoglobin (10 μM) or transferrin (0.85 mM) solution, which was 50 or 100% iron-saturated (holotransferrin), was added onto a paper disk placed on top of the plate. The 50% saturated transferrin was obtained by mixing the holotransferrin (Sigma) and the apotransferrin (Sigma) at a ratio of 1:2. The iron saturation level of the transferrin in the mixture was determined by measuring the iron content (Fe) and the total iron-binding capacity (TICB) of the mixture using a kit from Vitro (Rochester, N.Y.) and was expressed as Fe/TIBC × 100. The plate was incubated at 37°C for 24 h, and the diameter of the zone of bacterial growth, including the disk, was measured. An inorganic iron source, FeSO4, was included as a positive control.
Assays for protease and cytolysin activity.
Protease activity in the culture supernatant was determined as described by Kreger and Lockwood (17). Briefly, bacterial culture supernatant was mixed with an equal volume of azocasein (Sigma) solution (5 mg/ml) and incubated at 37°C for 15 min. The protein in the reaction mixture was then precipitated with 3.2% trichloroacetic acid, and the optical density at 450 nm (OD450) of the supernatant, which represents the amount of azocasein digested, was measured after addition of an equal volume of 0.5 N NaOH. Cytolysin activity in the culture supernatant was assayed by mixing the sample with an equal volume of murine blood cell suspension (0.7 to 0.9% in PBS) and incubating the mixture at 37°C for 5 min. The unlysed murine blood cells as well as the cell debris were pelleted by centrifugation, and the OD545 of the supernatant, which represents the level of hemolysis, was measured. Cytolysin activity was expressed as OD545 of specimen/OD545 of complete hemolysis by Triton X-100 × 100.
Statistical analysis.
Comparisons of the various data between the V. vulnificus strains were made with the two-tailed Student t test.
RESULTS
Isolation of protease-deficient V. vulnificus mutant.
A suicide vector, pCP3, which carried a DNA fragment that contained the vvp gene with a 426-bp in-frame deletion (Fig. 1A) was constructed and used to isolate a PD V. vulnificus mutant by allelic exchange technique. Among the 50 sucrose-resistant, ampicillin-sensitive colonies tested, 39 exhibited proteaseless phenotype on the skim milk plate. One of these proteaseless mutants, CP104, was further confirmed for presence of the deletion by PCR using a pair of primers complementary to sequences flanking the deletion (data not shown) and by Southern blot analysis (Fig. 1B).
Compared with CS9133 (the wild-type strain), CP104 produced much smaller clear zone surrounding the colony on a skim milk plate after overnight incubation. The reconstituted strain, CP110, which expressed the protease from a recombinant plasmid, pIF3, restored this ability (data not shown). Results of protease assay of the overnight culture supernatants were consistent with those observed on the skim milk plate: the OD450 values, which represent the amounts of azocasein digested by the protease, were 0.46, 0.04, and 0.59 for CS9133, CP104, and CP110, respectively. The growth rate of CP104 in LB medium was comparable to that of its parental strain (data not shown), indicating that the protease is not essential for bacterial growth in rich medium.
Bacterial virulence in mice.
The LD50 values of the wild-type strain, CS9133, and the PD mutant, CP104, in the mouse were 1.2 × 105 and 2.5 × 105 CFU, respectively, for infection i.p. and were 8.0 × 105 and 8.2 × 104 CFU, respectively, for infection via the oral route.
Bacterial invasion from the peritoneal cavity into the bloodstream.
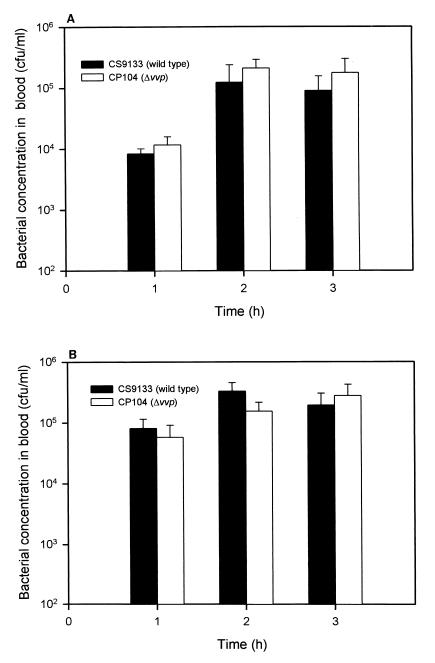
Invasion of V. vulnificus from the mouse peritoneal cavity into the bloodstream was not significantly different between CP104 and its parental strain, CS9133, in either C3H/HeN (P > 0.05) or C3H/HeJ mice (P > 0.05) (Fig. 2).
FIG. 2.
Invasion of bacteria from peritoneal cavity into the bloodstream in C3H/HeN (A) and C3H/HeJ (B) mice. Each mouse was injected i.p. with about 106 CFU of bacteria, and the bacterial concentration in the blood was determined by plate counts (n = 3 in C3H/HeN mouse group; n = 5 in C3H/HeJ mouse group).
Enhancement of vascular permeability by bacterial infection.
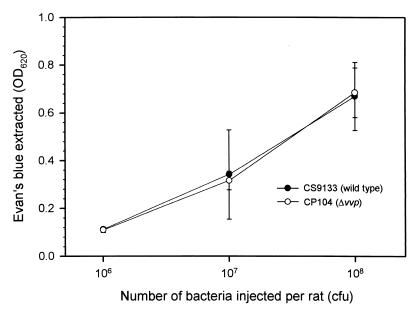
Rats administered with either the wild-type strain or the PD mutant showed an infective dose-dependent increase of vascular permeability, and no significant difference was observed between these two strains (P > 0.05) (Fig. 3).
FIG. 3.
Increase of vascular permeability by bacterial infection. Bacteria were injected intradermally into the dorsal skin of each Wistar rat that had been injected intravenously with Evans' blue. After 6 h, the rat was sacrificed and the dorsal skin was stripped off, and the amount of blue dye leaking out of the blood vessel was determined (n = 3).
Bacterial growth in the blood.
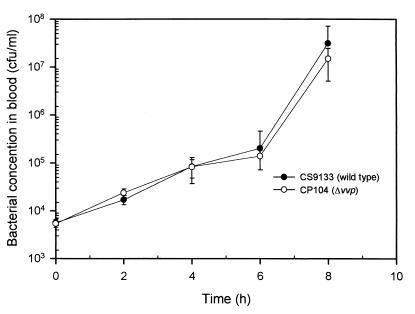
Both the wild-type strain and the PD mutant grew slowly for the first 6 h of incubation and faster thereafter. Overall, little difference in the growth rate was observed between these two strains (P > 0.05) (Fig. 4).
FIG. 4.
Growth of V. vulnificus strains in murine whole blood. Bacteria inoculated into the whole blood collected from the C3H/HeN mice was incubated at 37°C. Bacterial concentration in the blood was determined by plate counts (n = 4).
Utilization of hemoglobin and transferrin.
The PD mutant, the wild-type strain, and the reconstituted strain, CP110, were equally capable of utilizing FeSO4, hemoglobin, or 50 or 100% saturated transferrin (P > 0.05) (Table 2).
TABLE 2.
Utilization of various iron sources by V. vulnificus strains
| Iron source | Diam (mm) of zone of growth of various strains (mean ± SDa)
|
||
|---|---|---|---|
| CS9133 | CP104 | CP110 | |
| FeSO4 | 51.7 ± 0.3 | 51.8 ± 0.8 | 50.7 ± 0.8 |
| Hemoglobin | 36.8 ± 0.6 | 37.0 ± 0.0 | 37.2 ± 0.3 |
| Holotransferrin | 38.3 ± 0.3 | 39.9 ± 1.9 | 39.5 ± 0.9 |
| 50% iron-saturated transferrin | 30.5 ± 0.9 | 31.8 ± 1.2 | 29.7 ± 1.5 |
Calculated from the results of three experiments.
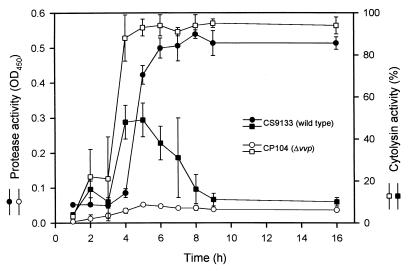
The cytolysin activity is enhanced in the vvp mutant.
The cytolysin activity (Fig. 5) in the culture supernatant of the PD mutant increased rapidly during the early growth phase, reached a maximum at 6 h of growth, and stayed at that level for up to 16 h. The cytolysin activity in the culture supernatant of the parental strain reached a maximum, which was about half of that of the vvp mutant, at 5 h and then declined rapidly. On the other hand, the protease activity in the culture supernatant of the parental strain was low until 5 h, when it increased rapidly to a high level that was maintained up to 16 h. The protease activity of the PD mutant was not detectable.
FIG. 5.
Protease and cytolysin activities in the culture supernatant of V. vulnificus strains. Bacteria were grown at 37°C after a 1:100 dilution of an overnight culture in fresh medium. The culture supernatant was then collected at intervals, and the protease and cytolysin activities were determined (n = 3).
DISCUSSION
The PD mutant of V. vulnificus was shown to be as virulent as its parental strain when administered to the mouse by i.p. injection. It also appeared to be as invasive as the parental strain in spreading from the peritoneal cavity into the bloodstream. Maruo et al. reported that the generation of bradykinin is important for V. vulnificus invasion into the bloodstream from the peritoneal cavity, because inhibition of bradykinin generation reduced bloodstream invasion (21). The bradykinin cascade is triggered by plasminogen activation, and two bacterial factors may be involved in this process. One is the extracellular protease which can activate plasminogen through its proteolytic activity (20); the other is the LPS that can convert the Hageman factor into a mature form, which in turn is the starting point of bradykinin cascade (20). We demonstrated that the PD mutant was similar to its parental strain in spread from the peritoneal cavity into the bloodstream in either the LPS-responsive C3H/HeN or the LPS-nonresponsive C3H/HeJ mouse strain. Because the PD mutant was still invasive in C3H/HeJ mice, some bacterial component(s) other than the protease and LPS might take part in bacterial invasion. Activation of bradykinin cascade also results in an increase of vascular permeability (20). Therefore, a similar explanation can apply to the insignificant difference in enhancement of vascular permeability observed between infection with the wild-type strain and with the PD mutant.
Surprisingly, the oral route LD50 of the PD mutant in the mouse was 10-fold lower than that of the wild-type strain. This suggests not only that the protease is not essential for bacterial virulence but also that some factors involved in pathogenesis via the oral route may be overreactive in the absence of protease and result in enhanced bacterial virulence.
Cytolysin activity in the culture supernatant of the wild-type strain declined rapidly during the late log growth phase when the protease activity was increasing rapidly. However, the cytolysin activity in the culture supernatant of the PD mutant increased to twice the level of that of the wild-type strain and remained at that level for a substantial period. It appeared that the cytolysin lost its activity in the presence of the metalloprotease, suggesting that the cytolysin might be a substrate of the protease. Furthermore, the protein profiles of a 9-h culture supernatant of the wild-type strain and the PD mutant examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were very different: the PD mutant gave rise to many more protein bands than did the wild-type strain (our unpublished data). Loss of protease, therefore, could lead to a high expression level, not only of the cytolysin but also of other factors, which may result in enhanced virulence in the host. The cytolysin, for example, which has been shown to enhance vascular permeability (11, 17, 34), may compensate for loss of protease in increasing vascular permeability when overexpressed in the PD mutant.
The role of Vvp in facilitating iron acquisition has been proposed by Nishina et al. (30) and Okujo et al. (31). They demonstrated that PD mutants isolated by chemical mutagenesis grew poorly on 30% iron-saturated transferrin, 15% iron-saturated lactoferrin, or heme proteins in a synthetic medium, but that the addition of purified V. vulnificus protease restored their growth to a wild-type level. In this study, we found that the PD mutant, its wild-type parental strain, and the reconstituted strain that overexpressed Vvp grew equally well on hemoglobin or 50 or 100% saturated transferrin. These results suggest that iron acquisition depends mainly on other factors, such as the siderophores (7, 18, 35) or a heme receptor on the outer membrane of V. vulnificus (19). The PD mutant showed little difference from the wild-type strain in growth in the murine blood, further demonstrating that the protease plays an insignificant role in iron acquisition from the blood.
The metalloprotease of V. vulnificus, Vvp, has high sequence homology with that of V. anguillarum and the hemagglutinin (HA)/protease of V. cholerae. By characterizing a metalloprotease mutant of V. anguillarum, Milton et al. found that this protease is not necessary for invasion in a fish model (23). Finkelstein et al. also suggested that the V. cholerae HA/protease has only an indirect, if any, role in virulence (10). An HA/protease mutant was shown to have no direct contribution to the virulence of V. cholerae in infant rabbits. However, Finkelstein et al. could not rule out the possibility that HA/protease may act as an accessory virulence factor by enhancing or regulating other virulence factors.
In summary, no conclusive evidence for the previously proposed roles of Vvp in the invasiveness of V. vulnificus can be inferred from this study. However, the data obtained from characterizing the PD mutant suggest a possible multifactor interaction in bacterial virulence, which may involve the protease but to an extent that is not yet clear. This protease is able to digest collagen and elastin and was thought to be associated with tissue damage and formation of skin lesions (28). A comparison of the abilities of the PD mutant and the parental strain to cause tissue damage is under way.
ACKNOWLEDGMENTS
This work was supported by grant NSC 85-2321-B-006-003 from National Science Council and grant DOH87-HR-606 from National Health Research Institute, Taiwan.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennt C E, Wright A C, Dutta S K, Morris J G., Jr Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J Infect Dis. 1991;164:1030–1032. doi: 10.1093/infdis/164.5.1030. [DOI] [PubMed] [Google Scholar]
- 4.Bullen J J, Spalding P B, Ward C G, Gutteridge J M. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 5.Chan T Y, Chow D P, Ng K C, Pan K W, McBride G A. Vibrio vulnificus septicemia in a patient with liver cirrhosis. Southeast Asian J Trop Med Public Health. 1994;25:215–216. [PubMed] [Google Scholar]
- 6.Cheng J C, Shao C P, Hor L I. Cloning and nucleotide sequencing of the protease gene of Vibrio vulnificus. Gene. 1996;183:255–257. doi: 10.1016/s0378-1119(96)00488-x. [DOI] [PubMed] [Google Scholar]
- 7.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51:275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Häse C C. Vibrio cholerae hemagglutinin-protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray L D, Kreger A S. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by Vibrio vulnificus infection. J Infect Dis. 1987;155:236–241. doi: 10.1093/infdis/155.2.236. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 13.Ianda J M, Powers C, Bryant R G, Abbott S L. Current perspectives on the epidemiology and pathogenesis of clinical significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama S, Shinoya H, Ohtake S. A new method for extraction of extravasated dye in the skin and the influence of fasting stress on passive cutaneous anaphylaxis in guinea pigs and rats. Microbiol Immunol. 1978;22:89–101. doi: 10.1111/j.1348-0421.1978.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 15.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy L M, Gunn R A. Syndromes of Vibrio vulnificus infections. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 16.Kothary M H, Kreger A S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987;133:1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- 17.Kreger A, Lockwood D. Detection of extracellular toxin(s) produced by Vibrio vulnificus. Infect Immun. 1981;33:583–590. doi: 10.1128/iai.33.2.583-590.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litwin C M, Bryne B L. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998;66:3134–3141. doi: 10.1128/iai.66.7.3134-3141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda H, Yamamoto T. Pathogenic mechanisms induced by microbial proteases in microbial infections. Biol Chem Hoppe Seyler. 1996;377:217–226. doi: 10.1515/bchm3.1996.377.4.217. [DOI] [PubMed] [Google Scholar]
- 21.Maruo K, Akaike T, Ono T, Maede H. Involvement of bradykinin generation in intravascular dissemination of Vibrio vulnificus and prevention of invasion by a bradykinin antagonist. Infect Immun. 1998;66:866–869. doi: 10.1128/iai.66.2.866-869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutants: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milton D L, Norqvist A, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi N, Shimizu C, Miyoshi S, Shinoda S. Purification and characterization of Vibrio vulnificus protease. Microbiol Immunol. 1987;31:13–25. doi: 10.1111/j.1348-0421.1987.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi N, Miyoshi S, Sugiyama K, Suzuki Y, Furuta H, Shinoda S. Activation of the plasma kallikrein-kinin system by Vibrio vulnificus protease. Infect Immun. 1987;55:1936–1939. doi: 10.1128/iai.55.8.1936-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi S, Shinoda S. Role of the protease in the permeability enhancement by Vibrio vulnificus. Microbiol Immunol. 1988;32:1025–1032. doi: 10.1111/j.1348-0421.1988.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi S, Shinoda S. Activation mechanism of human Hageman factor-plasma kallikrein-kinin system by Vibrio vulnificus metalloprotease. FEBS Lett. 1992;308:315–319. doi: 10.1016/0014-5793(92)81301-2. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi S, Nakazawa H, Kawata K, Tomochika K, Tobe K, Shinoda S. Characterization of the hemorrhagic reaction caused by Vibrio vulnificus metalloprotease, a member of the thermolysin family. Infect Immun. 1998;66:4851–4855. doi: 10.1128/iai.66.10.4851-4855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muench K H. Hemochromatosis and infection: alcohol and iron, oysters and sepsis. Am J Med. 1989;87:40N–43N. [PubMed] [Google Scholar]
- 30.Nishina Y, Miyoshi S, Nagase A, Shinoda S. Significant role of an exocellular protease in utilization of heme by Vibrio vulnificus. Infect Immun. 1992;60:2128–2132. doi: 10.1128/iai.60.5.2128-2132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okujo N, Akiyama T, Miyoshi S, Shinoda S, Yamamoto S. Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol Immunol. 1996;40:595–598. doi: 10.1111/j.1348-0421.1996.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 32.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, New York, N.Y.: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Shinoda S S, Miyoshi S, Yamanaka H, Miyoshi N. Some properties of Vibrio vulnificus hemolysin. Microbiol Immunol. 1985;29:583–590. doi: 10.1111/j.1348-0421.1985.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 35.Simpson L M, Oliver J D. Siderophore production by Vibrio vulnificus. Infect Immun. 1983;41:644–649. doi: 10.1128/iai.41.2.644-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 37.Warnock E W, MacMath T L. Primary Vibrio vulnificus septicemia. J Emerg Med. 1993;11:153–156. doi: 10.1016/0736-4679(93)90510-e. [DOI] [PubMed] [Google Scholar]
- 38.Wear J E, Thomas M B, Warner M, Linder K. Production of extracellular enzymes and cytotoxicity by Vibrio vulnificus. Diagn Microbiol Infect Dis. 1986;5:99–111. doi: 10.1016/0732-8893(86)90112-4. [DOI] [PubMed] [Google Scholar]