Abstract
INTRODUCTION:
There is limited knowledge on whether eating foods that are part of the Western diet may offset the potential benefits of the Mediterranean diet (MedDiet) on cognitive decline.
METHODS:
Analyses included 5,001 participants (63% African American, 36% males, 74±6.0 years old) who completed a food frequency questionnaire and at least two cognitive assessments (a composite of 4 tests) over 6.3(±2.8) years of follow-up. We used mixed-effects models adjusted for age, sex, education, race, cognitive activities, physical activity, and calorie intake.
RESULTS:
In the stratified analysis, the association of higher MedDiet on cognitive decline was significant only among those with a low Western diet score (T3vs.T1: β= 0.020, p=0.002; ptrend=0.002) and not in those with a high Western diet score (T3vs.T1: β=0.010, p=0.11; ptrend=0.09).
CONCLUSION:
In this prospective study, we found that the high consumption of components of the Western diet attenuates the beneficial association of the MedDiet on cognition.
Keywords: Western diet, High-fat diet, Mediterranean diet, biracial, longitudinal
1. Introduction
With the aging population, Alzheimer’s dementia is common and poses a significant burden on social, economic, and public health1. There is no known cure, and primary prevention has emerged as a potential strategy to halt or postpone the occurrence of Alzheimer’s dementia.
Epidemiological studies have identified specific dietary patterns associated with cognitive impairment and Alzheimer’s dementia2. For example, the Mediterranean diet (MedDiet) rich in foods such as fruits, vegetables, legumes & nuts, olive oil, and fish is associated with a slower decline in cognitive abilities 3–6. In contrast, the Western diet characterized by more frequent intake of foods such as red and organ meat, butter, fried and sweet foods adversely affects cognition in aging7, 8. Therefore, existing evidence on diet, nutrition, and cognition suggests adherence to a dietary pattern including MedDiet4–6, DASH9, Mediterranean-DASH Intervention for Neurodegenerative Disorders (MIND)10 avoiding diets such as the Western diet and the Southern-Western diet11. However, in practice, although people try to consume MedDiet dietary components like fish/legumes, fruits, vegetables, olive oil, and whole grains more often, they may also consume food items that are characteristics of a Western diet such as refined grains, french-fries, fried chicken/fish or sweet foods for few other meals during the week.
Therefore, this study investigates whether the consumption of unfavorable components of the western diet modifies the known beneficial association of the MedDiet on cognitive change in the biracial population of the Chicago Health and Aging Project (CHAP).
2. Subjects and Methods
2.1. Study population
The analytical sample included individuals from the Chicago Health and Aging Project (CHAP, 1993–2012), a population-based cohort study of older adults residing in Chicago12. The cohort enrolled 10,802 individuals, 78.7% of residents aged ≥ 65 years or older from four geographically defined biracial communities of African Americans and non-Hispanic whites. Details about the design and objectives of CHAP have been reported previously12. Informed consent was obtained from all the participants. All enrolled CHAP participants had a 90-minute in-home interview for cognitive and risk factor assessments conducted every three years during the study interval. Of 10,802 participants, 6,997 participants had valid dietary assessment data (invalid assessments were those with < 600 kcal or > 3700 kcal for women and <700 kcal or >4200 kcal for men; with entire pages or more than half of the items missing on the questionnaire; baseline MMSE score less than 10). We excluded participants without follow-up cognitive assessments (either deceased or did not participate in follow-up assessments or lost to follow-up, n=1825) and those who completed the food frequency questionnaire (FFQ) > 2.5 years after the baseline assessments (n=171); thus, the analytical sample was 5001. The demographic characteristics of our analytical sample were similar to the overall cohort (supplementary table 1). The Institutional Review Board of Rush University Medical Center approved the study.
2.2. Dietary assessment
Diet was assessed using a validated modified Harvard FFQ, with 144 food items and questions on vitamins and mineral supplements13, 14. The daily nutrient intake was computed using the Harvard nutrient database. One serving size of all the food items was defined as per the average amount consumed in the United States as per the United States Department of Agriculture database. The frequency of food intake was multiplied by the nutrient composition of one serving of that food item and then summed over all the foods and supplements to estimate the total daily intake. The Mediterranean diet (MedDiet) score was based on 11 food groups, as previously described15. The scoring ranged from 0–5 for each food item, and the total MedDiet score ranged from 0–55, where higher scores corresponded to greater adherence to the MedDiet (supplementary table 2). The scoring of MedDiet for the CHAP population has been previously described6.
The Western diet score was derived using principal component analysis (FFQ food items categorized into 40 food groups; supplementary table 3) as described previously16. The eigenvalue of more than 1 was considered and based on the food group consumption in the study population, our analysis retained two factors (factor loading > 0.20); factor 1 was labeled as a healthy diet pattern and factor 2 as a Western diet pattern. These two diet factors consisted of a distinct food group (Supplementary Figure.1). Factor 2, i.e., the Western diet pattern, was the primary score of interest for this study and included food items such as fried foods, refined grains, sweets, red and processed meats, and full-fat dairy, and pizza. The score was calculated by summing the selected food group intake (standardized), weighted by factor loading of the food groups. The Western diet scores generated help rank participants as per their adherence to this dietary pattern, such as a higher score indicating more consumption of unhealthy foods. Using the median score of the Western diet, we divided the study population into high (score > 3.5 ) and low (score ≤3.5) Western diet group.
2.3. Cognitive function assessment
During the in-home interviews, trained interviewers administered four cognitive tests at baseline, 3-year, 6-year, and 9-year follow-ups. The four cognitive tests included East Boston Tests of Immediate and Delayed Recall (Score range: 0 to 12)17, Mini-Mental State Examination (MMSE, Score range: 0 to 30)18, and Symbol Digit Modalities Test (Score range: 0 to 96)19. All four tests are highly correlated, and to reduce measurement errors along with any floor and ceiling artifacts, we computed a composite score for cognitive function. Using the baseline population means and standard deviation, we converted all the raw scores of each test to standardized z scores. We averaged them together to obtain the overall global measure of cognitive function20. Additionally, two cognitive domains, including episodic memory (based on averaged z scores for the two East Boston Tests) and perceptual speed (based on the z scores for the Symbol Digit Modalities Test), were also assessed 21–23.
2.4. Covariates
Information on age (based on the date of birth and date of the first cognitive assessment), sex, education (number of years of formal education), race (determined by questions and categories of the 1990 US census), and smoking status (nonsmokers vs. current or former smokers) was collected at participants’ baseline interview. Physical activity was based on self-reported minutes spent for walking, exercise, yard work, calisthenics, biking, and water exercise24. The mean frequency of participation in cognitive activities such as reading newspapers, books, playing board games or crossword puzzles, or going to religious services was constructed as a composite score 25. The measurements of weight (kg) and height (meters) were used to compute the body mass index (BMI) as kg/meters2 and modeled as two indicator variables, BMI≤20 and BMI≥30 26. Hypertension marked present if self-reported history or measured systolic/diastolic blood pressure of ≥160/95 mm Hg. Diabetes, stroke, and myocardial infarction was determined by self-report.
3. Statistical Analysis
Baseline characteristics of study participants were examined in low and high Western diet group by the tertile of MedDiet score. The correlation between the MedDiet and the Western diet score was assessed using Spearman’s rank correlation coefficient. We used linear mixed random-effects models to examine the MedDiet score’s association with the change in global cognitive function, episodic memory, and perceptual speed. The MedDiet Score was modeled as a continuous variable (linear) and an indicator variable (non-linear: as tertiles). The linear trend of the MedDiet association was also examined where all the records within the tertile were assigned the median value and modeled as a categorical variable. For both linear and non-linear sets of models, the study results were identical; thus, we primarily present the tertile estimates in the text, tables, and figures. We first analyzed the association in the basic multivariable model adjusted for established risk factors, including a term for age (years), sex, race, education (years), physical activity (hours/week), late-life cognitive activity, total calories (kcal) and an interaction term between time and each variable. Then we examined the effect modification due to high or low Western diet consumption by using a three-way interaction term between MedDiet, Western diet, and time. The significant interactions were then assessed in the stratified analyses for the difference in the association of MedDiet and cognition as per high and low Western diet groups. We further adjusted these models for smoking status and BMI.
Additionally, for secondary analyses, models were also adjusted for other potential confounders, i.e., cardiovascular conditions: hypertension, diabetes, stroke, and myocardial infarction (included simultaneously in the models). We also conducted a sensitivity analysis for the basic model after removing the participants whose baseline cognitive scores were in the lowest 10% of the distribution. Both high and Low Western diet groups were further tested for the effect modification by age (>75/≤75 years, sex (male/female), race (African Americans/others), education (>12/≤12 years) in separate multivariable basic models that included terms for tertile of MedDiet, potential effect modifier, other covariates of our basic model, and an interaction term between MedDiet and the specific effect modifier. Besides, we estimated the equivalent age differences in years between individuals with higher (third tertile) and lower (first tertile) MedDiet score (we used the beta coefficients [β (time*age) / β (time*tertile 3 M score) in the basic-adjusted models). Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
4. Results
The baseline characteristics of the 5001 study participants are presented in Table 1 (74.05(±6.0) years old, 63% women, 63% African Americans, 12.5(±3.6) years of education). The mean follow-up time was 6.3 (±2.8 years). The high Western diet group had more males, fewer African Americans, and fewer participants with a history of diabetes (may indicate people with preexisting diabetes who have already made dietary modifications) (Supplementary Table 4). Participants in the highest tertile of MedDiet score in both low and high Western diet groups were more likely to be white, had higher education, calorie intake, physical activity, and less stroke prevalence than those in the lowest tertile (Table 1). Overall MedDiet score was weakly associated with the Western diet score in our population (ρ=0.06). In the overall population, the median (IQR) Western diet score was similar as per the tertile of MedDiet (lowest tertile: 3.4 (2.3, 4.7); middle tertile: 3.5 (2.4, 4.9); highest tertile: 3.6 (2.6, 4.9)). Dietary components in the corresponding MedDiet tertiles of high and low Western diet groups were similar except for potato, dairy, and red meat (supplementary table 5).
Table 1:
Baseline characteristics of the 5001 study participants of the Chicago Health and Aging Project by tertile of MedDiet score stratified by low and high western diet score
| Overall | Low Western diet score | High Western diet | |||||
|---|---|---|---|---|---|---|---|
| Tertile of MedDiet score | Tertile of MedDiet score | ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | ||
| N | 5001 | 984 | 785 | 739 | 878 | 791 | 824 |
| MedDiet score, median (IQR) | 28 (25,32) | 24 (22, 25) | 28 (28, 29) | 33 (32, 36) | 24 (22, 25) | 29 (27, 29) | 33 (32, 36) |
| Western diet score, median (IQR) | 3.5 (2.4, 4.8) | 3.4 (2.3, 4.7) | 3.5 (2.4, 4.9) | 3.6 (2.6, 4.9) | 4.8 (4.0, 6.1) | 4.9 (4.1, 6.1) | 4.8 (4.1, 6.1) |
| Age, mean ± SD, years | 74.05 ± 6.0 | 74.3 ± 6.0 | 73.4 ± 5.6 | 73.0 ± 5.2 | 74.8 ± 6.5 | 74.6± 6.3 | 73.9 ± 5.8 |
| Male, % | 36 % | 31 % | 30 % | 32 % | 41 % | 44 % | 42 % |
| Race, % African Americans | 63 % | 76 % | 69 % | 52 % | 68 % | 59 % | 49 % |
| Education, mean ± SD, years | 12.5 ± 3.6 | 11.6 ± 3.4 | 12.6 ± 3.4 | 13.9 ± 3.4 | 11.6 ± 3.4 | 12.5 ± 3.6 | 13.5 ± 3.6 |
| Smokers, % past or current | 53% | 50 % | 47 % | 52 % | 58 % | 55 % | 54 % |
| Cognitive activity mean ± SD | 3.2 ± 0.7 | 3.0 ± 0.6 | 3.2 ± 0.6 | 3.4 ± 0.6 | 2.9 ± 0.7 | 3.2 ± 0.7 | 3.4 ± 0.7 |
| Physical activity, mean ±SD, hrs/week | 3.3 ± 5.3 | 2.6 ± 4.5 | 3.7 ± 5.9 | 4.3 ± 5.7 | 2.7 ± 5.1 | 3.2 ± 5.2 | 3.7 ± 5.4 |
| BMI, mean ±SD | 27.7 ± 5.7 | 28.6 ± 6.0 | 28.5 ± 5.6 | 27.5 ± 5.1 | 27.5 ± 6.1 | 27.1 ± 5.8 | 27.5 ± 5.4 |
| Total energy, mean ± SD, kcal/day | 1714 ± 625 | 1137 ± 340 | 1339 ± 347 | 1565 ± 365 | 1931 ± 548 | 2111 ± 545 | 2279 ± 570 |
| Diabetes, % | 19.3 % | 27 % | 22 % | 18 % | 15 % | 17 % | 15 % |
| Hypertension, % | 70.2 % | 74 % | 74 % | 68 % | 70 % | 68 % | 67 % |
| Myocardial infarctions, % | 14 % | 12 % | 13 % | 14.1 % | 12 % | 14 % | 15 % |
| Stroke, % | 10 % | 7 % | 6 % | 7.0 % | 11 % | 9 % | 7 % |
IQR: Interquartile range; SD: standard deviation; BMI: Body Mass Index
4.1. Diet and Cognitive decline
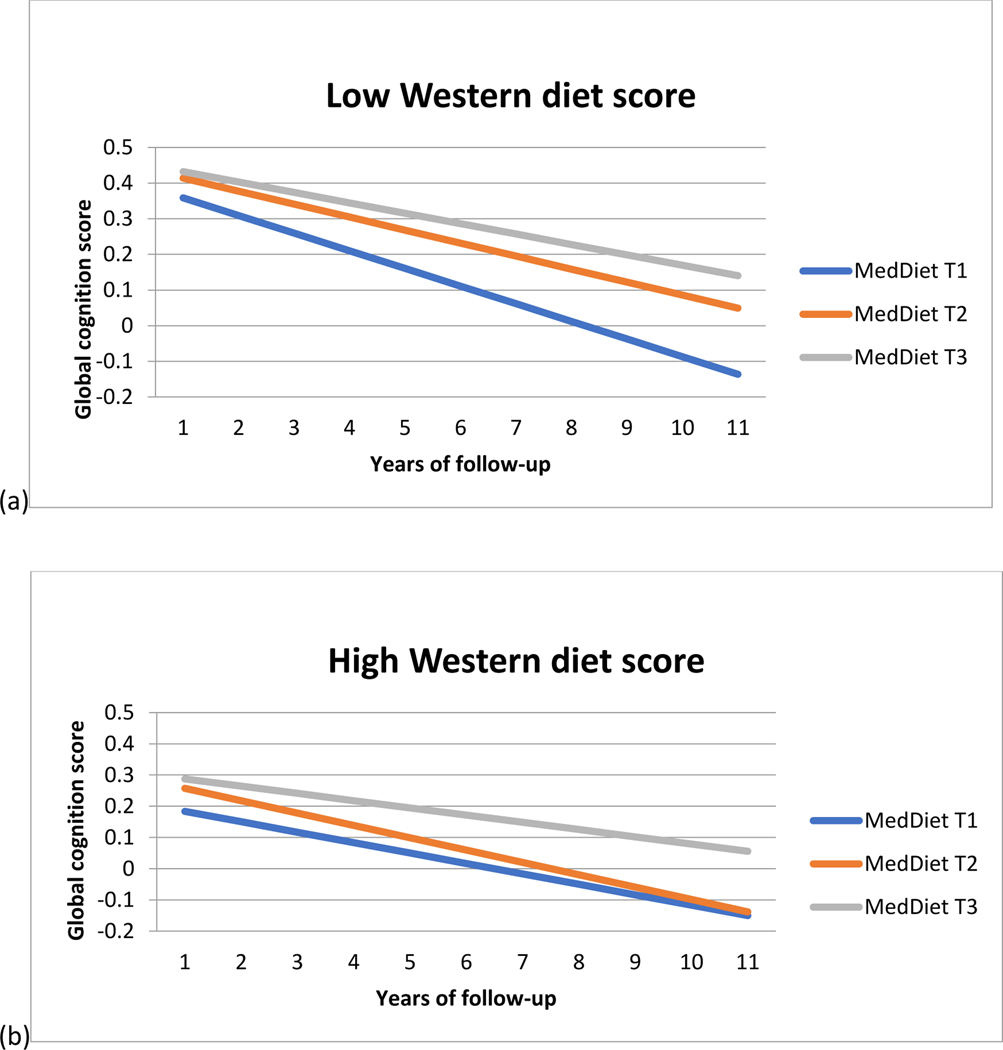
In the overall population, the MedDiet score was associated with slower cognitive decline (β =0.0014 (SE= 0.0004, p= 0.0005). Participants in the highest tertile of MedDiet had slower cognitive decline than those in the lowest tertile (Table 2). The difference in cognitive rates was almost equivalent to being 3.9 years younger in age. The Western diet score (β= 0.0001, p=0.96), and a healthy diet pattern ( (β= 0.00001, p=0.32), was not significantly associated with cognitive decline. To investigate whether the beneficial association of MedDiet is diminished by consuming a western diet (unhealthy foods), we conducted the interaction analysis for MedDiet and Western Diet. We found that those with moderate adherence to MedDiet had a significant effect modification based on their Western diet consumption (p-value for interaction=0.01, Table 2). Table 2 shows the association of MedDiet with cognitive decline among individuals with high and low Western diet scores. Among individuals consuming a low western diet, both second and third tertiles, i.e., both moderate and high adherence to the MedDiet was associated with slower cognitive decline (p for trend=0.002, Figure 1.a.). The difference in cognitive decline rates was approximately equivalent to being 5.8 years younger for those in the high MedDiet adherence group and 3.7 years younger for the moderate MedDiet adherence group compared to the low MedDiet adherence group. However, for those with a high Western diet score, the association between MedDiet and cognitive decline was not significant (p for trend=0.09, Figure 1.b.).
Table 2:
Estimated effects (β coefficient† (95% Confidence interval)) of MedDiet on the rate of change in global cognitive scores among Chicago Health and Aging Project participants, Interaction model and stratified by Low and High Western diet score over the average follow-up of 6.3 (± 2.8)years †‡§¶
| N | Tertile 1 | Tertile 2 β (95% CI) | Tertile 3 β (95% CI) | P for trend | |
|---|---|---|---|---|---|
| MedDiet association, overall | |||||
|
| |||||
| Basic model | 5001 | Ref | 0.004 (−0.004, 0.012) | 0.015 (0.007, 0.024) | 0.0005 |
|
| |||||
| Basic + CVD§ | 4959 | Ref | 0.003 (−0.004, 0.011) | 0.015 (0.006, 0.023) | 0.0006 |
|
| |||||
| Interaction model ‡ betas for the MedDiet*time and MedDiet*Western diet*time interaction term | |||||
|
| |||||
| MedDiet | 5001 | Ref | 0.014 (0.003, 0.025) | 0.022 (0.010,.033) | 0.0003 |
| MedDiet*Western diet | Ref | −0.020 (−0.037, −0.005) | −0.012 (−0.028,0.003) | 0.13 | |
|
| |||||
| Stratified Analysis | |||||
|
| |||||
| Low Western Diet group | |||||
|
| |||||
| Basic model | 2508 | Ref | 0.013 (0.002, 0.024) | 0.020 (0.007, 0.033) | 0.002 |
|
| |||||
| Basic + CVD§ | 2489 | Ref | 0.013 (0.002, 0.024) | 0.019 (0.007, 0.032) | 0.003 |
|
| |||||
| High Western Diet group | |||||
|
| |||||
| Basic model | 2493 | Ref | −0.006(−0.018, 0.005) | 0.010 (−0.002, 0.022) | 0.09 |
|
| |||||
| Basic + CVD§ | 2470 | Ref | −0.007 (−0.019, 0.005) | 0.010 (−0.002, 0.022) | 0.09 |
Basic model adjusted for age, sex, race, education, physical activity, late life cognitive activity and total calories intake, time and interaction term between time and each model covariate.
β=beta coefficient from the model for the interaction term between MIND diet score and time
Interaction model was basic model additionally controlled for western diet score and MedDiet*western diet
CVD: cardiovascular conditions included diabetes, hypertension, stroke and myocardial infarction.
Figure 1:
Rate of change in global cognitive score overtime among Chicago Health and Aging Project participants as per MedDiet score tertiles among participants with low and high Western diet score
Linear mixed models adjusted for age, sex, race, education, physical activity, cognitive activities, and total calories.
Some studies suggest that smoking may cause an increased risk of Alzheimer’s dementia 27, 28. BMI is affected by diet quality and has a more complex relation with dementia, both as a risk factor and an outcome. Further adjusting our models for smoking status and BMI did not change the results for the MedDiet association with cognitive decline in low (highest vs. lowest tertile: β =0.022 (SE=0.006,p=0.001) or high (highest vs. lowest tertile: β =0.012 (SE=0.006,p=0.06) Western diet group. We further examined whether the observed MedDiet associations with slower cognitive decline was mediated by its effects on cardiovascular conditions. After controlling for a history of diabetes, hypertension, myocardial infarction, and stroke, the effect estimates did not change much for either low or high Western diet groups (Table 2).
4.2. Diet and cognitive domains: Episodic Memory and Perceptual speed
Those in the highest tertile of MedDiet had a slower decline in episodic memory and perceptual speed (Table 3). When further controlled for smoking and BMI, there was no material change in these associations (Episodic Memory: highest vs. lowest MedDiet tertile: β = 0.015 (SE=0.005, p=0.006); Perceptual speed: highest vs. lowest MedDiet tertile: β = 0.012 (SE=0.005, p=0.01). These associations were not found to be mediated by cardiovascular conditions (Table 3).
Table 3:
Estimated effects (β coefficient †, (95% Confidence interval)) of MedDiet on the rate of change in episodic memory and perceptual speed scores among Chicago Health and Aging Project participants; Interaction model and stratified by Low and High Western diet score
| N | MedDiet Tertile 1 | MedDiet Tertile 2 β (95% CI) | MedDiet Tertile 3 β (95% CI) | P for trend | |
|---|---|---|---|---|---|
| MedDiet association, overall | |||||
|
| |||||
| Episodic Memory | |||||
|
| |||||
| Basic | 4965 | Ref | 0.001 (−0.009, 0.011) | 0.013 (0.002, 0.024) | 0.014 |
|
| |||||
| Basic + CVD§ | 4923 | Ref | 0.001 (−0.009, 0.011) | 0.013 (0.002, 0.023) | 0.016 |
|
| |||||
| Perceptual Speed | |||||
|
| |||||
| Basic | 4774 | Ref | −0.002(−0.010, 0.007) | 0.011 (0.002, 0.020) | 0.014 |
| Basic + CVD§ | 4738 | Ref | −0.002(−0.010, 0.006) | 0.011 (0.002, 0.020) | 0.015 |
|
| |||||
| Interaction model ‡ betas for the MedDiet*Western diet*time interaction term | |||||
|
| |||||
| Episodic Memory | 4965 | Ref | 0.046 (−0.049, 0.142) | 0.094 (−0.001, 0.190) | 0.06 |
| Perceptual speed | 4774 | Ref | 0.018 (−0.070, 0.105) | −0.001 (−0.088, 0.868) | 0.96 |
|
| |||||
| Stratified Analysis for Episodic Memory | |||||
|
| |||||
| Low Western Diet | |||||
|
| |||||
| Basic | 2489 | Ref | 0.012 (−0.002, 0.026) | 0.022 (0.006, 0.038) | 0.006 |
| Basic + CVD§ | 2470 | Ref | 0.012 (−0.001, 0.026) | 0.021 (0.006, 0.037) | 0.008 |
|
| |||||
| High Western diet | |||||
|
| |||||
| Basic | 2476 | Ref | −0.011 (−0.025, 0.004) | 0.004 (−0.011, 0.019) | 0.51 |
| Basic + CVD§ | 2453 | Ref | −0.011 (−0.026, 0.003) | 0.004 (−0.011, 0.019) | 0.52 |
Basic model adjusted for age, sex, race, education, physical activity, late life cognitive activity and total calories.
β=beta coefficient from the model for the interaction term between MedDiet score and time
Interaction models were basic model additionally controlled for western diet score and MedDiet*western diet
CVD: cardiovascular conditions included diabetes, hypertension, stroke, and myocardial infarction.
Further, the interaction model between MedDiet and Western diet indicated an effect modification only for Episodic memory (p=0.05) and not perceptual speed (Table 3). After stratifying by Western diet score, we found that MedDiet was associated with a slower decline in episodic memory in the low Western diet group only (Table 3).
4.3. Sensitivity Analyses
We also conducted a sensitivity analysis to consider any dietary changes or possible misreporting among people with cognitive impairment. We reanalyzed the data and repeated the core model after removing the participants whose baseline cognitive scores were in the lowest 10% distribution. The overall association of MedDiet with slower cognitive decline was similar in this sensitivity analysis (N=4476, highest vs. lowest tertile: β= 0.016, SE=0.005; p=0.0003; p for trend= 0.0003). The results were also similar for the low (N=2282, highest vs. lowest MedDiet tertile: β=0.021, SE=0.007, p=0.0015; p for trend= 0.002) and high (N=2194, highest vs. lowest MedDiet tertile: β=0.011, SE=0.007, p=0.10, p for trend=0.08) Western diet groups.
Additionally, we examined any effect modification for the association of MedDiet with cognition by age, sex, race, and education for both the high and low Western diet groups using the basic-adjusted models. There was no significant interaction between age, sex, race, or education and MedDiet score on the association with cognitive decline in either high or low Western diet scores (data not shown).
5. Discussion:
In this large prospective population-based cohort study, we found that the beneficial association of following the MedDiet with a slower decline in global cognition and episodic memory over time was attenuated with the higher consumption of foods that are part of a Western diet pattern. In our analyses, eating MedDiet high in fruits, vegetables, whole grain, legumes, nuts, olive oil, and fish intake may not slow cognitive decline in those with a high Western diet score, i.e., frequently consuming red & processed meat, sugar, refined grains, fried food, butter, and sweets. However, among those consuming less of the Westernized diet, the rate reduction of cognitive decline for an individual in the highest tertile of the MedDiet score compared to the lowest tertile was the equivalent to being 5.8 years younger in age. The PREDIMED trial29 and other studies4–6 suggest a beneficial effect of the Mediterranean dietary pattern on cognitive decline in older adults. Additionally, this study supports the possible effect modification in the association of healthy diet and cognitive decline due to a high intake of unhealthy foods in an older US community population.
No single healthy or unhealthy food is consumed in isolation. Thus, diet patterns may better reflect the complexity of diet as well as the interaction between various nutrients’ absorption and distribution. MedDiet, a plant-based diet, is a rich source of antioxidant nutrients, omega-3 fatty acid, monounsaturated fatty acid, B vitamins, and other bioactive, all of which are associated with cognition in the aging population30–37. On the other hand, the Western diet consists of foods such as red meat, sugar, sweets, fatty food, etc., that may adversely affect cognition. The Australian cohort reported a Western diet association with poorer scores in the visuospatial cognitive domain7. The Whitehall II study reported that higher red and processed meat, peas, fried food, and low whole grains intake are associated with higher inflammation and faster cognitive decline in older ages38. Similar findings on the Western diet and cognitive decline were reported by the NuAge cohort study, specifically in those with low socioeconomic position 32. One other study reported the role of mixed diet pattern on the cognitive decline over time and found that participants with low adherence to Prudent diet and high adherence to the Western diet had a more considerable decrease in mini-mental state examination scores than those with high Prudent and low Western diet scores8. The null association of the MedDiet on cognitive decline in the presence of high Western diet consumption in our study supports these previous findings.
Most of the dietary patterns that have shown improvement in cognitive function among older adults, including MedDiet, MIND, and DASH, have a unique scoring matrix based on the amount of servings consumed for each pattern-specific diet components39. The majority of these diet components are foods such as fruits, vegetables, whole grains, poultry, fish, dairy, legumes, nuts, olive oil. Although there is a reverse score for the unhealthy dietary components, this scoring may have some limitations. Overall, in our population, the Western diet had a negligible correlation with the MedDiet score (ρ=0.06) and a moderately negative correlation with the MIND diet score (ρ=−0.31). In a separate analysis, the MIND diet was also significantly associated with slower cognitive decline in this cohort. However, we did not find any significant effect modification between the second (p=0.13) or third (p=0.30) tertile of MIND diet with Western diet scores; hence MIND diet was not investigated in this analysis. Specifically, for the MedDiet, scoring is based on more healthy dietary components. The maximum score is given when that healthy food component is consumed as per the recommended serving size (as described by Panagiotakos et al. 15). The foods scored reverse in MedDiet include red meat and full-fat dairy, i.e., the perfect score is provided if these foods are consumed in limitation (red meat (≤ 1/week); full-fat dairy (≤10/week). However, other unhealthy food items such as sweets and fried foods that are essential components of the Western diet are not considered for this MedDiet scoring, thus limiting our scoring of these foods. Therefore, while conveying the public health message of consuming healthy dietary components of MedDiet to slow the rate of cognitive decline in older adults, it is also essential to emphasize the significance of limiting other unhealthy food options that are part of the Western diet.
There are various potential mechanisms through which diet may exert protective or adverse effects on cognition. The MedDiet pattern is thought to have a neuroprotective effect by reducing oxidative stress and inflammation40. There is growing evidence from animal studies indicating adverse effects of high fat, high carbohydrate diets, or diets labeled as Western diet on learning and memory41–43. A deleterious effect of a high fat/ cholesterol diet on cognition is via its effect on synaptic integrity, increased hippocampal insulin resistance, inflammation44, 45, and amyloid precursor protein46. The western diet also causes blood-brain barrier leakage based on the duration of exposure and obesity phenotype47. An animal study reported rats on a high-fat high-carbohydrate diet when fed vitamin E had reduced memory impairment and improved hippocampal oxidative stress 48. Thus, essential nutrients or food groups present in the healthy diet patterns with antioxidative and anti-inflammatory properties are vital for brain health. In contrast, unhealthy components, including bad fats and simple sugars, may affect cognition adversely in aging. However, the degree to which our results might reflect an intrinsic negative cognitive association of Western diet components themselves vs. a positive impact of MedDiet components’ substitution is unknown.
This prospective study’s strength is its large biracial sample of community older adults with long follow-up time, a validated dietary assessment questionnaire, and a composite measure of cognitive assessment at multiple time points. Additionally, analyses considered adjusting various demographic and lifestyle factors associated with cognition and found the results robust. The associations of MedDiet score with higher education and physical activity and lower stroke prevalence raise the possibility that the inclusion of additional, unmeasured socioeconomic and lifestyle measures may have weakened its association with cognition. Although this is an observational study in older adults and findings cannot be readily generalized to a younger population, maintaining a healthy diet throughout the life course is essential. Future longitudinal studies on diet and cognition among the middle-aged population are needed to extend these findings.
The Mediterranean diet has emerged as an important modifiable risk factor for dementia in older adults. Our results further suggest the value of simultaneously limiting dietary choices `that are components of the Western diet. When emphasizing healthy foods, including fish/legumes, fruits, vegetables, olive oil, and whole grains for cognitive health, dietary recommendations should include avoiding unhealthy foods like red meat, fried, sugary, and refined foods.
Supplementary Material
Research in Context.
Systemic review:
Mediterranean diet is associated with slower cognitive decline in many but not all longitudinal studies. People typically eat a combination of foods, including healthy components and other foods high in sugars and fats common in Western diets. This study aimed to determine whether frequent consumption of foods that are part of a Western diet may attenuate the beneficial effects of the Mediterranean diet on cognitive decline.
Interpretation:
The Mediterranean diet was associated with a slower rate of cognitive decline. However, this association was insignificant if, in combination, there is also higher consumption of foods that are part of Western diets, such as red and processed meat, sugar, refined grains, fried food, butter, and sweets.
Future direction:
For the most optimal benefit, dietary recommendations for cognition in older adults should emphasize both foods to include and to avoid. Future research is needed to replicate these results.
Acknowledgment:
We thank all the participants in the Chicago Health and Aging Project. We also thank all the study staff of the Chicago Health and Aging Project and the Rush Institute of Healthy Aging for this work.
Funding:
This work was supported by the National Institute of Aging at National Institutes of Health (RO1AG054476, RO1AG054057, R01AG021972, R01 AG011101). Funder of the study had no role in the study design; data collection, analyses, and interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
Dedication
The authors dedicate this manuscript to Dr. Martha Clare Morris, who passed away during the revisions of the drafts.
Declarations of interest: none
References:
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75.e62. [DOI] [PubMed] [Google Scholar]
- 2.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Advances in nutrition (Bethesda, Md) 2019;10:1040–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aridi YS, Walker JL, Wright ORL. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyama A, Houston DK, Simonsick EM, et al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci 2015;70:354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Archives of neurology 2009;66:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. The American journal of clinical nutrition 2011;93:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardener SL, Rainey-Smith SR, Barnes MB, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry 2015;20:860–866. [DOI] [PubMed] [Google Scholar]
- 8.Shakersain B, Santoni G, Larsson SC, et al. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement 2016;12:100–109. [DOI] [PubMed] [Google Scholar]
- 9.Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014;83:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson KE, Wadley VG, McClure LA, Shikany JM, Unverzagt FW, Judd SE. Dietary patterns are associated with cognitive function in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Journal of nutritional science 2016;5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). Journal of Alzheimer’s disease : JAD 2003;5:349–355. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. American journal of epidemiology 2003;158:1213–1217. [DOI] [PubMed] [Google Scholar]
- 14.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. The Journal of nutrition 2004;134:927–934. [DOI] [PubMed] [Google Scholar]
- 15.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–340. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. The American journal of clinical nutrition 1999;69:243–249. [DOI] [PubMed] [Google Scholar]
- 17.Scherr PA, Albert MS, Funkenstein HH, et al. Correlates of cognitive function in an elderly community population. American journal of epidemiology 1988;128:1084–1101. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19.Smith A. Symbol Digit Modalities Test manual-revised. Western Psychological; Los Angeles, CA: 1984. [Google Scholar]
- 20.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Archives of neurology 1999;56:1274–1279. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812–816. [DOI] [PubMed] [Google Scholar]
- 22.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology 2004;63:2322–2326. [DOI] [PubMed] [Google Scholar]
- 23.Weuve J, Rajan KB, Barnes LL, Wilson RS, Evans DA. Secular Trends in Cognitive Performance in Older Black and White U.S. Adults, 1993–2012: Findings From the Chicago Health and Aging Project. The journals of gerontology Series B, Psychological sciences and social sciences 2018;73:S73–s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. American journal of preventive medicine 1989;5:65–72. [PubMed] [Google Scholar]
- 25.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. The journals of gerontology Series B, Psychological sciences and social sciences 1999;54:P155–160. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology 2005;65:892–897. [DOI] [PubMed] [Google Scholar]
- 27.Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and Cognition. Current drug abuse reviews 2016;9:76–79. [DOI] [PubMed] [Google Scholar]
- 28.Durazzo TC, Mattsson N, Weiner MW, Alzheimer’s Disease Neuroimaging I. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2014;10:S122–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA internal medicine 2015;175:1094–1103. [DOI] [PubMed] [Google Scholar]
- 30.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006;67:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Archives of neurology 2002;59:1125–1132. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology 2004;62:1573–1579. [DOI] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Archives of neurology 2005;62:641–645. [DOI] [PubMed] [Google Scholar]
- 34.Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr 2013;52:1553–1567. [DOI] [PubMed] [Google Scholar]
- 35.Haan MN, Miller JW, Aiello AE, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. The American journal of clinical nutrition 2007;85:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin B, Xun P, Jacobs DR, Jr., et al. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. The American journal of clinical nutrition 2017;106:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tangney CC, Tang Y, Evans DA, Morris MC. Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline. Neurology 2009;72:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: The Whitehall II prospective cohort study. Clin Nutr 2017;36:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015;11:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagetta D, Maruca A, Lupia A, et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. European journal of medicinal chemistry 2020;186:111903. [DOI] [PubMed] [Google Scholar]
- 41.Attuquayefio T, Stevenson RJ, Oaten MJ, Francis HM. A four-day Western-style dietary intervention causes reductions in hippocampal-dependent learning and memory and interoceptive sensitivity. PloS one 2017;12:e0172645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray AJ, Knight NS, Cochlin LE, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2009;23:4353–4360. [DOI] [PubMed] [Google Scholar]
- 43.Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 2017;58:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnold SE, Lucki I, Brookshire BR, et al. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiology of disease 2014;67:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denver P, Gault VA, McClean PL. Sustained high-fat diet modulates inflammation, insulin signalling and cognition in mice and a modified xenin peptide ameliorates neuropathology in a chronic high-fat model. Diabetes, obesity & metabolism 2018;20:1166–1175. [DOI] [PubMed] [Google Scholar]
- 46.Thirumangalakudi L, Prakasam A, Zhang R, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem 2008;106:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hargrave SL, Davidson TL, Zheng W, Kinzig KP. Western diets induce blood-brain barrier leakage and alter spatial strategies in rats. Behavioral neuroscience 2016;130:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiology & behavior 2013;119:72–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.