Abstract
N-glycolylated carbohydrates are amino sugars with an N-glycolyl amide group. These glycans have not been well studied due to their surprising rarity in nature in comparison with N-acetylated carbohydrates. Recently, however, there has been increasing interest in N-glycolylated sugars because the non-human sialic acid N-glycolylneuraminic acid (Neu5Gc), apparently the only source of all N-glycolylated sugars in deuterostomes, appears to be involved in xenosialitis (inflammation associated with consumption of Neu5Gc-rich red meats). Xenosialitis has been implicated in cancers as well as other diseases including atherosclerosis. Furthermore, metabolites of Neu5Gc have been shown to be incorporated into glycosaminoglycans (GAGs), resulting in N-glycolylated GAGs. These N-glycolylated GAGs have important potential applications, such as dating the loss of the Neu5Gc-generating CMAH gene in humans and being explored as a xenosialitis biomarker and/or estimate of the body burden of diet-derived Neu5Gc, to understand the risks associated with the consumption of red meats. This review explores N-glycolylated carbohydrates, how they are metabolized to N-glycolylglucosamine and N-glycolylgalactosamine, and how these metabolites can be incorporated into N-glycolylated GAGs in human tissues. We also discuss other sources of N-glycolylated sugars, such as recombinant production from microorganisms using metabolic engineering as well as chemical synthesis.
Keywords: chondroitin, glycobiology, neuraminic acid, N-glycolyl, xenosialitis
Introduction
Structural glycomics has identified an incredible diversity of glycans and glycoconjugates in nature, and functional glycomics has led to an increased appreciation for the biological roles of these glycans. As the field of glycomics has matured, we have begun to identify rare glycans, glycoconjugates, and saccharide sequences in organisms and have sought to ascribe physiological or pathophysiological roles to these structures. New biotechnological tools including advances in carbohydrate analysis, molecular biology, metabolic engineering, and engineered model organisms have further accelerated these advances (Fenn and McLean 2013; Reinhold et al. 2013; Kailemia et al. 2014; Bergfeld et al. 2017; Lisacek et al. 2017; Schilling et al. 2020). This review examines one class of such rare glycans, N-glycolylated sugars. We discuss how these sugars are biosynthesized in mammalian and bacterial cells, and how they can be chemically synthesized. Furthermore, we describe their metabolism, and their incorporation into glycosaminoglycans. The presence or absence of N-glycolylated sugars in humans and their biological impact are also discussed. Finally, we discuss future applications of N-glycolylated monosaccharides in biomedicine and the study of evolution.
Metabolism of Neu5Gc in mammalian cells
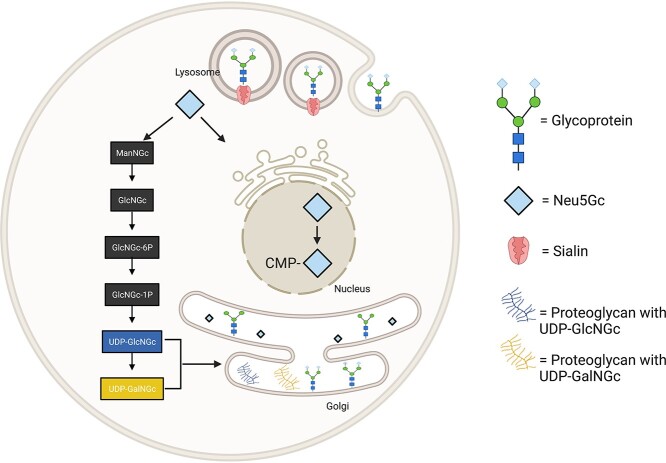
N-glycolylated carbohydrates are amino sugars that have an N-linked glycolyl group on the nitrogen of an amino sugar. Although rare in humans, there are various N-glycolylated amino sugars found in other animals. These sugars all appear to arise from the catabolism of N-glycolylneuraminic acid (Neu5Gc) (Bergfeld et al. 2017). Sialic acids, such as N-acetylneuraminic acid (Neu5Ac) and Neu5Gc, are usually found on the extracellular surface of cells at the terminal ends of glycoconjugates in vertebrates (Varki 2001; Bergfeld et al. 2012). Once these sialic acids are released from glycoconjugates (glycoproteins and proteoglycans) through enzymatic release in the lysosome, both Neu5Ac and Neu5Gc become available for reutilization and can be reincorporated into new glycoconjugates, catabolized, or excreted from the body (Tangvoranuntakul et al. 2003; Bardor et al. 2005). Sialic acids attached to glycoconjugates undergo enzymatic hydrolysis of their glycosidic linkage by sialidases in the lysozyme and then are released from the lysosome (Bardor et al. 2005; Varki et al. 2015). Lysosomal release of Neu5Gc is followed by delivery to the cytosolic compartment by a lysosomal sialic acid transporter called sialin (Bardor et al. 2005; Varki et al. 2015). On arriving inside the cytosol, Neu5Gc may be activated or broken down into its metabolites. In the nucleus, Neu5Gc can be activated by CMP-sialic acid synthesis to form cytidine-5′-monophospho-N-glycolylneuraminic acid (CMP-Neu5Gc) (Muchmore et al. 1989), which can then enter the Golgi apparatus, where it can be a donor for incorporation of Neu5Gc into newly synthesized glycoconjugates, such as glycoproteins. Concurrently, in the cytoplasm, Neu5Gc can be broken down and reassembled into the metabolites uridine diphosphate-N-glycolylgalactosamine (UDP-GalNGc) and uridine diphosphate-N-glycolylglucosamine (UDP-GlcNGc). These metabolites can then potentially act as donors for the addition of GlcNGc and GalNGc into glycoconjugates (Bardor et al. 2005; Bergfeld, Samraj, and Varki 2015). Once biosynthesis is complete, these glycoconjugates can then be exported or secreted to the cell surface (Fig. 1). The pathway for the metabolic incorporation of Neu5Gc into endogenous glycoproteins has been described in mammalian cells. There are other processes by which Neu5Gc can be transported into the cells, depending on the type of glycoconjugate the sialic acid is attached to. One example of such a process is clathrin-mediated endocytosis (Bardor et al. 2005). This is likely the major mechanism used in human cells.
Fig. 1.
Possible pathways for the uptake of Neu5Gc and its incorporation into glycoconjugates in mammalian cells. Free and conjugated sialic acid enter the cell by macropinocytosis. In the lysosome, a sialidase cleaves Neu5Gc from its glycan and exports it out of the lysosome via sialin. Once in the cytoplasm, Neu5Gc can break down into its catabolites or enter the nucleus for activation into CMP-Neu5Gc. In the Golgi, Neu5Gc can be incorporated into newly synthesized glycoconjugates or incorporate its metabolites from the cytoplasm into glycoconjugates. Created with BioRender.com.
CMP-Neu5Gc, the activated form of Neu5Gc is biosynthesized by the hydroxylation of cytidine-5′-monophospho-N-acetyllneuraminic acid (CMP-Neu5Ac) through the action of cytidine monophosphate N-acetylneuraminic acid hydroxylase (Cmah). This enzyme is encoded by the CMAH gene (Shaw et al. 1994; Takematsu et al. 1994; Peri et al. 2018). An Alu-mediated mutation caused a frameshift in the human CMAH gene leading to its inactivation (Chou et al. 2002; Hayakawa et al. 2006; Peri et al. 2018). This resulted in the loss of Neu5Gc biosynthesis capability in humans. CMAH loss is not limited to humans, as some other lineages have also independently lost the CMAH gene (Peri et al. 2018). Although humans cannot biosynthesize Neu5Gc, studies have shown that exogenous Neu5Gc from dietary sources can be metabolically incorporated into endogenous human glycoproteins (Tangvoranuntakul et al. 2003). This has led to research on how incorporated exogenous Neu5Gc is eliminated from humans over time. Furthermore, the catabolic pathway for Neu5Gc, leading to the synthesis of other N-glycolylated sugars has also been elucidated.
Neu5Gc can be reversibly converted to N-glycolylmannosamine (ManNGc) through the action of N-acetylneuraminate lyase (Schauer et al. 1999; Bateman et al. 2010; Bergfeld et al. 2015). ManNGc can then be epimerized to N-glycolylglucosamine (GlcNGc) through the action of UDP-GlcNAc-2′-epimerase (Luchansky et al. 2003; Bergfeld et al. 2012). GlcNAc kinase can phosphorylate GlcNGc, resulting in N-glycolylglucosamine-6-phosphate (GlcNGc-6P) (Bergfeld, Pearce, Diaz, Pham, et al. 2012), and then converted to N-glycolylglucosamine-1-phosphate (GlcNGc-1P) by phosphoacetylglucosamine mutase (Mio et al. 1999; Bergfeld et al. 2017). Alternatively, GlcNGc-6P can be irreversibly converted by GlcNAc-6-P deacetylase to GlcNH2–6-P and glycolate (Hall et al. 2007; Bergfeld et al. 2012). GlcNGc-1-P can react with UTP to form UDP-GlcNGc, catalyzed by the action of UDP-GlcNAc diphosphorylase (Wang-Gillam et al. 2000). UDP-GlcNGc can also be epimerized to UDP-GalNGc through the action of UDP-GlcNAc 4′-epimerase. UDP-GlcNGc and UDP-GalNGc can then be incorporated into glycosaminoglycans (GAGs)(Bergfeld et al. 2017; Awofiranye et al. 2020). Incorporation most commonly occurs in CS for unknown reasons (Fig. 2).
Fig. 2.
The Neu5Gc degradative pathway. Neu5Gc, when acted on by N-acetylneuraminate lyase (1), forms ManNGc, which in turn gets epimerized to GlcNGc UDP-N-acetylglucosamine 2-epimerase (2). GlcNGc gets phosphorylated at the 6-position by N-acetylglucosamine kinase (3) yielding GlcNGc-6P. Phosphoacetylglucosamine mutase (4) acts on GlcNGc-6P to yield GlcNGc-1P. Alternatively, GlcNGc-6P can be converted to GlcNH2–6-P and glycolate. Once GlcNGc-1P reacts with UDP-N-acetylglucosamine diphosphorylase (5) to form UDP-GlcNGc, UDP-GlcNGc will also be epimerized by UDP-N-acetylglucosamine 4-epimerase (6) to yield UDP-GalNGc. The final products UDP-GalNGc and UDP-GlcNGc get incorporated into glycosaminoglycans and glycoproteins, but incorporation most commonly occurs into CS for unknown reasons.
Non-animal sources of N-glycolyl groups
The N-glycolyl group is mostly described on animal glycoproteins originating solely from the CMAH enzyme or exogenous dietary Neu5Gc. However, a thorough search of a bacterial and fungal glycan database revealed that the N-glycolyl group is not just limited to animals (Toukach and Egorova 2016). The oligosaccharide component of the fungi Pleurocybella porrigens (Prionolomia porrigens) was noted to contain N-glycolylneuraminic acid in addition to other sugars (Takata et al. 2009). Furthermore, the capsular polysaccharide found on two different strains of the bacteria Haemophilus parasuis (H. parasuis) revealed two different structures possible (Perry et al. 2013). They both contain neuraminic acids with either an acetyl group or a glycolyl group attached to its N-5 position. This Gc substituent was seen in 50 percent of the studied sugar chains. The source of this fungal and bacterial Neu5Gc is unknown and may well also arise from exogenous vertebrate sources.
The Actinomycetota phylum contains some bacteria containing the N-glycolyl group. An example of this is the peptidoglycan of Mycobacterium smegmatis, which naturally has N-acetyl-glucosamine-1,4-N-glycolyl-muramic (Pedron et al. 1994) as shown in the Table I. Another similar species here is the Mycobacterium tuberculosis, which has linear chains containing GlcNAc and MurNAc/MurNGc in its peptidoglycan (Yao et al. 2012). The glycolyl group in this bacteria could serve to improve hydrogen bonding and therefore provide tighter binding of the peptidoglycan (Brennan and Nikaido 1995). Furthermore, it is also surprisingly known to trigger NOD2-mediated responses (Hansen et al. 2014). Some other related Actinomycetales like Rhodococcus, Tsukamurella, Gordonia, Nocardia, and Micromonospora are reported to contain N-glycolylmuramic acid (MurNGc) in their peptidoglycans (Collins et al. 1988; Linos et al. 1999; Raymond et al. 2005; Jongrungruangchok, Tanasupawat, and Kudo 2008; Coulombe et al. 2009). In Mycobacterium, MurNGc is biosynthesized through the conversion of N-acetylated muramic acid (UDP-MurNAc) to N-glycolylated muramic acid (UDP-MurNGc) by a mycobacterial UDP-MurNAc hydroxylase, encoded by the NAMH gene (Raymond et al. 2005). This extra fortification conferred by N-glycolyl muramic acid is believed to protect the bacteria from the effect of lactam antibiotics and lysozyme (Brennan 1995; Raymond et al. 2005; Wivagg, Bhattacharyya, and Hung 2014). Notably, the mycobacterial enzyme that makes UDP-MurNGc is encoded by NamH, a distant homolog of CMAH. Most interestingly, a particular type of fungus, Sporothrix schenckii from the Ascomycota division was found not to contain N-glycolylneuraminic, and not muramic acid as seen in other fungi (Alviano et al. 1982; Eneva et al. 2021).There are also some chemically synthesized sources of the N-glycolyl group, usually in the form of 2-N-glycolyl glucosamine. Methyl 2-N-glycolyl 2-deoxy-β-D-glucopyranoside and its 2-N-methyl analog were synthesized as inhibitors of the carbohydrate esterase family 4 deacetylase PgaB (Chibba et al. 2012). Furthermore, 2-N-glycolyl glucosamine was synthesized and fed to the cells. This study showed that yeast chitin synthase 2 can use 2-N-glycolyl glucosamine as an acceptor to synthesize novel chitin derivatives (Gyore et al. 2014).
Table I.
Non-animal sources of N-glycolylated compounds.
| Number | Source | N-glycolyl position | Compound | Reference |
|---|---|---|---|---|
|
1 |
Pleurocybella porrigens |
5-N-glycolyl Neuraminic acid |
|
|
|
2 |
Haemophilus parasuis |
5-N-glycolyl Neuraminic acid |
|
|
|
3 |
Mycobacterium smegmatis |
2-N-glycolyl Muramic acid |
|
|
|
4 |
Mycobacterium tuberculosis |
2-N-glycolyl Muramic acid |
|
|
|
5 |
Synthesized |
2-N-glycolyl glucosamine |
|
|
|
6 |
Synthesized |
2-N-glycolyl glucosamine |
|
Notably, unlike acetyl-CoA, which is widely used to generate N-acetyl groups, glycolyl-CoA never seems to be used to make N-glycolyl groups. In addition, N-glycolyl groups are generated via a chemically complex hydroxylation reaction that only works on N-acetylated-nucleotide sugars. Moreover, the metabolism of glycolyl-CoA, one of the metabolites of GABA (gamma-aminobutyric acid), does not contribute to the synthesis of N-glycolylated carbohydrates such as Neu5Gc (Vamecq and Poupaert 1990). Evidently, feeding CMAH-deficient mouse cells and multiple malignant human cell lines with high amounts of GABA did not facilitate the production of Neu5Gc (Bergfeld et al. 2012).
Chemical synthesis of Neu5Gc
The chemical synthesis of N-glycolylated sugars has been reported. Neu5Gc has been synthesized chemically and enzymatically from N-acetylglucosamine (GlcNAc), glucose, and D-arabinose (Choi et al. 1996; Kuboki et al. 1997; Hong et al. 2006; Pearce and Varki 2010; Yu et al. 2016; Kooner, Yu, and Chen 2019). Many derivatives of Neu5Gc have also been chemically synthesized (Ogura et al. 1987; Yu et al. 2016; Kooner et al. 2019). A few examples include 9-O-acetyl-Neu5Gc (Neu5Gc9Ac) and 4-O-acetyl-Neu5Gc (Neu4Ac5Gc). These are sialic acids that are naturally present in the bovine submandibular gland and horse glycoproteins, respectively (Reuter et al. 1983; Kooner et al. 2019). In addition, 8-O-methyl-N-glycolylneuraminic acid (Neu5Gc8Me) has been prepared from 5-O-methyl ManNGc (ManNGc5Me) and pyruvate (Yu et al. 2011). Other N-glycolylated sugars such as N-glycolylmannosamine (ManNGc), N-glycolylgalactosamine (GalNGc), and N-glycolylglucosamine (GlcNGc) have also been chemically synthesized to understand the effect of their incorporation into human cells (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a; Bergfeld, Pearce, Diaz, Pham, et al. 2012). N-glycolylhexosamines, N-glycolylhexosamine 6-phosphates, glycolyl coenzyme A, and glycolyl glutathione have been synthesized (Jourdian and Roseman 1962). Finally, N-glycolylated GAGs like N-glycolylated chondroitin sulfates have recently been synthesized enzymatically (Bergfeld et al. 2017) and through the metabolic engineering of bacteria (Awofiranye et al. 2020).
Neu5Gc and consequences of its presence or absence
The only known source of all N-glycolylated sugars in animals is Neu5Gc. Neu5Gc is a sialic acid found in most mammals except for Platypus, some birds, reptiles, ferrets, (Schauer et al. 2009; Ng et al. 2014), Western dogs (Hashimoto et al. 1984; Yasue et al. 1978; Ng et al. 2014), New World monkeys, and humans (Springer et al. 2014). Neu5Gc, along with other sialic acids, are usually found on the extracellular surface of cells and are attached to the non-reducing terminal ends of glycoconjugates, performing biological and pathological roles (Varki 2001, 2008; Bergfeld, Pearce, Diaz, Pham, et al. 2012).
One of the important pathophysiological roles of sialic acids is to serve as pathogen attachment site (Varki 2008). Their position at the non-reducing terminal end of glycan chains gives them an advantage in performing this function. While some pathogens show promiscuity in binding sialic acids (Li et al. 2022), some preferentially bind Neu5Gc over Neu5Ac and vice versa. Fortunately, since humans cannot synthesize Neu5Gc, they are protected from pathogens that preferentially bind Neu5Gc such as, Escherichia coli K99 (Smit et al. 1984), Simian Virus 40 (SV40) (Campanero-Rhodes et al. 2007) and Plasmodium reichenowi (Martin et al. 2005). In particular, Pitta reichenowi is the parasite responsible for causing malaria in chimpanzees and gorillas but not humans (Martin et al. 2005). Humans are not infected by this parasite likely because the erythrocyte binding protein of P. reichenowi infecting chimpanzee preferentially attaches to Neu5Gc residues causing infection (Okerblom and Varki 2017). In contrast, humans are still susceptible to infections from pathogens that preferentially bind Neu5Ac, such as close relative of P. reichenowi, P. falciparum (Martin et al. 2005; Rich et al. 2009). P. falciparum preferentially binds Neu5Ac over Neu5Gc contributing to the increased malaria susceptibility of humans (Martin et al. 2005; Rich et al. 2009). Some other examples of pathogens that preferentially bind Neu5Ac, thus increasing human susceptibility include, Streptococcus pneumoniae (Hentrich et al. 2016), Salmonella typhi (Deng et al. 2014), and Vibrio cholera (Alisson-Silva et al. 2018).
Another pathophysiological consequence of Neu5Gc loss is xenosialitis. Due to the loss of the CMAH gene, humans cannot synthesize Neu5Gc. However, low levels of Neu5Gc have been found in human tissues (Tangvoranuntakul et al. 2003; Diaz et al. 2009). Much of this Neu5Gc incorporation has been associated with red meat consumption. When red meat such as beef, pork, lamb, and veal, containing Neu5Gc is consumed, the sialic acids present are incorporated into human cells (Tangvoranuntakul et al. 2003). While dietary Neu5Gc is incorporated into normal human cells, it is more evident in cancer cells such as carcinomas (Hirabayashi et al. 1987; Devine et al. 1991; Marquina et al. 1996; Malykh, Schauer, and Shaw 2001). Neu5Gc metabolically incorporated into human cell-surface glycans generates xeno-autoantigens (Dhar, Sasmal, and Varki 2019). These antigens can elicit the production of xeno-autoantibodies (Padler-Karavani et al. 2013). Xeno-antibodies are believed to be present in all humans due to their postnatal introduction from a human-specific pathogen (Taylor et al. 2010). These antigen–antibody reactions trigger inflammation (xenosialitis) linked to carcinomas (De Visser et al. 2005; Hedlund et al. 2008; Andreu et al. 2010) (Fig. 3). Red meat consumption has also been linked to other diseases like atherosclerosis and certain carcinomas, suggesting additional pathophysiology of Neu5Gc (Micha et al. 2012; Koeth et al. 2013; Zelber-Sagi et al. 2018; Dhar et al. 2019).
Fig. 3.
Once incorporated into the human tissue, sources of Neu5Gc, such as red meats, are recognized as xeno-autoantigens. The body responds by producing anti-Neu5Gc antibodies (xeno-autoantibodies). The antibody–antigen interaction leads to a chronic inflammation (xenosialitis) in the tissues, contributing to diseases such as carcinomas, and atherosclerosis. Figure reproduced with permission from (Dhar et al. 2019).
Neu5Gc is present in rodents and, hence, would be found in their cell lines such as in Chinese hamster ovary (CHO) cells. CHO cells are widely used for the preparation of glycoprotein-based biopharmaceuticals, such as enzymes and antibodies (Datta, Linhardt, and Sharfstein 2013). These glycoproteins are glycosylated with host glycans containing a mixture of terminal Neu5Ac and Neu5Gc residues. There has been significant interest in the pharmaceutical industry to reduce the content of Neu5Gc to “humanize” the glycans of these biopharmaceuticals in an effort to decrease the risk of immunological side effects (Löfling et al. 2009; Salama et al. 2015).
The microbiome and its impact on N-glycolylated sugar metabolism
A particularly interesting finding from microbiome studies in the Hazda tribe was that these hunter gatherers had cyclically changing microbiomes. These changes were in line with a change in diet from a predominantly vegetarian diet to one rich in red meat (Samuel et al. 2017). Further studies have shown that consumption of red meat introduces bacteria that synthesize sialidases better equipped for N-glycolyl release (Zaramela et al. 2019).
Polyclonal anti-Neu5Gc antibody responses in humans
The anti-Neu5Gc antibody binding pocket holds more than just the glycolyl group. Most antibody binding sites can accommodate between 4 and 6 glycans (Kabat et al. 1988; Padlan and Kabat 1988). This suggests that an antibody response to Neu5Gc-containing epitopes would be a broad, polyclonal response against an array of these epitopes. This is indeed the case with a large diversity of such Neu5Gc-directed antibodies (Padler-Karavani et al. 2008). Moreover, it appears that the sum of these antibodies may be involved in the Xenosialitis phenomena as was shown in the link between colorectal cancer risk and Neu5Gc-directed antibodies (Samraj et al. 2018).
Evolutionary implications of CMAH loss
There are no orthologs or homologs of the CMAH gene in eukaryotes. The closest homolog is the NAMH gene found in the prokaryotic mycobacteria (Dhar et al. 2019). These genes are responsible for the conversion of an N-acetyl group to an N-glycolyl group. It is very interesting that this conversion has only been seen twice in nature, both times on a sugar nucleotide (Raymond et al. 2005; Davies and Vark 2013). A PubMed search for “N-glycolyl” yielded less than 350 publications while for “N-acetyl” there were more than 33,000 results (Fig. 4). The presence of N-glycolyl groups is particularly important in the context of evolution. Our closest evolutionary cousins, the chimpanzees and bonobos both have an active form of the Cmah enzyme and therefore decorate their cell surfaces with both Neu5Ac and Neu5Gc. All humans—on the other hand—have an inactivating mutation in the CMAH gene that renders them to have only Neu5Ac on their cell surfaces. It is likely that the mutation was initially synthesized as a pathogen avoidance mechanism; i.e. there was an evolutionary advantage if one had only Neu5Ac on their cell surfaces in avoiding pathogens that bound Neu5Gc. Alternatively, the subsequent fixation in the lineage leading to humans may have been due to reduced fertility of CMAH+ males with CMAH null females due to anti-Neu5Gc antibodies (Ghaderi et al. 2011).
Fig. 4.
A timeline of publications with the keyword “N-acetyl” and “N-glycolyl”.
Products of Neu5Gc breakdown are used in GAG biosynthesis
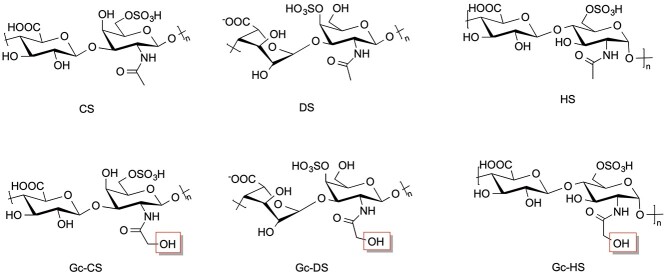
Neu5Gc constantly undergoes catabolism in humans during routine cell turnover. In mammals, one of the possible pathways for Neu5Gc catabolism occurs in 6 steps through the GalNAc pathway (Fig. 2) resulting in UDP-GalNGc and UDP-GlcNGc (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a). This is also supported by the proposed pathway for N-glycolyl chondroitin synthesis in metabolically engineered bacteria (Awofiranye et al. 2020). Recent studies have shown that UDP-GalNGc and one of its precursors, UDP-GlcNGc, produced from the breakdown of Neu5Gc, can be incorporated into GAGs such as chondroitin sulfate (CS), dermatan sulfate (DS) (sulfated [ 3) GalNAc (1
3) GalNAc (1 4) GlcA/IdoA]n where GlcA is glucuronic acid and IdoA is iduronic acid) and heparan sulfate (HS) (sulfated [
4) GlcA/IdoA]n where GlcA is glucuronic acid and IdoA is iduronic acid) and heparan sulfate (HS) (sulfated [ 4 GlcNAc/S (1
4 GlcNAc/S (1 4) GlcA/IdoA]n (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a; Bergfeld et al. 2017). Varki and co-workers fed mammalian cells GalNGc and hypothesized that it should be incorporated into cellular glycoconjugates. They found CS and DS with significant GalNGc incorporation (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a). GalNGc replaces GalNAc in CS/DS GAGs since they are galactosaminoglycans. The exogenously added GalNGc was converted into GlcNGc and incorporated into HS (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a), which is a glycosaminoglycan. N-glycolyl chondroitin sulfate (Gc-CS), N-glycolyl dermatan (Gc-DS), and N-glycolyl heparan sulfates (Gc-HS) are all rare GAG structures containing N-glycolylated sugar residues. (Fig. 5). It is important to note that only a small percentage of the hexosamine residues in the GAGs are N-glycolylated and that the vast majority of these hexosamine residues are N-acetylated. Gc-CS has since been detected in normal mouse liver and kidney, ovine, bovine, and porcine muscles, porcine intestinal mucosa, bovine trachea, humans, and fetal calf serum and in red meat consumers, as well as human-like Cmah null mice only when fed Neu5Gc-rich mucins (Bergfeld et al. 2017).
4) GlcA/IdoA]n (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a; Bergfeld et al. 2017). Varki and co-workers fed mammalian cells GalNGc and hypothesized that it should be incorporated into cellular glycoconjugates. They found CS and DS with significant GalNGc incorporation (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a). GalNGc replaces GalNAc in CS/DS GAGs since they are galactosaminoglycans. The exogenously added GalNGc was converted into GlcNGc and incorporated into HS (Bergfeld, Pearce, Diaz, Lawrence, et al. 2012a), which is a glycosaminoglycan. N-glycolyl chondroitin sulfate (Gc-CS), N-glycolyl dermatan (Gc-DS), and N-glycolyl heparan sulfates (Gc-HS) are all rare GAG structures containing N-glycolylated sugar residues. (Fig. 5). It is important to note that only a small percentage of the hexosamine residues in the GAGs are N-glycolylated and that the vast majority of these hexosamine residues are N-acetylated. Gc-CS has since been detected in normal mouse liver and kidney, ovine, bovine, and porcine muscles, porcine intestinal mucosa, bovine trachea, humans, and fetal calf serum and in red meat consumers, as well as human-like Cmah null mice only when fed Neu5Gc-rich mucins (Bergfeld et al. 2017).
Fig. 5.
UDP-GalNGc, when incorporated into CS and DS forms Gc-CS and Gc-DS, respectively, and UDP-GlcNGc when incorporated into HS forms Gc-HS. Representative structure for each GAG is shown.
The UDP sugar precursors required for GAG biosynthesis are synthesized in the cytoplasm and transported into the Golgi where glycosyltransferases co-polymerize GlcA and GlcNAc/GalNAc into GAG backbones and further modify these sugars (Hirschberg, Robbins, and Abeijon 1998; DeAngelis 2002; Handford, Rodriguez-Furlán, and Orellana 2006; Prydz 2015). The biosynthesis of N-glycolylated GAGs can take place because these glycosyltransferases are promiscuous and are able to accept UDP-N-glycolylhexosamines as substrates in place of UDP-N-acetylhexosamines (Thibodeaux, Melançon, and Liu 2007; Macauley et al. 2012). For example, the glycosyltransferases in animal cells that accept UDP-GalNAc as a donor substrate in the polymerization of chondroitin are also capable of accepting UDP-GalNGc. This is supported by the findings from experiments done in bacteria (Awofiranye et al. 2020). Chondroitin polymerase (KfoC) from E. coli strain K4 is a glycosyltransferase that catalyzes the polymerization of chondroitin using UDP-GlcA and UDP-GalNAc (Sugiura et al. 2002). This enzyme can also catalyze the co-polymerization of UDP-GlcA and UDP-GalNGc to form N-glycolylated chondroitin. Similarly, a similar glycosyltransferase (KifA, KifC), responsible for accepting UDP-GlcA and UDP-GlcNAc, can also accept UDP-GlcA and UDP-GlcNGc in the biosynthesis of N-glycolylated Heparosan, the Gc-HS backbone (Vaidyanathan et al. 2017).
Effects of N-glycolylation on GAGs
In animals, GAGs are typically biosynthesized as proteoglycans bound to a core protein and carry out signaling functions such as cell migration, differentiation, growth, and proliferation (Djerbal, Lortat-Jacob, and Kwok 2017). GAGs function primarily through their interaction with GAG-binding proteins that can result in protein activation, deactivation, oligomerization, stabilization, localization, or conformational changes (Capila and Linhardt 2002). The functional differences between N-glycolylated GAGs and N-acetylated GAGs are currently poorly understood. One of the effects of this replacement might be that interactions between GAGs and proteins facilitated by a hydrophobic N-acetyl group are reduced due to the presence of a hydrophilic N-glycolyl group. Hydrophilic GAGs such as CS contain hydrophobic domains due to C-H ring bonds as well as N-acetyl groups. This hydrophobicity can be responsible for biological and binding properties by facilitating hydrophobic interactions (Varki et al. 2015; De Paz and Nieto 2016). While most of the interaction energy between GAG and protein results from the positive charge of proteins interacting with the negative charge of the GAG (Esko, H. Prestegard, and Linhardt 2015), additional hydrophobic interaction from the N-acetyl groups might strengthen binding or enhance binding specificity. Therefore, if a hydrophobic N-acetyl group is replaced with a hydrophilic N-glycolyl group, the GAG–protein interaction may become less/more stable or less/more specific, modifying the signaling functions of GAGSs. Moreover, even minor modifications such as the presence or absence of an N-acetyl group of a GlcNAc residue can profoundly impact GAG conformation flexibility (Esko et al. 2015). It is important to remember, however, that the vast majority of the hexosamine residues in a GAG will be N-acetylated, and N-glycolylated residues will represent rare sequences; thus, many of these biological effects might be subtle.
Applications of N-glycolylated GAGs
From an evolutionary perspective, there is a variety of applications for N-glycolylated GAGs. One application is in hominin research through the dating of the human loss of Neu5Gc. The Alu-mediated event deleted a 92-bp exon of CMAH (Peri et al. 2018), leaving humans with a frameshift in the CMAH gene and, thus, a loss of the ability to biosynthesize Neu5Gc. Although Gc-CS has been detected in humans due to exogenous incorporation, the amount of GalNGc is insignificant compared to that in the CS of animals like chimpanzees that still have an intact CMAH gene (Bergfeld et al. 2017). Analyzing the presence of Gc-CS in hominin fossils should allow one to accurately pinpoint, which evolution event left humans with the loss of their ability to synthesize Neu5Gc. There have been previous attempts to perform this analysis using sialic acids for their content of Neu5Gc, but these were unsuccessful due to the relative instability of sialic acids in old fossils especially from tropical regions like Africa where many of the early fossils have been found (Wood and K. Boyle 2016). In contrast to Neu5Gc containing glycans, Gc-CS is sufficiently stable to be detectable in animal fossils as old as 4 million years old (Bergfeld et al. 2017). Thus, Gc-CS can be used to date the time of this evolutionary event. Detection in such old fossils still requires a large amount of starting material, so advances in carbohydrate analysis is still required to establish more specific and sensitive methods of analysis and detection. Efforts are also currently underway to metabolically engineer E. coli to produce Gc-CS to obtain standards for high sensitivity mass spectral analysis (Badri et al. 2019; (Awofiranye et al. 2020).
Furthermore, investigating N-glycolylated GAGs should improve our understanding of how red meat consumption can lead to xenosialitis and disease in humans. N-glycolylated GAGs, such as Gc-CS, can be extracted from human biofluids or tissues and used to investigate their functional impact on people who eat red meat. Such findings may lead to a method of detecting cancer susceptibility and for human food and dietary improvements. Measurements of N-glycolyl-CS may also provide an index of the body burden of Neu5Gc incorporated from dietary sources.
Conclusions and future prospects
Experiments have been carried out in CMAH −/− mice linking Neu5Gc presence to red meat and red meat consumption to carcinoma development via the xenosialitis pathway (Samraj et al. 2015). Neu5Gc link to cancer has not been explored in humans because its detection would have to be performed in intact and alive humans and is quite challenging because of difficulties associated with sialic acid detection (Tangvoranuntakul et al. 2003; Awofiranye et al. 2020). Gc-CS, a metabolite of Neu5Gc, can be used to circumvent these limitations. Detecting Neu5Gc in intact humans is very difficult because it is not present in the serum (Tangvoranuntakul et al. 2003), the use of antibodies is challenging (Padlan and Kabat 1988; Dhar et al. 2019), and Neu5Gc containing glycans are relatively unstable (Awofiranye et al. 2020). In contrast, Gc-CS is significantly more stable than sialic acid containing glycans and has been isolated from a four-million-year-old fossil. Furthermore, Gc-CS can be detected in the serum, and there have been examples of its successful detection (Bergfeld et al. 2017; Awofiranye et al. 2020). Gc-CS and other N-glycolylated GAGs, produced from dietary Neu5Gc can be used to detect the presence of Neu5Gc. This represents a more accurate method of detection and may be useful to test for a correlation between Neu5Gc incorporation to the incidence of carcinomas and other diseases.
CS exhibits chondroprotective properties by stimulating cartilage buildup and anti-inflammation (Jerosch 2011; Awofiranye et al. 2022). Thus, the impact of the N-glycolyl substitution should be tested, as it may abrogate its benefits in treating osteoarthritis, notwithstanding its low abundance.
Furthermore, it is surprising that human cell conserved the pathway for N-glycolylated sugar synthesis since it offers no known advantage. It is possible that there are other consequences of CMAH loss that have not yet been realized. To find these, additional studies are needed. One way this can be done is by searching for N-glycolylated GAG binding proteins and testing the effects of Gc-GAGs on human cells.
In conclusion, some light has recently been shed on N-glycolylated carbohydrates, particularly N-glycolylated GAGs. Here, we describe how Gc-GAGs are produced from the degradation of Neu5Gc, synthesized differently in certain bacteria, and chemically synthesized. The ability to biosynthesize Neu5Gc has been lost in humans, and with this loss comes both disadvantages and advantages. One of the major disadvantages is xenosialitis, which is an inflammation that is caused by ingesting exogenous Neu5Gc. This inflammation is linked to cancer. Further studies are needed to elucidate how N-glycolylated GAGs are synthesized, their application, and the possible biological impact of the N-glycolyl substitution.
Funding
Support for this work was provided by the National Science Foundation (MCB-1448657, CBET-1604547 to R.J.L and R01GM32373 to A.V.).
Conflict of interest statement. None declared.
Supplementary Material
Contributor Information
Adeola E Awofiranye, Department of Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA.
Chirag Dhar, Glycobiology Research and Training Center, University of California, San Diego, CA 92093, USA; Venn Biosciences Corporation d/b/a InterVenn Biosciences, South San Francisco, CA 94080, USA.
Peng He, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA.
Ajit Varki, Glycobiology Research and Training Center, University of California, San Diego, CA 92093, USA.
Mattheos A G Koffas, Department of Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA; Department of Chemical & Biological Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA.
Robert J Linhardt, Department of Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA; Department of Chemical & Biological Engineering, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA; Department of Chemistry & Chemical Biology, Center for Biotechnology and Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, NY 12180, USA.
References
- Alisson-Silva F, Liu JZ, Diaz SL, Deng L, Gareau MG, Marchelletta R, Chen X, Nizet V, Varki N, Barrett KE et al. “Human evolutionary loss of epithelial Neu5Gc expression and species-specific susceptibility to cholera” edited by K. J F Satchell PLOS Pathogens. 2018:14(6):e1007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alviano CS, Pereira MEA, Souza W, Oda LM, Travassos LR. Sialic acids are surface components of Sporothrix Schenckii yeast forms. FEMS Microbiol Lett. 1982:15(3):223–228. [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG et al. FcRγ activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010:17(2):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awofiranye AE, Baytas SN, Xia K, Badri A, He W, Varki A, Koffas M, Linhardt RJ. N-Glycolyl chondroitin synthesis using metabolically engineered E. Coli. AMB Express. 2020:10(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awofiranye AE, Hudson J, Tithi AD, Linhardt RJ, Vongsangnak W, Koffas MAG. Chondroitin sulfate and its derivatives: A review of microbial and other production methods. Fermentation. 2022:8(7):323. [Google Scholar]
- Badri A, Williams A, Xia K, Linhardt RJ, Koffas MAG. Increased 3′-Phosphoadenosine-5′-phosphosulfate levels in engineered Escherichia Coli cell lysate facilitate the in vitro synthesis of chondroitin Sulfate A. Biotechnol J. 2019:14(9):1800436. [DOI] [PubMed] [Google Scholar]
- Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005:280(6):4228–4237. [DOI] [PubMed] [Google Scholar]
- Bateman AC, Karamanska R, Busch MG, Dell A, Olsen CW, Haslam SM. Glycan analysis and influenza a virus infection of primary swine respiratory epithelial cells. J Biol Chem. 2010:285(44):34016–34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld AK, Pearce OMT, Diaz SL, Lawrence R, Vocadlo DJ, Choudhury B, Esko JD, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: Incorporation of N-glycolylhexosamines into mammalian glycans by feeding N-glycolylgalactosamine. J Biol Chem. 2012a:287(34):28898–28916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld AK, Pearce OMT, Diaz SL, Pham T, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: Elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem. 2012b:287(34):28865–28881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld AK, Samraj AN, Varki A. Metabolism of N-glycolylneuraminic acid in human and nonhuman cells, and potential relationships to human disease. In: Glycoscience: biology and medicine. Tokyo: Springer Japan; 2015. [Google Scholar]
- Bergfeld AK, Lawrence R, Diaz SL, Pearce OMT, Ghaderi D, Gagneux P, Leakey MG, Varki A. N-Glycolyl groups of nonhuman chondroitin sulfates survive in ancient fossils. Proc Natl Acad Sci. 2017:114(39):E8155–E8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995:64(1):29–63. [DOI] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Smith A, Chai W, Sonnino S, Mauri L, Childs RA, Zhang Y, Ewers H, Helenius A, Imberty A et al. N-Glycolyl GM1 ganglioside as a receptor for Simian virus 40. J Virol. 2007:81(23):12846–12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed. 2002:41(3):390–412. [DOI] [PubMed] [Google Scholar]
- Chibba A, Poloczek J, Little DJ, Lynne Howell P, Nitz M. Synthesis and evaluation of inhibitors of E. Coli PgaB, a polysaccharide de-N-acetylase involved in biofilm formation. Org Biomol Chem. 2012:10(35):7103–7107. [DOI] [PubMed] [Google Scholar]
- Choi S-K, Lee S, Whitesides GM. Synthesis of C-5 analogs of N-acetylneuraminic acid via indium-mediated allylation of N-substituted 2-amino-2-deoxymannoses. J Organomet Chem. 1996:61(25):8739–8745. [DOI] [PubMed] [Google Scholar]
- Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci. 2002:99(18):11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Smida J, Dorsch M, Stackebrandt E. Tsukamurella gen. Nov. harboring Corynebacterium Paurometabolum and Rhodococcus Aurantiacus. Int J Syst Bacteriol. 1988:38(4):385–391. [Google Scholar]
- Coulombe F, Divangahi M, Veyrier F, de Léséleuc, Gleason JL, Yang Y, Kelliher MA, Pandey AK, Sassetti CM, Reed MB et al. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009:206(8):1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P, Linhardt RJ, Sharfstein ST. An omics approach towards CHO cell engineering. Biotechnol Bioeng. 2013:110(5):1255–1271. [DOI] [PubMed] [Google Scholar]
- Davies LRL, Vark A. Why is N-glycolylneuraminic acid rare in the vertebrate brain? Top Curr Chem. 2013:366:31–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paz JL, Nieto PM. Improvement on binding of chondroitin sulfate derivatives to midkine by increasing hydrophobicity. Org Biomol Chem. 2016:14(14):3506–3509. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL. Microbial glycosaminoglycan glycosyltransferases. Glycobiology. 2002:12(1):9R–16R. [DOI] [PubMed] [Google Scholar]
- Deng L, Song J, Gao X, Wang J, Hai Y, Chen X, Varki N, Naito-Matsui Y, Galán JE, Varki A. Host adaptation of a bacterial toxin from the human pathogen Salmonella typhi. Cell. 2014:159(6):1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine PL, Clark BA, Birrell GW, Layton GT. The breast tumor-associated epitope defined by monoclonal antibody 3E1.2 is an O-linked mucin carbohydrate containing N-glycolylneuraminic acid. Cancer Res. 1991:51(21):5826–5836. [PubMed] [Google Scholar]
- Dhar C, Sasmal A, Varki A. From ‘serum sickness’ to ‘Xenosialitis’: Past, present, and future significance of the non-human sialic acid Neu5Gc. Front Immunol. 2019:10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Hai Y, Chen X, Els CM, der Linden, Varki A, Varki NM. “Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products” edited by MA. Batzer. PLoS One. 2009:4(1):4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerbal L, Lortat-Jacob H, Kwok J. Chondroitin sulfates and their binding molecules in the central nervous system. Glycoconj J. 2017:34(3):363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneva R, Engibarov S, Abrashev R, Krumova E, Angelova M. Sialic acids, Sialoconjugates and enzymes of their metabolism in fungi. Biotechnol Biotechnol Equip. 2021:35(1):346–357. [Google Scholar]
- Esko JD, Prestegard JH, Linhardt RJ. Proteins that bind sulfated glycosaminoglycans 3rd ed. Cold Spring Harbor (NY): Cold spring Harbor Laboratory Press 2015 [PubMed]
- Fenn LS, McLean JA. Structural separations by ion mobility-MS for glycomics and glycoproteomics. Methods in Molecular Biol (Clifton, NJ). 2013:951:171–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi D, Springer SA, Ma F, Cohen M, Secrest P, Taylor RE, Varki A, Gagneux P. Sexual selection by female immunity against paternal antigens can fix loss of function alleles. Proc Natl Acad Sci. 2011:108(43):17743–17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyore J, Parameswar AR, Hebbard CFF, Younghoon O, Bi E, Demchenko AV, Price NP, Orlean P. 2-Acylamido analogues of N-acetylglucosamine prime formation of chitin oligosaccharides by yeast chitin synthase 2. J Biol Chem. 2014:289(18):12835–12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RS, Xiang DF, Chengfu X, Raushel FM. N-acetyl-d-glucosamine-6-phosphate deacetylase: Substrate activation via a single divalent metal ion †. Biochemistry. 2007:46(27):7942–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford M, Rodriguez-Furlán C, Orellana A. Nucleotide-sugar transporters: Structure, function and roles in vivo. Braz J Med Biol Res. 2006:39(9):1149–1158. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Golchin SA, Veyrier FJ, Domenech P, Boneca IG, Azad AK, Rajaram MVS, Schlesinger LS, Divangahi M, Reed MB et al. N-Glycolylated peptidoglycan contributes to the immunogenicity but not pathogenicity of mycobacterium tuberculosis. J Infect Dis. 2014:209(7):1045–1054. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yamakawa T, Tanabe Y. Further studies on the red cell glycolipids of various breeds of dogs. A possible assumption about the origin of Japanese dogs. J Biochem. 1984:96(6):1777–1782. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Aki I, Varki A, Satta Y, Takahata N. Fixation of the human-specific CMP-N-acetylneuraminic acid hydroxylase pseudogene and implications of haplotype diversity for human evolution. Genetics. 2006:172(2):1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Vered Padler-Karavani NM, Varki, and Ajit Varki. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci. 2008:105(48):18936–18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentrich K, Löfling J, Pathak A, Nizet V, Varki A, Henriques-Normark B. Streptococcus pneumoniae senses a human-like sialic acid profile via the response regulator CiaR. Cell Host Microbe. 2016:20(3):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Kasakura H, Matsumoto M, Higashi H, Kato S, Kasai N, Naiki M. Specific expression of unusual GM2 ganglioside with Hanganutziu-Deicher antigen activity on human colon cancers. Jpn J Cancer Res. 1987:78(3):251–260. [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, Atp, and nucleotide Sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998:67(1):49–69. [DOI] [PubMed] [Google Scholar]
- Hong Z, Liu L, Hsu C-C, Wong C-H. Three-step synthesis of sialic acids and derivatives. Angew Chem Int Ed. 2006:45(44):7417–7421. [DOI] [PubMed] [Google Scholar]
- Jerosch J. Effects of glucosamine and chondroitin Sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. International Journal of Rheumatology. 2011:2011:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongrungruangchok S, Tanasupawat S, Kudo T. Micromonospora Chaiyaphumensis Sp. Nov., isolated from Thai soils. Int J Syst Evol Microbiol. 2008:58(4):924–928. [DOI] [PubMed] [Google Scholar]
- Jourdian GW, Roseman S. The sialic acids. II. Preparation of N-glycolylhexosamines, N-glycolylhexosamine 6-phosphates, glycolyl coenzyme A, and glycolyl glutathione. J Biol Chem. 1962:237(8):2442–2446. [PubMed] [Google Scholar]
- Kabat EA, Liao J, Osserman EF, Gamian A, Michon F, Jennings HJ. The epitope associated with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia Coli K1 to a human monoclonal macroglobulin, IgMNOV. J Exp Med. 1988:168(2):699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailemia MJ, Renee Ruhaak L, Lebrilla CB, Jonathan Amster I. Oligosaccharide analysis by mass spectrometry: A review of recent developments. Anal Chem. 2014:86(1):196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Xiaoming F, Yuping W, Li L et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013:19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooner AS, Hai Y, Chen X. Synthesis of N-glycolylneuraminic acid (Neu5Gc) and its glycosides. Front Immunol. 2019:10:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki A, Okazaki H, Sugai T, Ohta H. An expeditious route to N-glycolylneuraminic acid based on enzyme-catalyzed reaction. Tetrahedron. 1997:53(7):2387–2400. [Google Scholar]
- Li Z, Liu L, Unione L, Lang Y, de Groot RJ, Boons G-J. Synthetic O-acetyl-N-glycolylneuraminic acid oligosaccharides reveal host-associated binding patterns of coronaviral glycoproteins. ACS Infectious Diseases. 2022:8(5):1041–1050. [DOI] [PubMed] [Google Scholar]
- Linos A, Steinbüchel A, Spröer C, Kroppenstedt RM. Gordonia Polyisoprenivorans Sp. Nov., a rubber-degrading actinomycete isolated from an automobile Tyre. Int J Syst Bacteriol. 1999:49(4):1785–1791. [DOI] [PubMed] [Google Scholar]
- Lisacek F, Mariethoz J, Alocci D, Rudd PM, Abrahams JL, Campbell MP, Packer NH, Ståhle J, Widmalm G, Mullen E et al. Databases and associated tools for glycomics and glycoproteomics. In: Methods in molecular biology; Humana Press, New York, NY. 2017 [DOI] [PubMed]
- Löfling JC, Paton AW, Varki NM, Paton JC, Varki A. A dietary non-human sialic acid may facilitate hemolytic-uremic syndrome. Kidney Int. 2009:76(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003:278(10):8035–8042. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Chan J, Zandberg WF, He Y, Whitworth GE, Stubbs KA, Yuzwa SA, Bennet AJ, Varki A, Davies GJ et al. Metabolism of vertebrate amino sugars with N-glycolyl groups. J Biol Chem. 2012:287(34):28882–28897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001:83(7):623–634. [DOI] [PubMed] [Google Scholar]
- Marquina G, Waki H, Fernandez LE, Kon K, Carr A, Valiente O, Perez R, Ando S. Gangliosides expressed in human breast cancer. Cancer Res. 1996:56(22):5165–5171. [PubMed] [Google Scholar]
- Martin MJ, Rayner JC, Gagneux P, Barnwell JW, Varki A. Evolution of human-chimpanzee differences in malaria susceptibility: Relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci. 2005:102(36):12819–12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—An updated review of the evidence. Curr Atheroscler Rep. 2012:14(6):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T, Yamada-Okabe T, Arisawa M, Yamada-Okabe H. Saccharomyces cerevisiae GNA1, an essential gene encoding a novel acetyltransferase involved in UDP-N-acetylglucosamine synthesis. J Biol Chem. 1999:278(10):8035–8042. [DOI] [PubMed] [Google Scholar]
- Muchmore EA, Milewski M, Varki A, Diaz S. Biosynthesis of N-glycolyneuraminic acid. The primary site of hydroxylation of N-acetylneuraminic acid is the cytosolic sugar nucleotide pool. J Biol Chem. 1989:264(34):20216–20223. [PubMed] [Google Scholar]
- Ng PSK, Böhm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AEO, Coloe PJ, Grimmond SM et al. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza a virus receptors. Nat Commun. 2014:5(1):5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H, Furuhata K, Sato S, Anazawa K, Itoh M, Shitori Y. Synthesis of 9-O-acyl- and 4-O-acetyl-sialic acids. Carbohydr Res. 1987:167:77–86. [DOI] [PubMed] [Google Scholar]
- Okerblom J, Varki A. Biochemical, cellular, physiological, and pathological consequences of human loss of N-glycolylneuraminic acid. ChemBioChem. 2017:18(13):1155–1171. [DOI] [PubMed] [Google Scholar]
- Padlan EA, Kabat EA. Model-building study of the combining sites of two antibodies to alpha (1----6)dextran. Proc Natl Acad Sci. 1988:85(18):6885–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, Hai Y, Cao H, Chokhawala H, Felix Karp N, Varki XC, Varki A. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology. 2008:18(10):818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, Tremoulet AH, Hai Y, Chen X, Burns JC, Varki A. A simple method for assessment of human anti-Neu5Gc antibodies applied to Kawasaki disease. PLoS One. 2013:8(3):e58443–e58443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce OMT, Varki A. Chemo-enzymatic synthesis of the carbohydrate antigen N-glycolylneuraminic acid from glucose. Carbohydr Res. 2010:345(9):1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedron T, Girard R, Turco SJ, Chaby R. Phosphatidylinositol-anchored molecules and inducible lipopolysaccharide binding sites of human and mouse bone marrow cells. J Biol Chem. 1994:269(4):2426–2432. [PubMed] [Google Scholar]
- Peri S, Kulkarni A, Feyertag F, Berninsone PM, Alvarez-Ponce D. Phylogenetic distribution of CMP-Neu5Ac hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biology and Evolution. 2018:10(1):207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MB, MacLean LL, Gottschalk M, Aragon V, Vinogradov E. Structure of the capsular polysaccharides and lipopolysaccharides from Haemophilus Parasuis strains ER-6P (Serovar 15) and Nagasaki (Serovar 5). Carbohydr Res. 2013:378:91–97. [DOI] [PubMed] [Google Scholar]
- Prydz K. Determinants of glycosaminoglycan (GAG) structure. Biomol Ther. 2015:5(3):2003–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JB, Mahapatra S, Crick DC, Pavelka MS. Identification of the NamH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem. 2005:280(1):326–333. [DOI] [PubMed] [Google Scholar]
- Reinhold V, Zhang H, Hanneman A, Ashline D. Toward a platform for comprehensive glycan sequencing. MCP. 2013:12(4):866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G, Pfeil R, Stoll S, Schauer R, Kamerling JP, Versluis C, Vliegenthart JFG. Identification of new sialic acids derived from glycoprotein of bovine submandibular gland. Eur J Biochem. 1983:134(1):139–143. [DOI] [PubMed] [Google Scholar]
- Rich SM, Leendertz FH, Guang X, LeBreton M, Djoko CF, Aminake MN, Takang EE, Diffo JLD, Pike BL, Rosenthal BM et al. The origin of malignant malaria. Proc Natl Acad Sci. 2009:106(35):14902–14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A, Evanno G, Harb J, Soulillou J-P. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015:22(2):85–94. [DOI] [PubMed] [Google Scholar]
- Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci. 2015:112(2):542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samraj AN, Bertrand KA, Luben R, Khedri Z, Hai Y, Nguyen D, Gregg CJ, Diaz SL, Sawyer S, Chen X et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS One. 2018:13(6):e0197464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Jeff L, Sonnenburg ED, Gonzalez CG, Lichtman JS, Gregor R, Rob K, Alphaxard M, John C, Elias JE et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. 2017:357(6353):802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R, Sommer U, Krüger D, van Unen H, Traving C. The terminal enzymes of sialic acid metabolism: acylneuraminate pyruvate-lyases. Biosci Rep. 1999:19(5):373–383. [DOI] [PubMed] [Google Scholar]
- Schauer R, Vinayaga Srinivasan G, Coddeville B, Zanetta J-P, Guérardel Y. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res. 2009:344(12):1494–1500. [DOI] [PubMed] [Google Scholar]
- Schilling C, Badri A, Sieber V, Koffas M, Schmid J. Metabolic engineering for production of functional polysaccharides. Curr Opin Biotechnol. 2020:66:44–51. [DOI] [PubMed] [Google Scholar]
- Shaw L, Schneckburger P, Schlenzka W, Carlsem J, Christiansen K, Jurgensen D, Schauer R. CMP-N-acetylneuraminic acid hydroxylase from mouse liver and pig submandibular glands. Interaction with membrane-bound and soluble cytochrome B5-dependent electron transport chains. Eur J Biochem. 1994:219(3):1001–1011. [DOI] [PubMed] [Google Scholar]
- Smit H, Gaastra W, Kamerling JP, Vliegenthart JF, de Graaf FK. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia Coli K99 Fimbrial Adhesin. Infect Immun. 1984:46(2):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Diaz SL, Gagneux P. Parallel evolution of a self-signal: Humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014:66(11):671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura N, Tawada A, Sugimoto K, Watanabe H. Molecular cloning and characterization of chondroitin polymerase from Escherichia Coli strain K4. J Biol Chem. 2002:277(24):21567–21575. [DOI] [PubMed] [Google Scholar]
- Takata T, Hasegawa T, Tatsuno T, Date J, Ishigaki Y, Nakamura Y, Tomosugi N, Takano F, Ohta T. Isolation of N-acetylneuraminic acid and N-glycolylneuraminic acid from Pleurocybella Porrigens. J Health Sci. 2009:55(3):373–379. [Google Scholar]
- Takematsu H, Kawano T, Koyama S, Kozutsumi Y, Suzuki A, Kawasaki T. Reaction mechanism underlying CMP-N-acetylneuraminic acid hydroxylation in mouse liver: Formation of a ternary complex of cytochrome B5, CMP-N-acetylneuraminic acid, and a hydroxylation enzyme1. J Biochem. 1994:115(3):381–386. [DOI] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci. 2003:100(21):12045–12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Hai Y, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V et al. Novel mechanism for the generation of human Xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010:207(8):1637–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux CJ, Melançon CE, Liu HW. Unusual sugar biosynthesis and natural product glycodiversification. Nature. 2007:446(7139):1008–1016. [DOI] [PubMed] [Google Scholar]
- Toukach PV, Egorova KS. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016:44(D1):D1229–D1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan D, Williams A, Dordick JS, Koffas MAG, Linhardt RJ. Engineered heparins as new anticoagulant drugs. Bioengineering & Translational Medicine. 2017:2(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamecq J, Poupaert JH. Studies on the metabolism of glycolyl-CoA. Biochem Cell Biol. 1990:68(5):846–851. [DOI] [PubMed] [Google Scholar]
- Varki A. Loss of N-Glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. American J Physical Anthropology Suppl. 2001:33(Suppl):54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008:14(8):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schnaar RL, Schauer R. Sialic acids and other Nonulosonic acids; 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015 [PubMed]
- Visser D, Karin E, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005:7(5):411–423. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A, Pastuszak I, Stewart M, Drake RR, Elbein AD. Identification and modification of the uridine-binding site of the UDP-GalNAc (GlcNAc) pyrophosphorylase. J Biol Chem. 2000:275(2):1433–1438. [DOI] [PubMed] [Google Scholar]
- Wivagg CN, Bhattacharyya RP, Hung DT. Mechanisms of β-lactam killing and resistance in the context of Mycobacterium tuberculosis. J Antibiot. 2014:67(9):645–654. [DOI] [PubMed] [Google Scholar]
- Wood B, Boyle EK. Hominin Taxic diversity: Fact or fantasy? Am J Phys Anthropol. 2016:159(S61):37–78. [DOI] [PubMed] [Google Scholar]
- Yao Y, Barghava N, Kim J, Niederweis M, Marassi FM. Molecular structure and peptidoglycan recognition of Mycobacterium tuberculosis ArfA (Rv0899). J Mol Biol. 2012:416(2):208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasue S, Handa S, Miyagawa S, Inoue J, Hasegawa A, Yamakawa T. Difference in form of sialic acid in red blood cell glycolipids of different breeds of dogs. J Biochem. 1978:83(4):1101–1107. [DOI] [PubMed] [Google Scholar]
- Yu H, Cao H, Tiwari VK, Li Y, Chen X. Chemoenzymatic synthesis of C8-modified sialic acids and related Α2–3- and Α2–6-linked sialosides. Bioorg Med Chem Lett. 2011:21(17):5037–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li Y, Zeng J, Thon V, Nguyen DM, Ly T, Kuang HY, Ngo A, Chen X. Sequential one-pot multienzyme chemoenzymatic synthesis of glycosphingolipid glycans. J Org Chem. 2016:81(22):10809–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaramela LS, Martino C, Alisson-Silva F, Rees SD, Diaz SL, Chuzel L, Ganatra MB, Taron CH, Secrest P, Zuñiga C et al. Gut bacteria responding to dietary change encode sialidases that exhibit preference for red meat-associated carbohydrates. Nat Microbiol. 2019:4(12):2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelber-Sagi S, Ivancovsky-Wajcman D, Isakov NF, Webb M, Orenstein D, Shibolet O, Kariv R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018:68(6):1239–1246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.