Abstract
Background
Multiple non-arthroplasty surgical techniques are described for the management of large and massive irreparable rotator cuff tears. There is currently no consensus on the best management strategy. Our aim was to compare clinical outcomes following arthroscopic debridement, arthroscopic partial cuff repair, superior capsule reconstruction, balloon spacers or graft interposition for the management of large and massive irreparable rotator cuff tears.
Methods
A comprehensive search was performed of the following databases: Medline, Embase, CINAHL and Cochrane Database of Systematic Reviews. Data were extracted from relevant studies published since January 2000 according to the pre-specified inclusion criteria. The primary outcome was the post-operative improvement in shoulder scores. Meta-analysis of the primary outcome was performed. Secondary outcomes included retear rates and complications.
Results
Eighty-two studies were included reporting the outcomes of 2790 shoulders. Fifty-one studies were included in the meta-analysis of the primary outcome. The definition of an irreparable tear varied. All procedures resulted in improved shoulder scores at early follow-up. Shoulder scores declined after 2 years following balloon spacers, arthroscopic debridement and partial cuff repair. High retear rates were seen with partial cuff repairs (45%), graft interposition (21%) and superior capsule reconstruction (21%).
Conclusions
Large initial improvements in shoulder scores were demonstrated for all techniques despite high retear rates for reconstructive procedures. Shoulder scores may decline at mid- to long-term follow-up.
Keywords: Rotator cuff; Rotator cuff tears; Injuries, tendon; Shoulder
Background
Rotator cuff tears are a common cause of shoulder pain and can lead to significant functional impairment [1]. A primary consideration for large and massive tears is whether the tendon can be repaired. A tendon may be considered irreparable when it cannot be mobilised to the anatomical footprint despite mobilisation techniques such as interval slide and marginal convergence [2]. Poor tendon architecture with a high degree of fatty infiltration will also reduce the likelihood of a successful repair.
Several surgical techniques have evolved for the management of large and massive irreparable tears in adult patients without significant arthritic disease. Partial cuff repair (PCR) is an option where complete repairs are not possible [3]. Reconstruction of infraspinatus and teres minor is prioritised to re-establish the transverse force couple. Further techniques have evolved including subacromial balloon spacers, superior capsule reconstruction (SCR) and the use of autografts, allografts, xenografts or synthetic grafts to bridge residual cuff defects [2].
SCR aims to prevent superior migration of the humeral head and restore normal shoulder biomechanics; it involves passing a graft from the superior tubercle of the glenoid to the greater tuberosity [4, 5]. Similarly, balloon spacers are designed to prevent superior migration by deploying biodegradable saline-filled spacers underneath the acromion. Several large medical device companies have invested in the technology, and there has been a rapid expansion in data from case series [6].
Several tendon transfer techniques have been described to restore shoulder function according to tear configuration [7]. Posterosuperior tears may be managed with latissimus dorsi or trapezius tendon transfers [7, 8]. These have been shown to be effective in select groups; they are most commonly used in younger, high-demand patients and require specialised rehabilitation programmes [7, 8]. As a consequence, the patient populations managed with tendon transfers will likely be different compared to the other procedures in this review and tendon transfers will not be considered further. Reverse total shoulder replacement can lead to significant improvement in function and pain in patients with concurrent arthritis; however, concern about the complication and revision rates has limited its application in those without advanced arthritis, particularly in younger patients [9].
Despite the significant impact on patients’ quality of life, socio-economic considerations and protracted rehabilitation periods, a consensus on the optimal strategy has not been reached. Studies have demonstrated positive patient-reported outcome measures (PROM), functional scores and reduced pain for arthroscopic debridement, arthroscopic PCR, SCR, subacromial balloon spacers and graft interposition.
Aim
The aim of this review is to answer the following research question: In adult patients with a large or massive irreparable tear of the rotator cuff does arthroscopic debridement, arthroscopic PCR, SCR, subacromial balloon spacer or graft interposition lead to the greatest improvement in shoulder scores and the lowest risk of complications? Randomised trials and observational studies were considered. Our hypothesis is that SCR, balloon spacer and graft interposition do not result in superior shoulder scores compared to arthroscopic PCR.
Methods
The review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database. The review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [10].
Search strategy
The following databases were used: Medline, EMBASE, CINAHL and the Cochrane database of systematic reviews, from inception until May 2021. Trial registries were searched to highlight ongoing work. A scoping literature review was performed prior to protocol submission to optimise the search terms and inclusion criteria. The search terms are related to the population (rotator cuff tear, supraspinatus, infraspinatus, subscapularis, teres minor tears, AND massive, large, irreparable) and the interventions (superior capsule reconstruction, partial cuff repair, balloon spacer, graft bridging, debridement). Reference lists were scrutinised for further work. The full search terms are available in the Appendix.
Eligibility criteria
The review included adult patients with large or massive rotator cuff tears that were considered irreparable. The reparability of the cuff was determined by the authors of each study based on pre-operative imaging and intra-operative assessment. Across all studies, this was defined as a tear where the tendon could not be brought back to the tendon insertion without excessive tension on the tendon.
A minimum tear size was required; large or massive tears were included. Large and massive tears were defined as 3 cm or greater in any dimension or involving two or more tendons. The studies must report on pre-operative and post-operative shoulder scores for any of the included procedures: arthroscopic debridement, arthroscopic PCR, SCR, subacromial balloon spacers and graft interposition of incomplete repairs. Graft interposition was defined as cases where a graft was used to bridge a remaining tendon defect. It did not include cases where grafts were used in addition to a complete repair. In SCR, the graft is anchored into the superior glenoid and the greater tuberosity. Articles published no earlier than 2000 were considered. All types of graft material for SCR and graft interposition were accepted. Randomised trials and observational studies with multiple or single intervention groups of ten or more patients were included. Where trials compared one or more techniques that matched our inclusion criteria against alternative procedures, data from the relevant treatment arm were extracted.
Articles were excluded if they reported on tears associated with additional trauma including fractures or dislocations and patients with documented cuff tear arthropathy, SLAP tears, or advanced glenohumeral osteoarthritis. Small and medium tears (< 3 cm) were excluded alongside procedures that involved additional bone marrow infiltration, complete repair or augmentation of a complete repair. Animal or in vitro work, editorials, conference abstracts, case reports, case series of less than 10 patients and letters were also excluded. We included studies where patients may have undergone a previous failed rotator cuff repair. Finally, we required a minimum of 12 months of follow-up. We excluded studies with less than 12 months follow-up, where earlier outcome data could not be separated.
Outcomes
The primary outcomes were PROMs and functional scores including, but not limited to the Constant-Murley score, American Shoulder and Elbow Score (ASES), Oxford Shoulder Score (OSS), University of California in Los Angeles (UCLA) Shoulder Score, the Disabilities of Arm, Shoulder and Hand score (DASH), Subjective Shoulder Value (SSV), Simple Shoulder Test (SST) or the Japanese Orthopaedic Association (JOA) shoulder score. Secondary outcomes include retear rates and complications.
Study selection
Two authors (AD and PS) independently assessed trials for eligibility. Search results were organised using Covidence® systematic review management software. Duplicates were removed, and articles were selected according to the inclusion criteria. Conflicts were discussed between reviewers; a third reviewer (SS) was available as required to arbitrate.
Data extraction
A standardised form guided data extraction in four areas: study characteristics, pre-operative information, details of the interventions and post-operative outcomes (Table 1). Data were extracted by AD and PS, and the results were checked independently by both authors. In order to be included in the quantitative synthesis, studies must have reported PROMs as mean (± standard deviation). To avoid error, data were not extracted from graphs. To pool PROMs across studies, outcome duration was grouped into 1 year, 2 years, 3 years and 5 or more years. The durations of follow-up were frequently presented as a mean and range. To standardise the analysis, all patients within a group must have achieved the minimum follow-up duration in order to be included.
Table 1.
Data extraction. OSS—Oxford Shoulder Score, JOA—Japanese Orthopaedic Association score
| Study characteristics |
Study design, institutions, no. of relevant procedures, no. of centres, date of publication, number of patients, funding source, sampling methods |
|---|---|
| Pre-operative information |
Patient demographics and co-morbidities, pre-operative functional scores, quality of life assessments, diagnostic criteria, pre-operative imaging |
|
Intervention details |
The surgical approaches, types of grafts and implants, patient position, method of insertion |
|
Post-operative outcomes |
PROMs and functional scores, complication rates, post-operative management |
Quality and bias assessment
A risk-of-bias assessment was performed in all studies by two authors (AD and PS) with consensus reached by discussion where necessary (Appendix 4). The Methodological Index for Non-randomized Studies (MINORS) instrument was chosen following publication of the protocol because all except 1 study were observational and the majority were case series [11]. This instrument includes an eight-item assessment of non-comparative studies. The instrument can be extended to 12 items in order to include comparative studies.
Data analysis
Pre-operative and post-operative PROMs and functional scores were extracted. The difference between the pre-operative and post-operative scores was calculated at 1, 2, 3 and 5 years post-procedure. Direct comparison between procedures was not made given the quality of the available literature, outcomes for each procedure were pooled independently. To account for the different scores, the standardised mean difference (SMD) was calculated and weighted according to sample size. A random effects model was selected due to the variability in study design.
To facilitate interpretation of the SMD, this was re-expressed in the units of the Constant-Murley score. This was the score most commonly used across included studies. Transformation was performed according to the Cochrane handbook [12]. We used the pooled standard deviation of all studies included in the quantitative synthesis that reported the Constant-Murley score. Analysis was performed using Review Manager 5.3. Mean differences were calculated for the continuous data. Heterogeneity was assessed using the I2 Chi2 and Tau2 statistics.
Results
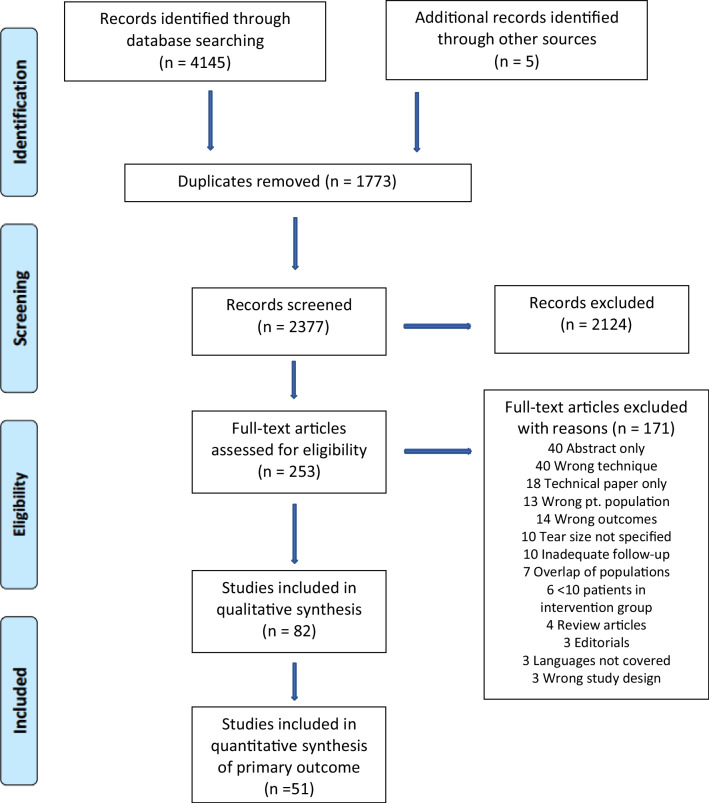
The search strategy revealed 4145 results; 5 further potential studies were identified through screening of references. After removal of duplicates, 2377 articles were available for screening (Fig. 1). Screening of titles and abstracts excluded 2124 articles. One hundred and seventy-one articles were excluded on full-text review leaving 82 studies for qualitative synthesis. Fifty-one studies were included in the quantitative synthesis of the primary outcome.
Fig. 1.
PRISMA study flow diagram
Study characteristics
Of the 82 eligible studies, 60 were case series, 21 were cohort studies and 1 was a randomised controlled trial (Tables 2, 3, 4, 5 and 6). The surgical management of 2790 shoulders was reported. The majority of studies were performed in a single centre; 5 involved patients from two or more institutions. Sufficient data for quantitative synthesis were provided in 51 studies. This includes 37 case series and 14 comparative studies (13 cohort studies and 1 randomised trial), where one or more of the treatment groups were included. In four cohort studies and in the randomised trial, one or more groups did not meet the inclusion criteria and data were extracted for the relevant management arm.
Table 2.
Partial cuff repair—included studies
| Author and date | Design | Mean age | No. of shoulders at f/u—PROMs | Follow-up duration (months) | Study results pooled | Functional/PROMs outcomes measured |
|---|---|---|---|---|---|---|
| Baverel [13] | Cohort–PCR vs LDTT | 68.5 | 26 | 16–29 | Yes | Constant, ASES, SSV, SST |
| Berth [14] | Cohort–PCR vs debridement | 63.4 | 21 | 21–28 | Yes | Constant, QuickDASH |
| Besnard [15] | Cohort–PCR vs complete | 59.3 | 20 | 24–120 | Yes | Constant |
| Cavalier [16] | Cohort–PCR vs debridement | 67 | 61 | 12 | No | Constant, ASES, SSV |
| Chen [17] | Case series | 60.3 | 37 | 25–59 | Yes | ASES |
| Cuff [18] | Case series | 65.2 | 28 | 60–90 | Yes | ASES, SST |
| Di Benedetto [19] | Case series | 67 | 31 | 79 | Yes | Constant |
| Farazdaghi [20] | Cohort–PCR vs complete | 63.7 | 14 | 95 | No | ASES, Penn |
| Franceschi [21] | Cohort–PCR vs debridement | 62 | 34 | 60–108 | Yes | UCLA |
| Galasso [22] | Case series | 62.7 | 95 | 24–148 | Yes | Constant, SST |
| Greiner [23] | Cohort–PCR vs SCR | 62.3 | 20 | 47–79 | No | Constant, DASH |
| Heuberer [24] | Cohort–PCR vs debridement | 67 | 22 | 23–70 | No | Constant, QuickDASH |
| Holtby [25] | Cohort–PCR vs complete | 67 | 73 | 24 | No | Constant, ASES |
| Jeong [26] | Cohort–PCR vs complete | 61.9 | 33 | 60–120 | Yes | UCLA, ASES, SSV |
| Kim [27] | Case series | 62.3 | 27 | 36–52 | Yes | Constant, SST |
| Kim [28] | Cohort–PCR vs complete | 61.5 | 19 | 24 | Yes | UCLA, ASES, SST |
| Lee [29] | Case series | 61.2 | 42 | 31–78 | Yes | UCLA, constant |
| Malahias [30] | Cohort–PCR vs PCR and balloon | 69.7 | 16 | 12 | Yes | Constant, ASES |
| Mori [31] | Cohort–PCR vs graft interposition | 65.7 | 24 | 35.6 | No | UCLA, Constant, ASES |
| Moser [32] | Cohort–PCR vs complete vs debridement (< 10 patients) | 62.5 | 11 | 24 | No | SPADI |
| Pandey [33] | Cohort–PCR vs graft interposition | 58 | 13 | 24–60 | Yes | Constant, OSS |
| Park [34] | Cohort–PCR vs augmentation | 63.8 | 37 | 24–51 | Yes | Constant, ASES, KSS |
| Paribelli [35] | Cohort–PCR vs LDTT | 64.9 | 20 | 12–60 | Yes | UCLA |
| Porcellini [36] | Case series | 63 | 67 | 60 | Yes | Constant, SST |
| Shon [37] | Case series | 65.9 | 31 | 40 ± 14.9 | Yes | ASES, SST |
| Wellmann [38] | Case series | 65 | 38 | 12 | No | Constant |
Partial cuff repair—included studies. PCR—partial cuff repair, LDTT—latissimus dorsi tendon transfer, complete–complete cuff repair. Constant—Constant-Murley Score, QuickDASH—Disabilities of the Arm, Shoulder and Hand Score, ASES—American Shoulder and Elbow Score, SSV—Subjective Shoulder Value, SST—Simple Shoulder Test, SPADI—Shoulder Pain and Disability Index, UCLA—The University of California at Los Angeles Shoulder Score, KSS—Korean Shoulder Score, OSS—Oxford Shoulder Score, JOA—Japanese Orthopaedic Association score. For comparative studies, the number of shoulders for the relevant treatment arm is reported
Table 3.
Graft interposition—included studies. OSS—Oxford Shoulder Score, JOA—Japanese Orthopaedic Association score
| Author and date | Design | Mean age | No. of shoulders at f/u—PROMs | Follow-up duration (months) | Study results pooled | Graft type used | Functional/PROMs outcomes measured |
|---|---|---|---|---|---|---|---|
| Audenaert [39] | Case series | 67 | 39 | 24–86 | No | Mersilene mesh | Constant |
| Badhe [40] | Case series | 66 | 10 | 36–60 | Yes | Porcine dermal xenograft | Constant |
| Bond [41] | Case series | 54.4 | 16 | 12–38 | Yes | Human dermal allograft | UCLA, Constant |
| Dukan [42] | Case series | 60.5 | 23 | 29.3 | Yes | Porcine dermal xenograft | Constant |
| Gupta [43] | Case series | 63 | 24 | 29–40 | No | Human dermal allograft | Constant |
| Gupta [44] | Case series | 60 | 27 | 24–40 | No | Porcine dermal xenograft | Constant |
| Kim [45] | Case series | 56 | 24 | 24–48 | No | Human dermal allograft | UCLA, ASES, SST |
| Kokkalis [46] | Case series | 58 | 21 | 33–72 | Yes | Human dermal allograft | ASES |
| Modi [47] | Case series | 62.6 | 61 | 12–72 | No | Human dermal allograft | OSS |
| Mori [31] | Cohort–graft interposition vs PCR | 65.7 | 24 | 35.6 | No | Fascia lata autograft | UCLA, Constant, ASES |
| Nada 2010[48] | Case series | 66.5 | 21 | 36 | No | Polyester fibre mesh | Constant |
| Pandey [33] | Cohort–graft interposition vs PCR | 58 | 13 | 24–60 | Yes | Human dermal allograft | Constant, OSS |
| Petrie [49] | Case series | 67.2 | 31 | 24 | No | Polyester fibre mesh | OSS |
| Rhee [50] | Case series | 61 | 31 | 24–67 | No | Biceps autograft | UCLA, Constant |
| Rhee [51] | Case series | 66.9 | 24 | 12 | Yes | Biceps autograft | ASES, QuickDASH |
| Sano [52] | Case series | 64 | 14 | 12–51 | Yes | Biceps autograft | JOA |
| Dimitrios [53] | Case series | 64.9 | 68 | 31–77 | Yes | Fascia lata autograft | Constant |
| Wong [54] | Case series | 53.6 | 45 | 24–68 | No | Human dermal allograft | UCLA |
Table 4.
Superior capsule reconstruction—included studies
| Author and date | Design | Mean age | No. of shoulders at f/u—PROMs | Follow-up duration (months) | Study results pooled | Graft type used | Functional/PROMs outcomes measured |
|---|---|---|---|---|---|---|---|
| Alarcon [55] | Case series | 61 | 31 | 24–51 | No | Fascia lata autograft | Constant |
| Barth [56] | Cohort—SCR vs complete vs augmentation | 60 | 24 | 24–29 | Yes | LHB autograft | Constant, ASES, SST |
| Burkhart [57] | Case series | 64 | 41 | 24–50 | Yes | Human dermal allograft | ASES, SSV |
| Denard [58] | Case series | 62 | 59 | 12–29 | Yes | Human dermal allograft | ASES, SSV, |
| Ferrando [59] | Case series | 65 | 52 | 34 | Yes | Porcine dermal xenograft | ASES, SSV |
| Greiner [23] | Cohort–SCR vs PCR | 62.3 | 20 | 47–79 | No | Porcine dermal xenograft | Constant, DASH |
| Kim [60] | Case series | 58 | 45 | 24–48 | No | LHB autograft | Constant, ASES |
| Kocaoglu [61] | Cohort–SCR vs SCR and PCR | 63.7 | 26 | 18–40 | Yes | Fascia lata autograft | Constant, QuickDASH |
| LaBelle [62] | Case series | 62.5 | 28 | 24–41 | Yes | Dermal allograft | SST, ASES |
| Lacheta [63] | Case series | 56 | 21 | 24–36 | No | Human dermal allograft | ASES, QuickDASH |
| Lee and Min [64] | Case series | 60.9 | 36 | 24.8 | No | Fascia lata autograft and dermal allograft | Constant, ASES |
| Lim [65] | Case series | 65.3 | 31 | 12–24 | Yes | Fascia lata autograft | Constant, ASES |
| Mihata [5] | Case series | 65.1 | 24 | 24–51 | Yes | Fascia lata autograft | ASES, JOA |
| Okamura [66] | Cohort–SCR 1 layer graft vs 3 layers | 76 | 35 | 24–69 | No | Teflon graft | ASES |
| Ohta [67] | Case series | 75.3 | 35 | 12–62 | Yes | Fascia lata autograft | UCLA, JOA |
| Ozturk [68] | RCT–SCR vs LDTT | 62.8 | 20 | 31 | Yes | Fascia lata autograft | Constant, ASES |
| Pashuck Pashuck 2020 | Case series | 58.9 | 14 | 23–25 | Yes | Human dermal allograft | ASES |
| Pennington [70] | Case series | 59.4 | 88 | 16–28 | Yes | Human dermal allograft | ASES |
| Polacek [71] | Case series | 60 | 20 | 12 | Yes | Porcine dermal xenograft | SPADI |
| Polacek [72] | Case series | 61.3 | 24 | 12 | Yes | Fascia lata autograft | SPADI |
| Takayama [73] | Cohort–SCR mini open vs arthroscopic | 69.7 | 46 | 24–66 | Yes | Fascia lata autograft | ASES |
Table 5.
Arthroscopic debridement—included studies
| Author and date | Design | Mean age | No. of shoulders at f/u—PROMS | Follow-up duration [months] | Study results pooled | Functional/PROMs outcomes measured |
|---|---|---|---|---|---|---|
| Berth [14] | Cohort–Debridement vs PCR | 63.4 | 21 | 21–28 | Yes | Constant, QuickDASH |
| Blanke [74] | Case series | 63.4 | 59 | 24–36 | No | Constant |
| Boileau [75] | Case series | 68 | 72 | 24–76 | Yes | Constant |
| Cavalier [16] | Cohort–Debridement vs PCR | 67 | 26 | 12 | No | Constant, ASES, SSV |
| Franceschi [21] | Cohort–Debridement vs PCR | 62 | 34 | 60–108 | Yes | UCLA |
| Heuberer [24] | Cohort–Debridement vs PCR | 67 | 23 | 23–70 | No | Constant, QuickDASH |
| Klinger [76] | Case series | 69 | 33 | 24–46 | No | Constant |
| Lee [77] | Case series | 62.4 | 32 | 24–63 | No | Constant, UCLA |
| Mirzaee [78] | Case series | 65 | 12 | 12–24 | Yes | UCLA |
| Park [79] | Case series | 64 | 16 | 84–126 | Yes | UCLA, constant |
| Scheibel [80] | Case series | 69 | 22 | 20–58 | No | Constant |
| Vad [81] | Case series | 61.3 | 32 | 24–84 | Yes | Shoulder rating questionnaire |
| Veado [82] | Case series | 72 | 12 | 12–60 | Yes | UCLA |
| Verhelst [83] | Case series | 69.9 | 32 | 21–52 | Yes | Constant |
Table 6.
Balloon spacer—included studies
| Author and Date | Design | Mean age | No. of shoulders at f/u—PROMs | Follow-up duration (months) | Study results pooled | Functional/PROMs outcomes measured |
|---|---|---|---|---|---|---|
| Basat [84] | Case series | 64.3 | 12 | 38 | Yes | Constant |
| Bakti [85] | Case series | 67 | 26 | 12–60 | No | OSS |
| Deranlot [86] | Case series | 69.8 | 39 | 12–36 | Yes | Constant |
| Familiari [87] | Case series | 63 | 51 | 24–56 | Yes | Constant |
| Gervasi [88] | Case series | 74.6 | 15 | 12–14 | No | Constant, ASES |
| Gervasi [89] | Case series | 73 | 40 | 24 | Yes | Constant, ASES |
| Iban [90] | Case series | 69 [median] | 10 | 24 | No | Constant, SST, QuickDASH |
| Lorente [91] | Case series | 69.4 | 15 | 12 | No | Constant |
| Maman [92] | Case series | Unknown | 42 | 12–40 | No | Constant |
| Senekovic [93] | Case series | 70.5 | 18 | 18–36 | Yes | Constant |
Twenty-six of the included studies reported shoulder scores following partial cuff repair, and 21 studies reported scores following SCR. Eighteen articles recorded results for graft interposition and outcomes for arthroscopic debridement were reported in 15 studies. However, in one cohort study comparing PCR and arthroscopic debridement, the series in the debridement arm was not included due to there being fewer than 10 patients in this treatment group. Ten studies reported shoulder scores for balloon spacers. The criteria used to define large and massive tears were documented in 58 studies. In the remaining articles, the authors described the tears as “large” or “massive” without further details. Magnetic resonance imaging (MRI) was the most favoured imaging modality; 74 papers reported the use of pre-operative MRI or magnetic resonance arthrogram in diagnosis and classification. The remaining either used ultrasound imaging or an intra-operative assessment. The age range of participants across all studies was 33–90. The Hamada classification of rotator cuff arthropathy was the most frequently used. Several studies were excluded due to lack of reporting of tear size, the majority of these were for balloon spacers.
The terminology used to describe graft interposition varied between studies and included “bridging”, “interposition” and “augmentation”. Careful review of the description of the technique was necessary to ensure the grafts were used to fill the remaining defect after the best possible repair. The most popular interposition graft was human dermal allograft (7 studies). In contrast, fascia lata autograft was preferred in superior capsule reconstruction (9 studies). Post-operative management was well reported in the majority of studies; 70 described a progressive rehabilitation programme.
The pre-operative management of patients was described in 28 studies, the majority of these documented a minimum of 6 months of failed conservative therapy. All surgical procedures were performed in the beach chair or lateral decubitus position. In the case of bridging repairs and superior capsule reconstruction, allografts, autografts, xenografts and synthetic grafts were used (Tables 2, 3, 4, 5 and 6).
In all cases of debridement and balloon spacer insertion, pendular and passive range of motion exercises were commenced within 4 weeks, with the majority initiating range of motion exercises from the first day post-op. Sling use varied, it was used for a maximum of 3–4 weeks following these two procedures. In contrast, following arthroscopic PCR, SCR or graft interposition, patients were immobilised in a sling for up to 6 weeks followed by passive and active range of motion exercises. Full strengthening work may not begin for 6 months.
Partial cuff repair
See Table 2.
Graft interposition
See Table 3.
Superior capsule reconstruction
See Table 4.
Arthroscopic debridement
See Table 5.
Balloon spacer
See Table 6.
Function scores and patient-reported outcome measures
In total, 49 studies reported pre-operative and post-operative Constant-Murley scores. The ASES was the next most reported score (32 studies) followed by the UCLA Shoulder Score (15 studies).
In all studies, there was an improvement in post-operative PROMs data compared to pre-operative scores. Synthesis of the difference in pre-operative and post-operative PROMs scores was possible across 51 studies. The pooled standardised mean difference for each implant is given in Table 7. Data were available at 1 year and 2 years for all 5 procedures. Three-year data were available for balloon spacers only and 5-year data for arthroscopic partial cuff repair and arthroscopic debridement. The number of studies contributing to the pooled SMD is shown in brackets. The greatest number was available for partial cuff repair and superior capsule reconstruction. A study by Vad et al. demonstrated a very high risk of bias and an SMD far greater than similar studies [81]. Results were pooled with and without inclusion of this study and omitted in the results given in Table 7. The full series of forest plots are available in the Appendix.
Table 7.
SMDs in the difference in pre-operative and post-operative PROMs scores. Number of articles pooled in brackets
| Procedure | 1 year | 2 years | 3 years | 5 years |
|---|---|---|---|---|
| Partial cuff repair |
3.18 (8) |
3.92 (10) |
3.42 (6) |
|
| Graft interposition |
3.58 (7) |
5.00 (3) |
||
| Superior capsule recon |
3.12 (10) |
4.04 (9) |
||
| Arthro. debridement |
4.48 (5) |
2.63 (4) |
3.21 (2) |
|
| Balloon spacer |
1.36 (2) |
4.53 (3) |
1.66 (2) |
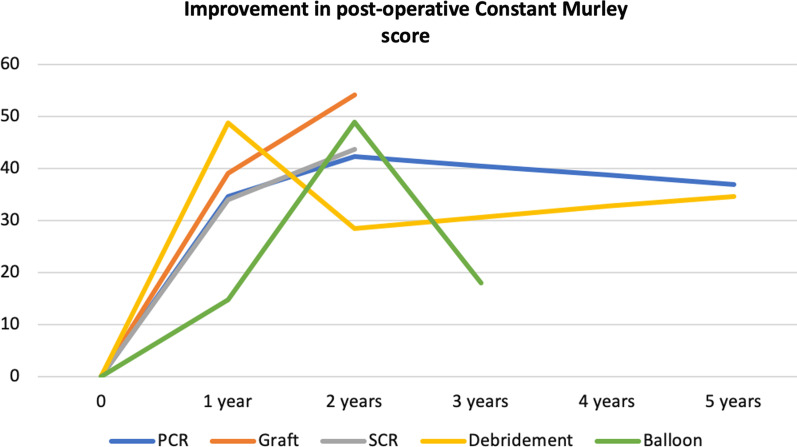
The SMD was re-expressed as a Constant-Murley score (Fig. 2); the mean standard deviation for the pre-operative Constant-Murley score was 10.9.
Fig. 2.
Standardised mean difference for each procedure, transformed to the Constant-Murley score
Secondary outcomes
The quality of reporting of revision procedures, retear rates and complications was mixed (results summarised in Tables 8 and 9 and the Appendix). Post-operative imaging was performed to a different extent across the studies. This may include a routine MRI or ultrasound (US) in all cases, a proportion of cases, post-operative imaging only when the patient was symptomatic or not at all.
Table 8.
Revision procedures, retears and infections following PCR, graft interposition and SCR
| Procedure | No. of shoulders | Revision procedures for failure of procedure (%) | Post-op imaging performed | Retears | Infections |
|---|---|---|---|---|---|
| Partial cuff repair | 733 | 2.9 | 170 | 45.3% | 0.55% |
| Graft interposition | 526 | 1.9 | 302 | 21.2% | 0.39% |
| Superior capsule reconstruction | 732 | 3.0 | 469 | 21.3% | 0.14% |
Table 9.
Revision procedures and infections following arthroscopic debridement and balloon spacer
| Procedure | No. of shoulders | Revision procedures for failure of procedure (%) | Infections |
|---|---|---|---|
| Arthroscopic debridement | 438 | 2.9 | 0.2% |
| Balloon spacer | 276 | 7.2 | 0% |
Six studies reporting outcomes of PCR performed an MRI or US in all shoulders. Of 170 shoulders, 77 showed evidence of a retear (45%). Following graft interposition, nine studies reported post-operative imaging in all cases and further 6 studies scanned a proportion of patients. In total, 302 shoulders were scanned and 64 tears of the graft or tendon were identified (21%). After SCR, 12 studies reported imaging for all shoulders, and a further 5 scanned a majority of shoulders for a total of 469; 100 retears were found (21%).
Revision procedures for failure or symptom recurrence were poorly documented. Some studies may have only reported on patients that achieved the documented minimum follow-up period, resulting in an underestimation of the true revision rate. The greatest proportion of revision procedures was performed after balloon spacers (7.2%). The fewest revisions were reported following graft interposition (1.9%).
Reporting of additional complications was sparse; these are documented in the Appendix. Polacek et al. reported three cases of acute porcine dermal xenograft rejection which required urgent revision [71]. Two cases of donor site morbidity were reported in two studies where fascia lata autograft was used for a SCR, and one required revision surgery at the donor site [72, 73]. Twenty studies reported VAS scores. In all studies, there was improvement in the post-operative pain scores. The limited data on pain scores preclude meaningful comparison between techniques.
Risk of bias
A risk-of-bias assessment of the included studies was performed according to the MINORS instrument and is summarised in Table 10 [11]. The cohort studies were generally of low quality. In order to maximise the opportunity for comparison between procedures, we extracted each relevant arm of the comparison separately and treated them as individual series. Consequently, the abbreviated 7-point MINORS criteria for non-comparative studies were calculated for all included studies. The results are summarised in Table 10. The full tables, including the results of the extended MINORS instrument for comparative studies, are available in the Appendix. For each procedure the mean risk of bias score across all seven domains was calculated.
Table 10.
Risk-of-bias scores
| Procedure | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation sample size | Mean score |
|---|---|---|---|---|---|---|---|---|
| Partial cuff repair | 2 | 1.46 | 1.62 | 2 | 2 | 1.54 | 0.23 | 1.55 |
| Graft interposition | 1.72 | 1.78 | 1.83 | 2 | 2 | 1.83 | 0 | 1.60 |
| Superior capsule recon | 2 | 1.86 | 1.62 | 2 | 2 | 1.67 | 0.19 | 1.62 |
| Arthro. debridement | 2 | 1.15 | 1.62 | 2 | 2 | 1.46 | 0 | 1.46 |
| Balloon spacer | 2 | 1.3 | 2 | 2 | 2 | 0.7 | 0 | 1.43 |
The greatest risk of bias was seen in studies on balloon spacers. These frequently described significant numbers lost to follow-up, and similar to all studies in this review, outcome scores were only presented for patients that completed follow-up.
Discussion
This systematic review and meta-analysis compared common surgical techniques for the management of large and massive irreparable rotator cuff tears. Management of this pathology in the absence of glenohumeral arthritis is challenging. This work demonstrates that arthroscopic debridement, arthroscopic PCR, SCR, subacromial balloon spacers and graft interposition all lead to improved shoulder scores at early- to mid-term follow-up. It is encouraging for surgeons and patients that there are several potential surgical strategies which lead to improvements in shoulder function. However, we show that clinical improvement may decline over time for arthroscopic debridement, PCR and balloon spacers, and this is most apparent for balloon spacers. Across the included case series, the improvement in shoulder scores for PCR was comparable with graft interposition and SCR. Despite the additional cost of graft augmentation and the additional expertise required for SCR, they may not provide further clinical benefit in the early to mid-term period compared to PCR.
The number of studies and participants available for synthesis at each time point varied. Three hundred and ninety participants from 10 studies were included in the synthesis of SCR 12-month outcomes compared to 50 in two studies for arthroscopic debridement at 5 years. We required a minimum post-operative duration of all patients within a series to be included in the quantitative synthesis. The greatest volume of evidence was available for graft interposition, partial cuff repair and superior capsule reconstruction. The pooled PROMs scores for each of these procedures were similar up to 2 years.
The results of arthroscopic debridement and subacromial spacers should be interpreted with caution. The trajectory of improvement in the post-operative scores for arthroscopic debridement shown in Fig. 2 demonstrates a decline in scores at a minimum of 24 months followed by an improvement at 5 years. In the 5-year group, there were two studies with a moderate to high risk of bias and a total of only 52 patients. The studies reporting on subacromial spacers had a higher failure rate and loss to follow-up. A randomised trial comparing balloon spacer to arthroscopic debridement demonstrated superior shoulder scores following arthroscopic debridement at 12 months [94]. A further trial comparing subacromial spacers to PCR demonstrated non-inferiority of subacromial spacers at 12 months [95]. However, despite improvement in the first 12–24 months after PCR and subacromial spacers, our work shows a decline in PROMs after this point, which is most prominent following subacromial spacers.
Reverse shoulder arthroplasty (RSA) was not included in this review. The use of RSA is rapidly expanding. However, they are performed infrequently in the absence of glenohumeral osteoarthritis, particularly in younger patients. RSA can be effective at mid- to long-term follow-up in patients with massive tears, with or without arthritic disease [96–98]. Ernstbrunner et al. reported that improvement in shoulder scores may be sustained over 10 years in the presence of massive tears; however, survivorship was 85% at 5 years and 70% at 15 years [97]. Other series demonstrate a sustained improvement in shoulder scores and a lower incidence of complications in the absence of glenohumeral osteoarthritis; however, these remain a concern [98, 99]. The National Joint Registry of England, Wales and Northern Ireland report a risk of revision of 3.85% at 8 years in all patients who receive a RSA, with a median patient age of 76 years [100]. Current evidence would suggest caution and shared decision-making when considering RSA in younger patients without osteoarthritis.
Latissimus dorsi and trapezius tendon transfers show encouraging results in specific patient groups. Recent reviews report large increases in shoulder scores across several series [16, 101, 102]; improved shoulder function may persist over 10 years[103]. A comparative study by Cavalier demonstrated an inferior Constant score at 12 months with latissimus dorsi tendon transfer compared to PCR and RSA, and a randomised trial of 42 patients reported inferior functional scores for LDTT compared to SCR [16, 68].
This review included a broad and comprehensive search strategy and included a large number of patients not included in earlier work. Previous systematic reviews have attempted to compare procedures for irreparable or massive rotator cuff tears [104, 105]. We have captured multiple studies not included in previous reviews. This is the first study to include a quantitative synthesis across a large number of studies accounting for different outcome scores.
This study has limitations. The quality of included studies varied, and reliable conclusions cannot be made about the comparative effectiveness of each procedure. Although further well-constructed and appropriately powered randomised trails comparing these techniques are warranted, they are practically challenging given the varied nature of tendon reparability and tendon quality in patients with large and massive tears. Despite an accepted definition of an irreparable tendon as one that cannot be brought back to the tendon footprint without undue tension, intra-operative assessments may differ and the techniques used to bring the tendon back to the footprint, such as interval slide and marginal convergence, are not routinely described—the decision of whether a tendon can be brought to the greater tuberosity may influence outcomes in borderline cases. We did not classify studies according to pre-operative management. This was poorly reported; dedicated anterior deltoid strengthening work can improve shoulder function in patients with irreparable cuff tears [106]. We did investigate the change in shoulder scores, rather than post-operative score alone which would account for any large differences in pre-operative function.
Conclusion
In adult patients with large and massive irreparable rotator cuff tears arthroscopic debridement, arthroscopic PCR, SCR, subacromial balloon spacers and graft interposition lead to improved early- and mid-term patient-reported outcomes and functional scores. Retear rates are 21% for SCR, 21% for graft interposition, and 45% for arthroscopic PCR. Mid- to long-term follow-up is necessary to further investigate whether there is a significant decline in shoulder function with time.
Appendix
Complete results tables: shoulder scores
Partial cuff repair
See Table 11.
Table 11.
Partial cuff repair—included studies
| Author and Date | No. of shoulders at f/u—PROMS | Study results pooled | Functional/PROMs outcomes pooled | Pre-op scores | 12-month scores | 24-month scores | X-month scores (specified minimum duration in months) |
|---|---|---|---|---|---|---|---|
| Baverel [13] | 26 | Yes |
Constant, ASES, SSV, SST |
36.7 (16.6) 33.5 (16.4) 37.5 (15.4) 3.5 (2.6) |
64.8 (13.7) 78.3 (19.3) 73.3 (17.5) 8.3 (2.4) |
||
| Berth [14] | 21 | Yes |
Constant, QuickDASH |
45.9 (9.2) 64.6 (11.9) |
79.4 (17.5) 16 (16.1) |
72.8 (16) 23.8 (16.8) |
|
| Besnard [15] | 20 | Yes | Constant | 31 (9.2) | 77.1 (13) | 72.8 (14.4)–85 m | |
| Cavalier [16] | 67 | No |
Constant, ASES, SSV |
51 (n/r) 34 (n/r) 42 (n/r) |
72 (n/r) 77.9 (n/r) 73 (n/r) |
||
| Chen [17] | 37 | Yes | Constant, | 45.95 (20.56) | 78.59 (14.29) | ||
| Cuff [18] | 28 | Yes |
ASES, SST |
46.6 (6.9) 5.6 (1.3) |
84.2 (4.1) 10.2 (0.8) |
79.3 (7.8)–60 m 9.1 (1.4)–60 m |
|
| Di Benedetto [19] | 31 | Yes | Constant | 46.5 (11.5) | 70.82 (14.66)–79 m | ||
| Farazdaghi [20] | 14 | No |
ASES Penn |
41.2 (10.1) 42.0 (12.5) Not PCR group alone |
70.6 (32.9)–60 m 71.1 (30.4)–60 m |
||
| Franceschi [21] | 34 | Yes | UCLA | 8.6 (4.1) | 32.2 (3.6) | 28.8 (4.2)–60 m | |
| Galasso [22] | 95 | Yes |
Constant, SST |
39.1 (8.4) n/r |
76.3 (9.7) 9.1 (2.2) |
||
| Greiner [23] | 20 | No |
Constant, DASH |
50.7 (n/r) n/r |
82.7 (8.4) 7.8 (11.1) |
||
| Heuberer [24] | 41 | No |
Constant QuickDASH |
In graph form only |
67.5 (9.9) 20.5 (14.4) |
||
| Holtby [25] | 73 | No |
Constant, ASES |
44.03 (n/r) 42.69 (n/r) |
73.73 (n/r) 71.42 (n/r) |
||
| Jeong [26] | 33 | Yes |
UCLA, ASES, SSV, |
15.1 (3) 40.9 (8.9) 38.4 (8.4) |
27.5 (2.8)–60 m 82.6 (7.1)–60 m 83.1 (6.8)–60 m |
||
| Kim [27] | 27 | Yes |
Constant, SST |
43.6 (7.9) 5.1 (1.2) |
74.1 (10.6) 8.8 (2.1) |
||
| Kim [28] | 19 | Yes |
UCLA, ASES, SST |
15.4 (2.9) 40.3 (9.3) 4.7 (1.4) |
28.4 (3.2) 85.6 (8.3) 8.8 (1.8) |
||
| Lee [29] | 42 | Yes |
UCLA, Constant |
20.5 (4.2) 41.2 (6.7) |
30.9 (2.3) 88.8 (7.9) |
||
| Malahias [30] | 16 | Yes |
Constant, ASES |
41.7 (15.6) 51 (16.5) |
69.6 (19.7) 79.8 (18.8) |
||
| Mori [31] | 48 | No |
UCLA, Constant, ASES |
13.7 (3.1) 36.3 (9.9) 41.8 (11.3) |
27.3 (6.1) 72.9 (16.8) 84.2 (19.7) |
||
| Moser [32] | 11 | No | SPADI | Collected but not reported | 29.5 (n/r) | ||
| Pandey [33] | 13 | Yes |
Constant, OSS |
43.1 (3.9) 17.8 (3.6) |
70.8 (5.3) 37.1 (2.4) |
||
| Park [34] | 37 | Yes |
Constant, ASES, KSS |
78 (11.6) 51.5 (22.7) 62.2 (14.1) |
87.4 (8.3) 72.8 (19.2) 77.4 (12.6) |
91.0 (7.4) 78.5 (18.5) 82.2 (13.2) |
|
| Paribelli [35] | 20 | Yes | UCLA | 7.3 (2.5) | 30.3 (4.2) | ||
| Porcellini [36] | 67 | Yes |
Constant, SST |
44 (14.1) 4.6 (2.3) |
73 (11.9)–60 m 9.0 (1.8) –60 m |
||
| Shon [37] | 31 | Yes |
ASES, SST |
41.97 (15.08) 3.61 (2.58) |
76.37 (17.01) 6.33 (2.58) |
73.78 (21.55) 6.07 (3.4) |
|
| Wellmann [38] | 38 | No | Constant | 56 (n/r) | 71 (n/r) |
PCR–partial cuff repair, LDTT—latissimus dorsi tendon transfer, Constant—Constant-Murley Score, QuickDASH–Disabilities of the Arm, Shoulder and Hand Score, ASES—American Shoulder and Elbow Score, SSV—Subjective Shoulder Value, SST—Simple Shoulder Test, SPADI—Shoulder Pain and Disability Index, UCLA—The University of California at Los Angeles Shoulder Score, KSS—Korean Shoulder Score, OSS—Oxford Shoulder Score
Graft interposition
See Table 12.
Table 12.
Graft interposition—included studies
| Author and Date | No. of shoulders at f/u | Study results pooled | Outcomes measured | Pre-op scores (SD) | 12-month scores (SD) | 24-month scores (SD) | X-month scores—specified duration (SD) |
|---|---|---|---|---|---|---|---|
| Audenaert [39] | 39 | No | Constant | 25.7 (n/r) | 72.1 (n/r) | ||
| Badhe [40] | 10 | Yes | Constant | 41.5 (17.5) | 62.5 (14.2) | 62.2 (14.5)–36 m | |
| Bond [41] | 16 | Yes |
Constant UCLA |
53.9 (10.6) 18.4 (4.2) |
84 (8.9) 30.4 (4.0) |
||
| Dukan [42] | 23 | Yes | Constant | 34.7 (7.8) | 78.3 (16.5) | ||
| Gupta [43] | 24 | No | Constant | 66.6 (n/r) | 88.7 (17.7) | ||
| Gupta [44] | 27 | No | Constant | 62.7 (n/r) | 91.8 (13.3) | ||
| Kim [45] | 24 | No |
UCLA, ASES, SST |
17 (n/r) 50 (n/r) |
30 (n/r) 83 (n/r) |
||
| Kokkalis [46] | 21 | Yes | ASES | 25.2 (6.78) | 74.4 (16.13) | 14 pts 24 months (pre-op 25.5, 6.38) (72.4, 14.23) | |
| Modi [47] | 61 | No | OSS | 26 median (n/r) | 42 median (n/r) | ||
| Mori [31] | 48 | No | UCLA, Constant, ASES |
14.3 (2.9) 37.4 (8.1) 40.8 (13) |
28.6 (4.3) 73.6 (6.6) 84.9 (8.1) |
||
| Nada [48] | 21 | No | Constant | 46.7 (n/r) | 36 months–84.5 (n/r) | ||
| Pandey [33] | 13 | Yes | Constant, OSS |
41.2 (3.1) 14.9 (3.5) |
83.9 (6) 43.9 (2.4) |
||
| Petrie [49] | 31 | No | OSS (old score) | 46.7 (n/r) | 30.6 (n/r) | ||
| Rhee [50] | 31 | No | UCLA, Constant |
12.5 (n/r) 48.4 (n/r) |
31.1 (n/r) 81.8 (n/r) |
||
| Rhee [51] | 24 | Yes | ASES, QuickDASH, |
45.4 (19.1) 50 (17.9) |
81.6 (17.6) 14.2 (20) |
||
| Sano [52] | 14 | Yes | JOA | 54.6 (9.3) | 83.1 (7.5) | ||
| Dimitrios [53] | 68 | Yes | Constant | 32.5 (8.74) | 88.7 (7.44) | ||
| Wong [54] | 45 | No | UCLA | 18.4 (n/r) | 27.5 (n/r) |
JOA—Japanese Orthopaedic Association Score
Superior capsule reconstruction
See Table 13.
Table 13.
Superior capsule reconstruction—included studies
| Author and Date | No. of shoulders at f/u—PROMS | Study results pooled | Functional/PROMs outcomes measured | Pre-op scores | 12-month scores | 24-month scores | X-month scores (specified) |
|---|---|---|---|---|---|---|---|
| Alarcon [55] | 31 | No | Constant | 36.0 (n/r) | 78.7 (n/r) | ||
| Barth [56] | 24 | Yes |
Constant, ASES, SST |
50 (13) 45 (19) 9 (2) |
77 (10) 80 (15) 8 (3) |
||
| Burkhart [57] | 41 | Yes |
ASES, SSV |
52 (3) 39 (n/r) |
90 (1) 88 (n/r) |
89 (2) 83 (n/r) |
|
| Denard [58] | 59 | Yes |
ASES, SSV, |
43.6 (18.6) 35.0 (19.9) |
77.5 (22) 76.3 (25.2) |
||
| Ferrando [59] | 52 | Yes |
ASES, SSV |
41 (19) 39 (17) |
86 (16) 80 (18) |
90 (9) 80 (11) |
|
| Greiner [23] | 20 | No |
Constant, DASH |
49.7 (n/r) n/r |
77.1 (10.5) 15.6 (15.4) |
||
| Kim [60] | 45 | No |
Constant, ASES |
64.9 (10.9) 60.9 (12.7) |
80.0 (9.4) 82.2 (9.2) |
80.0 (9.1) 82.7 (9.3) |
Healed and unhealed reported separately throughout paper |
| Kocaoglu [61] | 26 | Yes |
ASES QuickDASH |
48.5 (15.5) 53.6 (15.2) |
82.6 (15) 12.5 (5) |
||
| LaBelle [62] | 28 | Yes |
SST, ASES |
21.6 (17.6) 28.3 (10.1) |
66.6 (22.6) 68.2 (19.2) |
||
| Lacheta [63] | 21 | No |
ASES, QuickDASH |
54 (n/r) 37.6 (n/r) |
83.9 (n/r) 16.2 (n/r) |
||
| Lee and Min [64] | 36 | No |
Constant, ASES |
56.3 (8.8) 50.9 (8.9) |
84.3 (4.5) 85.1 (4.4) |
Healed and unhealed reported separately throughout paper | |
| Lim [65] | 31 | Yes |
Constant, ASES |
51.7 (13.9) 54.4 (17.9) |
63.7 (8.1) 73.7 (10.8) |
||
| Mihata [5] | 24 | Yes |
ASES, JOA |
23.5 (14.4) 48.3 (13) |
92.9 (11.3) 92.6 (9) |
||
| Okamura [66] | 35 | No | ASES | 42.4 (n/r) | 63.2 (n/r) | ||
| Ohta [67] | 35 | Yes |
UCLA, JOA |
15.3 (3.77) 62.3 (9.49) |
30.1 (3.11) 84.6 (5.66) |
||
| Ozturk [68] | 20 | Yes |
Constant, ASES |
36.6 (12.5) 23.2 (12.7) |
81.1 (11.3) 81.7 (12.3) |
||
| Pashuck [69] | 14 | Yes | ASES | 55 (17) | 83.3 (16) | 86.5 (9) | |
| Pennington [70] | 88 | Yes | ASES | 52.2 (19.3) | 81.56 (10.21) | 85.3 (n/r) | |
| Polacek [71] | 20 | Yes | SPADI | 51.3 (19.2) | 10.4 (8.8) | ||
| Polacek [72] | 24 | Yes | SPADI | 59.0 (19.4) | 9.7 (12.3) | ||
| Takayama [73] | 46 | Yes | ASES | 52.4 (12.6) | 86.1 (13.8) |
Arthroscopic debridement
See Table 14.
Table 14.
Arthroscopic debridement—included studies
| Author and Date | No. of shoulders at f/u—PROMS | Study results pooled | Functional/PROMs outcomes measured | Pre-op scores | 12-month scores | 24-month scores | X-month scores (specified) |
|---|---|---|---|---|---|---|---|
| Berth [14] | 21 | Yes | Constant, | 37 (13.6) | 61.3 (19.9) | 24.2 m 50.4 (15.3) | |
| QuickDASH | 69.6 (10.5) | 29.7 (19.7) | 35.3 (18.6) | ||||
| Blanke [74] | 59 | No | Constant | 33.9 (range only) | 54.5 (range only) | ||
| Boileau [75] | 72 | Yes | Constant | 46.3 (11.9) | 66.5 (16.3) | ||
| Cavalier [16] | 67 | No |
Constant, ASES, SSV |
52 (n/r) 30.5 (n/r) 43(n/r) |
62 (n/r) 59.9 (n/r) 65 (n/r) |
||
| Franceschi [21] | 34 | Yes | UCLA | 7.6 (2.6) | 23.2 (2.8) | 21.4 (3.7)–60 m | |
| Heuberer [24] | 41 | No |
Constant QuickDASH |
In graph form only |
65.8 (14.7) 24.1 (20.6) |
||
| Klinger [76] | 33 | No | Constant | 37 (n/r) | 67 (n/r) | ||
| Lee [77] | 32 | No |
Constant, UCLA |
47.6 (n/r) 15.4 (n/r) |
70.4 (n/r) 27.1 (n/r) |
||
| Mirzaee [78] | 12 | Yes | UCLA | 9.2 (0.8) | 27.5 (0.8) | ||
| Park [79] | 16 | Yes |
UCLA, Constant |
10.3 (2.7) 39.6 (7.6) |
26.8 (5.4) 58.8 (9.7) |
28.3 (4.6)–60 m 60.3 (10.2)–60 m |
|
| Scheibel [80] | 22 | No | Constant | 65.9 (n/r) | 90.6 (n/r) | ||
| Vad [81] | 32 | Yes | Shoulder rating questionnaire | 42.3 (1.4) | 81.4 (1.3) | ||
| Veado [82] | 12 | Yes | UCLA | 14.9 (4.6) | 29.9 (3.2) | 23.7 (3.3) | |
| Verhelst [83] | 32 | Yes | Constant | 34.9 (11.6) | 84 (11.6) |
Balloon spacer
See Table 15.
Table 15.
Balloon spacer—included studies
| Author and Date | No. of shoulders at f/u—PROMS | Study results pooled | Functional/PROMs outcomes measured | Pre-op scores | 12-month scores | 24-month scores | X-month scores (specified) |
|---|---|---|---|---|---|---|---|
| Basat [84] | 12 | Yes | Constant | 25.8 (5.31) | 75.4 (6.05) | ||
| Bakti [85] | 26 | No | OSS—in graph form only | ||||
| Deranlot [86] | 39 | Yes | Constant | 40 (14.6) | 59 (13.7) | 64 (3 year) (13.6) | |
| Familiari [87] | 51 | Yes | Constant | 27 (7.4) | 77 (15) | ||
| Gervasi [88] | 15 | No |
Constant, ASES |
31.9 (n/r) 24.5 (n/r) |
69.8 (n/r) 76 (n/r) |
||
| 72.5 (n/r) | |||||||
| Gervasi [89] | 40 | Yes |
Constant, ASES |
28.6 (11.6) 24.4 (11.8) |
67.9 (16.7) 84.2 (21.0) |
||
| Iban [90] | 10 | No |
Constant, SST, QuickDASH |
35 median (n/r) 3 median (n/r) 37 median (n/r) |
62.5 (n/r) 5 (n/r) 30 (n/r) |
53.5 (n/r) 6 (n/r) 27.5 (n/r) |
|
| Lorente [91] | 15 | No | Constant | 30 (range) | 47 (range) | ||
| Maman [92] | 42 | No | Constant | 36 (n/r) | 65.8 (n/r) | 70.8 (n/r) | |
| Senekovic [93] | 18 | Yes | Constant |
33.41(13.34) 20 pts |
60.46 (22.98) 18 pts |
65.42 (25.23)–36 m 16 pts |
Forest plots
PCR 12 months
PCR 24 months
PCR 5 years
Graft interposition 12 months
Graft interposition 24 months
SCR 12 months
SCR 24 months
Arthroscopic debridement 12 months
Arthroscopic debridement 24 months
Arthroscopic debridement 5 years
Balloon 12 months
Balloon 24 months
Balloon 3 years
Complications
Partial cuff repair
| Author and Date | Number of shoulders in PCR group for which f/u data available | No. of revision procedures for failure procedure | Post-op imaging performed—no. of patients | Number retears on imaging | Infections | Additional complications |
|---|---|---|---|---|---|---|
| Baverel [13] | 26 | 0 | 0 | n/a | 0 | 0 |
| Berth [14] | 21 | 1 | 21 | 11 | 0 | 0 |
| Besnard [15] | 20 | 4 | 0 | n/a | n/r | n/r |
| Cavalier [16] | 61 | 0 | 0 | n/a | 1 | 1 anchor migration |
| Chen [17] | 37 | 0 | Unknown | Unknown | n/r | n/r |
| Cuff [18] | 28 | 3 | 0 | n/a | 0 | 0 |
| DiBenedetto [19] | 31 | 0 | 0 | n/a | n/r | n/r |
| Farazdaghi [20] | 14 | 4 | 0 | n/a | 0 | 0 |
| Franceschi [21] | 34 | n/r | 0 | n/a | n/r | n/r |
| Galasso [22] | 95 | 8 | 0 | n/a | n/r | n/r |
| Greiner [23] | 20 | 0 | 0 | n/a | 0 | 0 |
| Heuberer [24] | 22 | 1 | Unknown | Unknown | 2 (washouts) | 0 |
| Holtby [25] | 73 | n/r | 0 | n/a | n/r | n/r |
| Jeong [26] | 33 | 0 | 33 | 28 | n/r | n/r |
| Kim [27] | 27 | 0 | 0 | n/r | n/r | n/r |
| Kim [28] | 19 | 0 | 0 | n/r | n/r | n/r |
| Lee [29] | 42 | 0 | 42 | 10 | 0 | 0 |
| Malahias [30] | 16 | 0 | 0 | n/a | 1 | 0 |
| Mori [31] | 24 | 0 | 24 | 10 | 0 | 0 |
| Moser [32] | 11 | 0 | 0 | n/a | 0 | 0 |
| Pandey [33] | 13 | 0 | 13 | 4 | 0 | 0 |
| Park [34] | 37 | 0 | 37 | 14 | 0 | 0 |
| Paribelli [35] | 20 | 0 | 0 | n/a | n/r | n/r |
| Porcellini [36] | 67 | 0 | 0 | n/a | 0 | 2 stiffness (capsular release) |
| Shon [37] | 31 | 0 | 0 | n/a | n/r | n/r |
| Wellmann [38] | 38 | 0 | 0 | n/a | n/r | n/r |
Graft interposition
| Author and Date | Number of shoulders in graft group for which f/u data available | No. of revision procedures | Post-op imaging performed—no. of patients | Number of retears on imaging | Infections | Additional complications |
|---|---|---|---|---|---|---|
| Audernaert [39] | 39 | 0 | 39 | 4 | 0 | 0 |
| Badhe [40] | 10 | 0 | 10 | 2 | 0 | 0 |
| Bond [41] | 16 | 0 | 16 | 3 | 0 | 0 |
| Dukan [42] | 23 | 5 | 18 | 9 | 0 | 2 intraop partial glenoid fractures |
| Gupta [43] | 24 | 0 | 19 | 5 | 0 | 0 |
| Gupta [44] | 27 | 0 | 22 | 6 | 0 | 0 |
| Kim [45] | 24 | 0 | 24 | 5 | 0 | 0 |
| Kokkalis [46] | 21 | 0 | 0 | n/a | 0 | 0 |
| Modi [47] | 61 | 1 | 14 | 2 | 1 (washout) | 0 |
| Mori [31] | 24 | 0 | 24 | 5 | 0 | 0 |
| Nada [48] | 21 | 1 | 21 | 0 | 0 | 0 |
| Pandey [33] | 13 | 0 | 13 | 1 | 0 | 0 |
| Petrie [49] | 31 | 2 | 0 | n/a | 0 | 0 |
| Rhee [50] | 31 | 1 | 14 | 5 | 0 | 0 |
| Rhee [51] | 24 | 0 | 24 | 13 | n/r | n/r |
| Sano [52] | 14 | 0 | 14 | 1 | 0 | 0 |
| Dimitrios [53] | 68 | 0 | 30 | 3 | 0 | 0 |
| Wong [54] | 45 | 0 | 0 | n/a | 1 (washout) | 0 |
Superior capsule reconstruction
| Author and Date | Number of shoulders in SCR group for which f/u data available | No. of revision procedures | Post-op imaging performed—no. of patients | Number of retears on imaging | Infections | Additional complications |
|---|---|---|---|---|---|---|
| Alarcon [55] | 31 | 0 | 29 | 4 | 0 | 1 haematoma |
| Barth [56] | 24 | 0 | 24 | 2 | n/r | n/r |
| Burkhart [57] | 41 | 2 (post-falls) | 26 | 4 | 0 |
1 stroke 1 tear biceps tenodesis |
| Denard [58] | 59 | 0 | 20 | 11 | 1 | 1 biceps pain |
| Ferrando [59] | 56 | 4 | 56 | 14 | n/r | n/r |
| Greiner [23] | 20 | 0 | 20 | 1 | n/r | n/r |
| Kim [60] | 45 | 0 | 45 | 6 | 0 | 1 skin dehiscence, 2 Popeye sign |
| Kocaoglu [61] | 26 | 0 | 12 | 2 | n/r | n/r |
| LaBelle [62] | 35 | 4 | 21 | 13 | n/r | n/r |
| Lacheta [63] | 22 | 1 | 21 | 5 | n/r | n/r |
| Lee and Min [64] | 36 | 0 | 36 | 13 | n/r | n/r |
| Lim [65] | 31 | 0 | 31 | 9 | 0 | 1 PE |
| Mihata [5] | 24 | 0 | 24 | 4 | 0 | 0 |
| Okamura [66] | 35 | 0 | 35 | 2 | 0 | 1 synovitis |
| Ohta [67] | 35 | 1 | 35 | 7 | 0 |
1 severe synovitis 1 anchor dislodgement |
| Ozturk [68] | 20 | 0 | 20 | 1 | 0 | 1 significant stiffness |
| Pashuck [69] | 14 | 1 | 14 | 2 | 0 | 0 |
| Pennington [70] | 88 | 1 | Only with Sx | - | n/r | n/r |
| Polacek [71] | 20 | 5 | Only with Sx | - | 0 | 3 acute graft rejection |
| Polacek [72] | 24 | 3 incl. 1 fascia lata site revision | Only with Sx | - | 0 | 1 fascia lata muscle prolapse. 1 haematoma |
| Takayama [73] | 46 | 0 | Only with Sx | - |
1 swelling at donor site 1 shoulder swelling |
Debridement
| Author and Date | Number of shoulders in debridement group for which f/u data available | No. of revision procedures | Infections | Additional complications |
|---|---|---|---|---|
| Berth [14] | 21 | 1 | 0 | 0 |
| Blanke [74] | 59 | 0 | 0 | 0 |
| Boileau [75] | 72 | 3 | 1 (washout) | 2 persistent pain. 1 shoulder stiffness, 2 GHJ OA, 3 pseudo paralysis |
| Cavalier [16] | 26 | 0 | 0 | 0 |
| Franceschi [21] | 34 | n/r | n/r | n/r |
| Heuberer [24] | 23 | 1 | 0 | 0 |
| Klinger [76] | 41 | 0 | 0 | 3 pseudoparalysis 2 pain 1 stiffness. 2 GHJ OA. 1 humeral head migration |
| Lee [77] | 32 | 4 | n/r | n/r |
| Mirzaee [78] | 12 | 0 | 0 | 2 GHJ OA |
| Park [79] | 16 | 1 | 0 | 0 |
| Scheibel [80] | 23 | 1 | 0 | 1 haematoma |
| Vad [81] | 32 | 0 | n/r | n/r |
| Veado [82] | 15 | 0 | n/r | n/r |
| Verhelst [83] | 32 | 2 | n/r | n/r |
Balloon spacer
| Author and Date | Number of shoulders in balloon spacer group for which f/u data available | No. of revision procedures | Infections | Additional complications |
|---|---|---|---|---|
| Basat [84] | 12 | 0 | n/r | n/r |
| Bakti [85] | 26 | 1 | 0 | 0 |
| Deranlot [86] | 39 | 1 | 0 | 0 |
| Familiari [87] | 51 | 6 | 0 | 0 |
| Gervasi [88] | 15 | 1 | 0 | 0 |
| Gervasi [89] | 40 | 3 | 0 | 0 |
| Iban [90] | 16 | 5 | 0 | 0 |
| Lorente 2017 [91] | 15 | 3 | n/r | n/r |
| Maman [92] | 42 | 0 | n/r | n/r |
| Senekovic [93] | 20 | 0 | 0 | 0 |
Risk of bias
All arms of relevant non-comparative and comparative studies were treated as individual series. The risk-of-bias results for the abbreviated 7-point MINORS instrument for non-comparative studies were applied to all articles and presented here. The results of the extended MINORS instrument for comparative studies are presented in the final section of this document.
PCR
| Author and Date | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size |
|---|---|---|---|---|---|---|---|
| Baverel [13] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Berth [14] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Besnard [15] | 2 | 2 | 1 | 2 | 2 | 0 | 0 |
| Cavalier [16] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Chen [17] | 2 | 1 | 1 | 2 | 2 | 1 | 0 |
| Cuff [18] | 2 | 1 | 1 | 2 | 2 | 1 | 0 |
| DiBenedetto [19] | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| Farazdaghi [20] | 2 | 1 | 1 | 2 | 2 | 1 | 2 |
| Franceschi [21] | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Galasso [22] | 2 | 2 | 2 | 2 | 2 | 0 | 0 |
| Greiner [23] | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Heuberer [24] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Holtby [25] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Jeong [26] | 2 | 1 | 1 | 2 | 2 | 1 | 0 |
| Kim [27] | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| Kim [28] | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| Lee [29] | 2 | 1 | 1 | 2 | 2 | 0 | 0 |
| Malahias [30] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Mori [31] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Moser [32] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Pandey [33] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Park [34] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Paribelli [35] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Porcellini [36] | 2 | 2 | 2 | 2 | 2 | 1 | 0 |
| Shon [37] | 2 | 1 | 1 | 2 | 2 | 2 | 0 |
| Wellmann [38] | 2 | 1 | 1 | 2 | 2 | 2 | 0 |
Graft interposition
| Author and Date | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size |
|---|---|---|---|---|---|---|---|
| Audernaert [39] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Badhe [40] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Bond [41] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Dukan [42] | 2 | 2 | 2 | 2 | 2 | 1 | 0 |
| Gupta [43] | 1 | 2 | 2 | 2 | 2 | 2 | 0 |
| Gupta [44] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Kim [45] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Kokkalis [46] | 1 | 2 | 1 | 2 | 2 | 2 | 0 |
| Modi [47] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Mori [31] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Nada [48] | 1 | 0 | 2 | 2 | 2 | 2 | 0 |
| Pandey [33] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Petrie [49] | 2 | 2 | 2 | 2 | 2 | 1 | 0 |
| Rhee [50] | 2 | 1 | 2 | 2 | 2 | 1 | 0 |
| Rhee [51] | 1 | 2 | 2 | 2 | 2 | 2 | 0 |
| Sano [52] | 2 | 1 | 0 | 2 | 2 | 2 | 0 |
| Varvitsiotis [53] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Wong [54] | 1 | 2 | 2 | 2 | 2 | 2 | 0 |
Superior capsule reconstruction
| Author and Date | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size |
|---|---|---|---|---|---|---|---|
| Alarcon [55] | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| Barth [56] | 2 | 2 | 1 | 2 | 2 | 1 | 0 |
| Burkhart [57] | 2 | 2 | 1 | 2 | 2 | 1 | 0 |
| Denard [58] | 2 | 1 | 2 | 2 | 2 | 1 | 0 |
| Ferrando [59] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Greiner [23] | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Kim [60] | 2 | 2 | 1 | 2 | 2 | 1 | 0 |
| Kocaoglu [61] | 2 | 2 | 1 | 2 | 2 | 2 | 0 |
| LaBelle [62] | 2 | 2 | 1 | 2 | 2 | 1 | 0 |
| Lacheta [63] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Lee and Min [64] | 2 | 2 | 1 | 2 | 2 | 1 | 0 |
| Lim [65] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Mihata [5] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Okamura [66] | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Ohta [67] | 2 | 1 | 1 | 2 | 2 | 1 | 0 |
| Ozturk [68] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Pashuck [69] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Pennington [70] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Polacek [71] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Polacek [72] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Takayama [73] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
Balloon spacer
| Author and Date | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size |
|---|---|---|---|---|---|---|---|
| Basat [84] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Batki [85] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Deranlot [86] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Familiari [87] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Gervasi [88] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Gervasi [89] | 2 | 1 | 2 | 2 | 2 | 1 | 0 |
| Iban [90] | 2 | 2 | 2 | 2 | 2 | 0 | 0 |
| Lorente [91] | 2 | 2 | 2 | 2 | 2 | 1 | 0 |
| Maman [92] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Senekovic [93] | 2 | 1 | 2 | 2 | 2 | 1 | 0 |
Debridement
| Author and Date | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size |
|---|---|---|---|---|---|---|---|
| Berth [14] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Blanke [74] | 2 | 1 | 1 | 2 | 2 | 2 | 0 |
| Boileau [75] | 2 | 1 | 1 | 2 | 2 | 2 | 0 |
| Cavalier [16] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Franceschi [21] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Heuberer [24] | 2 | 1 | 2 | 2 | 2 | 0 | 0 |
| Klinger [76] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Lee [77] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Mirzaee [78] | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Park [79] | 2 | 1 | 1 | 2 | 2 | 1 | 0 |
| Scheibel [80] | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Vad [81] | 2 | 0 | 0 | 2 | 2 | 0 | 0 |
| Veado [82] | 2 | 0 | 2 | 2 | 2 | 1 | 0 |
| Verhelst [83] | 2 | 1 | 2 | 2 | 2 | 1 | 0 |
Additional full risk-of-bias table for comparative studies
| Author and Date | Comparisons | Clearly state aim | Inclusion of consecutive patients | Prospective data collection | Unbiased endpoint assessment | Follow-up appropriate | Loss to f/u < 5% | Prospective calculation of sample size | Adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baverel [13] | PCR vs LDTT | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Berth [14] | PCR vs debridement | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Besnard [15] | PCR vs complete | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 2 |
| Cavalier [16] | PCR vs debridement | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 2 |
| Farazdaghi [20] | PCR vs complete | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 2 |
| Franceschi [21] | PCR vs debridement | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Greiner [23] | PCR vs SCR | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Heuberer [24] | PCR vs debridement | 2 | 1 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 2 |
| Holtby [25] | PCR vs complete | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 2 |
| Jeong [26] | PCR vs complete | 2 | 1 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 1 | 2 |
| Kim [28] | PCR vs complete | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Malahias [30] | PCR vs PCR and balloon | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 |
| Mori [31] | PCR vs graft interposition | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Moser [32] 2007 | PCR vs complete vs debridement | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Pandey [33] | PCR vs graft interposition | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 |
| Park [34] | PCR vs augmentation | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Paribelli [35] | PCR vs LDTT | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Barth [56] | SCR vs complete vs augmentation | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 |
| Kocaoglu [61] | SCR vs SCR and PCR | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
| Okamura [66] | SCR 1 layer graft vs 3 layers | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Ozturk [68] | SCR vs LDTT | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 |
Search strategy
Rotator cuff/
Rotator Cuff Injuries/
1 or 2
(massive or large or irreparable).ti,ab.
3 and 4
((massive or large or irreparable) adj3 (rotator cuff* adj3 (tear* or rupture* or injur*))).ti,ab.
((massive or large or irreparable) adj3 (supraspinatus adj3 (tear* or rupture* or injur*))).ti,ab.
((massive or large or irreparable) adj3 (infraspinatus adj3 (tear* or rupture* or injur*))).ti,ab.
((massive or large or irreparable) adj3 (subscapularis adj3 (tear* or rupture* or injur*))).ti,ab.
((massive or large or irreparable) adj3 (teres minor adj3 (tear* or rupture* or injur*))).ti,ab.
((massive or large or irreparable) adj3 (posterosuperior adj3 (tear* or rupture* or injur*))).ti,ab.
6 or 7 or 8 or 9 or 10 or 11
5 or 12
Arthroscopy/
Debridement/
arthroscop*.ti,ab.
debridement*.ti,ab.
Superior capsul* reconstruction*.ti,ab.
capsul* reconstruction*.ti,ab.
Reconstructive Surgical Procedures/
Reconstruct*.ti,ab.
Repair*.ti,ab.
Tenotomy/
Tenodesis/
tenotom*.ti,ab.
tenodesis.ti,ab.
partial*.ti,ab.
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
balloon*.ti,ab.
spacer*.ti,ab.
29 or 30
graft*.ti,ab.
graftjacket.ti,ab.
patch*.ti,ab.
allografts/
autografts/
surgical mesh/
bioprosthesis/
tissue scaffolds/
extracellular matrix/
acellular dermis/
allograft*.ti,ab.
autograft*.ti,ab.
surgical mesh.ti,ab.
bioprosthe*.ti,ab.
tissue scaffold*.ti,ab.
extracellular matrix.ti,ab.
acellular dermal matrix.ti,ab.
bioartificial tendon*.ti,ab.
augment*.ti,ab.
"Zimmer Collagen Repair Patch".ti,ab.
"Permacol".ti,ab.
"TissueMend".ti,ab.
"BioBlanket".ti,ab.
"Conexa".ti,ab.
"AlloPatch".ti,ab.
"Shelhigh Encuff Patch".ti,ab.
"OrthADAPT".ti,ab.
"Restore".ti,ab.
"CuffPatch".ti,ab.
"Polytape".ti,ab.
"SportMesh".ti,ab.
"Arthelon".ti,ab.
"Gore-tex".ti,ab.
"BioFiber".ti,ab.
"STR Grafts".ti,ab.
"Lars Ligament".ti,ab.
"X-repair".ti,ab.
32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68
13 and 28
13 and 31
13 and 69
70 or 71 or 72
exp animals/ not humans.sh.
73 not 7472 13 and 69
73 70 or 71 or 72
74 exp animals/ not humans.sh.
75 73 not 74
Acknowledgements
Not applicable.
Abbreviations
- CINAHL
Cumulative index to nursing and allied health literature
- CT
Computed tomography
- MRA
Magnetic resonance arthrogram
- PCR
Partial cuff repair
- SCR
Superior capsule reconstruction
- PROM
Patient-reported outcome measures
- PROSPERO
International prospective register of systematic reviews
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SLAP
Superior labral tear from anterior to posterior
- ASES
American Shoulder and Elbow Score
- OSS
Oxford Shoulder Score
- UCLA
University of California in Los Angeles Shoulder Score
- DASH
Disabilities of arm, shoulder and hand score
- JOA
Japanese Orthopaedic Association Shoulder Score
- MINORS
Methodological index for non-randomized studies
- SMD
Standardised mean difference
- MRI
Magnetic resonance imaging
- LDTT
Latissimus dorsi tendon transfer
- QuickDASH
Disabilities of the arm shoulder and hand score
- SSV
Subjective shoulder value
- SST
Simple shoulder test
- SPADI
Shoulder pain and disability index
- UCLA
The University of California at Los Angeles shoulder score
- KSS
Korean Shoulder Score
- SD
Standard deviation
- US
Ultrasound
- VAS
Visual analogue scale
- RSA
Reverse shoulder arthroplasty
Author’s contributions
AD developed the research question, performed the study screening, extracted the data, analysed the data and wrote the review. PS performed the study screening, extracted the data, analysed the data and wrote the review. PR developed the research question and edited the manuscript. SS performed the study screening, analysed the data and edited the manuscript. AM developed the research question and edited the manuscript. All authors read and approved the final manuscript.
Funding
No funding was required in the construction of this review.
Availability of data and materials
Further data are provided in the Appendix and full datasets can be provided by the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
According to the United Kingdom Health Research Authority, ethical approval was not required for this systematic review.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Linsell L, Dawson J, Zondervan K, Rose P, Randall T, Fitzpatrick R, et al. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology. 2006;45(2):215–221. doi: 10.1093/rheumatology/kei139. [DOI] [PubMed] [Google Scholar]
- 2.Woodmass JM, Wagner ER, Chang MJ, Welp KM, Elhassan BT, Higgins LD, et al. Arthroscopic treatment of massive posterosuperior rotator cuff tears. JBJS Rev. 2018;6(9):e3. doi: 10.2106/JBJS.RVW.17.00199. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clin Orthop Relat Res. 1991;267:45–56. doi: 10.1097/00003086-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 5.Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 2013;29(3):459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Catapano M, de SA D, Ekhtiari S, Lin A, Bedi A, Lesniak BP. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff tears: a systematic review of modern literature. J Arthrosc Jt Surg. 2019;35(4):1243–1253. doi: 10.1016/j.arthro.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Omid R, Lee B. Tendon transfers for irreparable rotator cuff tears. J Am Acad Orthop Surg. 2013;21(8):492–501. doi: 10.5435/JAAOS-21-08-492. [DOI] [PubMed] [Google Scholar]
- 8.Castagna A, Garofalo R, Cesari E. No prosthetic management of massive and irreparable rotator cuff tears. Shoulder Elb. 2014;6(3):147–155. doi: 10.1177/1758573214535369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ek ETH, Neukom L, Catanzaro S, Gerber C, Hon F. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old : results after five to fifteen years. J Shoulder Elb Surg. 2013;22(9):1199–1208. doi: 10.1016/j.jse.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J, Li T, Deeks J. Chapter 6: Choosing effect measures and computing estimates of effect. In: cochrane handbook for systematic reviews of interventions. 2021. p Section 6-5-2-2.
- 13.Baverel LP, Bonnevialle N, Joudet T, Valenti P, Kany J, Grimberg J, et al. Short-term outcomes of arthroscopic partial repair vs. latissimus dorsi tendon transfer in patients with massive and partially repairable rotator cuff tears. J Shoulder Elb Surg. 2021;30(2):282–289. doi: 10.1016/j.jse.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Berth A, Neumann W, Awiszus F, Pap G. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11(1):13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besnard M, Freychet B, Clechet J, Hannink G, Saffarini M, Carrillon Y, et al. Partial and complete repairs of massive rotator cuff tears maintain similar long-term improvements in clinical scores. Knee Surg Sport Traumatol Arthrosc. 2021;29(1):181–191. doi: 10.1007/s00167-020-05907-8. [DOI] [PubMed] [Google Scholar]
- 16.Cavalier M, Jullion S, Kany J, Grimberg J, Lefebvre Y, Oudet D, et al. Management of massive rotator cuff tears: prospective study in 218 patients. Orthop Traumatol Surg Res. 2018;104(8):S193–S197. doi: 10.1016/j.otsr.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen KH, Chiang ER, Wang HY, Ma HL. Arthroscopic partial repair of irreparable rotator cuff tears: factors related to greater degree of clinical improvement at 2 years of follow-up. Arthrosc J Arthrosc Relat Surg. 2017;33(11):1949–1955. doi: 10.1016/j.arthro.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Cuff DJ, Pupello DR, Santoni BG. Partial rotator cuff repair and biceps tenotomy for the treatment of patients with massive cuff tears and retained overhead elevation: midterm outcomes with a minimum 5 years of follow-up. J Shoulder Elb Surg. 2016;25(11):1803–1809. doi: 10.1016/j.jse.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Di Benedetto ED, Di Benedetto P, Fiocchi A, Beltrame A, Causero A. Partial repair in irreparable rotator cuff tear: our experience in long-term follow-up. Acta Biomed. 2017;88(4S):69–74. doi: 10.23750/abm.v88i4-S.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farazdaghi A, Paschos NK, Kelly JD. Comparison between partial and full coverage repair in massive rotator cuff tears. A minimum five year follow-up. Orthop Traumatol Surg Res. 2021;107(4):102911. doi: 10.1016/j.otsr.2021.102911. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi F, Papalia R, Vasta S, Leonardi F, Maffulli N, Denaro V. Surgical management of irreparable rotator cuff tears. Knee Surg Sports Traumatol Arthrosc. 2015;23(2):494–501. doi: 10.1007/s00167-012-2317-7. [DOI] [PubMed] [Google Scholar]
- 22.Galasso O, Riccelli DA, De Gori M, De Benedetto M, Orlando N, Gasparini G, et al. Quality of life and functional results of arthroscopic partial repair of irreparable rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 2017;33(2):261–268. doi: 10.1016/j.arthro.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Greiner S, Kaeaeb M, Voss A, Lawton R, Bhide P, Achenbach L. Comparison of superior capsular reconstruction and partial infraspinatus repair: a matched-pair analysis of irreparable rotator cuff tears. Orthop J Sport Med. 2021;9(2):1–8. doi: 10.1177/2325967120984264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuberer PR, Kolblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A, et al. Arthroscopic management of massive rotator cuff tears: an evaluation of debridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3828–3837. doi: 10.1007/s00167-015-3739-9. [DOI] [PubMed] [Google Scholar]
- 25.Holtby R, Razmjou H. Relationship between clinical and surgical findings and reparability of large and massive rotator cuff tears: a longitudinal study. BMC Musculoskelet Disord. 2014;15:180. doi: 10.1186/1471-2474-15-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong J, Kim S, Yoon T, Eum K, Chun Y. Arthroscopic repair of large and massive rotator cuff tears. J Bone Jt Surg Am. 2020;15(102):1248–1254. doi: 10.2106/JBJS.19.01014. [DOI] [PubMed] [Google Scholar]
- 27.Kim S-J, Lee I-S, Kim S-H, Lee W-Y, Chun Y-M. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761–768. doi: 10.1016/j.arthro.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Kim SJ, Kim SH, Lee SK, Seo JW, Chun YM. Arthroscopic repair of massive contracted rotator cuff tears: aggressive release with anterior and posterior interval slides do not improve cuff healing and integrity. J Bone Jt Surg Ser A. 2013;95(16):1482–1488. doi: 10.2106/JBJS.L.01193. [DOI] [PubMed] [Google Scholar]
- 29.Lee KW, Lee GS, Yang DS, Park SH, Chun YS, Choy WS. Clinical outcome of arthroscopic partial repair of large to massive posterosuperior rotator cuff tears: medialization of the attachment site of the rotator cuff tendon. CiOS Clin Orthop Surg. 2020;12(3):353–363. doi: 10.4055/cios19126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malahias MA, Brilakis E, Avramidis G, Trellopoulos A, Antonogiannakis E. Arthroscopic partial repair with versus without biodegradable subacromial spacer for patients with massive rotator cuff tears: a case–control study. Musculoskelet Surg. 2020 doi: 10.1007/s12306-020-00649-9. [DOI] [PubMed] [Google Scholar]
- 31.Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthrosc J Arthrosc Relat Surg. 2013;29(12):1911–1921. doi: 10.1016/j.arthro.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Moser M, Jablonski MV, Horodyski MB, Wright TW. Functional outcome of surgically treated massive rotator cuff tears: a comparison of complete repair, partial repair, and debridement. Orthopedics. 2007;30(6):479–482. doi: 10.3928/01477447-20070601-05. [DOI] [PubMed] [Google Scholar]
- 33.Pandey R, Tafazal S, Shyamsundar S, Modi A, Singh HP. Outcome of partial repair of massive rotator cuff tears with and without human tissue allograft bridging repair. Shoulder Elb. 2017;9(1):23–30. doi: 10.1177/1758573216665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SR, Sun DH, Kim J, Lee HJ, Bin KJ, Kim YS. Is augmentation with the long head of the biceps tendon helpful in arthroscopic treatment of irreparable rotator cuff tears? J Shoulder Elb Surg. 2018;27(11):1969–1977. doi: 10.1016/j.jse.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Paribelli G, Boschi S, Randelli P, Compagnoni R, Leonardi F, Cassarino AM. Clinical outcome of latissimus dorsi tendon transfer and partial cuff repair in irreparable postero-superior rotator cuff tear. Musculoskelet Surg. 2015;99(2):127–132. doi: 10.1007/s12306-015-0353-4. [DOI] [PubMed] [Google Scholar]
- 36.Porcellini G, Castagna A, Cesari E, Merolla G, Pellegrini A, Paladini P. Partial repair of irreparable supraspinatus tendon tears: Clinical and radiographic evaluations at long-term follow-up. J Shoulder Elb Surg. 2011;20(7):1170–1177. doi: 10.1016/j.jse.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Shon MS, Koh KH, Lim TK, Kim WJ, Kim KC, Yoo JC. Arthroscopic partial repair of irreparable rotator cuff tears: preoperative factors associated with outcome deterioration over 2 years. Am J Sports Med. 2015;43(8):1965–1975. doi: 10.1177/0363546515585122. [DOI] [PubMed] [Google Scholar]
- 38.Wellmann M, Lichtenberg S, Da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 2013;29(8):1275–1282. doi: 10.1016/j.arthro.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Audenaert E, VanNuffel J, Schepens A, Verhelst M, Verdonk R. Reconstruction of massive rotator cuff lesions with a synthetic interposition graft: a prospective study of 41 patients. Knee Surg Sport Traumatol Arthrosc. 2006;14(4):360–364. doi: 10.1007/s00167-005-0689-7. [DOI] [PubMed] [Google Scholar]
- 40.Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elb Surg. 2008;17(1 SUPPL.):35–39. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a graftjacket allograft: technique and preliminary results. Arthrosc J Arthrosc Relat Surg. 2008;24(4):403.e1–403.e8. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 42.Dukan R, Bommier A, Rousseau MA, Boyer P. Superior capsule reconstruction for irreparable rotator cuff tear with a porcine dermal graft: preliminary results at 2 years minimum follow-up. Muscles Ligaments Tendons J. 2019;9(4):571–578. [Google Scholar]
- 43.Gupta AK, Hug K, Berkoff DJ, Boggess BR, Gavigan M, Malley PC, et al. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med. 2012;40(1):141–147. doi: 10.1177/0363546511422795. [DOI] [PubMed] [Google Scholar]
- 44.Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872–879. doi: 10.1177/0363546512475204. [DOI] [PubMed] [Google Scholar]
- 45.Kim JO, Lee JH, Kim KS, Ji JH, Koh SJ, Lee JH. Rotator cuff bridging repair using acellular dermal matrix in large to massive rotator cuff tears: histologic and clinical analysis. J Shoulder Elb Surg. 2017;26(11):1897–1907. doi: 10.1016/j.jse.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Kokkalis ZT, Mavrogenis AF, Scarlat M, Christodoulou M, Vottis C, Papagelopoulos PJ, et al. Human dermal allograft for massive rotator cuff tears. Orthopedics. 2014;37(12):e1108–e1116. doi: 10.3928/01477447-20141124-59. [DOI] [PubMed] [Google Scholar]
- 47.Modi A, Singh HP, Pandey R, Armstrong A. Management of irreparable rotator cuff tears with the graftjacket allograft as an interpositional graft. Shoulder Elb. 2013;5(3):188–194. doi: 10.1111/sae.12021. [DOI] [Google Scholar]
- 48.Nada AM, Debnath UK, Robinson DA, Jordan C. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J Bone Jt Surg Ser B. 2010;92(10):1397–1402. doi: 10.1302/0301-620X.92B10.24299. [DOI] [PubMed] [Google Scholar]
- 49.Petrie MJ, Ismaiel AH. Treatment of massive rotator-cuff tears with a polyester ligament (LARS) patch. Acta Orthop Belg. 2013;79(6):620–625. [PubMed] [Google Scholar]
- 50.Rhee YG, Nam SC, Chan TL, Jin WY, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511–1518. doi: 10.1177/0363546508316020. [DOI] [PubMed] [Google Scholar]
- 51.Rhee SM, Oh JH. Bridging graft in irreparable massive rotator cuff tears: autogenic biceps graft versus allogenic dermal patch graft. CiOS Clin Orthop Surg. 2017;9(4):497–505. doi: 10.4055/cios.2017.9.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. doi: 10.1007/s00776-010-1453-5. [DOI] [PubMed] [Google Scholar]
- 53.Dimitrios V, Athanasios P, Eleni A, Xenofon P, George F, John F. Results of reconstruction of massive irreparable rotator cuff tears using a fascia lata allograft. Indian J Orthop. 2015;49(3):304–311. doi: 10.4103/0019-5413.156202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elb Surg. 2010;19(2 SUPPL.):104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Alarcon J, Uribe-Echevarria B, Clares C, Apablaza D, Vargas J, Benavente S, et al. Superior capsular reconstruction with autologous fascia lata using single lateral row technique is an effective option in massive irreparable rotator cuff tears. Minimum two-year follow-up. Sci Total Environ. 2021;142972. [DOI] [PubMed]
- 56.Barth J, Olmos MI, Swan J, Barthelemy R, Delsol P, Boutsiadis A. Superior capsular reconstruction with the long head of the biceps autograft prevents infraspinatus retear in massive posterosuperior retracted rotator cuff tears. Am J Sports Med. 2020;48(6):1430–1438. doi: 10.1177/0363546520912220. [DOI] [PubMed] [Google Scholar]
- 57.Burkhart SS, Pranckun JJ, Hartzler RU. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: clinical outcomes are maintained 2 years after surgery. Arthrosc - J Arthrosc Relat Surg. 2020;36(2):373–380. doi: 10.1016/j.arthro.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 58.Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthrosc J Arthrosc Relat Surg. 2018;34(1):93–99. doi: 10.1016/j.arthro.2017.08.265. [DOI] [PubMed] [Google Scholar]
- 59.Ferrando A, Kingston R, Delaney RA. Superior capsular reconstruction using a porcine dermal xenograft for irreparable rotator cuff tears: outcomes at minimum two-year follow-up. J Shoulder Elb Surg. 2021;30(5):1053–1059. doi: 10.1016/j.jse.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 60.Kim D, Jaewoong U, Lee J, Kim J. Improved clinical and radiographic outcomes seen after superior capsule reconstruction using long head biceps tendon allograft. Arthrosc J Arthrosc Relat Surg. 2021;37(9):2756–2767. doi: 10.1016/j.arthro.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Kocaoglu B, Firatli G, Ulku TK. Partial rotator cuff repair with superior capsular reconstruction using the biceps tendon is as effective as superior capsular reconstruction using a tensor fasciae latae autograft in the treatment of irreparable massive rotator cuff tears. Orthop J Sport Med. 2020;8(6):1–7. doi: 10.1177/2325967120922526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LaBelle MW, Mengers S, Strony J, Peck M, Flannery R, Cupp S, et al. Evaluating the role of graft integrity on outcomes: clinical and imaging results following superior capsular reconstruction. J Shoulder Elbow Surg. 2021;30(9):2041–2047. doi: 10.1016/j.jse.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 63.Lacheta L, Horan MP, Schairer WW, Goldenberg BT, Dornan GJ, Pogorzelski J, et al. Clinical and imaging outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears: a minimum 2-year follow-up. Arthrosc - J Arthrosc Relat Surg. 2020;36(4):1011–1019. doi: 10.1016/j.arthro.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 64.Lee SJ, Min YK. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? Preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018 doi: 10.1007/s00167-018-4912-8. [DOI] [PubMed] [Google Scholar]
- 65.Lim S, AlRamadhan H, Kwak JM, Hong H, Jeon IH. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome. Arch Orthop Trauma Surg. 2019;139(2):231–239. doi: 10.1007/s00402-018-3025-7. [DOI] [PubMed] [Google Scholar]
- 66.Okamura K, Abe M, Yamada Y, Makihara T, Yoshimizu T, Sakaki Y, et al. Arthroscopic superior capsule reconstruction with Teflon felt synthetic graft for irreparable massive rotator cuff tears: clinical and radiographic results at minimum 2-year follow-up. J Shoulder Elb Surg [Internet] 2021;30(3):625–634. doi: 10.1016/j.jse.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 67.Ohta S, Komai O, Onochi Y. Outcomes of superior capsule reconstruction for massive rotator cuff tears and risk factors for postoperative retear. Arch Orthop Trauma Surg. 2020;140(10):1319–1325. doi: 10.1007/s00402-019-03316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozturk BY, Ak S, Gultekin O, Baykus A, Kulduk A. Prospective, randomized evaluation of latissimus dorsi transfer and superior capsular reconstruction in massive, irreparable rotator cuff tears. J Shoulder Elb Surg. 2021;30(7):1561–1571. doi: 10.1016/j.jse.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 69.Pashuck TD, Hirahara AM, Cook JL, Cook CR, Andersen WJ, Smith MJ. Superior capsular reconstruction using dermal allograft is a safe and effective treatment for massive irreparable rotator cuff tears: 2-year clinical outcomes. Arthrosc J Arthrosc Relat Surg. 2021;37(2):489–496.e1. doi: 10.1016/j.arthro.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34(6):1764–1773. doi: 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Polacek M. Arthroscopic superior capsular reconstruction with acellular porcine dermal xenograft for the treatment of massive irreparable rotator cuff tears. Arthrosc Sport Med Rehabil. 2019;1(1):e75–84. doi: 10.1016/j.asmr.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polacek M, Nyegaard CP. Superior capsular reconstruction using 3-layered fascia lata autograft reinforced with a nonresorbable suture mesh. Arthrosc Sport Med Rehabil. 2020;2(5):e489–e497. doi: 10.1016/j.asmr.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takayama K, Yamada S, Kobori Y. Clinical effectiveness of mini-open superior capsular reconstruction using autologous tensor fascia lata graft. J Shoulder Elb Surg. 2021;30(6):1344–1355. doi: 10.1016/j.jse.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Blanke F, Sachser J, Majewski M. Operative techniques for treatment of chronic massive rotator cuff lesions: deltoid flap transfer versus arthroscopic debridement. Acta Orthop Belg. 2017;83(3):428–432. [PubMed] [Google Scholar]
- 75.Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747–757. doi: 10.2106/00004623-200704000-00008. [DOI] [PubMed] [Google Scholar]
- 76.Klinger HM, Steckel H, Ernstberger T, Baums MH. Arthroscopic debridement of massive rotator cuff tears: negative prognostic factors. Arch Orthop Trauma Surg. 2005;125(4):261–266. doi: 10.1007/s00402-004-0738-6. [DOI] [PubMed] [Google Scholar]
- 77.Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthrosc J Arthrosc Relat Surg. 2011;27(10):1341–1350. doi: 10.1016/j.arthro.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 78.Mirzaee F, Aslani M, Zafarani Z, Aslani H. Treatment of massive irreparable rotator cuff tear. Arch Bone Jt Surg. 2019;7(3):263–268. [PMC free article] [PubMed] [Google Scholar]
- 79.Park JG, Cho NS, Song JH, Baek JH, Rhee YG. Long-term outcome of tuberoplasty for irreparable massive rotator cuff tears: Is tuberoplasty really applicable? J shoulder Elb Surg. 2016;25(2):224–231. doi: 10.1016/j.jse.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 80.Scheibel M, Lichtenberg S, Habermeyer P. Reversed arthroscopic subacromial decompression for massive rotator cuff tears. J Shoulder Elb Surg. 2004;13(3):272–278. doi: 10.1016/j.jse.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 81.Vad VB, Warren RF, Altchek DW, O’Brien SJ, Rose HA, Wickiewicz TL. Negative prognostic factors in managing massive rotator cuff tears. Clin J Sport Med. 2002;12(3):151–157. doi: 10.1097/00042752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 82.de Castro Veado MA, Rodrigues AU. Functional evaluation of patients who have undergone arthroscopic debridement to treat massive and irreparable tears of the rotator cuff. Rev Bras Ortop (English Ed.) 2010;45(5):426–431. doi: 10.1016/S2255-4971(15)30431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verhelst L, Vandekerckhove PJ, Sergeant G, Liekens K, Van Hoonacker P, Berghs B. Reversed arthroscopic subacromial decompression for symptomatic irreparable rotator cuff tears: mid-term follow-up results in 34 shoulders. J Shoulder Elb Surg. 2010;19(4):601–608. doi: 10.1016/j.jse.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Basat H, Kirçil C, Armangil M, Demirtş M. Treatment alternative for irreparable rotator cuff ruptures: arthroscopic biodegradable balloon. Niger J Clin Pract. 2017;20(8):952–957. doi: 10.4103/1119-3077.196061. [DOI] [PubMed] [Google Scholar]
- 85.Bakti N, Bhat M, Gulihar A, Prasad V, Singh B, Princess O, et al. Subacromial balloon interpositional arthroplasty for the management of irreparable rotator cuff tears : five-year results. Open Orthop J. 2019;13(1):89–96. doi: 10.2174/1874325001913010089. [DOI] [Google Scholar]
- 86.Deranlot J, Herisson O, Nourissat G, Zibili D, Werthel JD, Vigan M, et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospective cases. Arthrosc J Arthrosc Relat Surg. 2017;33(9):1–6. doi: 10.1016/j.arthro.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 87.Familiari F, Nayar SK, Russo R, De Gori M, Ranuccio F, Mastroianni V, et al. Subacromial balloon spacer for massive, irreparable rotator cuff tears is associated with improved shoulder function and high patient satisfaction. Arthrosc J Arthrosc Relat Surg. 2021;37(2):480–486. doi: 10.1016/j.arthro.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 88.Gervasi E, Maman E, Dekel A, Cautero E. Fluoroscopy-guided biodegradable spacer implantation using local anesthesia: safety and efficacy study in patients with massive rotator cuff tears. Musculoskelet Surg. 2016;100:19–24. doi: 10.1007/s12306-016-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gervasi E, Maman E, Dekel A, Markovitz E, Cautero E. Fluoroscopically guided subacromial spacer implantation for massive rotator cuff tears: two years of prospective follow-up. Orthop J Sport Med. 2021;9(4):2325967121993469. doi: 10.1177/2325967121993469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruiz Ibán MA, Lorente Moreno R, Ruiz Díaz R, Álvarez Sciamanna R, Paniagua Gonzalez A, Lorente Gómez A, et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sport Traumatol Arthrosc. 2018;26(12):3848–3854. doi: 10.1007/s00167-018-5083-3. [DOI] [PubMed] [Google Scholar]
- 91.Lorente Gómez A, Ruiz Ibán MÁ, Ruiz Díaz R, Vega Rodríguez RM, Álvarez R, Paniagua A, et al. Malos resultados a corto plazo del balón subacromial InSpace®. Resultados de 15 casos consecutivos con un año de seguimiento. Rev Española Artrosc y Cirugía Articul. 2017;24(3):197–203. [Google Scholar]
- 92.Maman E, Safran O, Beyth S, Mozes G, Dekel A, Michael B. Biceps tenotomy does not affect the functional outcomes of patients treated with spacer implantation due to massive irreparable rotator cuff tears. Open Orthop J. 2017 doi: 10.2174/1874325001711011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Dekel A. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol. 2013;23(3):311–316. doi: 10.1007/s00590-012-0981-4. [DOI] [PubMed] [Google Scholar]
- 94.Metcalfe A, Parsons H, Parsons N, Brown J, Fox J, Gemperlé Mannion E, et al. Subacromial balloon spacer for irreparable rotator cuff tears of the shoulder (START:REACTS): a group-sequential, double-blind, multicentre randomised controlled trial. Lancet. 2022;399(10339):1954–1963. doi: 10.1016/S0140-6736(22)00652-3. [DOI] [PubMed] [Google Scholar]
- 95.Verma NN, Srikumaran U, Roden C, Rogusky E, Lapner P, Heather N, Abboud J. Inspace implant compared with partial repair for the treatment of full-thickness massive rotator cuff tears. J Bone Jt Surg. 2022;104(14):1250–1262. doi: 10.2106/JBJS.21.00667. [DOI] [PubMed] [Google Scholar]
- 96.Sevivas N, Ferreira N, Andrade R, Moreira P, Alves D. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears : a systematic review with meta-analysis and meta-regression. J Shoulder Elb Surg. 2017;26(9):e265–e277. doi: 10.1016/j.jse.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 97.Ernstbrunner L, Andronic O, Grubhofer F, Camenzind RS, Wieser K, Gerber C. Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: A systematic review of longitudinal outcomes. J Shoulder Elb Surg. 2019;28(4):774–781. doi: 10.1016/j.jse.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Kääb MJ, Kohut G, Irlenbusch U, Joudet T, Reuther F. Reverse total shoulder arthroplasty in massive rotator cuff tears: Does the Hamada classification predict clinical outcomes? Arch Orthop Trauma Surg. 2021 doi: 10.1007/s00402-609021-03755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viswanath A, Bale S, Trail I. Reverse total shoulder arthroplasty for irreparable rotator cuff tears without arthritis: a systematic review. J Clin Orthop Trauma. 2021;17:267–272. doi: 10.1016/j.jcot.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reed M, Brittain R, Howard P, Laurence S, Stonadge J, Wilkinson M et al. 16th Annual Report. The National Joint Registry 18th Annual Report 2021. London: National Joint Registry; 2021 Sep.
- 101.Adam JR, Nanjayan SKT, Johnson M, Rangan A. Tendon transfers for irreparable rotator cuff tears. J Clin Orthop Trauma. 2021;17:254–260. doi: 10.1016/j.jcot.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clark NJ, Elhassan BT. The role of tendon transfers for irreparable rotator cuff tears. Curr Rev Musculoskelet Med. 2018;11(1):141–149. doi: 10.1007/s12178-018-9468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Jt Surg Ser A. 2013;95(21):1920–1926. doi: 10.2106/JBJS.M.00122. [DOI] [PubMed] [Google Scholar]
- 104.Kovacevic D, Suriani RJ, Grawe BM, Yian EH, Gilotra MN, Hasan SA, et al. Management of irreparable massive rotator cuff tears: a systematic review and meta-analysis of patient-reported outcomes, reoperation rates, and treatment response. J Shoulder Elb Surg. 2020;29(12):2459–2475. doi: 10.1016/j.jse.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kooistra B, Gurnani N, Weening A, van den Bekerom M, van Deurzen D. Low level of evidence for all treatment modalities for irreparable posterosuperior rotator cuff tears. Knee Surg Sport Traumatol Arthrosc. 2019;27(12):4038–4048. doi: 10.1007/s00167-019-05710-0. [DOI] [PubMed] [Google Scholar]
- 106.Levy O, Mullett H, Roberts S, Copeland S. The role of anterior deltoid reeducation in patients with massive irreparable degenerative rotator cuff tears. J Shoulder Elb Surg. 2008;17(6):863–870. doi: 10.1016/j.jse.2008.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further data are provided in the Appendix and full datasets can be provided by the corresponding author upon reasonable request.