Abstract
Trypanosoma cruzi currently infects 18 million people, and 30% of those infected develop a chronic inflammatory process that causes significant morbidity or mortality. The major histocompatibility complex class II (MHC-II)-restricted T-cell response is critical to the control of the infection and to the ensuing inflammatory pathology. The specific epitopes or major antigens of this response have not been identified. The parasite simultaneously expresses variant members of the trans-sialidase superfamily. To begin to analyze the MHC-II response to these variant proteins, the response to a single surface protein, SA85-1.1, was initiated. These studies have demonstrated that a biased gamma interferon (IFN-γ) response to the SA85-1.1 protein develops during T. cruzi infection. In addition, adoptive transfer of a CD4 clone that recognizes an SA85-1.1 epitope, named epitope 1, and immunization with a peptide encoding epitope 1 were protective and suggested that epitope 1 may be immunodominant. In this report IFN-γ intracellular staining demonstrated that splenocytes from acutely and chronically infected mice, incubated with SA85-1.1 protein or peptides that encode epitope 1, result in IFN-γ synthesis by 4 to 6% of the splenic CD4 cells. These data indicate that during T. cruzi infection epitope 1 is a major epitope and that 4 to 6% of the CD4 cells are stimulated by a single trans-sialidase superfamily epitope and suggest that a combination of trans-sialidase superfamily proteins combines to stimulate a majority of CD4 cells. These data suggest that during T. cruzi infection the CD4 response to the trans-sialidase superfamily is critical to the protective response and to the ensuing chronic inflammatory pathology.
Trypanosoma cruzi, an obligate intracellular parasite, is the causative agent of Chagas' disease. During the acute phase of the infection parasites replicate within cells and are easily detected in the bloodstream as they disseminate throughout the mammalian host. A lifelong chronic phase ensues in which parasites are difficult to detect, and debilitating inflammatory pathology develops in 30% of the infected individuals (25). The major histocompatibility complex class II (MHC-II) CD4 T-cell response contributes to the control of the acute infection and the ensuing chronic pathology; however, T. cruzi antigens that stimulate this critical CD4 T-cell response have not been identified (1, 5, 7, 19–22).
The T. cruzi trans-sialidase superfamily includes the large SA85-1 surface protein family and many other parasite surface proteins (2, 8–10). None of the proteins of the SA85-1 family and very few of the trans-sialidase superfamily proteins are trans-sialidases (4, 9). Why the parasite maintains and expresses proteins of the trans-sialidase superfamily is unknown. The SA85-1 surface proteins and many other trans-sialidase superfamily surface proteins are simultaneously expressed and shed from trypomastigotes, the extracellular mammalian stage parasites, making these proteins available to stimulate a robust MHC-II CD4 T-cell response (4, 8, 10, 11, 13, 23, 24). Each SA85-1 T-cell epitope, however, appears to be encoded as a variant or altered epitope by many other members of the superfamily, and this epitope variation may influence or inhibit the CD4 T-cell response (12). Therefore, during T. cruzi infection the investigation of the trans-sialidase superfamily-specific CD4 response was initiated (15).
To begin to investigate the trans-sialidase superfamily MHC-II CD4 response during T. cruzi infection, the response to the SA85-1.1 protein, a protein of this superfamily that was initially selected due to the robust antibody response it stimulates during the infection, was pursued (8, 10, 12, 15). These studies have demonstrated that during acute and chronic T. cruzi infection SA85-1.1-specific CD4 T cells expand; however, in in vitro assays these SA85-1.1-specific CD4 cells secrete gamma interferon (IFN-γ) but fail to proliferate (15). The failure of these cells to proliferate in vitro suggests that their function is compromised and may explain why these antigen-specific cells have not been previously identified by proliferation assays (15). T-cell epitopes that stimulate this SA85-1.1-specific IFN-γ response during T. cruzi infection have not been identified. Additional studies, however, have identified an epitope of the SA85-1.1 protein, named epitope 1, and demonstrated that adoptive transfer of epitope 1-specific Th1 clones or immunization with epitope 1 protects mice during T. cruzi infection (12, 15). These data suggest that epitope 1 is immunodominant during T. cruzi infection.
In this report the SA85-1.1-specific and epitope 1-specific responses during T. cruzi infection are further analyzed by IFN-γ intracellular staining. These data indicate that the SA85-1.1 protein or epitope 1 stimulates 4 to 6% of the splenic CD4 cells derived from T. cruzi-infected mice, demonstrating that the SA85-1.1 surface protein and epitope 1 are a major antigen and epitope. The possible ramifications of this robust response during T. cruzi infection are discussed.
MATERIALS AND METHODS
Mice.
C57BL/6 female mice (8 to 10 weeks old) were used (Bantin & Kingman, Fremont, Calif.).
Parasites.
T. cruzi CL strain subclone 3 trypomastigotes were obtained from culture supernatants of infected 3T3 cells grown in Dulbecco's modified Eagle medium (DMEM) (BioWhittaker, Walkersville, Md.) supplemented with 10% heat-inactivated calf serum (BioWhittaker) and 50,000 U of penicillin-streptomycin (BioWhittaker) (18). Each mouse received 105 trypomastigotes intraperitoneally in 200 μl of DMEM (15). In this report, the acute and chronic infections are defined as occurring before day 29 and after day 80, respectively.
Antigens.
The SA85-1.1 protein in these experiments is encoded by a 462-bp fragment expressed as a histidine fusion protein and purified with nickel chromatography columns (Novagen) (12). Glutathione S-transferase (GST) was expressed in Escherichia coli and purified as previously described (12). Peptides b, c, d, e, f, g, and h (Table 1) were synthesized on a Multiple Synthesizer (Gilson, Inc., Middleton, Wis.) (12).
TABLE 1.
Synthetic peptides used to stimulate splenocytes
| Protein or peptide |
Amino acid sequencea |
|---|---|
| SA85-1.1 | RHISLSHNFTLVASVIIEEAPSGNTPLLTAVLVDAG |
| b | RHISLSHNFTLVASV |
| c | SHNFTLVASVIIEEA |
| d | LVASVIIEEAPSGNT |
| e | IIEEAPSGNTPLLTA |
| f | PSGNTPLLTAVLVDAG |
| g | NHNFTLVASVTIEEA |
| h | FTLVASVTIEEAPSEKT |
The top line displays a 36-amino-acid region of the SA85-1.1 protein that includes epitope 1; the 20 amino acids that define epitope 1 are in boldface type (12). The amino acid sequence of each synthetic peptide used is displayed below the SA85-1.1 sequence. Peptides g and h encode epitope 1 variants, and the amino acid differences from epitope 1 are in boldface type and underlined (12).
Supernatant containing shed surface proteins was prepared by incubating 1.5 × 108 washed trypomastigotes/ml in DMEM without serum overnight and removing the parasites by centrifugation at 16,000 × g for 5 min as previously described (13).
Splenocyte isolation.
Spleens were mashed between glass slides, and the cells were suspended in 5 ml of DMEM supplemented with penicillin-streptomycin and glutamine. Five milliliters of 1.66% NH4Cl was added, and the cell suspensions were incubated for 10 min at room temperature, washed three times, and suspended in RPMI 1640 supplemented with 5% heat-inactivated calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 50 μM β-mercaptoethanol, and 50,000 U of penicillin-streptomycin (complete medium).
Intracellular staining.
Cells (2 × 106/well), in 1 ml of complete medium, were incubated in vitro for 8 h with either no additional antigen, SA85-1.1 or GST protein (20 μg/ml), or peptide(s) (5 μg/ml) in 0.05% dimethyl sulfoxide. Splenocytes were also incubated with shed surface proteins or live trypomastigotes as indicated in the text. When stated, either Y3P (anti-I-A) monoclonal antibody (MAb) or 14.4.4s (anti-I-E) MAb (150 μg/ml) was added. GolgiPlug (1 μl/well; PharMingen) was added and cells were incubated (12 h), harvested, suspended in 50 μl of staining buffer (4% bovine serum albumin–phosphate-buffered saline–0.09% sodium azide [pH 7.4]), and stained on ice (30 min) with Tricolor-anti-B220 (1 μl/sample; RA3-6B2; Caltag Laboratories, Burlingame, Calif.) and fluorescein isothiocyanate-stained anti-mouse CD4 (2 μl/sample; GK1.5). Cells were washed twice with staining buffer, suspended in Cytofix/Cytoperm (PharMingen) (200 μl; 20 min at 4°C), washed twice with Perm/Wash (PharMingen), and suspended in 50 μl of Perm/Wash. Phycoerithrin (PE)-labeled anti-IFN-γ (XMG1.2; PharMingen) or PE-labeled isotype control (R3-34; PharMingen) was added (2 μl/sample), and samples were incubated (30 min at 4°C), washed twice with Perm/Wash, and suspended in 200 μl of staining buffer followed by 400 μl of 1% paraformaldehyde–phosphate-buffered saline and then were analyzed on a FACScan (Becton Dickinson, San Jose, Calif.) within 1 h. A minimum of 40,000 events were collected, and the data were analyzed with WinMDI 2.7 (developed by Joseph Trotter; available at http://facs.scripps.edu/software.html).
RESULTS
Intracellular staining of CD4 T cells from T. cruzi-infected mice detects a large population of CD4 cells that are SA85-1.1 specific.
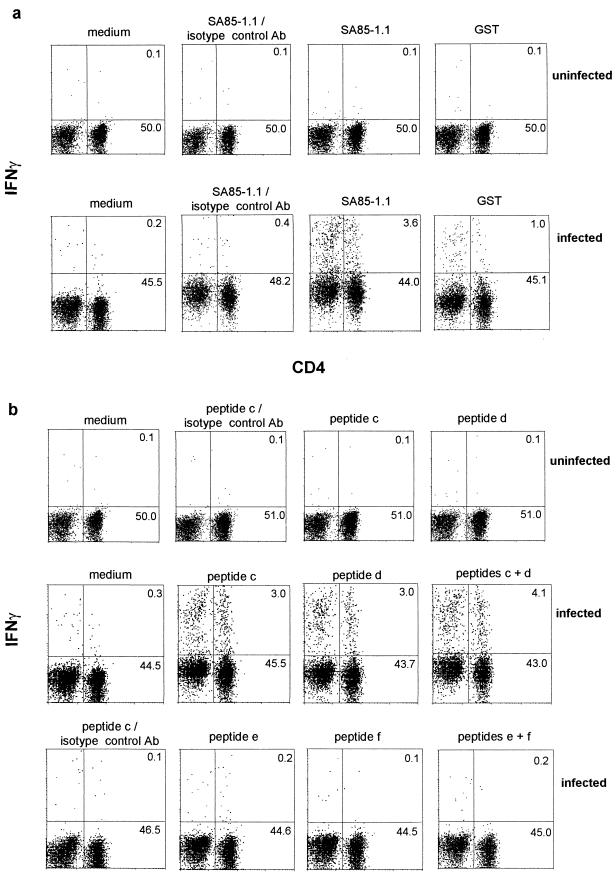
Previous studies have demonstrated that a SA85-1.1-specific CD4 response develops during T. cruzi infection and that these cells, following in vitro incubation with SA85-1.1 recombinant protein, secrete IFN-γ but not interleukin 4 (IL-4) (15). To further examine and quantitate this response, unfractionated splenocytes were analyzed for intracellular IFN-γ and IL-4. Initially, splenocytes from uninfected mice were incubated with SA85-1.1 protein or GST protein and no increase in IFN-γ production (Fig. 1a) or IL-4 (data not shown) was detected. Polyclonal stimulation of CD4 T cells, which may prevent the detection of the SA85-1.1-specific response, occurs during the acute infection; because this polyclonal response decreases during the chronic phase, initially splenocytes from chronically infected mice were analyzed (Fig. 1a) (16). Compared to the control medium a robust IFN-γ response was detected following incubation with SA85-1.1 protein (Fig. 1a, infected). In addition, following incubation with GST and the SA85-1.1 protein, an increase in CD4− IFN-γ+ cells was observed (Fig. 1a, infected). No IL-4+ cells were detected (data not shown). The specificity of the IFN-γ response was supported by the minimal binding of the IFN-γ isotype control MAb following SA85-1.1 incubation (Fig. 1a) and the small increase in CD4+ IFN-γ+ cells following incubation with the non-T. cruzi control protein, GST (Fig. 1a). If one considers the CD4+ IFN-γ+ cells present following GST incubation as background (1%), and therefore subtracts 1% from the 3.6% CD4+ IFN-γ+ cells in the SA85-1.1-incubated sample, then 2.6% of the cells analyzed are SA85-1.1-specific CD4+ IFN-γ+ cells (approximately 5% of the CD4 cells) (Fig. 1a, infected).
FIG. 1.
The SA85-1.1-specific and epitope 1-specific response in mice chronicallly infected with T. cruzi is detected by IFN-γ intracellular staining. Splenocytes were isolated from mice and incubated with medium alone or SA85-1.1 protein or GST protein (20 μg/ml) (a) or with medium alone or peptides c and d, which encode epitope 1, or peptides e and f, which are derived from SA85-1.1 protein but do not encode epitope 1 (5 μg of total peptide/ml) (b). Splenocytes were labeled for flow cytometry analysis with Tricolor-anti-B220, fluorescein isothiocyanate-stained anti-CD4, and either PE-labeled anti-IFN-γ or a PE-labeled isotype control MAb. To simplify the presentation, the B220+ cells are not shown. The dot plots are annotated with the percentages of cells in the upper and lower right quadrants. The B220+ cells are not included in these calculations. These data are representative of more than five independent experiments.
SA85-1.1 epitope is a major epitope during T. cruzi infection.
Previous studies have shown that either adoptive transfer of SA85-1.1 epitope 1-specific Th1 clones or immunization with a peptide encoding epitope 1 provided protection during T. cruzi infection, suggesting that epitope 1 is a dominant epitope during the infection (15). CD4 cells isolated from T. cruzi-infected mice and cultured with peptides that encode epitope 1 did not stimulate epitope 1-specific CD4 cells in limiting dilution analysis, cytokine enzyme-linked immunosorbent assays (ELISAs), IFN-γ enzyme-linked immunospot (ELISPOT) assays, or proliferation assays (15). These data suggested either that an epitope 1-specific response did not occur during T. cruzi infection or that these assays were not sufficiently sensitive to detect such a response. Because IFN-γ intracellular staining detected a greater number of SA85-1.1-specific CD4 cells than limiting dilution analysis or IFN-γ ELISPOT assays, it appeared to be a more sensitive assay, and therefore intracellular staining was used in an attempt to detect the epitope 1-specific response (15).
Splenocytes isolated from mice chronically infected with T. cruzi were cultured in vitro with peptides that encode epitope 1 (Fig. 1b and Table 1). Two different peptides that encode epitope 1 (peptides c and d) stimulated a robust IFN-γ response (approximately 6% of the CD4 cells) (Fig. 1b [infected] and Table 1). Again, following incubation with peptide c or d an increase in the CD4− IFN-γ+ cells were observed (Fig. 1b, infected). No IL-4-producing cells were detected (data not shown). Both peptide c and peptide d encode the essential region of epitope 1 but differ in the amino acids that flank this region (Table 1) (12). In contrast, incubation with peptide e or peptide f (peptides that encode 15 amino acids of SA85-1.1 protein adjacent to epitope 1 [Table 1]) failed to stimulate a detectable CD4+ IFN-γ+ response (Fig. 1b, infected). The specificity of the epitope 1 response was further demonstrated by (i) the lack of a detectable response by splenocytes isolated from uninfected mice following incubation with peptide c or peptide d (Fig. 1b, uninfected) and (ii) the lack of binding by the IFN-γ isotype control MAb following incubation with peptide c or peptide d (Fig. 1b and data not shown). The magnitude of the IFN-γ responses stimulated by either the SA85-1.1 protein or the peptides that encode epitope 1 were similar, suggesting that epitope 1 is the only epitope encoded by the truncated SA85-1.1 protein and that epitope 1 is a major epitope during the infection (Fig. 1).
The epitope 1-specific response is MHC-II dependent.
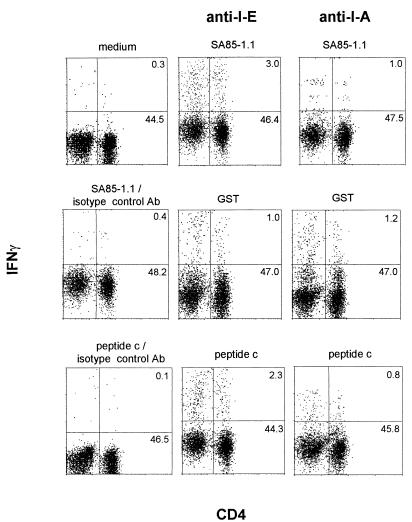
To further examine this epitope 1-specific intracellular IFN-γ response, the effect of blocking MHC-II epitope presentation with the anti-MHC-II I-A MAb, Y3P, was analyzed (Fig. 2). Splenocytes from chronically infected C57BL/6 mice were incubated with various antigens and either the anti-I-A MAb or the anti-I-E control MAb, 14.4.4s (Fig. 2). The number of CD4+ IFN-γ+ cells generated following incubation with SA85-1.1 protein or peptide c was decreased by the anti-I-A MAb compared to the anti-I-E MAb (Fig. 2). Although incubation with GST protein again resulted in a modest increase in CD4+ IFN-γ+ cells above the background levels, this increase was not inhibited by the anti-I-A MAb, indicating that the GST response did not represent a MHC-II-dependent response (Fig. 2).
FIG. 2.
The SA85-1.1-specific and epitope 1-specific responses are MHC-II restricted. Splenocytes were isolated from chronically infected mice and incubated with medium alone, SA85-1.1 or GST proteins (20 μg/ml), or peptide c (5 μg/ml). In addition, either anti-MHC-II I-A MAb or anti-MHC-II I-E MAb, at a final concentration of 150 μg/ml, was added to the cultures. Cells were stained for flow cytometry and analyzed as described in the legend to Fig. 1. These data are representative of two independent experiments.
Epitope 1-specific CD4 cells are also detected during acute T. cruzi infection.
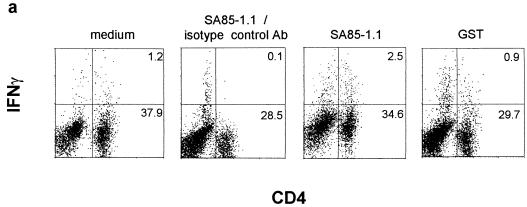
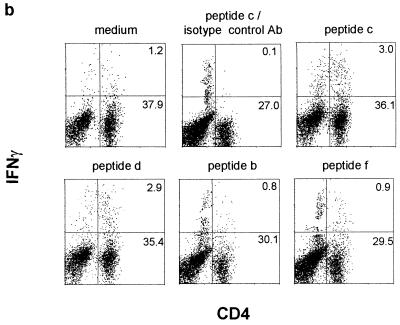
Since limiting dilution analysis and ELISAs had demonstrated the expansion of IFN-γ-secreting SA85-1.1 protein-specific CD4 cells during acute T. cruzi infection, intracellular staining was used to analyze the SA85-1.1 protein-specific and epitope 1-specific response during the acute infection (15). Although the background level of CD4+ IFN-γ+ cells was higher in the acutely infected mice (incubation with medium), increased levels of CD4+ IFN-γ+ cells could be detected following incubation with SA85-1.1 protein (∼4% of the CD4 cells) (Fig. 3a) or peptides that encode epitope 1 (∼4% of the CD4 cells) (Fig. 3b). Incubation with GST did not result in an increase in CD4+ IFN-γ+ cells as compared to the medium control (Fig. 3a). Again, no IL-4-producing CD4 cells were detected (data not shown). Because epitope 1 stimulates a very large number of CD4 cells (4 to 6%) to produce IFN-γ during acute and chronic T. cruzi infection, these data strongly argue that epitope 1 is a major epitope of the CD4 response (Fig. 1 and 3).
FIG. 3.
The SA85-1.1-specific and epitope 1-specific response is detected by intracellular IFN-γ staining during the acute phase of T. cruzi infection. Splenocytes were isolated from a day 10 infected mouse, and incubated with medium alone or proteins (20 μg/ml) (a) or medium alone or peptides (5 μg/ml) (b). Cells were stained for flow cytometry and analyzed as described in the legend to Fig. 1. These data are representative of three independent experiments.
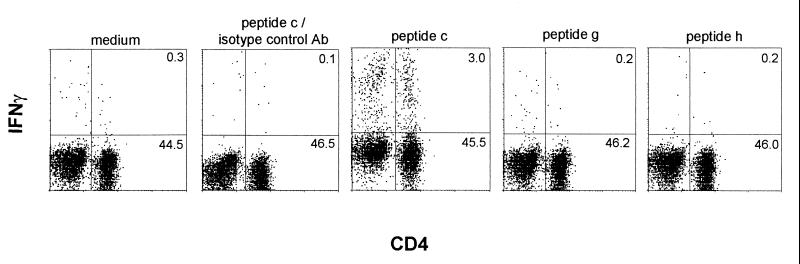
Epitope 1 variants do not stimulate a detectable response.
Previous studies have indicated that T. cruzi trypomastigotes express a superfamily of variant surface proteins and have specifically demonstrated that the MHC-II SA85-1.1 epitope 1 is encoded in other SA85-1 proteins as a series of variant epitopes (12). The ability of two epitope 1 variants, encoded by the SA85-1.3 and SA85-1.4 proteins, to stimulate CD4 splenocytes from mice chronically infected with T. cruzi was examined by intracellular staining. Previous studies indicate that these proteins are expressed by each trypomastigote, that these variant epitopes are appropriately processed and presented by splenocytes to T cells, and that they are able to stimulate an SA85-1.1-specific T cell line in vitro (12). In addition, using an in vitro binding assay these variant epitopes and epitope 1 have been shown to bind to MHC I-Ab with very similar affinities (unpublished data). Peptides g and h that encode these variant epitopes did not, however, stimulate a detectable IFN-γ response (Fig. 4 and Table 1) (12). Again, peptide c stimulated a robust IFN-γ response (∼6% of the CD4 cells) (Fig. 4). These data suggest that many of the surface protein variant epitopes do not stimulate a CD4 IFN-γ response during T. cruzi infection, and therefore the variant epitopes may function as passive or active antagonists during the infection.
FIG. 4.
Intracellular IFN-γ staining does not detect a response to variants of epitope 1. Splenocytes were isolated from chronically infected mice and incubated with different peptides at 5 μg/ml. Cells were stained for flow cytometry and analyzed as described in the legend to Fig. 1. These data are representative of three independent experiments.
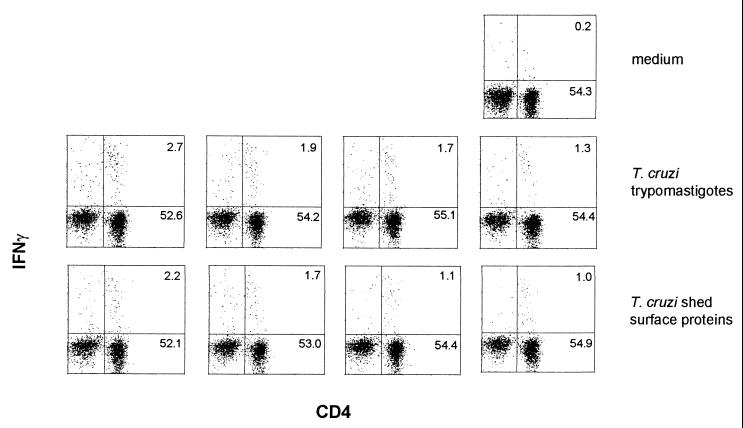
Intracellular IFN-γ staining detects a smaller response to live trypomastigotes than to synthetic peptides encoding epitope 1.
The large magnitude of the epitope 1 response was surprising and suggested that if one epitope of the T. cruzi trans-sialidase superfamily stimulated 6% of the CD4 cells, then other unrelated epitopes of the trans-sialidase superfamily may combine to dominate the CD4 response. On the other hand, the simultaneous expression of trans-sialidase superfamily proteins may prevent a robust CD4 response by limiting the amount of each epitope expressed by the parasite or by the expression of antagonistic epitopes (12, 15). To begin to address these questions and to investigate the CD4 response to the entire trans-sialidase superfamily, the intracellular IFN-γ response was used to assay the response of splenic CD4 cells from chronically infected mice incubated with different amounts of live trypomastigotes (from 107 to 106) or trypomastigote-shed surface proteins (from 24 to 2.4 μg of protein per ml) (Fig. 5). Both samples stimulated CD4 cells to produce IFN-γ: in this experiment, at their highest concentration live trypomastigotes stimulated 4.5% of the CD4+ cells (subtracting the 0.2% from the medium control as background) and shed surface proteins stimulated 3.7% of the CD4+ cells (Fig. 5). Again, peptide c stimulated 6.2% of the CD4 cells (data not shown). The CD4 IFN-γ responses following incubation with trypomastigotes or shed trypomastigote proteins were not quite as large as the response following incubation with SA85-1.1 protein (Fig. 1) or with peptides encoding epitope 1 (Fig. 1 and 5), suggesting that the parasite may express insufficient amounts of epitope 1 and other trans-sialidase superfamily epitopes to stimulate maximal responses (12, 15).
FIG. 5.
IFN-γ intracellular staining detects a CD4 response to live trypomastigotes or preparations of shed trypomastigote surface proteins. Splenocytes were isolated from chronically infected mice and incubated with medium alone, T. cruzi trypomastigotes (from left to right, 107, 5 × 106, 2.5 × 106, and 106) or T. cruzi-shed surface proteins (from left to right, 24, 12, 6, and 2.4 μg/ml). Cells were stained for flow cytometry and analyzed as described in the legend to Fig. 1. These data are representative of two independent experiments.
DISCUSSION
In previous studies adoptive transfer of SA85-1.1 epitope 1-specific clones or immunization with epitope 1 provided protection against T. cruzi, suggesting that epitope 1 is a major or dominant epitope during the infection (15). In addition, several assays have demonstrated that SA85-1.1 protein-specific CD4 T cells produce IFN-γ and expand during T. cruzi infection (15). These assays, however, failed to detect a response to synthetic peptides that encoded epitope 1 (unpublished data). In this report, IFN-γ intracellular staining detected an epitope 1-specific CD4 T-cell response during T. cruzi infection (Fig. 1). SA85-1.1 protein or peptides (peptide c or peptide d) encoding epitope 1 stimulated 4 to 6% of the CD4 cells derived from acutely or chronically infected mice (Fig. 1 to 3). In contrast, variants of epitope 1 that are encoded by genes for other trans-sialidase proteins failed to stimulate a detectable response by CD4 cells derived from chronically infected mice (Fig. 4 and Table 1), whereas live trypomastigotes or shed surface proteins stimulated approximately 3.7 to 4.5% of the CD4 cells to produce IFN-γ (Fig. 5).
IFN-γ intracellular staining of unfractionated splenocytes appears to be a more sensitive assay than limiting dilution analysis and IFN-γ ELISAs of CD4-enriched populations, because intracellular staining detected the epitope 1-specific response whereas the other assays did not (16; data not shown). In addition, another benefit of the intracellular staining technique was that it analyzed the SA85-1.1- and epitope 1-responding CD4 cells without fractionation of the splenocytes and therefore without the risk of examining a biased population of CD4 cells.
Most surface proteins of T. cruzi are members of the trans-sialidase superfamily, and in the CL strain two subfamilies, the SA85-1 and FL160 families, have been characterized (2, 4, 8, 10, 23). The proteins of these two subfamilies are encoded by >850 genes and appear to represent a small fraction of the trans-sialidase superfamily (10, 23). Each parasite appears to simultaneously express many members of the trans-sialidase superfamily (10, 11, 23). Epitope 1 has been shown to be encoded as a variant epitope in a minimum of 10 other expressed SA85-1 proteins, and these 10 variant epitopes are processed and presented by antigen-presenting cells and are able to stimulate T cells (12). These data suggest that each trans-sialidase protein epitope will be encoded as a series of variant epitopes in many other trans-sialidase proteins (12). Variant epitopes have been shown to inhibit T-cell proliferation, to stimulate cytokine production without cellular proliferation, or to induce T-cell anergy (6). In addition, epitope variation can decrease the amount of each epitope expressed; this decrease in epitope amount can prevent T-cell stimulation or can influence the development of Th cells into Th1 or Th2 cells (3). Several studies indicate that a decrease in the amount of an epitope favors the development of Th1 cells (3). It remains unclear if, during T. cruzi infection, the trans-sialidase superfamily epitope variation affects the CD4 T-cell response. The epitope variation may contribute to the IFN-γ bias of the response or the failure of the CD4 T cells to proliferate in vitro (15). The failure of the variant epitopes, encoded by peptide g and peptide h (Table 1), to stimulate a detectable response (Fig. 4) and the observation that trypomastigotes or shed trypomastigote proteins stimulate a smaller response than peptides that encode epitope 1 (Fig. 5) are consistent with the possibility that many variant epitopes of the superfamily may function as passive antagonists during T. cruzi infection and affect the CD4 T-cell response.
As shown in Fig. 1, 5 to 6% of the CD4 T cells are stimulated to produce IFN-γ following incubation with either SA85-1.1 recombinant protein or peptide c. The fact that 1 in 20 CD4 cells is responding to epitope 1 argues that this is an immunodominant response. It is possible that this large IFN-γ-biased response is parasite strain specific, mouse strain specific, or mammalian host specific. It is also not clear that all the responding T cells are directly stimulated by SA85-1.1 protein or peptide c or d (Fig. 1 to 3). A subset of the IFN-γ+ cells may be initially activated by MHC-II-restricted epitope presentation, and these activated cells may then stimulate other cells by non-MHC mechanisms or “bystander” activation. The contribution of bystander activation to this response may be analyzed using adoptive transfer of a clone of naive epitope 1-specific and naive non-epitope 1-specific CD4 T cells and comparing the development and activation of these different clones during T. cruzi infection. Alternatively, if they can be developed, epitope 1-specific MHC tetramer reagents can be used to analyze the contribution of bystander activation. Our previous adoptive transfer studies demonstrated protection during T. cruzi infection from epitope 1-specific Th1 clones and no protection from control keyhole limpet hemocyanin-specific Th1 clones, suggesting that bystander activation of the keyhole limpet hemocyanin IFN-γ-producing clone did not occur and that bystander activation is not a major contributor to T-cell activation (15). Bystander activation may also explain the IFN-γ production by CD4− cells in these experiments (Fig. 1 to 4). Alternatively, the CD4− IFN-γ+ cells may represent MHC-II-restricted CD4− cells that have been stimulated by presentation of SA85-1.1 epitopes or epitope 1. MHC-II-restricted CD4− T cells can exist in peripheral lymphoid organs (14). Further supporting this possibility is a previous study that identified the development of a large population of CD4− CD8− NK− T cells that produce IFN-γ during T. cruzi infection (17). Investigations of the CD4− IFN-γ+ population indicate that these cells are composed of TCR+ NK1.1− CD8+ and TCR+ NK1.1− CD8− cells (unpublished data).
To our knowledge these are the first major MHC-restricted antigen and epitope to be identified during T. cruzi infection. Typically, major epitopes are identified using proliferation assays. If only proliferation assays had been used in these studies, then the observation that the SA85-1.1 protein and epitope 1 are a major antigen and epitope would not have been made (15).
IFN-γ is critical in the control of acute T. cruzi infection and is likely to contribute to the chronic inflammatory pathology (1, 5, 7, 19–22). Results presented here indicate that during acute and chronic T. cruzi infection, 4 to 6% of the CD4 cells (∼1 out of 20 CD4 T cells) are stimulated by epitope 1, a single trans-sialidase superfamily epitope, to produce IFN-γ (Fig. 1). We have been unable to find studies that quantitate the T-cell responder frequency of other protozoan MHC-II-restricted immunodominant epitopes. The epitope 1 response is large whether it is stimulated directly by epitope 1 or in part by nonspecific mechanisms (Fig. 1 to 3). In addition, each parasite appears to express thousands of trans-sialidase proteins (4, 10, 11, 23). Therefore, it is reasonable to propose that several more major epitopes may be encoded within the trans-sialidase superfamily. If this is correct, then the trans-sialidase superfamily may dominate the host CD4 response. Therefore, T. cruzi, by focusing the CD4 T-cell response at the trans-sialidase proteins that are shed into the extracellular milieu, may protect itself from the host immune response. Furthermore, the focusing of the IFN-γ response on antigens that are secreted into the host tissues may contribute to the inflammatory damage that occurs during T. cruzi infection (8, 13, 24).
ACKNOWLEDGMENTS
We thank Wes Van Voorhis and Dave Lewis for critically reviewing the manuscript, Dave Coder for advice on the flow cytometry figures, Jim Blake and Wes Cosand of Bristol-Meyers Squibb (Seattle, Wash.) for the synthetic peptides, Alexander Rudensky and Paul de Roos for the MHC binding assays, Andy Farr for the 14.4.4s MAb, Alexander Rudensky for the Y3P MAb, and Monika Wleklinski-Lee for preparation of T. cruzi antigens.
This work was supported by a grant from the National Institutes of Health (1 R29 AI33663-01A2) and by the American Heart Association and the March of Dimes. Stuart Kahn is a Burroughs Wellcome New Investigator in Molecular Parasitology.
REFERENCES
- 1.Araujo F G. Development of resistance to Trypanosoma cruzi in mice depends on a viable population of L3T4+ (CD4+) T lymphocytes. Infect Immun. 1989;57:2246–2248. doi: 10.1128/iai.57.7.2246-2248.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campetella O, Sanchez D, Cazzulo J J, Frasch A C C. A superfamily of Trypanosoma cruzi surface antigens. Parasitol Today. 1992;8:378–381. doi: 10.1016/0169-4758(92)90175-2. [DOI] [PubMed] [Google Scholar]
- 3.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 4.Cross G A, Takle G B. The surface trans-sialidase family of Trypanosoma cruzi. Annu Rev Microbiol. 1993;47:385–411. doi: 10.1146/annurev.mi.47.100193.002125. [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos R R, Rossi M A, Laus J L, Silva J S, Savino W, Mengel J. Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med. 1992;175:29–39. doi: 10.1084/jem.175.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evavold B D, Sloan-Lancaster J, Allen P M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 7.Hontebeyrie J-M, Said G, Milon G, Marchal G, Eisen H. L3T4+ T cells able to mediate parasite-specific delayed-type hypersensitivity play a role in the pathology of experimental Chagas' disease. Eur J Immunol. 1987;17:1027–1033. doi: 10.1002/eji.1830170720. [DOI] [PubMed] [Google Scholar]
- 8.Kahn S, Colbert T G, Wallace J C, Hoagland N A, Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci USA. 1991;88:4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahn S, Kahn M, Van Voorhis W C, Goshorn A, Strand A, Hoagland N, Eisen H, Pennathur S. SA85-1 proteins of Trypanosoma cruzi lack sialidase activity. Mol Biochem Parasitol. 1993;60:149–152. doi: 10.1016/0166-6851(93)90038-y. [DOI] [PubMed] [Google Scholar]
- 10.Kahn S, Van Voorhis W C, Eisen H. The major 85-kD surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J Exp Med. 1990;172:589–597. doi: 10.1084/jem.172.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn S J, Nguyen D, Norsen J, Wleklinski M, Granston T, Kahn M. Trypanosoma cruzi: monoclonal antibodies to the surface glycoprotein superfamily differentiate subsets of the 85-kDa surface glycoproteins and confirm simultaneous expression of variant 85-kDa surface glycoproteins. Exp Parasitol. 1999;92:48–56. doi: 10.1006/expr.1998.4394. [DOI] [PubMed] [Google Scholar]
- 12.Kahn S J, Wleklinski M. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J Immunol. 1997;159:4444–4451. [PubMed] [Google Scholar]
- 13.Kahn S J, Wleklinski M, Ezekowitz R A B, Coder D, Aruffo A, Farr A. The major surface glycoproteins of Trypanosoma cruzi amastigotes are ligands of the human serum mannose binding protein. Infect Immun. 1996;64:2649–2656. doi: 10.1128/iai.64.7.2649-2656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locksley R M, Reiner S L, Hatam F, Littman D R, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 15.Millar A E, Wleklinski-Lee M, Kahn S J. The surface protein superfamily of Trypanosoma cruzi stimulates a polarized Th1 response that becomes anergic. J Immunol. 1999;162:6092–6099. [PubMed] [Google Scholar]
- 16.Minoprio P M, Eisen H, Forni L, D'Imperio L-M R, Joskowicz M, Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand J Immunol. 1986;24:661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 17.Nabors G S, Tarleton R L. Differential control of IFN-gamma and IL-2 production during Trypanosoma cruzi infection. J Immunol. 1991;146:3591–3598. [PubMed] [Google Scholar]
- 18.Plata F, Garcia-Pons F, Eisen H. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur J Immunol. 1984;14:392–399. doi: 10.1002/eji.1830140503. [DOI] [PubMed] [Google Scholar]
- 19.Rottenberg M E, Bakhiet M, Olsson T, Kristensson K, Mak T, Wigzell H, Orn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottenberg M E, Rodriguez D A, Orn A. Control of Trypanosoma cruzi infection in mice deprived of T-cell help. Scand J Immunol. 1992;36:261–268. doi: 10.1111/j.1365-3083.1992.tb03098.x. [DOI] [PubMed] [Google Scholar]
- 21.Russo M, Starobinas N, Minoprio P, Coutinho A, Hontebeyrie J-M. Parasitic load increases and myocardial inflammation decreases in Trypanosoma cruzi-infected mice after inactivation of helper T cells. Ann Inst Pasteur Immunol. 1988;139:225–236. doi: 10.1016/0769-2625(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 22.Tarleton R L, Grusby M J, Postan M, Glimcher L H. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Van Voorhis W C, Barrett L, Koelling R, Farr A G. FL-160 proteins of Trypanosoma cruzi are expressed from a multigene family and contain two distinct epitopes that mimic nervous tissues. J Exp Med. 1993;178:681–694. doi: 10.1084/jem.178.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Voorhis W C, Eisen H. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J Exp Med. 1989;169:641–652. doi: 10.1084/jem.169.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Control of Chagas disease 811. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]