Abstract
Background
Sepsis is the most common cause of death in hospitals, and intra-abdominal infection (IAI) accounts for a large portion of the causes of sepsis. We investigated the clinical outcomes and factors influencing mortality of patients with sepsis due to IAI.
Methods
This post-hoc analysis of a prospective cohort study included 2126 patients with sepsis who visited 16 tertiary care hospitals in Korea (September 2019–February 2020). The analysis included 219 patients aged > 19 years who were admitted to intensive care units owing to sepsis caused by IAI.
Results
The incidence of septic shock was 47% and was significantly higher in the non-survivor group (58.7% vs 42.3%, p = 0.028). The overall 28-day mortality was 28.8%. In multivariable logistic regression, after adjusting for age, sex, Charlson Comorbidity Index, and lactic acid, only coagulation dysfunction (odds ratio: 2.78 [1.47–5.23], p = 0.001) was independently associated, and after adjusting for each risk factor, only simplified acute physiology score III (SAPS 3) (p < 0.001) and continuous renal replacement therapy (CRRT) (p < 0.001) were independently associated with higher 28-day mortality.
Conclusions
The SAPS 3 score and acute kidney injury with CRRT were independently associated with increased 28-day mortality. Additional support may be needed in patients with coagulopathy than in those with other organ dysfunctions due to IAI because patients with coagulopathy had worse prognosis.
Keywords: Intensive care unit, Intra-abdominal infection, Mortality rate, Organ dysfunction, Sepsis, Source control
Introduction
Sepsis is an uncontrolled reaction of the host to infection, which can be potentially life-threatening. Accurate calculation of the global burden of sepsis is difficult. A recent study has reported approximately 48.9 million cases and 11 million sepsis-related deaths in 2017. This accounts for nearly 20% of all deaths worldwide, and sepsis is the most common cause of death in hospitals in the United States [1, 2]. Sepsis is a major public health issue, and the costs for treating sepsis in hospitals have increased, exceeding US $24 billion per year [2, 3]. Likewise, the incidence of sepsis and the treatment costs in Korea have steadily increased, particularly among older adults [4].
Intra-abdominal infection (IAI) is defined as an inflammatory reaction to bacteria and their toxins in the peritoneum, resulting in a purulent exudate in the peritoneal cavity [5–8]. Among all the causes of sepsis, IAI is reportedly the second most common cause, with a relatively high mortality rate of nearly 30.0% [4, 9, 10]. In particular, most sepsis cases in surgical intensive care units (ICUs) are caused by IAI. Source control and antibiotic use are essential in the treatment of these patients. Various forms of IAIs may exist, including those in the hepatobiliary tract, stomach, small bowel, and colon. In addition, there are differences in mortality and clinical outcomes according to the type of organ, degree of anatomical disruption, and duration of IAI [5, 7, 9]. Treatment of IAI can be difficult because the spectrum of infection is wide compared with that of other infection causes and source control, wherein drainage or surgical treatment is often required.
However, studies on the clinical outcomes and impact of organ dysfunction in patients with sepsis due to IAI are limited. Therefore, the primary objective of our study was to investigate the clinical outcomes and the factors affecting the mortality in patients with sepsis due to IAI. The secondary objective was to determine the impact of organ dysfunction on mortality rates.
Materials and methods
Study design, setting, and definition
This post-hoc analysis of a prospective cohort study was performed by the Korean Sepsis Alliance (KSA) encompassing 16 tertiary or university-affiliated hospitals in Korea. The Steering Committee developed the research data collection methods to manage the sepsis data platform, periodically reviewed the progress of each study, and supervised the overall research progress in association with the Korea Disease Control and Prevention Agency (KDCA). The data used in this study were screened from all consecutive patients who visited the participating hospitals for six months (September 1, 2019, to February 29, 2020).
This prospective cohort study analyzed data from the Korean sepsis registry, and this study was already deliberated as a service project by the KDCA. The study was approved by the institutional review boards (IRB) of all participating hospitals, including the IRB of Asan Medical Center (approval number 2018-0675). Data were collected and analyzed in an ethical manner while protecting the patients' right to privacy. The requirement for informed consent was waived owing to the non-interventional, observational characteristics by the IRB of all participating hospitals, including the IRB of Asan Medical Center (approval number 2018-0675).
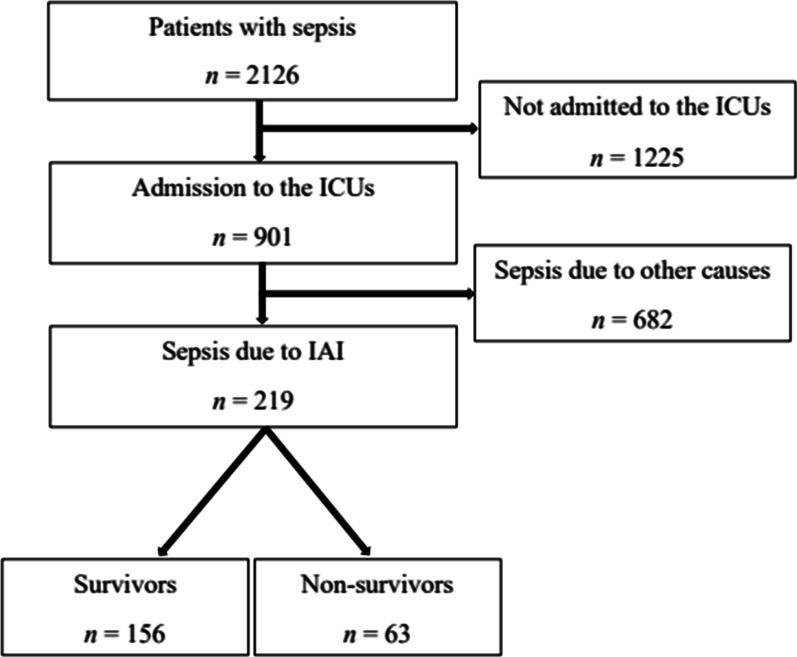
Our study enrolled a total of 2126 patients with sepsis who were admitted to the participating hospitals for 6 months. Of these patients, 901 were treated in ICUs. Finally, the study included 219 patients who were admitted to the ICU due to sepsis caused by IAI (Fig. 1). Sepsis patients aged ≥ 19 years were included and followed up until death or discharge. As defined by the Clinical Criteria of the Third International Consensus Definition (Sepsis-3), sepsis was defined as a life-threatening organ dysfunction resulting from an uncontrolled host response to infection [11]. Organ dysfunction was included in the definition of sepsis, and the presence or absence of organ dysfunction was determined using a Sequential Organ Failure Assessment (SOFA) score. In this study, sepsis was diagnosed if the patient met the following two conditions: (1) suspicion or confirmation of infection and (2) increase in SOFA score by two points or more when an event occurred. The base SOFA score of each organ was assumed to be zero for patients with no known pre-existing organ dysfunction, and patients with a SOFA score of two or higher at the time of the event were enrolled in the study. Peritonitis caused by perforation of a hollow viscus, intra-abdominal abscess, biliary tract infection, pancreatic infection, enteritis, and colitis were defined as having IAI. The Eastern Cooperative Oncology Group (ECOG) scale is a measurement index created to check performance status, determine appropriate treatment policy, and predict prognosis in cancer patients. Using the ECOG scale, it is possible to objectively evaluate the patient's level of functioning in terms of their ability to care for themselves, perform activities of daily living, and their physical ability. It is divided into six scales: (0) fully active and no performance restrictions; (1) strenuous physical activity restricted, fully ambulatory and able to carry out light work; (2) capable of all self-care but unable to carry out any work activities; up and about > 50% of waking hours; (3) capable of only limited self-care and confined to bed or chair > 50% of waking hours; (4) completely disabled and cannot carry out any self-care; and (5) death. Continuous renal replacement therapy (CRRT) refers to patients who underwent CRRT for the treatment of acute kidney injury, and excludes patients who underwent conventional hemodialysis as renal replacement therapy. In community-acquired sepsis, time zero was defined as the time of an emergency room triage visit. In-hospital-acquired sepsis, time zero was defined as the first time the rapid response team recognized the sepsis. Source control was defined as non-surgical treatments such as percutaneous drainage, and surgical treatment such as debridement or laparotomy. Patients who were treated with antibiotics and received intensive care, without any such procedures, were considered not to have received source control.
Fig. 1.
Flow chart of patients’ enrollment. ICU intensive care unit, IAI intra-abdominal infection
Data collection
All trained research coordinators in each participating hospital completed their entry into the shared data platform, and the coordinating hospital evaluated the quality of the data for completeness and logical errors. The data collected retrospectively in each hospital were as follows: (1) patient characteristics, including age, sex, body mass index (BMI), Charlson Comorbidity Index (CCI), physiological status, medical history, SOFA score, simplified acute physiology score III (SAPS 3), and laboratory data at time zero; (2) clinical results, including the duration from time zero to antibiotic administration, source control implementation, and duration from time zero to source control implementation; (3) infection and microbiological data, including the type of isolated bacteria and fungi, occurrence of bacteremia and multidrug resistance (MDR), and location of infection; and (4) organ dysfunction data, including the type of organ dysfunction results and analysis of the number of organ dysfunctions. Additional data compared with those in previous studies were collected. However, these were not used in our study.
Statistical analysis
Statistical analysis was conducted by the staff of the coordinating center and by a professor at the Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, who did not participate in data collection.
Data are presented as numbers (percentages) or as mean ± standard deviation. The characteristics and clinical data of the survivor and non-survivor groups were compared using the chi-square test or Fisher’s exact test (for categorical variables) and Student’s t-test (for continuous variables). Risk factors associated with 28-day mortality were analyzed by univariate and then multivariate logistic regression, and the degree of association with 28-day mortality was presented as an independent factor by means of odds ratios (ORs) with their corresponding 95% confidence intervals. Infection profile data were analyzed using the number (percentage) or mean ± standard deviation. The microbiological data were described by total numbers and proportions, which showed the distribution of bacteria and fungi in patients with sepsis due to IAI. Multivariate logistic regression analysis was used to estimate the association between organ dysfunction and 28-day mortality, with unadjusted and adjusted evaluations. We adjusted 28-day mortality for age, sex, CCI, and lactic acid levels. We used OR to present the impact of organ dysfunction on 28-day mortality and analyzed the impact of the number of organ dysfunctions on 28-day mortality using ORs in the logistic regression analysis. Also, the multiple logistic regression by stepwise selection was used to explain the 28-day mortality using independent variables. The logistic regression performance is denoted by c-statistics and Hosmer–Lemeshow tests. Differences were considered statistically significant at P < 0.05. All analyses were conducted using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA).
Results
Patient characteristics and clinical data
During the 6-month study period, total 2126 patients were admitted owing to sepsis at the participating hospitals; 219 (10.3%) were admitted to the ICU owing to sepsis caused by IAI according to collected data review. The 28-day mortality rate of all enrolled patients with sepsis was 28.1% (n = 598/2126). Among these patients, the mortality rate was 28.8% (n = 63/219) for sepsis due to IAI in the ICU.
Patient characteristics and clinical data at the time of sepsis diagnosis are summarized in Table 1. The mean age of the patients was 69.4 ± 13.1 years, and 53.9% were men. The mean BMI was 23.0 ± 4.2 kg/m2; no significant difference was noted in BMI between the survivor and non-survivor groups. The mean CCI in the non-survivor group was higher than that of the survivor group, with significant differences (5.9 ± 3.0 vs. 5.1 ± 2.3, p = 0.011). The initial Eastern Cooperative Oncology Group scores ranged from 0 to 4. However, there was no significant difference in the scores between the two groups (p = 0.075). The incidence of septic shock was 47%, and was significantly higher in the non-survivor group than in the survivor group (58.7% vs. 42.3%, p = 0.028). The initial lactate level was significantly higher in the non-survivor group than in the survivor group (7.2 ± 5.0 vs. 4.5 ± 3.1, p < 0.001). In addition, the initial SOFA score was significantly higher in the non-survivor group than in the survivor group (12.2 ± 4.6 vs. 8.4 ± 3.1, p < 0.001). Moreover, patients who underwent CRRT had a higher mortality rate than those who did not undergo CRRT (68.3% vs. 16%, p < 0.001). By contrast, no significant difference was noted in the number of patients who were prescribed steroids to treat sepsis between the two groups (26.9% vs. 34.9%, p = 0.239). In the initial laboratory tests at time zero, total bilirubin, C-reactive protein, procalcitonin, and brain natriuretic peptide levels were higher in the non-survivor group.
Table 1.
Patient characteristics and clinical data
| All patients (n = 219) |
Survivors (n = 156) |
Non-survivors (n = 63) |
P-value | |
|---|---|---|---|---|
| Age (years), mean (± SD) | 69.4 ± 13.1 | 69.1 ± 13.0 | 70.2 ± 13.5 | 0.886 |
| Sex, n (%) | 0.538 | |||
| Male | 118 (53.9) | 82 (52.6) | 36 (57.1) | |
| BMI (kg/m2), mean (± SD) | 23.0 ± 4.2 | 22.8 ± 3.9 | 23.6 ± 4.8 | 0.091 |
| Charlson comorbidity index, mean (± SD) | 5.3 ± 2.6 | 5.1 ± 2.3 | 5.9 ± 3.0 | 0.011 |
| Initial ECOG score, n (%) | 0.075 | |||
| 0 | 52 (23.7) | 39 (25.0) | 13 (20.6) | |
| 1 | 59 (26.9) | 46 (29.5) | 13 (20.6) | |
| 2 | 47 (21.5) | 31 (19.9) | 16 (25.4) | |
| 3 | 38 (17.4) | 21 (13.5) | 17 (27.0) | |
| 4 | 23 (10.5) | 19 (12.2) | 4 (6.3) | |
| Septic shock, n (%) | 103 (47.0) | 66 (42.3) | 37 (58.7) | 0.028 |
| Initial lactate level (mmol/L), mean (± SD) | 5.3 ± 4.0 | 4.5 ± 3.1 | 7.2 ± 5.0 | < 0.001 |
| Initial SOFA score, mean (± SD) | 9.5 ± 4.0 | 8.4 ± 3.1 | 12.2 ± 4.6 | < 0.001 |
| SAPS 3 score, mean (± SD) | 73.9 ± 16.4 | 70.1 ± 14.4 | 83.4 ± 17.3 | 0.098 |
| CRRT, n (%) | 68 (31.1) | 25 (16.0) | 43 (68.3) | < 0.001 |
| Steroid use, n (%) | 64 (29.2) | 42 (26.9) | 22 (34.9) | 0.239 |
| Initial laboratory results | ||||
| Platelet count (103/uL) | 170.7 ± 125.6 | 184.5 ± 130.0 | 136 ± 107.2 | 0.299 |
| Creatinine level (mg/dL) | 2.1 ± 1.9 | 1.96 ± 2.0 | 2.40 ± 1.5 | 0.796 |
| Total bilirubin level (mg/dL) | 3.0 ± 5.1 | 2.2 ± 2.8 | 4.9 ± 8.2 | < 0.001 |
| CRP level (mg/dL) | 14.2 ± 10.3 | 14.3 ± 9.5 | 14.0 ± 12.2 | 0.027 |
| Procalcitonin level (ng/ml) | 37.0 ± 67.2 | 31.7 ± 50.0 | 50.6 ± 97.9 | 0.006 |
| BNP level (pg/mL) | 699.0 ± 1207.3 | 458.6 ± 886.0 | 1312.0 ± 1659.0 | < 0.001 |
| Time zero to time of antibiotics administration (min) | 184.7 ± 303.0 | 171.4 ± 238.8 | 217.5 ± 422.8 | 0.495 |
| Source control, n (%) | 90 (41.1) | 73 (46.8) | 17 (27.0) | 0.007 |
| Source control time (h) | 22.1 ± 37.0 | 22.9 ± 39.4 | 18.5 ± 24.9 | 0.489 |
| Number of organ dysfunction, mean (± SD) | 1.7 ± 1.3 | 1.4 ± 1.21 | 2.3 ± 1.4 | 0.019 |
Data are shown as mean ± standard deviation or number (percentage)
SD standard deviation, BMI body mass index, ECOG Eastern Cooperative Oncology Group, SOFA Sequential Organ Failure Assessment, SAPS 3 simplified acute physiology score III, CRRT continuous renal replacement therapy, CRP C-reactive protein, BNP brain natriuretic peptide
In addition, there was no significant difference between the two groups in the duration from time zero to the administration of antibiotics and source control. However, the number of patients who underwent source control through non-surgical treatment such as percutaneous drainage, or surgical treatment was significantly higher in the survivor group than in the non-survivor group (46.8% vs. 27%, p = 0.007). The mean number of organ dysfunction was significantly higher in the non-survivor group than in the survivor group (2.3 ± 1.4 vs. 1.4 ± 1.2, p = 0.019).
Microbiological pathogens (or spectrum) and characteristics
Table 2 shows the distribution of the isolated microbiological pathogens expressed by per species percentage of bacteria and fungi from patients with sepsis due to IAI. Of the 219 patients, 157 (71.7%) were identified with a causative pathogen. Among the patients with isolated pathogens, gram-negative bacteria were found in 81.5%, gram-positive bacteria in 32.5%, and fungi in 4.5% of patients. Among the isolated causative pathogens in our study, the most common pathogen was Escherichia coli (48.4%), followed by Klebsiella pneumoniae (22.9%), Enterococcus faecium (8.9%), and Enterococcus faecalis (5.7%). Additionally, Acinetobacter baumannii (4.5%), Enterobacter cloacae (3.8%), Klebsiella oxytoca (3.2%), and Pseudomonas aeruginosa (3.2%) were other gram-negative bacteria, and Staphylococcus aureus (1.8%) and Corynebacterium striatum (0.5%) were other gram-positive bacteria. Moreover, Candida albicans (3.2%), Candida glabrata (2.5%), and Candida krusei (0.6%) were the identified fungi.
Table 2.
Distribution of the microbiological pathogens isolated from cultures in patients with sepsis due to IAI
| No. of patients (total, n = 157) | % Total | |
|---|---|---|
| Gram-positive | 51 | 32.5 |
| Enterococcus faecium | 14 | 8.9 |
| Enterococcus faecalis | 9 | 5.7 |
| Staphylococcus aureus | 4 | 1.8 |
| Corynebacterium striatum | 1 | 0.5 |
| Others | 26 | 11.9 |
| Gram-negative | 128 | 81.5 |
| Escherichia coli | 76 | 48.4 |
| Klebsiella pneumoniae | 36 | 22.9 |
| Acinetobacter baumannii | 7 | 4.5 |
| Enterobacter cloacae | 6 | 3.8 |
| Klebsiella oxytoca | 5 | 3.2 |
| Pseudomonas aeruginosa | 5 | 3.2 |
| Others | 24 | 15.3 |
| Fungus | 7 | 4.5 |
| Candida albicans | 5 | 3.2 |
| Candida glabrata | 4 | 2.5 |
| Candida krusei | 1 | 0.6 |
Bacteremia occurred in 49.3% of all patients, and did not differ significantly between the survivor and non-survivor groups (48.7% vs. 50.8%, p = 0.781). Among the identified pathogens, there were no statistically significant differences in the types of bacteria and fungus between the survivor and the non-survivor groups. Mixed growth was defined as the detection of more than one type of gram-positive, gram-negative, and fungal pathogens. Of the patients with identified causative pathogens, 19.1% had a mixed growth on culture. The proportion of patients with mixed growth was higher in the non-survivor group than in the survivor group, although the difference was not statistically significant (27.5% vs. 16.2%, p = 0.304). MDR was defined as the antimicrobial resistance of a microorganism to at least one antibiotic in three or more antimicrobial categories [12]. The overall prevalence of MDR pathogens did not differ significantly between the survivor and non-survivor groups (47.9% vs. 40.0%, p = 0.134). Among the MDR pathogens, the most common were Enterobacteriaceae (54.2%), followed by Enterococcus spp. (12.5%), Acinetobacter spp. (4.2%), Staphylococcus aureus (2.8%), and Pseudomonas aeruginosa (2.8%). There was no difference in the mortality rate according to the location of infection (Table 3).
Table 3.
Microbiological profile on survivors and non-survivors
| All patients (n = 219) |
Survivors (n = 156) |
Non-survivors (n = 63) |
P-value | |
|---|---|---|---|---|
| Identified pathogens, n (%) | 157 (71.1) | 117 (75.0) | 40 (63.5) | 0.087 |
| Bacteremia, n (%) | 108 (49.3) | 76 (48.7) | 32 (50.8) | 0.781 |
| Type of bacteria, n (%) | ||||
| Gram-positive | 51 (32.5) | 34 (29.1) | 17 (42.5) | 0.112 |
| Gram-negative | 128 (81.5) | 95 (81.2) | 33 (82.5) | 0.322 |
| Fungus, n (%) | 7 (4.5) | 6 (5.1) | 1 (2.5) | 0.390 |
| Mixed growth, n (%) | 30 (19.1) | 19 (16.2) | 11 (27.5) | 0.304 |
| MDR, n (%) | 72 (45.9) | 56 (47.9) | 16 (40.0) | 0.134 |
| Enterobacteriaceae | 39 (54.2) | 30 (53.6) | 9 (56.3) | 0.387 |
| Enterococcus spp. | 9 (12.5) | 5 (8.9) | 4 (25.0) | 0.289 |
| Acinetobacter spp. | 3 (4.2) | 2 (3.6) | 1 (6.3) | 0.860 |
| Staphylococcus aureus | 2 (2.8) | 1 (1.8) | 1 (6.3) | 0.505 |
| Pseudomonas aeruginosa | 2 (2.8) | 2 (3.6) | 0 | 0.367 |
| Others | 21 (29.2) | 18 (32.1) | 3 (18.8) | 0.123 |
| Location of infection, n (%) | ||||
| Community-acquired | 135 (61.6) | 96 (61.5) | 39 (61.9) | 0.960 |
| Healthcare-acquired | 84 (38.4) | 60 (38.5) | 24 (38.1) | 0.960 |
MDR multidrug resistance
Organ dysfunction
In the organ dysfunction analysis, the most common type of organ dysfunction in patients with sepsis due to IAI was respiratory dysfunction (36.5%), followed by renal (36.1%), coagulation (34.2%), cardiovascular (25.6%), central nervous system (CNS) (19.6%), and liver (18.3%) dysfunctions. Among the dysfunctional organs, the mortality rate associated with each organ dysfunction was the highest in patients with CNS dysfunction, followed by coagulation, renal, respiratory, cardiovascular, and liver dysfunctions (Table 4).
Table 4.
Organ dysfunction analysis data in sepsis due to IAI
| All patients (n = 219) |
Survivors (n = 156) |
Non-survivors (n = 63) |
P-value | |
|---|---|---|---|---|
| Organ dysfunction, n (%) | ||||
| Respiratory | 80 (36.5) | 50 (62.5) | 30 (37.5) | 0.030 |
| Coagulation | 75 (34.2) | 42 (56.0) | 33 (44.0) | < 0.001 |
| Liver | 40 (18.3) | 29 (72.5) | 11 (27.5) | 0.845 |
| Cardiovascular | 56 (25.6) | 36 (64.3) | 20 (35.7) | 0.183 |
| CNS | 43 (19.6) | 24 (55.8) | 19 (44.2) | 0.013 |
| Renal | 79 (36.1) | 49 (62.0) | 30 (38.0) | 0.024 |
IAI intra-abdominal infection, CNS central nervous system
Multivariate logistic regression analysis was used to estimate the association between organ dysfunction and 28-day mortality. Among the organ dysfunctions, the 28-day mortality rate was more significantly affected with coagulation (OR = 2.99 [1.63–5.48], p < 0.001), CNS (OR = 2.38 [1.19–4.75], p = 0.014), renal (OR = 1.99 [1.09–3.61], p = 0.024), and respiratory (OR = 1.93 [1.06–3.5], p = 0.031) dysfunctions. Furthermore, after adjusting for age, sex, CCI, and lactic acid level, only coagulation dysfunction appeared to affect 28-day mortality significantly (OR = 2.78 [1.47–5.23], p = 0.001) (Table 5).
Table 5.
Multivariable logistic regression analysis for each organ dysfunction in patients with sepsis due to IAI
| Variable | Crude | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P-value | |
| Respiratory | 1.93 (1.06–3.5) | 0.031 | 1.71 (0.9–3.25) | 0.102 |
| Coagulation | 2.99 (1.63–5.48) | < 0.001 | 2.78 (1.47–5.23) | 0.001 |
| Liver | 0.93 (0.43–1.99) | 0.845 | 0.99 (0.44–2.25) | 0.979 |
| Cardiovascular | 1.55 (0.81–2.96) | 0.185 | 1.35 (0.67–2.71) | 0.398 |
| Central nervous system | 2.38 (1.19–4.75) | 0.014 | 1.79 (0.85–3.79) | 0.127 |
| Renal | 1.99 (1.09–3.61) | 0.025 | 1.45 (0.76–2.76) | 0.260 |
Adjusting was performed according to age, sex, CCI, and lactic acid level
IAI intra-abdominal infection, OR odds ratio, CI confidence interval, CCI Charlson Comorbidity Index
On studying the association between the number of organ dysfunctions and the 28-day mortality rate, mortality was found to be 24.7% (n = 20/81, p = 0.307) in patients with one organ dysfunction and 18.4% (n = 9/49, p = 0.068) in patients with two organ dysfunctions. The 28-day mortality was 55.6% in patients with three organ dysfunctions (OR = 9.06 [2.64–31.15], p = 0.001) and 50% in patients with four or more organ dysfunctions (OR = 7.25 (1.85–28.36], p = 0.004). In patients with three or more organ dysfunctions, 28-day mortality was more than twice that of single organ dysfunction (Table 6). In time, the mortality rate in patients with more than three organ dysfunctions distinctly increased.
Table 6.
OR of multiple organ dysfunctions for 28-day mortality in logistic regression analysis
| Number of organ dysfunction | n | Mortality | OR (95% CI) Crude | P-value |
|---|---|---|---|---|
| 0 | 33 | 4/33 (12.1) | Reference | |
| 1 | 81 | 20/81 (24.7) | 2.38 (0.74–7.59) | 0.144 |
| 2 | 49 | 9/49 (18.4) | 1.63 (0.46–5.81) | 0.451 |
| 3 | 36 | 20/36 (55.6) | 9.06 (2.64–31.15) | 0.001 |
| ≥ 4 | 20 | 10/20 (50.0) | 7.25 (1.85–28.36) | 0.004 |
OR odds ratio, CI confidence interval
C-statistics = 0.69, Hosmer–Lemeshow test p = 1.000
Predictive factors for 28-day mortality
CCI, septic shock, initial lactate level, initial SOFA score, SAPS 3 score, CRRT, source control, and the number of dysfunctional organs were associated with 28-day mortality in the univariate analyses. In the multivariate logistic regression analysis, after adjusting for each risk and confounding factor, only the SAPS 3 score (p < 0.001) and CRRT (p < 0.001) were independently associated with higher 28-day mortality (Table 7).
Table 7.
Multivariable logistic regression analysis for 28-day mortality in patients with sepsis due to IAI
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (years), mean (± SD) | 1.01 (0.98–1.03) | 0.581 | ||
| BMI (kg/m2), mean (± SD) | 1.04 (0.97–1.12) | 0.239 | ||
| Charlson comorbidity index, mean (± SD) | 1.13 (1.00–1.26) | 0.039 | ||
| Septic shock, n (%) | 1.94 (1.07–3.51) | 0.029 | ||
| Initial lactate level (mmol/L), mean (± SD) | 1.19 (1.09–1.3) | < 0.001 | ||
| Initial SOFA score, mean (± SD) | 1.24 (1.12–1.36) | < 0.001 | ||
| SAPS 3 score, mean (± SD) | 1.05 (1.03–1.08) | < 0.001 | 1.04 (1.02–1.07) | < 0.001 |
| CRRT, n (%) | 109.58 (24.89–482.48) | < 0.001 | 7.71 (3.76–15.82) | < 0.001 |
| Bacteremia, n (%) | 1.09 (0.61–1.95) | 0.780 | ||
| Time zero to time of antibiotic administration (min) | 1.00 (1.00–1.01) | 0.330 | ||
| Source control, n (%) | 2.38 (1.26–4.51) | 0.008 | 2.02 (0.95–4.30) | 0.069 |
| Source control time (h), mean (± SD) | 1.00 (1.00–1.00) | 0.660 | ||
| Number of organ dysfunction, n (%) | 1.66 (1.30–2.13) | < 0.001 | ||
| MDR, n (%) | 0.61 (0.32–1.17) | 0.136 | ||
IAI intra-abdominal infection, OR odds ratio, CI confidence interval, BMI body mass index, SOFA Sequential Organ Failure Assessment, SAPS 3 simplified acute physiology score III, CRRT continuous renal replacement therapy, MDR multidrug resistance
Multiple logistic: C-statistics = 0.83, Hosmer–Lemeshow test p = 0.53
Discussion
Despite the increased incidence of sepsis and septic shock, recent studies have consistently shown a decrease in sepsis mortality over time, owing to advances in medical technology [1, 13]. However, the overall in-hospital mortality rate due to sepsis was still quite high at 29.0% in some recent studies in Korea [10, 14]. Similarly, our study showed that the overall in-hospital mortality rate with sepsis was 28.1%, and the mortality rate for sepsis caused by IAI was 23.3%. In addition, in the ICU, the overall sepsis mortality rate was 35.1%, and the mortality rate of sepsis due to IAI was 28.8%. The finding of higher sepsis mortality rates in the ICU is not surprising because patients treated in the ICU tend to have more severe disease than patients treated in the wards, but more efforts should be made to reduce the high mortality rates.
There have been many studies on tools for predicting the progress and prognosis of the disease, which are still underway [15–17]. One of the most important factors affecting the mortality and clinical outcomes in sepsis is comorbidity, and the CCI can quantify a patient's comorbidity. In this time of aging populations, the CCI of patients is increasing. The CCI is widely used as a tool to predict the mortality rate of patients with sepsis due to IAI and determine their prognosis in advance [14, 18, 19]. In our study, the mean CCI in the non-survivor group was higher than that in the survivor group, with significant differences (5.9 ± 3.0 vs. 5.1 ± 2.3, p = 0.011). In addition, CCI (p = 0.039, OR = 1.13 [1.00–1.26]) was an independent risk factor for mortality prediction in the univariate logistic regression analysis.
Multicenter research and efforts are being conducted worldwide to improve the clinical outcomes of patients with sepsis. For instance, the Surviving Sepsis Campaign released new guidelines for the treatment of sepsis and a new updated “Hour-1 bundle” for sepsis treatment [20, 21]. Immediate resuscitation, initial early screening, antibiotic treatment, and source control are imperative to improve patient outcomes [14]. In addition, initial lactate levels and initial SOFA scores can be used to predict clinical progress and prognosis of sepsis. A large number of studies have already shown that, the higher the initial lactate level and initial SOFA score, the higher the mortality rate in patients with sepsis [22, 23]. In our study, initial lactate level and SOFA score were also independent risk factors in predicting the mortality rate in patients with sepsis caused by IAI (p < 0.001).
In the treatment of patients with sepsis due to IAI, the usage of antibiotics and source control is very important. Adequate and swift antibiotic administration and coverage from the time of recognition of sepsis are vital [24–27]. Although no significant difference was noted, the non-survivor group showed a tendency of delayed antibiotic administration in our study compared with that in the survivor group (217.5 ± 422.8 min vs. 171.4 ± 238.8 min, p = 0.495). Sepsis caused by IAI due to biliary sepsis, intestinal perforation, postoperative leakage, and intra-abdominal abscess can be controlled using open laparotomy, percutaneous transhepatic biliary drainage, and percutaneous catheter drainage insertion. Ultimately, the prognosis of patients with sepsis depends on the source controls, and the faster the source control, the lower is the mortality rate [28, 29]. Our study showed a significantly higher number of survivors in the source control group (46.8% vs. 27.0%, p = 0.007).
In several studies on IAI, E. coli and K. pneumoniae were the most common gram-negative causative pathogens. In gram-positive pathogens, most of them were E. faecalis and E. faecium [6, 9]. In our study, the same pathogens were also detected and the proportion was similar, as previously mentioned. In several studies, identifying causative pathogens were not significantly correlated to mortality [10, 30, 31]. Likewise, in our study, there was no significant difference in the identification of causative pathogens between the survivor group and the non-survivor group. However, a study by Gupta et al. [32] showed that the mortality rate was higher in patients in whom the causative pathogen was not identified, and that non-identification of the pathogen was an independent predictor of death. Additionally, some studies on IAI have shown that MDR is an independent risk factor for mortality [6, 9, 33]. However, the impact of MDR on mortality was not significant in our study (p = 0.136).
Organ dysfunction is a useful prognostic indicator for mortality in patients with sepsis, and the mortality rate increases significantly as the number of organ dysfunctions increases [34–36]. In one study by Umegaki et al. [34], patients with three (23.5%) and four or more organ dysfunctions (38.9%) had over two and four times the ICU mortality rates, respectively, compared with that of single organ dysfunction (8.9%). In addition, the hazard ratios were 1.6, 2.0, and 2.7 in 2-, 3-, and 4 or more organ dysfunctions, respectively, showing an increasing trend as the number of organ dysfunctions increases. In our study, the mortality rate was more than two-fold higher in patients with three organ dysfunctions (55.6%) or four or more organ dysfunctions (50.0%) than that of patients with a single organ dysfunction (24.7%) or two organ dysfunctions (18.4%). The ORs were also 9.06 (p = 0.001) and 7.25 (p = 0.004) in patients with three and four or more organ dysfunctions, respectively. Similarly, the dysfunction of three or more organs in our study could be considered as an independent risk factor for mortality.
The impact of each organ dysfunction on mortality and the proportion of organ dysfunction occurring in patients with sepsis varies between the studies. In several studies of organ dysfunction in patients with sepsis, respiratory and cardiovascular dysfunction were the most common [13, 34]. However, in our study of patients with sepsis due to IAI, respiratory and renal dysfunction were the most common. In some studies of patients with sepsis, respiratory and cardiovascular dysfunctions had a higher mortality rate than that of patients with other organ dysfunctions [13, 34–36]. However, in our study, the mortality rate was higher in patients with CNS or coagulation dysfunction than in patients with other organ dysfunctions. There are few studies on the impact of each organ dysfunction on mortality in sepsis caused by IAI, so the exact mechanisms by which different organ dysfunctions are associated with death are poorly understood. However, some other studies have shown a higher mortality rate in patients with CNS or coagulation dysfunction than in patients with respiratory or cardiovascular dysfunction [36, 37], as in our study.
The coagulation cascade may be abrogated owing to the aberrant expression of cytokines and tissue factors in response to sepsis with systemic inflammation. Microvascular thrombosis and ischemia are the most important processes in sepsis, causing tissue damage and multiple organ dysfunctions [38, 39]. As the coagulation system is disordered, sepsis-induced coagulopathy occurs, and manifestations of bleeding increase [38–40]. Several studies have shown that coagulopathy in patients with sepsis is associated with high mortality rates [41, 42]. In our study, coagulation dysfunction was closely related to mortality, which may have been due to sepsis-induced coagulopathy or bleeding tendency. However, in this study, data were not collected on the cause of death, so it is unknown whether the deaths were caused by bleeding.
This study has several limitations. First, this was a retrospective study based on medical records conducted in multiple institutions. Each hospital differed in its treatment policy, level, and system, including its facilities. As this study did not only focus on sepsis caused by IAI, the data may also be inadequate. To overcome these limitations, a prospective study of patients with sepsis caused by IAIs may be needed.
Conclusions
In our study, CCI, septic shock, initial lactate level, initial SOFA score, SAPS 3 score, acute kidney injury with CRRT, source control, and the number of dysfunctional organs were found to be independent risk factors affecting 28-day mortality. Among the organ dysfunctions in sepsis caused by IAI, coagulopathy was found to be an independent risk factor for 28-day mortality. Therefore, more intensive care may be needed because the prognosis could be worse in patients with coagulopathy than in those with other organ dysfunctions due to IAI.
Acknowledgements
Not applicable.
Consortium list: The following persons and institutions participated in the Korean Sepsis Alliance (KSA): Steering Committee—Chae-Man Limc (Chair), Sang-Bum Hongc, Dong Kyu ohc, Gee Young Suhd, Kyeongman Jeond, Ryoung-Eun Kod, Young-Jae Choe, Yeon Joo Leee, Sung Yoon Lime, Sunghoon Parkf
Participated Persons and Centers: Chae-Man Limc, Suk-Kyung Hongb, Sang Hyun Kwakg, Song-I Leeh, Jae Young Mooni, Kyung Chan Kimj, Sunghoon Parkf, Tai Sun Parkk, Youjin Changl, Gil Myeong Seongm, Heung Bum Leen, Jeongwon Heoo, Jae-myeong Leep, Woo Hyun Choq, Kyeongman Jeond, Yeon Joo Leee, Sang-Min Leer, Su Hwan Lees, Jong-Joon Ahnt, Eun Young Choiu.
bDivision of Acute Care Surgery, Department of Surgery, Asan Medical center, University of Ulsan College of Medicine, Seoul, Republic of Korea; cDepartment of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; dDepartment of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; eDepartment of Pulmonary and Critical Care Medicine, Seoul National University Bundang Hospital, Seongnam, Republic of Korea; fDepartment of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Republic of Korea; gDepartment of Anesthesiology, Chonnam National University Hospital, Gwangju, Republic of Korea; hDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Republic of Korea; iDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chungnam National University Sejong Hospital, Sejong, Republic of Korea; jDepartment of Internal Medicine, Daegu Catholic University Medical Center, Daegu Catholic University College of Medicine, Daegu, Republic of Korea; kDivision of Pulmonology, Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Republic of Korea; lDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Inje University Sanggye Paik Hospital, Seoul, Republic of Korea; mDepartment of Internal Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Republic of Korea; nDepartment of Internal Medicine, Research Center for Pulmonary Disorders, Jeonbuk National University Medical School, Jeonju, Republic of Korea; oDepartment of Internal Medicine, Kangwon National University Hospital, Chuncheon, Republic of Korea; pDepartment of Acute Care Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea; qDivision of Allergy, Pulmonary and Critical Care Medicine, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea; rDepartment of Pulmonary and Critical Care Medicine, Seoul National University Hospital, Seongnam, Republic of Korea; sDepartment of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; tDepartment of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Republic of Korea; uDivision of Respiratory and Critical Care Medicine, Department of Internal Medicine, Yeungnam University Medical Center, Daegu, Republic of Korea
Abbreviations
- IAI
Intra-abdominal infection
- ICUs
Intensive care units
- KSA
Korean Sepsis Alliance
- KDCA
Korea Disease Control and Prevention Agency
- SOFA
Sequential Organ Failure Assessment
- BMI
Body mass index
- CCI
Charlson Comorbidity Index
- SAP 3
Simplified acute physiology score III
- MDR
Multidrug resistance
- ORs
Odds ratios
- CRRT
Continuous renal replacement therapy
- CNS
Central nervous system
Author contributions
CH analyzed and interpreted the patient data regarding sepsis patients. JW and HJ reviewed the literature and contributed to manuscript drafting. All authors read and approved the final manuscript.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (fund code 2020E280700).
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Asan Medical Center (approval number 2018-0675). And this study also approved by the Institutional Review Boards of all participating hospitals; Chonnam National University Hospital (approval number CNUH2029075), Chungnam National University Hospital (approval number 2019-11-048-001), Daegu Catholic University Medical Center (approval number CR-18-163-L), Hallym University Sacred heart hospital (approval number 2018-09-004), Hanyang University Guri Hospital (approval number 2020-08-015), Inje University Sanggye Paik Hospital (approval number 2018-08-014), Jeju National University Hospital (approval number 2018-06-012), Jeonbuk national university hospital (approval number CUH 2018-10-027), Kangwon national university hospital (approval number 2018-08-004-001), Korea University Anam Hospital (approval number 2 0 1 9 A N 0 0 2 7), Pusan National University Yangsan Hospital (approval number 05-2019-092), Samsung Medical Center (approval number 2018-05-108), Seoul National University Bundang Hospital (approval number B-1810-500-402), Seoul national university hospital (approval number H-1808-135-967), Ulsan university hospital (approval number UUH 2018-08-003). Data were collected and analyzed in an ethical manner while protecting the patients' right to privacy. The requirement for informed consent was waived by the Institutional Review Boards (IRB) of all participating hospitals owing to the non-interventional, observational study design.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suk-Kyung Hong, Email: skhong94@amc.seoul.kr.
the Korean Sepsis Alliance (KSA) investigators:
Chae-Man Lim, Sang-Bum Hong, Dong Kyu Oh, Gee Young Suh, Kyeongman Jeon, Ryoung-Eun Ko, Young-Jae Cho, Yeon Joo Lee, Sung Yoon Lim, Sunghoon Park, Chae-Man Lim, Suk-Kyung Hong, Sang Hyun Kwak, Song-I. Lee, Jae Young Moon, Kyung Chan Kim, Sunghoon Park, Tai Sun Park, Youjin Chang, Gil Myeong Seong, Heung Bum Lee, Jeongwon Heo, Jae-myeong Lee, Woo Hyun Cho, Kyeongman Jeon, Yeon Joo Lee, Sang-Min Lee, Su Hwan Lee, Jong-Joon Ahn, and Eun Young Choi
References
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3.Martin AB, Hartman M, Benson J, Catlin A, National Health Expenditure Accounts Team National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood) 2016;35:150–160. doi: 10.1377/hlthaff.2015.1194. [DOI] [PubMed] [Google Scholar]
- 4.Oh SY, Cho S, Kim GH, Jang EJ, Choi S, Lee H, et al. Incidence and outcomes of sepsis in Korea: a nationwide cohort study from 2007 to 2016. Crit Care Med. 2019;47:e993–e998. doi: 10.1097/CCM.0000000000004041. [DOI] [PubMed] [Google Scholar]
- 5.Lopez N, Kobayashi L, Coimbra R. A comprehensive review of abdominal infections. World J Emerg Surg. 2011;6:7. doi: 10.1186/1749-7922-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. doi: 10.1186/s13017-015-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–164. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 8.Wittmann DH, Schein M, Condon RE. Management of secondary peritonitis. Ann Surg. 1996;224:10–18. doi: 10.1097/00000658-199607000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blot S, Antonelli M, Arvaniti K, Blot K, Creagh-Brown B, de Lange D, et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: "AbSeS", a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019;45:1703–1717. doi: 10.1007/s00134-019-05819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon K, Na SJ, Oh DK, Park S, Choi EY, Kim SC, et al. Characteristics, management and clinical outcomes of patients with sepsis: a multicenter cohort study in Korea. Acute Crit Care. 2019;34:179–191. doi: 10.4266/acc.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen PB, Henriksen DP, Brabrand M, Lassen AT. Prevalence of organ failure and mortality among patients in the emergency department: a population-based cohort study. BMJ Open. 2019;9:e032692. doi: 10.1136/bmjopen-2019-032692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Kim K, Lee H, Ahn S. Epidemiology of sepsis in Korea: a population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin Exp Emerg Med. 2019; 6:49–63. 10.15441/ceem.18.007. [DOI] [PMC free article] [PubMed]
- 15.Balcan B, Olgun S, Torlak F, Sagmen SB, Eryuksel E, Karakurt S. Determination of factors affecting mortality of patients with sepsis in a tertiary intensive care unit. Turk Thorac J. 2015;16:128–132. doi: 10.5152/ttd.2015.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassyap CK, Abraham SV, Krishnan SV, Palatty BU, Rajeev PC. Factors affecting early treatment goals of sepsis patients presenting to the emergency department. Indian J Crit Care Med. 2018;22:797–800. doi: 10.4103/ijccm.ijccm_27_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Mesa JD, Marquez-Gomez I, Sena G, Buonaiuto VA, Mora-Ordoñez J, Salido M, et al. Factors associated with severe sepsis or septic shock in complicated pyelonephritis. Medicine. 2017;96:e8371. doi: 10.1097/md.0000000000008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emami-Razavi SH, Mohammadi A, Alibakhshi A, Jalali M, Ghajarzadeh M. Incidence of post-operative sepsis and role of Charlson Co-Morbidity Score for predicting postoperative sepsis. Acta Med Iran. 2016;54:318–322. [PubMed] [Google Scholar]
- 19.Sinapidis D, Kosmas V, Vittoros V, Koutelidakis IM, Pantazi A, Stefos A, et al. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect Dis. 2018;18:242. doi: 10.1186/s12879-018-3156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavallee JF, Gray TA, Dumville J, Russell W, Cullum N. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci. 2017;12:142. doi: 10.1186/s13012-017-0670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teles F, Rodrigues WG, Alves M, Albuquerque CFT, Bastos SMO, Mota MFA, et al. Impact of a sepsis bundle in wards of a tertiary hospital. J Intensive Care. 2017;5:45. doi: 10.1186/s40560-017-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freund Y, Lemachatti N, Krastinova E, Van Laer M, Claessens YE, Avondo A, et al. Prognostic accuracy of Sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA. 2017;317:301–308. doi: 10.1001/jama.2016.20329. [DOI] [PubMed] [Google Scholar]
- 23.Lie KC, Lau CY, Van Vinh Chau N, West TE, Limmathurotsakul D, for Southeast Asia Infectious Disease Clinical Research N. Utility of SOFA score, management and outcomes of sepsis in Southeast Asia: a multinational multicenter prospective observational study. J Intensive Care. 2018; 6:9. 10.1186/s40560-018-0279-7. [DOI] [PMC free article] [PubMed]
- 24.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/ccm.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 25.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742–1748. doi: 10.1128/aac.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136:1237–1248. doi: 10.1378/chest.09-0087. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.ccm.0000217961.75225.e9. [DOI] [PubMed] [Google Scholar]
- 28.Bloos F, Thomas-Rüddel D, Rüddel H, Engel C, Schwarzkopf D, Marshall JC, et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18:R42. doi: 10.1186/cc13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18:R87. doi: 10.1186/cc13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JK, Lee J, Park YS, Lee CH, Yim JJ, Yoo CG, et al. Clinical manifestations of pneumonia according to the causative organism in patients in the intensive care unit. Korean J Intern Med. 2015;30:829–836. doi: 10.3904/kjim.2015.30.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigakis MJG, Jewell E, Maile MD, Cinti SK, Bateman BT, Engoren M. Culture-negative and culture-positive sepsis: a comparison of characteristics and outcomes. Anesth Analg. 2019;129:1300–1309. doi: 10.1213/ANE.0000000000004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture-negative severe sepsis: nationwide trends and outcomes. Chest. 2016;150:1251–1259. doi: 10.1016/j.chest.2016.08.1460. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Nunes J, Cardoso T. Intra-abdominal infections: the role of different classifications on the selection of the best antibiotic treatment. BMC Infect Dis. 2019;19:980. doi: 10.1186/s12879-019-4604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umegaki T, Ikai H, Imanaka Y. The impact of acute organ dysfunction on patients’ mortality with severe sepsis. J Anaesthesiol Clin Pharmacol. 2011;27:180–184. doi: 10.4103/0970-9185.81816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lone NI, Walsh TS. Impact of intensive care unit organ failures on mortality during the five years after a critical illness. Am J Respir Crit Care Med. 2012;186:640–647. doi: 10.1164/rccm.201201-0059OC. [DOI] [PubMed] [Google Scholar]
- 36.Nfor TK, Walsh TS, Prescott RJ. The impact of organ failures and their relationship with outcome in intensive care: analysis of a prospective multicentre database of adult admissions. Anaesthesia. 2006;61:731–738. doi: 10.1111/j.1365-2044.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakr Y, Lobo SM, Moreno RP, Gerlach H, Ranieri VM, Michalopoulos A, et al. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care. 2012;16:R222. doi: 10.1186/cc11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Iba T, Levi M, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Semin Thromb Hemost. 2020;46:89–95. doi: 10.1055/s-0039-1694995. [DOI] [PubMed] [Google Scholar]
- 40.Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 41.Lyons PG, Micek ST, Hampton N, Kollef MH. Sepsis-associated coagulopathy severity predicts hospital mortality. Crit Care Med. 2018;46:736–742. doi: 10.1097/CCM.0000000000002997. [DOI] [PubMed] [Google Scholar]
- 42.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.