Abstract
Background
Childhood adiposity is inversely associated with young adult percent dense breast volume (%DBV) and absolute dense breast volume (ADBV), which could contribute to its protective effect for breast cancer later in life. The objective of this study was to identify metabolites in childhood serum that may mediate the inverse association between childhood adiposity and young adult breast density.
Methods
Longitudinal data from 182 female participants in the Dietary Intervention Study in Children (DISC) and the DISC 2006 (DISC06) Follow-Up Study were analyzed. Childhood adiposity was assessed by anthropometry at the DISC visit with serum available that occurred closest to menarche and expressed as a body mass index (BMI) z-score. Serum metabolites were measured by untargeted metabolomics using ultra-high-performance liquid chromatography–tandem mass spectrometry. %DBV and ADBV were measured by magnetic resonance imaging at the DISC06 visit when participants were 25–29 years old. Robust mixed effects linear regression was used to identify serum metabolites associated with childhood BMI z-scores and breast density, and the R package mediation was used to quantify mediation.
Results
Of the 115 metabolites associated with BMI z-scores (FDR < 0.20), 4 were significantly associated with %DBV and 6 with ADBV before, though not after, adjustment for multiple comparisons. Mediation analysis identified 2 unnamed metabolites, X-16576 and X-24588, as potential mediators of the inverse association between childhood adiposity and dense breast volume. X-16576 mediated 14% (95% confidence interval (CI) = 0.002, 0.46; P = 0.04) of the association of childhood adiposity with %DBV and 11% (95% CI = 0.01, 0.26; P = 0.02) of its association with ADBV. X-24588 also mediated 7% (95% CI = 0.001, 0.18; P = 0.05) of the association of childhood adiposity with ADBV. None of the other metabolites examined contributed to mediation of the childhood adiposity–%DBV association, though there was some support for contributions of lysine, valine and 7-methylguanine to mediation of the inverse association of childhood adiposity with ADBV.
Conclusions
Additional large longitudinal studies are needed to identify metabolites and other biomarkers that mediate the inverse association of childhood adiposity with breast density and possibly breast cancer risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-022-01588-y.
Introduction
Maturation of the breasts occurs during adolescence, and the developing breast may be particularly susceptible to exposures related to later cancer risk. Childhood adiposity is associated with decreased breast cancer risk in adulthood [1–6], as well as decreased percent breast density [7–15]. Breast density is one of the strongest breast cancer risk factors. Risk increases monotonically with increasing density, and women with extremely dense breasts are at a fourfold excess risk of breast cancer compared to those with mostly fatty breasts [16, 17]. Approximately 4.7 million US women aged 40–74 years are estimated to have extremely dense breasts [18]. Results from cohort studies suggest that decreased breast density may mediate, in part, the protective effect of childhood adiposity on breast cancer risk [6, 13].
In our earlier analysis from the Dietary Intervention Study in Children (DISC) and the DISC 2006 (DISC06) Follow-Up Study, adiposity in childhood was significantly inversely associated with dense breast volume measured by magnetic resonance imaging (MRI); women who were heavier as children and adolescents had lower percent dense breast volume (%DBV) and less absolute dense breast volume (ADBV) in young adulthood independent of adult adiposity and several additional covariates [19]. Associations were strongest at the 3-year and 5-year DISC follow-up visits when participants were 11–15 years old, or around the age of menarche, which averaged 12.9 years in this cohort.
Overweight and obesity have a multitude of physiologic effects that could influence breast morphogenesis during childhood and subsequent breast density. To better understand early life determinants of adult breast density, we conducted an exploratory study that used untargeted metabolomics profiling in serum collected in childhood during DISC to identify potential mediators of the association of childhood adiposity with young adult breast density phenotypes measured during the DISC06 Follow-Up Study.
Materials and methods
Design
DISC was a multicenter randomized controlled clinical trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) to test the safety and efficacy of a dietary intervention to reduce serum low-density lipoprotein cholesterol (LDL-C) in children with elevated LDL-C. The trial’s design and results have been described previously [20–23]. Briefly, between 1988 and 1990, 301 healthy, prepubertal 8–10-year-old girls (and 362 boys) with elevated LDL-C were recruited into DISC at 6 clinical centers1 and randomized by the data coordinating center2 to a behavioral dietary intervention or usual care control group. Planned intervention continued until 1997 when the mean age of participants was 16.7 years. Assent was obtained from DISC participants and informed consent was obtained from parents/guardians prior to randomization. In 2006–2008 when participants were 25 to 29 years old, the DISC06 Follow-Up Study was conducted to evaluate the longer-term effects of the diet intervention on biomarkers associated with breast cancer in DISC female participants [24]. Informed consent was obtained from participants again prior to the DISC06 follow-up visit.
Participants
DISC participants originally were recruited through schools, health maintenance organizations and pediatric practices. A total of 301 8–10-year-old girls with elevated serum LDL-C who met several additional eligibility criteria were enrolled [20].
All female DISC participants were invited to participate in the DISC06 Follow-Up Study and 260 (86.4%) attended visits. Those who were pregnant or breast feeding at or within 12 weeks before visits (n = 30), who had breast augmentation or reduction surgery (n = 13), or whose breast MRI was missing or not technically acceptable (n = 35) were excluded leaving a total of 182 participants with breast density measurements.
DISC participants provided blood samples on multiple occasions during childhood. Because of limited volume of serum remaining, we measured metabolites in a single sample that had adequate volume (> 0.1 ml) and was collected at the DISC visit that occurred closest in time before or after menarche.
Data collection
Data and serum were collected previously in DISC or the DISC06 Follow-Up Study. DISC data were collected at baseline, before randomization and annually thereafter. Height and weight were measured, and a brief physical examination including Tanner staging of sexual maturation was performed. Data on demographics, medical history, medication use, physical activity and onset of menses were collected. At baseline, Year-1, Year-3, Year-5 and last visits, a venous blood sample was collected. Girls who were postmenarcheal completed menstrual cycle calendars to estimate the day of the menstrual cycle when blood was collected. At these visits, three non-consecutive 24-h dietary recalls were collected over 2 weeks and averaged to estimate nutrient intake [25].
For the DISC06 Follow-Up Study, participants attended a single visit between 2006 and 2008. Visits occurred within 14 days of onset of next menses whenever possible. Participant data, including demographics, medical and reproductive history, hormonal contraceptive and medication use, and physical activity, were collected on the same day, while 24-h dietary recalls were collected over two weeks.
In both studies, centralized data collection training was conducted prior to data collection. Data were collected by staff masked to treatment group assignment.
Anthropometry
Height and weight were measured annually in DISC and again in DISC06 using the same procedures. Height was measured using a stadiometer, and weight was measured on an electronic or beam balance scale. Measurements were taken twice. A third measurement was taken if the first two measurements were not within allowable tolerances (0.5 cm for height and 0.2 kg for weight) and the two closest values were averaged. BMI was calculated as wt(kg)/ht(m2) and for DISC visits during childhood expressed as a z-score relative to Centers for Disease Control and Prevention (CDC) 2000 Growth Charts [26] to account for changes with age. The BMI z-score at the DISC visit when blood used for metabolomics assays was collected was used in all analyses.
Blood collection and processing
Blood was collected at DISC and DISC06 follow-up visits in the morning after an overnight fast by venipuncture using standard procedures. Blood was allowed to clot for 45 min at room temperature and centrifuged at 1500×g for 20 min before separating serum, which was aliquoted into glass vials in DISC and cryovials in DISC06 and stored continuously at − 80 °C.
Breast density assessment
Breast density was measured at the DISC06 follow-up visit using non-contrast MRI. Imaging was performed using a whole-body 1.5 Tesla or higher-field-strength MRI scanner and dedicated breast imaging radiofrequency coil. A standard protocol was followed consisting of a 3D T1-weighted fast gradient echo pulse sequence performed with and without fat suppression and in transaxial and coronal orientations. A 32–40 cm field of view was used for bilateral coverage.
MRI technologists at the clinical centers were individually trained to recognize and correct failures due to incomplete fat suppression, motion artifacts and inadequate breast coverage. Acceptable image quality on 3 volunteers was required for site certification. Participant scans that were inaccurate due to artifacts, motion or technique were excluded (n = 21).
All MRI image data were processed at the University of California San Francisco using customized software to identify the chest wall–breast tissue boundary and skin surface, and to separate breast fibroglandular and fatty tissue using a segmentation method based on fuzzy C-means (FCM) clustering [27]. FCM segmentation was performed using fat-suppressed images; nonfat-suppressed images were used when incorrect or failed segmentation occurred due to poor fat suppression. In problematic cases that could not be segmented with automated FCM methods, manual delineation was used.
Separately for each breast, total breast volume and ADBV were measured and absolute non-dense breast volume (ANDBV) was estimated by subtraction. %DBV was calculated as the ratio ADBV:total breast volume × 100. All breast density measures on the two breasts were highly correlated (r > 0.94). Results for the two breasts were averaged to provide single measures of %DBV, ADBV and ANDBV for each participant.
Metabolomics assays
Untargeted metabolomic profiling was performed by Metabolon (Durham, NC). DISC serum samples were randomly ordered with 10% blind quality control (QC) samples integrated throughout to monitor laboratory performance. A pooled matrix sample served as a technical replicate throughout analyses, extracted water samples served as process blanks, and a cocktail of QC standards that was spiked into every sample allowed instrument performance monitoring and aided chromatographic alignment. Forty-two technical replicates from DISC06 samples that had previously been analyzed by Metabolon were re-assayed to facilitate comparison of metabolite levels in serum collected in childhood and young adulthood. A limited dataset with recalibrated levels of named metabolites measured in 180 participants with both DISC and DISC06 samples was created to allow adjustment for adult metabolite levels when analyzing associations of child levels with breast density.
Samples were prepared using the automated MicroLab StAR system (Hamilton Co.). Proteins were precipitated with methanol and the resulting extract was divided into 5 fractions for analysis by four ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) methods with one sample reserved for backup. Samples were placed briefly on a TurboVap (Zymark) to remove the organic solvent and were stored overnight under nitrogen before preparation for analysis.
All methods used a Waters ACQUITY ultra-high-performance liquid chromatography and a Thermo Scientific Q-Exactive high-resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. After drying, sample extracts were reconstituted in solvents compatible with each of the four analytical methods. Details of the methods have been reported previously [28, 29]. Briefly, two aliquots were analyzed under acidic positive ion conditions using a C18 column. One was chromatographically optimized for more hydrophilic compounds, whereas the other chromatographically optimized for more hydrophobic compounds. The third aliquot was analyzed under basic negative ion optimized conditions following gradient elution on a dedicated C18 column. The fourth aliquot also was analyzed under negative ionization following gradient elution from a HILIC column. The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion and a scan range of 70–1000 m/z.
Compounds were identified by comparison to library entries of purified standards or recurrent unknowns. Biochemical identifications were based on three criteria: retention index, accurate mass match to the library and the MS/MS forward and reverse scores between the experimental data and authentic standards. At the time DISC samples were analyzed, more than 3300 commercially available purified standard compounds had been acquired and characterized.
Proprietary visualization and interpretation software were used to confirm the consistency of peak identification among samples. Peaks were quantified using area under the curve. A data normalization step corrected for day-to-day variation from instrument tuning differences.
Biochemicals are named according to the following guidelines. Biochemicals without any symbols appended to the end of their name were confirmed based on an authentic chemical standard. Biochemicals with a single asterisk appended to the end of their name were not confirmed based on a standard, but Metabolon is confident in their identify. Biochemicals with a double asterisk appended to the end of their name do not have a standard available, but Metabolon is reasonably confident in their identity. Biochemicals with a number appended to the end of their name are structural isomers of another biochemical in Metabolon’s library.
Statistical analysis
A total of 880 biochemicals including 650 named biochemicals of known identify and 230 unnamed biochemicals of unknown structural identity were semi-quantified as relative peak intensity by Metabolon. Metabolites with ≥ 30% of values less than the limit of detection or with coefficients of variation ≥ 25% calculated from masked quality control samples were dropped, leaving 571 metabolites for analysis. For metabolites with < 30% of values below the limit of detection, undetected values were imputed at the lowest observed value. Metabolites were transformed to the natural log scale, and extreme values were winsorized using the median absolute deviation [30].
Statistical models
The hypothesized associations among childhood BMI z-scores, childhood serum metabolites and young adult breast density phenotypes are shown in Fig. 1. Childhood BMI z-score could directly influence adult breast density and/or act indirectly via childhood serum metabolites. We, therefore, conducted a series of analyses to evaluate associations of BMI z-scores with breast density and serum metabolites, and serum metabolites with breast density. Associations were evaluated using robust mixed effects multivariable linear regression implemented using the R package robustlmm [31]. P-values, which are not reported by robustlmm, were estimated by borrowing degrees of freedom (df) from the same model fit with R package lmerTest [32]. All analyses were conducted with 2 levels of adjustment. Initial models adjusted for age at childhood BMI measurement (years, continuous) and treatment group assignment as fixed effects and DISC clinic as a random effect. When breast density phenotypes were the dependent variables, BMI and BMI2 at time of breast density assessment (continuous) also were included as fixed effects. Fully adjusted models with serum metabolites as the dependent variables also included fixed effects for race (white/nonwhite), whereas fixed effects for race, college graduate (yes/no), duration hormone use (continuous), number live births (0/1+) and current smoker (yes/no) also were included when breast density phenotypes were the dependent variables. These covariates were identified by backward stepwise elimination. When serum metabolites were included in models either as dependent or independent variables, menstrual cycle phase at blood collection was adjusted for by including a factor with 4 levels—premenarche/follicular phase/luteal phase/postmenarche unknown phase. Associations of BMI z-score with serum metabolites and serum metabolites with breast density were adjusted for multiple comparisons using the Benjamini Hochberg false discovery rate (FDR).
Fig. 1.
Hypothesized associations among BMI z-scores, serum metabolites and breast density. Childhood adiposity (BMI z-score) could affect adult breast density directly or serum metabolites could mediate this association
BMI z-score and breast density
The association of childhood BMI z-score with breast density was assessed separately for %DBV, ADBV and ANDBV. ADBV and ANDBV were natural log transformed prior to analysis. Breast density phenotype was modeled as a continuous dependent variable while childhood BMI z-score was included as a continuous fixed effect. Percent difference in ADBV and ANDBV for each unit increase in BMI z-score was estimated from the model coefficient for BMI z-score as (exp(β) − 1) × 100 [33].
BMI z-score and serum metabolites
Associations of childhood BMI z-score with serum metabolites were evaluated similarly to ADBV and ANDBV except natural log transformed serum metabolite levels were included as the dependent variable. Percent difference in metabolite levels for each unit increase in BMI z-score were calculated by back transforming the model coefficient for BMI z-score as shown above for ADBV and ANDBV.
Serum metabolites and breast density phenotypes
Metabolites that were associated with BMI z-score at FDR < 0.20 were evaluated in association with %DBV and ADBV. For these analyses, breast density phenotype was modeled as a continuous dependent variable and natural log transformed serum metabolite levels and BMI z-scores were included as continuous fixed effects. The difference in %DBV for a 10% increase in serum metabolite was estimated from the model coefficient for the metabolite as β × ln(1.10) [33]. Percent difference in ADBV for a 10% increase in serum metabolite was estimated from the model coefficient for the metabolite as (1.10β − 1) * 100 [33].
To explore the influence of dietary intake of nutrients on associations of nutrient metabolites with breast density phenotypes, average intakes from foods and supplements from three 24-h dietary recalls collected at the DISC visit when blood was collected were included as fixed effects in fully adjusted models described above.
Spearman correlations were used to estimate associations between metabolite levels in serum from childhood (DISC) and adulthood (DISC06) using the limited dataset described under Metabolomics Assays. Models described earlier were refit including fixed effects for both child and adult metabolite levels to evaluate whether these correlations explained associations between child metabolite levels and young adult breast density.
Mediation analysis
Mediation analysis was performed using the model-based approach as implemented in R package mediation [34]. Because childhood BMI z-scores were inversely associated with %DBV and ADBV, mediation analysis was performed for metabolites associated in opposite directions with BMI z-score (FDR < 0.20) and these breast density phenotypes (P < 0.05). Two multivariable linear regression models were fit for each metabolite–breast density phenotype combination evaluated. The first model included the metabolite as the dependent variable and BMI z-score, age, treatment group assignment, race and menstrual cycle phase at blood collection as fixed effects. The second model included the breast density phenotype as the dependent variable and metabolite, BMI z-score, age, treatment group assignment and several additional potential confounders measured at the DISC06 visit described above as fixed effects. Mediation was evaluated by applying the function mediate to these two models with BMI z-score as the ‘treatment’ and the metabolite as the ‘mediator,’ using bootstrap variances estimated with 5000 simulations.
All tests of statistical significance were two-sided. All analyses were conducted using SAS 9.4 and R 4.1 statistical software.
Results
Table 1 summarizes participant characteristics during childhood (at the DISC visit when blood used for metabolomic assays was collected) and young adulthood (at the DISC06 follow-up visit). Ninety percent of participants were white, their mean age at blood collection was 11.63 ± 2.13 years, and their mean BMI z-score was 0.34 ± 0.93. Participants’ mean age at menarche was 12.90 ± 1.26 years, and 26% were postmenarcheal at the visit. At the DISC06 follow-up visit, participants’ mean age was 27.17 ± 1.02 years and their mean BMI was 25.39 ± 5.36 kg/m2. Over half (66%) were college graduates, 71% were nulliparous, 58% were currently using hormonal contraceptives, and 24% were current smokers. Mean breast density phenotypes were 27.64 ± 20.48% for %DBV, 104.18 ± 70.64 cm3 for ADBV and 413.25 ± 364.27 cm3 for ANDBV.
Table 1.
Characteristics of participants (N = 182)
| Child characteristics | Mean (sd) |
|---|---|
| Age (year) | 11.63 (2.13) |
| BMI Z-score | 0.34 (0.93) |
| Age at menarche (year) | 12.90 (1.26) |
| N (%) | |
|---|---|
| Race | |
| White | 164 (90%) |
| Nonwhite | 18 (10%) |
| Days until start of next menses at blood collection | |
| Premenarche | 134 (74%) |
| Postmenarche | |
| ≤ 14 days (luteal) | 14 (8%) |
| > 14 days (follicular) | 19 (10%) |
| Unknown | 15 (8%) |
| Treatment group | |
| Intervention | 87 (48%) |
| Usual care | 95 (52%) |
| Young adult characteristics | Mean (sd) |
|---|---|
| Age (year) | 27.17 (1.02) |
| BMI (kg/m2) | 25.39 (5.36) |
| Duration hormone use (year) | 5.27 (3.65) |
| N (%) | |
|---|---|
| Education | |
| High school or equivalent | 18 (10%) |
| Some college | 44 (24%) |
| College graduate | 95 (52%) |
| Graduate school | 25 (14%) |
| Hormonal contraceptive use | |
| Current | 105 (58%) |
| Former | 66 (36%) |
| Never | 11 (6%) |
| Number live births | |
| 0 | 129 (71%) |
| 1 | 30 (16%) |
| 2+ | 23 (13%) |
| Smoking status | |
| Current | 44 (24%) |
| Former | 38 (21%) |
| Never | 100 (55%) |
| Breast phenotypes | Mean (sd) |
|---|---|
| Breast density (%) | 27.64 (20.48) |
| Dense breast volume (cm3) | 104.18 (70.64) |
| Non-dense breast volume (cm3) | 413.25 (364.27) |
BMI z-score and breast density
Associations of BMI z-scores with breast density phenotypes are shown in Table 2. BMI z-score was significantly inversely associated with %DBV and ADBV. In fully adjusted models, for each unit increase in BMI z-score, %DBV decreased by 3.43 (95% CI = − 6.04, − 0.82, P = 0.01) while ADBV decreased by 24.44% (95% CI = − 34.27%, − 13.14%, P = 0.0001). BMI z-score was not significantly associated with ANDBV, and this phenotype was not examined further.
Table 2.
Association of childhood BMI Z-score with young adult breast density phenotypes
| Phenotype | Minimally adjusted modela | Fully adjusted modelb | ||||
|---|---|---|---|---|---|---|
| Δ/unit BMI | 95% CI | P-value | Δ/unit BMI | 95% CI | P-value | |
| Breast density (%) | − 3.60 | − 6.30, − 0.90 | 9.70E−03 | − 3.43 | − 6.04, − 0.82 | 1.09E−02 |
| Dense breast Volume (cm3) | − 24.36% | − 34.66%, − 12.43% | 2.52E−04 | − 24.44% | − 34.27%, − 13.14% | 1.18E−04 |
| Non-dense breast volume (cm3) | − 8.59% | − 17.02%, 0.69% | 7.05E−02 | − 8.57% | − 17.04%, 0.78% | 7.30E−02 |
aEstimates are from robust mixed effects multivariable linear regression models including breast density phenotype as the dependent variable; childhood BMI z-score (continuous), age at childhood BMI measurement (continuous), treatment group assignment, BMI and BMI2 at breast density measurement in young adulthood (continuous) as fixed effects; and DISC clinic as a random effect
bEstimates are from robust mixed effects multivariable linear regression models as described above under (1) plus fixed effects for race (white/nonwhite), college graduate (yes/no), duration hormone use (continuous), number live births (0/1+) and current smoker (yes/no)
BMI z-score and serum metabolites
Figure 2 illustrates associations of BMI z-scores with serum metabolites from fully adjusted models by metabolite superclass. Detailed results for all metabolites are shown in Additional file 1: Table S1. One hundred and seven known and unknown metabolites representing diverse superclasses were associated with BMI z-scores at a FDR < 0.20 in fully adjusted models. Sphingomyelin (d18:2/14:0, d18:1/14:1)*, a lipid, was the most significantly associated (FDR = 7.45e−08); for each one unit increase in BMI z-score, sphingomyelin increased by 13.65% (95% CI = 9.49, 17.97).
Fig. 2.
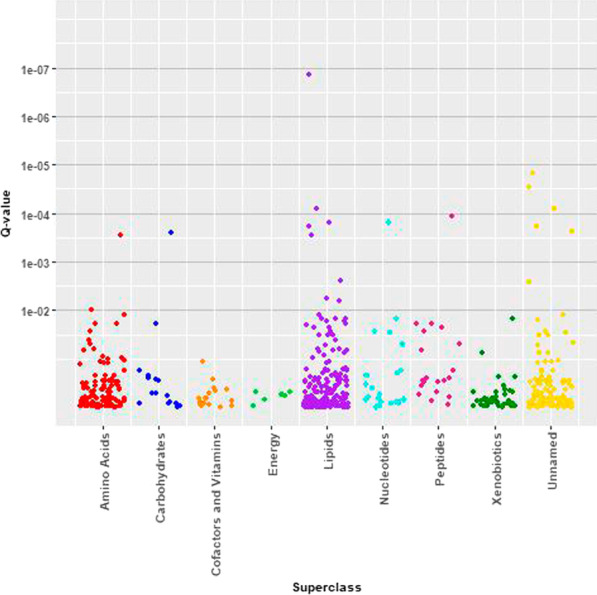
Manhattan plot—Associations of childhood adiposity (BMI z-scores) with serum metabolites by metabolite superclass membership
Serum metabolites and breast density phenotypes
Associations of %DBV and ADBV with serum metabolites that were associated with childhood BMI Z-score are shown in Tables 3 and 4, respectively. Associations of these metabolites with childhood BMI Z-score also are shown. Detailed results for all metabolites are shown in Additional file 2: Table S2 and Additional file 3: Table S3. Two unnamed metabolites, X-16576 and X-12104, were significantly positively associated with both %DBV and ADBV, while the amino acid lysine was significantly inversely associated with both these breast density phenotypes. For each 10% increase in serum lysine, %DBV decreased by 1.13 (95% CI = − 2.23, − 0.03) and ADBV decreased by 7.68% (95% CI = − 12.76%, − 2.31%). The nucleotide N1-methylinosine also was significantly positively associated with %DBV, while the unnamed metabolite X-24588, the nucleotide 7-methylquanine and the amino acid valine were significantly inversely associated with ADBV.
Table 3.
Association of %DBV with serum metabolites associated with childhood BMI z-score (FDR < 0.20) and association of these metabolites with BMI Z-score
| Metabolite | Superclass | Difference in %DBV associated with 10% increase in serum metabolitea | Percent difference in serum metabolites associated with a unit increase in BMI z-scoreb | ||||
|---|---|---|---|---|---|---|---|
| Δ | 95% CI | P-value | Δ | 95% CI | P-value | ||
| X-16576 | Unknown | 0.39 | 0.08, 0.71 | 1.57E−02 | − 13.65 | − 21.53, − 4.99 | 3.01E−03 |
| X-12104 | Unknown | 0.50 | 0.03, 0.97 | 3.88E−02 | 9.66 | 3.48, 16.22 | 2.15E−03 |
| lysine | Amino acid | − 1.13 | − 2.23, − 0.03 | 4.57E−02 | 3.05 | 0.50, 5.65 | 1.98E−02 |
| N1-methylinosine | Nucleotide | 0.76 | 0.01, 1.51 | 4.83E−02 | 6.92 | 2.95, 11.05 | 6.67E−04 |
| X-15492 | Unknown | − 0.31 | − 0.64, 0.01 | 6.11E−02 | 29.01 | 17.12, 42.10 | 6.56E−07 |
| X-17340 | Unknown | − 0.31 | − 0.66, 0.03 | 7.96E−02 | 28.52 | 17.89, 40.11 | 5.17E−08 |
| Androstenediol (3beta,17beta) monosulfate (2) | Lipid | − 0.23 | − 0.50, 0.04 | 9.51E−02 | 33.61 | 19.66, 49.19 | 6.96E−07 |
| 2-Aminoheptanoate | Lipid | 0.37 | − 0.06, 0.81 | 9.60E−02 | 8.25 | 0.90, 16.14 | 2.85E−02 |
aResults shown for metabolites associated with %DBV at P < 0.10. Estimates are from robust mixed effects multivariable linear regression models including %DBV as the dependent variable; childhood fixed effects – ln(metabolite level) (continuous), BMI z-score (continuous), age at BMI measurement (continuous), treatment group assignment, race (white/nonwhite) and menstrual cycle phase at blood collection (premenarche/luteal/follicular/unknown); adult fixed effects—BMI and BMI2 at breast density (continuous), college graduate (yes/no), duration hormone use (continuous), number live births (0/1 +), current smoker (yes/no); and DISC clinic as a random effect
bEstimates are from robust mixed effects multivariable linear regression models including ln(metabolite level) (continuous) as the dependent variable; childhood fixed effects—BMI z-score (continuous), age at BMI measurement (continuous), treatment group assignment, race (white/nonwhite) and menstrual cycle phase at blood collection (premenarche/luteal/follicular/unknown); and DISC clinic as a random effect
Table 4.
Association of ADBV with serum metabolites associated with childhood BMI Z-score (FDR < 0.20) and association of these metabolites with BMI Z-score
| Metabolite | Superclass | Percent difference in ADBV associated with 10% increase in serum metabolitea | Percent difference in serum metabolites associated with a unit increase in BMI z-scoreb | ||||
|---|---|---|---|---|---|---|---|
| Δ | 95% CI | P-value | Δ | 95% CI | P-value | ||
| X-16576 | Unknown | 2.91 | 1.25, 4.60 | 6.92E−04 | − 13.65 | − 21.53, − 4.99 | 3.01E−03 |
| X-12104 | Unknown | 3.81 | 1.24, 6.45 | 4.06E−03 | 9.66 | 3.48, 16.22 | 2.15E−03 |
| lysine | Amino acid | − 7.68 | − 12.76, − 2.31 | 6.26E−03 | 3.05 | 0.50, 5.65 | 1.98E−02 |
| X-24588 | Unknown | − 2.84 | − 5.05, − 0.57 | 1.53E−02 | 8.18 | 0.73, 16.19 | 3.21E−02 |
| 7-Methylguanine | Nucleotide | − 5.13 | − 9.63, − 0.39 | 3.57E−02 | 4.77 | 1.45, 8.20 | 5.16E−03 |
| Valine | Amino acid | − 5.28 | − 10.01, − 0.31 | 3.90E−02 | 4.13 | 1.65, 6.66 | 1.20E−03 |
| Homoarginine | Amino acid | − 2.71 | − 5.40, 0.06 | 5.64E−02 | 7.22 | 1.63, 13.12 | 1.16E−02 |
| X-17340 | Unknown | − 1.70 | − 3.51, 0.14 | 7.13E−02 | 28.52 | 17.89, 40.11 | 5.17E−08 |
| 5alpha-androstan-3alpha,17beta-diol monosulfate (1) | Lipid | 0.99 | − 0.08, 2.06 | 7.14E−02 | 37.90 | 17.09, 62.41 | 1.66E−04 |
| N-acetylvaline | Amino acid | − 4.55 | − 9.25, 0.39 | 7.21E−02 | 3.68 | 0.63, 6.83 | 1.87E−02 |
| Glycochenodeoxycholate 3-sulfate | Lipid | − 1.01 | − 2.10, 0.10 | 7.63E−02 | − 14.80 | − 24.58, − 3.75 | 1.09E−02 |
| Kynurenine | Amino acid | − 2.72 | − 5.76, 0.42 | 9.10E−02 | 6.20 | 1.74, 10.85 | 6.65E−03 |
aResults shown for metabolites associated with ADBV at P < 0.10. Estimates are from robust mixed effects multivariable linear regression models including ADBV as the dependent variable; childhood fixed effects – ln(metabolite level) (continuous), BMI z-score (continuous), age at BMI measurement (continuous), treatment group assignment, race (white/nonwhite) and menstrual cycle phase at blood collection (premenarche/luteal/follicular/unknown); adult fixed effects—BMI and BMI2 at breast density (continuous), college graduate (yes/no), duration hormone use (continuous), number live births (0/1 +), current smoker (yes/no); and DISC clinic as a random effect
bEstimates are from robust mixed effects multivariable linear regression models including ln(metabolite level) (continuous) as the dependent variable; childhood fixed effects—BMI z-score (continuous), age at BMI measurement (continuous), treatment group assignment, race (white/nonwhite) and menstrual cycle phase at blood collection (premenarche/luteal/follicular/unknown); and DISC clinic as a random effect
Associations of lysine and valine with %DBV and ADBV were not materially changed after adjustment for dietary intake of the respective amino acid (data not shown). Levels of lysine, valine, N1-methylinosine and 7-methylguanine in serum from DISC and DISC06 were only weakly correlated, and adjustment for DISC06 levels also did not materially alter associations of DISC levels with breast density phenotypes (data not shown). Unnamed metabolites were not measured in DISC06 samples.
Mediation analysis
Mediation analysis identified the unnamed metabolite X-16576 as a potential mediator of the childhood adiposity–breast density association. This metabolite significantly mediated 14% (95% CI = 0.002, 0.46; P = 0.04) of the association with %DBV and 11% (95% CI = 0.01, 0.26; P = 0.02) of the association with ADBV. The unnamed metabolite X-24588 also mediated 7% of the childhood adiposity–ADBV association (95% CI = 0.001, 0.18; P = 0.05). No other metabolites contributed significantly to mediation of the childhood adiposity–%DBV association. Lysine, valine and the nucleotide 7-methylguanine each mediated 6–7% of the association of childhood adiposity with ADBV, though the effect was only borderline significant.
Discussion
This analysis confirmed the previously reported inverse association of childhood adiposity with adult breast density and identified two unnamed metabolites, X-16576 and X-24588, as potential mediators of this association. X-16576 significantly mediated 14% of the inverse association of childhood adiposity with %DBV and 11% of its inverse association with ADBV. X-24588 also significantly mediated 7% of the inverse association of childhood adiposity with ADBV. None of the other metabolites examined contributed to mediation of the inverse childhood adiposity–%DBV association, though there was some support for contributions of lysine, valine and 7-methylguanine to mediation of the inverse association of childhood adiposity with ADBV.
To our knowledge, this is the first study to examine associations of untargeted metabolite profiles in serum from childhood with adult breast density phenotypes. Because the goal of the analysis was to identify mediators of the inverse association between childhood adiposity with %DBV and ADBV, only metabolites that were associated with childhood BMI z-score were evaluated in association with breast density phenotypes. Nonetheless, we identified several named and unnamed metabolites associated with %DBV and ADBV at P < 0.05, though none remained significant after adjusting for multiple comparisons. The amino acid lysine was inversely associated with %DBV and ADBV, and valine was inversely associated with ADBV, as well. However, support for these amino acids mediating the inverse association between childhood adiposity and breast density was weak. Inverse associations of lysine and valine with breast density phenotypes suggest greater uptake from the circulation into tissues where they can be incorporated into proteins, regulate cellular processes or be metabolized. Lysine’s highly reactive terminal ε-amino group contributes importantly to protein stability and makes it a target for numerous posttranslational modifications that alter DNA–protein interactions and transcriptional activity with consequences for cancer, including breast cancer [35]. Collagen is rich in glycated lysine [36] and is a major constituent of dense breast stroma [37]. Extracellular lysine and other essential amino acids including valine are higher in dense compared to non-dense breast tissue where they are available to promote growth and proliferation [38]. Conversely, valine is a branched chain amino acid (BCAA) involved in regulation of protein synthesis, glucose homeostasis and the phosphoinositide 3-kinase-protein kinase B-mammalian target of rapamycin (PI3K-AKT-mTOR) signaling pathway [39]. mTOR regulates cellular proliferation and growth [40], and the PI3K-AKT-mTOR pathway is dysregulated in several cancers including breast cancer [41]. The PI3K-AKT signaling network also has been reported to integrate mechanical and biochemical signaling to control branching morphogenesis of mammary epithelial cells [42]. Specifically, PI3K-AKT is a positive regulator of mammary epithelial cell branching [43].
Few epidemiologic studies have evaluated serum/plasma amino acids with breast density or breast cancer risk. In the Mexican Teachers’ Cohort, lysine and valine in plasma from premenopausal women were not associated with percent mammographic density [44]. Regardless, disruption of BCAA biosynthesis and degradation was associated with breast cancer risk in the Korean Cancer Prevention Study-II [45], and higher plasma levels of valine were associated with increased breast cancer risk in the SU.VI.MAX prospective cohort [46]. Neither lysine nor valine were associated with breast cancer risk in 4 additional prospective cohorts [47–50].
7-Methylguanine was inversely associated with ADBV in our analysis, though similar to the amino acids, support for mediation of the inverse association of childhood adiposity with breast density was weak. 7-methylguanine is a marker of DNA damage caused by endogenous and exogenous methylating agents [51]. It is elevated in the urine of smokers [52–54], though at the time of blood collection in childhood only 2 DISC participants included in the current analysis reported smoking cigarettes. In a study of steel workers, urinary 7-methylguanine also was positively associated with age and inversely associated with BMI and an index of diet quality [53]. 7-Methylguanine also is a marker of RNA turnover and metabolic rate [55]. In the Alpha-Tocopherol Beta-Carotene (ATBC) Cancer Prevention Study, 7-methylguanine was associated with all-cause and cardiovascular disease mortality [56]. We are not aware of any reports of associations of 7-methylguanine with breast density or breast cancer risk.
Our study had several strengths. To ensure scientific rigor and data quality centralized data collection training was conducted prior to DISC and DISC06, and all questionnaire data, anthropometry and breast images were collected by trained personnel according to strict protocols. Serum was collected after an overnight fast and continuously stored at − 80 °C. Metabolites were measured by Metabolon, a leader in the field, using UPLC-MS/MS with inclusion of multiple quality control samples to monitor performance. Breast density was measured by MRI, which is not impaired by high parenchymal breast density, making it especially effective for younger women with dense breast tissue. MRI technologists at the clinical sites were individually trained, and acceptable image quality on 3 volunteers was required for site certification. The median %DBV in DISC06 was 21.9% with a range of 1.5–77.0%. Thus, even though young women, on average, have dense breasts, there was wide variation across women.
Our study also has some limitations. Most notably, our study was exploratory and the sample size of 182 women limited power to detect even moderate associations. Several factors could potentially limit generalizability of results—16% of otherwise eligible participants had missing or technically unacceptable breast MRIs and could not be included in analyses, most participants were Caucasian and well educated and DISC eligibility required elevated LDL-C at baseline. However, similar to others [57], we did not observe an association of LDL-C with breast density in DISC06. DISC was a clinical trial of a diet intervention aimed at blood cholesterol lowering during preadolescence with no expectations of influence on breast density. Regardless, we adjusted for treatment group assignment in all analyses. As a precaution due to the sensitivity of the young breast to radiation, in DISC06 we measured breast density by MRI, while most studies quantify breast density from mammographic images. Because breast density measured by MRI and mammography are highly correlated with r ≥ 0.75 [27, 58], associations of metabolites with breast density would not be expected to differ depending on the modality used to measure density. Though we adjusted for several covariates in analysis, uncontrolled confounding cannot be ruled out. Many of the metabolites evaluated, including the two most strongly associated with %DBV and ADBV, were unnamed, which hampers interpretation of results.
Conclusion
Childhood adiposity is inversely associated with adult breast density. Identification of serum metabolites that mediate this association could lead to discovery of underlying metabolic pathways and improve understanding of breast development in relation to breast density, an established breast cancer risk factor. Support from the current analysis for mediation of the inverse association of childhood adiposity with %DBV and ADBV was strongest for the unnamed metabolites X-16576 and X-24588, while it was more limited for the amino acids lysine and valine and the nucleotide metabolite 7-methylguanine. Larger studies in more diverse populations are needed.
Supplementary Information
Additional file 1. Percent difference in metabolite levels associated with a one unit increase in BMI z-score.
Additional file 2. Difference in %DBV associated with a 10% increase in metabolite.
Additional file 3. Difference in ADBV associated with a 10% increase in metabolite.
Acknowledgements
We thank all Dietary Intervention Study in Children 2006 Follow-Up Study participants. We also thank the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center Biostatistics and Bioinformatics Shared Service for its support.
Abbreviations
- ADBV
Absolute dense breast volume
- AKT
Protein kinase B
- ATBC
Alpha-Tocopherol Beta-Carotene Cancer Prevention Study
- BCAA
Branched chain amino acids
- BMI
Body mass index
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- DHEAS
Dehydroepiandrosterone sulfate
- DISC
Dietary Intervention Study in Children
- DISC06
Dietary Intervention Study in Children 2006 Follow-Up Study
- DNA
Deoxyribonucleic acid
- FCM
Fuzzy c-means
- FDR
False discovery rate
- HESI-II
Heated electrospray ionization
- LDL-C
Low-density lipoprotein cholesterol
- MRI
Magnetic resonance imaging
- mTOR
Mammalian target of rapamycin
- NHLBI
National Heart Lung and Blood Institute
- PI3K
Phosphoinositide 3-kinase
- QC
Quality control
- RIA
Radioimmunoassay
- SHBG
Sex hormone-binding globulin
- UPLC-MS/MS
Ultra-high-performance liquid chromatography–tandem mass spectroscopy
- %DBV
Percent dense breast volume
Author contributions
JFD, VJS, LGS and LVH contributed to study design and data collection; JFD, LSM and YZ contributed to data analysis; all authors contributed to data interpretation and manuscript preparation. All authors read and approved the final manuscript.
Funding
This work was supported by the National Institutes of Health (R01CA214783, R01CA104670, P30CA134274) and the Maryland Department of Health’s Cigarette Restitution Fund Program. The sponsors had no role in the study design, analysis, collection and interpretation of the data, the preparation of the manuscript or the decision to submit the manuscript for publication.
Availability of data and materials
All data from participants who consented to data sharing will be deposited in the NHLBI biorepository at BioLINCC.
Declarations
Ethics approval and consent to participate
Assent was obtained from DISC participants and informed consent was obtained from parents/guardians prior to randomization. Informed consent was obtained from participants again prior to the DISC06 follow-up visit. Institutional review boards at all participating clinical centers and the data coordinating center approved the original DISC protocol. The DISC06 protocol was also approved by the institutional review board at the Fox Chase Cancer Center.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Children’s Hospital, New Orleans, LA; Johns Hopkins Hospital, Baltimore, MD, Kaiser Permanente Center for Health Research, Portland, OR; University of Medicine and Dentistry of New Jersey, Newark, NJ; Northwestern University Medical School, Chicago, IL; University of Iowa Hospital and Clinics, Iowa City, IA.
Maryland Medical Research Institute, Baltimore, MD.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magnusson CM, Roddam AW, Pike MC, et al. Body fatness and physical activity at young ages and the risk of breast cancer in premenopausal women. Br J Cancer. 2005;93(7):817–824. doi: 10.1038/sj.bjc.6602758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardia A, Vachon C, Olson J, et al. Relative weight at age 12 and risk of postmenopausal breast cancer. Cancer Epidemiol Biomark Prev. 2008;17:374–378. doi: 10.1158/1055-9965.EPI-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer H, Tworoger S, Hankinson S, Willett W. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagherazzi G, Guillas G, Boutron-Ruault M, Clavel-Chapelon F, Mesrine S. Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur J Cancer Prev. 2013;22(1):29–37. doi: 10.1097/CEJ.0b013e328355ec04. [DOI] [PubMed] [Google Scholar]
- 5.Shawon M, Eriksson M, Li J. Body size in early life and risk of breast cancer. Br Cancer Res. 2017;19(1):84. doi: 10.1186/s13058-017-0875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice M, Bertrand K, VanderWeele T, et al. Mammographic density and breast cancer risk: a mediation analysis. Br Cancer Res. 2016;18(1):94. doi: 10.1186/s13058-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samimi G, Colditz G, Baer H, Tamimi R. Measures of energy balance and mammographic density in the Nurses' Health Study. Breast Cancer Res Treat. 2008;109(1):113–122. doi: 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 8.Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–64. doi: 10.1093/aje/kwm112. [DOI] [PubMed] [Google Scholar]
- 9.Harris H, Tamimi R, Willett W, Hankinson S, Michels K. Body size across the life course, mammographic density, and risk of breast cancer. Am J Epidemiol. 2011;174(8):909–918. doi: 10.1093/aje/kwr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack V, dos Santos SI, De Stavola B, et al. Life-course body size and perimenopausal mammographic parenchymal patterns in the MRC 1946 British birth cohort. Br J Cancer. 2003;89(5):852–859. doi: 10.1038/sj.bjc.6601207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffreys M, Warren R, Gunnell D, McCarron P, Smith G. Life course breast cancer risk factors and adult breast density (United Kingdom) Cancer Causes Control. 2004;15:947–955. doi: 10.1007/s10552-004-2473-2. [DOI] [PubMed] [Google Scholar]
- 12.Lope V, Pérez-Gómez B, Moreno MP, et al. Childhood factors associated with mammographic density in adult women. Breast Cancer Res Treat. 2011;130(3):965–974. doi: 10.1007/s10549-011-1664-2. [DOI] [PubMed] [Google Scholar]
- 13.Andersen Z, Baker J, Bihrmann K, Vejborg I, Sorensen T, Lynge E. Birthweight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16(1):R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoemaker M, Jones M, Allen S, et al. Childhood body size and pubertal timing in relation to adult mammographic density phenotype. Br Cancer Res. 2017;19(1):13. doi: 10.1186/s13058-017-0804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Berkey C, Herman C, et al. Adiposity change over the life course and mammographic breast density in postmenopausal women. Cancer Prev Res. 2020;13(5):475–482. doi: 10.1158/1940-6207.CAPR-19-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 17.Boyd N, Martin L, Yaffe M, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Br Cancer Res. 2011;13(6):223. doi: 10.1186/bcr2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprague B, Gangnon R, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255. doi: 10.1093/jnci/dju255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DISC Collaborative Research Group Dietary intervention study in children (DISC) with elevated low-density- lipoprotein cholesterol. Design and baseline characteristics. Ann Epidemiol. 1993;3(4):393–402. doi: 10.1016/1047-2797(93)90067-E. [DOI] [PubMed] [Google Scholar]
- 20.Bertrand K, Baer H, Orav E, et al. Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res. 2015;17(1):95. doi: 10.1186/s13058-015-0601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Writing Group for the DISC Collaborative Research Group Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low-density lipoprotein cholesterol. The Dietary Intervention Study in Children (DISC) JAMA. 1995;273(18):1429–35. doi: 10.1001/jama.1995.03520420045036. [DOI] [PubMed] [Google Scholar]
- 22.Obarzanek E, Hunsberger S, Van Horn L, et al. Safety of a fat-reduced diet: the Dietary Intervention Study in Children (DISC) Pediatrics. 1997;100(1):51–59. doi: 10.1542/peds.100.1.51. [DOI] [PubMed] [Google Scholar]
- 23.Obarzanek E, Kimm S, Barton B, et al. Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: seven-year results of the Dietary Intervention Study in Children (DISC) Pediatrics. 2001;107(2):256–64. doi: 10.1542/peds.107.2.256. [DOI] [PubMed] [Google Scholar]
- 24.Dorgan JF, Liu L, Klifa C, et al. Adolescent diet and subsequent serum hormones, breast density, and bone mineral density in young women: results of the Dietary Intervention Study in Children. Cancer Epidemiol Biomark Prev. 2010;19(6):1545–1556. doi: 10.1158/1055-9965.EPI-09-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Horn LV, Stumbo P, Moag-Stahlberg A, et al. The Dietary Intervention Study in Children (DISC): dietary assessment methods for 8- to 10-year-olds. J Am Diet Assoc. 1993;93(12):1396–1403. doi: 10.1016/0002-8223(93)92241-O. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski R, Ogden C, Guo S, et al. 2000 CDC growth charts for the United States: methods and development. National Center for Health Statistics. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 27.Klifa C, Carballido-Gamio J, Wilmes L, et al. Quantitation of breast tissue index from MR data using fuzzy clustering. In: Conference proceedings IEEE engineering in Medicine and Biology Society, vol. 3. 2004, p. 1667–1670. [DOI] [PubMed]
- 28.Dorgan J, Jung S, Dallal C, et al. Alcohol consumption and serum metabolite concentrations in young women. Cancer Causes Control. 2020;31(2):113–126. doi: 10.1007/s10552-019-01256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford L, Kennedy A, Goodman K, et al. Precision of a clinical metabolomics profiling platform for use in the identification of inborn errors of metabolism. J Appl Lab Med. 2020;5(2):342–356. doi: 10.1093/jalm/jfz026. [DOI] [PubMed] [Google Scholar]
- 30.Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. JESP. 2013;49(4):764–766. [Google Scholar]
- 31.Koller M. robustlmm: an R package for robust estimation of linear mixed-effects models. J Stat Softw. 2016;75(6):1–24. doi: 10.18637/jss.v075.i06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juju. Obtaining p-values in a robustlmm mixed model via Satterthwaite-approximated DFs of the equivalent lme4 model. 10 Aug 2020. https://stats.stackexchange.com/q/456032.
- 33.UCLA Statistical Consulting Group. https://stats.idre.ucla.edu/other/mult-pkg/faq/general/faqhow-do-i-interpret-a-regression-model-when-some-variables-are-log-transformed/.
- 34.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014 doi: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 35.Azevedo C, Saiardi A. Why lysine? The ongoing tale of one of the most modified amino acids. Adv Biol Regul. 2016;60:144–150. doi: 10.1016/j.jbior.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Ansari N, Moinuddin, Ali R. Glycated lysine residues: a marker for non-enzymatic protein glycation in age-related diseases. Dis Mark. 2011;30(6):317–24. doi: 10.1155/2011/718694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taroni P, Quarto G, Pifferi A, et al. Breast tissue composition and its dependenc on demographic risk factors for breast cancer: non-invasive assessment by time domain diffuse optical spectroscopy. PLoS ONE. 2015;10(6):e0128941. doi: 10.1371/journal.pone.0128941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahamsson A, Rzepecka A, Dabrosin C. Increased nutrient availability in dense breast tissue of postmenopausal women in vivo. Sci Rep. 2017;7:42733. doi: 10.1038/srep42733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19(4):954. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morita M, Grave S, Hulea L, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle. 2015;14(4):473–480. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014 doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu W, Nelson C. PI3K regulates branch initiation and extension of cultured mammary epithelia via Akt and Rac1 respectively. Dev Biol. 2013;379(2):235–245. doi: 10.1016/j.ydbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu W, Nelson C. PI3K signaling in the regulation of branching morphogenesis. BioSystems. 2012;109:402–411. doi: 10.1016/j.biosystems.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 44.His M, Lajous M, Liliana Gómez-Flores-Ramos L, et al. Biomarkers of mammographic density in premenopausal women. Br Cancer Res. 2021;23:75. doi: 10.1186/s13058-021-01454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo H, Kim M, Kim M, et al. Analysis of metabolites and metabolic pathways in breast cancer in a Korean prospective cohort: the Korean Cancer Prevention Study-II. Metabolomics. 2018;14:85. doi: 10.1007/s11306-018-1382-4. [DOI] [PubMed] [Google Scholar]
- 46.Lécuyer L, Dalle C, Lyan B, et al. Long-term breast cancer risk in the SU.VI.MAX prospective cohort. Cancer Epidemiol Biomark Prev. 2019;28(8):1300–7. doi: 10.1158/1055-9965.EPI-19-0154. [DOI] [PubMed] [Google Scholar]
- 47.His M, Viallon V, Dossus L, et al. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019;17(1):178. doi: 10.1186/s12916-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeleznik O, Balasubramanian R, Zhao Y, et al. Circulating amino acids and amino acid-related metabolites and risk of breast cancer among predominantly premenopausal women. NPJ Breast Cancer. 2021;7(1):54. doi: 10.1038/s41523-021-00262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore S, Mazzilli K, Sampson J, et al. A metabolomics analysis of postmenopausal breast cancer risk in the Cancer Prevention Study II. Metabolites. 2021;11:95. doi: 10.3390/metabo11020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore S, Playdon M, Sampson J, et al. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. J Natl Cancer Inst. 2018;110(6):588–597. doi: 10.1093/jnci/djx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ames B. Endogenous DNA damage as related to cancer and aging. Mutat Res. 1989;214:41–46. doi: 10.1016/0027-5107(89)90196-6. [DOI] [PubMed] [Google Scholar]
- 52.Loft S, Svoboda P, Kasai H, et al. Prospective study of urinary excretion of 7-methylguanine and the risk of lung cancer: effect modification by mu class glutathione-S-transferases. Int J Cancer. 2007;121(7):1579–1584. doi: 10.1002/ijc.22863. [DOI] [PubMed] [Google Scholar]
- 53.Tamae K, Kawai K, Yamasaki S, et al. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci. 2009;100(4):715–721. doi: 10.1111/j.1349-7006.2009.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ichiba M, Matsumoto A, Kondoh T, Horita M, Tomokuni K. Decreasing urinary PAH metabolites and 7-methylguanine after smoking cessation. Int Arch Occup Environ Health. 2006;79(7):545–549. doi: 10.1007/s00420-005-0071-6. [DOI] [PubMed] [Google Scholar]
- 55.Topp H, Schoch G. Whole-body degradation rates of transfer-, ribosomal-, and messenger ribonucleic acids and resting metabolic rate in 3- to 18-year-old humans. Pediatr Res. 2000;47(1):163–168. doi: 10.1203/00006450-200001000-00027. [DOI] [PubMed] [Google Scholar]
- 56.Huang J, Weinstein S, Moore S, et al. Serum metabolomic profiling of all-cause mortality: a prospective analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study Cohort. Am J Epidemiol. 2018;187(8):1721–1732. doi: 10.1093/aje/kwy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamburrini A, Woolcott C, Boyd N, et al. Associations between mammographic density and serum and dietary cholesterol. Breast Cancer Res Treat. 2011;125(1):181–189. doi: 10.1007/s10549-010-0927-7. [DOI] [PubMed] [Google Scholar]
- 58.Boyd N, Martin L, Chavez S, et al. Breast-tissue composition and other risk factors for breast cancer in young women: a cross-sectional study. Lancet Oncol. 2009;10(6):569–580. doi: 10.1016/S1470-2045(09)70078-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Percent difference in metabolite levels associated with a one unit increase in BMI z-score.
Additional file 2. Difference in %DBV associated with a 10% increase in metabolite.
Additional file 3. Difference in ADBV associated with a 10% increase in metabolite.
Data Availability Statement
All data from participants who consented to data sharing will be deposited in the NHLBI biorepository at BioLINCC.


