Abstract
Background
To investigate the association between low-carbohydrate-diet (LCD) score and cognitive performance based on a nationally representative sample aged ≥ 60 years from National Health and Nutrition Examination Survey (NHANES) database.
Methods
This cross-sectional study included 2,537 eligible older adults from the NHANES database 2011–2014. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) word learning subtest, Animal Fluency Test (AFT), and Digit Symbol Substitution Test (DSST) were used to assess the cognitive performance. All participants were categorized into the low and normal cognitive performance groups. The univariate and multivariate logistic regression analyses were utilized to evaluate the association of LCD score with cognitive performance. Stratified analyses based on age, body mass index (BMI), gender, marital status, education level was conducted.
Results
After adjusting age, education level, marital status, household income, history of diabetes, history of hypertension, history of congestive heart failure, history of coronary heart disease, history of heart disease, history of stroke, magnesium and the using of psychotropic medication, LCD score was correlated with the CERAD word learning subtest. The associations between LCD score and AFT, DSST were not statistically significant. Moreover, LCD score was also related to cognitive performance among individuals who were aged < 65 years or BMI 25–30 kg/m2 or was married/separated, or had an education level of high school or above.
Conclusion
The adherences to LCD might be associated with the risk of cognitive performance among older adults. Further large-scale cohort studies are needed to test the causal relationship of LCD and cognitive performance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03607-1.
Keywords: Low-carbohydrate-diet score, Cognitive performance, Older adults, NHANES
Background
Nowadays, global aging is increasing as life expectancy increases, and at the same time, cognitive decline related to age may be a primary health issue for elderly population [1]. It is estimated that the number of people in the United States suffered from cognitive impairment increased from 12.23 million in 2020 to 21.55 million in 2060 [2]. The irreversibility of cognitive impairment made the prevention and treatment of low cognitive performance a top priority [3]. Therefore, it is important to explore modifiable lifestyle and some risk factors to prevent low cognitive performance.
Diet is a kind of modifiable factor. It has previously been proposed that diet might play an important role in the intervention strategy for cognitive performance [4, 5]. Fan, et al. reported that adherence to higher Dietary Guidelines for Americans (DGA) was associated with a better cognitive performance (such as processing speed and executive function) for American adults aged ≥ 60 years [6]. Carbohydrates, fats, and proteins are considered as the important nutrients required for brain health, which has received much attention in a healthy diet [7]. Carbohydrates are the main source of energy in the diet and consumed mainly through foods such as rice, potatoes and grains [8]. In the study of Muth AK et al., they pointed out that carbohydrate consumption was associated with the cognitive function [7]. Additionally, Li Y, et al. found that dietary protein intake was positively correlated with cognitive function in older adults [9]. In the recent years, a low-carbohydrate-diet (LCD) score characterized by a diet with lower intake of carbohydrates and higher intakes of proteins and fats has been proposed [10]. LCD score has received widespread attention as a viable option for the treatment of losing weight and preventing obesity [11, 12]. Previous studies have showed that LCD score was linked to many aspects of health, such as diabetes mellitus, coronary artery calcium progression, psychological disorders, and so on [10, 13, 14]. Sangsefidi, et al. reported that LCD score might be associated with lower chance of metabolic syndrome in Iranian adults based on a cross-sectional study [11]. However, to the best of our knowledge, few studies have explored the associations of LCD score and cognitive performance in the older adults so far.
Our hypothesis in this study is that LCD score may be associated with cognitive performance for older adults due to oxidative stress and inflammation. We investigated the association of LCD score and cognitive performance based on a nationally representative sample of aged ≥ 60 years from National Health and Nutrition Examination Survey (NHANES) database.
Methods
Data sources and study population
In this cross-sectional study, all information of study population derived from the NHANES database. The NHANES data is a representative sample of the non-institutionalized population of the USA. It is a two-year-cycle program performed by the Centers for Disease Control and Prevention (CDC) of America using a multistage, probability sampling methods [1, 15]. NHANES examines a nationally representative sample of approximately 5,000 people from 15 different counties each year [3]. Its primary goal is to assess the health and nutritional status of adults and children in the United States. CDC obtained ethical approval and consented all the participants.
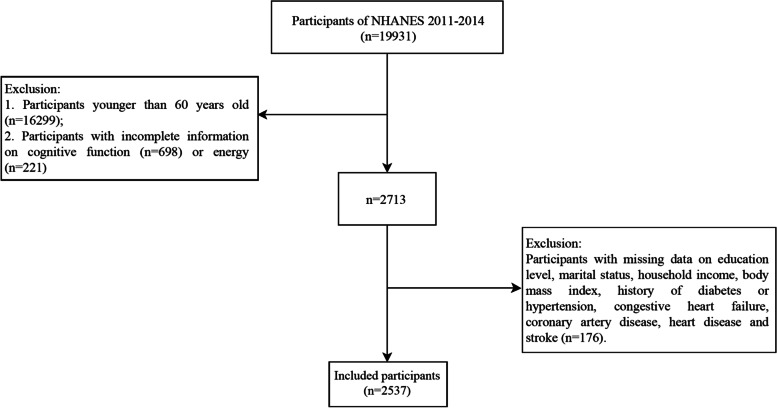
In the current study, we selected two NHANES cycles, namely, NHANES 2011–2012 and 2013–2014; because some cognitive function tests were specially conducted in these two NHANES cycles. A total of 19,931 people participated in the NHANES from 2011–2014. Our study was limited to 2,731 adults aged 60 years or older with complete information on cognitive function and energy. Among them, we excluded some individuals with missing information on education level, marital status, household income, body mass index (BMI), history of diabetes or hypertension, congestive heart failure, coronary artery disease, heart disease and stroke. Finally, 2,537 eligible older adults were included (Fig. 1). This study only obtained public data, so ethical review was not required by Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University.
Fig. 1.
Flowchart showing the selection of study population
Data collection
The following variables were extracted in this study [1, 16]: age (years), gender, race, education level, marital status, household income (5$), BMI (kg/m2), waistline (cm), history of diabetes, history of hypertension, history of congestive heart failure, history of coronary heart disease, history of heart disease, history of stroke, magnesium (mg), calcium (mg), vitamin D (mcg), the using of psychotropic medication, carbohydrate intake score, fat intake score, protein intake score. BMI was calculated as weight divided by height squared. History of diabetes or hypertension was defined as self-reported physician diagnosis.
Dietary assessment
All NHANES participants were asked to undergo a first dietary recall interview at the Mobile Examination Center, and a second interview data via telephone 3 to 10 days later [17]. For participants of NHANES database, dietary intake data were used to estimate the types and amounts of foods and beverages consumed during the 24‐hour period before the interview (midnight to midnight), and to estimate the energy, nutrition, and other food components consumed from these foods and beverages. In this study, dietary intake was estimated using the average of data from two 24-h dietary recall.
Calculation of the low-carbohydrate-diet score
The average dietary data from two 24-h dietary recall interview was used to calculate the intakes of fat, protein, carbohydrate and energy. LCD score was calculated based on a comprehensive assessment of fat, protein and carbohydrate [12]. The intakes per gram of fat, protein, and carbohydrate were first converted to kilocalories (conversion ratio: 1:9, 1:4, and 1:4, respectively); then we calculated the percentage of carbohydrate (kilocalories) and total energy, the percentage of protein (kilocalories) and total energy, and the percentage of fat (kilocalories) and total energy. For fat and protein, the highest percentage of intake was scored 10 points, and the lowest was 0 points. For carbohydrates, those with the lowest percentage of intake scored 10 points, and the highest intake scored 0 points (Supplemental Table 1). LCD score is the sum of the scores for the three nutrients, ranging from 0 to 30. The higher score reflects a higher intake of fat and protein and a lower intake of carbohydrate. In this study, LCD score was divided into five groups according to 20% quantiles, 40% quantiles, 60 quantiles and 80% quantiles: < 4 points group; 4–8 points group; 8–12 points group; 12–17 points group and > 17 points group.
Cognitive performance assessment
A series of cognitive function testing in the adults aged ≥ 60 years from NHANES were used in the 2011–2014, including the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) word learning subtest, Animal Fluency Test (AFT), Digit Symbol Substitution Test (DSST) [1].
CERAD word learning subtest [6], which consists of three consecutive learning trials as well as a delayed recall trial, is used to evaluate immediate and delayed memory. In the learning trials, participants were asked to read 10 unrelated words one at a time. After completing the DSST and AFT assessments, the delayed recall trial asked participants to recall 10 words used in the learning experiment. With scores ranging from 0 to 10 for each trial, and the final score of the CERAD test was the sum of three consecutive learning trials and a delayed recall trial.
AFT [1], as a component of executive function, is performed to assess the categorical verbal fluency. Participants were asked to name as many animals as possible in one minute with each named animal receiving one point, and the final score of AFT was the sum for the number of correctly named animals.
DSST [15] is used to assess processing speed, sustained attention and working memory. The test was performed by using a paper form with a key at the top pairing numbers with nine symbols. Participants were asked to match the corresponding symbols from the 133 boxes that held adjacent numbers in two minutes. The final score of DSST was the sum for the number of correct matches.
The outcome of this study was the cognitive performance, which contained CERAD word learning subtest, AFT, and DSST. Currently, there is no standard for the CERAD word learning subtest, AFT, and DSST tests to identify low cognitive performance. Therefore, we used the lowest quartile as the cut-off point; below or equal to the lower quartile was considered as low cognitive population, and above the lower quartile was normal population, which was also consistent with some literature [1, 18].
Statistical analysis
In the present study, we described the normally distributed variables by mean ± standard error (mean ± SE), and comparison between the low cognitive performance group and the normal cognitive performance group adopted Student’s t-test. The number of cases and the composition ratio [n (%)] was used to describe the categorical data, and comparison between two groups adopted χ2 test. “n” was considered as unweighted number, and “(%)” was considered as weighted percentage.
For the current study, all participants were categorized into the low cognitive performance group and the normal cognitive performance group. We conducted a descriptive analysis between two groups. Then we conducted the univariate and multivariate logistic regression analyses to examine the association between LCD score and cognitive performance. We adopted restricted cubic spline (RCS) curves to assess the dose–response relationship of LCD score and cognitive performance. Additionally, we also performed stratified analyses based on age, BMI, gender, marital status, education level to further examine the associations between LCD score and cognitive performance. The missing values were deleted in this study, and the proportion of missing values was shown in the Supplemental Table 2. In addition, we also performed the sensitivity analysis before and after deletion of missing values (Supplemental Table 3). SAS 9.4 and R 4.0.3 software were used for statistical analyses, and we calculated odds ratio (OR) and 95% confidence interval (CI). P < 0.05 was considered as statistically significant difference.
Results
Baseline characteristics
The baseline characteristics of all eligible participants were shown in Table 1. We found that there were some significant differences between the low cognitive performance group and the normal cognitive performance group according to CERAD word learning subtest in the distribution of carbohydrate intake score, age, education level, marital status, household income, height, history of diabetes, history of hypertension, history of congestive heart failure, history of coronary heart disease, history of heart disease, history of stroke, magnesium and the using of psychotropic medication. Likewise, there were some differences between the low cognitive performance group and the normal cognitive performance group according to AFT: gender, age, race, education level, marital status, household income, BMI, history of diabetes, history of hypertension, history of congestive heart failure, history of coronary heart disease, history of heart disease, history of stroke, magnesium, calcium and the using of psychotropic medication. Additionally, compared to participants with normal cognitive performance, people with low cognitive performance were more likely to have higher age, lower household income and carbohydrate intake score. The number of the history of hypertension and history of diabetes in people with low cognitive performance was lower than that of people with normal cognitive performance. Detailed information was shown in Table 1.
Table 1.
The characteristics of all eligible participants
| Variables | CERAD word learning subtest | AFT | DSST | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal cognitive performance group (N = 1526) | Low cognitive performance group (N = 1011) | P | Normal cognitive performance group (N = 1517) | Low cognitive performance group (N = 1020) | P | Normal cognitive performance group (N = 1535) | Low cognitive performance group (N = 1002) | P | |
| LCD score, Mean (S.E) | 11.38 (0.23) | 10.38 (0.29) | 0.001 | 11.16 (0.23) | 10.75 (0.36) | 0.295 | 11.18 (0.24) | 10.66 (0.34) | 0.174 |
| Carbohydrate intake score, Mean (S.E) | 3.73 (0.11) | 3.32 (0.14) | 0.005 | 3.67 (0.11) | 3.43 (0.16) | 0.152 | 3.69 (0.11) | 3.36 (0.15) | 0.046 |
| Fat intake score, Mean (S.E) | 4.63 (0.11) | 4.38 (0.15) | 0.108 | 4.52 (0.10) | 4.58 (0.16) | 0.675 | 4.58 (0.11) | 4.44 (0.16) | 0.388 |
| Protein intake score, Mean (S.E) | 3.02 (0.10) | 2.68 (0.14) | 0.038 | 2.98 (0.09) | 2.74 (0.13) | 0.122 | 2.91 (0.09) | 2.86 (0.14) | 0.757 |
| Gender, n (%) | 0.865 | < 0.001 | 0.003 | ||||||
| Male | 713 (46.09) | 532 (46.49) | 802 (49.97) | 443 (39.23) | 755 (48.96) | 490 (40.16) | |||
| Female | 813 (53.91) | 479 (53.51) | 715 (50.03) | 577 (60.77) | 780 (51.04) | 512 (59.84) | |||
| Age, years, Mean (S.E) | 68.06 (0.21) | 70.63 (0.37) | < 0.001 | 68.43 (0.21) | 70.03 (0.35) | < 0.001 | 68.45 (0.21) | 70.18 (0.37) | < 0.001 |
| Race, n (%) | 0.255 | < 0.001 | < 0.001 | ||||||
| Mexican American | 123 (2.80) | 91 (3.70) | 143 (3.12) | 71 (3.13) | 108 (2.27) | 106 (5.02) | |||
| Other Hispanic | 141 (2.98) | 108 (4.13) | 147 (3.02) | 102 (4.08) | 109 (2.13) | 140 (6.21) | |||
| Non-Hispanic White | 743 (81.45) | 526 (80.41) | 806 (83.69) | 463 (76.17) | 846 (84.58) | 423 (73.27) | |||
| Non-Hispanic Black | 370 (7.84) | 222 (7.83) | 304 (6.08) | 288 (11.14) | 306 (5.84) | 286 (12.29) | |||
| Other Race: Including Multi-Racial | 149 (4.93) | 64 (3.93) | 117 (4.09) | 96 (5.48) | 166 (5.18) | 47 (3.22) | |||
| Education, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Less than high school | 269 (10.33) | 339 (23.47) | 283 (10.84) | 325 (23.01) | 185 (8.54) | 423 (29.61) | |||
| High school and above | 1257 (89.67) | 672 (76.53) | 1234 (89.16) | 695 (76.99) | 1350 (91.46) | 579 (70.39) | |||
| Marital status, n (%) | 0.008 | 0.007 | < 0.001 | ||||||
| Married | 870 (65.58) | 544 (59.55) | 876 (65.64) | 538 (59.22) | 917 (67.10) | 497 (55.16) | |||
| Divorced | 244 (13.72) | 226 (19.65) | 243 (13.71) | 227 (19.89) | 235 (13.23) | 235 (21.71) | |||
| Separated | 249 (13.25) | 125 (11.92) | 226 (12.79) | 148 (12.74) | 228 (12.57) | 146 (13.21) | |||
| Othera | 163 (7.45) | 116 (8.88) | 172 (7.87) | 107 (8.15) | 155 (7.09) | 124 (9.92) | |||
| Household income, $, Mean (S.E) | 48,459.93 (1147.74) | 40,649.94 (1155.73) | < 0.001 | 48,165.23 (1176.77) | 40,912.60 (1118.19) | < 0.001 | 48,623.77 (1220.00) | 39,005.55 (1162.59) | < 0.001 |
| BMI, kg/m2, Mean (S.E) | 28.95 (0.24) | 29.38 (0.31) | 0.125 | 28.85 (0.21) | 29.59 (0.36) | 0.023 | 28.83 (0.24) | 29.71 (0.38) | 0.039 |
| Waistline, cm, Mean (S.E) | 101.99 (0.61) | 103.49 (0.84) | 0.102 | 102.22 (0.53) | 103.13 (0.90) | 0.272 | 102.11 (0.61) | 103.50 (1.00) | 0.211 |
| Diabetes, n (%) | 0.037 | < 0.001 | < 0.001 | ||||||
| Yes | 322 (17.39) | 260 (21.94) | 309 (16.47) | 273 (23.84) | 291 (16.01) | 291 (25.76) | |||
| No | 1132 (78.47) | 705 (73.74) | 1133 (79.24) | 704 (72.11) | 1169 (79.81) | 668 (69.99) | |||
| Borderline | 72 (4.14) | 46 (4.32) | 75 (4.28) | 43 (4.05) | 75 (4.18) | 43 (4.25) | |||
| Hypertension, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Yes | 902 (53.11) | 676 (66.66) | 875 (53.72) | 703 (66.02) | 889 (52.99) | 689 (69.15) | |||
| No | 624 (46.89) | 335 (33.34) | 642 (46.28) | 317 (33.98) | 646 (47.01) | 313 (30.85) | |||
| Congestive heart failure, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| Yes | 71 (4.22) | 102 (10.60) | 77 (4.61) | 96 (10.10) | 74 (4.51) | 99 (11.00) | |||
| No | 1455 (95.78) | 909 (89.40) | 1440 (95.39) | 924 (89.90) | 1461 (95.49) | 903 (89.00) | |||
| Coronary heart disease, n (%) | 0.003 | 0.036 | 0.052 | ||||||
| Yes | 113 (7.56) | 120 (13.12) | 125 (8.40) | 108 (11.76) | 127 (8.47) | 106 (12.00) | |||
| No | 1413 (92.44) | 891 (86.88) | 1392 (91.60) | 912 (88.24) | 1408 (91.53) | 896 (88.00) | |||
| Heart disease, n (%) | < 0.001 | 0.040 | < 0.001 | ||||||
| Yes | 120 (7.41) | 100 (10.92) | 122 (7.82) | 98 (10.29) | 110 (7.23) | 110 (11.90) | |||
| No | 1406 (92.59) | 911 (89.08) | 1395 (92.18) | 922 (89.71) | 1425 (92.77) | 892 (88.10) | |||
| Stroke, n (%) | < 0.001 | 0.005 | < 0.001 | ||||||
| Yes | 75 (4.73) | 91 (7.97) | 73 (4.83) | 93 (7.91) | 66 (4.31) | 100 (9.44) | |||
| No | 1451 (95.27) | 920 (92.03) | 1444 (95.17) | 927 (92.09) | 1469 (95.69) | 902 (90.56) | |||
| Magnesium, mg, Mean (S.E) | 305.39 (6.22) | 269.36 (5.16) | < 0.001 | 303.64 (5.88) | 266.84 (6.86) | < 0.001 | 306.24 (6.48) | 264.74 (5.46) | < 0.001 |
| Calcium, mg, Mean (S.E) | 915.89 (20.49) | 858.58 (20.75) | 0.029 | 912.27 (20.29) | 858.26 (24.52) | 0.081 | 914.23 (20.80) | 861.38 (24.13) | 0.087 |
| Vitamin D, mcg, Mean (S.E) | 5.05 (0.17) | 4.84 (0.22) | 0.511 | 5.00 (0.14) | 5.00 (0.31) | 0.994 | 4.98 (0.17) | 5.07 (0.28) | 0.803 |
| Psychotropic medication, n (%) | < 0.001 | < 0.001 | < 0.001 | ||||||
| No | 1346 (99.79) | 777 (97.76) | 1318 (99.78) | 805 (97.75) | 1346 (99.75) | 777 (97.51) | |||
| Yes | 3 (0.21) | 16 (2.24) | 2 (0.22) | 17 (2.25) | 4 (0.25) | 15 (2.49) | |||
Note: Othera, represents widowed, never married and living with partner; BMI Body mass index, CERAD, Consortium to Establish a Registry for Alzheimer’s Disease, AFT Animal Fluency Test, DSST Digit Symbol Substitution Test
The association between LCD score and cognitive performance
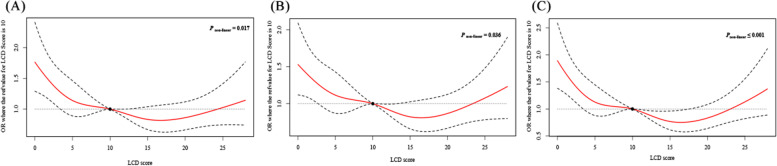
Table 2 displays the association between LCD score and cognitive performance. Three model was used in this study. For the CERAD word learning subtest, after adjusting age, education level, marital status, household income, history of diabetes, history of hypertension, history of congestive heart failure, history of coronary heart disease, history of heart disease, history of stroke, magnesium and the using of psychotropic medication (Model 3), the multivariate logistic regression analysis (Model 3) showed that compared with LCD score < 4 points, OR with 95% CI was 0.56 (0.42–0.75, P = 0.003) among LCD score 4–8 points; OR with 95% CI was 0.60 (0.42–0.86, P = 0.019) among LCD score 8–12 points; OR with 95% CI was 0.56 (0.38–0.82, P = 0.014) among LCD score 12–17 points; OR with 95% CI was 0.78 (0.55–1.10, P = 0.185) among LCD score 12–17 points. These results indicated that LCD score was correlated with the CERAD word learning subtest. The associations between LCD score and AFT, DSST were not statistically significant in multivariate logistic regression analysis. Furthermore, the result of RCS curves suggested that there might be a “U-shaped” in the associations of LCD score and cognitive performance (Fig. 2).
Table 2.
The association between LCD score and cognitive performance
| Variables | LCD score level | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| CERAD word learning subtest # | < 4 | Ref | Ref | Ref | |||
| 4–8 | 0.58 (0.43–0.78) | 0.001 | 0.59 (0.44–0.80) | 0.003 | 0.56 (0.42–0.75) | 0.003 | |
| 8–12 | 0.58 (0.44–0.78) | 0.001 | 0.58 (0.43–0.79) | 0.002 | 0.60 (0.42–0.86) | 0.019 | |
| 12–17 | 0.49 (0.33–0.71) | < 0.001 | 0.53 (0.37–0.77) | 0.003 | 0.56 (0.38–0.82) | 0.014 | |
| > 17 | 0.68 (0.50–0.91) | 0.016 | 0.74 (0.55–1.01) | 0.071 | 0.78 (0.55–1.10) | 0.185 | |
| AFT* | < 4 | Ref | Ref | Ref | |||
| 4–8 | 0.72 (0.53–0.97) | 0.041 | 0.76 (0.55–1.04) | 0.098 | 0.72 (0.52–1.00) | 0.186 | |
| 8–12 | 0.65 (0.47–0.90) | 0.014 | 0.68 (0.48–0.95) | 0.034 | 0.70 (0.48–1.03) | 0.213 | |
| 12–17 | 0.61 (0.46–0.81) | 0.002 | 0.70 (0.53–0.93) | 0.023 | 0.73 (0.54–0.98) | 0.170 | |
| > 17 | 0.84 (0.57–1.23) | 0.374 | 0.96 (0.65–1.43) | 0.856 | 1.00 (0.65–1.52) | 0.984 | |
| DSST& | < 4 | Ref | Ref | Ref | |||
| 4–8 | 0.72 (0.53–0.97) | 0.041 | 0.76 (0.55–1.04) | 0.098 | 0.72 (0.52–1.00) | 0.186 | |
| 8–12 | 0.65 (0.47–0.90) | 0.014 | 0.68 (0.48–0.95) | 0.034 | 0.70 (0.48–1.03) | 0.213 | |
| 12–17 | 0.61 (0.46–0.81) | 0.002 | 0.70 (0.53–0.93) | 0.023 | 0.73 (0.54–0.98) | 0.170 | |
| > 17 | 0.84 (0.57–1.23) | 0.374 | 0.96 (0.65–1.43) | 0.856 | 1.00 (0.65–1.52) | 0.984 | |
Note: LCD Low-carbohydrate-diet, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, AFT Animal Fluency Test, DSST Digit Symbol Substitution Test, OR Odds ratio, CI Confidence interval
Model 1, did not adjust for any confounders;
Model 2, adjusted for age, gender and race;
Model 3 (for CERAD word learning subtest #), adjusted for age, education level, marital status, household income, history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium and the using of psychotropic medication;
Model 3 (for AFT*), adjusted for age, gender, race, education level, marital status, household income, body mass index; history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium, calcium and the using of psychotropic medication;
Model 3 (for DSST&), adjusted for age, gender, race, education level, marital status, household income, body mass index; history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium and calcium
Fig. 2.
A The dose–response relationship between LCD score and CERAD word learning subtest; (B) The dose–response relationship between LCD score and AFT; (C) The dose–response relationship between LCD score and DSST. The solid line represents the odds ratios, and the dotted line represents the 95% confidence interval; LCD = low-carbohydrate-diet; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; AFT = Animal Fluency Test; DSST = Digit Symbol Substitution Test
Stratified analyses by age, BMI, gender, marital status and education level
In the present study, we also performed stratified analyses by age, BMI, gender, marital status and education level. The results were shown in Table 3. We found that there was still a correlation between LCD score and the CERAD word learning subtest based on age, BMI, gender, marital status, education level. These results showed that the cognitive performance was associated with LCD score among population who aged < 65 years or BMI 25–30 kg/m2 or was married/separated, or had an education level of high school or above, which indicated the association of LCD score and cognitive performance might be more robust for these individuals.
Table 3.
Stratified analyses by age, BMI, gender, marital status and education level
| Variables | LCD score level | CERAD word learning subtest # | AFT* | DSST& | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||||
| Age, years | |||||||||
| 60 ≤ and < 65 | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.22 (0.09–0.52) | 0.005 | 0.45 (0.24–0.85) | 0.071 | 0.39 (0.20–0.74) | 0.045 | |||
| 8–12 | 0.37 (0.19–0.71) | 0.012 | 0.57 (0.25–1.31) | 0.255 | 0.56 (0.24–1.30) | 0.247 | |||
| 12–17 | 0.42 (0.20–0.89) | 0.043 | 0.88 (0.42–1.81) | 0.740 | 0.65 (0.32–1.31) | 0.292 | |||
| > 17 | 0.49 (0.20–1.20) | 0.145 | 1.07 (0.54–2.13) | 0.850 | 1.00 (0.45–2.20) | 0.994 | |||
| ≥ 65 | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.78 (0.54–1.11) | 0.194 | 0.83 (0.60–1.15) | 0.341 | 0.81 (0.52–1.26) | 0.410 | |||
| 8–12 | 0.71 (0.48–1.05) | 0.115 | 0.71 (0.48–1.06) | 0.190 | 0.68 (0.48–0.96) | 0.115 | |||
| 12–17 | 0.59 (0.36–0.96) | 0.059 | 0.64 (0.44–0.92) | 0.097 | 0.63 (0.40–0.98) | 0.133 | |||
| > 17 | 0.84 (0.61–1.15) | 0.299 | 0.94 (0.61–1.46) | 0.797 | 0.95 (0.57–1.56) | 0.848 | |||
| Gender | |||||||||
| Male | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.37 (0.21–0.64) | 0.005 | 0.74 (0.43–1.30) | 0.356 | 0.80 (0.46–1.40) | 0.486 | |||
| 8–12 | 0.54 (0.29–1.00) | 0.075 | 0.70 (0.39–1.24) | 0.289 | 0.67 (0.36–1.25) | 0.276 | |||
| 12–17 | 0.54 (0.33–0.88) | 0.031 | 0.77 (0.51–1.16) | 0.275 | 0.77 (0.48–1.26) | 0.358 | |||
| > 17 | 0.82 (0.48–1.40) | 0.483 | 1.30 (0.72–2.37) | 0.434 | 1.54 (0.89–2.67) | 0.194 | |||
| Female | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.73 (0.50–1.07) | 0.139 | 0.71 (0.49–1.04) | 0.175 | 0.57 (0.36–0.92) | 0.105 | |||
| 8–12 | 0.67 (0.42–1.07) | 0.127 | 0.75 (0.45–1.26) | 0.357 | 0.65 (0.42–1.00) | 0.146 | |||
| 12–17 | 0.53 (0.36–0.79) | 0.010 | 0.71 (0.47–1.08) | 0.204 | 0.55 (0.33–0.92) | 0.106 | |||
| > 17 | 0.70 (0.43–1.13) | 0.172 | 0.79 (0.46–1.37) | 0.465 | 0.69 (0.42–1.13) | 0.233 | |||
| BMI, kg/m2 | |||||||||
| ≤ 25 | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 1.13 (0.58–2.21) | 0.726 | 0.97 (0.55–1.71) | 0.924 | 0.97 (0.44–2.15) | 0.941 | |||
| 8–12 | 0.95 (0.43–2.07) | 0.892 | 0.78 (0.43–1.40) | 0.447 | 0.88 (0.38–2.03) | 0.774 | |||
| 12–17 | 0.61 (0.29–1.28) | 0.218 | 0.44 (0.20–0.98) | 0.116 | 0.78 (0.36–1.69) | 0.561 | |||
| > 17 | 1.53 (0.76–3.09) | 0.259 | 1.12 (0.55–2.26) | 0.768 | 1.33 (0.59–3.01) | 0.535 | |||
| > 25 and ≤ 30 | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.34 (0.18–0.63) | 0.006 | 0.73 (0.43–1.23) | 0.325 | 0.43 (0.23–0.81) | 0.079 | |||
| 8–12 | 0.39 (0.21–0.73) | 0.014 | 0.53 (0.31–0.90) | 0.102 | 0.42 (0.24–0.76) | 0.062 | |||
| 12–17 | 0.38 (0.18–0.80) | 0.028 | 0.86 (0.53–1.40) | 0.598 | 0.50 (0.26–0.94) | 0.121 | |||
| > 17 | 0.52 (0.34–0.82) | 0.017 | 0.84 (0.48–1.49) | 0.595 | 0.62 (0.30–1.27) | 0.282 | |||
| > 30 | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.55 (0.29–1.05) | 0.102 | 0.63 (0.38–1.07) | 0.185 | 0.86 (0.50–1.45) | 0.604 | |||
| 8–12 | 0.61 (0.36–1.04) | 0.100 | 0.77 (0.43–1.40) | 0.454 | 0.78 (0.42–1.46) | 0.499 | |||
| 12–17 | 0.64 (0.38–1.06) | 0.115 | 0.80 (0.46–1.40) | 0.492 | 0.83 (0.44–1.57) | 0.614 | |||
| > 17 | 0.70 (0.36–1.35) | 0.316 | 1.10 (0.64–1.91) | 0.756 | 1.34 (0.74–2.43) | 0.406 | |||
| Education | |||||||||
| Less than high school | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.60 (0.37–0.99) | 0.065 | 0.85 (0.52–1.40) | 0.555 | 0.77 (0.36–1.63) | 0.513 | |||
| 8–12 | 1.18 (0.63–2.22) | 0.618 | 0.77 (0.51–1.14) | 0.239 | 0.89 (0.43–1.86) | 0.772 | |||
| 12–17 | 0.88 (0.54–1.45) | 0.631 | 0.42 (0.26–0.69) | 0.013 | 0.68 (0.36–1.28) | 0.275 | |||
| > 17 | 1.61 (0.88–2.97) | 0.147 | 0.63 (0.35–1.13) | 0.170 | 0.77 (0.28–2.14) | 0.629 | |||
| High school or above | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.55 (0.37–0.80) | 0.008 | 0.68 (0.48–0.97) | 0.079 | 0.65 (0.40–1.04) | 0.122 | |||
| 8–12 | 0.51 (0.34–0.77) | 0.007 | 0.67 (0.43–1.03) | 0.119 | 0.63 (0.39–1.02) | 0.110 | |||
| 12–17 | 0.50 (0.31–0.80) | 0.012 | 0.77 (0.54–1.11) | 0.212 | 0.65 (0.41–1.04) | 0.125 | |||
| > 17 | 0.67 (0.44–1.02) | 0.080 | 1.05 (0.67–1.63) | 0.852 | 1.07 (0.66–1.74) | 0.781 | |||
| Marital status | |||||||||
| Married | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.56 (0.34–0.93) | 0.041 | 0.70 (0.42–1.18) | 0.217 | 0.65 (0.42–1.03) | 0.104 | |||
| 8–12 | 0.63 (0.40–0.99) | 0.061 | 0.68 (0.44–1.04) | 0.115 | 0.57 (0.35–0.92) | 0.050 | |||
| 12–17 | 0.62 (0.36–1.06) | 0.099 | 0.89 (0.56–1.39) | 0.614 | 0.76 (0.45–1.28) | 0.332 | |||
| > 17 | 0.84 (0.53–1.34) | 0.479 | 1.05 (0.67–1.66) | 0.834 | 0.96 (0.62–1.48) | 0.856 | |||
| Divorced | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.63 (0.31–1.30) | 0.236 | 0.68 (0.34–1.36) | 0.315 | 0.81 (0.35–1.86) | 0.634 | |||
| 8–12 | 0.97 (0.47–1.99) | 0.930 | 1.04 (0.43–2.51) | 0.929 | 1.90 (0.91–3.97) | 0.138 | |||
| 12–17 | 0.54 (0.29–1.02) | 0.078 | 0.79 (0.39–1.61) | 0.544 | 0.71 (0.32–1.60) | 0.439 | |||
| > 17 | 0.45 (0.24–0.86) | 0.030 | 0.38 (0.15–0.98) | 0.092 | 0.96 (0.41–2.23) | 0.929 | |||
| Separated | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 0.46 (0.20–1.08) | 0.095 | 0.71 (0.28–1.79) | 0.490 | 0.52 (0.25–1.09) | 0.127 | |||
| 8–12 | 0.29 (0.11–0.74) | 0.021 | 0.40 (0.20–0.82) | 0.041 | 0.29 (0.12–0.71) | 0.030 | |||
| 12–17 | 0.35 (0.13–0.93) | 0.052 | 0.27 (0.09–0.82) | 0.055 | 0.29 (0.08–1.08) | 0.107 | |||
| > 17 | 0.76 (0.23–2.43) | 0.645 | 1.64 (0.55–4.93) | 0.405 | 1.10 (0.33–3.68) | 0.876 | |||
| Other# | < 4 | Ref | Ref | Ref | |||||
| 4–8 | 1.10 (0.49–2.50) | 0.818 | 0.82 (0.33–2.01) | 0.679 | 1.32 (0.46–3.83) | 0.626 | |||
| 8–12 | 1.33 (0.52–3.40) | 0.558 | 1.75 (0.73–4.20) | 0.267 | 1.19 (0.41–3.49) | 0.758 | |||
| 12–17 | 0.73 (0.23–2.28) | 0.592 | 0.23 (0.09–0.57) | 0.025 | 0.30 (0.11–0.86) | 0.076 | |||
| > 17 | 0.96 (0.35–2.66) | 0.935 | 0.99 (0.38–2.58) | 0.979 | 0.47 (0.14–1.58) | 0.275 | |||
Note: Other#, represents widowed, never married and living with partner; BMI, body mass index; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; AFT, Animal Fluency Test; DSST, Digit Symbol Substitution Test
For CERAD word learning subtest #: adjusted for age, education level, marital status, household income, history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium and the using of psychotropic medication;
For AFT*: adjusted for age, gender, race, education level, marital status, household income, body mass index; history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium, calcium and the using of psychotropic medication;
For DSST&: adjusted for age, gender, race, education level, marital status, household income, body mass index; history of diabetes, hypertension, congestive heart failure, coronary heart disease, heart disease and stroke, magnesium and calcium
Discussion
A better understanding of the link between diet and cognitive performance has important implications to prevent and better manage cognitive decline. In this cross-sectional study, we combined data from the 2011–2012 and 2013–2014 NHANES database with 2,537 older adults, which aimed at investigating the associations of LCD score and cognitive performance. After adjusted confounders, the findings displayed that LCD score might be associated with the CERAD word learning subtest and a possible “U-shaped” dose–response relationships were also detected, which indicated that the LCD score was related to the cognitive performance for older adults. These findings might highlight the importance of the higher intakes of fat, protein, and the lower intake of carbohydrate for maintaining specific aspects of cognitive performance among older adults.
The association between cognitive performance and diet has been considered as a hot topic in recent years. Previous researches reported that nutrients of food could regulate the immune system and alter neuroinflammatory processes associated with the pathogenesis of Alzheimer's disease and cognitive impairment [19, 20]. In the study of Wengreen et al., they explored the associations between Dietary Approaches to Stop Hypertension (DASH)-Mediterranean-style dietary patterns and age-related cognitive change, and the result showed that older adults who followed the DASH- Mediterranean-style dietary patterns had higher levels of cognitive function [21]. Furthermore, Coelho-Júnior et al., also pointed out that high adherence to Mediterranean diet was cross-sectionally related to the cognitive function [22]. A ketogenic diet as a very high-fat, low-carbohydrate diet, which was considered as a fasting-like effect putting the body into a state of ketosis [23]. Davis and colleagues expounded that the ketogenic diet may delay, ameliorate, or prevent progression of cognitive decline [24], but older adults with Alzheimer's disease may have difficulty complying with ketogenic diet interventions. LCD, which have a similar composition with the ketogenic diet, have aroused wide concern in the recent years [25].
To the best of our knowledge, this is the first study to have examined the relationship between LCD score and the risk of cognitive performance for older adults based on the NHANES database. Of the three cognitive tests, higher LCD score displayed a protective effect when evaluated by the CERAD word learning subtest, implying the prominent role of LCD score in older adults' ability to learn both immediate and delayed learning ability for new verbal information [26]. In other words, a lower carbohydrate intake and higher fat or protein intake could might be related to a decreased risk of low cognitive performance in moderation. The mechanism about the relationship between LCD score and cognitive performance is unclear, the possible reason was that proper lower carbohydrate intake and high fat and protein intake may cause a state of ketosis, which helped to improve oxidative stress and inflammation, and in turn improved cognitive performance [27]. However, it is worth mentioning that the association of LCD score with AFT, DSST were not statistically significant. The result might indicate that the effect of LCD score on verbal fluency, processing speed, sustained attention and working memory in the older population was less significant. Of course, more research is needed in the future to explain these associations.
In addition, LCD score was associated with the cognitive performance, especially for population who aged < 65 years or BMI 25–30 kg/m2 or was married/separated, or had an education level of high school or above. The findings might provide a reference in reducing the risk of low cognitive performance by decreasing the carbohydrate intake and increasing the fat or protein intake for older adults in moderation, especially in population who aged < 65 years or BMI 25–30 kg/m2 or was married/separated, or had an education level of high school or above. Additionally, it is worth mentioning that the relationship of LCD score (12–17 points) and cognitive performance was significant in both men and women. However, when the LCD score was between 4–8 points, the relationship was robust only in the older male population. The current literature has not provided a clear explanation for sex differences in the association between LCD score and cognitive performance. This difference between men and women may be due to the fact that men are more affected by LCD scores than women [28, 29]. More prospective studies are needed to further elucidate the sex differences in the association between LCD score and cognitive performance among older adults.
The present study has several advantages. This is the first study to show the associations of LCD score and cognitive performance among a representative sample of the US population aged ≥ 60 years. LCD score are easy to calculate and more practical. Additionally, this study included a relatively large sample size, which makes the conclusions more reliable and makes it possible to perform subgroup analysis according to age, BMI, gender, marital status, education.
Nevertheless, there were some limitations that should be pointed out. Firstly, given that this research designed as a cross-sectional study, we cannot be able to ascertain a causal relationship of LCD score and cognitive performance. Secondly, the 24-h dietary recall interview is based on a questionnaire, which may lead to misclassification of food intake. Lastly, we excluded some participants who had incomplete cognitive tests or had missing information, we couldn't be sure whether these people swayed the results. Thus, the results should be interpreted with caution.
Conclusion
In summary, we observed that the adherences to LCD score might be associated with the risk of cognitive performance among a representative sample of the US elderly population. The association between LCD score and cognitive performance was stronger in older adults who aged < 65 years or BMI 25–30 kg/m2 or was married/separated, or had an education level of high school or above. However, further large-scale cohort studies are needed to test the causal relationship of LCD and cognitive performance.
Supplementary Information
Additional file 1: Supplemental Table 1. The criteria for determining the LCD score. Supplemental Table 2. The proportion of missing values. Supplemental Table 3. The sensitivity analysis before and after deletion of missing values.
Acknowledgements
Not applicable.
Abbreviations
- DGA
Dietary Guidelines for Americans
- NHANES
National Health and Nutrition Examination Survey
- CDC
Centers for Disease Control and Prevention
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
- AFT
Animal Fluency Test
- DSST
Digit Symbol Substitution Test
Authors’ contributions
HW and GR designed the study. HW wrote the manuscript. YL and GT collected, analyzed and interpreted the data. GR critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Declarations
Ethics approval and consent to participate
This study did not need to be approved by the Institutional Review Board of Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University because the data was accessed from NHANES (a publicly available database). All methods were carried out in accordance with relevant guidelines and regulations (declaration of Helsinki). All individuals provided written informed consent before participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong X, Li S, Sun J, Li Y, Zhang D. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020–2060) Alzheimers Dement. 2021;17:1966–1975. doi: 10.1002/alz.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong X, Li S, Chen J, Li Y, Wu Y, Zhang D. Association of dietary ω-3 and ω-6 fatty acids intake with cognitive performance in older adults: National Health and nutrition examination Survey (NHANES) 2011–2014. Nutr J. 2020;19:25. doi: 10.1186/s12937-020-00547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59:815–849. doi: 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]
- 5.Gauci S, Young LM, White DJ, Reddan JM, Lassemillante AC, Meyer D, et al. Diet may moderate the relationship between arterial stiffness and cognitive performance in older adults. J Alzheimers Dis. 2022;85:815–828. doi: 10.3233/JAD-210567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Zhang Y, Li J, Liu Y, Chang H, Jiang Y, et al. Association between healthy eating index-2015 and various cognitive domains in US adults aged 60 years or older: the National Health and Nutrition Examination Survey (NHANES) 2011–2014. BMC Public Health. 2021;21:1862. doi: 10.1186/s12889-021-11914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muth AK, Park SQ. The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr. 2021;40:3999–4010. doi: 10.1016/j.clnu.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Kim Y, Lim H. Glycaemic indices and glycaemic loads of common Korean carbohydrate-rich foods. Br J Nutr. 2019;121:416–425. doi: 10.1017/S0007114518003446. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Li S, Wang W, Zhang D. Association between dietary protein intake and cognitive function in adults aged 60 years and older. J Nutr Health Aging. 2020;24:223–229. doi: 10.1007/s12603-020-1317-4. [DOI] [PubMed] [Google Scholar]
- 10.Sangsefidi ZS, Salehi-Abarghouei A, Sangsefidi ZS, Mirzaei M, Hosseinzadeh M. The relation between low carbohydrate diet score and psychological disorders among Iranian adults. Nutr Metab (Lond) 2021;18:16. doi: 10.1186/s12986-021-00546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangsefidi ZS, Lorzadeh E, Nadjarzadeh A, Mirzaei M, Hosseinzadeh M. The association between low-carbohydrate diet score and metabolic syndrome among Iranian adults. Public Health Nutr. 2021;24:6299–6308. doi: 10.1017/S1368980021003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 13.Namazi N, Larijani B, Azadbakht L. Low-carbohydrate-diet score and its association with the risk of diabetes: a systematic review and meta-analysis of cohort studies. Horm Metab Res. 2017;49:565–571. doi: 10.1055/s-0043-112347. [DOI] [PubMed] [Google Scholar]
- 14.Gao JW, Hao QY, Zhang HF, Li XZ, Yuan ZM, Guo Y, et al. Low-carbohydrate diet score and coronary artery calcium progression: results From the CARDIA Study. Arterioscler Thromb Vasc Biol. 2021;41:491–500. doi: 10.1161/ATVBAHA.120.314838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng FW, Ford NA, Taylor MK. US older adults that consume avocado or guacamole have better cognition than non-consumers: national health and nutrition examination survey 2011–2014. Front Nutr. 2021;8:746453. doi: 10.3389/fnut.2021.746453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng J, Deng L, Qiu S, Bian H, Cai B, Li Y, et al. Low lean mass and cognitive performance: data from the national health and nutrition examination surveys. Aging Clin Exp Res. 2021;33:2737–2745. doi: 10.1007/s40520-021-01835-w. [DOI] [PubMed] [Google Scholar]
- 17.Mazidi M, Katsiki N, Mikhailidis DP, Sattar N, Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. 2019;40:2870–2879. doi: 10.1093/eurheartj/ehz174. [DOI] [PubMed] [Google Scholar]
- 18.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135:963–970. doi: 10.1001/jamaophthalmol.2017.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17:1006–1015. doi: 10.1016/S1474-4422(18)30338-7. [DOI] [PubMed] [Google Scholar]
- 20.Vauzour D, Camprubi-Robles M, Miquel-Kergoat S, Andres-Lacueva C, Bánáti D, Barberger-Gateau P, et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res Rev. 2017;35:222–240. doi: 10.1016/j.arr.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, et al. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County study on memory, health and aging. Am J Clin Nutr. 2013;98:1263–1271. doi: 10.3945/ajcn.112.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho-Júnior HJ, Trichopoulou A, Panza F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2021;70:101395. doi: 10.1016/j.arr.2021.101395. [DOI] [PubMed] [Google Scholar]
- 23.Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic diet in Alzheimer's disease. Int J Mol Sci. 2019;20:3892. doi: 10.3390/ijms20163892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis JJ, Fournakis N, Ellison J. Ketogenic diet for the treatment and prevention of dementia: a review. J Geriatr Psychiatry Neurol. 2021;34:3–10. doi: 10.1177/0891988720901785. [DOI] [PubMed] [Google Scholar]
- 25.Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients. 2019;11:962. doi: 10.3390/nu11050962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Qiao S, Zhuang L, Xu S, Chen L, Lai Q, et al. Choline intake correlates with cognitive performance among elder adults in the United States. Behav Neurol. 2021;2021:2962245. doi: 10.1155/2021/2962245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grammatikopoulou MG, Goulis DG, Gkiouras K, Theodoridis X, Gkouskou KK, Evangeliou A, et al. To keto or not to keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on alzheimer disease. Adv Nutr. 2020;11:1583–1602. doi: 10.1093/advances/nmaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SA, Lim K, Shin S. Associations between low-carbohydrate diets from animal and plant sources and dyslipidemia among Korean adults. J Acad Nutr Diet. 2019;119:2041–2054. doi: 10.1016/j.jand.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Cardoso BR, Hare DJ, Macpherson H. Sex dependent association between selenium status and cognitive performance in older adults. Eur J Nutr. 2021;60:1153. doi: 10.1007/s00394-020-02384-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. The criteria for determining the LCD score. Supplemental Table 2. The proportion of missing values. Supplemental Table 3. The sensitivity analysis before and after deletion of missing values.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.