Abstract
Aim
To quantify the areas of burden experienced by patients requiring repeated intravitreal injections (IVI) in the management of exudative retinal diseases.
Methods
The validated Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections survey was administered to patients at four retina clinical practices across four US states. The primary outcome measure was Treatment Burden Score (TBS), a single score assessing overall burden.
Results
Of 1416 (n=657 age-related macular degeneration; n=360 diabetic macular oedema/diabetic retinopathy; n=221 retinal vein occlusion; n=178 other/uncertain) patients, 55% were women with an average age of 70 years. Patients most frequently reported receiving IVI every 4–5 weeks (40%). The mean TBS was 16.1±9.2 (range 1–48; scale of 1–54), and the TBS was higher in patients with diabetic macular oedema and/or diabetic retinopathy (DMO/DR) (17.1) compared with those with age-related macular degeneration (15.5) or retinal venous occlusive (15.3) (p=0.028). Though the mean level of discomfort was quite low (1.86) (scale 0–6), 50% of patients reported experiencing side effects more than half of the visits. Patients having received fewer than 5 IVI reported higher mean anxiety levels before (p=0.026), during (p=0.050) and after (p=0.016) treatment compared with patients having received more than 50 IVI. After the procedure, 42% of patients reported restrictions from usual activities due to discomfort. Patients reported a high mean satisfaction rating of 5.46 (scale 0–6) with the care of their diseases.
Conclusions
The mean TBS was moderate and highest among patients with DMO/DR. Patients with more total injections reported lower levels of discomfort and anxiety but higher disruption to daily life. Despite the challenges related to IVI, the overall satisfaction with treatment remained high.
Keywords: Retina, Treatment other
WHAT IS ALREADY KNOWN ON THIS TOPIC
Current treatment for exudative retinal diseases requires frequent intravitreal injections (IVI) and visual outcome greatly depends on adherence to treatment regimen. We use the validated Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII) survey to quantitatively measure treatment burden associated with IVI.
WHAT THIS STUDY ADDS
Our study finds that though the overall reported level of discomfort and anxiety associated with IVI was low, patients report experiencing side effects frequently and for prolonged periods of time. The mean Treatment Burden Score (TBS) was moderate among surveyed patients, but the overall satisfaction with treatment remained high.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The QUALITII survey and the TBS are validated tools to quantitatively measure patient burden of treatment and can provide a standardised measure of patient experience with emerging treatment options.
Introduction
Intravitreal injections (IVI) are one of the most common medical procedures performed globally.1 2 It is currently estimated that over 7 million IVIs are administered annually in the USA and this figure is projected to continue to grow.1 Currently, IVI of anti-vascular endothelial growth factor (VEGF) pharmaceuticals are the first-line treatment for many exudative retinal diseases, including neovascular age-related macular degeneration (AMD),2 diabetic macular oedema (DMO)3 4 and retinal venous occlusive (RVO) disease.5 Treatment of these pathologies typically involves repeated anti-VEGF IVIs as frequently as every 4 weeks over an indefinite course.6 Given the high demand and indefinite nature of treatment, an understanding of the treatment-related burden that patients experience is of great interest in order to optimise patient understanding, experience and adherence to examination and treatment schedules.
Standardised tools such as the retinopathy treatment satisfaction questionnaire (RetTSQ)7 published in 2005 and macular disease treatment satisfaction questionnaire (MacTSQ)8 published in 2017 have been designed to measure patient satisfaction among patients receiving therapies for macular diseases. The purpose of these quantitative surveys was focused on patient satisfaction and though several authors have highlighted the challenging aspects of patient experiences with IVI,9–11 no single quantitative model of patient treatment burden has been reported. Insightfully, Boyle et al found that though MacTSQ surveys demonstrated high satisfaction scores, patient interviews revealed incongruity between MacTSQ score and treatment-related anxiety.12
In a single-centre retrospective study, 39.8% of 314 participating patients did not comply with treatment on a pro re nata (PRN) regimen for 1 year. In this study, the fear of injection and disbelief in the benefit of treatment were the most common reasons cited for discontinuation.13 In another study analysing adherence barriers to IVIs, the top reported barriers to compliance were time commitment, depression, and associated costs of travel.14 Therefore, careful consideration of the many factors impacting patient-perceived burden may yield insights valuable towards guiding best practices in the management of exudative retinal diseases.
The Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII) was intentionally developed to provide a quantitative and qualitative measure of patient burden.15 The 50-item questionnaire was conceptualised based on literature review, expert input and informal interviews of patients receiving IVI. The questionnaire was validated in a single centre pilot study involving 142 patients with categorical principal components analysis (CATPCA) analysis revealing five dimensions of patient burden, including disruption of normal routine or capacity, anxiety, frequency of visits, chronicity of disease and perceived treatment value; these dimensions were estimated to account for 67% of variance with Cronbach’s alpha of 0.97, and a refined subset of nine questions was identified as the most salient aspects of treatment whose response scale could be used as a direct single measure of the patient burden of treatment; this subset was used to construct the Treatment Burden Score (TBS).15 The purpose of the current study was to characterise the burden of repeated IVI using the QUALITII survey within four retina clinical practices across the USA.
Methods
Participants
Participants from four retinal practices (Retina Consultants of Texas, Houston TX; Mid-Atlantic Retina, Philadelphia PA; Sierra Eye Associates, Reno NV and Retina Vitreous Associates of Florida, Tampa FL) were invited to voluntarily self-administer the QUALITII survey electronically. No prior medical record review was performed and no patient-specific identifying information was monitored or recorded. It was deemed by the Houston Methodist Research Institute Institutional Review Board that this study did not meet the definition of Human Subject Research per 45 CFR 46 and did not require IRB review or approval.
Study design
Parameters for inclusion were patients receiving ongoing IVIs for exudative retinal diseases. The full 50-item QUALITII survey (online supplemental appendix 1) was administered via emailed survey or electronically at the site of clinical care. The QUALITII survey was previously validated and includes questions on demographics, disease and treatment history, patient perceptions of discomfort, anxiety, inconvenience and satisfaction associated with treatment.15 Response scales were a combination of multiple-choice items ranked on a 7-point Likert scale (scale 0–6) with graded continuum, multiple-choice items without ranked scales and free response. Nine Likert scale questions were incorporated into the survey and these response scales were intermittently reversed, such that 0 represented the highest rating at times and the lowest rating at other times, to control for tendency of respondents to select answers reflexively. During the analysis, the Likert scale questions were converted such that a maximum score of 6 always represents maximum value (ie, 6 represented very significant discomfort). Of the 50 items on the QUALITII survey, a subset of nine items identified by CATPCA as the most salient aspects of treatment burden formed the TBS with a theoretical range of 1–54, where 54 represents maximum burden (online supplemental appendix 2).
bmjophth-2022-001188supp001.pdf (94.7KB, pdf)
bmjophth-2022-001188supp002.pdf (62.3KB, pdf)
Statistical analysis
All variables were individually reviewed and converted to numerical values for statistical analysis where relevant. The distributions of continuous variables were assessed with histograms and tests for normality. Associations between categorical variables were tested using χ2 tests. Differences in the medians of continuous variables were tested using the Wilcoxon Rank-Sum test or Kruskal-Wallis test with pairwise group comparisons adjusted using the Dwass, Steel, Critchlow-Fligner method, which controls for experiment error rate and maximum type I error rate.16 Multivariable analyses using linear regression of the mean were conducted to further determine individual effects of the variables of interest while adjusting for the effects of the other variables in the model. A set of predictor variables including age, location, disease, time of diagnosis, frequency of IVI and total number of IVI were picked for inclusion in the model based on statistical and clinical significance. Prior to creating the multivariable model, all predictor variables were tested for multicollinearity with one another using variance inflation factor (VIF) statistics. Any predictor variables with VIF statistics >5 would be excluded, but all were ≤5. A significance level of 0.05 was used for all comparisons to determine statistical significance. SAS statistical software V.9.4 (SAS Instituted, Cary, North Carolina) was used for all analyses.
Results
Demographics
A total of 1416 patients completed the QUALITII survey across four clinical sites (n=12, 185, 536 and 683) with 98% completing the survey in English. The demographics and other characteristics of participants are included in table 1.
Table 1.
Respondent demographics
| Factor | N (%) |
| Language | |
| English | 1390 (98) |
| Spanish | 26 (2) |
| Age, Mean (Range) | 71 (25–100) |
| <63 years | 337 (24) |
| 63–71 years | 331 (23) |
| 72–79 years | 388 (27) |
| >80 years | 360 (25) |
| Gender | |
| Men | 644 (45) |
| Women | 772 (55) |
| Ethnicity | |
| Caucasian (white) | 1198 (85) |
| Latino (Hispanic) | 95 (7) |
| African American (black) | 69 (5) |
| Asian | 30 (2) |
| American Indian | 3 (0) |
| Pacific Islander | 6 (0) |
| Other | 15 (1) |
| Insurance | |
| Private health insurance | 886 (48) |
| Marketplace insurance | 10 (1) |
| Military healthcare | 41 (3) |
| Public health insurance (Medicare or Medicaid) | 861 (61) |
| No insurance/self-pay | 30 (2) |
| Diagnosis | |
| Age-related macular degeneration (AMD) | 657 (46) |
| Diabetic macular oedema (DMO) and/or diabetic retinopathy (DR) | 360 (25) |
| Retinal vein occlusion (RVO) | 221 (16) |
| Other | 93 (7) |
| Uncertain | 85 (6) |
Multiple selections were allowed for insurance policies.
n=1416 for all factors listed.
Patient-reported details of IVI treatment
The greatest proportion of patients reported being diagnosed with their respective retinal disease within the last 1 to 4 years (47.7%) with a range of diagnosis within 1 year (14.0%) and over 10 years (10.0%). Anti-VEGFs were the most commonly administered medication and 36.6% of patients reported having tried more than a single medication. The median frequency of injection was once every 4 to 5 weeks, with a range from every 4 to 5 weeks (40.5%) to every 13 to 16 weeks (2.4%), and 5.2% of patients reported injections on a as-needed/PRN basis. The highest proportion of patients reported having received 20–50 total IVI (28.4%) with a range from 0 to 5 IVI (15.4%) to greater than 50 IVI (17.7%).
Patient burden associated with treatment
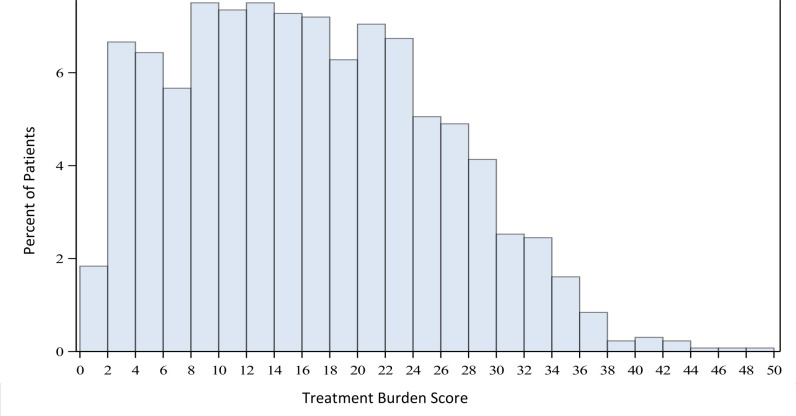
The mean TBS was 16.1±9.2 (actual range of 1–48) and the TBS distribution is displayed in figure 1. The mean TBS across the four sites was 14.8, 15.0, 16.0 and 20.6, suggesting the burden of treatment may not be equal across all sites. The two sites with the highest number of patients surveyed demonstrating mean TBS of 14.8 and 16.0 (p=0.005). Patients younger than 63 years old were found to have a mean TBS of 18.8 compared with 14.4 found in patients greater than 80 years old (p=<0.001). The mean TBS was highest for patients with DMO/DR (17.1) compared with patients with AMD (15.5) and RVO (15.3) (p=0.028). Mean TBS was lower with higher total number of injections such that patients who had received more than 50 IVI reported a lower TBS (15.0) than patients who had received less than 5 IVI (17.6) though this was not statistically significant (p=0.148). Linear regression analysis was fitted to solve for the TBS at the mean of the data (table 2). Age, location and disease exerted a significant effect on TBS while time of diagnosis, frequency of diagnosis and total number of injections did not.
Figure 1.
Bar graph of distribution of TBS. TBS, Treatment Burden Score.
Table 2.
Treatment Burden Score linear regression model: Mean
| Factors | Difference of means | 95% CI | P value | |
| Lower | Upper | |||
| Age | ||||
| < 63 years old (vs > 80 years old) | −4.33 | −6.21 | −2.45 | <0.001 |
| Location | ||||
| Northeast (vs Midwest) | 4.26 | 2.54 | 5.98 | <0.001 |
| Midwest (vs South) | 5.99 | 4.21 | 7.76 | <0.001 |
| South (vs Northeast) | 1.73 | 0.51 | 2.94 | 0.005 |
| Disease | ||||
| AMD (vs RVO) | 1.29 | −0.22 | 2.79 | 0.093 |
| DMO/DR (vs AMD) | 1.63 | −0.93 | 2.18 | 0.028 |
| RVO (vs DMO/DR) | −1.66 | −2.31 | 0.99 | 0.042 |
| Time of Diagnosis | ||||
| < 5 years (vs > 5 years) | −0.85 | −2.25 | 0.54 | 0.23 |
| Frequency of treatment | ||||
| Every 4–5 weeks (vs every 10–16 weeks) | −1.58 | −3.27 | 0.11 | 0.066 |
| Every 10–16 weeks (vs PRN) | 0.83 | −2.24 | 3.9 | 0.596 |
| PRN (vs every 4–5 weeks) | −0.75 | −3.62 | 2.12 | 0.608 |
| Total number of injections | ||||
| < 10 (vs > 50) | −1.44 | −3.4 | 0.51 | 0.148 |
Patients with no missing data for any of the variables were included in the regression analysis; n=1120.
P value < 0.05 considered to be significant.
AMD, age-related macular degeneration; DMO, diabetic macular oedema; DR, diabetic retinopathy; PRN, pro re nata; RVO, retinal vein occlusion.
Despite the burden associated with treatment, the majority of patients (90.1%) reported they would recommend IVI to others with similar eye conditions. Overall, patients reported a mean satisfaction of 5.46, which was found to be higher among patients who had received more than 50 IVI (mean 5.55) compared with those having received less than 5 IVI (mean 5.19) (p=0.044). The top motivations to continue treatment noted by patients include preservation of vision (54%), improvement of vision (19%) and prevention of disease progression and blindness (17%).
Patient-reported factors of discomfort related to treatment
The most commonly reported side effects of IVI were eye pain (49.7%) and increased light sensitivity (37.1%) while 26.8% of patients reported not routinely experiencing any side effects. The mean score related to IVI associated discomfort was quite low (1.86), but a sizeable proportion of patients reported experiencing side effects more than half the visits (50%) and discomfort lasting greater than 4 hours (38%). Of note, 25% of patients reported post-IVI discomfort lasting greater than 8 hours. Patient age was associated with perception of pain during IVI; the mean score related to IVI associated discomfort was 2.22 among patients younger than 63 years compared with 1.56 among patients older than 80 years (p=<0.001). The most uncomfortable aspects of treatment reported by patients were injection of the medication into the eye (29%), the feeling after the topical anaesthetic wears off (17%) and the use of betadine (12%). Top patient-reported strategies to manage the discomfort of treatment include rest (51%), use of artificial tears (32%) and pain medication (13%).
Patient-reported details on anxiety related to treatment
Levels of anxiety experienced before, during and after treatment were rated at a mean value of 1.5, 1.8 and 0.9. Patients who received IVI on an as needed/PRN basis reported a higher mean level of anxiety (2.0) prior to treatment compared with patients who received IVI at fixed intervals of monthly (1.5) or every 10–16 weeks (1.5) (p=0.048). Notably, patients with a history of greater total number of injections received reported lower anxiety levels during treatments, including before (p=0.026), during (p=0.050) and after (p=0.016) injection. The use of antianxiety medication such as benzodiazepines to cope with anxiety related to IVI was reported by 4.1% of patients. Patient reported factors related to anxiety are listed in table 3.
Table 3.
Most frequent responses on anxiety related free-response questions
| Causes of anxiety | Factors lessening anxiety | Factors worsening anxiety | Anxiety managing strategies |
| (Response rate: 26%) | (Response rate: 19%) | (Response rate: 14%) | (Response rate: 20%) |
| Idea of intravitreal injection | Confidence in physician | Long wait time | Breathing exercise/meditation |
| 31% | 16% | 35% | 22% |
| Fear of loss of vision | Supportive staff | Delays in procedure | No coping mechanism |
| 8% | 9% | 7% | 16% |
| Pain associated with injection | Breathing exercises/meditation | Crowded office space | Positive thinking |
| 8% | 9% | 5% | 10% |
| Anxiety before injection | Shorter wait time | Patient concerns not addressed | Anti-anxiety medication |
| 6% | 8% | 5% | 8% |
| Previous bad experience | Completing visit | Anticipation of injection | Faith/prayer |
| 5% | 8% | 4% | 6% |
Responses were coded from free-text responses.
Patient-reported burden related to treatment
Following IVI, 42.1% of patients reported subsequent restrictions from usual activities due to the discomfort associated with the procedure. Patients with more than 50 IVI (51%) reported a higher incidence of restrictions from usual activity compared with patients with fewer than 5 IVI (32%) (p=<0.001). Consistent with this pattern, patients who had more than 50 IVI reported a longer duration of restriction, with 28.5% reporting restrictions lasting between 9 hours and 24 hours compared with 17.1% among patients who had fewer than 5 IVI (p=0.005).
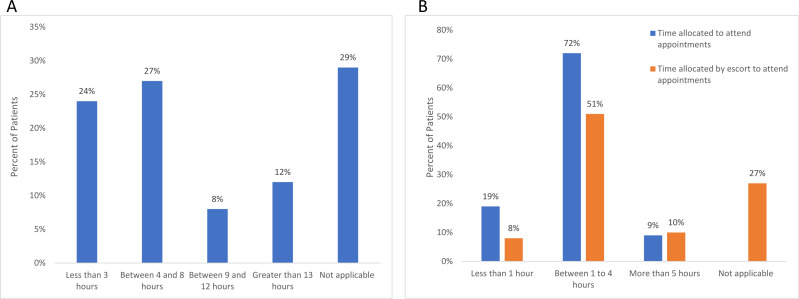
Although most patients rated the mean time consumption of their treatment as low to moderate (2.41), 62.7% of patients reported scheduling appointments at specific times to minimise disruptions to daily activity. Additionally, 62.6% of patients reported requiring an escort to attend appointments and rated the mean level of convenience as 3.91. The duration of restriction and time allocated by patient and escort to attend appointments are conveyed in figure 2.
Figure 2.
(A) Patient reported duration of restriction before returning to usual activity. (B) Patient reported time allocated to attend appointments and time allocated by escort to attend appointments.
A subset of questions specifically addressed the burdens associated with bilateral IVI, which 33.0% of participating patients reported receiving. Increased inconvenience (12.8%) was the most commonly reported effect of bilateral IVI, whereas 46% of respondents reported no difference in the inconvenience associated with bilateral compared with unilateral IVI. Of patients who received bilateral IVI, those who had been diagnosed for more than 5 years earlier (6.7%) noted increased inconvenience compared with patients who had been diagnosed within 5 years (2.9%) (p=0.001). Additionally, of patients who received bilateral IVI, those who had received more than 50 IVI (3.2%) reported increased anxiety level on visits to receive bilateral IVI than those having received fewer than 5 IVI (0.0%) (p=0.011).
Discussion
The current study provides an analysis of the details of treatment burden perceived by patients who are receiving ongoing IVI for exudative retinal diseases. The TBS, a single score measurement assessing overall treatment burden, was moderate on average across participating centres. The mean TBS was the highest among patients with DMO/DR compared with patients with AMD or RVO. This difference could be due to a variety of factors specific to diabetes, including increased physical and psychosocial burden associated with complications of diabetes,17–19 the younger working population of patients with diabetes or the increased out-of-pocket cost that many patients with diabetes bear for IVI treatment.20 In this study, the TBS exhibited an inverse relationship with age and total number of injections, suggesting that perhaps patients adapt to the demands of IVI and gain a better understanding of the benefits of treatment as they continue their treatment course.
Most patients reported high levels of satisfaction with low levels of discomfort associated with treatment despite the frequency of IVI many patients were receiving. Older respondents reported lower levels of discomfort than younger respondents, but treatment duration and total number of IVI did not have an effect on perceived discomfort level. This finding supports that there may be a component of decrease in tactile sensitivity associated with age. Additionally, desensitisation with consecutive IVI which had been previously reported was not observed in our study.21 Despite the overall mean low level of discomfort, a substantial proportion of respondents reported that they had experienced prolonged periods of discomfort and frequent side effects. Advising patients of not only potential side effects of IVI but also frequency and duration of possible discomfort can help set realistic expectations for patients in their treatment course. The relationship between the low levels of discomfort and sustained levels of discomfort was an interesting finding from the survey.
Overall anxiety was low throughout the procedure and the main factors reported to induce fear were the injection itself, fear of losing sight and fear of the pain associated with the IVI, consistent with prior studies.11 13 Patients who received IVI on an as needed/PRN basis reported greater anxiety compared with other treatment regimens, which could be associated with uncertainty of not knowing whether they will require an IVI at that visit.22 Similar to the overall TBS, anxiety decreased with increasing total number of IVI received, which suggest that many patients can become accustomed to the side effects of treatments as well as gain an understanding of treatment benefit. As such, it may be valuable to direct specific attention to adaptability and importance early in the treatment course to minimise loss to treatment non-adherence.
The main factors patients identified as exacerbators of anxiety were long wait times, delays in procedure and crowded office space. Accordingly, optimising clinic workflow efficiencies and minimising treatment delays may help reduce patient anxiety. Patients reported breathing exercise and meditation as the most common management strategies for anxiety, while 16% of patients reported not having any specific coping mechanisms. The beneficial effects of meditation have been studied in primary open-angle glaucoma patients and were found to reduce intraocular pressure, improve quality of life and normalise stress biomarkers.23 With the advent of several meditation apps to reduce stress, providing patients with these tools may be a simple intervention to consider.24
The overall perceived level of inconvenience was low to moderate with most patients allocating between 1 hours and 4 hours for total appointment. A substantial proportion of patients required an escort to attend appointments supporting that the burden of treatment is not only confined to the patient but also the care provider being predominantly family and friends. This supports that there is indeed a socioeconomic component of burden in patients receiving IVI. Patients who received greater number of IVI reported higher rates of disruption to daily activities, which appears to be associated with slower recovery after treatment associated with older age.
Approximately, a third of patients were receiving bilateral IVI. Patients who had received more IVI or were diagnosed more than 5 years earlier reported increased levels of anxiety and increased inconvenience during visits where bilateral IVI were administered compared with unilateral IVI. This finding suggests that some patients who have been receiving bilateral IVI may desire to opt out of bilateral IVI at some point during their treatment course, warranting discussion of the possibility.
There are several limitations of the current analysis. First, while patients were included from four geographically distinct retina practices in four US states, the number of patients was not distributed evenly across sites. Patients also self-administered the survey either at home or while at their clinic visit, which could introduce variability in perceived burden as patients who completed the survey during their clinic visit may have a heightened perception of their treatment experience.
In summary, the overall level of discomfort and anxiety with receiving IVI was low among the patients included in this analysis, even though patients experienced side effects often and for prolonged durations. Addressing patient concerns and setting up expectations at the early visits as well as maintaining discussions during the treatment course may improve patient experiences and lead to improved adherence over time. Still, despite the various burdens associated with treatment, it is reassuring that overall satisfaction and belief in the effectiveness of the treatment was high. Furthermore, the TBS used in this study is a validated, quantitative estimate of patient treatment burden, which provides a standardised measure of treatment burden across different clinics and provides a basis on which to compare patient experiences between IVI and emerging treatment options for exudative retinal diseases, including surgical devices, extended-release treatments and gene therapies.
Acknowledgments
The authors would like to acknowledge the contribution of the research and administrative staff at the various clinical sites for assisting with survey logistics and administration of survey.
Footnotes
Presented at: The American Society of Retina Specialists (ASRS) 2021. San Antonio TX, October 8-12, 2021. Results of a Multicenter Survey to Measure Treatment Burden: Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII).
Contributors: RW, CKC and CCW designed the study, acquired and interpreted the work, drafted and revised the manuscript. SL, RM, MS, MA, JV, AAA, AE, AVC acquired the data and were involved in critical revision of the content. EBL and SF analysed and interpreted the work as well as were involved in critical revision of the content. VW and AMS substantially contributed to the conception and design of the survey and were involved in critical revision of the content. NS was involved in conception of the design, interpretation of the data and critical revision of the content. AMK, DAE, CR were involved in interpretation of the data, drafting and revision of the content and final approval of the content. CCW is the guarantor of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Martin DF. Evolution of intravitreal therapy for retinal Diseases-From CMV to CNV: the LXXIV Edward Jackson memorial lecture. Am J Ophthalmol 2018;191:xli–lviii. 10.1016/j.ajo.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema. Ophthalmology 2015;122:2044–52. 10.1016/j.ophtha.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 4.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313–9. 10.1016/j.ophtha.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra R, Preston GC, Keenan TDL, et al. Intravitreal injections: past trends and future projections within a UK tertiary hospital. Eye 2022;36:1373–8. 10.1038/s41433-021-01646-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brose LS, Bradley C. Psychometric development of the retinopathy treatment satisfaction questionnaire (RetTSQ). Psychol Health Med 2009;14:740–54. 10.1080/13548500903431485 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell J, Bradley C. Design and development of the MacTSQ measure of satisfaction with treatment for macular conditions used within the IVAN trial. J Patient Rep Outcomes 2017;2:5. 10.1186/s41687-018-0031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 2013;27:787–94. 10.1038/eye.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal O, Segal-Trivitz Y, Nemet AY, et al. Anxiety levels and perceived pain intensity during intravitreal injections. Acta Ophthalmol 2016;94:203–4. 10.1111/aos.12802 [DOI] [PubMed] [Google Scholar]
- 11.Boyle J, Vukicevic M, Koklanis K, et al. Experiences of patients undergoing anti-VEGF treatment for neovascular age-related macular degeneration: a systematic review. Psychol Health Med 2015;20:296–310. 10.1080/13548506.2014.936886 [DOI] [PubMed] [Google Scholar]
- 12.Boyle J, Vukicevic M, Koklanis K, et al. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med 2018;23:127–40. 10.1080/13548506.2016.1274040 [DOI] [PubMed] [Google Scholar]
- 13.Polat O, İnan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Türk Oftalmoloji Dergisi 2017;47:205–10. 10.4274/tjo.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller S, Junker S, Wilke T, et al. Questionnaire for the assessment of adherence barriers of intravitreal therapy: the ABQ-IVT. Int J Retina Vitreous 2021;7:43. 10.1186/s40942-021-00311-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClard CK, Wang R, Windham V, et al. Questionnaire to assess life impact of treatment by intravitreal injections (QUALITII): development of a patient-reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol 2021;6:e000669. 10.1136/bmjophth-2020-000669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douglas CE, Michael FA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Methods 1991;20:127–39. 10.1080/03610929108830487 [DOI] [Google Scholar]
- 17.Kalra S, Jena BN, Yeravdekar R. Emotional and psychological needs of people with diabetes. Indian J Endocrinol Metab 2018;22:696. 10.4103/ijem.IJEM_579_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Brazeal M, Choi H, et al. Physical and psychosocial factors associated with depression among adults with type 2 diabetes mellitus at a federally qualified healthcare center. Soc Work Health Care 2018;57:834–50. 10.1080/00981389.2018.1508113 [DOI] [PubMed] [Google Scholar]
- 19.Weinger K, Lee J. Psychosocial and psychiatric challenges of diabetes mellitus. Nurs Clin North Am 2006;41:667–80. 10.1016/j.cnur.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Chua K-P, Lee JM, Conti RM. Out-Of-Pocket spending for insulin, diabetes-related supplies, and other health care services among privately insured us patients with type 1 diabetes. JAMA Intern Med 2020;180:1012–4. 10.1001/jamainternmed.2020.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rifkin L, Schaal S. Factors affecting patients' pain intensity during in office intravitreal injection procedure. Retina 2012;32:696–700. 10.1097/IAE.0b013e3182252ad3 [DOI] [PubMed] [Google Scholar]
- 22.Martel A, Nahon-Esteve S, Martini K, et al. Feelings, preoperative anxiety, and need for information in patients undergoing intravitreal injections. Graefes Arch Clin Exp Ophthalmol 2020;258:1395–403. 10.1007/s00417-020-04699-4 [DOI] [PubMed] [Google Scholar]
- 23.Dada T, Mittal D, Mohanty K, et al. Mindfulness meditation reduces intraocular pressure, lowers stress biomarkers and modulates gene expression in glaucoma: a randomized controlled trial. J Glaucoma 2018;27:1061–7. 10.1097/IJG.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 24.Champion L, Economides M, Chandler C. The efficacy of a brief app-based mindfulness intervention on psychosocial outcomes in healthy adults: a pilot randomised controlled trial. PLoS One 2018;13:e0209482. 10.1371/journal.pone.0209482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjophth-2022-001188supp001.pdf (94.7KB, pdf)
bmjophth-2022-001188supp002.pdf (62.3KB, pdf)
Data Availability Statement
No data are available.