Abstract
Background
Predicting progression to clinical arthritis in individuals at-risk of developing rheumatoid arthritis is a prerequisite to developing stratification groups for prevention strategies. Selecting accurate predictive criteria is the critical step to define the population at-risk. While positivity for anti-citrullinated protein antibodies (ACPA) remains the main recruitment biomarker, positivity for other autoantibodies (AutoAbs) identified before the onset of symptoms, may provide additional predictive accuracy for stratification.
Objective
To perform a multiple AutoAbs analysis for both the prediction and the time of progression to inflammatory arthritis (IA).
Methods
392 individuals were recruited based on a new musculoskeletal complaint and positivity for ACPA or rheumatoid factor (RF). ELISAs were performed for ACPA, RF, anti-nuclear Ab, anti-carbamylated protein (anti-CarP) and anti-collagen AutoAbs. Logistic and COX regression were used for analysis.
Results
Progression to IA was observed in 125/392 (32%) of cases, of which 78 progressed within 12 months. The AutoAbs ACPA, RF, anti-CarP were individually associated with progression (p<0.0001) and improved prediction when combined with demographic/clinical data (Accuracy >77%; area under the curve (AUC) >0.789), compared with prediction using only demographic/clinical data (72.9%, AUC=0.760). Multiple AutoAbs testing provided added value, with +6.4% accuracy for number of positive AutoAbs (AUC=0.852); +5.4% accuracy for AutoAbs levels (ACPA/anti-CarP, AUC=0.832); and +6.2% accuracy for risk-groups based on high/low levels (ACPA/RF/anti-CarP, AUC=0.837). Time to imminent progression was best predicted using ACPA/anti-CarP levels (AUC=0.779), while the number of positive AutoAbs was/status/risk were as good (AUC=0.778).
Conclusion
We confirm added value of multiple AutoAbs testing for identifying progressors to clinical disease, allowing more specific stratification for intervention studies.
Keywords: Arthritis, Rheumatoid; Anti-Citrullinated Protein Antibodies; Rheumatoid Factor
What is already known about this subject?
Rheumatoid arthritis is now recognised as a continuum of disease with a preclinical phase identified by the presence of Autoantibodies highlighting a breach of tolerance. Both rheumatoid factor and anti-citrullinated protein antibodies have been associated with progression to inflammatory arthritis from this at-risk, preclinical stage.
What does this study add?
Other autoantibodies have been described but have not yet been assessed for their additional value in improving prediction models. Whether combination of autoantibodies may have added value also remains to be investigated.
How might this impact on clinical practice or further developments?
Being able to predict the highest risk of progression with better accuracy and/or the imminence of it, would allow the design of clinical trials testing interventions aiming at preventing progression to disease, notably by shortening the follow-up time needed to observe progression.
Introduction
Rheumatoid arthritis (RA) is a chronic disease with substantial impact on the lives of people worldwide, with >1 million patients having severe disability from the disease in Europe alone. Despite the recent progress achieved with ‘treat-to-target’ therapeutic strategies1 and the earlier access to treatment enabled by the European Alliance of Associations for Rheumatology (EULAR)-2010 diagnostic criteria,2 the outcome of RA is still a major concern as well as a financial burden on health services, patients and society with considerable socioeconomic cost.3–5
Over a decade ago, research highlighted a preclinical phase of the disease with the recognition of an inflammatory arthritis continuum (IAC),6 moving the knowledge gap to the earliest stages of disease progression. Although the exact pathogenesis of RA remains unclear, autoimmune processes are believed to play a role and recent research has proposed a series of events underpinning the IAC.6 A breach of tolerance needs to occur (ie, development of autoimmunity) and this assumption has largely been verified by the presence of specific autoantibodies (AutoAb), years before the development of symptoms,7–11 occurring in association with an major histocompatitbility complex/T-cell signalling genetic background.12 13 Systemic autoimmunity is not sufficient to initiate clinical arthritis and a second series of events (the second hit) needs to happen, for which the cells and the possible triggers are not yet known, although several hypotheses have been proposed (implicating cytokines, netosis, pain, osteoclast and/or T-cell or Th17 cells).14–18 This understanding offers an unprecedented opportunity to study ‘at-risk’ individuals and ultimately intervene with the ambition of preventing the progression to arthritis by means of life-style modification or pharmacotherapy.
Several risk stratification models have been developed including demographic, genetic, clinical, imaging, serological and immunological biomarkers with variable predictive value.19–26 Reported predictive biomarkers however have low sensitivity for the prediction of progression to disease stages while specificities are relatively high (>75%). Other studies have reported potential biomarkers, while not modelling for the actual outcome.8 10 11 27–30
The presence of disease specific AutoAbs (notably anti citrullinated protein antibodies, ACPA) prior to disease development remains the best-known risk-factor,7 8 although it is also the main biomarker used to recruit most at-risk cohorts, (including arthralgia and first degree relationship to RA patients). Different ACPA tests also showed different results (positivity and levels), based on the repertoire of peptides used in second and third generation kits.31 Other AutoAbs have been detected years before the onset of inflammatory symptoms including anti-carbamylated protein (anti-CarP), rheumatoid factor (RF) (of the IgM class, further referred to as RF), anti-native and anti-glycated collagen-II AutoAbs.9–11
In this study, we aimed to investigate the added value of testing for the presence of multiple AutoAbs for the prediction of progression to clinical synovitis, in a cohort of at-risk individuals selected on the basis of positivity for ACPA and/or RF associated with a new onset of non-specific musculoskeletal symptoms. Several AutoAbs were explored, notably using in-house ELISA for anti-CarP and anti-collagen AutoAbs. Our data suggest added-value for ACPA, RF, anti-CarP and anti-collagen-II AutoAbs when tested individually either as positive/negative status, continuous levels or dichotomised as high-risk/low-risk groups. Detecting positivity of multiple AutoAbs improved predictive accuracy, which may facilitate more precise selection of study populations as well as determination of high risk of progression in a short time frame to prioritise intervention.
Methods
Subjects and samples
Serum samples were obtained from participants attending an at-risk research clinic at Chapel Allerton Hospital in Leeds between 2008 and 2018.
Briefly, individuals aged ≥18 years, with a new non-specific musculoskeletal joint symptom presenting to their primary care physician or other health professional were referred as previously described.22 23 A new musculoskeletal symptom was defined as pain in any joint and/or musculoskeletal symptom (including but not limited to rotator cuff tendonitis, subacromial bursitis, carpal tunnel syndrome, tendonitis, or epicondylitis), which was not previously reported. Individuals were then recruited to attend the research clinic if they tested positive for ACPA or RF as tested by National Health Service (NHS) services as described below. Exclusion criteria were previous diagnosis of an inflammatory condition, exposure to DMARDs and presence of a swollen joint at first visit. Participants were followed 3 monthly for 1 year and then yearly, until progression to inflammatory arthritis (IA) developed (ie, clinical swelling of at least one joint), evaluated by a rheumatologist/rheumatology research nurse. Progression could occur at any time, and patients had open access to immediate appointment if experiencing a new symptoms. Classification for RA criteria were recorded at study visit only and progression to RA was observed although later during follow-up.
The dataset used in this report was frozen in late 2019 and do not include visits, samples or data collected over the pandemic. We only included participants up to the point in time for which they had visit data. The strategy for data analysis was to use (1) the exact time of progression for participants who progressed at any time during follow-up; (2) for non-progressors, we only included those having at least 12-month follow-up (up to 10 years), while follow-up duration was calculated from baseline until their last available appointment.
A serum sample was collected in clotting tube at inclusion in the cohort, spun for 10 min, no shorter than 30 min and no longer than 1 hour after collection, aliquoted and stored at −30°C.
Autoantibody status and levels
In the absence of absolute standards for AutoAbs assays, arbitrary units (AU) or optical densities (OD) values were used to describe levels observed. Hospital services were used to initially assess positivity (yes/no) for ACPA and/or RF.
RF levels (IgM) were measured by nephrology and considered positive above a cut-off of 20 AU/mL. Anti-nuclear Ab (ANA) were detected as part of our routine assessment and detected by immunofluorescence (homogeneous, nucleolar or specked staining) and reported as negative/positive. Levels of ACPA and RF measured in 15 HCs were all below cut-off and ANA staining were all negative.
ACPA IgG levels were then measured using a seconnd-generation research ELISA (CCP-2, immunoCap Phadia). Cut-off for positivity was set according to manufacturers at 10 AU/ml. Patients with borderline results were retested at 3 months follow-up (mostly confirming negative).
In-house ELISA was developed for IgG autoantibodies for Anti-CarP and anti-native and anti-glycated collagen. In addition, IgG-RF was measured using a commercial ELISA (EuroImmune, Germany) as per manufacturer’s instruction (cut-off 20 RU/mL).
Anti-CarP Abs were measured in the Leiden University Medical Centre (Leiden, The Netherland) as previously described,10 using carbamylated foetal calf serum (10 µg/mL) as antigen. Positivity for Anti-CarP Abs in HC (n=175, median age 51, range 27–65) was previously described32 33 and a cut-off set at 235 UA/mL.
In-house ELISA for the detection of anti-native collagen (CI and CII) and glycated collagen (GLY-CI and GLY-CII) was performed as previously described.11 33 Briefly, collagen CI (Cellsystems) and CII (MD Biosciences) were chemically modified by Maillard reaction in a single batch to generate post-translationally modified GLY-CI/GLY-CII. ELISA plates were coated with 10 µg/mL of GLY-CI/GLY‐CII or native-CI/CII. ELISA OD values were recorded following normalisation to BSA and GLY-BSA (10 µg/mL). Ranges of results in HC were previously established33 (n=98, median age 51, range 27–65) and set at 1.8 and 3 OD/mL for native CI/CII, and 2 and 2.4 for GLY-CI/GLY-CII respectively.
Enough serum was available from only for 152 and 71 individuals for RF-IgG and the collagen related AutoAb testing respectively. ANA were tested in 290 participants. We had missing data for a few samples for the main three autoAbs tested. This affected mainly RF with 11 missing data (status and levels). For ACPA and anti-CarP, this affected 29 (levels only) and 20 cases, respectively (status and level).
Statistical analysis
Frequencies were compared using Pearson’s χ2 test. Numerical variables were not normally distributed (Kolmogorov-Smirnov test) and compared using non-parametric Mann‐Whitney U tests. A value of p<0.05 was considered statistically significant. Where needed, corrections for multiple testing were applied.
Missing data frequencies ranged from none to 31/451 (6.9%) for both clinical and AutoAbs data. Multiple data imputation was performed using five cycles. Logistic regressions were used to model the added value of multiple AutoAb testing for the prediction of progression, using a stepwise Forward approach. A Cox regression was also used to model time to progression. Data were presented using GraphPad Prism V.8 and analysed using SPSS V.26 and /or R V.4.1.2 packages.
Results
Population description
Individuals at-risk of RA, followed since 2008 and up to December 2019, was included in our AutoAbs analysis, when progression to IA could be established over at least 12 months of follow-up, excluding recently recruited patients with less than 12 months of follow-up data, withdrawal by choice. Patients with whom we lost contact (over 12 year) were included to the last appointment available. This allowed us to include 392 participants. Participants had a median age of 51 years (IRQ 18 years, range 18–82) and 70% were women. Participant’s demographic and clinical characteristics (including missing data) according to their progression status are shown in table 1.
Table 1.
Overall cohort clinical description (n=392)
| Univariate statistics | Unadjusted | Adjusted model (logistic regression) |
Adjusted model (COX regression) |
|||||||
| Missing data n/451 | Progressor n=125 | Non-progressor n=267 | P value | OR (95% CI) |
P value | OR (95% CI) |
P value | HR (95% CI) |
P value | |
| Age* (years) |
None | 53 (21) (22–80) |
50 (19) (18–82) |
0.108 | 1.018 (0.998 to 1.030) |
0.030 | ||||
| Sex (F/M) |
None | 89/36 | 195/72 | 0.718 | 0.940 (0.638 to 1.577) |
0.081 | ||||
| Smoking (never/ever) | 1 | 36/89 | 122/144 | <0.0001 | 3.072 (1.994 to 4.733) |
<0.0001 | 2.626 (1.718 to 4.331) |
<0.0001 | 1.813 (1.228 to 3.106) |
0.015 |
| HLA SE (yes/no) |
1 | 93/32 | 121/145 | <0.0001 | 3.239 (2.102 to 4.990) |
<0.0001 | 3.204 (1.902 to 4.832) |
<0.0001 | 2.575 (1.522 to 3.976) |
<0.0001 |
| EMS* (minutes) |
1 | 30 (60) (0–480) |
5 (30) (0–560) |
<0.0001 | 1.003 (1.000 to 1.006) |
0.031 | ||||
| ESR* (minutes) |
1 | 14 (19) (1–83) |
10 (15) (1–71) |
<0.0001 | 1.042 (1.018 to 1.051) |
<0.0001 | 1.040 (1.015 to 1.050) |
<0.0001 | 1.031 (1.015 to 1.044) |
<0.0001 |
| CRP (hs)* (mg/L) |
1 | 2.6 (8) (0–80) |
1.5 (4) (0–83) |
0.015 | 1.037 (0.009 to 1.064) |
0.011 | ||||
| TJC78* | 1 | 2 (4) (0–21) |
1 (3) (0–16) |
0.002 | 1.138 (1.045 to 1.199) |
0.001 | 1.162 (1.064 to 1.243) |
<0.0001 | 1.135 (1.066 to 1.199) |
<0.0001 |
| Family relatives (yes/no) |
None | 37/88 | 80/187 | 0.778 | Accuracy 72.9% SEN 37% (31–47) SPE 89% (85–92) PPV 63% (53–71) NPV 75% (72–77) AUC 0.760 (0.709 to 0.811) |
AUC 0.725 (0.723 0.727) |
||||
| Diabetes (yes/no) |
12 | 20/105 | 33/234 | 0.325 | ||||||
*Median (IQR) (range).
AUC, area under curve; CRP, C reactive protein (high sensitivity); EMS, early morning stiffness; ESR, erythrocyte sedimentation rate; PPV/NPV, positive/negative predictive value; SE, share epitope; SEN, sensitivity; SPE, specificity; TJC78, tender joint count in 78 joints.
We observed progression to clinical IA in 125/392 (32%). The median time of progression was 12 months (IQR 21 months and range 1–113) and the follow-up in non- progressors had a median time of 38 months (IQR 38 and range 12–144). Progression over years is illustrated in online supplemental figure 1. Age was not different between progressors and non-progressors but female gender tended to be more frequent in progressors (p=0.108). A patient-reported case of first degree arthritis in the family was also not associated with progression. Previously reported risk factors from this cohort22 were associated with progression, including smoking history, early morning stiffness, erythrocyte sedimentation rate (ESR), C reactive protein and painful joint counts (all p<0.001). Comorbidities such as diabetes were not associated with progression.
rmdopen-2022-002512supp001.pdf (228.8KB, pdf)
There was randomness of missing data at low frequencies (maximum 5.1% missing data excluding RF-IgG, ANA and anti-collagen, see below), therefore, missing data were imputed for both clinical as well as for AutoAbs. Unadjusted OR for each variable were calculated from the pooled 5 cycles of imputation and were not significantly different from the original dataset.
AutoAbs determination
Because of limited amount of serum, all AutoAbs could not be tested in all samples (missing tests detailed in table 2), notably for the anti-collagen AutoAbs and RF-IgG, while ANA were not performed routinely in 36% of participants, resulting in 7 AutoAbs tested.
Table 2.
AutoAbs individual predictive performance (n=392)
| (A) AutoAbs status | ||||||
|
Univariate
p value |
OR (95% CI) |
AUC
(95% CI) |
SEN/SPE
(95% CI) |
PPV/NPV
(95% CI) |
||
| ACPA 29 missing |
<0.0001 | 10.95 (6.20 to 19.35) |
0.748 (0.699 to 0.798) | 86% (79 to 92) 62% (59 to 67) |
52% (48 to 56) 90% (86 to 93) |
|
| RF eleven missing |
<0.0001 | 4.80 (3.03 to 7.57) |
0.687 (0.621 to 0.734) | 68% (60 to 75) 68% (62 to 73) |
51% (45 to 55) 81% (77 to 85) |
|
| anti-CarP 20 missing |
<0.0001 | 4.54 (2.88 to 7.18) |
0.670 (0.610 to 0.730) | 55% (45 to 63) 74% (69 to 80) |
53% (46 to 59) 76% (63 to 72) |
|
| anti-native CII 71 cases |
0.111 | |||||
| anti-GLY-CII 71 cases |
0.075 | |||||
| (B) AutoAbs levels | ||||||
|
Univariate
p value |
OR (95% CI) | Cut-off (AU/mL) | AUC (95% CI) |
SEN/SPE
(95% CI) |
PPV/NPV
(95% CI) |
|
| ACPA 29 missing |
<0.0001 | 1.005 (1.004 to 1.006) | 150 | 0.770 (0.716 to 0.824) | 56% (46 to 66) 75% (70 to 80) |
43% (37 to 50) 84% (80 to 86) |
| RF eleven missing |
<0.0001 | 1.002 (1. to 1.003) | 60 | 0.702 (0.645 to 0.750) | 5% (1 to 8) 98% (95 to 99) |
54% (21 to 38) 67% (66 to 68) |
| Anti-CarP 20 missing |
<0.0001 | 1.001 (1.001 to 1.003) | 300 | 0.720 (0.666 to 0.775) | 26% (19 to 35) 95% (92 to 97) |
70% (57 to 80) 72% (67 to 75) |
| (C) Risk categories | ||||||
|
Univariate
p value |
OR (95% CI) | AUC (95% CI) |
SEN/SPE
(95% CI) |
PPV/NPV
(95% CI) |
||
| ACPA 29 missing |
<0.0001 | 6.230 (3.971 to 9.776) |
0.710 (0.649 to 0.766) | 61% (51 to 69) 80% (75 to 85) |
60% (53 to 66) 81% (77 to 84) |
|
| RF eleven missing |
<0.0001 | 4.020 (2.617 to 6.176) |
0.641 (0.579 to 0.702) | 52% (43 to 60) 78% (73 to 83) |
54% (47 to 61) 77% (74 to 80) |
|
| Anti-CarP 20 missing |
<0.0001 | 4.169 (2.695 to 6.449) |
0.675 (0.615 to 0.735) | 51% (42 to 59) 80% (75 to 85) |
57% (50 to 63) 76% (73 to 79) |
|
ACPA, anticitrullinated protein antibody; AUC, area under the curve; PPV/NPV, positive and negative predictive value; RF, rheumatoid factor; SEN/SPE, sensitivity/specificity.
Most participants were ACPA+ (206/392, 52%), accounting for individual with initial borderline levels, found negative on retesting, and 61 were ACPA+only. 167 participants were RF+ (figure 1A, median 65 UA/mL, IQR 140 UA/mL) of which, 39 participants were RF+only. RF-IgG and ANA were positive in 50% and 20% of samples tested respectively. Positivity for anti-CarP was observed in 126/372 (33.9%) of participants, 10 being anti-CarP+only. Anti-native-CI and CII AutoAbs were detected at low frequencies (9.9% and 29.6%, respectively) while anti-GLY-CII (50.7%) was more frequent but not anti-GLY-CI (24%).
Figure 1.
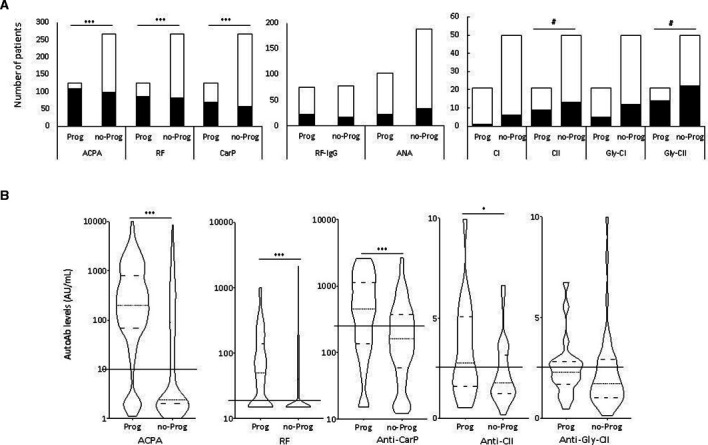
Positivity (A), as number of patients and (B) levels of AutoAb in at-risk individuals. (A) Bars represent the number of individual tested positive (black) or negative (white) for each individual. X2 tests were used individually to assess associations with progression (***p<0.0001, #p<0.100). ACPA n=363, RF n=381, anti-CarP n=372, RF-IgG n=152, ANA n=290, all anti-collagen autoAbs n=71. (B) Violin plots represent the distribution of AutoAb levels observed. Solid lines across the plot indicate the positivity cut-off for each test. Medians and quartiles of distribution are indicated by dotted and dashed lines within violin plot respectively. MWU tests comparing levels were used individually to assess associations with progression (*p<0.05, ***p<0.001, #p<0.100). ACPA, anticitrullinated protein antibody; ANA, anti-nuclear Ab; RF, rheumatoid factor.
Using positivity status (figure 1A), ACPA, RF and anti-CarP were highly associated with progression (table 2A, all p<0.0001). Although the assays could only be performed in a small number of samples (n=71), anti-GLY-CII and anti-native-CII showed a trend for association with progression (p=0.075 and p=0.111, respectively). RF-IgG, ANA and Anti-CI AutoAbs were not associated with progression.
Using continuous levels of AutoAbs (figure 1B), there was significantly higher levels of ACPA (irrespective of the test used), RF and anti-CarP AutoAbs in progressors (table 2B, p<0.0001), while levels were significant higher for anti-native CII (p=0.048) but not for anti-GLY-CII.
Results presented below account for the cohort of 392 patients following imputations for ACPA, RF and anti-CarP, while RF-Ig and ANA AutoAbs were not pursued further due to lack of association with progression and anti-collagen AutoAbs as it could only be tested in 71 patients.
Prediction of progression: individual AutoAbs
Altogether, ACPA, RF, anti-CarP and anti-CII AutoAbs were taken forward, while RF-IgG, ANA, and CI AutoAbs were no longer included in the analysis as not associated with progression. The rate of progression in ACPA+, RF+ and anti-CarP+individual are illustrated in figure 2A, confirming clear separation between status.
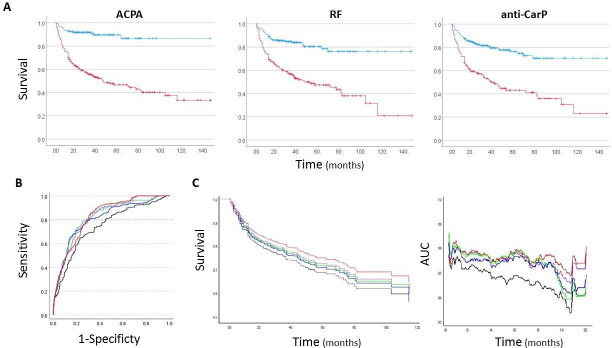
Figure 2.
Modelling the predictive value of autoAbs in at-risk individuals. (A) Individual time of progression with respect to positivity for the three main autoAbs tested (red positive, blue negative). (B) Logistic regression: AUC for prediction models using clinical data and multiple autoAbs based on status, levels, high and low risk groups or autoAbs count. (C, D) Cox regression: Survival and AUC for prediction models using clinical data and multiple autoAbs based on status, levels, risk groups or autoAbs count. (B–D) Black clinical data only; green clinical data+status; red clinical data+levels; blue clinical data+risk group; purple clinical data+autoAbs count. ACPA, anticitrullinated protein antibody; AUC, area under the curve; RF, rheumatoid factor.
ACPA positivity performed best, the OR being 10.95 (table 2A), with best sensitivity (86%) and 90% negative predictive value (NPV) notably compared with RF (81%) and anti-CarP (76%). RF and anti-CarP had high OR>4.5 compared with other variable (table 1).
An analysis performed on continuous AutoAbs levels (table 2B) confirmed the predictive performance of the different AutoAbs with area under the curve (AUC) values ranging from 0.702 for RF to 0.770 for ACPA. This allowed for levels to be dichotomised for a more practical high versus a low risk of progression, using a cut-off set at 80% specificity for progression. Individual predictive performances for each AutoAbs were calculated for high vs low risk (table 2C). ORs were not better than when using AutoAbs status but AUC were improved for anti-CarP (0.675) while but not for ACPA or RF. All positive predictive value (PPV) were relatively similar (range 54%–60%), while NPV were best for CCP-res assays (81%).
AUC is individually presented for ACPA/RF/anti-CarP in online supplemental figure 2 for status, levels and risk groups.
Prediction of progression: added-value of Individual autoAbs
To demonstrate the possible added value of AutoAbs testing, we first established which demographic and clinical variables were predictive of progression (from eight parameters routinely recorded). A regression model built from the clinical data retained 4-variables (table 1, AUC=0.760 and displayed in online supplemental figure 3), including a genetic risk factor (shared epitope SE), an environmental risk factor (smoking), pain (tender joint count in 78 joints, TJC78) and an inflammation marker (ESR) which reproduced previously reported results22 and allowed 72.9% of individual’s outcome to be predicted correctly (Accuracy) and all four variable being highly predictive (all p<0.0001). This 4-variables model was considered the reference for further comparison.
We evaluated the added value of AutoAbs individually (table 3 and online supplemental figure 3) for status (A), levels (B) and risk categories (C). All models retained the four original clinical variables with each AutoAb, suggesting that they all had individual added value, improving accuracy (+4.1% to +4.4%) over the reference model and better AUC (+0.029 to +0.076). Overall, the AutoAb status with the best prediction accuracy were ACPA or anti-Carp (7.3%), best specificity-PPV was with anti-Carp (90% and 70%) although the AUC=0.789 was less good than ACPA (0.836) or RF (0.800).
Table 3.
Added-value of individual autoAbs (n=392)
| (A) | +ACPA status | +RF status | +Anti CarP status | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Smoking | 1.842 (1.18 to 3.215) | <0.0001 | 2.372 (1.538 to 4.012) | <0.0001 | 2.412 (1.552 to 3.981) | <0.0001 |
| SE | 2.606 (1.533 to 4.137) | 0.006 | 3.084 (1.791 to 4.713) | <0.0001 | 2.767 (1.711 to 4.418) | <0.0001 |
| ESR | 1.032 (1.007 to 1.045) | <0.0001 | 1.031 (1.007 to 1.043) | 0.005 | 1.033 (1.009 to 1.045) | 0.004 |
| TJC78 | 1.167 (1.063 to 1.262) | <0.0001 | 1.173 (1.074 to 1.268) | <0.0001 | 1.157 (1.057 to 1.239) | <0.0001 |
| AutoAb | 7.820 (3.937 to 12.048) | <0.0001 | 3.935 (2.2666 to 5.663) | <0.0001 | 3.198 (1.706 to 4.219) | <0.0001 |
| Accuracy | 77.3% | 77% | 77.3% | |||
| SEN SPE |
60% (50–67) 84% (79–88) |
52% (43–59) 89% (85–93) |
50.5% (39–55) 90 (84–92) |
|||
| PPV NPV |
66% (58–71) 82% (77–84) |
68.5% (61–77) 80% (76–81) |
70% (59–72) 79% (75–80) |
|||
| AUC (95% CI) |
0.836 (0.793 to 0.879) | 0.800 (0.753 to 0.846) | 0.789 (0.740 to 0.839) | |||
| (B) | + ACPA levels | + RF levels | + anti CarP levels | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Smoking | 2.122 (1.375 to 3.613) | 0.001 | 2.439 (1.479 to 3.978) | <0.0001 | ||
| SE | 2.752 (1.605 to 4.239) | <0.0001 | 3.109 (1.849 to 4.818) | <0.0001 | ||
| ESR | 1.037 (1.010 to 1.047) | 0.002 | 1.031 (1.011 to 1.053) | 0.003 | ||
| TJC78 | 1.195 (1.082 to 1.272) | <0.0001 | 1.150 (1.056 to 1.234) | 0.002 | ||
| AutoAb | 1.005 (1.004 to 1.007) | <0.0001 | 1.001 (1.001 to 1.002) | <0.0001 | ||
| Accuracy | 77.6% | No added value | 77.7% | |||
| SEN SPE |
52% (42–58) 89.5% (85–92) |
46.5% (35–51) 92% (87–94) |
||||
| PPV NPV |
70% (58–74) 79% (76–82) |
73.5% (61–78) 77.5% (74–79) |
||||
| AUC (95% CI) |
0.824 (0.780 to 0.868) |
0.787 (0.736 to 0.838) |
||||
| (C) | + ACPA risk group | + RF risk group | + Anti CarP risk group | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Smoking | 2.060 (1.378 to 3.644) | <0.0001 | 2.2735 (1.478 to 3.802) | 0.002 | 2.378 (1.498 to 3.841) | <0.0001 |
| SE | 2.822 (1.672 to 4.459) | <0.0001 | 3.208 (1.850 to 4.756) | <0.0001 | 2.761 (1.701 to 4.116) | <0.0001 |
| ESR | 1.041 (1.011 to 1.048) | 0.001 | 1.033 (1.009 to 1.045) | 0.001 | 1.032 (1.007 to 1.044) | 0.003 |
| TJC78 | 1.202 (1.091 to 1.283) | <0.0001 | 1.156 (1.060 to 1.240) | <0.0001 | 1.144 (1.050 to 1.226) | 0.002 |
| AutoAb | 6.906 (4.012 to 11.890) | <0.0001 | 2.770 (1.641 to 4.677) | <0.0001 | 3.741 (2.200 to 6.361) | <0.0001 |
| Accuracy | 78.3% | 75.8% | 78.3% | |||
| SEN SPE |
57% (46–63) 89% (85–92) |
42% (34–50) 91% (87–94) |
49.5% (38–55) 92% (86–93) |
|||
| PPV NPV |
70% (62–77) 81% (76–83) |
69% (60–77) 77% (73–78) |
74% (61–77) 79.5% (75–80) |
|||
| AUC (95% CI) |
0.832 (0.789 to 0.875) |
0.770 (0.718 to 0.813) |
0.792 (0.728–0.823) |
|||
ACPA, anticitrullinated protein antibody; AUC, area under thecurve; ESR, erythrocyte sedimentation rate; PPV/NPV, positive/negative predictive value; RF, rheumatoid factor; SE, share epitope; SEN, sensitivity; SPE, specificity; TJC78, tender joint count in 78 joints.
For continuous levels (table 3B), ACPA and anti-CarP but not RF, demonstrated added value over the reference model. The best performing model was with anti-CarP with+5.5% accuracy but the best AUC was for ACPA (0.824).
Using AutoAbs risk categories (table 3C), the best models were similar for ACPA or anti-Carp (+5.4% accuracy) but the best AUC was for ACPA (0.832). All model had similar specificity (anti-CarP being the best with 92%) while ACPA had highest sensitivity (57%).
Added value of multiple autoantibody testing
We used combination ACPA with RF and anti-CarP to evaluate the added value of multiple AutoAbs testing. A progression rate is presented in online supplemental figure 4 with respect to combined positivity for the three autoAbs.
First, Status were combined in a single variable, counting the number of positive AutoAbs. Data suggested significant association of higher number of positive AutoAbs with progression (p<0.0001) with triple negativity (123/392 (28% of the cohort) poorly associated with progression (5/123 (4.0%)), 1 AutoAb+ (117/392 (30% of cohort)) showing increased progression rate (28/117 (23%)), 2 AutoAbs+ (83/392 (21% of cohort)) a further increase (44/83 (53%)) and triple positivity (69/392 (17.6% of the cohort)) the highest progression rate (48/63 (76%). The number of positive AutoAbs had an individual OR=3.422 and AUC=0.813.
The AutoAbs count was then analysed with the variables of the reference model to address its added-value (table 4A, figure 2B). The combination provided a clear improvement on accuracy (+6.4%) and AUC=0.852). To account more for the importance of each AutoAbs to the prediction, models were then constructed using status, levels and risk groups. Using status, the model retained both ACPA and RF but removed anti-CarP. It increased slightly the prediction (77.8% accuracy) of the models with only ACPA or anti-Carp (table 3, 77.3%), while showing an increased AUC=0.855, but not better specificity over anti-Carp-alone (90%). There was added accuracy (+5.4%) of multiple AutoAbs testing using continuous levels keeping ACPA and anti-CarP above the model using anti-CarP levels alone (77.7%), as well as improved AUC=0.832. Using risk categories, the model retained again ACPA and anti-CarP, and showed good accuracy (79.1%) although slightly bellow AutoAb counts (−0.2%) but above autoAb status (+1.3%).
Table 4.
Added value of multiple autoAb testing: logistic and COX regression T(est of proportional hazards (PH) models overtime were valid (p>0.05) for all predictors)
| (A) Logistic regression, n=392 | ||||||||
| Counting autoAbs | AutoAb model (status) |
AutoAb model (levels) | AutoAb model (risk) |
|||||
| OR (95% CI) |
P value | OR (95% CI) |
P value | OR (95% CI) |
P value | OR (95% CI) |
P value | |
| Smoking | 1.806 (1.100 to 3.034) | 0.037 | 1.772 (1.121 to 3.089) | 0.045 | 2.180 (1.268 to 3.345) |
0.003 | 1.959 (1.129 to 3.052) | 0.015 |
| SE | 2.340 (1.519 to 4.166) | <0.0001 | 2.495 (1.531 to 4.224) | 0.002 | 2.894 (1.678 to 4.484) | <0.0001 | 2.608 (1.612 to 4.381) | <0.0001 |
| ESR | 1.024 (1.073 to 1.275) | 0.033 | 1.025 (1.003 to 1.047) | 0.024 | 1.004 (1.002 to 1.006) | 0.006 | 1.036 (1.014 to 1.059) | 0.001 |
| TJC78 | 1.167 (1.073 to 1.275) | 0.002 | 1.178 (1.080 to 1.292) | 0.001 | 1.171 (1.075 to 1.264) | <0.0001 | 1.188 (1.081 to 1.272) | <0.0001 |
| AutoAbs count | 2.898 (2.126 to 3.465) | <0.0001 | ||||||
| ACPA | 6.751 (3.550 to 11.039) | <0.0001 | 1.004 (1.002 to 1.005) | <0.0001 | 5.487 (3.095 to 9.728) | <0.0001 | ||
| RF | 3.220 (1.959 to 5.104) | <0.0001 | ||||||
| Anti-CarP | 1.001 (1.000 to 1.001) |
0.006 | 2.156 (1.196 to 3.888) |
0.011 | ||||
| Accuracy SEN SPE PPV NPV AUC (95% CI) p value |
79.3% 70% (61–79) 82.5% (77–86) 60% (53–66) 88% (84–91) 0.852 (0.813–0.891) <0.0001 |
77.8% 67.5% (49–66) 85% (78–88) 60% (56–70) 86.5% (77–83) 0.855 (0.817–0.893) <0.0001 |
78.3% 73% (63–82) 80% (75–84) 50% (44–57) 92% (88–94) 0.832 (0.789–0.875) <0.0001 |
79.1% 71% (61–79) 82.5% (77–86) 59% (52–65) 88% (85–91) 0.837 (0.797–0.880) <0.0001 |
||||
| (B) Cox regression for imminent progression (next 12 months), n=344 | ||||||||
| Counting autoAbs | AutoAb model (status) |
AutoAb model (levels) |
AutoAb model (risk) |
|||||
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| SE | 2.409 (1.430 to 3.751) | 0.001 | 2.729 (1.428 to 3.044) | <0.0001 | 2.671 (1.424 to 3.075) | <0.0001 | 2.304 (1.450 to 4.100) | 0.002 |
| ERS | 1.017 (1.082 to 1.215) | 0.043 | 1.024 (1.008 to 1.050) | 0.004 | 1.026 (1.010 to 1.044) | 0.002 | ||
| EMS | 1.003 (1.001 to 1.005) |
0.005 | 1.003 (1.001 to 1.005) | 0.010 | 1.002 (1.001 to 1.004) | 0.043 | ||
| TJC78 | 1.107 (1.082 to 1.215) |
0.003 | 1.132 (1.065 to 1.200) | <0.0001 | 1.120 (1.044 to 1.198) | 0.001 | 1.155 (1.078 to 1.238) | <0.0001 |
| AutoAb count | 2.217 (1.803 to 2.789) | <0.0001 | ||||||
| ACPA | 4.441 (2.342 to 6.323) | <0.0001 | 1.003 (1.002 to 1.004) | <0.0001 | 3.589 (2.372 to 6.086) | <0.0001 | ||
| RF | 2.754 (1.500 to 3.060) | <0.0001 | ||||||
| Anti-CarP | 1.001 (1.000 to 1.003) |
<0.0001 | 1.778 (1.076 to 2.766) |
0.025 | ||||
| AUC (95% CI) p value |
0.778 (0.776 0.781) <0.0001 |
0.778 (0.776 0.781) <0.0001 |
0.779 (0.777 0.782) <0.0001 |
0.778 (0.777 0.782) <0.0001 |
||||
| Nagelkerke R2 | 0.285 | 0.273 | 0.287 | 0.269 | ||||
ACPA, anticitrullinated protein antibody; AUC, area under the curve; EMS, early morning stiffness; ESR, erythrocyte sedimentation rate; PPV/NPV, positive/negative predictive value; RF, rheumatoid factor; SE, share epitope; SEN, sensitivity; SPE, specificity; TJC78, tender joint count in 78 joints.
We then performed an analysis of time to progression using a COX regression (table 4B). We chose to look at imminent progression within 12 months which did not include patients who progressed late (n=344, as illustrated in online supplemental figure 1). The proportional hazards assumption of Cox model was checked and found satisfied (p>0.05). The reference model selected the different variables (SE, ESR EMS and TJC78) and had an AUC=0.725 (table 1). The COX models did not retain smoking but showed clear increase in AUCs (table 4B, figure 2C). The status and risk category models kept ACPA and RF and were as good as counting autoAbs model (all same AUC=0.778). The best improvement in AUC was observed for AutoAbs levels (AUC=0.779, figure 2D) with ACPA and anti-CarP, suggesting value for utilising levels for predicting time of imminent progression.
Discussion
Multiple AutoAbs have been associated with RA, including triple positivity for ACPA/RF/anti-CarP34 35 and more recently with its pre-clinical stages.7–11 Here, we show the individual and combined value of AutoAbs used for RA classification for the prediction of progression to clinical disease. In this large cohort of 392 individuals selected on the basis of having a new non-specific musculoskeletal complain and being ACPA+and/or RF+, progression to IA was associated with ACPA, RF, anti-CarP and trends for anti-collagen-II Abs (likely due to small numbers), while not with anti-collagen-I and ANA. These associations were individually predictive improving the performance of the prediction based solely on demographic and clinical characteristics.22 Levels (although not for RF) consistently provided higher specificity and better AUC, and PPV, while sensitivity was more variable and NPV were similar. Here, the combination of AutoAbs levels with demographic and clinical data showed improvement over an individual AutoAb model, while dichotomising levels into high/low risk groups was better than using positive status.
Our data aligned with a meta-analysis of 12 studies,36 showing evidence of the value of triple positivity, ACPA, RF and anti-CarP, in identifying individuals at-risk from the healthy population (however not including demographic and clinical data in the models). We further demonstrate that triple positivity as well as analysis by status, levels or risk groups all suggested clear added value of using anti-CarP AutoAb for a better prediction of progression overall as well as its timing.
Participants included in our cohort based on NHS-ACPA positivity showed a high rate of false positive compared with research ELISA test (also previously observed).31It may be beneficial to use a higher cut-off (at 10 AU/mL for example) for recruitment of individual with non-specific musculoskeletal symptoms. This would allow >75% of false positive cases, not to be referred for clinical follow-up. On the other hand, these individuals still present with a new MSK pain complaint and may actually offer an interesting ACPA-negative research control group. This had notably allowed for RF+only participants (n=39, 8 progressed) and anti-CarP+only (n=10, 1 progressed) participants to be identified (see online supplemental figure 2 for illustration). Altogether, 123 participants had a triple negative status and five progressed (1.2%) compared with 26/110 (23.6%) in the group with at least one auto-Ab positive (p<0.0001). Importantly for the 186 ACPA-negative participant 14 of the 17 that progressed were either RF+ (n=9) or anti-CarP+ (n=1) or both (n=4), leaving three progressors currently not identified by an autoantibody (although one was positive for anti-Gly-CII+).
Currently, many biomarker have been proposed in individual at-risk of RA. From a single blood test, our study has confirmed the previously reported better individual predictive value of ACPA (using second or third generation tests,37 38 while still demonstrating that combinations of autoantibodies are more informative that each autoantibody alone. In addition to serological testing, other biomarkers such as high resolution imaging (ultrasound and MRI)39–41 or T-cell subsets23 24 or the combination of some, may still provide increased predictive value. The impact of certain biomarkers may also be greatest in those with ‘imminent’ arthritis, given most individuals develop subclinical joint and tendon inflammation prior to the onset of clinical joint swelling41 while other may provide additional value for understanding pathogenesis.
Limitations to the study, despite a large number of participants was the uniqueness of recruitment criteria, which currently limits any replication. There are different recognised populations considered at-risk of RA, notably as most recently redefined by a EULAR taskforce.42 These include seropositive arthralgia, clinically suspected arthralgia (CSA), first degree relatives of RA patients and ACPA+individuals with non-specific MSK symptoms. In Leeds, we chose to recruit the later, as at-risk individuals often initially present to primary care clinicians while CSA requires specialist assessment (ie, secondary care). Furthermore, there is a much higher overall rate of progression in these individual than reported in CSA, while ACPA+positive RA is usually more severe compared with seronegative disease.43 44 As criteria are needed to define an at-risk population and to balance the specificity of the recruitment with the number of cases needed to develop models. The overall cohort tested over 9000 potential participants since 2008, while about >750 were included being ACPA+in line with reported prevalence of ACPA-positivity.45 The populations and models developed for ACPA+arthralgia22 46 are therefore not directly transferable to cohort of patients with CSA (as defined by EULAR taskforce42 47 48), or in first degree relatives of patients with IA,49–51 which are the other main characteristics used for selecting at risk individuals. In addition, progression rate in these different groups are different and if 32% for IA in our cohort, progression to RA was only observed following from the development of IA in less than 25% of cases.
In conclusion, our data confirm the value of multiple AutoAbs testing for three particular antigens in preclinical RA, while suggesting that others may also have value (anti-collagen-II AutoAbs notably) and allowing for the exclusion of some (ANA, RF-IgG, anti-Collagen-I but not II). A baseline assessment of multiple AutoAbs may, therefore, inform a follow-up strategy for individual complaining of non-specific musculoskeletal symptoms. A research strategy based on an observational design may direct participants for 3 monthly vs annual review, using an overall prediction of progression (logistic models) based on counting AutoAbs or using risk groups. Alternatively, a strategy aiming at modifying the risk of progression (ie, intervention) may be better tested using a stratification based on time to progression (Cox regression) reducing the need for long-term follow-up.
Acknowledgments
XX received support from a joint PhD scholarship between the University of Leeds and the China Research Council (No. 201708330243). LAT received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 724517).
Footnotes
Contributors: Design of the study: FP and PE. Data acquisition, data analysis: FP, LD, XX, FS, LAT and DC. Drafting of the manuscript: FP, LAT, KM and LD. Review/editing of the manuscript: all authors.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health
Competing interests: LAT is mentioned as an inventor on a patent describing a method to detect anti-CarP antibodies.
Patient and public involvement statement: Patient and Public were involved in the study design, recruitment and samples collection. Biomarker research was included in the study design but the lab work was not directly involving pPPI.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The at-risk of RA study (coordinated Programme to Prevent Arthritis) was approved under references REC-06/Q1205/169. Healthy controls (HC) were also included (REC-09/H1307/98). All participants provided written informed consent for their blood samples to be used in research.
References
- 1.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international Task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis & Rheumatism 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 3.Frank C, Nason E. Health research: measuring the social, health and economic benefits. CMAJ 2009;180:528–34. 10.1503/cmaj.090016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481–94. 10.1002/art.1780370408 [DOI] [PubMed] [Google Scholar]
- 5.Pincus T, Callahan LF. The 'side effects' of rheumatoid arthritis: joint destruction, disability and early mortality. Br J Rheumatol 1993;32 Suppl 1:28–37. [PubMed] [Google Scholar]
- 6.Gerlag DM, Raza K, van Baarsen LGM, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the study Group for risk factors for rheumatoid arthritis. Ann Rheum Dis 2012;71:638–41. 10.1136/annrheumdis-2011-200990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastbom A, Strandberg G, Lindroos A, et al. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004;63:1085–9. 10.1136/ard.2003.016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Stadt LA, de Koning MHMT, van de Stadt RJ, et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum 2011;63:3226–33. 10.1002/art.30537 [DOI] [PubMed] [Google Scholar]
- 9.Shi J, van de Stadt LA, Levarht EWN, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 2014;73:780–3. 10.1136/annrheumdis-2013-204154 [DOI] [PubMed] [Google Scholar]
- 10.Brink M, Verheul MK, Rönnelid J, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 2015;17:25. 10.1186/s13075-015-0536-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strollo R, Ponchel F, Malmström V, et al. Autoantibodies to posttranslationally modified type II collagen as potential biomarkers for rheumatoid arthritis. Arthritis Rheum 2013;65:1702–12. 10.1002/art.37964 [DOI] [PubMed] [Google Scholar]
- 12.Padyukov L, Seielstad M, Ong RTH, et al. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis 2011;70:259–65. 10.1136/ard.2009.126821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre S, Bowes J, Diogo D, et al. High-Density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 2012;44:1336–40. 10.1038/ng.2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mankia K, Emery P. Preclinical rheumatoid arthritis: progress toward prevention. Arthritis Rheumatol 2016;68:779–88. 10.1002/art.39603 [DOI] [PubMed] [Google Scholar]
- 15.McInnes Ib and S. G., The pathogenesis of rheumatoid arthritis. New England Journal of Medicine 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis - shaping the immunological landscape. Nat Rev Rheumatol 2016;12:63–8. 10.1038/nrrheum.2015.171 [DOI] [PubMed] [Google Scholar]
- 17.Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 2012;122:1791–802. 10.1172/JCI60975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catrina AI, Ytterberg AJ, Reynisdottir G, et al. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:645–53. 10.1038/nrrheum.2014.115 [DOI] [PubMed] [Google Scholar]
- 19.Chibnik LB, Keenan BT, Cui J, et al. Genetic risk score predicting risk of rheumatoid arthritis phenotypes and age of symptom onset. PLoS One 2011;6:e24380. 10.1371/journal.pone.0024380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott IC, Seegobin SD, Steer S, et al. Predicting the risk of rheumatoid arthritis and its age of onset through modelling genetic risk variants with smoking. PLoS Genet 2013;9:e1003808. 10.1371/journal.pgen.1003808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks JA, Chen C-Y, Jiang X, et al. Improved performance of epidemiologic and genetic risk models for rheumatoid arthritis serologic phenotypes using family history. Ann Rheum Dis 2015;74:1522–9. 10.1136/annrheumdis-2013-205009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakieh C, Nam JL, Hunt L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 2015;74:1659–66. 10.1136/annrheumdis-2014-205227 [DOI] [PubMed] [Google Scholar]
- 23.Hunt L, Hensor EM, Nam J, et al. T cell subsets: an immunological biomarker to predict progression to clinical arthritis in ACPA-positive individuals. Ann Rheum Dis 2016;75:1884–9. 10.1136/annrheumdis-2015-207991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponchel F, Burska AN, Hunt L, et al. T-cell subset abnormalities predict progression along the Inflammatory Arthritis disease continuum: implications for management. Sci Rep 2020;10:3669–78. 10.1038/s41598-020-60314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Stadt LA, Witte BI, Bos WH, et al. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis 2013;72:1920–6. 10.1136/annrheumdis-2012-202127 [DOI] [PubMed] [Google Scholar]
- 26.Ten Brinck RM, van Dijk BT, van Steenbergen HW, et al. Development and validation of a clinical rule for recognition of early inflammatory arthritis. BMJ Open 2019;8:e023552. 10.1136/bmjopen-2018-023552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulie PG, Van Snick J. Rheumatoid factor (RF) production during anamnestic immune responses in the mouse. III. Activation of RF precursor cells is induced by their interaction with immune complexes and carrier-specific helper T cells. J Exp Med 1985;161:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane KD. Preclinical rheumatoid arthritis (autoantibodies): an updated review. Curr Rheumatol Rep 2014;16:419. 10.1007/s11926-014-0419-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aho K, Heliövaara M, Maatela J, et al. Rheumatoid factors antedating clinical rheumatoid arthritis. J Rheumatol 1991;18:1282–4. [PubMed] [Google Scholar]
- 30.Deane KD, O'Donnell CI, Hueber W, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62:3161–72. 10.1002/art.27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Matteo A, Mankia K, Duquenne L, et al. Third-Generation anti-cyclic citrullinated peptide antibodies improve prediction of clinical arthritis in individuals at risk of rheumatoid arthritis. Arthritis Rheumatol 2020;72:1820–8. 10.1002/art.41402 [DOI] [PubMed] [Google Scholar]
- 32.Ponchel F, van Delft MAM, Xie X, et al. Anti-carbamylated protein antibodies: are they useful for the diagnosis of rheumatoid arthritis? Clin Exp Rheumatol 2021;39:146–50. 10.55563/clinexprheumatol/u891rd [DOI] [PubMed] [Google Scholar]
- 33.Xie X, van Delft MAM, Shuweihdi F, et al. Auto-Antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthritis Cartilage 2021;29:924–33. 10.1016/j.joca.2021.03.008 [DOI] [PubMed] [Google Scholar]
- 34.Montes A, Regueiro C, Perez-Pampin E, et al. Anti-Carbamylated protein antibodies as a reproducible independent type of rheumatoid arthritis autoantibodies. PLoS One 2016;11:e0161141. 10.1371/journal.pone.0161141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamacchia C, Courvoisier DS, Jarlborg M, et al. Predictive value of anti-CarP and anti-PAD3 antibodies alone or in combination with rf and AcpA for the severity of rheumatoid arthritis. Rheumatology 2021;60:4598–608. 10.1093/rheumatology/keab050 [DOI] [PubMed] [Google Scholar]
- 36.Verheul MK, Böhringer S, van Delft MAM, et al. Triple positivity for Anti-Citrullinated protein autoantibodies, rheumatoid factor, and Anti-Carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals. Arthritis Rheumatol 2018;70:1721–31. 10.1002/art.40562 [DOI] [PubMed] [Google Scholar]
- 37.Ten Brinck RM, van Steenbergen HW, van Delft MAM, et al. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017;56:2145–53. 10.1093/rheumatology/kex340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wouters F, Maurits MP, van Boheemen L, et al. Determining in which pre-arthritis stage HLA-shared epitope alleles and smoking exert their effect on the development of rheumatoid arthritis. Ann Rheum Dis 2022;81:48–55. 10.1136/annrheumdis-2021-220546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt L, Eugénio G, Grainger AJ. Magnetic resonance imaging in individuals at risk of rheumatoid arthritis. Best Pract Res Clin Rheumatol 2017;31:80–9. 10.1016/j.berh.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 40.Boer AC, Burgers LE, Mangnus L, et al. Using a reference when defining an abnormal MRI reduces false-positive MRI results-a longitudinal study in two cohorts at risk for rheumatoid arthritis. Rheumatology 2017;56:1700–6. 10.1093/rheumatology/kex235 [DOI] [PubMed] [Google Scholar]
- 41.Di Matteo A, Duquenne L, Cipolletta E, et al. Ultrasound subclinical synovitis in anti-CCP-positive at-risk individuals with musculoskeletal symptoms: an important and predictable stage in the rheumatoid arthritis continuum. Rheumatology 2022;61:3192–200. 10.1093/rheumatology/keab862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mankia K, Siddle HJ, Kerschbaumer A, et al. EULAR points to consider for conducting clinical trials and observational studies in individuals at risk of rheumatoid arthritis. Ann Rheum Dis 2021;80:1286–98. 10.1136/annrheumdis-2021-220884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nam JL, Hensor EMA, Hunt L, et al. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann Rheum Dis 2016;75:2060–7. 10.1136/annrheumdis-2015-208235 [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Montoya L, Nam JL, Duquenne L, et al. Prioritising referrals of individuals at-risk of RA: guidance based on results of a 10-year national primary care observational study. Arthritis Res Ther 2022;24:26. 10.1186/s13075-022-02717-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finckh A, Courvoisier D, Lamacchia C. Measuring AcpA in the general population or primary care: is it useful? RMD Open 2020;6:e001085. 10.1136/rmdopen-2019-001085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bos WH, Wolbink GJ, Boers M, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 2010;69:490–4. 10.1136/ard.2008.105759 [DOI] [PubMed] [Google Scholar]
- 47.van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis 2017;76:491–6. 10.1136/annrheumdis-2016-209846 [DOI] [PubMed] [Google Scholar]
- 48.Burgers LE, Siljehult F, Ten Brinck RM, et al. Validation of the EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Rheumatology 2017;56:2123–8. 10.1093/rheumatology/kex324 [DOI] [PubMed] [Google Scholar]
- 49.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000;43:30–7. [DOI] [PubMed] [Google Scholar]
- 50.Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (rA) using first-degree relatives of probands with RA. Arthritis Rheum 2009;61:1735–42. 10.1002/art.24833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bemis EA, Demoruelle MK, Seifert JA, et al. Factors associated with progression to inflammatory arthritis in first-degree relatives of individuals with RA following autoantibody positive screening in a non-clinical setting. Ann Rheum Dis 2021;80:154–61. 10.1136/annrheumdis-2020-217066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002512supp001.pdf (228.8KB, pdf)
Data Availability Statement
No data are available.