Abstract
Background
Endometriosis is a progressive disease, and early detection and early treatment are particularly important. The purpose of this study was to investigate the effect of the timing of laparoscopy on the spontaneous pregnancy rate of primary infertile patients complicated with pelvic effusion within 6 months after surgery.
Material/Methods
We enrolled 330 primary infertile patients with pelvic effusion and bilateral patent fallopian tubes. They were divided into 3 groups based on retrospective analysis of clinical data. Study Group 1 underwent laparoscopy 1 month after hysterosalpingography (HSG), Study Group 2 received laparoscopy after trying to conceive for 3 months, and the Control Group did not undergo laparoscopy. According to the specific conditions during laparoscopy, repair and plastic surgery of fallopian tube, electrocautery of endometriosis and uterine suspension were performed. The main observation indicators were proportions of retrograde menstruation, peritoneal endometriosis, and tubal adhesions in laparoscopy groups, and spontaneous pregnancy rates within 6 months.
Results
The proportions of retrograde menstruation were 92.2% and 93.1% in Study Group 1 and Study Group 2, respectively, with no statistical significance. The proportions of peritoneal endometriosis were 51.0% and 64.7%, with a statistically significant difference. The proportions of tubal adhesions were 31.4% and 36.2%, with no statistical significance. The pregnancy rates within 6 months were 73.9%, 52.6%, and 13.1%, with a statistically significant difference for pairwise comparisons.
Conclusions
The pregnancy rate of primary infertile patients with patent fallopian tubes complicated with pelvic effusion can be significantly improved through early laparoscopic surgery (exploration and treatment).
Keywords: Endometriosis; Infertility; Laparoscopy; Pelvic Inflammatory Disease; Pregnancy; Ultrasonography, Doppler
Background
Endometriosis (referred to as “EMs”) is a common sex hormone-dependent disease in which endometrial tissue (glands and stroma) grows and infiltrates outside the body of the uterus, subsequently causing pain and infertility. According to epidemiological surveys, the incidence of EMs in women of reproductive age is about 10% to 15%, and 30% to 50% of them are complicated with infertility [1], which seriously affects female reproductive function.
EMs includes peritoneal, ovarian, deeply infiltrative, and other types, of which peritoneal and ovarian types are the most common Ems [2]. Peritoneal EMs is a precursor (early) lesion of pelvic Ems [3]. Ovarian endometriosis cysts can be diagnosed by ultrasonography [4], but it is difficult to diagnose early EMs, that is, peritoneal EMs by ultrasonography [5]. In addition, the value of hysterosalpingography (HSG) for diagnosis of early EMs is limited because peritoneal EMs associated with only a few pelvic adhesions rarely cause lumen obstruction [6]. Therefore, minimally invasive, visualized laparoscopy has become the criterion standard method for diagnosing EMs, especially for early EMs [7]. Early diagnosis and treatment greatly improve the reproductive function of patients, since EMs is a common cause of infertility and a progressive disease. Accordingly, the purpose of this study was to investigate whether early laparoscopic exploration for primary infertile patients with pelvic effusion, fluid in the female pelvic cavity revealed by imaging examination [8], and patent fallopian tubes revealed by HSG is capable of detecting and eliminating pelvic EMs lesions and improving the pelvic microenvironment in the early stage, thereby increasing the postoperative pregnancy rate of patients.
Material and Methods
Study Subjects
The clinical data of primary infertile patients who visited the Department of Reproductive Endocrinology, West China Second University Hospital, Sichuan University from January 2017 to June 2021 were collected and screened retrospectively according to the following inclusion and exclusion criteria.
Inclusion criteria:
Women of reproductive age from 20 to 40 years old with fertility demand;
Primary infertile patients without previous pregnancy history, who had not been pregnant (without contraception) for more than 1 year;
Women with regular normal menstruation, a menstrual cycle of 21 to 35 days, ovulation observed in ovulation monitoring and biphasic basal body temperature;
Bilateral patent fallopian tubes as revealed by HSG;
Existence of pelvic effusion as revealed by transvaginal ultrasonography.
Exclusion criteria:
All males received a reproductive health examination, and women whose husbands had abnormal serum sex hormone or semen test results were excluded;
Those complicated with ovulatory disorders, such as premature ovarian insufficiency (POI), polycystic ovary syndrome (PCOS), or hyperprolactinemia;
Those with congenital uterine dysplasia;
Those with a history of pelvic surgery or taking EMs medications;
Those who underwent assisted reproduction or drug-induced ovulation were excluded to avoid the effects of other treatment methods on pregnancy rate;
Those complicated with contraindications for laparoscopic surgery in study groups.
The sample size of this retrospective study was calculated by using the Power and Sample Size Calculations (version 3.1.6) on the basis of a previous study by Marcoux [9]. To attain statistical significance (α=0.05, power=80%), a minimum sample size of 60 subjects was required for each group.
According to our statistics, 1392 primary infertile patients had visited the outpatient department during the study period, among which 551 patients had pelvic effusion revealed by ultrasound examination, and the prevalence of pelvic effusion was 39.58% (551/1392). After consulting the relevant clinical data, 221 subjects were excluded from the study for the following reasons:
Incomplete medical records (n=63);
Fallopian tube obstruction or uterine anomalies (n=42);
Complicated with reproductive endocrine diseases (n=36);
Abnormal results of husband’s sex hormone or semen examination (n=29);
Received assisted reproduction or drug-induced ovulation therapy (n=51).
Finally, a total of 330 patients were included. The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of West China Second University Hospital. Written informed consent was obtained from individuals or their guardians.
Methods
After strict screening, a total of 330 patients were included, and their clinical data were comprehensively collected for retrospective analysis. All had undergone HSG, the result of which revealed that the uterine cavity was normal with the bilateral fallopian tubes unobstructed. They were divided into 3 groups: Study Group 1 (153 cases), Study Group 2 (116 cases), and Control Group (61 cases), based on whether they underwent laparoscopic exploration and the timing of surgery. Patients in Study Group 1 underwent laparoscopic exploration 1 month after HSG, patients in Study Group 2 received laparoscopic exploration after trying to conceive for 3 months without fertilization after taking an HSG examination. According to the specific conditions during laparoscopy, repair and plastic surgery of fallopian tube, electrocautery of endometriosis and uterine suspension were performed. Patients in the Control Group, who refused surgery, only underwent expectant management or ovulation monitoring. Furthermore, for primary infertile patients with patent fallopian tubes complicated with pelvic effusion, we investigated the effects of diagnostic/therapeutic laparoscopy and the timing of surgery on the spontaneous pregnancy rate within 6 months after surgery.
General Conditions
The general conditions of all patients were recorded, including age, infertility duration, menstrual cycle, anti-Mullerian hormone (AMH), and body mass index (BMI).
Laparoscopic Exploration
The patients in the laparoscopic exploration groups (Study Group 1 and Study Group 2) underwent surgery under general anesthesia within 3 to 7 days after the end of 1 period, without sexual activity. The pelvic organs and peritoneal surface were comprehensively examined for EMs lesions to observe the distribution and characteristics of pelvic effusion, while the following operations were performed:
Absorption of pelvic effusion, and repeated flushing of the pelvic cavity with a large amount of normal saline until the flushing fluid is clear;
Removal of EMs lesions, if any, as thoroughly as possible, including electrocoagulation for peritoneal chromatosis nodules, electrocoagulation for peritoneal defects, and electrocoagulation for neoplastic vessels;
Release of adhesions, if any, to restore the anatomical position;
Uterine suspension to correct uterine retroversion;
Hydrotubation with methylene blue solution to confirm bilateral patent fallopian tubes. All surgical operations were successfully completed without complications.
The surgical records were searched for relevant surgical data and compare the 2 groups of patients from:
The proportion of patients with pelvic effusion shown by B-mode ultrasound and confirmed as retrograde menstrual blood by laparoscopy;
The proportion of patients with peritoneal EMs;
The proportion of patients with tubal adhesions;
The EMs score (revised American Fertility Society Scoring, r-AFS) of the American Society for Reproductive Medicine (ASRM) recorded for the patients diagnosed with EMs during surgery [10].
Comparison of Spontaneous Pregnancy Rates
Patients in Study Group 1 and Study Group 2 were observed and expected to have spontaneous pregnancy for 6 months after surgery, and the patients in the Control Group were also observed for 6 months. Their pregnancy situation was determined by inquiring outpatient cases and telephone follow-up, and the spontaneous pregnancy rates within 6 months were compared among the 3 groups.
Statistics Processing
SPSS 26.0 software was used for statistical analysis of data, where quantitative data were expressed as (χ̆±s), with one-way analysis of variance used for comparisons among groups, and qualitative data were expressed as percentages (%) with the chi-square test used for comparisons among groups. Unless otherwise specified, the test level α was set at 0.05.
Results
Comparison of General Conditions of Patients in the 3 Groups
The differences in the general information of the patients in the 3 groups, such as age, infertility duration, menstrual cycle, AMH level, and BMI, were not statistically significant (P>0.05), suggesting that the baseline data between the groups were comparable, as shown in Table 1.
Table 1.
Comparison of the baseline data of the 3 groups (χ̆±s).
| Study Group 1 | Study Group 2 | Control Group | F | P | |
|---|---|---|---|---|---|
| Age (year) | 28.98±3.24 | 29.83±3.70 | 29.41±3.88 | 1.910 | 0.150 |
| Infertility duration (year) | 2.73±1.47 | 2.76±1.50 | 2.92±1.72 | 0.353 | 0.703 |
| Menstrual cycle (day) | 29.88±2.92 | 29.95±3.12 | 29.79±2.97 | 0.059 | 0.943 |
| AMH (ng/ml) | 3.78±1.99 | 3.93±2.16 | 3.68±1.87 | 0.344 | 0.709 |
| BMI (kg/m2) | 22.91±3.04 | 22.58±3.01 | 22.97±3.28 | 0.495 | 0.610 |
AMH – anti-Müllerian hormone; BMI – body mass index.
Imaging Data
For bilateral patent fallopian tubes revealed by hysterosalpingography, see Figure 1.
For pelvic effusion shown by B-mode ultrasound, see Figure 2.
For retrograde menstruation revealed during laparoscopic surgery, see Figure 3.
For bilateral patent fallopian tubes revealed through hydrotubation with methylene blue solution under laparoscopy, see Figures 4 and 5.
For adhesion of fallopian tube revealed during laparoscopy, see Figure 6.
For congested and swollen fallopian tubes immersed in retrograde menstrual blood, see Figure 7.
For peritoneal EMs, see Figure 8 (before electrocautery) and Figure 9 (after electrocautery).
Figure 1.
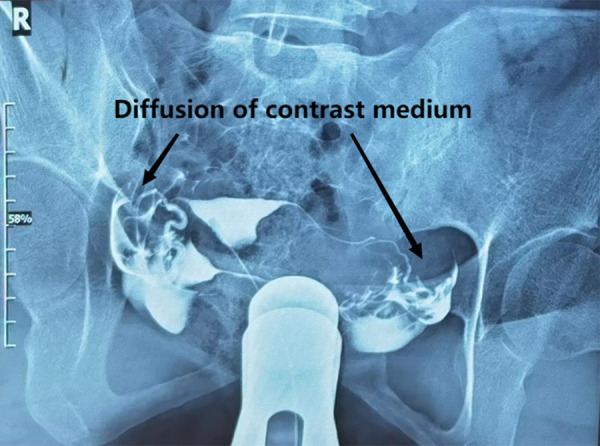
Bilateral patent fallopian tubes revealed by hysterosalpingography (the arrows mean diffusion of contrast medium). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 2.
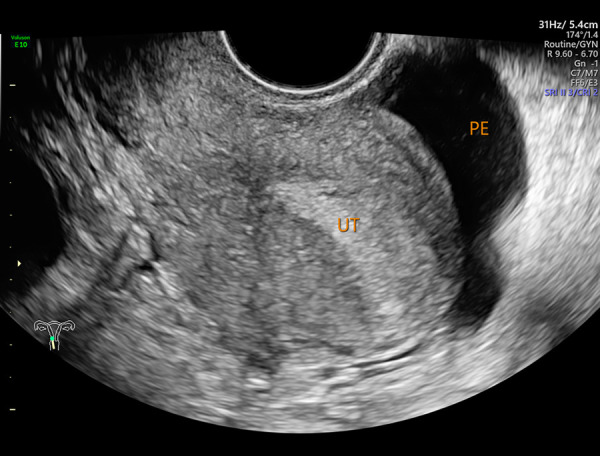
Pelvic effusion shown by transvaginal B-mode ultrasound (PE– pelvic effusion; UT – uterus). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 3.
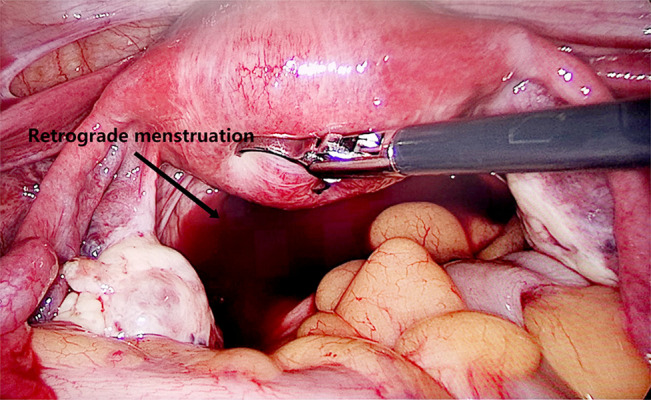
Retrograde menstrual blood revealed during laparoscopic surgery (the arrow means retrograde menstruation). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 4.
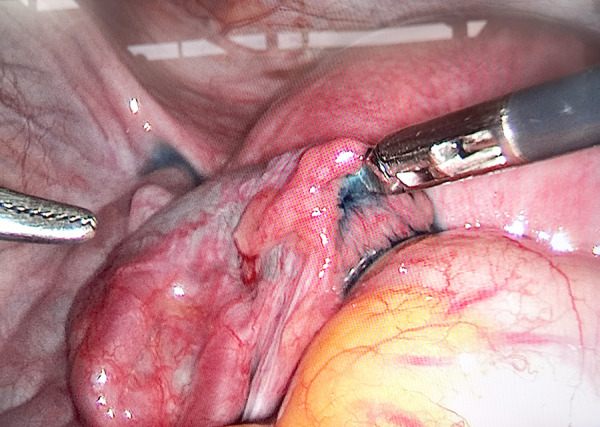
Left patent fallopian tube revealed under laparoscopy (outflow of methylene blue solution). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 5.
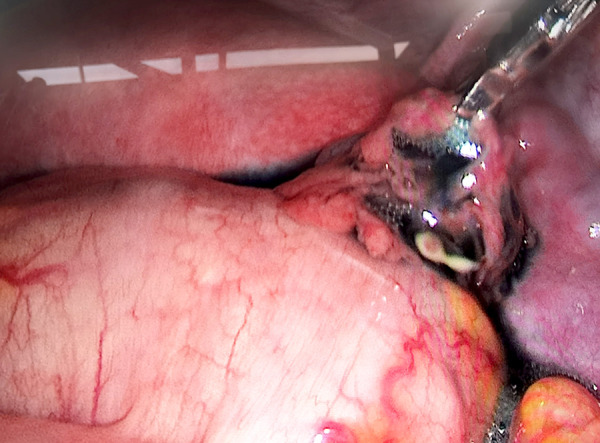
Right patent fallopian tube revealed under laparoscopy (outflow of methylene blue solution). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 6.
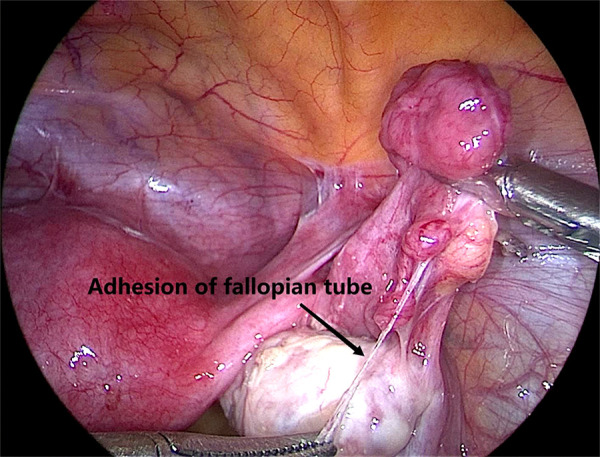
Adhesion of fallopian tube revealed during laparoscopy (the arrow means adhesion of fallopian tube). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 7.
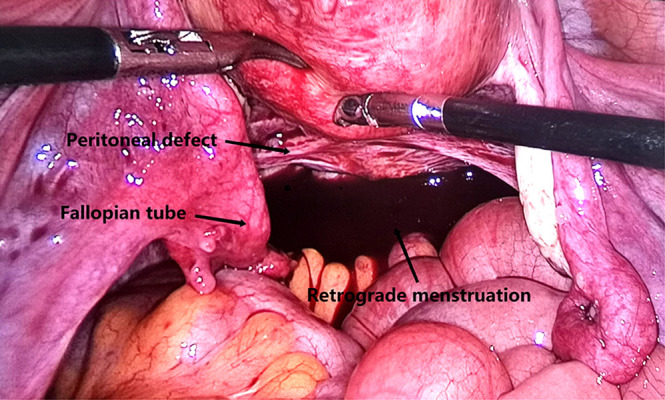
Fallopian tubes immersed in bloody peritoneal fluid (the arrows mean peritoneal defect, fallopian tube and retrograde menstruation respectively). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 8.
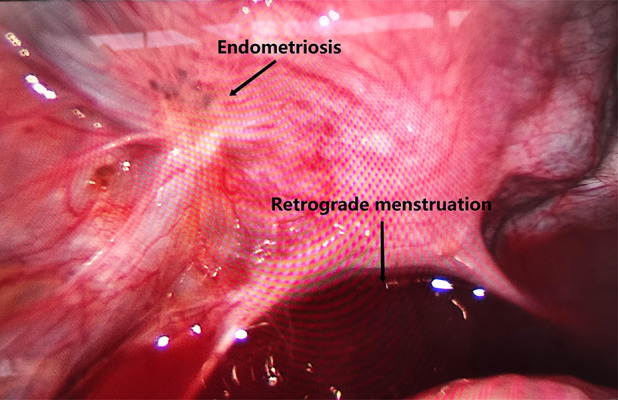
Peritoneal endometriosis before laparoscopic electrocautery (the arrows mean endometriosis lesion and retrograde menstruation respectively). Image software: Adobe Photoshop, CS6, Adobe Systems.
Figure 9.
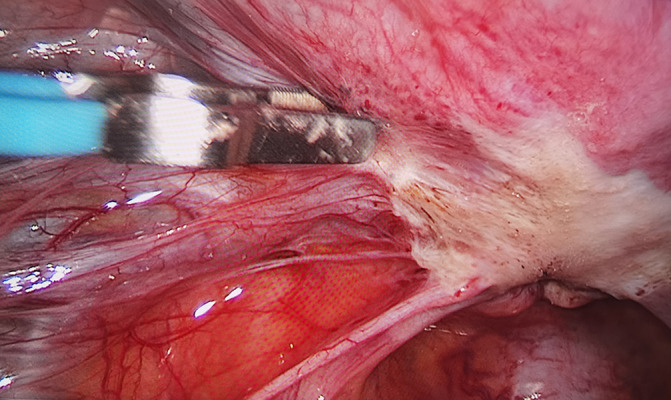
Peritoneal endometriosis after laparoscopic electrocautery. Image software: Adobe Photoshop, CS6, Adobe Systems.
Comparison of Intraoperative Conditions Between Study Group 1 and Study Group 2
The proportion of patients with pelvic effusion shown by B-mode ultrasound and confirmed as retrograde menstrual blood by laparoscopy was 92.2% and 93.1% in Study Group 1 and Study Group 2, respectively, with no statistically significant difference (P>0.05).
-
The presentation of peritoneal EMs under laparoscopy includes:
red lesions (early lesions): the peritoneum presents red lesions, flamboyancy, dense blood vessels, or glandular vegetations;
brown lesions (typical lesions): shrinking black lesions, presenting cinder-like spots, plaques, and purple nodules, see Figure 8;
white lesions (old lesions): the peritoneum is white and muddy, with peritoneal defects and scarring. The proportions of patients with peritoneal EMs revealed under laparoscopy were 51.0% and 64.7% in Study Group 1 and Study Group 2, respectively, with a statistically significant difference (P<0.05).
The proportions of patients with tubal adhesions were 31.4% and 36.2% in Study Group 1 and Study Group 2, respectively, showing an increasing trend, with no statistically significant difference (P>0.05).
Comparing the r-AFS scores of patients diagnosed with EMs during surgery in Study Group 1 and Study Group 2, the severity of EMs was in an upward trend, but the difference was not statistically significant (P>0.05) (Table 2).
Table 2.
Comparison of intraoperative conditions between Study Group 1 and Study Group 2.
| Retrograde menstruation % (n) |
Peritoneal endometriosis % (n) |
Tubal adhesions % (n) |
r-AFS (χ̆±s) |
|
|---|---|---|---|---|
| Study Group 1 | 92.2 (141/153) | 51.0 (78/153) | 31.4 (48/153) | 7.07±3.75 |
| Study Group 2 | 93.1 (108/116) | 64.7 (75/116) | 36.2 (42/116) | 7.47±3.88 |
| χ2 | 0.086 | 5.030 | 0.693 | |
| t | 0.655 | |||
| P | 0.769 | 0.025 | 0.405 | 0.513 |
r-AFS – revised American Fertility Society Scoring.
Comparison of Spontaneous Pregnancy Among the 3 Groups of Patients
Comparing spontaneous pregnancy rates within 6 months among the 3 groups, the ratios of Study Group 1, Study Group 2, and Control Group were 73.9%, 52.6%, and 13.1%, respectively, and the difference between groups was statistically significant (P<0.05) when using the Bonferroni adjustment method for pairwise comparison (Table 3). In our study, the reported spontaneous pregnancy rates after surgery were higher compared to other international studies, which may be related to the following factors:
Table 3.
Comparison of spontaneous pregnancy among the 3 groups of patients, % (n).
| Study Group 1 | Study Group 2 | Control Group | χ2 | P | |
|---|---|---|---|---|---|
| Spontaneous pregnancy rate within 6 months | 73.9 (113/153) | 52.6 (61/116) | 13.1 (8/61) | 65.530 | <0.001 |
The subjects included were all young primary infertile patients, whose menstruation was regular with no ovulatory disorders;
The HSG test results revealed that the bilateral fallopian tubes were unobstructed, with a low proportion of tubal adhesions found during surgery, which provided good conditions for pregnancy;
The husband’s sex hormone and semen examination results were normal.
Comparison of Pregnancy Outcomes Among the 3 Groups of Patients
The pregnancy outcomes of the 3 groups were recorded or tracked until miscarriage or delivery, and the pregnancy outcome indicators (abortion rate, live birth rate, and ectopic pregnancy rate) were compared. Among all the pregnant cases, the miscarriage rates of Study Group 1, Study Group 2, and Control Group were 15.0% (17/113), 19.7% (12/61), and 25.0% (2/8), respectively, and the live birth rates of the 3 groups were 85.0% (96/113), 80.3% (49/61), and 75.0% (6/8), respectively, with no statistically significant difference (P=0.646). The ectopic pregnancy rates of the 3 groups were 2.7% (3/113), 3.3% (2/61), and 12.5% (1/8), respectively. The ectopic pregnancy rate in the Control Group was obviously higher than that in the 2 study groups, but the difference was not statistically significant (P=0.381).
Discussion
Pathogenesis of EMs – Led by the Implantation Theory of Retrograde Menstruation
The pathogenesis of EMs is not clearly understood and its occurrence may be related to various factors such as sex hormones, immunity, inflammation, and genetics. Sampson’s implantation theory of retrograde menstruation is currently the dominant theory. During menstruation, endometrial gland epithelial and stromal cells can flow backwards with menstrual blood, enter the pelvic cavity via the fallopian tubes, and be implanted in the ovary and the adjacent pelvic peritoneum for continuous growth and spreading, forming the pelvic EMs. In addition, the characteristics of the eutopic endometrium play a key role, that is, “eutopic endometrial determinism”, and the biological properties of the eutopic endometrium are the determinants of the development of EMs. Compared with non-EMs patients, the characteristics of eutopic endometrium of EMs patients, such as adhesion and invasiveness, have stronger ability to stimulate neovascularization. Other pathogenic mechanisms include coelomic metaplasia, vascular and lymphatic metastasis theory, and induction theory. According to the results of this study, for primary infertile patients with pelvic effusion and patent fallopian tubes revealed by B-mode ultrasound, more than 90% were found to have pelvic effusion retrograde menstrual blood during surgery (Figures 1–5). The proportion of patients with peritoneal EMs was 50%, which is much higher than the incidence of EMs (around 20%) reported in the literature. The possible reasons are that: 1) It is difficult to carry out early diagnosis for early EMs, which mainly includes peritoneal EMs through imaging such as ultrasound, which can easily lead to missed diagnosis. 2) The peritoneal EMs reported in this study was detected by laparoscopy, which overcomes the shortcomings of imaging, greatly improves the (early) diagnosis rate of EMs, and provides an objective basis for early treatment of EMs.
Effect of EMs on Fertility
EMs is closely associated with infertility. According to epidemiological survey findings [11], there are currently about 176 million EMs patients in the world, and 20% to 50% of infertile women are complicated with EMs, which seriously affects the fertility of women of reproductive age. The concept of “EMs-associated infertility”, first introduced by Buyalos et al [12] in 2000, states that infertility and EMs are mutually influenced, where EMs may cause infertility or spontaneous abortion due to its influence on all aspects of pregnancy and, conversely, infertility is one of the risk factors for EMs.
The influencing mechanism of EMs on female fertility is complex and may cover the followings:
The abnormal pelvic microenvironment with retrograde menstrual blood and exudation of EMs lesions make patient’s peritoneal fluid contain excessive amounts of activated macrophages, immune inflammatory cytokines, and other abnormal substances, which are toxic to sperm, ovum, and fertilized ovum and cause infertility [13–15];
Abnormal ovarian function: including abnormal follicle development, ovulation disorders, impaired corpus luteal function, decreased quality of oocytes, and dysfunction of granulosa cells in synthesizing sex hormones [16,17];
Formation of abdominopelvic adhesions that affects ovulation and fertilization: adhesions around the fallopian tubes interfere with the function of ovum pick-up and gamete transport in the fallopian tubes, and if adhesions around the ovary forms, the release of ovum also would be inhibited. In this study, tubal adhesions were found in more than 30% of patients in the study groups who underwent laparoscopic exploration. Even if no adhesions were found, the fallopian tubes immersed in bloody peritoneal fluid were often congested and swollen, and the function of ovum pick-up and gamete transport may be severely affected (Figure 7).
Abnormal immune function: the ectopic endometrium is recognized as a “foreign body” by the immune system in the body, which activates the antigen-antibody system and complement system in the body, resulting in an increase in cytokines. The damage response caused by a variety of cytokines and the complement system may lead to infertility [18];
Endometrial receptivity changes: the patients with EMs have abnormal local immune regulation in the endometrium and a higher occurrence rate of endometrial lesions such as endometrial polyps, which decreases endometrial receptivity and interferes with blastocyst implantation, resulting in predisposition to implantation failure [19–21].
Significance of Laparoscopic Surgery in Improving the Pregnancy Rate of Patients with EMs
Laparoscopic exploration is a surgical diagnostic method commonly used for EMs. Laparoscopy allows exploration of lesion site and range and access to lesion tissue for histopathological diagnosis. According to the literature [22], laparoscopic removal of the endometrial lesion helps significantly improve the postoperative pregnancy rate of patients. The possible reason is that surgical removal of EMs lesions is accompanied by restoration of normal anatomical relationship of abdominopelvic cavity through separation of adhesions and flushing of abdominopelvic cavity with large amounts of normal saline to improve the fallopian tube function and ovarian ovulation function and the abdominopelvic microenvironment, thereby increasing the postoperative pregnancy rate.
Significance of Early Laparoscopic Exploration for Primary Infertile Patients Complicated with Pelvic Effusion
EMs is a common disease in women of reproductive age and a common cause of infertility. EMs is a progressive disease with a rapid rate of progress. In the early stage, it mainly invades peritoneum (Figure 8), ovarian cortex, and uterosacral ligament, with a focal nature. As the disease develops, the lesions invade deeper and merge with each other, resulting in the formation of pelvic adhesions and chocolate cysts, eventually forming a “frozen pelvis”, which seriously affects the patient’s reproductive function and quality of life. Despite treatment, the pregnancy rate decreases, and relevant study findings have shown that the pregnancy rate of patients after treatment for old low-activity ectopic lesions (non-early stage) is significantly lower than that of the patients with fresh (early) active lesions [23]. Therefore, EMs should be diagnosed as early as possible for timely treatment to restore and improve fertility. However, imagological examination of early endometriosis lesions is mostly devoid of specific findings, and laparoscopic exploration has special significance as it provides a comprehensive understanding of a patient’s pelvic cavity under direct vision for early detection of punctate and flaky tissues and understanding of pelvic adhesions for early corresponding treatment. Removal of retrograde menstrual blood and flushing of the pelvic cavity with large amounts of normal saline during surgery also effectively improve the pelvic microenvironment and raise the pregnancy rate. The findings of this study also prove the viewpoints above that:
For the primary infertile patients with pelvic effusion revealed by B-mode ultrasound included in this study who had no uncomfortable symptoms, no positive findings in gynecological examination, and no abnormal ovulation function, more than 90% had pelvic effusion with retrograde menstrual blood and were found to have early EMs, over 50% of which were peritoneal EMs, through laparoscopic exploration. Exploratory laparoscopy enables early diagnosis of EMs.
The pregnancy rate of Study Group 1 who underwent laparoscopic exploration as early as possible was 73.9%, which was significantly higher than the pregnancy rate of Study Group 2 (52.6%) who underwent laparoscopic exploration after 3 months of expectant treatment (P<0.05), suggesting that early diagnosis and treatment of EMs significantly improved the pregnancy rate.
The pregnancy rate of the Control Group who did not undergo laparoscopic exploration and failed to remove retrograde menstruation and EMs lesions as early as possible was much lower than those of Study Group 1 and Study Group 2 (P<0.05). Therefore, the primary infertile patients with normal ovulation function and patent fallopian tube complicated with pelvic effusion should undergo laparoscopic exploration as early as possible for early diagnosis and timely treatment of EMs, which is capable of effectively improving the pregnancy rate.
The literature [22] also indicates that laparoscopic resection of EMs lesions can improve the postoperative pregnancy rate of patients. The ASRM expert consensus [24,25] recommends that for patients with mild EMs at r-ASRM stage I–II, therapeutic laparoscopic surgery (resection or electrocautery of EMs lesions, and release of adhesions) is more effective than diagnostic laparoscopic surgery. Therapeutic laparoscopic surgery obeys the principle of “taking immediate treatment for lesions on sight”, that is, removing the lesions visible to the naked eye during surgery, and improving the abdominopelvic microenvironment and restoring the normal anatomical relationship by separating adhesions and flushing the abdominopelvic cavity with a large amount of normal saline, to improve the fallopian tube function and ovarian ovulation function, thereby increasing the postoperative pregnancy rate.
EMs is a progressive disease, and early detection and treatment is particularly important. According to our results, the prevalence of pelvic effusion in primary infertility patients was 39.58% (551/1392). After screening by consulting clinical data, the proportion of participants with only pelvic effusion was 23.71% (330/1392), which indicated the prevalence of “healthy” patients who would benefit from laparoscopy. For primary infertile patients with normal ovarian ovulation function and patent fallopian tubes complicated with pelvic effusion, the appropriate relaxation of laparoscopic surgery guidelines enables early removal of retrograde menstruation and early detection and treatment of pelvic EMs to improve the pelvic microenvironment for the purpose of the increasing pregnancy rate. The weaknesses of the study are as follows:
The type of patients was very selected and subsequent infertile patients could also be included as a subgroup for further research;
Multi-center randomized controlled trials (RCT) with larger sample sizes will be more convincing.
Conclusions
The pregnancy rate of primary infertile patients with patent fallopian tubes complicated with pelvic effusion can be significantly improved through early laparoscopic surgery (exploration and treatment). In the expectant treatment (non-surgery) group, the pregnancy rate is significantly lower than that in the laparoscopic surgery groups. Endometriosis is a progressive disease, and early detection and early treatment are particularly important. Moreover, the results of our study indicated that the pregnancy outcomes (abortion rate, live birth rate, and ectopic pregnancy rate) were not significantly different among the 3 groups. Theoretically, it is possible that there is a greater number of ectopic pregnancies in non-operatively managed patients with tubal adhesions. Therefore, to explore and analyze the effect of timing of laparoscopic surgery on pregnancy outcomes in primary infertile patients, we will consider expanding the sample size and conducting subgroup analysis (tubal adhesion group vs non-tubal adhesion group) in future research.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Done
Department of Gynecology and Obstetrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, PR China; Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, PR China.
Ethics Approval and Consent to Participate
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved by the Human Ethics Committee of West China Second University Hospital. Written informed consent was obtained from individuals or guardians of participants.
Availability of Figures
The pictures in our manuscript are all original and originated from the Imaging Department and Operating Room of West China Second University Hospital, the use of which was approved by the institution.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Vallerie A, Hsieh T, Baxi L. Peritoneal inclusion cyst: Effects on fertility and antepartum course. Obstet Gynecol. 2008;112:498–500. doi: 10.1097/AOG.0b013e3181809e5c. [DOI] [PubMed] [Google Scholar]
- 2.Olkowska-Truchanowicz J, Białoszewska A, Zwierzchowska A, et al. Peritoneal fluid from patients with ovarian endometriosis displays immunosuppressive potential and stimulates Th2 response. Int J Mol Sci. 2021;22(15):8134. doi: 10.3390/ijms22158134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anger D, Foster W. The link between environmental toxicant exposure and endometriosis. Front Biosci. 2008;13:1578–93. doi: 10.2741/2782. [DOI] [PubMed] [Google Scholar]
- 4.Johnson N, Hummelshoj L, Adamson G, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24. doi: 10.1093/humrep/dew293. [DOI] [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil Steril. 2012;98(3):591–98. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Niskar A, Needham L, Rubin C, et al. Serum dioxins, polychlorinated biphenyls, and endometriosis: A case-control study in Atlanta. Chemosphere. 2009;74(7):944–49. doi: 10.1016/j.chemosphere.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Members of the Endometriosis Guideline Core Group. Becker C, Bokor A, et al. ESHRE guideline: Endometriosis. Hum Reprod Open. 2022;2022(2):hoac009. doi: 10.1093/hropen/hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JA, Gosink BB. Fluid in the female pelvis: Cyclic patterns. J Ultrasound Med. 1986;5(2):75–79. doi: 10.7863/jum.1986.5.2.75. [DOI] [PubMed] [Google Scholar]
- 9.Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–22. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher C, Mäkinen N, Harris H, et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat Commun. 2019;10(1):4857. doi: 10.1038/s41467-019-12536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buyalos RP, Agarwal SK. Endometriosis-associated infertility. Curr Opin Obstet Gynecol. 2000;12(5):377–81. doi: 10.1097/00001703-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Broi M, Jordo-Jr AA, Rui AF, Navarro PA. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol Reprod Dev. 2018;85(2):128–36. doi: 10.1002/mrd.22943. [DOI] [PubMed] [Google Scholar]
- 14.Bao H, Qu Q, Huang X, et al. Impact of hydrosalpinx fluid on early human embryos. Syst Biol Reprod Med. 2017;63(4):279–84. doi: 10.1080/19396368.2017.1319993. [DOI] [PubMed] [Google Scholar]
- 15.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- 16.Cahill DJ, Hull MGR. Pituitary-ovarian dysfunction and endometriosis. Hum Reprod Update. 2000;(1):56–66. doi: 10.1093/humupd/6.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Doody MC, Gibbons WE, Zamah NM. Linear regression analysis of ultrasound follicular growth series: statistical relationship of growth rate and calculated date of growth onset to total growth period. Fertil Steril. 1987;47(3):436–40. doi: 10.1016/s0015-0282(16)59051-7. [DOI] [PubMed] [Google Scholar]
- 18.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 19.The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril. 2004;86:S156–60. doi: 10.1016/j.fertnstert.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Wei Q, Clair J, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91(5):1686–91. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaghay M, Lessey B. Uterine receptivity: Alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25(6):461–75. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- 22.Campo S, Campo V, Gambadauro P. Is a positive family history of endometriosis a risk factor for endometrioma recurrence after laparoscopic surgery? Reprod Sci. 2014;21(4):526–31. doi: 10.1177/1933719113503413. [DOI] [PubMed] [Google Scholar]
- 23.Vimercati A, Achilarre M, Scardapane A, et al. Accuracy of transvaginal sonography and contrast-enhanced magnetic resonance-colonography for the presurgical staging of deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2012;40(5):592–603. doi: 10.1002/uog.11179. [DOI] [PubMed] [Google Scholar]
- 24.Saridogan E. ESHRE guideline: Management of women with endometriosis. Hum Reprod. 2014;29(3):400–12. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Dai Y, Zhang J, et al. Pregnancy outcomes in women with infertility and coexisting endometriosis and adenomyosis after laparoscopic surgery: A long-term retrospective follow-up study. BMC Pregnancy Childbirth. 2021;21(1):383. doi: 10.1186/s12884-021-03851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


