Abstract
Rhodococcus equi is a facultative intracellular bacterium of macrophages which can infect immunocompromised humans and young horses. In the present study, we examine the mechanism of host defense against R. equi by using a murine model. We show that bacterial killing is dependent upon the presence of gamma interferon (IFN-γ), which activates macrophages to produce reactive nitrogen and oxygen intermediates. These two radicals combine to form peroxynitrite (ONOO−), which kills R. equi. Mice deficient in the production of either the high-output nitric oxide pathway (iNOS−/−) or the oxidative burst (gp91phox−/−) are more susceptible to lethal R. equi infection and display higher bacterial burdens in their livers, spleens, and lungs than wild-type mice. These in vivo observations, which implicate both nitric oxide (NO) and superoxide (O2−) in bacterial killing, were reexamined in cell-free radical-generating assays. In these assays, R. equi remains fully viable following prolonged exposure to high concentrations of either nitric oxide or superoxide, indicating that neither compound is sufficient to mediate bacterial killing. In contrast, brief exposure of bacteria to ONOO− efficiently kills virulent R. equi. The intracellular killing of bacteria in vitro by activated macrophages correlated with the production of ONOO− in situ. Inhibition of nitric oxide production by activated macrophages by using NG-monomethyl-l-arginine blocks their production of ONOO− and weakens their ability to control rhodococcal replication. These studies indicate that peroxynitrite mediates the intracellular killing of R. equi by IFN-γ-activated macrophages.
Rhodococcus equi is a gram-positive, facultative intracellular coccobacillus which can cause pneumonia in immunocompromised people, especially individuals with AIDS or those undergoing immunosuppressive therapy. The organism is thought to be delivered to the lungs via inhalation and grows primarily, if not exclusively, within host macrophages (15, 30). In young horses (foals), R. equi can cause severe pneumonia or, occasionally, septic arthritis or osteomyelitis (13). A number of in vitro studies have demonstrated that resident macrophages isolated from different species and from various anatomical locations can support the intracellular growth of R. equi (reviewed in reference 15).
Some previous studies have suggested that humoral immune responses can contribute to host defense against R. equi. Antibody levels in adult horses generally correlate with resistance to clinical infection (31), and passive immunization with immune sera can protect susceptible foals against infection (28). However, adoptive transfer experiments and studies in mice which have been genetically or experimentally depleted of T cells or cytokines (22, 23, 36, 39) argue for the importance of cell-mediated immunity in host defense against R. equi. Furthermore, the emergence of this organism as an opportunistic pathogen in AIDS patients (15) confirms the importance of cell-mediated immunity. Thus, in both the murine model of experimental infection and the human experience, the generation of Th1-type immune responses correlates with disease resolution and a failure to mount these responses is generally associated with poor clinical outcomes (23).
It has been well established in a variety of model systems that immunological activation of macrophages results in enhanced microbicidal activities (35). Activated macrophages exhibit an increase in the secretion of both reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates (RNIs) (reviewed in reference 37). ROIs are produced during a process called the respiratory burst, which involves an increase in cellular oxygen consumption and the reduction of molecular oxygen to superoxide (O2−). Activated macrophages can also produce RNIs via the high-output inducible nitric oxide synthase enzyme (iNOS), which oxidizes l-arginine to yield nitric oxide (NO) and citrulline (45). Resident or gamma interferon (IFN-γ)-primed macrophages do not express iNOS and therefore do not produce measurable levels of RNIs. In contrast, activated macrophages constitutively secrete high levels of RNIs (35). Both ROIs and RNIs are individually capable of mediating microbial killing by macrophages. Several early studies have pointed to a primary role for oxygen radicals in host defense. In several different experimental systems, including cell-free oxygen-generating enzyme systems and, more recently, gene-knockout mice lacking a component of the respiratory burst enzyme complex, oxygen radical formation has been shown to participate in the killing of a diverse group of pathogens, including the promastigote form of Leishmania donovani (32), Toxoplasma gondii (33), Plasmodium falciparum (44), and Staphylococcus aureus (19, 27).
More recent studies have focused on the role of nitrogen radicals as the primary macrophage microbicidal mediator. Again, using a variety of experimental approaches, NO and its congeners have been shown to kill both intracellular and extracellular, as well as both gram-negative and -positive, bacteria. Consistent with these observations, mice lacking the inducible NO synthase by gene targeting or mice treated with inhibitors of iNOS are generally much more susceptible to infection by a diverse variety of microorganisms (34). This increased susceptibility implicates NO in microbial killing by macrophages; however, it does not indicate precisely which molecules generated via the iNOS pathway are responsible for microbial killing.
Evidence is now developing that the combination of the two radical-generating systems may exert a synergistic effect in the killing of some microorganisms (10). Macrophages can generate superoxide simultaneously with NO, yielding the more reactive peroxynitrite (ONOO−) (18). This compound has been shown to possess greater toxicity than NO for Escherichia coli (5), Salmonella enterica serovar Typhimurium (8), Mycoplasma pulmonis (14), and Candida albicans (43). In these experimental model systems, both oxygen and nitrogen radicals are required to mediate maximal microbicidal effects. In other experimental systems, however, NO appears to be more toxic than ONOO−. This is true of Leishmania major (1), Giardia lamblia (11), and Cryptococcus neoformans (42). All of these organisms are susceptible to NO, and the addition of superoxide to NO to yield peroxynitrite lowers NO concentrations and reduces toxicity against these organisms.
In the present studies, we investigate the killing of R. equi by activated macrophages. We examine the requirement for macrophage activation and the relative potency of oxygen and nitrogen radicals in mediating bacterial killing. We demonstrate that mice deficient in either of the two radical-generating pathways are hypersusceptible to infection and that neither O2− nor NO is sufficient to kill R. equi in vitro. The combination of both radicals, however, to form peroxynitrite results in the efficient and rapid killing of R. equi cells by activated macrophages. We visualize ONOO− formation in situ in activated macrophages phagocytizing R. equi.
MATERIALS AND METHODS
Bacteria.
R. equi 238 is a clinical isolate obtained from a pneumonic foal and provided by the Veterinary Microbiology Laboratory, New Bolton Center, University of Pennsylvania, Kennett Square. Bacteria were stored in 15% glycerol at −70°C. Prior to use, bacteria were streaked onto chocolate agar plates and grown at 37°C for 36 h. For all assays, one isolated colony of bacteria was inoculated into 10 ml of Mueller-Hinton broth and cultured overnight at 37°C. Bacterial cultures were grown to a density of approximately 108 per ml. Before their addition to macrophages, bacteria were washed with phosphate-buffered saline (PBS) and resuspended in phagocytosis buffer, which consisted of 1% gelatin in equal parts Dulbecco's modified Eagle medium and Media 199 (Mediatech, Herndon, Va.) buffered with 12.5 M HEPES.
Mice.
Four- to 6-week-old female BALB/c, C57BL/6, C57BL/6-NOS2tm1Lau (iNOS−/−) (26), and C57BL/6-Ifngtm1Ts (IFN-γ−/−) (7) mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). The gp91phox−/− mice (19) were a generous gift from Mary C. Dinauer (Indiana University, Indianapolis).
Mouse infections.
Frozen stocks of R. equi were thawed in 3 ml of Mueller-Hinton broth at 37°C for 3 h and then washed and resuspended in cold Hanks' balanced salt solution. Mice were infected intravenously with a 100-μl volume of PBS containing 3 × 106 to 9 × 106 viable bacteria. Bacterial burdens in the liver, spleen, and lung were determined at various days postinfection. Organs were harvested from infected mice, homogenized in 10 ml of cold water, diluted, and plated onto chocolate agar plates. After incubation of plates at 37°C for 36 h, the CFU recovered from each organ were determined. For viability assays, infected mice were monitored daily and the days on which mice succumbed to infection were recorded.
Cell-free bactericidal assays.
For all cell-free bactericidal assays, virulent R. equi was grown overnight at 37°C, washed once, and resuspended in 50 mM potassium phosphate buffer (pH 7.4). Bacteria were exposed to 1 mM diethylamine-nitric oxide (DEA/NO; Calbiochem, San Diego, Calif.). DEA/NO is a stable complex in solid form which decomposes quickly (t1/2 = 2.1 min) at 37°C in phosphate buffer (pH 7.4) to release 1.5 mol of NO per mol of compound (29). A fresh, 40 mM solution of DEA/NO was prepared in ice-cold 0.01 M sodium hydroxide to minimize decomposition. DEA/NO was diluted 40-fold into 1 ml of phosphate buffer containing 106 viable bacteria. NO release from DEA/NO was measured by using the Griess reagent as previously described (9). Inactive DEA/NO was decomposed in phosphate buffer for 30 min before addition to bacteria. One unit of xanthine oxidase (Sigma Chemical Co., St. Louis, Mo.) was used to convert 5 mM xanthine (Sigma) to uric acid, releasing superoxide anion (O2−) (2). Bacteria, xanthine, and xanthine oxidase were combined in a 1.5-ml reaction volume and incubated at 37°C for 30 min. O2− generation was measured by the reduction of cytochrome c as previously described (20). Peroxynitrite (ONOO−) was synthesized in a quenched-flow reactor from nitrite and hydrogen peroxide as previously described (3) and stored at −70°C until used. The millimolar concentration of ONOO− was determined before each experiment by using the following calculation: [(OD302 in 1 M NaOH − OD302 in 50 mM phosphate buffer) × dilution × 1,000]/1,670 (3), where OD302 is the optical density at 302 nm. ONOO− was added to bacteria in a 1-ml reaction volume at a final concentration of 1 mM for 1 min at 37°C. Because the half-life of ONOO− is less than 1 s in phosphate buffer (pH 7.4) at 37°C, inactive ONOO− was prepared by allowing ONOO− decomposition to nitrate in phosphate buffer for 5 min before addition to bacteria. Following exposure to each compound (DEA/NO, X-XO, ONOO−), bacteria were centrifuged, resuspended in phosphate buffer, and exposed for a second and third time prior to dilution plating for CFU quantitation.
The intracellular growth of R. equi in macrophages.
Bone marrow-derived macrophages (BMMϕ) were established as described previously (41). Briefly, bone marrow was flushed from the femurs of mice by using 4 ml of cold PBS (Mediatech) and a 23-gauge needle. Cells were resuspended in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (D-10) and 20% L929-conditioned media as a source of colony-stimulating factor. Cells were incubated 5 to 7 days at 37°C with 5% CO2 in plastic petri dishes until monolayers became confluent. BMMϕ were removed from plates by using 5 mM EDTA, resuspended in D-10, and adhered to glass coverslips in 24-well plates. Following a 1-h incubation, cells were washed with antibiotic-free D-10 and incubated overnight. Macrophages (approximately 105 per coverslip) were either unstimulated or stimulated in vitro for 18 to 24 h with 100 U of IFN-γ (Genzyme, Cambridge, Mass.) per ml and 50 ng of lipopolysaccharide (LPS) (E. coli 0127:B8) (Sigma) per ml in the presence or absence of 500 μM NG-monomethyl-l-arginine (NMLA) (Calbiochem).
Bacteria were added to washed macrophage monolayers at a multiplicity of infection (MOI) of 5 to 10 bacteria per macrophage in the presence of 5% fresh normal mouse serum as a source of complement. After a 30-min incubation with bacteria, monolayers were washed with warm phagocytosis buffer to remove any unbound bacteria from wells. An additional 30-min incubation was performed to allow bacteria sufficient time to be internalized by macrophages, followed by washing and replacement of media with D-10 supplemented with 5 to 10 μg of gentamicin sulfate (GIBCO/BRL, Rockville, Md.) per ml. This concentration of gentamicin sulfate kills extracellular bacteria while minimally affecting intracellular bacteria, as described previously (16). At various times postinfection, macrophage monolayers were fixed in 100% methanol (20 min, 4°C) and the bacteria associated with macrophages were stained by immunofluorescence using rabbit antiserum to R. equi, as previously described (16). The total number of bacteria associated with 200 macrophages per monolayer were quantitated by using fluorescence microscopy. Because of the difficulty in counting individual bacteria within large clusters, macrophages containing large clusters of bacteria were recorded simply as having 10 bacteria per cell. Results are presented as the total number of bacteria per 200 macrophages and/or the number of macrophages containing 10 or more bacteria.
The visualization of ONOO− production by macrophages.
Intracellular ONOO− production was visualized by using dihydrorhodamine (DHR)-123 (Molecular Probes, Eugene, Ore.) as previously described (17). DHR was prepared as a 10-mg/ml stock in dimethylformamide, purged with nitrogen, and stored in aliquots at −20°C. BMMϕ were cultured overnight in 24-well plates in antibiotic-free, phenol red-free D-10 containing 100 U of IFN-γ/ml and 50 ng of LPS/ml with or without 500 μM NMLA (Calbiochem). Cells were washed and media was replaced with antibiotic-free, phenol red-free D-10 containing 1 mM DHR and 5% normal mouse serum. Bacteria from an overnight culture were added to macrophage monolayers at an MOI of 50:1 for 15 min at 37°C. This relatively high MOI was used to achieve a higher level of infection in the majority of cells in the monolayer. Intracellular ONOO−-mediated oxidation of DHR was visualized as rhodamine fluorescence in an Olympus inverted microscope. The number of fluorescing macrophages was counted and expressed as the percent of positive cells in the population.
RESULTS
Rhodococcus infection in IFN-γ−/−, iNOS−/−, and gp91phox−/− mice.
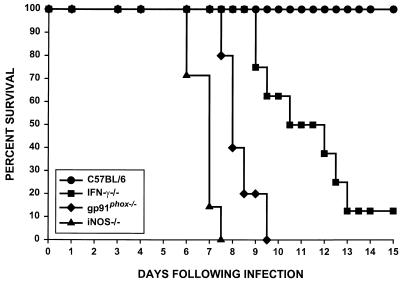
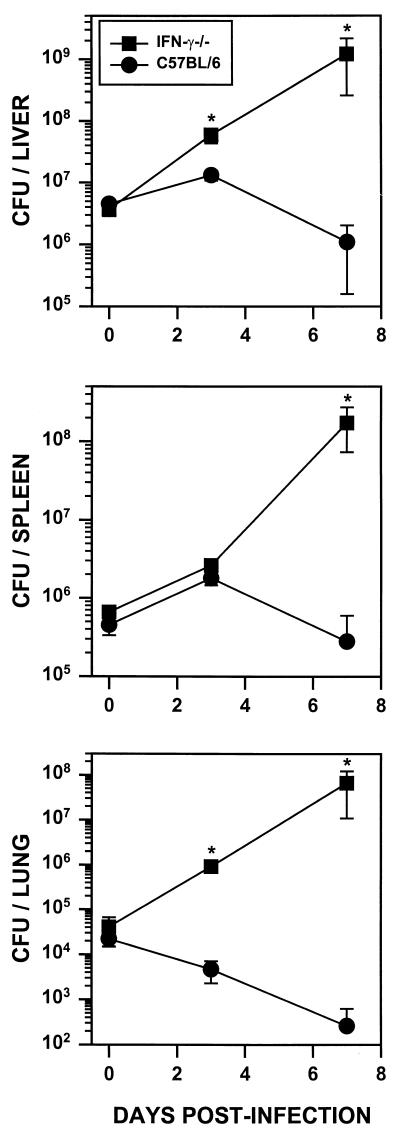
R. equi infections were examined in mice lacking key components of host defense. Infections in mice lacking IFN-γ production (IFNγ−/−), high-output nitric oxide production (iNOS−/−), or superoxide production (gp91phox−/−) were compared to infections in normal C57BL/6 mice. The survival of mice injected intravenously with 3 × 106 virulent R. equi cells was followed over 15 days. This inoculum is approximately 1/10 of the 50% lethal dose (data not shown), and consequently, all wild-type C57BL/6 mice survived the infection (Fig. 1). In contrast, knockout mice were highly susceptible to this relatively low inoculum of R. equi. iNOS−/− mice were most susceptible, with all of the mice succumbing by 7.5 days postinfection. Half of the gp91phox−/− mice succumbed to infection by day 8 and all were dead by 9.5 days postinfection. Although IFN-γ−/− mice survived slightly longer than the iNOS−/− or gp91phox−/− mice, by 10 days, half of the IFN-γ knockout (GKO) mice were dead, and by 13 days postinfection, 80% had succumbed to R. equi infection (Fig. 1). The increased mortality observed in knockout mice corresponded with an increase in bacterial burdens in their visceral organs (Fig. 2). Organ burdens from wild-type and IFN-γ−/− mice were determined at 0, 3, and 7 days following intravenous infection. By 7 days, CFU recovered from liver, spleen, and lungs of IFN-γ−/− mice were approximately 600-, 1,100-, and 250,000-fold higher, respectively, than those recovered from organs of wild-type mice (Fig. 2). At 5 days postinfection, organ burdens from iNOS−/− and gp91phox−/− mice were also dramatically increased relative to those of wild-type mice (data not shown). These mice did not survive for 7 days, precluding an analysis of this later time point.
FIG. 1.
R. equi infection in IFN-γ−/−, gp91phox−/−, and iNOS−/− mice. C57BL/6 (n = 7), IFN-γ−/− (n = 8), gp91phox−/− (n = 5), and iNOS−/− (n = 7) mice were infected intravenously with virulent R. equi (3 × 106 cells). Data are taken from a single experiment in which all groups were included in parallel. The data are representative of three separate experiments including a total of ≥18 mice per group.
FIG. 2.
CFU recovered from organs of IFN-γ−/− and C57BL/6 mice following R. equi infection. IFN-γ−/− mice (squares) and wild-type littermates (circles) were infected intravenously with virulent R. equi (9 × 106 cells). Bacterial burdens in the liver (top), spleen (middle), and lung (bottom) were determined at 0, 3, and 7 days postinfection. Each data point represents CFU (± standard deviation) of five mice per group. ∗, significantly different from control mice (P ≤ 0.008; Mann-Whitney U test).
The in vitro generation of NO and O2−.
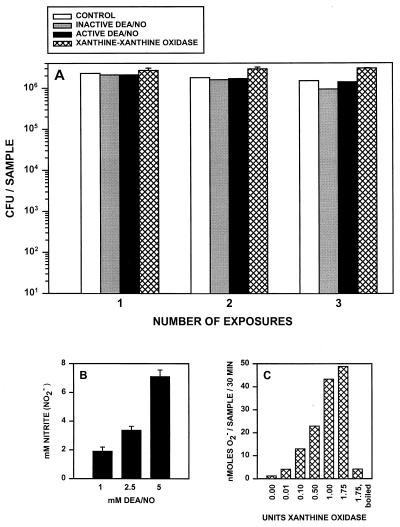
Our in vivo observations suggested that both oxygen and nitrogen radicals can contribute to host defense to R. equi. We directly measured bacterial susceptibility to oxygen and nitrogen radicals utilizing cell-free radical-generating systems. The NO-releasing compound DEA/NO (29) was used to test the susceptibility of R. equi to NO, while the xanthine-xanthine oxidase (X-XO) enzymatic system (2) was used to generate O2−. The viability of R. equi following one, two, or three 30-min exposures to either NO or O2− was determined. No decrease in bacterial viability was observed even after three consecutive prolonged exposures to either DEA/NO or X-XO (Fig. 3A). Bacterial numbers remained relatively constant throughout and were not decreased relative to those of unexposed bacteria or to bacteria exposed to inactive DEA/NO, which does not release NO (Fig. 3A). Radical generation by these cell-free systems was quantitated in parallel to assure that the lack of bacterial killing was not due to a failure to generate radicals in these systems. A concentration of 1 mM DEA/NO yielded >1.5 mM NO (measured as nitrite accumulation) and 1 U of xanthine oxidase yielded >40 nmol of O2− from 5 mM xanthine over 30 min in these systems (Fig. 3B and C, respectively). In similar, cell-free experiments, acidified nitrite or hydrogen peroxide at concentrations as high as 10 mM also failed to kill R. equi (data not shown). The interpretation drawn from these experiments is that neither nitrogen nor oxygen radicals alone were sufficient to mediate a bactericidal effect.
FIG. 3.
R. equi exposure to NO released by DEA/NO or O2− generated by X-XO. (A) R. equi (106 cells) was exposed to 1 mM inactive or active DEA/NO or 1 U of xanthine oxidase and 5 mM xanthine (hatched bar). Following a 30-min incubation at 37°C, bacteria were centrifuged, resuspended, and reexposed to X-XO or DEA/NO for a second and third time. Control samples were incubated in phosphate buffer alone. Cell viability was determined by a CFU assay. The data are representative of three separate experiments. (B) The amount of NO released by DEA/NO was determined by using the Griess reagent (9) following the incubation of 1, 2.5, or 5 mM DEA/NO in phosphate buffer at 37°C for 30 min. (C) Increasing amounts of xanthine oxidase were added to phosphate buffer containing 5 mM xanthine in the presence of cytochrome c. The amount of O2− generated was determined following a 30-min incubation at 37°C by measuring the reduction of cytochrome c (20). Xanthine oxidase which had been boiled for 15 min was used as a control.
The in vitro killing of R. equi by ONOO−.
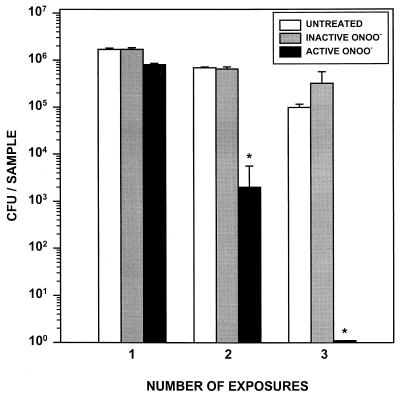
Peroxynitrite (ONOO−) is a strong oxidant that is generated from the spontaneous reaction of NO and O2− (18). We examined the susceptibility of R. equi to ONOO− in a cell-free system (Fig. 4). The viability of R. equi following one, two, or three 1-min exposures to 1 mM active or inactive ONOO− was examined. This concentration of ONOO− is biologically relevant based on calculations from the formation of ONOO− by rat alveolar macrophages in the lung lining fluid and in phagolysosomes of macrophages (18). Following a single 1-min exposure to active ONOO−, bacterial viability was not significantly decreased relative to that of untreated controls. However, following a second exposure to ONOO−, bacterial viability had decreased by >2 logs. No bacterial CFU were recovered after the third 1-min exposure to active ONOO−, indicating complete bacterial killing by ONOO− (Fig. 4). As expected, exposure of bacteria to inactive ONOO− did not affect their viability (Fig. 4). These data demonstrate that even brief exposures of R. equi to ONOO− in a cell-free system can result in complete bacterial killing.
FIG. 4.
Exposure of R. equi to ONOO−. R. equi (106 cells) in 50 mM potassium phosphate buffer was exposed to 1 mM active peroxynitrite. Following a 1-min incubation at 37°C, bacteria were centrifuged, resuspended, and reexposed to ONOO− for a second and third time. Control (untreated) samples were incubated in phosphate buffer alone or with inactive ONOO− which had completely decomposed prior to addition to bacteria. Cell viability was determined by a CFU assay. Data shown are means (± standard deviation) of triplicate samples and are representative of three independent experiments. ∗, significantly different from untreated samples (P < 0.001; Student's t test).
Quantitation of Rhodococcus replication inside activated macrophages.
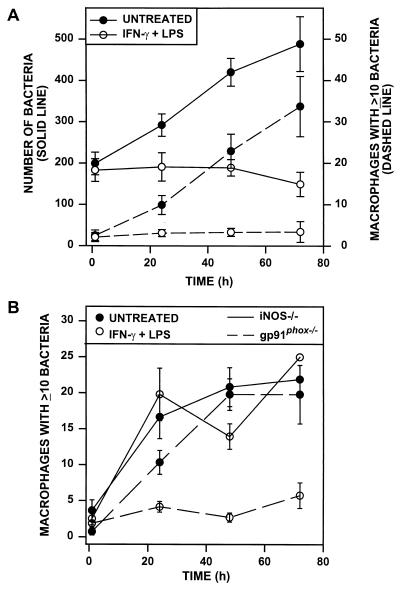
We performed in vitro studies to measure R. equi killing by activated macrophages. BMMϕ monolayers were activated with IFN-γ and LPS and infected with R. equi. Infection at an MOI of 5 to 10 bacteria per macrophage resulted in approximately 50% of macrophages being infected with an average of 1 or 2 bacteria per cell. Bacterial growth in macrophages was examined over a 72-h period. Nonactivated (untreated) macrophages supported the replication of R. equi cells (Fig. 5A), as previously described (16). Bacterial replication resulted in a progressive increase both in the total number of bacteria per 200 macrophages (Fig. 5A) and the in number of macrophages infected with 10 or more bacteria (Fig. 5A). In activated macrophages, in contrast, both the total number of bacteria and the number of macrophages with ≥10 bacteria remained relatively constant throughout the observation period (Fig. 5A). These data show that macrophages activated with IFN-γ and LPS restrict the intracellular growth of R. equi in vitro.
FIG. 5.
Quantitation of R. equi growth within activated wild-type, iNOS−/−, and gp91phox−/− macrophages. BMMϕ were either untreated or activated overnight with 100 U of IFN-γ/ml plus 50 ng of LPS/ml before infection with R. equi at an MOI of between 5 and 10 bacteria per macrophage. At 1, 24, 48, and 72 h postinfection, parallel macrophage monolayers were washed, fixed, and stained for fluorescence microscopy. (A) R. equi replication inside untreated and activated BALB/c BMMϕ was quantitated. Bacterial growth was expressed as both the total number of bacteria per 200 macrophages (solid lines, left axis) and the number of macrophages containing ≥10 bacteria (dashed lines, right axis). Each data point shown represents the mean (± standard error of the mean) of three separate experiments done in triplicate. At all time points (24 to 72 h), bacterial numbers in activated macrophages were statistically different from those in untreated macrophages (P ≤ 0.001; Mann-Whitney U test). (B) The replication of R. equi inside untreated or activated macrophages from iNOS−/− or gp91phox−/− mice was quantitated. Data represent the number of macrophages infected with 10 or more bacteria. Each point represents the mean (± standard error of the mean) of ≥3 experiments counted in triplicate except for 72-h iNOS−/−, which represents a triplicate sample from a single experiment.
Because the generation of ONOO− requires the production of both NO and O2−, we examined the capacity of BMMϕ from iNOS−/− or gp91phox−/− mice to inhibit R. equi replication in vitro following activation with IFN-γ and LPS (Fig. 5B). As expected, bacterial replication occurred in nonactivated macrophages from both mice and the extent of replication was comparable to that observed in nonactivated wild-type macrophages (Fig. 5A and B). Macrophages which cannot produce NO (iNOS−/−) were unable to restrict R. equi replication, even after IFN-γ and LPS activation (Fig. 5B, solid lines). Surprisingly, however, activated gp91phox−/− macrophages were fully capable of restricting intracellular R. equi replication (Fig. 5B, dashed lines), despite their inability to mount a respiratory burst. We hypothesized that other sources of cellular oxygen radicals were contributing to ONOO− formation and bacterial killing, but our attempts to reverse bacterial killing by treating macrophages with a variety of oxygen radical inhibitors were not consistently successful (data not shown).
The production of ONOO− by activated macrophages.
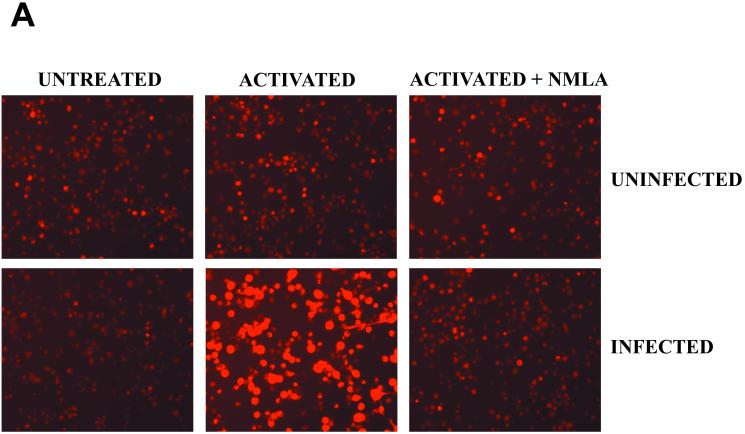
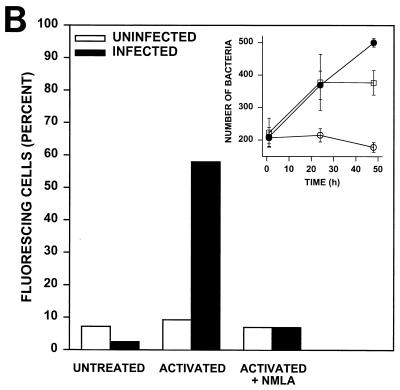
To visualize ONOO− production by macrophages phagocytizing R. equi, monolayers of resident or activated macrophages were incubated with DHR prior to the addition of opsonized R. equi. In the presence of ONOO−, DHR is oxidized to fluorescent rhodamine (17). This technology was used to visualize ONOO− formation in situ, during bacterial phagocytosis. A dull and equivalent level of background fluorescence was evident in all of the control monolayers that were tested, including resident uninfected cells (Fig. 6A). However, activated macrophages to which R. equi was added exhibited a rapid increase in fluorescence intensity, indicating ONOO− production. This production was evident even in low-magnification photomicrographs in which virtually all of the cells phagocytizing R. equi cells were brightly fluorescent (Fig. 6A). More than 50% of the cells in the monolayer stained positively for ONOO− (Fig. 6B). In contrast, the phagocytosis of R. equi cells by resident (nonactivated) macrophages failed to produce ONOO−. The magnitude of fluorescence was indistinguishable from background levels (Fig. 6A), and the number of cells producing ONOO− was not different from that of uninfected cells. Activated, infected cells treated with NMLA to inhibit NO production failed to produce ONOO− (Fig. 6B), and these cells failed to restrict R. equi growth in vitro (Fig. 6B, inset).
FIG. 6.
Peroxynitrite formation by activated, R. equi-infected macrophages. (A) Monolayers of BMMϕ were either untreated or activated overnight with 100 U of IFN-γ/ml and 50 ng of LPS/ml in the presence or absence of NMLA. R. equi was added to monolayers at an MOI of 50:1 in the presence of 1 mM DHR for 15 min at 37°C. Uninfected cells were treated similarly but were not infected with R. equi. Monolayers were analyzed for rhodamine fluorescence and photographed on an inverted microscope. (B) The number of cells positive for rhodamine fluorescence was quantitated and expressed as a percent positive. These data are representative of three independent experiments. (Inset) The intracellular growth of R. equi in resident cells (closed circles), activated cells (open circles), or activated cells treated with 500 μM NMLA (squares). Bacterial numbers are statistically different between macrophages activated with or without NMLA at 24 and 48 h (P ≤ 0.04; Student's t test).
DISCUSSION
R. equi is an intracellular bacterium that resides primarily within host macrophages. Previous studies have demonstrated that R. equi can replicate in nonactivated resident macrophages in vitro (16). Because the murine model has been established as an appropriate system to study R. equi infection (15), both in vivo and in vitro studies were developed to provide a better understanding of the role of the activated macrophage in R. equi infection. Studies in mice lacking the gene for IFN-γ were undertaken to address the requirement of IFN-γ for R. equi clearance in vivo. GKO mice were hypersusceptible to lethal R. equi infection and had increased bacterial burdens in their livers, spleens, and lungs at 3 and 7 days postinfection relative to those of wild-type mice. The most significant differences were seen in the lung at 7 days postinfection, when bacterial burdens in GKO mice were 5 logs higher than those observed in normal mice. Thus, IFN-γ plays a central role in host defense to R. equi. This observation is similar to that seen when GKO mice were infected with other intracellular pathogens, including another nocardioform actinomycete, Mycobacterium tuberculosis (6, 12). These studies confirm and extend the previous results of Kanaly et al., which showed that treatment of mice with anti-IFN-γ exacerbated disease (22). The requirement for IFN-γ confirms initial correlations between Th1-type immune responses and efficient resolution of experimental R. equi disease in mice (22, 24) and establishes the activated macrophage as the principal cell type involved in host defense against R. equi.
Studies were undertaken to identify the mechanism whereby activated macrophages killed R. equi. Oxygen and nitrogen radicals were the focus of this work. The generation of ROIs by macrophages occurs during the phagocytosis of many bacteria, fungi, and protozoa (25). Resident macrophages have some capacity to undergo a respiratory burst, but immunologic activation of macrophages augments this process substantially. The production of NO occurs only in activated macrophages. IFN-γ is the primary signal for iNOS transcription; however, secondary signals, such as tumor necrosis factor alpha, that are induced following the exposure of macrophages to bacterial products are generally also required for NO production. The role of oxygen and nitrogen radicals in bacterial killing was examined in vivo in animals deficient in the production of either of these radicals and in vitro in cell-free radical-generating systems. Because mice lacking iNOS failed to clear bacteria and succumbed to what should have been a sublethal infection, our initial studies focused on NO and its congeners in killing R. equi. Several subsequent observations, however, were not consistent with NO alone being directly bactericidal to R. equi. The failure of NO generated in a cell-free system to kill R. equi and the in vivo observation that gp91phox−/− mice were also susceptible to lethal R. equi infection suggested a more complex mechanism of bacterial killing by activated macrophages, which involved oxygen as well as nitrogen radicals. These observations prompted us to examine the role of peroxynitrite in bacterial killing.
To examine peroxynitrite-mediated killing of R. equi, small amounts of ONOO− (1 mM) were added directly to viable R. equi organisms in a cell-free system. This concentration represents a low net exposure because, at physiological pH, ONOO− rapidly decomposes to nitrate. Taking into account the short half-life of ONOO−, a single bolus of 1 mM ONOO− is equivalent to a steady-state concentration of approximately 28 μM (46). This level of ONOO− is therefore comparable to levels produced in situ, since rat alveolar macrophages within the lung epithelial lining fluid can produce up to 1 mM peroxynitrite min−1 (18). Furthermore, concentrations of peroxynitrite surrounding a bacterium in the phagolysosome may actually be higher (18). The toxicity of ONOO− for bacteria has been well documented (10). This strong oxidant can cause membrane damage via lipid peroxidation (40) and can damage DNA (21), oxidize sulfhydryl groups (38), and mediate tyrosine nitration (4). In the present work, we not only show that R. equi is very sensitive to brief exposures to ONOO− but also demonstrate that activated (but not resident) macrophages produce ONOO− during bacterial phagocytosis. This is a direct demonstration of ONOO− production by macrophages during bacterial phagocytosis. The production of ONOO− occurred only in macrophages undergoing phagocytosis and only when the macrophages were activated by IFN-γ.
In summary, we have shown that while NO is required for R. equi killing, this radical alone cannot mediate bacterial killing. Even large concentrations of NO (5 mM [data not shown]) for extended periods of time under a variety of conditions (acidic and neutral pH [data not shown]) were unable to inhibit bacterial growth. The combination of NO with O2− to form ONOO−, however, was potently bactericidal to R. equi, demonstrating a synergy between ROIs and RNIs. These studies reinforce a two-step model for the efficient killing R. equi by activated macrophages. The first step is macrophage activation which is typically mediated by IFN-γ and tumor necrosis factor alpha (24). This activation induces the transcription of iNOS mRNA and results in constitutive NO production. The second signal is provided by the bacteria themselves, which stimulate the respiratory burst during the process of phagocytosis. The high levels of O2− produced by cells undergoing bacterial phagocytosis combines with NO produced by activated macrophages to form ONOO−, which kills R. equi.
ACKNOWLEDGMENTS
This work was supported in part by a grant from The Grayson Jockey Club Research Foundation.
We are grateful to John Chan (Albert Einstein College of Medicine, Bronx, N.Y.) for his assistance with the generation of peroxynitrite.
REFERENCES
- 1.Assreuy J, Cunha F Q, Epperlein M, Noronha-Dutra A, O'Donell C A, Liew F Y, Moncada S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol. 1994;24:672–676. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- 2.Babior B M, Curnette J T, Kipnes R S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975;85:235–244. [PubMed] [Google Scholar]
- 3.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman J S, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin J C, Tsai M H. Kinetics of superoxide dismutase and iron catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli L, Crow J P, Beckman J S. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.DeGroote M A, Granger D, Xu Y, Cambell G, Prince R, Fang F C. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 10.Fang F C. Mechanisms of nitric oxide-related anti-microbial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandes P D, Assreuy J. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz J Med Biol Res. 1997;30:93–99. doi: 10.1590/s0100-879x1997000100015. [DOI] [PubMed] [Google Scholar]
- 12.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giguère S, Prescott J S. Clinical manifestations, diagnosis, treatment and prevention of R. equi infections in foal. Vet Microbiol. 1997;56:313–334. doi: 10.1016/s0378-1135(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 14.Hickman-Davis J, Gibbs-Erwin J, Lindsey J R, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc Natl Acad Sci USA. 1999;96:4953–4958. doi: 10.1073/pnas.96.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hondalus M K. Pathogenesis and virulence of Rhodococcus equi. Vet Microbiol. 1997;56:257–268. doi: 10.1016/s0378-1135(97)00094-1. [DOI] [PubMed] [Google Scholar]
- 16.Hondalus M K, Mosser D M. Survival and replication of Rhodococcus equi in macrophages. Infect Immun. 1994;62:4167–4175. doi: 10.1128/iai.62.10.4167-4175.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ischiropoulos H, Gow A, Thom S R, Kooy N W, Royall J A, Crow J P. Detection of reactive nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Methods Enzymol. 1999;301:367–373. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- 18.Ischiropoulos H, Zhu L, Beckman J S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 19.Jackson S H, Gallin J I, Holland S M. The gp91phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston R B, Godzik C A, Cohn Z A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978;148:115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juedes M J, Wogan G N. Peroxynitrite-induced mutation spectra of pSP189 following replication in bacteria and in human cells. Mutat Res. 1996;349:51–61. doi: 10.1016/0027-5107(95)00152-2. [DOI] [PubMed] [Google Scholar]
- 22.Kanaly S T, Hines S A, Palmer G H. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatuous pneumonia. Infect Immun. 1995;63:3037–3041. doi: 10.1128/iai.63.8.3037-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanaly S T, Hines S A, Palmer G H. Transfer of a CD4+ Th1 cell line to nude mice effects the clearance of Rhodococcus equi from the lung. Infect Immun. 1996;64:1126–1132. doi: 10.1128/iai.64.4.1126-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasuga-Aoki H, Takai S, Sasaki Y, Tsubaki S, Madarame H, Nakane A. Tumour necrosis factor and interferon-γ are required in host resistance against virulent Rhodococcus equi infection in mice: cytokine production depends on the virulence levels of R. equi. Immunology. 1999;96:122–127. doi: 10.1046/j.1365-2567.1999.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff S J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 26.Laubach V E, Shesely E G, Smithies O, Sherman P A. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leijh P C J, Nathan C F, van den Barselaar M T H, van Furth R. Relationship between extracellular stimulation of intracellular killing and oxygen-dependent microbicidal systems of monocytes. Infect Immun. 1985;47:502–507. doi: 10.1128/iai.47.2.502-507.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madigan J E, Hietala S, Mueller N. Protection against naturally-acquired Rhodococcus equi pneumonia in foals by administration of hyperimmune plasma. J Reprod Fertil Suppl. 1991;44:571–578. [PubMed] [Google Scholar]
- 29.Maragos C M, Morley D, Wink D A, Dunams T M, Saavedra J E, Hoffman A, Bove A A, Isaac L, Hrabie J A, Keefer L K. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. J Med Chem. 1991;34:3243–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 30.Martens R J, Fiske R A, Renshaw H W. Experimental subacute foal pneumonia induced by aerosol administration of Corynebacterium equi. Equine Vet J. 1982;14:111–116. doi: 10.1111/j.2042-3306.1982.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 31.Martens R J, Martens J G, Fiske R A, Hietala S K. Rhodococcus equi foal pneumonia. Protective effects of immune plasma in experimentally infected foals. Equine Vet J. 1989;21:249–255. doi: 10.1111/j.2042-3306.1989.tb02161.x. [DOI] [PubMed] [Google Scholar]
- 32.Murray H W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981;153:1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray H W, Cohn Z A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979;150:938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Investig. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan C, Hibbs J B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–75. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 36.Nordmann P, Ronco E, Nauciel C. Role of T-lymphocyte subsets in R. equi infection. Infect Immun. 1992;60:2748–2752. doi: 10.1128/iai.60.7.2748-2752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulnock D M. The molecular biology of macrophage activation. Immunol Ser. 1994;60:47–62. [PubMed] [Google Scholar]
- 38.Radi R, Beckman J S, Bush K M, Freeman B A. Peroxynitrite mediated sulfhydryl oxidation: the cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 39.Ross T L, Balson G A, Miners J S, Smith G D, Shewen P E, Prescott J F, Yager J A. Role of CD4+, CD8+, and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with Rhodococcus equi. Can J Vet Res. 1996;60:186–192. [PMC free article] [PubMed] [Google Scholar]
- 40.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman B A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 41.Sutterwala F S, Noel G J, Clynes R, Mosser D M. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tohyama M, Kawakami K, Futenma M, Saito A. Enhancing effect of oxygen radical scavengers on murine macrophage anticryptococcal activity through the production of nitric oxide. Clin Exp Immunol. 1996;103:436–441. doi: 10.1111/j.1365-2249.1996.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasquez-Torres A, Jones-Carson J, Balish E. Peroxynitrite contributes to the candidacidal activity of nitric oxide producing macrophages. Infect Immun. 1996;64:3127–3133. doi: 10.1128/iai.64.8.3127-3133.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wozencraft A O, Dockrell H M, Taverne J, Targett G A T, Playfair J H L. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984;43:664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Q, Nathan C. The high output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 46.Zhu L, Gunn C, Beckman J S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]