Abstract
Background
Bronchiectasis is characterized by neutrophilic inflammation and frequent exacerbations often associated with infections. Lipid mediators play critical roles in the inflammatory response, and the balance between anti-inflammatory and pro-inflammatory mediators could drive to chronic inflammation. The aim of this study was to evaluate the metabolites of docosahexaenoic acid and arachidonic acid in sputum of adults with bronchiectasis defining their associations with clinical data, bacterial load and neutrophil elastase.
Methods
An observational, cross-sectional study was conducted at the bronchiectasis program of the Policlinico Hospital in Milan, Italy, where patients were enrolled. Active neutrophil elastase was measured by enzyme-linked immunosorbent assay, pro-resolving and pro-inflammatory fatty acid-derived mediators were evaluated by mass spectrometry and respiratory pathogens were assessed by real-time PCR. Analysis were performed on sputum collected during stable state and clinical data were also collected.
Results
Levels of pro-inflammatory mediators derived from arachidonic acid metabolism showed association with neutrophil elastase, were proportional to Pseudomonas aeruginosa identifications and were linked with radiological gravity index, while the concentrations of pro-resolution mediators derived from docosahexaenoic acid were associated with a better health status, highlighted by the inverse correlation with radiological gravity index, bacterial infections and sputum volume production.
Conclusion
Pro-inflammatory mediators derived from FA metabolisms are associated with severity of bronchiectasis while DHA-derived metabolites are inversely associated with severity of the disease, which may be used for personized treatment of bronchiectasis.
Keywords: Bronchiectasis, Docosahexaenoic acid, Arachidonic acid
Introduction
Bronchiectasis is a chronic and debilitating respiratory disease characterized by lung inflammation and permanent bronchi dilatation. This condition affects peoples of all ages profoundly affecting the quality of life: subjects with bronchiectasis have daily cough, excessive sputum production and frequent pulmonary exacerbations [1], often associated with lung bacterial infections [2, 3]. Secretion of pro-inflammatory mediators from epithelial and immune cells typically causes a massive neutrophil influx within the airways, as often observed in frequent exacerbators colonized by Pseudomonas aeruginosa (Psa) [4]. Early neutrophil involvement with release of reactive oxygen species (ROS), pro-inflammatory lipid mediators (LMs) and protease such as neutrophil elastase (NE) is directly involved in lung damage in bronchiectasis [5]. Studies on cystic fibrosis (CF) have reported the presence of fatty acid alteration in the blood and tissues of patients, such as decreased levels of docosahexaenoic acid (DHA), and of linoleic acid (LA) [6] together with increased levels of arachidonic acid (AA). Fatty acids metabolites play multiple roles in the inflammatory response: AA metabolites possess both potent pro-inflammatory and anti-inflammatory activities, while DHA-derived molecules, such as maresins, protectins and resolvins, are involved in the resolution of inflammation and are collectively defined as specialized pro-resolving mediators (SPMs) [7–10]. We hypothesized that changes in the relative availability of polyunsaturated fatty acids (PUFAs) may lead to an altered balance of pro-inflammatory and pro-resolution lipid mediators, either resulting in, or as a result of chronic colonization by different types of bacteria, leading to chronic inflammation.
Based on this assumption the aim of this study was to evaluate the presence of metabolites derived from DHA and AA into the airways of adults with bronchiectasis during stable state, analyzing potential correlation with clinical and radiological parameters, neutrophil elastase activity and bacterial colonization.
Material and methods
Study design and population
An observational cross-sectional study was carried out at the Bronchiectasis Program of the Respiratory Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy, between March 2017 and March 2019. Adults (aged ≥ 18 years) with clinically (daily sputum production) and radiologically (at least one lobe involvement on chest computed tomography) bronchiectasis were recruited during clinical stability (⩾1 month from the last exacerbation and antibiotic course). Patients with cystic fibrosis or bronchiectasis due to pulmonary fibrosis were excluded. The study was performed in accordance with the declaration of Helsinki, was approved by the ethical committee of the hospital (Ethics committee Milano Area 2, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 255_2020) and all subjects provided written informed consent to participate.
Study procedures
Spontaneous sputum samples were obtained, and mucus plugs were isolated. Deoxyribonucleic acid (DNA) was extracted according to a published technique [11, 12]. Aliquots of mucus plug were also diluted 8 × in PBS, vortexed and centrifuged at 4 °C for 15 min at 3000 g. Supernatants and DNA were stored at − 80 °C until analysis.
Mass spectrometry analysis of AA and DHA metabolites
After thawing of sputum supernatant samples (0.2-1 mL), aliquots (0.2 mL) were added with stable isotope labeled internal standards (leukotriene B4 [d4]LTB4; prostaglandin E2 [d4]PGE2; 5-hydroxyeicosatetraenoic acid [d8]5-HETE, and lipoxin A4 [d5]LXA4; 2.5 ng each), centrifuged, acidified with acetic acid (final concentration 0.01%) and extracted using preconditioned polymeric solid phase extraction cartridges (Strata-X, 33 µm Polymeric Reversed Phase; Phenomenex, Torrance, CA). After washing with ultrapure water, DHA- and AA-derived metabolites were eluted using methanol/water, 90/10, v/v (0.5 ml), and the eluate taken to dryness using a rotary vacuum evaporator (SpeedVac; Thermo Scientific, Waltham, MA). Upon reconstitution in 40 µL HPLC solvent A (8.3 mM acetic acid buffer to pH 5.7 with ammonium hydroxide) plus 20 µL of HPLC solvent B (acetonitrile/methanol, 65:35, v/v), an aliquot of each sample (20 µL) was injected onto a C18 HPLC column (Ascentis 150 × 2 mm, 3 µm; Supelco, Bellefonte, PA) and eluted at the rate of 400 µL/min with a linear gradient from 45% solvent B, which was increased to 75% in 12 min, to 98% in 2 min, then held for 11 min before re-equilibration at 45% B for 10 min. The HPLC effluent was directly infused into a triple quadrupole mass spectrometer (API4000, Applied Biosystem, Foster City, CA, U.S.A.) equipped with electrospray ion source for mass spectrometric analysis in the negative ion mode using multiple reaction monitoring for the specific m/z transitions: 343–281 for 17-hydroxy-docosahexaenoic acid (17OH-DHA, the precursor of both resolvins and protectin), 359–206 for protectin D1(PD1), 375–141 for resolvin D1 (RvD1), 343–205 for 14-hydroxy-docosahexaenoic acid (14OH-DHA, the precursor of maresin), 359–250 for maresin 1 (MaR1), 335–195 for leukotriene B4 (LTB4), 319–219 for 15-hydroxy-eicosatetraenoic acid (15-HETE), 351–271 for prostaglandin E2 (PGE2), 438–333 for leukotriene E4 (LTE4), 327–116 for [d8]5-HETE, 339–197 for [d4]LTB4, 359–275 for [d4]PGE2, 443–338 for [d5]LTE4, and 356–222 for [d5]LXA4, that was used as IS for resolvin D1 RvD1. Quantification was performed using area ratios to the corresponding internal standards, and data were analyzed using MassHunter software. Standard curves were obtained using synthetic PD1 (a gift from Dr. Thierry Durand, CNRS, Montpellier, France), LTB4, PGE2, RvD1, MaR1, 15-HETE, LTE4, 14OH-DHA and 17OH-DHA (Cayman Chem, Ann Arbor, MI). The peak–area ratios of every compound to the relevant deuterated internal standard was calculated and plotted against the amount of the synthetic standards. Calibration lines were calculated by the least squares linear regression method and the correlation coefficient r2 was always better than 0.99. To calculate the concentration of any given analyte, the peak–area ratio to the relevant internal standard was calculated and read off the corresponding calibration line. Detection limit varied between 1 and 25 pg injected (3 to 75 pg in the sample), depending on the analyte. Optimization of declustering potential, collision energy and CXP, was carried out for each metabolite directly injecting 1 to 5 ng of synthetic standard using the same eluent used for the analysis.
Neutrophil elastase evaluation
Sputum supernatants were used also for NE evaluation by means of ProteaseTag® Active Neutrophil Elastase Immunoassay (Proaxis, Belfast, UK) as per manufacturer’s instructions [13].
Real-time PCR bacterial identification
Bacterial DNA was quantified using real-time polymerase chain reaction (PCR) assay for Pseudomonas aeruginosa (Psa; gyrB gene), Staphylococcus aureus (Sa; nuc gene), Streptococcus pneumoniae (Spn; lytA gene) and Haemophilus influenzae (Hi; fucK gene) using AB7900HT Fast Real-Time PCR System (Applied Biosystems) with primers and probes previously published [14]. Quantitative PCR was performed in 20-µL reaction mixture containing 2 × QuantiFast Multiplex PCR Master Mix (Qiagen), primers and probes and 2 µL of DNA extracted from sputum samples. Moreover a real-time PCR targeting human RNaseP gene was used to detect PCR inhibition or extraction failure. All amplification were performed with parameters as follow: 95 °C for 5 min, followed by 45 cycles of 95 °C for 45 s and 60 °C for 1 min. To quantify bacterial DNA, standard curves were prepared using genomic DNA from S. pneumoniae (ATCC® 700669DQTM), P. aeruginosa (ATCC® 47085DQTM), S. aureus (ATCC® 29213DQTM) and H. influenzae (ATCC® 51907DQTM). Standard DNA were analyzed with Qubit and Quant-iT dsDNA Assay Kit High Sensitivity (Thermo Fisher Scientific) followed by dilution at 1 ng/µL. Then, standard curves were prepared by tenfold dilution from 1 ng/µL to 1*10–7 ng/µL, samples and curves were tested in triplicate and samples was assumed to be positive if Ct is < 38. Target genes (gyrB, nuc, lytA and fucK) were in a single copy in the genome, copies measured was assumed to be equivalent to the bacterial load [15]. The number of genome copies were calculated based on 7.22 femtograms, 3.01 femtograms, 2.28 femtograms and 1.95 femtograms of DNA per Psa, Sa, Spn and Hi genomes respectively. Quantification of bacteria in the samples were based on standard curve generate by plotting the Ct values against known genome copies. Conversion of genome copies/reaction to genome copies/mL was based on a 2µL input per reaction derived from 100µL of eluate extracted from 200µL of treated specimen. Sa positive samples were also analyzed by means of RIDA GENE MRSA test (r-biopharm), a multiplex real-time PCR for the identification of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA).
Clinical variables
Demographics, comorbidities, disease severity, respiratory symptoms, sputum evaluation, radiological assessment, quality of life and biological characteristics were recorded. The severity of bronchiectasis was evaluated according to both Bronchiectasis Severity Index (BSI) and the cumulative score based on five parameters: forced expiratory volume in 1 s [FEV1], age, chronic colonization, extension, and dyspnea (FACED score).
Statistical analysis
Qualitative variables were summarized with absolute and relative (percentage) frequencies, while quantitative variables were shown with medians [interquartile ranges, (IQR)] (Table 1). Kruskal–Wallis Test, Mann–Whitney and Spearman’s correlations were used for analysis performed with SPSS statistical software, version 26.
Table 1.
Clinical characteristics of the study population
| Variables | Total cohort | Group aNE < 20 µg mL−1 | Group aNE ⩾20 µg mL−1 |
|---|---|---|---|
| n = 40 | n = 15 | n = 25 | |
| Demographics | |||
| Median (IQR) age, years | 61 (53–69.5) | 65 (53–74) | 60 (53–67) |
| Females, n (%) | 28 (70) | 14 (93) | 14 (56) |
| Median (IQR) BMI, kg/m2 | 23.6 (20.1–25.9) | 24 (19.6–26.5) | 23.5 (21–25.4) |
| Median (IQR) Weight, kg | 61 (52–73) | 61 (51–73) | 62 (53–73) |
| Underweight (BMI < 18.5 kg/m2), n (%) | 4 (10) | 2 (13.3) | 2 (8) |
| Former or current smoker, n (%) | 10 (25) | 6 (40) | 4 (16) |
| Disease severity | |||
| Median (IQR) BSI score | 8 (5–12) | 9 (6–11) | 8 (5–12) |
| Median (IQR) FACED score | 3 (2–4) | 2 (1.5–4) | 3 (2–3) |
| Radiology | |||
| Median (IQR) Reiff score | 4 (3–6) | 3 (3–6) | 4 (3–6) |
| Mean (SD) No. of lobes | 3.68 (1.4) | 3.66 (1.6) | 3.68 (1.3) |
| 4 + lobes involvement, n (%) | 20 (50) | 7 (46.6) | 13 (52) |
| Clinical status and diagnostic results | |||
| Median (IQR) total exacerbation previous year | 2 (1.5–4) | 3 (2–4) | 2 (1–4) |
| ≥ 2 exacerbations previous year, n (%) | 30 (75) | 12 (80) | 18 (72) |
| ≥ 3 exacerbations previous year, n (%) | 19 (45.7) | 10 (66.6) | 9 (36) |
| ≥ 1 hospitalization previous year, n (%) | 7 (17.5) | 2 (13.3) | 5 (20) |
| Emphysema, n (%) | 4 (10) | 1 (6.66) | 3 (12) |
| Quality of life | |||
| Median (IQR) QoL-B questionnaire—Physical | 53.3 (38.3–66.7) | 50 (35–73.3) | 56.6 (40–66.7) |
| Median (IQR) QoL-B questionnaire—Role | 56.6 (45–73.3) | 53.3 (46.7–66.7) | 66.6 (41.6–73.3) |
| Median (IQR) QoL-B questionnaire—Vitality | 44.4 (33.3–55.6) | 44.4 (33.3–55.6) | 55.6 (25–55.6) |
| Median (IQR) QoL-B questionnaire—Emotion | 75 (50–91.7) | 70.8 (52–83.3) | 79.1 (52–91.7) |
| Median (IQR) QoL-B questionnaire—Social | 52.8 (33.3–75) | 55.6 (43.7–72.9) | 45.8 (33.3–72.9) |
| Median (IQR) QoL-B questionnaire – Treatment Burden | 55.6 (44.4–66.7) | 55.6 (44.4–66.7) | 55.6 (44.4–66.7) |
| Median (IQR) QoL-B questionnaire—Health | 33.3 (20.8–52) | 33.3 (27–54.8) | 33.3 (16.7–48) |
| Median (IQR) QoL-B questionnaire—Respiration | 68.5 (48.1–77.8) | 68.5 (50.6–77.8) | 70.4 (50–74.1) |
| Relevant comorbidities | |||
| Median (IQR) BACI | 0 (0–3) | 3 (0–3) | 0 (0–3) |
| COPD, n (%) | 4 (10) | 2 (13.3) | 2 (8) |
| MRGE, n (%) | 14 (35) | 7 (46.6) | 7 (28) |
| Asthma, n(%) | 7 (17.5) | 4 (26.6) | 3 (12) |
| Pulmonary function | |||
| Mean (SD) FEV1, % | 71.3 (23.1) | 71.8 (18) | 68.2 (29.4) |
| FEV1 ≤ 35, n (%) | 4 (10) | 1 (6.6) | 3 (12) |
| FEV1 ≤ 50, n (%) | 7 (17.5) | 1 (6.6) | 6 (24) |
| Chronic therapy | |||
| PPI, n (%) | 16 (40) | 8 (53.3) | 8 (32) |
| LABA, n (%) | 31 (77.5) | 12 (80) | 19 (76) |
| LAMA, n (%) | 22 (55) | 10 (66.6) | 12 (48) |
| ICS, n (%) | 22 (55) | 7 (46.6) | 15 (60) |
| Sputum biomarkers | |||
| Median (IQR) Active neutrophil elastase, µg/ml | 24.7 (13–38.5) | 7 (2.1–15.2) | 35.3 (28–48) |
| Median (IQR) LTB4, ng/mL | 1.4 (0.6–4) | 0.9 (0.3–1.7) | 2 (0.8–4.6) |
| Median (IQR) PGE2, ng/mL | 3.6 (1.4–5.5) | 1.5 (0.9–3.8) | 4.1 (2.6–5.7) |
| Median (IQR) 15-HETE, ng/mL | 16.6 (8.6–26.6) | 18.4 (6.8–25.4) | 14.9 (8.9–26.3) |
| Median (IQR) 14-OH DHA, ng/mL | 1.6 (0.91–2.6) | 2.1 (1.1–5.4) | 1.4 (0.8–2.1) |
| Median (IQR) 17-OH DHA, ng/mL | 6.8 (4.1–11.7) | 7.3 (4.8–21.5) | 5.9 (2.7–10.2) |
| Median (IQR) LTE, ng/mL | 0.17 (0.0–0.4) | 0.17 (0.0–0.4) | 0.18 (0.0–0.4) |
| Real Time PCR Bacterial Identification | |||
| Positive to Psa, n (%) | 21 (52.5) | 5 (33.3) | 16 (64) |
| Median (IQR) Psa genome copies*mL−1 | 2,583,621.9 (0–106,128,808.9) | 0 (0–10,530,297.8) | 42,243,767 (0.0–145,083,102.5) |
| Positive to Hi, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Median (IQR) Hi genome copies*mL−1 | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Positive to Sa, n (%) | 16 (40) | 6 (40) | 10 (40) |
| MRSA, n (%) | 4 (10) | 1 (6.6) | 3 (12) |
| Median (IQR) Sa genome copies*mL−1 | 0 (0–5031.1) | 0 (0.0–5602.1) | 0 (0.0–4053.1) |
| Positive to Spn, n (%) | 11 (27.5) | 4 (26.6) | 7 (28) |
| Median (IQR) Spn genome copies*mL−1 | 0 (0–276.9) | 0 (0.0–268.2) | 0 (0.0–245.4) |
BMI body mass index, BSI bronchiectasis severity index, FACED F: Forced expiratory volume in 1 s [FEV1], A age, C chronic colonization by Pseudomonas aeruginosa, E radiological extension [number of pulmonary lobes affected], D dyspnea, BACI bronchiectasis aetiology comorbidity index, COPD chronic obstructive pulmonary disease, MRGE gastro-esophageal reflux disease, FEV1 forced expiratory volume in the 1st second, PPI proton pump inhibitors, LABA long-acting β2-agonists, LAMA long-acting muscarinic antagonists, ICS inhaled corticosteroids
Results
Forty adults [28, (70%) female, age median (IQR): 61 (53–69.5)] were enrolled. Characteristics of the cohort, including active neutrophil elastase (aNE), bacteria identification and related genome copies, and AA and DHA metabolites in sputum samples are reported in Table 1. Mass spectrometry analysis showed the presence of pro-inflammatory mediators such as LTB4 [median (IQR): 1.4 ng/mL (0.6–4)], PGE2 [median (IQR): 3.6 ng/mL (1.4–5.5)], 15-HETE [median (IQR): 16.6 ng/mL (8.6–26.6)] and LTE4 [median (IQR): 0.17 ng/mL (0.0–0.4)] as well as pro-resolving mediators precursors 14OH-DHA [median (IQR): 1.6 ng/mL (0.91–2.6)] and 17OH-DHA [median (IQR): 6.8 ng/mL (4.1–11.7)]. Active neutrophil elastase had a median (IQR) concentration of 24.7 µg/mL (13–38.5). Real-time PCR allowed to identify bacteria and related genome copies (% of detection; median copies with IQR) such as Psa [52.5%; 2,583,621.9 (0–106,128,808.9)], Hi [0%; 0 (0–0)], Sa [40%, 0 (0–5031.1); 10% MRSA] and Spn [27.5%, 0 (0–276.9)].
Fatty acid pro-resolving/pro-inflammatory mediators versus clinical and laboratory data
Patients were divided into two groups based on the concentration of aNE as defined by Chalmers and collegues [16] of being most associated with worse outcomes in bronchiectasis: aNE ⩾20 µg·mL−1 (high aNE) versus aNE < 20 µg mL−1 (low aNE). Analysis of pro-inflammatory biomarkers, in particular LTB4 [low aNE: 0.92 ng/mL (0.30–1.71) vs. high aNE: 2.07 ng/mL (0.84–4.59); p = 0.0006, Fig. 1A] and PGE2 [low aNE: 1.51 ng/mL (0.96–3.87) vs. high aNE: 4.14 ng/mL (2.60–5.75); p = 0.015, Fig. 1B] revealed significantly higher concentrations in the high aNE group. Moreover PGE2 was also statistically higher in samples positive for Psa [negative PCR: 1.95 ng/mL (0.93–4.26) vs. positive PCR: 4.51 ng/mL (3.36–5.79); p = 0.002, Fig. 1C], and showed a significantly correlated with radiological severity as measured by Reiff score (ρ:0.438, p = 0.0046, Fig. 1D). Finally, 15-HETE correlated with the number of exacerbations in the previous year (ρ:0.343, p = 0.029), and taken together all these evidence suggested a close link between AA-derived lipid mediators and clinical inflammatory markers.
Fig. 1.
LTB4 (Panel A) and PGE2 (Panel B) in sputum samples with high or low levels of active neutrophil elastase (aNE). Panel C: PGE2 in sputum samples with or without Pseudomonas aeruginosa infection. Panel D: Correlation between PGE2 in sputum samples and radiological severity
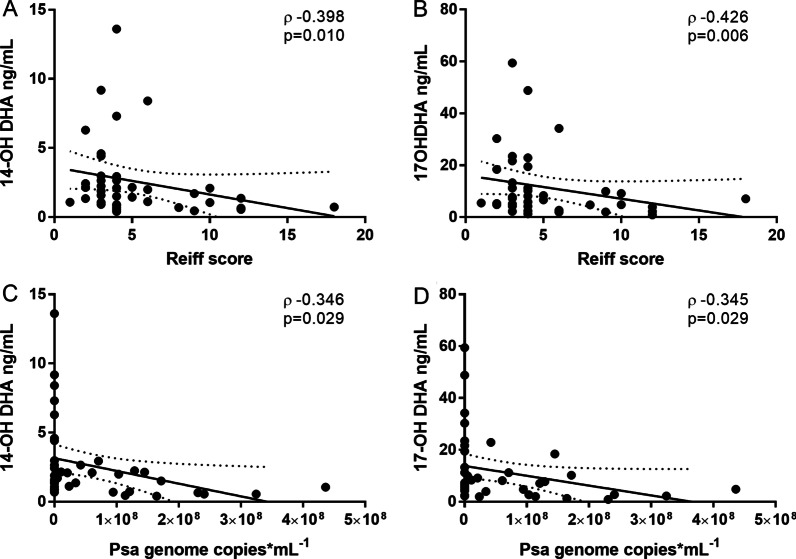
On the contrary, the precursor of SPMs 14OH-DHA and 17OH-DHA showed statistically significant inverse correlations with Reiff score [Reiff score 14OH-DHA (ρ: − 0.398, p = 0.010), Fig. 2A; Reiff score 17OH-DHA (ρ: − 0.426, p = 0.006), Fig. 2B] and with detected Psa genome copies [Psa 14OH-DHA (ρ: − 0.346, p = 0.029), Fig. 2C; Psa 17OH-DHA (ρ: − 0.345, p = 0.029), Fig. 2D], and 14OH-DHA concentrations also showed inverse correlation with daily sputum volume production (ρ: − 0.445, p = 0.004).
Fig. 2.
Inverse correlation between 14OH-DHA or 17OH-DHA, and radiological severity as assessed by Reiff score (Panels A and B, respectively), or PSA genome copies (Panels C and D, respectively)
Discussion
In agreement with previously published data [16–18], the findings of the present work provide evidence that the presence of Psa in sputum of bronchiectasis patients may drive airway neutrophilic inflammation possibly through the formation of the potent neutrophil chemotactic factor LTB4 [19], leading to a significant influx and activation of neutrophils with theassociated release of active neutrophil elastase. PGE2 may also contribute to the chemotactic effect of LTB4 by inducing vasodilation, and its correlation with the Reiff score also suggests a role in radiological severity. These results are well in agreement with a recent report assessing eicosanoids in bronchoalveolar lavage fluids of patients with mild to severe bronchiectasis [20], and are consistent with the known role of AA-derived lipid mediators in the inflammatory response [21]. Results from the analysis of precursors of pro-resolving mediators such as 14OH-DHA and 17OH-DHA showed significant inverse correlations of their concentrations in sputum with clinical parameters such as the Reiff score, with detected Psa genome copies, and with daily sputum volume production. Bedi and colleagues [20] also reported the measurement of eicosanoids in plasma, suggesting that in bronchiectasis patients lower concentrations of LXA4, a trihydroxyderivative of AA endowed with anti-inflammatory activity [22], may contribute to the severity of the disease. This evidence is also in line with our findings, but it must be noted that plasma determination of biologically active AA-derived metabolites may not be appropriate to assess the formation of these metabolites in vivo [23]. Eicosanoids are local mediators produced on-demand, that do not circulate, as hormones do, in order to reach their targets, but act directly in the local environment, typically in the immediate surroundings of the cells synthesizing it. Furthermore, they are very often locally metabolized either by spontaneous chemical degradation, as for Prostaglandin I2 and Thromboxane A2 [24], or by enzymatic activities, such as for the formation of 20-hydroxy and 20-carboxy derivative of LTB4 by neutrophil themselves [25] or macrophages. When entering the circulation PGE2, for example, is completely metabolized by a single pulmonary transit [26], and it is generally accepted that when local sampling is not possible, systemic formation of eicosanoids should be assessed measuring the concentration of their urinary metabolites [27–29].
While we believe this study is of interest in defining critical factors for the evolution of the pathology in different subjects, we are also aware that it comes with some limitations: (1) the number of subjects is reduced and sample size cannot be calculated due to absence of other studies on fatty acid mediators using sputum from bronchiectasis patients; (2) data on diet was not recorded during observation and this might be important because omega-3 and omega-6 fatty acids intake could change the availability of AA and DHA as substrates of pro-inflammatory or pro-resolution mediators; (3) sputum has been selected as matrix of interest because of the local nature of the mediators studied but significant variability may be present; (4) the study is monocentric and data may not be generalized.
Conclusion
The observation that SPM precursor molecules could be detected locally, in sputum samples from bronchiectasis patients, and inversely correlate to severity, as in the case of the Reiff score, suggest that DHA-derived metabolites may play a role helping the resolution of the inflammatory reaction and limiting pulmonary damage. Evidence that SPMs can enhance clearance of microorganisms and stimulate tissue repair, as well as modulate viral and bacterial infections by increasing phagocytosis and the ability to kill bacteria [30, 31] support the observed inverse correlation of SPM precursor molecules with detected Psa genome copies.
This study can be taken as a pilot for additional longitudinal studies in which subjects could be included based on (i.e.) stable versus exacerbation state; additionally the effect of DHA supplementation could also be evaluated with respect to both the formation of DHA-derived oxygenated metabolites and clinical parameters including Reiff score and Psa colonization. This approach could be useful to better understand the specific weight of pro-inflammatory vs. pro-resolution mediators, and to assess the ability of DHA supplementation to affect the duration of exacerbation and total number of exacerbations during year.
Acknowledgements
Not applicable.
Abbreviations
- Psa
Pseudomonas aeruginosa
- ROS
Reactive oxygen species
- Lms
Lipid mediators
- NE
Neutrophil elastase
- CF
Cystic fibrosis
- DHA
Docosahexaenoic acid
- LA
Linoleic acid
- AA
Arachidonic acid
- SPMs
Specialized pro-resolving mediators
- PUFAs
Polyunsaturated fatty acids
- LTB4
Leukotriene B4
- PGE2
Prostaglandin E2
- 5-HETE
5-Hydroxyeicosatetraenoic acid
- LXA4
Lipoxin A4
- 17OH-DHA
17-Hydroxy-docosahexaenoic acid
- PD1
Protectin D1
- 15-HETE
15-Hydroxyeicosatetraenoic acid
- LTE4
Leukotriene E4
- RvD1
Resolvin D1
- 14OH-DHA
14-Hydroxy-docosahexaenoic acid
- MaR1
Maresin 1
- DNA
Deoxyribonucleic acid
- PCR
Polymerase chain reaction
- Sa
Staphylococcus aureus
- Spn
Streptococcus pneumoniae
- Hi
Haemophilus influenzae
- ct
Cycle threshold
- aNE
Active neutrophil elastase
- gyrB
Subunit B DNA gyrase
- nuc
Nuclease
- lytA
Autolysin
- fucK
Fuculokinase
- BMI
Body mass index
- BSI
Bronchiectasis severity index
- BACI
Bronchiectasis aetiology comorbidity index
- COPD
Chronic obstructive pulmonary disease
- MRGE
Gastro-esophageal reflux disease
- FEV1
Forced expiratory volume in the 1st second
- PPI
Proton pump inhibitors
- LABA
Long-acting β2-agonists
- LAMA
Long-acting muscarinic antagonists
- ICS
Inhaled corticosteroids
Author contributions
Conceptualization: LT, AS, FB, SA; Formal analysis: LT, AS, PR and CP; Investigations: LT, AS, SA, AG, FA; Resources: FB, AS, PM, CA; Data curation: LT, AS, FA, SA and AG; Writing (original draft preparation): LT and AS; Writing (review and editing): PR, AG, CP, CA, MLS, ST, PM, FA, SA and FB; Supervision: SA; Project administration: AS; Funding acquisition: FB. All authors have read and approved the final manuscript.
Funding
This study was partially funded by Italian Ministry of Health—Current research IRCCS.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of the hospital (Ethics committee Milano Area 2, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 255_2020) and all subjects provided written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
L.T., P.R., C.P., M.L.S, S.T., F.A., A.G., C.A., P.M., have no competing interest. A.S. reports grants from Chiesi, and personal fees from Chiesi and Boheringer Ingelheim. S.A. reports grants from INSMED, Chiesi and Fisher & Paykel; personal fees from McGRAW HILL, INSMED, ZAMBON, AstraZeneca, CSL Behring GmbH, Grifols, Fondazione Charta, Boehringer Ingelheim, Chiesi, ZCUBE, Menarini and GlaxoSmithKline. F.B. reports grant from Astrazeneca, Chiesi and INSMED; personal fees from Menarini, Astrazeneca, Chiesi, GSK, Guidotti, Grifols, INSMED, Novartis, Viatris, Vertex and Zambon.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017 doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 2.Amati F, Simonetta E, Gramegna A, et al. The biology of pulmonary exacerbations in bronchiectasis. Eur Respir Rev. 2019 doi: 10.1183/16000617.0055-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole PJ. Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6–15. [PubMed] [Google Scholar]
- 4.Sibila O, Perea L, Cantó E, et al. Antimicrobial peptides, disease severity and exacerbations in bronchiectasis. Thorax. 2019 doi: 10.1136/thoraxjnl-2018-212895. [DOI] [PubMed] [Google Scholar]
- 5.Gramegna A, Amati F, Terranova L, et al. Neutrophil elastase in bronchiectasis. Respir Res. 2017 doi: 10.1186/s12931-017-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Turkmani MR, Freedman SD, Laposata M. Fatty acid alterations and n-3 fatty acid supplementation in cystic fibrosis. Prostaglandins Leukot Essent Fatty Acids. 2007 doi: 10.1016/j.plefa.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Schwab JM, Chiang N, Arita M, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007 doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Dalli J, Colas RA, et al. Protectins and maresins: new pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Gupta SK, Perretti M, et al. The Atlas of Inflammation Resolution (AIR) Mol Aspects Med. 2020 doi: 10.1016/j.mam.2020.100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018 doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oriano M, Terranova L, Teri A, et al. Comparison of different conditions for DNA extraction in sputum—a pilot study. Multidiscip Respir Med. 2019 doi: 10.1186/s40248-018-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terranova L, Oriano M, Teri A, et al. How to process sputum samples and extract bacterial DNA for microbiota analysis. Int J Mol Sci. 2018 doi: 10.3390/ijms19103256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oriano M, Terranova L, Sotgiu G, et al. Evaluation of active neutrophil elastase in sputum of bronchiectasis and cystic fibrosis patients: a comparison among different techniques. Pulm Pharmacol Ther. 2019 doi: 10.1016/j.pupt.2019.101856. [DOI] [PubMed] [Google Scholar]
- 14.Gadsby NJ, McHugh MP, Russell CD, et al. Development of two real-time multiplex PCR assays for the detection and quantification of eight key bacterial pathogens in lower respiratory tract infections. Clin Microbiol Infect. 2015 doi: 10.1016/j.cmi.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Lin S, Khalil A, et al. Quantitative PCR assay using sputum samples for rapid diagnosis of pneumococcal pneumonia in adult emergency department patients. J Clin Microbiol. 2005 doi: 10.1128/JCM.43.7.3221-3226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201605-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keir HR, Fong CJ, Crichton ML, et al. Personalised anti-inflammatory therapy for bronchiectasis and cystic fibrosis: selecting patients for controlled trials of neutrophil elastase inhibition. ERJ Open Res. 2019 doi: 10.1183/23120541.00252-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oriano M, Gramegna A, Terranova L, et al. Sputum neutrophil elastase associates with microbiota and Pseudomonas aeruginosa in bronchiectasis. Eur Respir J. 2020 doi: 10.1183/13993003.00769-2020. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 20.Bedi P, Ziegler K, Whitfield PD, et al. Dysregulation of prostaglandins, leukotrienes and lipoxin A 4 in bronchiectasis. Thorax. 2021 doi: 10.1136/thoraxjnl-2020-216475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder PC. Eicosanoids. Essays Biochem. 2020 doi: 10.1042/EBC20190083. [DOI] [PubMed] [Google Scholar]
- 22.Romano M, Cianci E, Simiele F, et al. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.03.083. [DOI] [PubMed] [Google Scholar]
- 23.Granström E, Samuelsson B. Quantitative measurement of prostaglandins and thromboxanes: general considerations. Adv Prostaglandin Thromboxane Res. 1978;5:1–13. [PubMed] [Google Scholar]
- 24.Samuelsson B, Goldyne M, Granstrom E, et al. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978 doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- 25.Hansson G, Lindgren JA, Dahlén SE, et al. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- 26.Said SI. Pulmonary metabolism of prostaglandins and vasoactive peptides. Annu Rev Physiol. 1982 doi: 10.1146/annurev.ph.44.030182.001353. [DOI] [PubMed] [Google Scholar]
- 27.Catella F, Nowak J, Fitzgerald GA. Measurement of renal and non-renal eicosanoid synthesis. Am J Med. 1986 doi: 10.1016/0002-9343(86)90905-8. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch N. Urinary Leukotriene E4. Immunol Allergy Clin North Am. 2007 doi: 10.1016/j.iac.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Song WL, Wang M, Ricciotti E, et al. Tetranor PGDM, an abundant urinary metabolite reflects biosynthesis of prostaglandin D2 in mice and humans. J Biol Chem. 2008 doi: 10.1074/jbc.M706839200. [DOI] [PubMed] [Google Scholar]
- 30.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017 doi: 10.1016/j.mam.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014 doi: 10.1111/imm.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.