Graphical abstract
Keywords: Remdesivir, Apixaban, Synchronous fluorescence, Green chemistry
Abstract
Remdesivir and apixaban have been included in the treatment guidelines of several countries for severe COVID-19 infections. To date, no analytical method has been developed for the determination of remdesivir and apixaban in plasma matrix. The main objective of this work was to develop a highly sensitive, green-adapted spectrofluorometric method for the determination of remdesivir and apixaban at the Nanoscale. Remdesivir and apixaban showed overlapping fluorescence emission spectra at 403 nm and 456 nm when excited at 246 nm and 285 nm, respectively. This overlap was resolved in two steps. The first step was synchronous fluorescence scanning of remdesivir and apixaban, and the second step was manipulation of the second-order derivative for the obtained spectra. These steps allowed complete resolution of the overlapping fluorescence spectra and selective determination of remdesivir and apixaban at 410 and 469 nm, respectively. The variables affecting the synchronous scanning of the aforementioned drugs were optimized in terms of sensitivity parameters and principles of green analytical chemistry. The described method allowed sensitive determination of remdesivir and apixaban over the concentration range of 5–200 ng/mL and 50–3000 ng/mL, respectively. The described method was validated and successfully applied for the simultaneous determination of the mentioned drugs in pure form and in spiked human plasma.
1. Introduction
The development of quantitative analytical methods with more sensitive detection limits and selective targeting for compounds of interest is a major goal for analytical chemists. In particular, the determination of drugs down to the Nano- gram range represents a potential approach for determination of drugs in biological fluids [1], [2], [3]. In addition, analytical chemists have recently devoted themselves to making analytical methods more environmentally friendly and safer for humans by using solvents and chemicals with low environmental impact, low energy consumption, and lower waste generation [4], [5], [6], [7]. Analytical methods based on fluorescence spectroscopy provide a more sensitive tool for determining compounds of interest that exhibit unique fluorescence features [8], [9], [10]. The high sensitivity of fluorescence spectroscopy is the main advantage, as it allows the determination of compounds down to the Nano gram range. In addition, spectrofluorometry consumes less than 0.1 kWh of energy per sample and produces little waste, which draws attention to the principles of green chemistry [11]. The selectivity of spectrofluorometric methods for multi-components resolution decreases when the native fluorescence spectra of the compounds of interest exhibit strong overlap. Integration of the synchronous fluorescence scanning mode usually results in, narrow sharp spectra which solve the overlap problem. In addition, the mathematical derivation of the synchronous spectra can help in spectral resolution of multi-components in different mixtures. Previous reports have described the use of synchronous fluorescence spectroscopy scanning for the determination of drugs in mixtures in different matrices, including pharmaceutical form or plasma matrix [12], [13], [14].
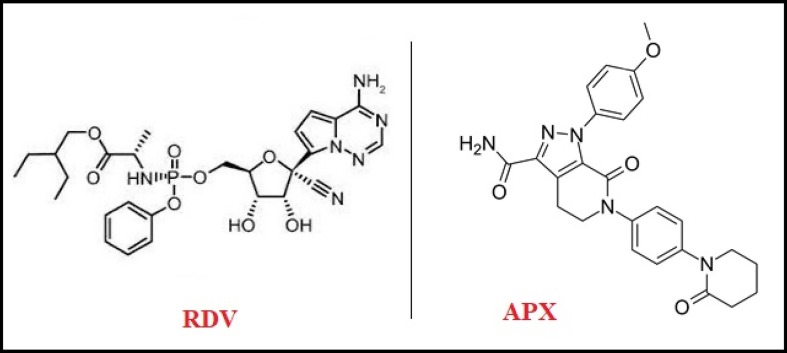
Remdesivir (RDV), Fig. 1 , is a commonly known antiviral agent that binds to viral RNA-dependent RNA polymerase. It impedes viral replication by premature termination of RNA transcription. Many reports have described the role of RRV against SARS-CoV-2 in vitro, and intravenous administration of RDV has been approved by the FDA and European emergency to be used for COVID-19 [15], [16], [17]. Several analytical methods have been developed for the determination of RDV, either alone or in combination with other drugs, in different matrices [18], [19], [20], [21], [22], [23], [24].
Fig. 1.
Structural formula of RDV and APX.
Apixaban [APX], Fig. 1, is an oral, potent, highly selective, reversible and direct inhibitor of activated coagulation factor X, which is the end point of the intrinsic and extrinsic coagulation pathways. In addition, APX has the ability to indirectly inhibit thrombin-induced platelet aggregation. Many reports have described the major role of direct oral anticoagulants such as APX in severe COVID-19 reducing the risk of venous and arterial thromboembolism and the incidence of severe thromboembolic complications [24], [25], [26], [27]. Various analytical methods have been developed for the determination of APX, either alone or in combination with other drugs, in different matrices [24], [25], [26], [27], [28], [29], [30], [31].
RDV is usually prescribed with APX for severe and hospitalized cases of COVID-19 aiming to reduce the risk of severe thromboembolic complications and death [32], [33]. To date, no analytical methods have been developed that allow determination of the drugs under study in spiked human plasma. The main objective of this work was to develop a highly sensitive, green fitted analytical method for the determination of RDV and APX in plasma matrix. To achieve sensitivity and green chemistry points, a spectrofluorometric method was developed and optimized to provide sensitive determination in the concentration range of 5–200 ng/mL for RDV and 50–3000 ng/mL for APX. The described method was based on the measurement of the second derivative of the fluorescence spectra of RDV and APX. This tool allowed selective determination of the mentioned drugs. The developed method was successful used for the simultaneous determination of RDV and APX in pure form and spiked human plasma.
2. Experimental
2.1. Materials and reagents
Pure samples of RDV and APX were provided by Pharmakeda Health Company (Egypt). A drug-free human plasma sample was provided by the National Egyptian Blood Bank and kept frozen until analysis. Remdesivir-EVA PHARMA® vial (100 mg RDV per 20 mL) and Eliquis® tablets (5 mg APX per tablet) were obtained from local pharmacy. All chemicals and solvents used throughout the analysis were of high pure analytical grade and provided by El Nasr Company (Egypt) and Sigma Aldrich (Germany). In accordance with US pharmacopoeia, several buffer solutions with varying pH ranges were prepared.
2.2. Instrumentation
A Jasco FP-6200 Spectrofluorometer was used for all spectral readings (Japan). Jasco Spectra Manager™ software was used to manipulate the measured spectra.
2.3. Standard solutions
A standard stock solution (100 µg/mL) of RDV and APX was prepared by separately transferring 10 mg of each drug powder with 60 mL ethanol into two 100-mL volumetric flasks, shaking till dissolved, and then completing the flasks to the mark with ethanol. Working solutions of the studied drugs with a concentration of 1 μg/mL were prepared from their stock solutions by further dilution with water.
2.4. Procedures
2.4.1. Development of calibration graphs for analytical procedure
Two separate serial dilution sets of RDV and APX were prepared by transferring aliquots of (0.05–2 µg) and (0.5–30 µg) of RDV and APX, respectively, from their working standard solutions into two separate sets of 10-mL volumetric flasks. The flasks were adjusted to volume with water. A blank sample was prepared under the same conditions. The solution of each flask was scanned in synchronous mode at Δλ = 150 nm. The recorded synchronous spectra were then transformed to the corresponding second-order derivative with data points = 7. The peak amplitudes of the second-derivative spectra were measured at 410 nm and 469 nm for RDV and APX, respectively. The measured values were plotted against the respective drug concentrations in ng/mL to obtain the calibration curves, and the corresponding regression equations were derived.
2.4.2. Analysis of synthetic mixtures
Five samples were prepared containing synthetic mixtures with different concentration ratios of drugs under study. The procedure mentioned under the development of calibration graphs for analytical procedures was applied and the concentration of each drug in each sample was calculated.
2.4.3. Analysis of pharmaceutical preparations
The contents of three vials of Remdesivir-EVA PHARM® (100 mg RDV per 20 mL) were well mixed. An aliquot of 10 mg RDV was transferred to a 100-mL volumetric flask, mixed well and made up to volume with water. The appropriate amounts of the sample fraction were transferred to a series of 10-mL volumetric flasks, and the amounts of analytes that fell within the calibration range were determined by adding water. Samples were analyzed according to procedure described under the development of calibration graphs for analytical procedures.
In contrast, ten Eliquis® tablets, each containing 5 mg APX, were weighed and finely crushed. The exact weight of powder corresponding to one tablet was added to a 100 mL volumetric flask containing 60 mL ethanol. The flask was shaken vigorously for 20 min before being filtered and made up to 100 mL with water. After further dilution with water, five samples with different concentrations were obtained and the process described under the development of calibration graphs for the analytical procedure was carried out.
2.4.4. Procedures for spiked human plasma matrix
Various spiked human plasma samples of RDV and APX were prepared by transferring aliquots of RDV and APX, corresponding to (0.034–2 µg) and (0.39–30 µg), respectively, from their respective working standard solutions (1 μg/mL) into two sets of centrifugation tubes containing 0.1 mL of human plasma. To these tubes, 5 mL of acetonitrile was added, followed by centrifugation at 5000 rpm for 20 min. The resulting supernatants were evaporated to dryness in a rotary evaporator. The residues obtained were dissolved in a given volume of ethanol, transferred to 10-mL volumetric flasks, and diluted to volume with water. A blank sample was prepared under the same conditions. Calibration curves were created as described under the development of calibration graphs for analytical procedures. To adopt the plasma matrix calibration curves, 3.4 ng/mL and 39 ng/mL were set as the lower limit of quantitation (LLOQ) for RDV and APX, respectively. In addition, 200 ng/mL and 3000 ng/mL were set as the upper limit of quantitation (ULQ). It was also recommended to set other quality control samples, including middle quantifiable concentration (MQC), the low quantifiable concentration (LQC), and the high quantifiable concentration (HQC). Careful trials revealed that the MQC was 100 ng/mL for RDV and 1500 ng/mL for APX, the LQC was 10 ng/mL for RDV and 100 ng/mL for APX, and the HQC was 150 ng/mL for RDV and 2300 ng/mL for APX. These samples were then determined three times to evaluate accuracy and precision. The matrix effect is efficiently evaluated by determining LOC and HQC samples six times with a plasma matrix.
3. Results and discussion
Although the common analytical methods are based on spectrophotometric [34], [35], [36] or spectrofluorometric scanning [12], [13], [14], analytical chemists have recently devoted themselves to the task of making analytical methods more environmentally friendly and safer for humans. This has been done by using solvents and chemicals that have a low impact on the environment while reducing energy consumption and waste generation. In the current study, we tried to follow the principles of green analytical chemistry by using an environmentally friendly solvent and energy efficient equipment while reducing waste generation. For this reason, we are conducting the study using a spectrofluorometer that consumes less than 0.1 kWh of energy per sample, produces little waste, and enables Nano-detection of compounds.
3.1. Spectral characteristics
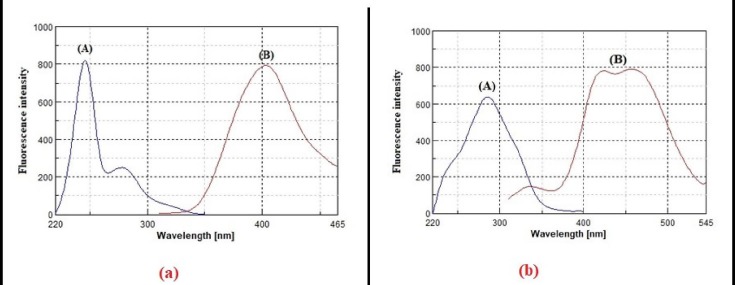
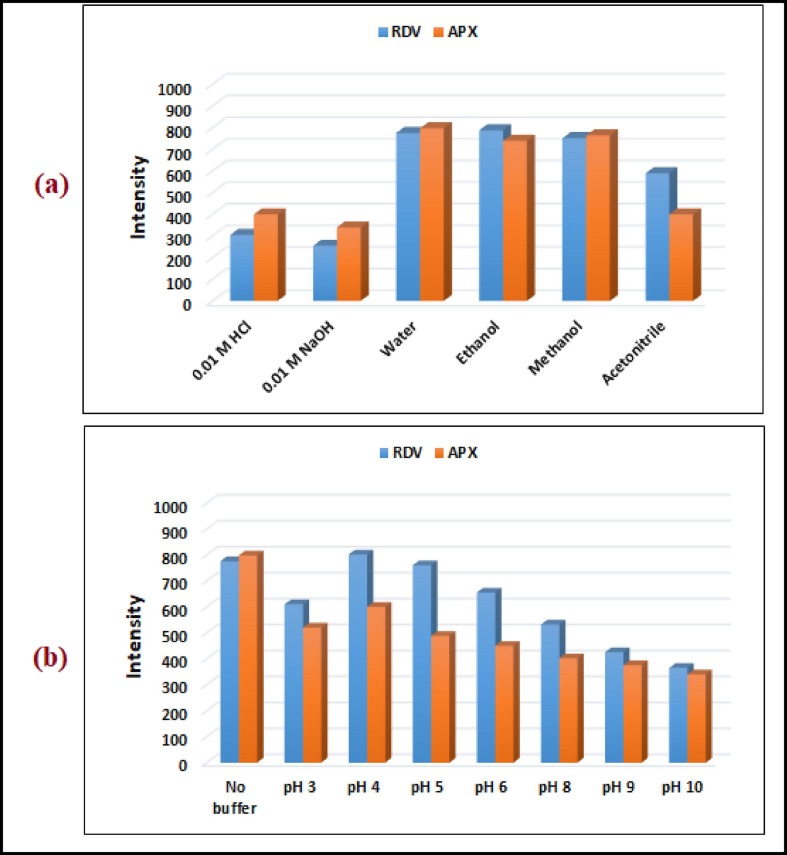
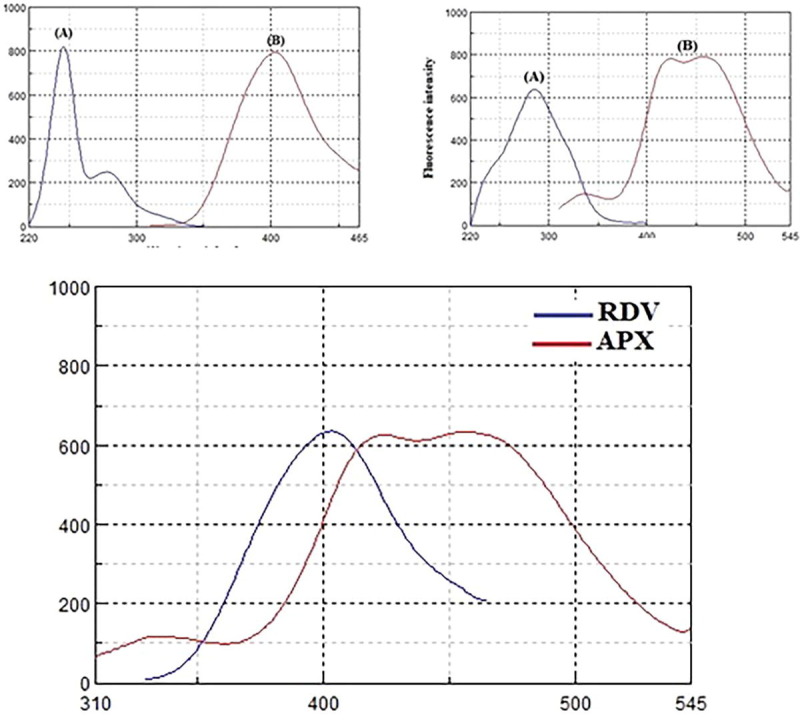
RDV and APX exhibit unique native emission spectra at 403 nm and 456 nm when excited at 246 nm and 285 nm, respectively (Fig. 2 ). It is extremely difficult to directly determine the studied drugs in synthetic mixtures or in the plasma matrix between their emission spectra overlap (Fig. 3 ). Derivatization of such spectra to higher orders also could not solve the problem of overlap. Normally, synchronous fluorescence spectrometry is a suitable method to resolve overlapping spectra in multicomponent analysis by narrowing spectral bands, increasing selectivity through spectral simplification, and shortening measurement time. The variables that affect the synchronous measurements of RDV and APX must first be optimized to achieve higher spectral resolution while maintaining green analytical chemistry standards. These variables include the selection of diluent solvent, buffer solution, and the best Δλ for the measurement. For such optimization studies, fixed concentrations of RDV and APX were used, and each variable was independently changed while the others remained constant. The synchronous spectra of RDV and APX were evaluated with water, ethanol, methanol, acetonitrile, 0.01 M HCl, and 0.01 M NaOH to investigate the effect of diluting solvents on the synchronous fluorescence intensities (Fig. 4 a). Dilution with water, ethanol, and methanol resulted in the highest intensities for both RDV and APX. In accordance with the concepts of green analytical chemistry, water was chosen as a diluting solvent because it poses no environmental risks.
Fig. 2.
Excitation(A) and emission (B) spectra of 125 ng/mL RDV (a), and 2000 ng/mL APX (b), in water.
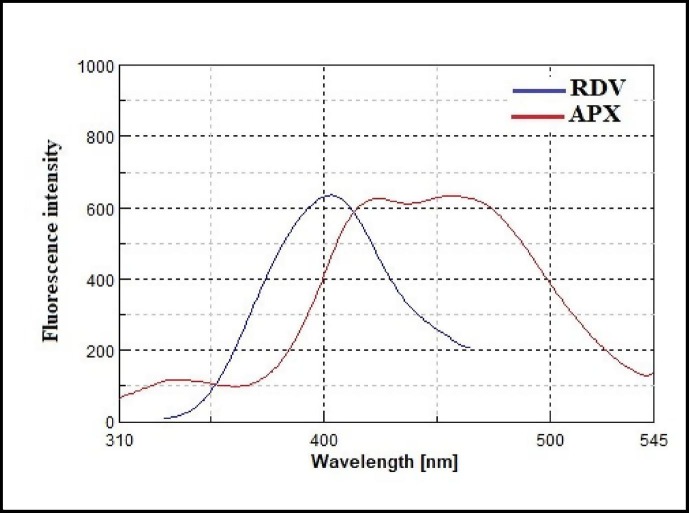
Fig. 3.
Emission spectra of RDV (125 ng/mL) and APX (2000 ng/mL) in water after excitation at 260 nm.
Fig. 4.
Optimization of experimental conditions for RDV and APX including diluting solvent(a) and buffer type (b).
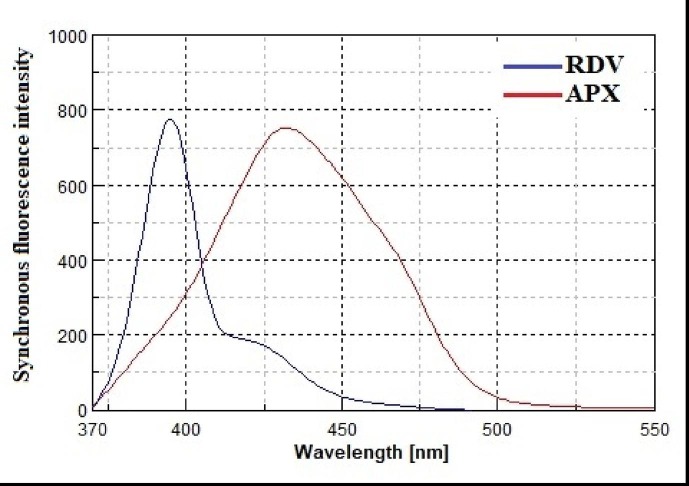
In addition, the synchronous intensities of RDV and APX were compared with and without the addition of buffer solutions with different pH values (Fig. 4b). At a pH of 4 and without the addition of buffer, RDV was shown to produce a high intensity that increased only slightly when buffer was used. However, it has been shown that APX can produce high intensity even without buffer. Therefore, the method was performed without the use of buffer solutions, which is consistent with the concepts of green analytical chemistry to minimize the use of hazardous chemicals. In addition, the synchronic spectra of RDV and APX were studied and compared over a wide range of Δλ (20–200 nm) to find the optimal Δλ that provides the highest resolution of such a combination. It was found that RDV and APX exhibited sharp, narrow synchronous peaks with acceptable sensitivity at Δλ = 150 nm, which roughly corresponds to the difference between the emission and excitation maxima of the studied drugs (Fig. 5 ).
Fig. 5.
Synchronous fluorescence spectra of RDV (125 ng/ml) and APX (2000 ng/ml) in water using Δλ = 150 nm.
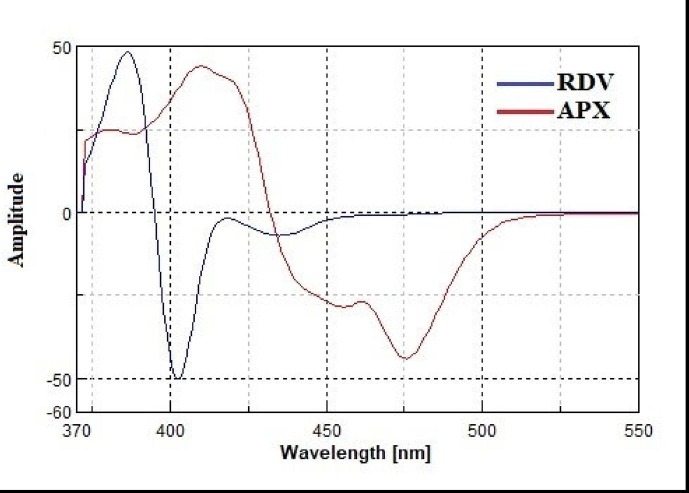
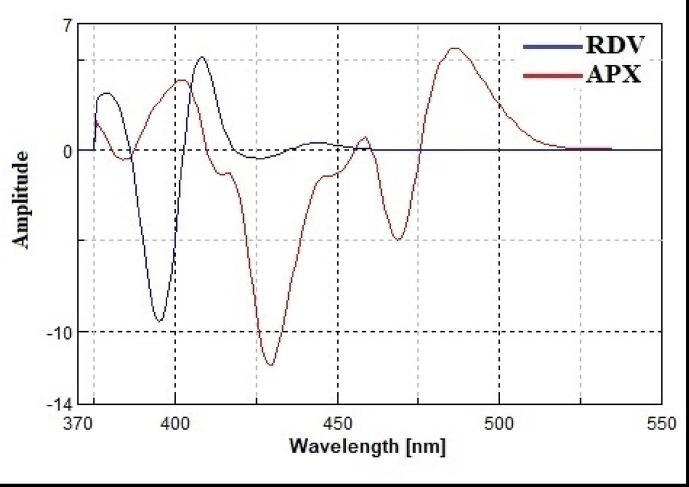
However, the problem of spectral overlap could not be completely solved. The use of a derivative technique in conjunction with a synchronous fluorescence approach allows complete resolution of the overlapping spectra. The overlapping spectra were not completely resolved by converting the synchronous spectra of the investigated drugs into first-order derivatives (Fig. 6 ). Complete resolution of the overlapping spectra was achieved by converting the synchronous spectra of the studied drugs into second-order derivatives. RDV and APX could be determined from their second-order derivative synchronous spectra at 410 and 469 nm, respectively, without interfering with each other (Fig. 7 ).
Fig. 6.
First derivative synchronous fluorescence spectra of RDV (125 ng/ml) and APX (2000 ng/ml) in water using Δλ = 150 nm.
Fig. 7.
Second derivative synchronous fluorescence spectra of RDV (125 ng/ml) and APX (2000 ng/ml) in water using Δλ = 150 nm.
3.2. Method validation
3.2.1. Validation of the analytical procedures
The method was validated in accordance with the ICH Q2 (R1) standards [37]. Linearity, range, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision, and selectivity were examined. The method used showed satisfactory linearity in the concentration ranges of 5–200 ng/mL for RDV and 50–3000 ng/mL for APX. The data from the regression equations are shown in Table 1 . The residual standard deviation (SD) of the regression line and the slope of the calibration curve were used to calculate LOD (3.3SD/slope) and LOQ (10SD/slope). The calculated LOD and LOQ values, listed in Table 1, describe the sensitivity of the method. Accuracy of the developed method, calculated as mean average percent error (%Er), was evaluated by determining three concentrations in triplicate covering the linearity range of each drug; (25, 100, 150 ng/mL) for RDV and (250, 1500, 2500 ng/mL) for APX. The precision of the method, calculated as relative standard deviation (RSD), was evaluated by determining the same concentrations in triplicate on one day for repeatability and on three consecutive days for mean precision. The results shown in Table 1 confirmed the accuracy and precision of the developed method. The described procedures showed successful applicability in terms of selectivity for the measurement of RDV and APX in their mixed laboratory solutions without mutual interference (Table 2 ) and in pharmaceutical dosage forms (Table 3 ), without interference from excipients, as confirmed by the results of the standard addition technique (Table 3). The obtained results were statistically compared with the reported methods [28] using Student's t-test and F-test. The results showed no statistically significant difference between the methods (Table 3).
Table 1.
Regression and validation data for the determination of RDV and APX by the proposed method.
| Parameters | RDV | APX |
|---|---|---|
| Wavelength (nm) | 410 | 469 |
| Linearity range (ng/mL) | 5–200 | 50–3000 |
| LOD (ng/mL) | 0.603 | 8.598 |
| LOQ (ng/mL) | 1.828 | 26.054 |
| Slope | 0.0349 | 0.0023 |
| Intercept | 0.2604 | 0.3678 |
| Coefficient of determination (r2) | 0.9992 | 0.9994 |
| Accuracy (%Er)a | −0.14 | 0.59 |
| Repeatability Precision (RSD)b | 0.771 | 0.624 |
| Intermediate precision (RSD)b | 0.925 | 0.815 |
The mean average percentage error of nine determinations (three concentrations repeated three times).
RSD of nine determinations (three concentrations repeated three times).
Table 2.
Determination of RDV and APX in synthetic mixed samples by the proposed method.
| RDV |
APX |
||||
|---|---|---|---|---|---|
| Added (ng/mL) | Founda (ng/mL) | %Recovery | Added (ng/mL) | Founda (ng/mL) | %Recovery |
| 50 | 49.79 | 99.58 | 50 | 49.43 | 98.87 |
| 100 | 98.56 | 98.56 | 50 | 49.65 | 99.30 |
| 50 | 50.28 | 100.55 | 100 | 100.09 | 100.09 |
| 100 | 98.24 | 98.24 | 100 | 101.00 | 101.00 |
| 200 | 198.53 | 99.26 | 200 | 200.52 | 100.26 |
| Mean ± RSD | 99.24 ± 0.915 | Mean ± RSD | 99.90 ± 0.836 | ||
Table 3.
Quantitative determination of RDV and APX in pharmaceutical preparations using the proposed methods, as well as the results of using the standard addition technique, with statistical comparison to the reported methods.
| Recovery ± RSD | |||||
|---|---|---|---|---|---|
| RDV | Reported method | APX | Reported method [28] | ||
| Remdesivir- vial a | 99.64 ± 0.888 | 99.82 ± 0.962 | Eliquis tablets a | 100.05 ± 0.769 | 100.11 ± 1.064 |
| Standard addition b | 101.12 ± 1.480 | Standard addition b | 100.52 ± 0.987 | ||
| t-test (2.306)c | 0.317 | t-test (2.306)c | 0.101 | ||
| F-test (6.388)c | 0.849 | F-test (6.388)c | 0.522 | ||
Average of five determinations.
Average of three determinations.
The values in parentheses are tabulated values of t and F at P = 0.05.
3.2.2. Validation of bioanalytical procedures
Validation of the developed methods was performed according to the guidelines ICH M10 [38], including linearity, lower limit of quantification (LLOQ), accuracy, precision, and matrix effect. Linearity was evaluated at seven calibration standard levels. The linearity ranges for RDV and APX were 3.4–200 ng/mL and 39–3000 ng/mL, respectively, with a coefficient of determination (r2) ≥ 0.9971 (Table 4 ). The LLOQ of RDV and APX were quantitated and calculated with acceptable precision and accuracy. As listed in Table 4, the LLOQ of RDV and APX were 3.4 ng/mL and 39 ng/mL, respectively. The accuracy of the described methods was performed by calculating the mean average percent error (%Er) of RDV and APX for four quality control samples including LLOQ, MQC, LQC, and HQC. Concentrations were measured in triplicate using the calibration plot of the spiked plasma sample and the mean percent error (%Er) was calculated (Table 4). The precision of the methods described was examined by calculating the percent relative standard deviation for triplicate determinations of LLOQ, LQC, MQC, and HQC values for each drug within one day for repeatability and for three consecutive days for intermediate precision. The small values of %RSD showed high precision of the proposed models, as shown in Table 4.
Table 4.
Bioanalytical validation parameters for quantitative analysis of RDV and APX by the proposed methods.
| Parameters | RDV | APX |
|---|---|---|
| LLOQ (ng/mL) | 3.4 | 39 |
| Linearity range (ng/mL) | 3.4–200 | 39–3000 |
| Accuracy (%Er)a | −3.88 | −4.12 |
| Repeatability precision (RSD)b | 1.266 | 1.189 |
| Intermediate precision (RSD)b | 1.889 | 1.661 |
| Matrix effect (Mean ± RSD) | 97.12 ± 1.259 | 95.07 ± 1.118 |
The mean average percentage error of triplicate determination of four quality control samples.
RSD of triplicate determination of four quality control samples.
The matrix effect is defined as the change in the determined response of the compound due to interfering components in the sample matrix. It was efficiently evaluated by analyzing LOC and HQC samples six times with a plasma matrix. The percent recovery and %RSD were calculated. The determined percent recovery was above 95 % for all samples, indicating that no unidentified compounds interfered in the sample matrix. In addition, the %RSD was less than 2, confirming the absence of a plasma matrix effect on the drugs studied and subsequent applicability in bioanalysis (see Table 4).
3.3. Greenness assessment
The evaluation of the described spectrofluorometric method in the context of environmentally friendly analytical chemistry was based on two metrics for evaluating environmental friendliness, namely the analytical eco-scale and the analytical greenness metric (AGREE). The analytical eco-scale is a semi-quantitative metric based on the concept that the ideal green analysis has a value of 100. Penalty points (PPs) are assigned for each analytical procedure parameter (amount of reagents, hazards, energy, and waste) that deviates from the ideal green analysis. Penalty points for all procedure parameters are added and subtracted from the base 100. More than 75 points indicate excellent green analysis, less than 50 points indicate inadequate green analysis, and between 50 and 75 points indicate acceptable green analysis [39]. The calculated analytical eco-score of the described spectrofluorometric method (97 points) supports the NEMI results and indicates excellent green analysis. (Table 5 ). The analytical greenness measure (AGREE) is a user-friendly software that uses the twelve-significance principle of green analytical chemistry as input criterion. Each of the twelve inputs is scored on a common scale from 0 to 1 and reflected the intuitive red-yellow-green color scale. In addition, the weight of each input criterion is assigned according to its importance in the process, which is reflected in the width of the corresponding segment. The output is a clock-like graph with the total score and the color representation in the center. The perfect analysis has a score of one and is represented by a dark green color [40], [41]. The pictogram AGREE in Table 5 indicates the highest score (0.77) for the spectrofluorometric method described, indicating an excellent green analysis.
Table 5.
Greenness assessment of the proposed method by Analytical Eco-scale and AGREE tools.
| Greenness assessment | ||
|---|---|---|
| Analytical Eco-scale | AGREE tool | |
| Parameters | PPs | |
| Reagents | 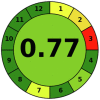 |
|
| Water | 0 | |
| Instrument (spectrofluorometer) | ||
| Energy: <0.1kWh per sample | 0 | |
| Occupational hazards | 0 | |
| Waste < 10 mL | 3 | |
| Total PPs | 3 | |
| Analytical Eco-scale score | 97 | |
4. Conclusion
Remdesivir and apixaban were prescribed concomitantly for the treatment of severe COVID-19 infections. Green fitted second derivative synchronous spectrofluorometric tool was developed for Nano level determination of remdesivir and apixaban. The method was optimized in terms of sensitivity parameters and principles of green analytical chemistry. In addition, the method allowed selective simultaneous determination of the investigated drugs in the pure form and spiked human plasma.
CRediT authorship contribution statement
Afnan S. Batubara: Methodology, Formal analysis. Ahmed H. Abdelazim: Methodology, Writing – original draft. Mohammed Gamal: Validation, Investigation. Ahmed A. Almrasy: Validation, Data curation. Sherif Ramzy: Conceptualization, Project administration, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the Deanship of scientific research at Umm Al-Qura University for supporting this work by grant code (22UQU4280448DSR02).
Data availability
Data will be made available on request.
References
- 1.Ghonim R., El-Awady M.I., Tolba M.M., Ibrahim F. Green quantitative spectrofluorometric analysis of rupatadine and montelukast at nanogram scale using direct and synchronous techniques. R. Soc. Open Sci. 2021;8:211196. doi: 10.1098/rsos.211196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gama M.R., Collins C.H., Bottoli C.B. Nano-liquid chromatography in pharmaceutical and biomedical research. J. Chromatogr. Sci. 2013;51:694–703. doi: 10.1093/chromsci/bmt023. [DOI] [PubMed] [Google Scholar]
- 3.Merone G.M., Tartaglia A., Rossi S., Santavenere F., Bassotti E., D’Ovidio C., Bonelli M., Rosato E., De Grazia U., Locatelli M. Fast Quantitative LC-MS/MS Determination of Illicit Substances in Solid and Liquid Unknown Seized Samples. Anal. Chem. 2021;93:16308–16313. doi: 10.1021/acs.analchem.1c03310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelazim A.H., Abourehab M.A., Abd Elhalim L.M., Almrasy A.A., Ramzy S. Green adherent spectrophotometric determination of molnupiravir based on computational calculations; application to a recently FDA-approved pharmaceutical dosage form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023;285:121911. doi: 10.1016/j.saa.2022.121911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelazim A.H., Abourehab M.A., Abd Elhalim L.M., Almrasy A.A., Ramzy S. Different spectrophotometric methods for simultaneous determination of lesinurad and allopurinol in the new FDA approved pharmaceutical preparation; additional greenness evaluation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023;285:121868. doi: 10.1016/j.saa.2022.121868. [DOI] [PubMed] [Google Scholar]
- 6.Ramzy S., Abdelazim A.H., Hasan M.A. Application of green first derivative synchronous spectrofluorometric method for quantitative analysis of fexofenadine hydrochloride and pseudoephedrine hydrochloride in pharmaceutical preparation and spiked human plasma. BMC Chem. 2022;16:1–11. doi: 10.1186/s13065-022-00855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelazim A.H., Ramzy S. Application of different quantitative analytical techniques for estimation of aspirin and omeprazole in pharmaceutical preparation. BMC Chem. 2022;16:1–8. doi: 10.1186/s13065-022-00854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attia K., El-Abassawi N., El-Olemy A., Abdelazim A. Second derivative spectrophotometric and synchronous spectrofluorometric determination of lesinurad in the presence of its oxidative degradation product. New J. Chem. 2018;42:995–1002. [Google Scholar]
- 9.Walash M.I., Belal F.F., El-Enany N.M., El-Maghrabey M.H. Synchronous fluorescence spectrofluorimetric method for the simultaneous determination of metoprolol and felodipine in combined pharmaceutical preparation. Chem. Cent. J. 2011;5:1–9. doi: 10.1186/1752-153X-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelwahab N.S., Abdelrahman M.M. Simultaneous determination of methocarbamol and ibuprofen by first derivative synchronous fluorescence spectroscopic method in their binary mixture and spiked human plasma. J. Fluoresc. 2014;24:129–135. doi: 10.1007/s10895-013-1276-9. [DOI] [PubMed] [Google Scholar]
- 11.Hicks M.B., Farrell W., Aurigemma C., Lehmann L., Weisel L., Nadeau K., Lee H., Moraff C., Wong M., Huang Y. Making the move towards modernized greener separations: introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 2019;21:1816–1826. [Google Scholar]
- 12.Patra D., Mishra A. Recent developments in multi-component synchronous fluorescence scan analysis. TrAC Trends Anal. Chem. 2002;21:787–798. [Google Scholar]
- 13.Attia K.A., El-Olemy A., Ramzy S., Abdelazim A.H., Hasan M.A., Abdel-Kareem R.F. Simultaneous determination of elbasvir and grazoprevir in their pharmaceutical formulation by synchronous fluorescence spectroscopy coupled to dual wavelength method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;248:119157. doi: 10.1016/j.saa.2020.119157. [DOI] [PubMed] [Google Scholar]
- 14.Attia K.A., El-Olemy A., Ramzy S., Abdelazim A.H., Hasan M.A., Mohamed T.F., Nasr Z.A., Mohamed G.F., Shahin M. Development and validation of a highly sensitive second derivative synchronous fluorescence spectroscopic method for the simultaneous determination of elbasvir and grazoprevir in pharmaceutical preparation and human plasma. New J. Chem. 2020;44:18679–18685. [Google Scholar]
- 15.Jorgensen S.C., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19, Pharmacotherapy: The Journal of Human Pharmacology and Drug. Therapy. 2020;40:659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R.E. Ferner, J.K. Aronson, Remdesivir in covid-19, in: British Medical Journal Publishing Group, 2020.
- 17.Norrie J.D. Remdesivir for COVID-19: challenges of underpowered studies. Lancet. 2020;395:1525–1527. doi: 10.1016/S0140-6736(20)31023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelazim A.H., Ramzy S. Spectrophotometric quantitative analysis of remdesivir using acid dye reagent selected by computational calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022;276:121188. doi: 10.1016/j.saa.2022.121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramzy S., Abdelazim A.H., Osman A., Hasan M.A. Spectrofluorimetric quantitative analysis of favipiravir, remdesivir and hydroxychloroquine in spiked human plasma. Spectrochim. Acta Part A: Mol. Biomol. Spectroscopy. 2022:121625. doi: 10.1016/j.saa.2022.121625. [DOI] [PubMed] [Google Scholar]
- 20.Du P., Wang G., Yang S., Li P., Liu L. Quantitative HPLC-MS/MS determination of Nuc, the active metabolite of remdesivir, and its pharmacokinetics in rat. Anal. Bioanal. Chem. 2021;413:5811–5820. doi: 10.1007/s00216-021-03561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noureldeen D.A., Boushra J.M., Lashien A.S., Hakiem A.F.A., Attia T.Z. Novel environment friendly TLC-densitometric method for the determination of anti-coronavirus drugs “Remdesivir and Favipiravir”: Green assessment with application to pharmaceutical formulations and human plasma. Microchem. J. 2022;174:107101. doi: 10.1016/j.microc.2021.107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Awady M., Elmansi H., Belal F. Insights on the Quantitative Concurrent Fluorescence-Based Analysis of Anti-COVID-19 Drugs Remdesivir and Favipiravir. J. Fluoresc. 2022;32:1941–1948. doi: 10.1007/s10895-022-02998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim A.E., Deeb S.E., Abdelhalim E.M., Al-Harrasi A., Sayed R.A. Green Stability Indicating Organic Solvent-Free HPLC Determination of Remdesivir in Substances and Pharmaceutical Dosage Forms. Separations. 2021;8:243. [Google Scholar]
- 24.Pasupuleti R.R., Tsai P.-C., Ponnusamy V.K., Pugazhendhi A. Rapid determination of remdesivir (SARS-CoV-2 drug) in human plasma for therapeutic drug monitoring in COVID-19-Patients. Process Biochem. 2021;102:150–156. doi: 10.1016/j.procbio.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder S., Graff J. Novel oral anticoagulants: clinical pharmacology, indications and practical considerations. Eur. J. Clin. Pharmacol. 2013;69:1617–1633. doi: 10.1007/s00228-013-1510-z. [DOI] [PubMed] [Google Scholar]
- 26.Frost C., Nepal S., Wang J., Schuster A., Byon W., Boyd R.A., Yu Z., Shenker A., Barrett Y.C., Mosqueda-Garcia R. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor X a inhibitor, in healthy subjects. Br. J. Clin. Pharmacol. 2013;76:776–786. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masotti L., Campanini M. Pharmacology of new oral anticoagulants: mechanism of action, pharmacokinetics, pharmacodynamics. Italian J. Med. 2013;7:1–7. [Google Scholar]
- 28.Delavenne X., Mismetti P., Basset T. Rapid determination of apixaban concentration in human plasma by liquid chromatography/tandem mass spectrometry: application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2013;78:150–153. doi: 10.1016/j.jpba.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian V.B., Katari N.K., Dongala T., Jonnalagadda S.B. Stability-indicating RP-HPLC method development and validation for determination of nine impurities in apixaban tablet dosage forms. Robustness study by quality by design approach. Biomed. Chromatogr. 2020;34:e4719. doi: 10.1002/bmc.4719. [DOI] [PubMed] [Google Scholar]
- 30.Pursley J., Shen J.X., Schuster A., Dang O.T., Lehman J., Buonarati M.H., Song Y., Aubry A.-F., Arnold M.E. LC–MS/MS determination of apixaban (BMS-562247) and its major metabolite in human plasma: an application of polarity switching and monolithic HPLC column. Bioanalysis. 2014;6:2071–2082. doi: 10.4155/bio.14.66. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz E., Boonen K., Van Den Heuvel D., Van Dongen J., Schellings M., Emmen J., Van Der Graaf F., Brunsveld L., Van De Kerkhof D. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J. Thromb. Haemost. 2014;12:1636–1646. doi: 10.1111/jth.12702. [DOI] [PubMed] [Google Scholar]
- 32.Cerezo-Manchado J.J., Birlanga O.M., de Guadiana Romualdo L.G., Gil-Ortega I., Francés A.M., Iturbe-Hernandez T. Dabigatran in patients with atrial fibrillation after COVID-19 hospitalization: an update of the ANIBAL protocol. Drugs Context. 2022;11 doi: 10.7573/dic.2021-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schenker C., Hirzel C., Walti L.N., Zeerleder S.S., Andres M., Ramette A., Barbani M.T., Suter-Riniker F., Holbro A., Tritschler T. Convalescent plasma and remdesivir for protracted COVID-19 in a patient with chronic lymphocytic leukaemia: a case report of late relapse after rapid initial response. Br. J. Haematol. 2022;196:e27. doi: 10.1111/bjh.17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abourehab M., Shahin M., Sheikh R., Ellateif A., Fawzi S., Gouda A. Utilization of N-bromosuccinimide for the sensitive spectrophotometric determination of pipazethate HCl as antitussive drug in pure and dosage forms. Annales Pharmaceutiques Françaises, Elsevier. 2021:652–663. doi: 10.1016/j.pharma.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Kassem M.A., Guesmi N.E. Sensitive kinetic spectrophotometric determination of Cyclobenzaprine Hcl in pure form and pharmaceutical formulations. Analy. Chem. Lett. 2016;6:657–668. [Google Scholar]
- 36.Gouda A., Hamdi A., El Sheikh R., Abd Ellateif A., Badahdah N., Alzuhiri M., Saeed E. Development and validation of spectrophotometric methods for estimation of antimigraine drug eletriptan hydrobromide in pure form and pharmaceutical formulations. Annales Pharmaceutiques Françaises, Elsevier. 2021:395–408. doi: 10.1016/j.pharma.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Branch K. Guidelines from the international conference on harmonisation (ICH) J. Pharm. Biomed. Anal. 2005;38:798–805. doi: 10.1016/j.jpba.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 38.Guideline I.H. European Medicines Agency; Amsterdam, The Netherlands: 2019. Bioanalytical method validation M10. [Google Scholar]
- 39.Gałuszka A., Migaszewski Z.M., Konieczka P., Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012;37:61–72. [Google Scholar]
- 40.Pena-Pereira F., Wojnowski W., Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020;92:10076–10082. doi: 10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hameed A.M. An eco-friendly ultrasound-assisted deep eutectic solvent-based liquid–phase microextraction method for enrichment and quantification of nickel in environmental samples. J. Umm Al-Qura Univ. Appl. Sci. 2022:1–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.