Abstract
We studied the role of cytotoxic components (DAMPs) formed in the body of patients with COVID-19 in ensuring the long-term preservation of post-COVID-19 manifestations and the possibility of creating an experimental model by transferring DAMPs to rats. In patients with post-COVID-19 syndrome (PCS) 2 months after SARS-CoV-2 infection we determined the presence of cytotoxic components in the blood serum (Terasaki test, Dunaliella viridis test and content of DAMPs). In post-COVID-19 syndrome patients with a high content of serum cytotoxic oligopeptide fraction (selective group, n = 16) we determined the number of leukocytes, lymphocytes, neutrophil granulocytes and monocytes in the blood, the content of C-reactive protein (CRP), the concentration of C3 and C4 complement components and circulating immune complexes, the serum content of IL-6, IL −10, IL-18, TNF-α, phagocytic activity of neutrophils, presence of neutrophil traps and autoantibodies ANA.
It has been shown that in patients with PCS, there are components with cytotoxicity in the blood serum, form specific immunopathological patterns, which are characterized by: an increased content of CRP, complement system components C3 and C4 and cytokines (TNF-α, IL-6, IL-10, IL-18) activation, the formation of a wide range of autoantibodies ANA, the low efficiency of endocytosis in oxygen-independent phagocytosis; their phagocytic activity reaches its functional limit, and against this background, activation of neutrophil traps occurs, which can contribute to further induction of DAMPs. This self-sustaining cell-killing activation provided long-term preservation of PCS symptoms.
The transfer of blood serum components from selective group patients with PCS to rats was accompanied by the appearance of cytotoxic components in them which induced sensitization and immunopathological reactions. Preventive administration of a biologically active substance with polyfunctional properties MF to experimental animals “corrected” the initial functional state of the body's immune-metabolic system and eliminated or facilitated immuno-inflammatory reactions.
Keywords: post-COVID-19 syndrome, Immune-metabolic state, Cytotoxic components, Phagocytosis, The complement system, Autoantibodies
1. Introduction
The COVID-19 pandemic has become a real challenge to the medical community. Along with the problem of treating patients in critical conditions caused by SARS-CoV-2, the pandemic COVID-19 has exacerbated some environmental, social and biomedical problems. Specialists encounter special difficulties during emergency surgical care to patients infected with the virus in the acute and long-term period (Djaharuddin et al., 2021; Di Martino et al., 2020). It is obvious that COVID-19 cannot be eliminated without a deep understanding of the immune-metabolic disorders mechanisms formation and knowledge of the individual characteristics of the pathological processes manifestation the post-COVID-19 syndrome (PCS) course.
The study of the pathogenic mechanisms of the PCS symptoms development is of current interest. The formation and manifestations of this syndrome are an important part of the solving diagnosing and treating problem of COVID-19. An effective and relatively quick decision of these complex medical and biological problems is possible only on the basis of fundamental knowledge of the body's responses characteristics to infectious agents. This requires not only clinical, but also experimental studies with adequate laboratory models. Available data indicate that prolonged post-COVID-19 syndrome (PCS) occurs in more than 80% patients who have recovered from COVID-19 (Rosales-Castillo et al., 2020, Da Rosa Mesquita et al., 2021, Boix and Merino, 2022). The relevance of studying the mechanisms of the PCS development also consists in the fact that this will contribute to understanding the fundamental mechanisms of the organism adaptation to numerous biotic environmental factors.
As is known, the first stage of SARS-CoV-2 infection of the body is associated with the presence of pathogen-associated molecular patterns (PAMPs). PAMPs have immunogenic properties and are capable of triggering a cascade of immunological and metabolic changes in different body systems (Naqvi et al., 2022). In addition of the PAMPs' impact to the body in coronavirus infection, a large number of molecular cytotoxic patterns (damage-associated molecular patterns – DAMPs) are formed in the body (Parthasarathy et al., 2022). There is the activation of barrier innate immunity factors and a cytokine storm in some patients in the acute period of SARS-CoV-2. This leads to the formation of a multiple organ dysfunction syndrome (MODS) in most cases (Gupta et al., 2020, Sungnak et al., 2020, Nalbandian et al., 2021, Huang et al., 2021), which is characterized by the induction of various proteolytic enzymes in the immune response hyperactivation (Gioia et al., 2020). Activation of proteolytic enzymes in a viral infection is also accompanied by the formation of a wide range of cytotoxic compounds DAMPs.
There is data that proteases and DAMPs with the participation of viral proteins can cause the vascular endothelium inflammation, pronounced lymphocytic infiltration with the participation of complement proteins and the formation of a membrane attack complex (MAC) (Risitano et al., 2020).
Unfortunately, the study of the role of cytotoxic serum components DAMPs formed in COVID-19 and in the formation of PCS has not yet been given due attention (Land, 2021, Parthasarathy et al., 2022). With that, the formation of various cytotoxic compounds in infected patients may play a key role in the development and maintenance of PCS symptoms and the formation of comorbidities. To solve this problem, we took a working hypothesis, according to which, in patients with PCS, the resulting DAMPs can persist for a long time due to the launch of the sensitization induction process. As a result, specific metabolic patterns are formed that are associated with the manifestation of post-COVID-19 syndrome. Such conditions can be imprinted (remembered) in patients with PCS, and also because they lead to the formation of chronic inflammatory reactions.
If so, then can be expected that: 1 – cytotoxic components in SARS-CoV-2 viral infection, formed in the body will affect the functional activity of various systems, i.e. they are polyfunctional; 2 – even at low doses, DAMPs can induce a cascade of metabolic changes and are capable to auto-amplification of pathogenic effects; 3 – cytotoxic components do not have species and tissue specificity, i.e. if they are administered into experimental animals, then they may show new properties characteristic of PCS; 4 – biological substances with antioxidant properties and are a kind of “competitors” cytotoxic DAMPs, can accelerate the detoxification processes in the body through the mechanism of hormesis, thereby reducing or changing the symptoms and immune-metabolic disorders in PCS-patients.
Previously, it was shown that a biologically active substance (Mix factor, “MF”), which is a complex of low molecular weight proteins, polysaccharides and vitamins, obtained from Pleurotus ostreatus and Saccharomyces cerevisiae, has a pronounced antioxidant, detoxifying activity and affects antigen-presenting phagocytic cells (Kurguzova et al., 2015, Bozhkov et al., 2017a, Bozhkov et al., 2017b, Bozhkov et al., 2021). It can be assumed the administration of this substance to experimental animals will change the immune-metabolic response to cytotoxic components. If this is so, then this may serve as a confirmation of the stated hypothesis.
In patients operated in the acute period SARS-CoV-2 infection for urgent abdominal pathologies, a number of post-COVID-19 symptoms were revealed two months later COVID-19. To determine the role of cytotoxic components in the PCS development their innate barrier functions of immunity (leukogram, cytokines, complement components, phagocytosis, and the degree of serum cytotoxicity degree) were determined. Along with this, an attempt was made to develop an experimental model on rats. The animals were injected with blood serum, which was obtained from patients with PKS (selective group with the maximum increase in the oligopeptide fraction λ = 238 nm), and in such animals, changes in immunological and some physiological parameters were determined. In addition, the influence of the biological substance “MF” on the severity of immunological parameters after the administration of serum components from patients with PCS was studied.
For this, in PCS-patients, and such pathologies as coagulopathy, endotheliitis, pancreatitis, we determined: the number of immunocompetent cells in the blood, the concentration of cytokines, C3 and C4 complement components and circulating immune complexes, the activity of oxygen-independent and oxygen-dependent phagocytosis, the lymphocytotoxicity degree in the Terasaki’ test and the integral cytotoxicity in the cell test with Dunaliella viridis microalgae. The most significant immunological parameters associated with PCS were estimated in experimental animals after serum administration of PCS-patients, and after preventive administration of “MF”.
2. Materials and methods
2.1. Justification of the experimental approach
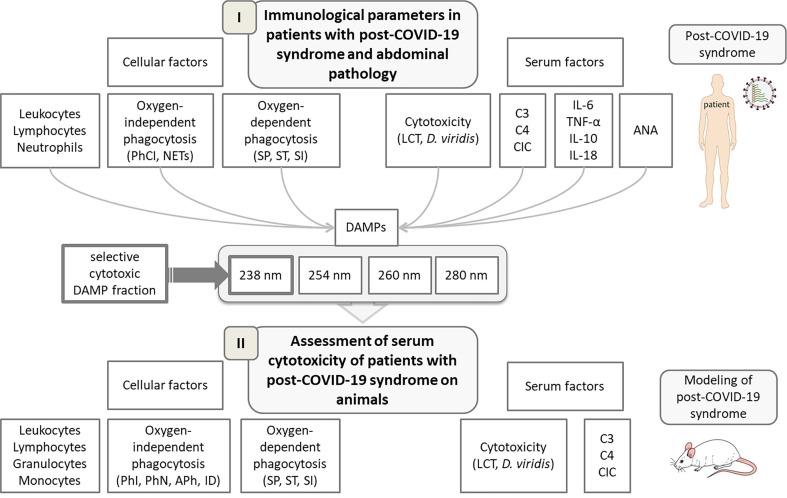
The study consisted of two stages: clinical and experimental (Fig. 1 ). At the first clinical stage of the study, some immunological parameters were evaluated in patients were treated at the State Institution “Zaycev V. T. Institute of general and urgent surgery of National academy of Medical sciences of Ukraine” with COVID-19-associated surgical abdominal pathology (pancreatitis, pancreatic necrosis, cholangitis, acute gastrointestinal bleeding) and were being identified SARS-CoV-2. Some hematological parameters, cellular and serum characteristics of the immune system were determined in these patients two months later (Fig. 1).
Fig. 1.
Scheme of the study design.
In the sample of examined patients (n = 16) with post-COVID-19 syndrome, there was a corresponding distribution by age and sex: 16 patients (10 men, 48–64 years old; 6 women, 52–78 years old). Examined patients with abdominal surgical pathology (pancreatitis, pancreatic necrosis, cholangitis, portal hypertension complicated by acute gastrointestinal bleeding) were treated in accordance with approved anesthesia protocols, local surgical protocols for emergency surgical care and rehabilitation protocols (personalized rehabilitation taking into account metabolic disorders, such as the presence of a respiratory burst, mitochondrial dysfunction, impaired hemostasis and the presence of enzymopathies). The diagnosis of COVID-19 was established by the presence of specific SARS-CoV-2 IgA and IgM antibodies in acute period.
These patients were previously treated at the State Institution “Zaycev V. T. Institute of general and urgent surgery of National academy of Medical sciences of Ukraine”. In patients with abdominal pathology, a leukogram, immunological parameters, and the presence of IgG antibodies specific to SARS-CoV-2 were determined in the long period after a viral infection (two months later).
For reference or standard values, we took the average values of the studied parameters obtained in our laboratory for a long time (at least a year) in healthy individuals (n = 97). And in the experiment on animals, the corresponding control groups were used.
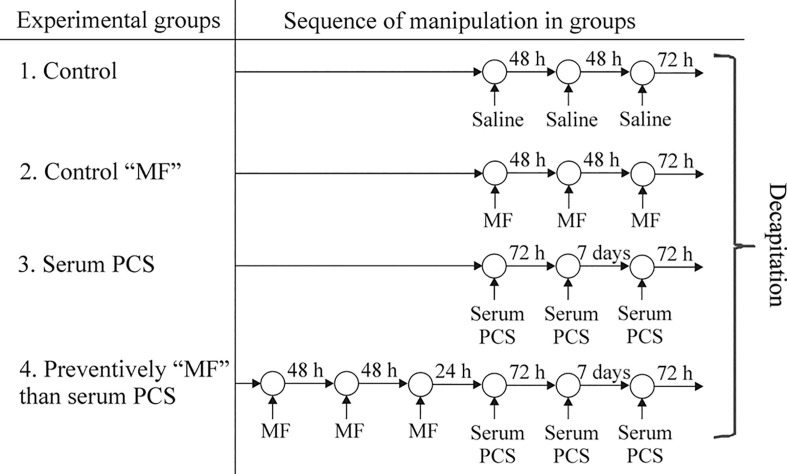
At the second experimental stage, Wistar rats were immunized with the blood serum of PCS-patients, whose some hematological parameters, cellular and serum characteristics were determined (Fig. 1). Animals were divided into four groups (Fig. 2 ). Animals of the control group were injected with saline volume 0.1 ml (group 1). To study the effect of a biologically active substance on the studied parameters, the second group (group 2) of animals was administered “MF” at a dose 0.05 ml/100 g of body weight according to the scheme (Fig. 2). The third group (group 3) of animals was injected subcutaneously at 0.1 ml/100 g of body weight serum PCS according to the scheme (Fig. 2). The fourth group (group 4) received preventive per os “MF” at a dose 0.05 ml/100 g of body weight, and 24 h after the last administration, they were injected with PCS serum according to the scheme (Fig. 2). There were 5 adult males, 3–4 months old age in each experimental group.
Fig. 2.
Scheme shows the time sequence of administration (arrows) in four groups’ experimental animals. The determination of the studied parameters was carried out in all groups simultaneously. Such a scheme of manipulation with animals made it possible to exclude the influence of circadian rhythms on the examined parameters.
All animals were kept under standard vivarium conditions and had free access to food and water. Experiments with animals were carried out in agreement with the bioethical committee of V.N. Karazin Kharkiv National University (protocol of the bioethical commission of Kharkiv University No. 3 of September 5, 2021), which is guided by the provisions of the “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes” (Strasbourg, March 18, 1986).
2.2. Quantitative analysis of immunocompetent cells
The absolute content of leukocytes, lymphocytes, monocytes, granulocytes and neutrophils was determined by cytometry. Blood was collected in tubes with K3 EDTA (3-substituted potassium salt of ethylenediaminetetraacetic acid) for further studies of hematological parameters on an automatic analyzer Mindray BC-2800 Vet (USA).
2.3. Activity of oxygen-independent phagocytosis
Oxygen-independent phagocytosis was determined by the degree of absorption and adhesion of antigens (Saccharomyces cerevisiae) by neutrophils; the effectiveness of phagocytosis was assessed by the digesting ability of neutrophils – the phagocytosis completion index (PhCI) (Muniz-Junqueira et al., 2003). We determined the phagocytic index (PhI) – the number of neutrophils involved in phagocytosis (as a percentage of the total number of neutrophils) and the phagocytic number (PhN) – the average number of S. cerevisiae cells absorbed by one neutrophil (in conventional units). Satellite sample endocytosis was assessed after 120 min. The phagocytosis completion index was calculated in arbitrary units by the ratio of PhN after 30 min to PhN after 120 min. In this method we used light microscopy (Olympus BX53, Japan).
To visualize the process in animals, we determined oxygen-independent phagocytosis by fluorescence microscopy (Olympus BX53, Japan) with acridine orange (AO), taking into account the intensity of absorption by neutrophils of intact S. cerevisiae cells with green-stained undenatured DNA. Green luminescence at AO staining upon complete digestion (after 45 min) of the absorbed S. cerevisiae yeast cells gradually changed from yellow to red upon DNA denaturation of the antigen cells during their destruction. Normally, the digestion process ended with complete lysis of the yeast. In undigested yeast cells, the luminescence does not change color from green to red. The number of absorbed S. cerevisiae cells with a changed luminescence spectrum from green (λmax = 530 nm) to red (λmax = 640 nm) was counted relative to undigested yeast cells that retained green luminescence. We determined: phagocytic index (PhI, %); phagocytic number (PhN, conventional units); number of active phagocytes (APh, %) and digestion index (DI, %) (Gorchakov et al., 2003).
2.4. Determination of neutrophil traps (NETs)
The proportion of transformed neutrophils was determined by fluorescence microscopy (Olympus BX53, Japan) in the form of neutrophil traps with chromatin released from cells, forming a network for antigen capture (Dicker et al., 2018).
2.5. Activity of oxygen-dependent phagocytosis
Oxygen-dependent phagocytosis was assessed by the intensity of spontaneous or induced activation of neutrophils NADP-N-oxidase reactions in the test for the reduction of the soluble dye nitroblue tetrazolium (NBT) absorbed by the phagocyte into insoluble diformazan under the influence of the resulting superoxide anion using light microscopy (Olympus BX53, Japan) (Segal and Levi, 1973). The number of blue-violet diformazan granules in the neutrophil corresponded to the localization of NADP-H-oxidase, and their size corresponded to the total activity of this enzyme and the formation of ROS (reactive oxygen species). We determined the number of cells containing diformazan granules. The number of diformazan granules was used to assess the activity of spontaneous (SP, up to 10%) and induced (ST, up to 70%) oxygen-dependent phagocytosis. When granules covered 50% or more of the cytoplasm area, the reaction was assessed as positive. The stimulation index (SI) was calculated using the formula: SI = ST/SP.
2.6. The content of C3 and C4 complement components
The content of C3 and C4 complement components was measured by immunoturbidimetry on a biochemical analyzer Stat-Fax 1900 (USA) using Dialab kits (Austria). The interaction of the protein with specific antibodies led to the formation of immunocomplexes. Their precipitation contributed to an increase in the turbidity of the solution. The measurements were carried out at λ = 340 nm wavelengths, the result was proportional to the concentration of complement components C3 and C4 in the sample, which was quantified in g/l (Nilsson and Nilsson, 2012).
2.7. The content of C-reactive protein (CRP)
The content of the CRP in blood serum was evaluated by agglutination in a latex-test. Quantitative determination was performed by multiple dilutions of blood serum and repeated agglutination reactions (Human kits, EU) (Alhabbab, 2018).
2.8. The concentration of circulating immune complexes (CIC)
The concentration of CIC was measured by spectrophotometry (Shimadzu UV-2600, Japan) as a result of the interaction of antigens, antibodies and proteins of the complement system after their precipitation in polyethylene glycol 6000 at room temperature. The optical density was measured at a 450 nm wavelength against borate buffer (Riha et al., 1979).
2.9. Serum levels of cytokines (IL-6, IL-10, IL-18, TNF-α)
The content of IL-6, IL-10, IL-18, and TNF-α in blood serum was evaluated using a test system (RayBiotech kits, USA) for ELISA with monoclonal antibodies adsorbed on polystyrene plates. The antigen–antibody complex was detected using a conjugate, the peroxidase of which catalyzes the cleavage of the substrate (hydrogen peroxide) and causes a change in the color of the indicator (de La Rica and Stevens, 2012). The optical density was measured at a 450 nm wavelength on a Stat-Fax 3200 (USA).
2.10. The content of blood serum cytotoxic components
The evaluation of molecular patterns associated with damage (DAMPs) was determined by spectrophotometry (Shimadzu UV-2600, Japan) at different wavelengths, which reflected the concentration of various fractions: λ = 238 nm – oligopeptides, λ = 254 nm – peptides, λ = 260 nm – nucleotides, λ = 280 nm – aromatic amino acids. The content of DAMPs fractions was expressed in optical units obtained after precipitation with a 10% solution of trichloroacetic acid (Shitov, 2013).
2.11. Blood serum components cytotoxicity (Terasaki test)
Determination of the blood serum components cytotoxicity was carried out in the in vitro system by the degree of autolymphocyte cell membranes degradation after the action of autoserum in the presence of exogenous complement. A suspension of lymphocytes was isolated in a ficol-verografin density gradient (density 1.077) and incubated with autoserum at 37 degreesC for 30 min. In stained preparations, the ratio of living intact cells and dead cells with a destroyed membrane was calculated using light microscopy (Olympus BX53, Japan) (Terasaki and Mcclelland, 1964).
2.12. Determination of the integral cytotoxicity of blood serum components using a single-cell bioindicator Dunaliella viridis
The totality of cytotoxic components of the DAMPs serum of patients was determined by light microscopy (Olympus BX53, Japan) using the D. viridis bioindicator according to the degree of impact on the viability (motility, the presence of a flagellum, aggregates, exometabolite release) of bioindicator cells. The integral coefficient of cytotoxicity (Cc) was calculated, taking into account all changes in the cells of the test system (shape, mobility, formation of micro- and macroaggregates, release of exometabolite) under the influence of various cytotoxic factors (Klimova et al., 2016).
2.13. The presence of ANA autoantibodies
Serum specific antinuclear antibodies (ANA) were detected by indirect immunofluorescent analysis (Olympus BX53, Japan) using monoclonal antibodies (MAbs) labeled with fluorescein (FITC) and glasses with biochip reaction zones, the surface of which is coated with a substrate: HEp-2 and primate liver sections (EUROIMMUN Reagent Kit, Germany). Serum of patients was applied to glasses with biochips and incubated. Specific antibodies of the IgA, IgG and IgM classes present in the positive samples bound to the chip antigens. Then, bound antibodies were detected by fluorescent staining after incubation of slides with FITC-labeled antibodies to the corresponding human immunoglobulins, antinuclear antibodies. Luminescence specificity was evaluated at x400 magnification.
2.14. Statistical analysis
Data are presented as mean (x) and error of the mean (SE). Significant differences between groups were determined using the nonparametric Mann-Whitney test (U test). Differences between the control and experimental groups were taken at the significance level of p < 0.05. Statistical analysis was performed using the STATISTICA 10 program (Statsoft, USA).
3. Results
3.1. Hematological and immunological parameters in patients with post-COVID-19 syndrome and abdominal pathology
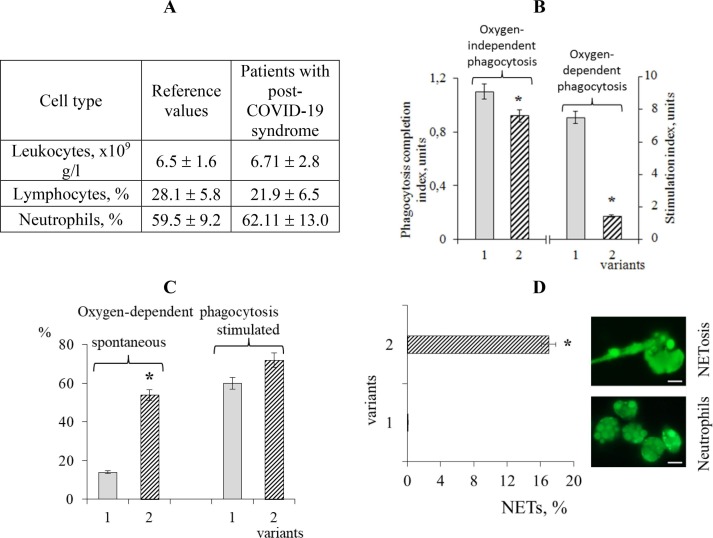
The content of leukocytes and neutrophils in patients with post-COVID-19 syndrome (PCS) two months after the acute period did not differ from the reference values (Fig. 3 A). Along with this, the number of lymphocytes was below the reference values (Fig. 3A). The efficiency of neutrophils in oxygen-independent phagocytosis was low, which is associated with a decrease in their endocytosis completion index by 20% compared with the reference values (Fig. 3 B-2).
Fig. 3.
Cellular immunopathological patterns: A – The number of leukocytes, lymphocytes and neutrophils in patients with post-COVID-19 syndrome; B – phagocytosis completion index and phagocytosis stimulation index by zymosan for the reference group (1) and the group of patients with post-COVID-19 syndrome (2); C – spontaneous and stimulated level of oxygen-dependent phagocytosis for the reference group (1) and the group of patients with post-COVID-19 syndrome (2); D – the number of neutrophil traps (NETs) in healthy donors (1) and NETs formation in patients with post-COVID-19 syndrome (2). Scale bar 10 μm. Average values for 16 patients and standard errors (x ± SE) are presented; * – P < 0.05 compared with the reference values (U test). As reference values we taken the average values of the studied parameters for healthy individuals (n = 97), obtained over a long period (at least a year) in our laboratory.
The spontaneous level in oxygen-dependent phagocytosis is an indicator of the ROS formation in the NADP-H reaction without zymosan stimulation, and in patients with PCS this indicator was increased by 3.7 times compared with the reference values (Fig. 3 C). The addition of zymosan to the suspension of phagocytic cells did not lead to further stimulation of phagocytosis; it remained almost at a spontaneous level (Fig. 3 C). The low level of the stimulation index (SI) in oxygen-dependent phagocytosis (1.44 ± 0.45) compared with the reference values (7.5 ± 1.87) also indicates the absence of the possibility of phagocytosis additional stimulation in PCS-patients (Fig. 3B).
A high incidence of neutrophil traps (NETs), which are absent in normal healthy donors, was found in patients with PCS (Fig. 3D). High frequency of NETs formation can lead to additional production of cytotoxic compounds – DAMPs (Papayannopoulos, 2018).
The observed features of changes in phagocytosis parameters in patients with PCS may be associated with the formation of new specific humoral patterns with lysis ability.
It was found that in patients with PCS, the content of C-reactive protein (Fig. 4 A), which is involved in the induction of a prolonged inflammatory process (Dhingra et al., 2007), was increased by 8 times compared to the reference values. In these patients, there are also increased the content of complement system components, which are active proteases (Forneris et al., 2012). Thus, the content of the complement component C3 was increased by 2 times and the concentration of the complement component C4 was increased by 1.5 times relative to the reference values (Fig. 4 A).
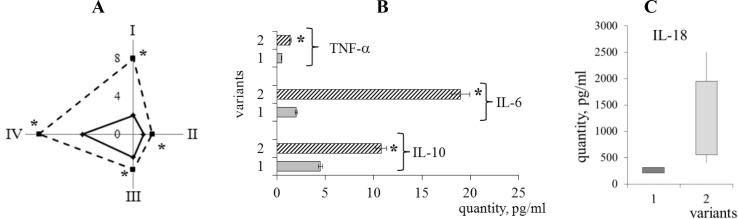
Fig. 4.
Humoral immunopathological patterns: A – content of C-reactive protein (I), C3 (II) and C4 (III) component of the complement system, circulating immune complexes (IV) in the reference group (—) and in patients with post-COVID-19 syndrome ( − − −); B – level of TNF-α, IL-6, IL-10 in the reference group (1) and in patients with post-COVID-19 syndrome (2); C – level of IL-18 in the reference group (1) and in patients with post-COVID-19 syndrome (2). Average values for 16 patients and standard errors (x ± SE) are presented. * – P < 0.05 compared with the reference values (U test). As reference values we taken the average values of the studied parameters for healthy individuals (n = 97), obtained over a long period (at least a year) in our laboratory.
An interesting fact is the increase in the amount of TNF-α in patients with PCS by 3 times (Fig. 4B). While in acute viral infection it did not exceed the reference values (Sancho et al., 2022). Pro-inflammatory IL-6 in patients with PCS was 15 times higher than the reference values and amounted to 30.2 pg/ml with a reference level of 2.0 pg/ml (Fig. 4C). There was a 2-fold increase in anti-inflammatory IL-10 also in these patients (Fig. 4B). The content of pro-inflammatory IL-18, which belongs to the IL-1 family, was significantly increased in comorbid conditions in patients with the abdominal pathology and post-COVID-19 syndrome in the long term (Fig. 4C). It should be noted that in the examined patients in the long term of the disease, a high variability of this cytokine from 400 to 2500 pg/ml with the reference values of 261.0 to 360.3 pg/ml was observed.
The results obtained indicate that development of all stages of chronic inflammation in patients in the long-term period after SARS-CoV-2 infection, and changes in humoral predictors of the development of inflammation may indicate the presence of a wide range of cytotoxic components in them. To assess the presence of cytotoxic components in PCS-patients, the spectral characteristics of blood serum were determined.
It was found that the absorption of the blood serum components of patients with PCS at λ = 238 nm (the oligopeptide fraction) was reduced by 23%; at λ = 254 nm (the peptide fraction) was increased by 33%; at λ = 260 nm (the nucleotide fraction) was increased by 30%, and at λ = 280 nm (the fraction of aromatic amino acids) was increased in optical density by 55% in comparison with the reference values (Table 1 ).
Table 1.
Absorption spectra of blood serum components in patients with post-COVID-19 syndrome (n = 16) those are potentially capable of exhibiting cytotoxic properties and their reference values (average values of the studied parameters for healthy individuals (n = 97), obtained over a long period (at least a year) in our laboratory); x ± SE are shown * – P < 0.05 compared with the reference values (U test).
| Wavelengths, nm (λ) | Molecular fractions | Absorption intervals of reference values of serum components | Absorption of various molecular fractions of serum components of patients with PCS, units E |
|---|---|---|---|
| 238 | Oligopeptides | 0.620 ± 0.012 | 0.502 ± 0.017* |
| 254 | Peptides | 0.240 ± 0.011 | 0.319 ± 0.078 |
| 260 | Nucleotides | 0.240 ± 0.010 | 0.312 ± 0.019* |
| 280 | Aromatic amino acids | 0.250 ± 0.018 | 0.383 ± 0.116 |
It should be noted that the observed increase in the peptide and nucleotide fractions in the blood serum of PCS patients may indicate dysfunction and structural disorders of mitochondria in them.
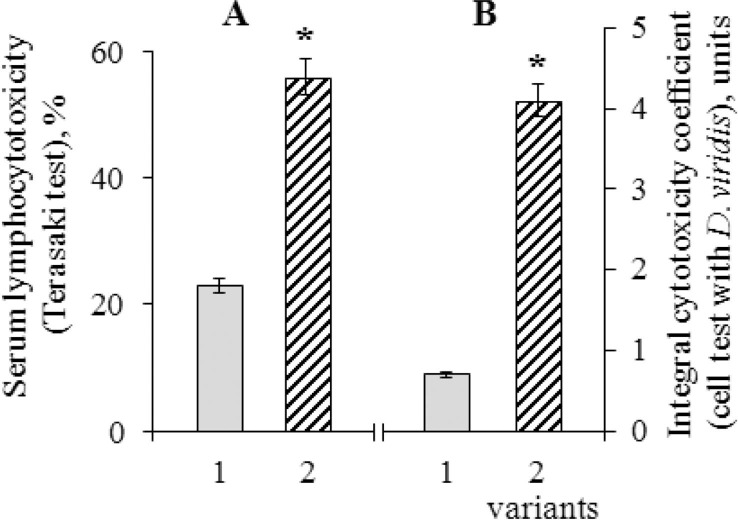
To assess the degree of cytotoxicity of blood serum components in patients with PCS the Terasaki test was used. We found that the serum components of PCS-patients have a pronounced lymphocytotoxicity in relation to the autolymphocytes membranes in the presence of exogenous complement. Thus, this indicator was increased by 2.4 times in comparison with the reference values (Fig. 5 A).
Fig. 5.
The degree of serum lymphocytotoxicity in the Terasaki test (A) and the integral cytotoxicity coefficient in the cell test with D. viridis (B) in healthy donors (1) and patients with post-COVID-19 syndrome (2). Average values for 16 patients and standard errors (x ± SE) are presented. * – P < 0.05 compared with the reference values (U test). As reference values we taken the average values of the studied parameters for healthy individuals (n = 97), obtained over a long period (at least a year) in our laboratory.
The use of a microalgae test system D. viridis as a bioindicator made it possible to reveal the integral cytotoxic effect of the serum components of patients with PCS. The integral coefficient of cytotoxicity was 4.1 at 0.7 units in healthy donors, i.e. was increased by 5.8 times after adding the blood serum of patients with PCS to the test culture of microalgae D. viridis (Fig. 5 B). This effect is evidence of the lack of species specificity for cytotoxic components formed in the PCS-patients, since microalgae are plant objects, and confirms the wide range of DAMPs toxicity contained in the blood serum.
The revealed changes in immunological parameters and the presence of cytotoxic components in patients with abdominal pathology and post-COVID-19 syndrome against the background of a chronic inflammatory process can lead to the development of autoimmune reactions, which can also aggravate the condition of such patients.
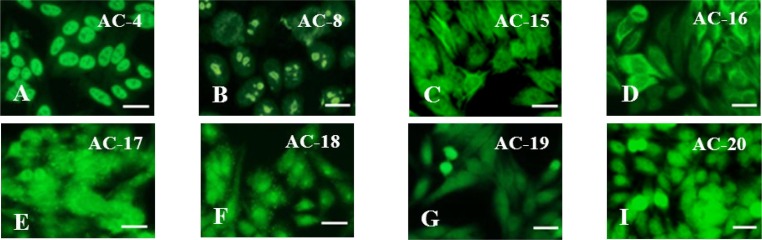
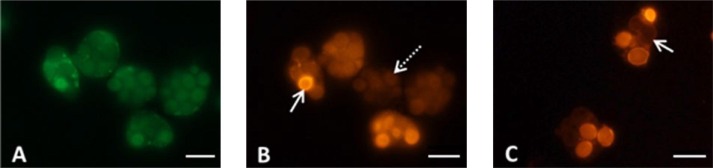
In all patients with PCS were found specific autoantibodies to various structures of cell nuclei (ANA) – to nucleoplasm components (target SS-A/Ro, SS-B/La, Mi-2), to the spliceosome complex PM/Scl-75, to F-actin, to cytoskeletal proteins (vimentin, cytokeratin, tropomyosin), to α-actin, vinculin, to lysosomes (target GW182), to PL7 (threonyl-tRNA synthetase) and PL12 (alanyl-tRNA synthetase), to Jo-1 (histidyl-tRNA synthetase) (Fig. 6 A-I).
Fig. 6.
Antinuclear antibodies (ANA) in patients with post-COVID-19 syndrome (n = 16): A (code AC-4) – to nucleoplasm (target antigens SS-A/Ro, SS-B/La, Mi-2), B (code AC-8) – to the spliceosome complex PM/Scl-75, C (code AC-15) – to F-actin, D (code AC-16) – to vimentin, cytokeratin, tropomyosin, E (code AC-17) – to α-actin, vinculin, F (code AC-18) – to lysosomes (target antigens GW182 protein), G (code AC-19) – to PL7 (threonyl-tRNA synthetase) and PL12 (alanyl-tRNA synthetase), I (code AC-20) – to Jo-1 (histidyl-tRNA-synthetase). Scale bar 20 μm. Codes AC (AC – anti-cell pattern) in accordance with the International Consensus on ANA Patterns (ICAP) are presented. FITC staining of HEp-2 standard antigenic substrates after interaction with serum antibodies; magnification × 1000.
Therefore, in patients with abdominal pathology and PCS in the long-term period after SARS-CoV-2 infection (two months later), a specific humoral pattern is formed, which also contains nonspecific cytotoxic components. These components can lead to damage of various tissues of the body and form imprinted pathogenic conditions. If this is the case, then it can be expected that the administration of the blood serum components of such patients into experimental animals will cause similar immune and physiological responses in them.
At the next stage of the work, the blood serum of patients with PCS was administered to experimental animals (Fig. 2) and significant immunophysiological parameters were determined.
3.2. Evaluation of the blood serum cytotoxicity of patients with post-COVID-19 on animals and the possibility of modifying of these serum action by preliminary administration of the biologically active substance “Mix factor” (MF) to animals
A change in some physiological characteristics was observed in animals after administration of blood serum from patients with PCS: an increase in body temperature by 1.5–2.5 degrees, loss of body weight and slight diarrhea. There are an increase in the number of leukocytes by 59%, lymphocytes by 51%, granulocytes by 76%, and monocytes by almost 2 times compared with the control group in these animals (Table 2 ). The administration of only MF substance to animals did not affect the number of leukocytes and lymphocytes, while the number of granulocytes and monocytes increased by 59% and 120%, respectively, compared to the control (Table 2). Preventive administration of MF substance to experimental animals before administration of blood serum from PCS-patients did not change the number of granulocytes, which were at the control level (group 1). And the number of other types of immunocompetent cells was at the same level as in animals that were injected only with the blood serum of PCS-patients (Table 2).
Table 2.
Hematological parameters of experimental animals: “C” – control group, “MF” – three times the administration of MF substance, “post-Covid-19″ – three times the administration of blood serum from patients with PCS, ”MF + post-Covid-19″ – three times the administration of MF substance and after that, a threefold injection of blood serum from patients with PCS. The mean values and standard errors (x ± SE) for 5 animals in each group are presented (U test).
| Index | Animal groups |
|||
|---|---|---|---|---|
| C | MF | Serum of post-COVID-19 | MF + Serum of post-COVID-19 | |
| Leukocytes, ⋅109 / l | 11.,9 ± 1.27 | 13.8 ± 0.58 | 18.9 ± 2.34* | 19.07 ± 5.22* |
| Lymphocytes, ⋅109 / l | 8.3 ± 0.78 | 7.87 ± 1.05 | 12.53 ± 1.03* | 13.47 ± 3.82* |
| Granulocytes, ⋅109 / l | 3.27 ± 0.49 | 5.2 ± 0.5 | 5.77 ± 1.19* | 4.93 ± 1.24 |
| Monocytes, ⋅109 / l | 0.33 ± 0.09 | 0.73 ± 0.28* | 0.63 ± 0.15* | 0.67 ± 0.18* |
Note: * – P < 0.05 compared with the control group (C).
Consequently, the components of the blood serum of patients with PCS induced an increase in the proliferation and differentiation of immunocompetent cells in the rats, and the preventive administration of MF had little effect on this process.
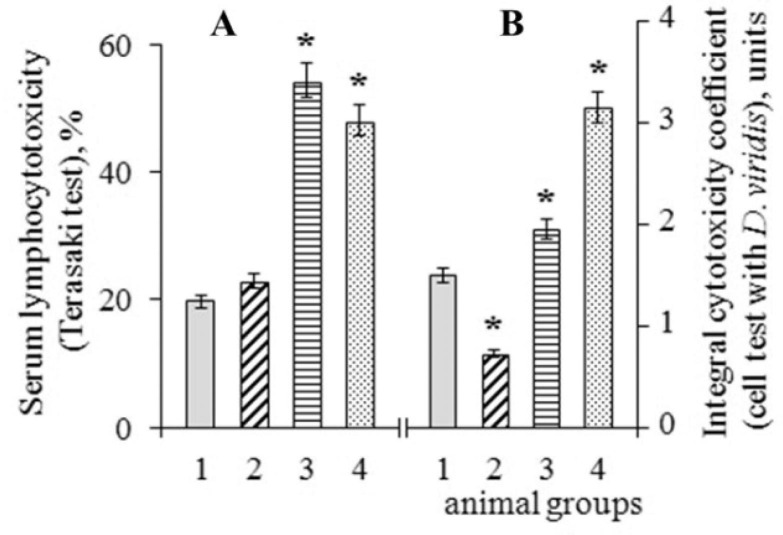
The presence of cytotoxic components in the serum of animals after immunization was assessed by the degree of destruction of the lymphocytes cell membranes via the action of autoantibodies in the complement presence in vitro (Terasaki test). There were no significant changes in the number of cells with a destroyed membrane after the administration of the MF substance to the animals per os (Fig. 7 A). At the same time, the administration of blood serum from patients with PCS to animals was accompanied by an increase in the degree of lymphocytotoxicity by 2.8 times compared with this indicator in control animals (group 1) (Fig. 7 A).
Fig. 7.
Cytotoxicity of blood serum of rats. A – The degree of lymphocytes membranes damage in the Terasaki test; B – Integral cytotoxicity coefficient (Cc) in the cell test with D. viridis. Groups of animals: 1 (C) – control group; 2 (MF) – animals that received Mix-factor per os; 3 (post-Covid-19) – animals immunized with sera from post-Covid-19 patients; 4 (MF + post-Covid-19) – animals that received Mix-factor per os before immunization. * – differences are significant with the control group (P < 0.05). The mean values and standard errors (x ± SE) for 5 animals in each group are presented (U test).
If the experimental animals (group 4) were preventively injected with the substance MF before the administration of the blood serum of PCS-patients, the degree of lymphocytotoxicity (the Terasaki test) in these animals decreased by 20% compared with the serum of animals, which were administered only serum of patients with PCS (group 3), but this indicator remained 2 times higher than the control values of group 1 (Fig. 7 A).
Since the “transfer” of toxic components was carried out from a human to a rat, it can be argued that these components do not really have species specificity, and possibly tissue specificity, and are capable of affecting various tissues and organs of the body.
To verify this, we tested the components of the serum of experimental animals that received the serum of PCS-patients on the D. viridis cell test culture. These microalgae do not have a cell wall and in this they are similar to animal cells (Masjuk, 1973), and their receptors on the cell membrane are able to perceive the entire set of blood serum components.
It turned out that the integral coefficient of cytotoxicity (Cc) after adding the blood serum of animals injected by the blood serum of patients with PCS to the D. viridis test culture was increased by 30–35% compared with the serum of control animals (Fig. 7 B, group 3).
If the rats were preventively injected with the MF substance followed by the administration of the blood serum of PCS-patients, then the blood serum of such animals had a more pronounced effect on the D. viridis cell test system compared to animals of the 3rd group that received only the blood serum of patients with PCS (Fig. 7B, group 4). This may be a consequence of the intercellular interactions activation under the influence of the MF substance due to the adhesive properties and activation of enzyme systems.
If the intact control animals were injected only with the MF substance, then the blood serum of such animals, on the contrary, had a stimulating effect on the behavior of the cell test culture, which was manifested in a decrease in Cc even compared to the control group of animals (Fig. 7 B, group 2).
Therefore: 1 – the existing cytotoxic molecular patterns that are formed in the body of patients with PCS, when they are “transferred” to the body of rats, also exhibit cytotoxicity, i.e. they are not species specific; 2 – since the cytotoxic components administered into the organism of animals are presented in very small quantities and cause a pronounced biological response, it can be assumed that they induce “self-formation”; 3 – biologically active substance MF modifies the body's response to the action of cytotoxic components of the blood serum of patients with PCS.
3.3. Influence of blood serum components of patients with PCS on the content of C3 and C4 complement components, circulating immune complexes (CIC) after their administration to experimental animals
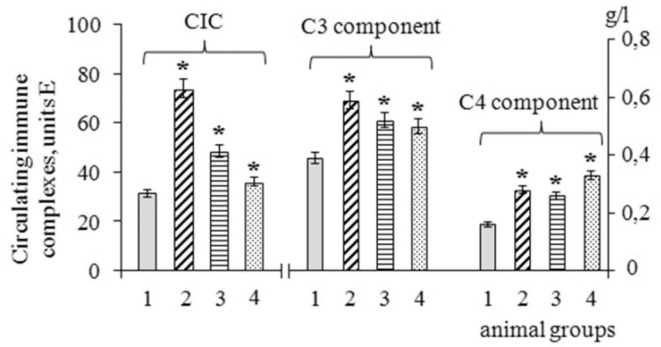
The concentration of C3 and C4 complement components in the blood serum of animals that received only MF were increased by 50% and 75%, respectively, compared with the control (Fig. 8 B, group 2). In animals that were injected with the blood serum of patients with PCS, the concentrations of C3 and C4 complement components were increased to a lesser extent than after administration of MF alone, but their content was increased compared to the control (Fig. 8 B, group 3). Preventive administration of the MF substance to animals, followed by the administration of blood serum from patients with PCS, inhibited the activation of the C3 component, and the content of the C4 component increased compared to group 3 animals that received only the blood serum of patients with PCS (Fig. 8 B, group 4).
Fig. 8.
Concentration of circulating immune complexes CIC, C3 and C4 complement components in the serum of experimental animals. Groups of animals: 1 (C) – control group; 2 (MF) – animals that received Mix-factor per os; 3 (post-Covid-19) – animals immunized with sera from post-Covid-19 patients; 4 (MF + post-Covid-19) – animals that received Mix-factor per os before immunization. * – differences are significant with the control group (P < 0.05). The mean values and standard errors (x ± SE) for 5 animals in each group are presented (U test).
It was found that the administration of MF substance to animals was accompanied by an increase in the amount of CIC 2.4 times compared to the control level (Fig. 8 A, group 2), which indicates an increase in the opsonizing effects of antibodies and complement. If animals were injected with the blood serum of patients with PCS, this was also accompanied by an increase in the content of CIC in the serum of such animals, but only by 50% compared with the control (Fig. 8 A, group 3).
If the experimental animals were previously injected per os with the MF substance, and next they were injected with the blood serum of PCS-patients, then in such animals the CIC content did not significantly change compared to the control (Fig. 8 A, group 4). The results obtained are indirect evidence that the decrease in the CIC content in animals after preventive administration of MF is associated not only with a decrease in the rate of CIC formation, but also with an increase in the rate of their elimination from the body, which can be ensured by the activation of phagocytosis.
3.4. Influence of blood serum components of patients with PCS on the phagocytic activity of neutrophils in experimental animals
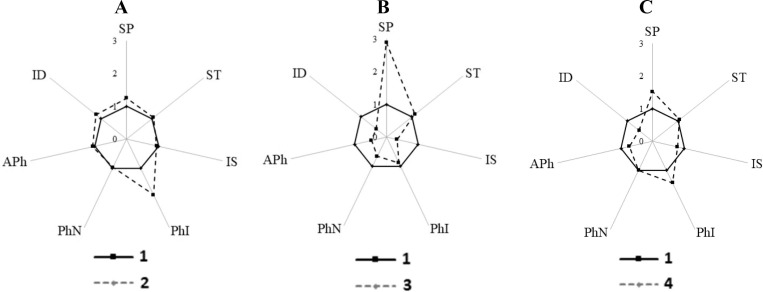
In all animals, the formation of NETs was not detected in the study of oxygen-independent phagocytosis with acridine orange. In animals that were injected with the MF substance, the number of cells that entered into phagocytosis increased. Partial or complete denaturation of the S. cerevisiae antigen DNA occurred in neutrophils (Fig. 9 B, C). The phagocytic number (the average number of S. cerevisiae in a neutrophil) and the number of active phagocytes that captured at least one cell did not differ from the control, the digestion index significantly increased (Fig. 10 A).
Fig. 9.
Denaturation of S. cerevisiae DNA at different stages of phagocytosis of granulocyte neutrophils: A – the start of incubation – native DNA (green color, λmax = 530 nm); B – preparation 90 min after the start of incubation, (red color λmax = 640 nm) – partial digestion S. cerevisiae antigen. Lack of complete digestion of microorganisms (dotted arrow). Bright red color (straight arrow) – intensive digestion of absorbed microorganisms, denaturation of antigen DNA. The absence of a color change of microorganisms inside the phagocytes from green to red – the phagocytosis process is not completed. C – preparation after 90 min after the start of incubation, complete digestion of the S. cerevisiae antigen, an example of the presence of an empty endovacuole (indicated by an arrow). Scale bar 10 μm. Fluorescence microscopy with acridine orange, magnification x1000.
Fig. 10.
Indicators (in arbitrary units) of oxygen-independent (PhI, PhN, APh, ID) and oxygen-dependent (SP, ST, IS) phagocytosis of studied groups animals relative to the control. Combinations of groups on diagrams: A – 1–2; B – 1–3; C – 1–4. Groups of animals: 1 (C) – control group; 2 (MF) – animals that received Mix-factor per os; 3 (post-Covid-19) – animals immunized with sera from post-Covid-19 patients; 4 (MF + post-Covid-19) – animals that received Mix-factor per os before immunization. PhI – phagocytic index – the number of neutrophils involved in phagocytosis; PhN – phagocytic number – the average number of yeast cells absorbed by one phagocyte; APh – the number of active phagocytes – the number of neutrophils containing at least one actively digested yeast cell; ID – index of digestion – the ratio of the number of actively digested yeast cells to all absorbed yeast cells by 100 neutrophils; SP is the number of positive cells that spontaneously absorbed the NBT dye; ST is the number of positive cells that absorbed the NBT dye after stimulation by the zymosan activator; IS – stimulation index. Average values obtained from 5 animals are shown. * – differences are significant with the control group (P < 0.05).
Administration of blood serum from patients with PCS to experimental animals inhibited adhesion, absorption of antigens by granulocyte neutrophils and their digestion against the background of an increase in the number of neutrophils capable of phagocytosis (5,77 ± 1,9 ⋅109 versus 3,27 ± 0,49 ⋅109 in control).
The average number of absorbed cells (S. cerevisiae) by one phagocyte (phagocytic number) in animals injected with the blood serum of patients with PCS was 50% less compared to the control (Fig. 9 A, 10B). The index of digestion of absorbed cells in animals injected with the blood serum of patients with PCS was reduced by 2.5 times compared with the control, and the number of active phagocytes was 2.2 times less control (Fig. 9A, 10B).
Preventive administration of the MF substance to animals (group 4) led to a significant increase in the phagocytic index and phagocytic number to the control level (group 1). And the denaturation of the antigen increased in comparison with the 3rd group of animals, which were injected with only PCS serum (Fig. 10 C). Consequently, the MF substance stimulated the absorption and digestion capacity of phagocytes, increasing the efficiency of DNA denaturation of antigen cells in granulocyte neutrophils in groups 2 and group 4 of experimental animals.
In oxygen-dependent phagocytosis in group 3, after administration of PCS serum, spontaneous NADP-H reactions were negatively increased, leading to an increase in ROS (Fig. 10B). This significantly reduced the overall phagocytosis stimulation index (SI) in the NBT test.
As is known, each metabolic process has its own limit of possible activity, which determines the adaptive capabilities of the biological system. In order to assess the potential additional reserve of the oxidative capacity of phagocytic cells, the NADP-H oxidase system was activated with zymosan (Makni-Maalej et al., 2013).
It turned out that zymosan did not lead to the activation of phagocytes obtained from animals that were injected with the blood serum of patients with PCS (Fig. 10C). The obtained results indicate that in animals that were injected with the blood serum of patients with PCS, oxygen-dependent phagocytosis reached its functional limit, which was also characteristic for patients with PCS.
An increase in the number of phagocytes and phagocyte index compared with the control was observed if the animals were injected with only the MF substance. In such animals, the activity of oxygen-dependent phagocytosis was slightly reduced (Fig. 10 A). Given that the MF substance induced an increase in the number of monocytes and granulocytes in animals, it can be argued that MF has a pronounced immunotropic effect, activating the proliferation and differentiation of blood cells.
The preventive administration of the MF substance before the serum of patients with PCS positively reduced spontaneous NADP-H reactions by 60% compared with group 3, which received only the serum of patients with PCS (Fig. 10 C). The stimulation index (SI) in oxygen-dependent phagocytosis after the preventive administration of the MF substance, followed by the administration of the serum of patients with PCS, increased almost to the control values, which indicates the restoration of the oxygen reserve of phagocytes (Fig. 10C).
Therefore, the transfer of blood serum components from patients with PCS to experimental animals was accompanied by the manifestation of PCS effects similar to those in patients. The biologically active MF substance influenced the functional parameters of the immune system, modifying the response of the blood serum components of patients with PCS so that most of the studied parameters approached the control values, i.e. it may be of interest in the prevention of infectious pathologies.
4. Discussion
The organism and the virus during the formation of “relationships” solve different tasks: the organism – to adapt and survive; virus – to multiply and leave. When solving this strategic task, the organism uses, first of all, the currently available (congenitally acquired) elements. These elements are able to maintain homeostasis in the infectious agent presence and the induction of new elements that form an adaptive strategy. The actions of the virus are aimed at “turning off” the body's defense mechanisms. As is known, the humoral and cellular factors of the immune system and the closely related redox system of the body play a leading role in the adaptive strategy formation of the body (Bartolini et al., 2021). It is important to note, in virus infection the proteins of the complement system interact with the virus proteins in the vascular endothelium, induce platelet, tissue, cellular and humoral factors of hemostasis (Maloney et al., 2020). Since the proteins of the complement system have protease activity, in the absence of specific inhibitors they lead to the destruction of cell membranes. This can induce the formation of DAMPs and an increase in cytotoxicity in the Terasaki test, which was observed experimentally (Fig. 5A).
The results obtained and the available data allow us to conclude that the body's response to a viral infection will depend on: 1 – the initial immune-metabolic state of the body, i.e. there is a pronounced individual nature of the response; 2 – the ability of the genome and the immune-metabolic system to ensure its reorganization in time, aimed at maintaining homeostasis against the background of infection; 3 – viral load and, as a result, DAMPs concentration.
A large amount of available data supports this. Thus, it was shown that the mortality rate of those infected with SARS-CoV-2 in the group over 80 years old was 14.8%, in the age group of 70 to 80 years – 8%, and in the age group of 10 to 40 years – only 0.2% (Wang et al., 2020a, Wang et al., 2020b). As is known, age is a temporal characteristic of the immune-metabolic system and it has ability to adequately respond to endo- and exogenous changes, i.e. the initial functional state in young and old people is different, which determines the differences in the adaptive response to infection (Bozhkov et al., 2017a, Bozhkov et al., 2017b).
Another fact in favor of the leading role of the initial functional state of the body in the adaptive response formation can be the data that SARS-CoV-2 infection in 80% of cases ends in recovery and no specific therapeutic measures are required. At the same time, in 1 of 6 cases, severe symptoms arise with the development of respiratory failure (Gupta et al., 2020, Wang et al., 2020a, Wang et al., 2020b, Lai et al., 2020). Along with this, post-COVID-19 syndrome with exacerbation or formation of concomitant pathologies persists in recovered patients (Huang et al., 2021). Such a pronounced individual feature of the body's response to infection is determined by differences in the initial immune-metabolic states of the body.
The results of this work showed that patients with abdominal surgical pathology and SARS-CoV-2 infection tolerated COVID-19 easily, but they had post-COVID-19 syndrome (PCS) in the long-term period. If the components of the blood serum of such patients are administered to animals, then they observed individual changes in physiological characteristics, indicators of humoral and cellular immunity, which were similar to those in patients with PCS. The preventive administration of the MF substance, which changes the functional immune-metabolic state, modified the subsequent response to the action of serum cytotoxic components of patients with PCS. This confirms the influence of the initial functional state on the subsequent response of the body.
We believe that the central problem in the treatment and prevention of the PCS development is the study of the relationship between the initial functional states of the body and the formation of cellular destruction factors (DAMPs), which can also determine the time of manifestation of the post-COVID-19 phenotype.
In contrast to an acute infectious process, which is usually accompanied by leukopenia (Rodriguez-Morales et al., 2020), leukocytosis (Huang et al., 2020), an acute inflammatory reaction (Carvalho-Schneider et al., 2020), and in some cases a cytokine storm (Coperchini et al., 2020), in patients two months after SARS-CoV-2 infection, i.e. in the post-COVID-19 period, the characteristics of the cellular component of the immune system did not differ from the control values (Fig. 3). In all patients, the index of stimulation in oxygen-dependent phagocytosis was very low, which indicates the absence of an oxygen reserve, i.e. the efficiency of phagocytosis was at the level of its potential limit, and further increase in its activity is impossible (Fig. 3). In this state of the cell link, the cytokine network formed a new pattern, different from that in the acute period (Fig. 3). The results obtained indicate the formation of chronic inflammation of “moderate strength” in such patients. Since the pattern of cytokines and indicators of the redox system perform regulatory and coordinating functions (Barciszewska, 2021), it can be assumed that they may have two scenarios for further development of events: slow recovery with the preservation (imprinting) of new immune-metabolic patterns, or the formation of systemic inflammation with exacerbation of concomitant chronic pathologies.
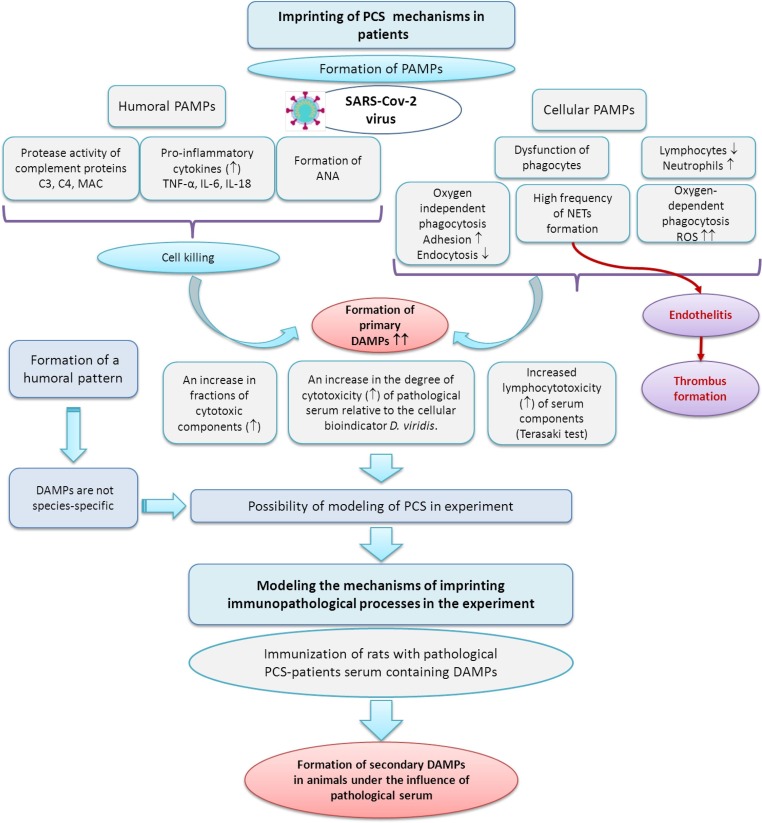
In the event that the body's activation of antibody-producing cells (initial state) is sufficient to completely inactivate the virus, then the infection may be asymptomatic. If the virus inactivation is partially completed, then the next level of adaptive response will be used – activation of the complement system, phagocytosis, excessive formation of ROS, formation of new specific patterns of the cytokine network and activation of a wide range of proteases, and as a result, the formation of cytotoxic components (DAMPs). This sequence of “events” took place in the studied patients (Fig. 11 ). It should be noted that in patients with PCS with low efficiency of phagocytosis, an additional reserve for the removal of cell components destruction products was observed – the formation of NETs (Fig. 11).
Fig. 11.
The scheme demonstrates the formation of specific humoral patterns, changes in the functional activity of phagocytosis and various pathways for the formation of DAMPs that do not have species specificity when transferred to animals, which may be accompanied by the secondary DAMPs induction.
We believe that the level of DAMPs, the formation of NETs, and the number of autoantibodies formed against this background is of fundamental importance in the development of relationships in the “organism – virus” system, which was shown in this work (Fig. 6). The formation of such combinations, as a result of the organism's adaptive response to the virus actions, will in turn lead to the formation of immune-metabolic memory or imprinting. This is explained by the fact that the formed pattern becomes a “self-sustaining” system, i.e. goes into a chronic state and “closes” on itself other metabolic processes, which ensures a relatively long time of its functioning (Fig. 11).
The formation of NETs can be provided by several processes: an increase in the number of DAMPs and products of free radical reactions (Wu, 2020), the state of the cytokine network (Vaz et al., 2011), and other factors against the background of a possible functional limit of the immunity cellular link, which took place in this study. The resulting NETs contribute to the development of hypercoagulation and thrombosis (Zhang et al., 2022). In turn, activated platelets further enhance the induction of NETs formation (Gibbs et al., 2022), i.e. a self-sustaining cycle is formed (Fig. 11).
It should be noted that the proteins of the complement system, the amount of which was increased in patients with PCS two months after SARS-CoV-2 infection (Fig. 4 A), along with participation in the formation of the CIC, can form the MAC (membrane attack complexes). Such complex activates proteases and other hydrolytic enzymes, which is accompanied by and an increase in the number of DAMPs (Grasselli et al., 2021). Other sources of DAMPs in the body are neutrophils and monocytes, the number of which was also increased in experimental animals after they were injected with the blood serum of patients with PCS (Table 2).
An important aspect of the study is the revealed induction of autoantibodies to lysosomal proteins and to the enzyme inosine monophosphate dehydrogenase-2, which is able to inhibit transcription and serve as confirmation that the SARS-CoV-2 virus can use the lysosomal exit route from host cells without destroy cell membranes, which may partially reduce its pathogenicity (Chen et al., 2021). The results obtained allow us to state that the post-COVID-19 syndrome is a manifestation of the body's adaptive response to the actions of the virus, and this response can be imprinted, which explains the duration of post-COVID-19 states (Fig. 11).
We believe that the DAMPs formed during SARS-CoV-2 infection play an important role in the relationship between the organism and the virus. Since, on the one hand, they are a consequence of this interaction, and, on the other hand, they are nonspecific regulators of the body's adaptive mechanisms to the virus. In the case when the rate of DAMPs formation is high and the concentration achieved per unit time is high, multiple organ dysfunction and severe course of COVID-19 will occur. And when the DAMPs formation in the organism occurs relatively slowly and due to the activation of the immune system they are removed from the organism, this will be accompanied by an additional induction of adaptive processes. These processes will manifest themselves in the post-COVID-19 syndrome with various outcomes, slow recovery and the formation of new immune-metabolic states.
As was shown in this work, the serum cytotoxic components of patients with PCS do not have species and, possibly, tissue specificity; their transfer to rats caused similar immune-metabolic responses in them (Fig. 11). This fact proves the absence of their specific action and makes it possible to simulate post-COVID-19 effects on experimental animals, and may serve as an indirect confirmation that DAMPs are “capable” of triggering a cascade of metabolic processes that enhance their cytotoxic effect to a certain limit.
As noted, another argument in favor of the influence of the body initial functional state on the formation of a response to cytotoxic components is the modification of the adaptive response to the cytotoxic effects of the PCS-patients serum with by the preventive administration of MF. This effect can be explained by the fact that the MF components, which are represented by various peptides and carbohydrates (Kurguzova et al., 2015), have a multifunctional effect on metabolism. Thus, they increased the content of monocytes in the blood by 2.2 times and to a lesser extent (by 60%) of granulocytes. As is known, monocytes produce a wide range of cytokines and chemokines (Ballou and Lozanski, 1992), activate the complement system, in particular C3 and C4 components (Fig. 8), and after 1–2 days, blood monocytes penetrate into various tissues and form a population of resident macrophages (Mulder et al., 2021). Modern studies have shown that the monocyte-macrophage system performs extremely wide and diverse regulatory functions, including autoregulation of the bone marrow. The revealed changes correlated with the same increase (by 2.2 times) in the content of CIC in the serum and the phagocyte number (by 2.4 times) (Fig. 10). It should be noted that the administration of the MF substance to animals did not show lymphocytotoxicity in the Terasaki test. It was previously shown that MF normalized pathologically altered liver function (Bozhkov et al., 2017a, Bozhkov et al., 2017b, Bozhkov et al., 2021). Consequently, the preliminary administration of the MF substance changes the immune-metabolic characteristics and forms a new immune-metabolic “landscape” in the body. And the cytotoxic components of the blood serum of PCS patients, which are administered to the model organism, are inactivated much faster and excreted from the organism.
Such an effect of the MF substance is comparable to the effect of hormesis (Agathokleous et al., 2018), and MF can be attributed to hermetins, substances capable of non-specific stimulation of the body's adaptive responses to the subsequent action of stress factors (Gems and Partridge, 2008). Further studies of PCS on the obtained experimental model will significantly expand our knowledge of the action of cytotoxic components formed against the background of infection and develop systems for the elimination of post-COVID-19 complications.
5. Conclusions
In patients with post-COVID-19 syndrome, cytotoxic components are present and specific immunopathological patterns are formed, which are characterized by: an increased content of C-reactive protein, complement system proteins activation, an increase in the concentration of TNF, IL-6, IL-10, IL-18 and the formation of a wide range of autoantibodies ANA, low efficiency of endocytosis in oxygen-independent phagocytosis, phagocytic activity reaches its functional limit, activation of NETs occurs, which can participate in additional DAMPs induction, which ensures their imprinting.
Long-term persistence of post-COVID-19 syndrome in infected SARS-CoV-2 is associated with the formation of cytotoxic components (DAMPs), which do not have species specificity and can be produced due to the constant presence of cell destruction factors and NETs in the body.
The administration into the body of experimental animals (rats) of the blood serum of patients with post-COVID-19 syndrome is accompanied by the induction of an adaptive immune response and the appearance of cytotoxic components in them, which made it possible to “simulate” post-COVID-19 manifestations in animals in order to study the mechanisms of its development and develop ways to eliminate it.
The nature and characteristics of the body's immune system response to toxic compounds, including those present in patients with COVID-19, to a large extent depends on the initial functional state of the body. The initial functional state is determined not only by genetic characteristics, but also by epigenetic factors, the most important of which are food components.
Mix-factor (MF) refers to functional food products and is a multicomponent natural substance with a multifunctional mechanism of action, can “correct” the body initial functional state such a way, in particular, it provides an increase in phagocytic activity, which eliminates or facilitates the course of post-COVID-19 syndrome.
Funding
This work is supported by the National academy of medical sciences of Ukraine (state registration No. 0121U113289).
CRediT authorship contribution statement
Elena M. Klimova: Conceptualization, Data curation, Methodology, Funding acquisition, Resources, Supervision, Writing – original draft. Anatoliy I. Bozhkov: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – original draft. Olena V. Lavinska: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Larisa A. Drozdova: Investigation, Visualization. Natalia I. Kurguzova: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Agathokleous E., Kitao M., Calabrese E.J. Environmental hormesis and its fundamental biological basis: Rewriting the history of toxicology. Environ. Res. 2018;165:274–278. doi: 10.1016/j.envres.2018.04.034. [DOI] [PubMed] [Google Scholar]
- Alhabbab R.Y. Basic Serological Testing. Techniques in Life Science and Biomedicine for the Non-Expert. Springer; Cham: 2018. C-reactive protein (CRP) latex agglutination test. [DOI] [Google Scholar]
- Ballou S.P., Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4(5):361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- Barciszewska A.M. Elucidating of oxidative distress in COVID-19 and methods of its prevention. Chem. Biol. Interact. 2021;1(344) doi: 10.1016/j.cbi.2021.109501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D., Stabile A.M., Bastianelli S., Giustarini D., Pierucci S., Busti C., Vacca C., Gidari A., Francisci D., Castronari R., Mencacci A., Di Cristina M., Focaia R., Sabbatini S., Rende M., Gioiello A., Cruciani G., Rossi R., Galli F. SARS-CoV2 infection impairs the metabolism and redox function of cellular glutathione. Redox Biol. 2021 doi: 10.1016/j.redox.2021.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix V., Merino E. Síndrome post-COVID. El desafío continúa. Med Clin (Barc) 2022;158:178–180. doi: 10.1016/j.medcli.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhkov A.I., Nikitchenko Y.V., Klimova E.M., Linkevych O.S., Lebid K.M., Al-Bahadli A.M.M., Alsardia M.M.A. Young and old rats have different strategies of metabolic adaptation to Cu-induced liver fibrosis. Adv. Gerontol. 2017;7:41–50. doi: 10.1134/S2079057017010040. [DOI] [PubMed] [Google Scholar]
- Bozhkov A.I., Nikitchenko Y.V., Lebid K.M., Ivanov E.G., Kurguzova N.I., Gayevoy S.S., Sharko M.O., Alsardia Mohammad M.A., Mohammad A.B., A.y., Low molecular weight components from various sources eliminate oxidative stress and restore physiological characteristic of animals at early stages of Cu-induced liver fibrosis development. Transl. Biomed. 2017;8:2. doi: 10.2167/2172-0479.1000107. [DOI] [Google Scholar]
- Bozhkov A.I., Bozhkov A.A., Ponomarenko I.E., Kurguzova N.I., Akzhyhitov R.A., Goltvyanskii A.V., Klimova E.M., Shapovalov S.O. Elimination of the toxic effect of copper sulfate is accompanied by the normalization of liver function in fibrosis. Regul. Mech. Biosyst. 2021;12(4):655–663. doi: 10.15421/022190. [DOI] [Google Scholar]
- Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., Gaudy-Graffin C., Grammatico-Guillon L., Bernard L. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2020;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Zheng Q., Sun L., Ji M., Li Y., Deng H., Zhang H. ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev. Cell56. 2021;(23):3250–3263.e5. doi: 10.1016/j.devcel.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Rosa Mesquita R., Francelino Silva Junior L.C., Santos Santana F.M., de Oliveira T.F., Alcântara R.C., Arnozo G.M., da Silva Filho E.R., dos Santos A.G.G., da Cunha E.J.O., de Aquino S.H.S., de Souza C.D.F. Clinical manifestations of COVID-19 in the general population: systematic review. Wien. Klin. Wochenschr. 2021;133:377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rica R., Stevens M.M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012;7(12):821–824. doi: 10.1038/nnano.2012.186. [DOI] [PubMed] [Google Scholar]
- Dhingra R., Gona P., Nam B.H., D'Agostino R.B., Sr, Wilson P.W., Benjamin E.J., O'Donnell C.J. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am. J. Med. 2007;120(12):1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino M., García Septiem J., Maqueda González R., Muñoz de Nova J.L., de la Hoz Rodríguez Á., Correa Bonito A., Martín-Pérez E., et al. Cirugía electiva durante la pandemia por SARS-CoV-2 (COVID-19): análisis de morbimortalidad y recomendaciones sobre priorización de los pacientes y medidas de seguridad. Cir. Esp. 2020 doi: 10.1016/j.ciresp.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A.J., Crichton M.L., Pumphrey E.G., Cassidy A.J., Suarez-Cuartin G., Sibila O., Furrie E., Fong C.J., Ibrahim W., Brady G., Einarsson G.G., Elborn J.S., Schembri S., Marshall S.E., Palmer C.N.A., Chalmers J.D. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018;141(1):117–127. doi: 10.1016/j.jaci.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaharuddin I., Munawwarah S., Nurulita A., Ilyas M., Tabri N.A., Lihawa N. Comorbidities and mortality in COVID-19 patients. Gac. Sanit. 2021;5(Suppl 2):S530–S532. doi: 10.1016/j.gaceta.2021.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forneris F., Wu J., Gros P. The modular serine proteases of the complement cascade. Curr. Opin. Struct. Biol. 2012;22(3):333–341. doi: 10.1016/j.sbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gems D., Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7(3):200–2003. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gibbs D.V., Shreenivas S.S., Hudock K.M. Role of Acute Thrombosis in Coronavirus Disease 2019. Crit. Care Clin. 2022;38(3):491–504. doi: 10.1016/j.ccc.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia M., Ciaccio C., Calligari P., De Simone G., Sbardella D., Tundo G., Fasciglione G.F., Di Masi A., Di Pierro D., Bocedi A., Ascenzi P., Coletta M. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem. Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorchakov A.M., Kruchinsky N.G., Gorchakova F.T., Korosteleva I.V. Institute of Environmental and Professional Pathology; Belarus: 2003. Metod kompleksnoj ocenki fagocitarnoj aktivnosti nejtrofilov krovi [Method of a comprehensive assessment of the phagocytic activity of blood neutrophils] p. 15. in Russian. [Google Scholar]
- Grasselli G., Tonetti T., Filippini C., Slutsky A.S., Pesenti A., Ranieri V.M. Pathophysiology of COVID-19-associated acute respiratory distress syndrome - Authors' reply. Lancet Respir. Med. 2021;9(1):e5–e6. doi: 10.1016/S2213-2600(20)30525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;15, 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;16, 397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimova E.M., Bozhkov A.I., Boyko V.V., Drozdova L.A., Lavinskaya E.V., Skok M.V. Endogenic cytotoxic compounds and formation of the clinic forms of myasthenia. Transl. Biomed. 2016;7(3):1–13. doi: 10.21767/2172-0479.100084. [DOI] [Google Scholar]
- Kurguzova N.I., Bozhkov A.I., Nikitchenko Y.V., Al Begai M.A.Y., Goltvyansky A.V., Alsardia M.M.A., Bozhkov A.A. Interconnection of antitoxic and antioxidant systems of the organism under the action of natural low molecular complex–fungidol. Am. J. Biomed. Life Sci. 2015;2(6–1):25–32. doi: 10.11648/j.ajbls.s.2014020601.15. [DOI] [Google Scholar]
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land W.G. Role of DAMPs in respiratory virus-induced acute respiratory distress syndrome – with a preliminary reference to SARS-CoV-2 pneumonia. Genes Immun. 2021;22:141–160. doi: 10.1038/s41435-021-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makni-Maalej K., Chiandotto M., Hurtado-Nedelec M., Bedouhene S., Gougerot-Pocidalo M.A., Dang P.M., El-Benna J. Zymosan induces NADPH oxidase activation in human neutrophils by inducing the phosphorylation of p47phox and the activation of Rac2: involvement of protein tyrosine kinases, PI3Kinase, PKC, ERK1/2 and p38MAPkinase. Biochem. Pharmacol. 2013;1, 85(1):92–100. doi: 10.1016/j.bcp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Maloney B.E., Perera K.D., Saunders D.R.D., Shadipeni N., Fleming S.D. Interactions of viruses and the humoral innate immune response. Clin. Immunol. 2020;212:08351. doi: 10.1016/j.clim.2020.108351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masjuk N.P. Morphology, systematics, ecology, geographical distribution of Dunaliella. Teod Kiev. Science. 1973;372 [Google Scholar]
- Mulder K., Patel A.A., Kong W.T., Piot C., Halitzki E., Dunsmore G., Khalilnezhad S., Irac S.E., Dubuisson A., Chevrier M., Zhang X.M., Tam J.K.C., Lim T.K.H., Wong R.M.M., Pai R., Khalil A.I.S., Chow P.K.H., Wu S.Z., Al-Eryani G., Roden D., Swarbrick A., Chan J.K.Y., Albani S., Derosa L., Zitvogel L., Sharma A., Chen J., Silvin A., Bertoletti A., Blériot C., Dutertre C.A., Ginhoux F. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021;10, 54(8):1883–1900.e5. doi: 10.1016/j.immuni.2021.07.007. [DOI] [PubMed] [Google Scholar]
- Muniz-Junqueira M.I., Peçanha L.M., Silva-Filho V.L., Cardoso M.C.A., Tosta C.E. Novel microtechnique for assessment of postnatal maturation of the phagocytic function of neutrophils and monocytes. Clin. Vaccine Immunol. 2003;10:1096–1102. doi: 10.1128/cdli.10.6.1096-1102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi I., Giroux N., Olson L., Morrison S.A., Llanga T., Akinade T.O., Zhu Y., Zhong Y., Bose S., Arvai S., Abramson K., Chen L., Que L., Kraftf B., Shen X., Lee J., Leong K.W., Nair S.K., Sullenger B. DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID-19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials. 2022;283 doi: 10.1016/j.biomaterials.2022.121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Nilsson E.K. Complement Diagnostics: Concepts, Indications, and Practical Guidelines. Clin. Dev. Immunol. 2012;962702:11. doi: 10.1155/2012/962702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Ling S., Hu K., Liu J., Xu J.W. Role of the renin-angiotensin system in NETosis in the coronavirus disease 2019 (COVID-19) Biomed. Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112718. 112718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. 2018. Nat Rev Immunol. 18(2), 134-147. http://doi.org/10.1038/nri.2017.105. [DOI] [PubMed]
- Parthasarathy U., Martinelli R., Vollmann E.H., Best K., Therien A.G. The impact of DAMP-mediated inflammation in severe COVID-19 and related disorders. Biochem. Pharmacol. 2022;195 doi: 10.1016/j.bcp.2021.114847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha I., Haskova V., Kaslik J., Maierova M., Stransky J. The use of polyethylene glycol for immune complex detection in human sera. Mol. Immunol. 1979;16:489–493. doi: 10.1016/0161-5890(79)90075-0. [DOI] [PubMed] [Google Scholar]
- Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales, A.J., Cardona-Ospina, J.A., Gutiérrez-Ocampo, E., Villamizar-Peña, R., Holguin-Rivera, Y., Escalera-Antezana, J.P., Alvarado-Arnez, L.E., Bonilla-Aldana, D.K., Franco-Paredes, C., Henao-Martinez, A.F., Paniz-Mondolfi, A., Lagos-Grisales, G.J., Ramírez-Vallejo, E., Suárez, J.A., Zambrano, L.I., Villamil-Gómez, W.E., Balbin-Ramon, G.J., Rabaan, A.A., Harapan, H., Dhama, K., Nishiura, H., Kataoka, H., Ahmad, T., Sah, R., 2020. Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 34, 101623. http://doi.org/10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed]
- Rosales-Castillo A., García de los Ríos C., Mediavilla García J.D. Persistencia de manifestaciones clínicas tras la infección COVID-19: importancia del seguimiento. Med. Clin. (Barc.) 2020;156:35–36. doi: 10.1016/j.medcli.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho F.E., Hanslin K., Hultström M., Larsson A., Frithiof R., Lipcsey M. Uppsala Intensive Care COVID-19 Research Group. Soluble TNF receptors predict acute kidney injury and mortality in critically ill COVID-19 patients: A prospective observational study. Cytokine. 2022;149 doi: 10.1016/j.cyto.2021.155727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal A.W., Levi A.J. The mechanism of the NBT (nitroblue tetrazolium) test. Clin. Sci. 1973;44(6):26P. doi: 10.1042/cs044026pa. [DOI] [PubMed] [Google Scholar]
- Shitov A.Y. Molecules of middle mass as an indicator of divers’ «hyperbaric intoxication». Almanac of Clinical Medicine. 2013;28:48–52. doi: 10.18786/2072-0505-2013-28-48-52. [DOI] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L. HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki P., Mcclelland J. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- Vaz A.R., Silva S.L., Barateiro A., Fernandes A., Falcão A.S., Brito M.A., Brites D. Pro-inflammatory cytokines intensify the activation of NO/NOS, JNK1/2 and caspase cascades in immature neurons exposed to elevated levels of unconjugated bilirubin. Exp. Neurol. 2011;229(2):381–390. doi: 10.1016/j.expneurol.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/s0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wu C., Xu J., Zhang B., Zhang X., Gao Z., Xia Z. Factors affecting the mortality of patients with COVID-19 undergoing surgery and the safety of medical staff: A systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020;1(102):39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.