Abstract
Objectives
Olfactory dysfunction is a common complaint in patients with chronic rhinosinusitis (CRS). The study aimed to evaluate the effectiveness of olfactory training (OT) in patients with CRS after sinonasal surgery.
Methods
We enrolled 111 patients with CRS who underwent sinonasal surgery. Prior to surgery and 3 months after starting OT, the participants were assessed by both an olfactory function test and endoscopy. The Korean version of the Sniffin' Stick II (KVSS‐II) was used to perform the olfactory function test. Over the course of 3 months, five odorants were used in OT (rose, lemon, cinnamon, orange, and peach).
Results
Over a 12‐week duration, 37% of the participants in the OT group showed clinically relevant increase in olfactory function. The OT group had significantly higher olfactory outcomes for the total KVSS‐II and identification scores than the non‐OT group between the initial and follow‐up assessments. The initial score influenced the degree of olfactory improvement after OT.
Conclusions
Patients with OT exhibited significantly higher total KVSS‐II scores compared with non‐OT patients following sinonasal surgery; in particular, the odor identification score was different between the two groups. The results of this study demonstrated that a 12‐week period of repeated short‐term exposure to various odors could be useful in enhancing olfactory activity in patients who underwent sinonasal surgery for the improvement of sensory‐neural olfactory impairment.
Level of evidence
2c.
Keywords: olfactory recovery, olfactory training, sinonasal surgery
The aim of this study was to evaluate the effectiveness of olfactory training (OT) in patients with CRS after sinonasal surgery. OT patients exhibited significantly higher total threshold, discrimination, and identification scores compared with non‐OT patients at the end of 3 months. In the subset analysis, the odor identification scores differed between the two groups after 3 months.

1. INTRODUCTION
Olfactory dysfunction (OD) can be classified into conductive, sensorineural, and mixed types. In conductive losses, such as nasal polyps and chronic rhinosinusitis (CRS), inspired odorants are unable to enter the olfactory cleft in the nasal cavity. In sensorineural loss, the damage to the olfactory receptor neurons or their central projection contributes to OD. 1 Attempted treatments have included medical (topical and systemic steroids, zinc, etc.) and surgical (sinonasal operation for CRS‐related OD and septoplasty, septorhinoplasty, etc., for non‐CRS‐related OD) treatment. 2 Hummel et al. 3 studied the effectiveness of olfactory training (OT) in a group of patients with olfactory loss owing to postinfectious, post‐traumatic, or idiopathic etiologies. Despite the limited studies in the patients with sinonasal disease, OT has shown promise as an alternative treatment modality for several causes of OD, with the exception of sinonasal disease.
Previous research demonstrated that exposing various odors in patients with postinfectious and post‐traumatic OD for 16 weeks increased their olfactory function. 4 According to a recent meta‐analysis, OT is a promising clinical therapy for patients with OD, and many other trials have shown that OT has good olfactory outcomes with no serious side effects. 5 Recently, we reported that OT resulted in olfactory improvement, which was reflected in the total threshold, discrimination, and identification (TDI) scores, in patients with postinfectious OD (PIOD) compared to a control group using five odorants familiar to Koreans. 6 Moreover, OT can be considered for patients with persistent coronavirus disease 2019‐related OD since this therapy is inexpensive and has negligible adverse effects. 7 OD is a common complaint in patients with CRS. 8 , 9 Although the main mechanism of CRS‐induced OD is unclear, it is thought to be a combination of mechanical obstruction from edematous mucosa or polyposis, as well as sensorineural damage arising from chronic inflammatory injury to the olfactory neuroepithelium. 10 Endoscopic sinonasal surgery (ESS) is used to improve the sinus function and access to topical medical treatment in patients with medically refractory CRS. However, olfactory function following ESS can be unpredictable. 11 , 12 An earlier study endorsed the effect of ESS in reducing CRS‐related OD. However, several recent studies on the outcomes of OD following ESS have reported conflicting results. 13 A study found that OT improved the olfactory activity of patients with sinonasal disease, but did not evaluate the effects of OT on post‐sinonasal‐operative patients. Another study reported no significant changes between the OT only group and the OT with steroids group in individual component values of TDI scores in patients with sinonasal disease; however, it did not include a control group. 14 Therefore, the effectiveness of OT in patients with CRS who experienced mixed (conductive and sensorineural) OD after resolving the conductive cause by sinonasal surgery has not been investigated.
1.1. Objectives
This study aimed to evaluate the effectiveness of OT in patients with CRS following sinonasal surgery compared with non‐OT patients.
2. MATERIALS AND METHODS
2.1. Ethical considerations
This study was performed in accordance with the principles of the Declaration of Helsinki. The study was reviewed by the Institutional Review Board of Konkuk University Medical Center (IRB No. KUH 11100063), and written informed consent was obtained from all the patients. The study design was approved by the ethics committee of the medical faculty at Konkuk University Medical Center.
2.2. Participants
Adult patients with CRS with or without nasal polyps who required sinonasal surgery and were unresponsive to medication treatment were recruited from a subspecialized rhinology clinic. The inclusion criteria were age ≥18 years with bilateral chronic or recurrent rhinosinusitis, and a Lund–MacKay (LM) computed tomography (CT) scan score difference ≤2 between the right and left sides of the nasal cavity. Patients were excluded if they were unable or unwilling to comply with the required postoperative visits for data collection, or if they were ineligible for informed consent, displayed unilateral disease, had an olfactory cleft obstruction on preoperative CT scan or pre/perioperative endoscopic finding, had an underlying bleeding disorder, or a CT scan revealed a significant difference in disease status between the nasal cavities (LM score difference >2).
2.3. Design
Bilateral ESS was performed in all the patients, and septoplasty was performed in some patients (total of 26 patients: 17 patients in the OT group and 9 patients in the non‐OT group) who had nasal septal deviation by one surgeon under general anesthesia to improve the cause of OD. Penicillin‐based antibiotics were intravenously administrated for 2 days. Patients were treated endoscopically with debridement once a week as outpatients for the first month after discharge from the hospital. Oral antibiotics and intranasal corticosteroids were administrated for 2 weeks to prevent postoperative infection and reduce postoperative mucosal swelling. Saline nasal irrigation was administrated once a day during postoperative care. Patients were followed up monthly in outpatient clinics for 3 months. The perioperative sinus endoscopy (POSE) scoring system was performed on all patients by a single experienced otorhinolaryngologist.
Following bilateral sinonasal surgery, patients started OT over a period of 12 weeks after their nasal packing (Merocel®, Medtronic XOMED, Jacksonville, FL, USA) was removed on Postoperative day 1. The details of the OT procedure were explained to all the patients who underwent bilateral ESS and they were offered the choice of continuing with the training program or awaiting spontaneous recovery. The OT protocol and counseling on behavior and lifestyle modification were performed in accordance with a previously published method. 6 Briefly, the patients were exposed to five different odorants twice a day: rose, lemon, cinnamon, orange, and peach. These odorants were chosen to represent odors that Koreans are familiar with to increase patient compliance. 15 They were instructed to sniff the odorants for 10 s each morning and evening, with a 10‐s break between each odorant. The non‐OT group patients were not assigned any sniffing task. With the exception of OT, the postoperative medical treatment did not differ between the groups. Both the groups were evaluated at 12 weeks after the operation.
2.4. Main outcome measures
The Korean version of the Sniffin' Stick II (KVSS‐II), 16 composed of the olfactory threshold, odor discriminatory, and odor identification test was used to perform the olfactory function test. In the KVSS‐II, the sums of the three tests are presented as a TDI score. Total scores of 0 to 20 are defined as “anosmia,” 20.25 to 27 as “hyposmia,” and 27.25 to 48 as “normosmia.” A visual analog scale (VAS) was used to evaluate self‐assessed smell function and the Korean version of the Mini‐Mental State Examination was used to assess cognitive impairment. Three months after the operation, all the participants were reviewed using endoscopy and the KVSS‐II.
The preoperative LM score, POSE score, VAS, and KVSS‐II scores were collected. The LM scoring system was used to compare the preoperative status. Similarly, the POSE scoring system was used for the comparison of the postoperative status. Olfactory function was evaluated using the KVSS‐II at 2 ± 1 weeks prior to surgery. The LM score was used to describe the CT findings.
2.5. Statistical analyses
SPSS version 21.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The mean ± standard deviation or percentage (%) was used to present the demographic and clinical data. The t‐test for independent samples was used to make comparisons between the training patients and controls. For categorical data, Pearson's chi‐square test was applied. Paired t‐test was used to compare olfactory function in each group prior to surgery and at 12 weeks afterward. Univariable linear regression analysis was performed to investigate the relationship between the changes in the TDI scores and age, sex, duration of disease, severity of OD, LM score, and OT. Factors with a p‐value of <.1 were subjected to multivariable linear regression analysis. Multivariable linear regression analysis was performed to investigate the relationship between the changes in the TDI scores and sex, severity of OD, and OT.
3. RESULTS
3.1. Participant demographics
We enrolled 68 patients in the OT group and 43 patients in the non‐OT group. There were no differences between the study groups at the preoperative assessment in terms of the LM scores, initial TDI scores, initial VAS scores, or POSE scores. Tables 1 and 2 show the descriptive statistics of the findings.
TABLE 1.
Demographic characteristics of patients
| Olfactory training group (N = 68) | Olfactory non‐training group (N = 43) | p‐value | |
|---|---|---|---|
| Age, years | 45.6 ± 15.8 | 44 ± 14.9 | .6093 |
| Female/male | 27 (39.7)/41 (60.3) | 20 (46.5)/23 (53.5) | .4796 |
| Median duration, months | |||
| <12 | 35 (51.5) | 23 (53.5) | .8358 |
| ≧12 | 33 (48.5) | 20 (46.5) | |
| Degree of dysfunction | |||
| Anosmia | 15 (22.1) | 16 (37.2) | .1744 |
| Hyposmia | 48 (70.6) | 23 (53.5) | |
| Normosmia | 5 (7.3) | 4 (9.3) | |
| Nasal polyp | |||
| CRSwNP | 49 (72.1) | 37 (86.0) | .0856 |
| CRSsNP | 19 (27.9) | 6 (14.0) | |
| Septoplasty | 17 (25.0) | 9 (20.9) | .6219 |
| L–M score | 12.8 ± 5.2 | 13 ± 5.2 | .8147 |
| POSE score (3 months) | 1.5 ± 2.5 | 1.8 ± 1.9 | .5463 |
Note: Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as N (%).
Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyp; CRSsNP, chronic rhinosinusitis without nasal polyp; L–M score, Lund–Mackay score; POSE score, perioperative sinus endoscopy score.
TABLE 2.
Comparison of KVSS II scores and VAS scores between the training and non‐training groups
| Olfactory training group (N = 68) | Olfactory non‐training group (N = 43) | p‐value | |
|---|---|---|---|
| KVSS II test score (initial) | 19.8 ± 7.7 | 18.8 ± 7.9 | .5194 |
| Threshold score (initial) | 3.8 ± 3 | 3.3 ± 3.1 | .3625 |
| Discrimination score (initial) | 7.3 ± 3.2 | 7.1 ± 3.2 | .7874 |
| Identification score (initial) | 8.6 ± 3.7 | 8.3 ± 3.6 | .7289 |
| KVSS II test score (3 months) | 23.4 ± 7.6 | 20.1 ± 7.9 | .034* |
| Threshold score (3 months) | 4.7 ± 3.6 | 4.3 ± 3.1 | .5488 |
| Discrimination score (3 months) | 8.5 ± 3.1 | 7.6 ± 3.3 | .142 |
| Identification score (3 months) | 9.9 ± 3.2 | 8.3 ± 3.7 | .0138* |
| Difference in KVSS II score (3 months—initial) | 3.6 ± 5.8 | 1.3 ± 6.5 | .0592 |
| Threshold score (3 months—initial) | 0.8 ± 2.8 | 1 ± 3.8 | .8374 |
| Discrimination score (3 months—initial) | 1.2 ± 3.7 | 0.5 ± 3.1 | .2755 |
| Identification score (3 months—initial) | 1.4 ± 3.1 | −0.1 ± 3.1 | .02* |
| VAS score (initial) | 5.1 ± 2.8 | 4.7 ± 2.5 | .43 |
| VAS score (3 months) | 5.6 ± 2.7 | 4.8 ± 2.5 | .0995 |
Note: Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as N (%).
Abbreviations: KVSS, Korean version of the Sniffin' Stick; VAS, visual analog scale.
p < .05.
3.2. The effects of OT after sinonasal surgery
In the OT group, approximately 22.1% (n = 15) of the patients had anosmia, 70.6% (n = 48) hyposmia, and 7.3% (n = 5) normosmia. In the non‐OT group, On the other hand, 37.2% (n = 16) of the patients had anosmia, 53.5% (n = 23) hyposmia, and 9.3% (n = 4) normosmia. There were no significant differences in the TDI scores between the two groups at preoperative evaluation, and there were no significant differences in the odor TDI subset tests.
Three months after the sinonasal surgery, patients who underwent OT showed a significant increase in the TDI scores. The subset analysis of the TDI scores showed that the odor TDI scores increased significantly in the OT group. The non‐training group also showed improvement in the threshold score compared with the other scores; however, the difference was not statistically significant. In either group, there were no significant variations in VAS scores between the test times. Table 3 shows the descriptive statistics for the outcomes.
TABLE 3.
Comparison of KVSS II scores between the training and non‐training groups
| Olfactory training group (N = 68) | Olfactory non‐training group (N = 43) | |||||
|---|---|---|---|---|---|---|
| Initial | 3 months later | p‐value | Initial | 3 months later | p‐value | |
| KVSS II test score | 19.8 ± 7.7 | 23.4 ± 7.6 | <.0001* | 18.8 ± 7.9 | 20.1 ± 7.9 | .1793 |
| Threshold score | 3.8 ± 3 | 4.7 ± 3.6 | .0175* | 3.3 ± 3.1 | 4.3 ± 3.1 | .0984 |
| Discrimination score | 7.3 ± 3.2 | 8.5 ± 3.1 | .0086* | 7.1 ± 3.2 | 7.6 ± 3.3 | .3326 |
| Identification score | 8.6 ± 3.7 | 9.9 ± 3.2 | .0006* | 8.3 ± 3.6 | 8.3 ± 3.7 | .8841 |
| VAS score | 5.1 ± 2.8 | 5.6 ± 2.7 | .1606 | 4.7 ± 2.5 | 4.8 ± 2.5 | .9271 |
Abbreviations: KVSS, Korean version of the Sniffin' Stick; VAS, visual analog scale.
p < .05.
Patients with OT exhibited significantly higher total TDI scores compared with the non‐OT patients at the end of 3 months. In the subset analysis, the odor identification scores differed between the two groups after 3 months. Considering the improvement on an individual level, 25 of 68 participants from the OT group (37%) showed a clinically relevant increase in the olfactory function (>5.5 increase in the TDI score 17 ), compared with 11 of 43 participants (26%) in the non‐OT group. Table 2 and Figure 1 show the descriptive statistics of the findings.
FIGURE 1.
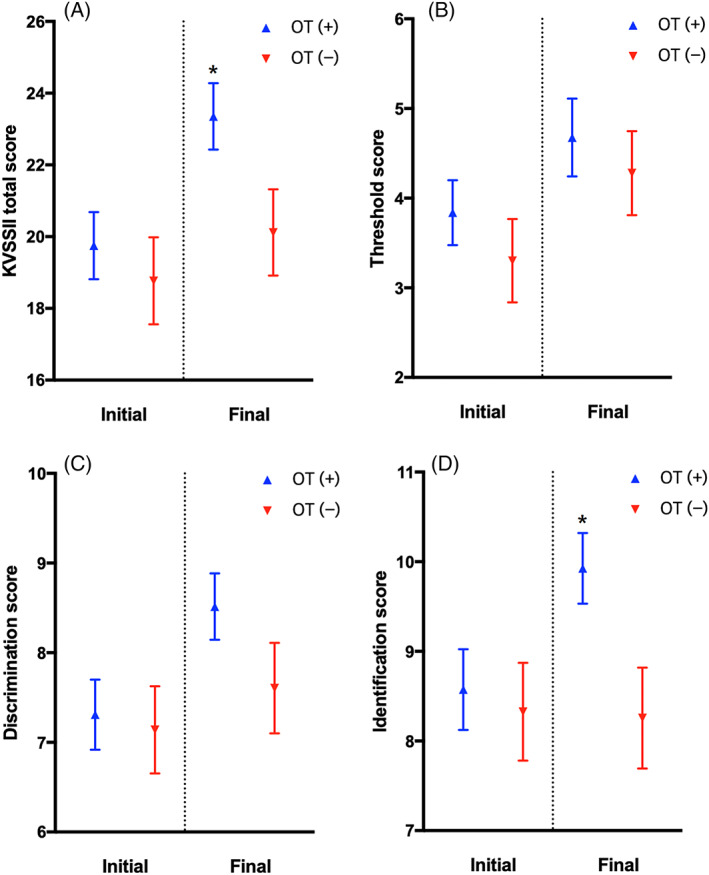
In the olfactory training (OT) and non‐OT groups, the total threshold, discrimination, and identification (TDI) score (A), threshold score (B), discrimination score (C), identification score (D), and difference between the initial and final evaluations are compared. KVSS‐II, Korean version of the Sniffin' Stick. *p < .05
3.3. Comparison of olfactory recovery by sex, age, severity, and duration of OD
The preoperative KVSS‐II scores adjusted with sex, degree of dysfunction, age, median duration and LM score were not correlated with recovery of olfactory function in the univariable linear regression analysis. Following adjustment for the degree of dysfunction and OT factors, sex showed no correlation with the difference in the KVSS‐II score. The group with anosmia at preoperative evaluation showed a significantly higher increase in the KVSS‐II test results compared with the patients with hyposmia and normosmia at preoperative evaluation. Table 4 shows the descriptive data for the outcomes.
TABLE 4.
Difference in the Korean version of the Sniffin' Stick II (KVSS II) scores between the patients in the olfactory training and non‐olfactory training groups
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Estimate | p‐value | Estimate | p‐value | R 2 | ||
| Age | −0.0024 | .9494 | 0.146 | |||
| Sex | Male | 2.4096 | .0405* | 1.7715 | .1191 | |
| Female | Ref | Ref | ||||
| Degree of dysfunction | Anosmia | 6.2401 | .0065* | 5.8734 | .0094* | |
| Hyposmia | 3.2801 | .1216 | 2.5356 | .2226 | ||
| Normosmia | Ref | Ref | ||||
| Median duration, months | <12 | −1.6005 | .1712 | |||
| ≧12 | Ref | |||||
| L–M score | −0.1673 | .1402 | ||||
| Olfactory training | Yes | 2.2541 | .0592 | 2.5898 | .0261* | |
| No | Ref | Ref | ||||
Abbreviation: L–M score, Lund–Mackay score.
p < .05.
4. DISCUSSION
The following are the significant findings among the patients with CRS from this study: (1) when compared with the non‐OT patients, patients who received OT showed statistically significant improvements in both total TDI score and identification score; (2) differences in the KVSS‐II scores were independent of age, sex, LM score, and duration of disease; and (3) patients with anosmia at preoperative evaluation showed a significantly higher increase in the KVSS‐II score compared with the patients with hyposmia and normosmia at preoperative evaluation.
In this study, the patients who underwent sinonasal surgery with OT showed a significant improvement in the TDI scores after 3 months. The subset analysis of the TDI scores showed that the odor TDI scores increased significantly in the OT group. The non‐training group also showed improvement in the threshold score compared with the other scores; however, the difference was not statistically significant.
According to a study of 20 trials published since 1991, olfaction generally improved following functional ESS. However, limited research exists on the impact of OT on individuals with sinonasal disorders. 18 Controversies also exist concerning whether olfactory function improves with ESS in patients with CRS. According to one prospective study, the University of Pennsylvania Smell Identification Test (UPSIT) score in the CRS with nasal polyposis (CRSwNP) subgroup improved considerably following endoscopic sinus surgery. The evaluated UPSIT olfactory function improved after surgery in 46.0% of patients, did not change after surgery in 12.7% of patients, and worsened in 41.3% of patients. 19 A 5‐year prospective research with 75% of the participants having CRSwNP found a considerable improvement in measured olfaction at 2 years after surgery; however, this became nonsignificant at 5 years. 20 There were also studies that reported negative olfactory outcomes following ESS. A study reported that the mean postoperative T&T recognition threshold test of the eosinophilic CRS group declined after 12 months. 21 In another study, 34% of the patients showed a decline in olfactory function following sinonasal surgery. 22 According to a meta‐analysis of olfactory outcomes following ESS for CRS, ESS improved subjective and objective olfactory parameters, with patients with nasal polyposis and prior OD demonstrating the greatest benefits. 23
In our analysis, the group with anosmia at preoperative evaluation showed significantly higher improvements in the KVSS‐II score compared with the other patients. With the exception of the initial KVSS‐II score, the difference in the KVSS‐II score was independent of age, sex, LM score, and duration of disease. There were no significant differences between the OT and non‐OT groups in terms of age, sex distribution, or duration of the condition in a prior study on patients with PIOD. 6 This is another significant difference compared to previous studies.
We also observed significantly higher total TDI and identification scores in the patients of the OT group compared to those of the non‐OT group. An analysis revealed that an increase in the identification score resulted in an increase in the total TDI score. A previous study on patients with PIOD showed a significantly increased total TDI score, threshold score, and identification score following OT. The majority of prior research found that OT improved composite TDI, identification, and discrimination scores in individuals with PIOD. 24
The odor threshold appears to be relatively unimpaired in central sources of OD and is poorly connected with cognitive tests. Therefore, the threshold score is more strongly tied to peripheral abnormalities in the olfactory system. 5 In this study, sinonasal surgery was performed in both groups, which improved the peripheral olfactory function and conduction of olfactory molecules. This could result in improvement in the threshold scores. Total KVSS‐II and identification scores were significantly increased in the OT group, similar to our previous study on patients with PIOD. Long‐term exposure to various odors enhances the survival of newly generated interneurons 3 and odor memory, 12 indicating that adult neurogenesis can play a role in olfactory memory. OT based on repeated stimulation by odors could promote the survival of immature new neurons and eliminate more mature neurons. 25 Based on this mechanism, patients could improve their olfactory function following OT.
Patients could assign themselves to the study groups in this non‐randomized study, and those who had severe OD in the preoperative state could have been included in the non‐OT group owing to less hope or motivation. These factors can be considered a selection bias. In fact, there were more patients with anosmia in the non‐OT group than the OT group (22.1% in the OT group / 37.2% in the non‐OT group). In Table 4, regardless of OT, the result indicates that the increase in the KVSS‐2 score must be greater in patients with anosmia compared to those with hyposmia and normosmia. According to this, the selection bias could be toward the non‐OT group since it was composed with more patients with anosmia; thus, a larger increase in their KVSS‐2 scores should be expected. However, our results indicated that the OT group, with less proportion of patients with anosmia compared to the non‐OT group, showed more statistically significant increase in the KVSS‐2 scores. This shows that the OT has a significant effect in improvement in the recovery of patients, despite the differences in the proportion of patients with anosmia between the two groups.
There were certain limitations to this study. First, only a small number of patients was involved. To better understand the effects of OT, larger studies are needed. Second, OT was applied for only 12 weeks. Therefore, it is unclear if long‐term OT exposure is helpful to olfactory function.
5. CONCLUSION
Patients with OT exhibit significantly higher total TDI scores than non‐OT patients following sinonasal surgery. In particular, odor identification scores were significantly different between the two groups. According to the findings of the study, a 12‐week course of repeated short‐term exposure to various odors could be useful in enhancing olfactory function in patients who have undergone sinonasal surgery for sensory‐neural OD.
AUTHOR CONTRIBUTIONS
Joon Yong Park contributed to the study conception and design; acquisition, analyses, and interpretation of the data; drafting of the article. Bo Yoon Choi, Hansol Kim, and Taesik Jung contributed to the design of the work; acquisition, analyses, and interpretation of the data. Jin Kook Kim contributed to the study conception and design; analysis and interpretation of the data; drafting of the article. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by Konkuk University Hospital Institutional Review Board (Approval number: KUH 11100063).
ACKNOWLEDGEMENT
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF‐2022R1F1A1068186, NRF‐2016R1A5A2012284).
Park JY, Choi BY, Kim H, Jung T, Kim JK. Olfactory training assists in olfactory recovery after sinonasal surgery. Laryngoscope Investigative Otolaryngology. 2022;7(6):1733‐1739. doi: 10.1002/lio2.955
Funding information National Research Foundation of Korea, Grant/Award Numbers: 2022R1F1A1068186, 2016R1A5A2012284
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Goncalves S, Goldstein BJ. Pathophysiology of olfactory disorders and potential treatment strategies. Curr Otorhinolaryngol Rep. 2016;4(2):115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katherine L, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction. JAMA Otolaryngol Head Neck Surg. 2019;145(9):846‐853. [DOI] [PubMed] [Google Scholar]
- 3. Hummel T, Rissom K, Redden J, Hahner A, Weidenbecher M, Huttenbrink K‐B. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496‐499. [DOI] [PubMed] [Google Scholar]
- 4. Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post‐traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123(12):E85‐E90. [DOI] [PubMed] [Google Scholar]
- 5. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2016;6(3):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi BY, Jeong H, Noh H, Park JY, Cho JH, Kim JK. Effects of olfactory training in patients with postinfectious olfactory dysfunction. Clin Exp Otorhinolaryngol. 2021;14(1):88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitcroft KL, Hummel T. Olfactory dysfunction in COVID‐19: diagnosis and management. JAMA. 2020;323(24):2512‐2514. [DOI] [PubMed] [Google Scholar]
- 8. Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23(2):139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008;22(4):445‐448. [DOI] [PubMed] [Google Scholar]
- 10. Raviv JR, Kern RC. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37(6):1143‐1157. [DOI] [PubMed] [Google Scholar]
- 11. Yamagishi M, Hasegawa S, Suzuki S, Nakamura H, Nakano Y. Effect of surgical treatment of olfactory disturbance caused by localized ethmoiditis. Clin Otolaryngol Allied Sci. 1989;14(5):405‐409. [DOI] [PubMed] [Google Scholar]
- 12. Klimek L, Moll B, Amedee RG, Mann WJ. Olfactory function after microscopic endonasal surgery in patients with nasal polyps. Am J Rhinol. 1997;11(4):251‐255. [DOI] [PubMed] [Google Scholar]
- 13. Pade J, Hummel T. Olfactory function following nasal surgery. Larygoscope. 2008;118(7):1260‐1264. [DOI] [PubMed] [Google Scholar]
- 14. Fleiner F, Lau L, Göktas Ö. Active olfactory training for the treatment of smelling disorders. Ear Nose Throat J. 2012;91(5):198‐203, 215. [DOI] [PubMed] [Google Scholar]
- 15. Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2021;164(2):244‐254. [DOI] [PubMed] [Google Scholar]
- 16. Cho JH, Jeong YS, Lee YJ, Hong SC, Yoon JH, Kim JK. The Korean version of the Sniffin' Stick (KVSS) test and its validity in comparison with the cross‐cultural smell identification test (CC‐SIT). Auris Nasus Larynx. 2009;36(3):280‐286. [DOI] [PubMed] [Google Scholar]
- 17. Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116(10):1858‐1863. [DOI] [PubMed] [Google Scholar]
- 18. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54(26):1‐30. [DOI] [PubMed] [Google Scholar]
- 19. Andrews PJ, Poirrier AL, Lund VJ, Choi D. Outcomes in endoscopic sinus surgery: olfaction, nose scale and quality of life in a prospective cohort study. Clin Otolaryngol. 2016;41(6):798‐803. [DOI] [PubMed] [Google Scholar]
- 20. Rowe‐Jones JM, Medcalf M, Durham SR, Richards DH, Mackay IS. Functional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double‐blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology. 2005;43(1):2‐10. [PubMed] [Google Scholar]
- 21. Oka H, Tsuzuki K, Takebayashi H, Kojima Y, Daimon T, Sakagami M. Olfactory changes after endoscopic sinus surgery in patients with chronic rhinosinusitis. Auris Nasus Larynx. 2013;40(5):452‐457. [DOI] [PubMed] [Google Scholar]
- 22. Kimmelman CP. The risk to olfaction from nasal surgery. Laryngoscope. 1994;104(8 Pt 1):981‐988. [DOI] [PubMed] [Google Scholar]
- 23. Kohli P, Naik AN, Farhood Z, et al. Olfactory outcomes after endoscopic sinus surgery for chronic rhinosinusitis: a meta‐analysis. Otolaryngol Head Neck Surg. 2016;155(6):936‐948. [DOI] [PubMed] [Google Scholar]
- 24. Sorokowska A, Drechsler E, Karwowski M, Hummel T. Effects of olfactory training: a meta‐analysis. Rhinology. 2017;55(1):17‐26. [DOI] [PubMed] [Google Scholar]
- 25. Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo‐Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28(45):11511‐11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


