Abstract
Objectives
Dysfunction in smell or taste is well recognized phenomenon in patients infected with SARS‐CoV‐2. This study aimed to quantify the incidence and associated co‐morbidities of reported olfactory or gustatory dysfunction in patients who tested positive for SARS‐CoV‐2.
Methods
From March 23, 2020 through July 31, 2020, 192,683 patients were tested for SARS‐CoV‐2 at Mayo Clinic. These patients with a positive test were contacted via telephone by physicians at Mayo Clinic and information gathered on patient demographics, comorbidities, symptoms and clinical risk stratification based on these factors.
Results
Two thousand two hundred and fifty patients tested positive for SARS‐CoV‐2 (1.2%). Six hundred and sixty‐seven (29.6%) of these patients reported loss of smell or taste. Factors found to be correlated with reporting loss of smell or taste on multivariate analysis were: younger age, female sex, or symptoms of chest pain or tightness, cough, or headache and lower clinical risk category. Coronary artery disease (CAD) was associated with not reporting loss of taste or smell.
Conclusion
Of 2250 patients testing positive for SARS‐CoV‐2 at Mayo Clinic, 667 reported loss of taste and smell. Patients who reported loss of smell or taste were younger, female and more likely to report cough, chest pain, headache, or history of chronic obstructive pulmonary disease (COPD), but overall had fewer high‐risk comorbidities. Those who were older, male, and a reported history of CAD were less likely to report chemosensory dysfunction. Our data are the largest single institution data reporting COVID‐19 associated loss of smell or taste, and the first to associate COPD and CAD as factors that affect rates of reported chemosensory dysfunction.
Level of evidence
IIB.
Keywords: ACE2, COVID‐19, SARS‐CoV‐2, smell, taste
Of 2250 patients testing positive for SARS‐CoV‐2 at Mayo Clinic, 667 reported loss of taste and smell. Patients who reported loss of smell or taste were younger, female and more likely to report cough, chest pain, headache, or history of COPD, but overall had fewer high‐risk comorbidities. Those who were older, male, and a reported history of CAD were less likely to report chemosensory dysfunction.

1. INTRODUCTION
The 2019 novel coronavirus (COVID‐19) global pandemic has caused widespread morbidity and mortality and has prompted investigation to better understand and characterize the disease. Smell and taste dysfunction are common symptoms in COVID‐19 patients. 1 , 2 One systemic review and meta‐analysis of smell and taste dysfunction in nearly 20,000 COVID‐19 patients places olfactory dysfunction at 48% and gustatory dysfunction at 41%. 3 Other systematic reviews and meta‐analyses publish similar results regarding the rates of smell and taste dysfunction. 4 , 5 , 6 However, there is little in the literature regarding comorbidities that are associated with reporting loss of smell or taste. Female sex and younger age have been associated with reporting loss of smell or taste in some smaller studies. 7 , 8 This study reports the incidence olfactory and gustatory symptoms and characterizes the associated attributes and comorbidities of 2250 patients who tested positive for SARS‐CoV‐2 at Mayo Clinic from March 23, 2020 to July 31, 2020. Additionally, there is discussion of relevant literature regarding the ACE2 receptor as the cellular entry point for SARS‐CoV‐2, variable expression of ACE2 in certain comorbid conditions, and the role these factors may play on rates of reported chemosensory dysfunction.
2. MATERIALS AND METHODS
2.1. IRB statement
This study was approved by the Mayo Clinic COVID‐19 Research Committee and the Mayo Clinic Institutional Review Board.
2.2. Subjects and setting
In response to COVID‐19, Mayo Clinic created a multidisciplinary telehealth team—COVID Frontline Care Team (CFCT). The CFCT team included physicians, nursing, and allied health staff. Nasopharyngeal PCR samples were collected at Mayo Clinic Rochester and Mayo Clinic Health System (MCHS) satellite sites across Minnesota and Wisconsin from local, regional, national, and international patients. Patients were tested for SARS‐CoV‐2 based on self‐reported symptoms, clinical suspicion, or screening prior to medical care involving aerosol‐generating procedures at Mayo Clinic.
Patients who tested positive were contacted by phone by a member of the CFCT team. As part of the enrollment under the care of the CFCT, demographic and clinical information was obtained by a physician during the initial contact. The physician‐led team recorded patient‐reported comorbidities including smoking status, diabetes, chronic obstructive pulmonary disease (COPD)/emphysema, asthma, chronic lung disease, congestive heart failure, coronary artery disease (CAD), end stage liver disease, and obesity. These comorbidities were analyzed for this study. Furthermore, a clinical risk group system triaged high‐risk patients and identified those most at risk for severe infection and poor clinical outcomes. These groups were established by the following risk factors: age > 65 years; history of diabetes, chronic lung disease (asthma, cystic fibrosis, COPD, emphysema, idiopathic pulmonary fibrosis, and bronchiectasis), congestive heart failure, liver cirrhosis, end‐stage renal disease, or bone marrow/organ transplant; active smoker; active chemotherapy; or obesity with BMI > 40. The low‐risk group was defined by 0 risk factors, medium had 1 risk factor, high had 2 or more, and ultra‐high had 4 or more risk factors.
Symptoms present at the time of contact after the initial positive test were also queried. These included yes or no answers to symptoms of loss of taste or smell, chest pain or tightness, lightheadedness and/or dizziness, cough, fever, chills, muscle aches, sore throat, and headache. The question used to investigate smell and taste was “have you noticed any loss of taste or smell?” There was no objective testing.
Patients included in this study were tested at Mayo Clinic Rochester and the Mayo Clinic Health System (MCHS) from March 23, 2020 to July 31, 2020. Inclusion criteria were any patient who tested positive and gave authorization to share data for research purposes. The only exclusion criteria were insufficient data collection and lack of authorization for research. The Mayo CFCT maintained a prospective clinical REDCap (Research Electronic Data Capture) database for all patients managed by the team. Patients who did not agree to the Minnesota Research Agreement were excluded. For this prospectively enrolled retrospective study, the CFCT clinical database was queried and data abstracted for patients who gave research authorization and were managed from March 23, 2020 to July 31, 2020.
2.3. Statistical methods
Demographic and clinical factors of those who reported loss of taste or smell and those who did not were compared under univariable and multivariable analysis. Univariable comparisons were performed using Chi‐square tests for categorical variables and Kruskal–Wallis tests for continuous explanatory variables, as appropriate. Multivariable logistic regression was performed to examine differences between groups based on variables that met statistical significance on univariable analysis, while accounting for baseline factors such as age and sex. All analyses were performed using SAS version 9.4 (SAS Institute Inc.). Study data were collected and managed using REDCap.
3. RESULTS
From March through July 2020, 192,683 tests for SARS‐CoV‐2 were performed at Mayo Clinic Rochester and across the MCHS. Approximately 4400 SARS‐CoV‐2 positive patients were managed by the CFCT program during this time frame, and 2250 patients met the inclusion criteria. Among these patients testing positive, 1877 were within the low clinical risk group, 155 in the medium clinical risk group, 210 high clinical risk group, and 8 in the ultra‐high clinical risk group. Six hundred and sixty‐seven out of 2250 (29.6%) who tested positive for SARS‐CoV‐2 reported loss of taste or smell (Table 1). There was variation in reporting of loss or taste of smell by risk group: low risk‐ 589/1877 (31.4%), medium risk‐ 35/155(22.6%), high risk‐ 42/210 (20.0%), and ultra‐high 1/8 (12.5%), with p = .0007. The risk groups also had significantly different average ages: low‐risk average age 37.0 years, medium‐risk average age 61.6 years, high‐risk average age 55.8 years, and ultra‐high 61.0 years (p = .0001).
TABLE 1.
Univariate analysis of demographics, comorbidities, and symptoms of patients with COVID‐19 associated loss of taste or smell
| Univariate analysis of patient demographics, comorbidities, and symptoms | ||||
|---|---|---|---|---|
| Loss of taste or smell | ||||
| Reported (N = 667) | Not reported (N = 1583) | Total (N = 2250) | p | |
| Sex, n (%) | .002 a | |||
| Female | 390 (58.5%) | 811 (51.2%) | 1201 (53.4%) | |
| Male | 277 (41.5%) | 772 (48.8%) | 1049 (46.6%) | |
| Age | <.001 b | |||
| N | 667 | 1583 | 2250 | |
| Mean (SD) | 37.2 (14.81) | 41.9 (17.32) | 40.5 (16.75) | |
| Median (IQR) | 34.1 (24.0, 47.7) | 39.3 (26.4, 54.9) | 37.4 (25.7, 53.2) | |
| Range | 17.2, 84.9 | 4.9, 96.1 | 4.9, 96.1 | |
| Race, n (%) | .046 a | |||
| American Indian/Alaskan Native | 1 (0.2%) | 4 (0.3%) | 5 (0.2%) | |
| Asian | 47 (7.2%) | 88 (5.6%) | 135 (6.1%) | |
| Black or African American | 97 (14.8%) | 263 (16.8%) | 360 (16.2%) | |
| Native Hawaii/Pacific Islander | 2 (0.3%) | 0 (0.0%) | 2 (0.1%) | |
| Other | 132 (20.1%) | 265 (16.9%) | 397 (17.9%) | |
| White | 377 (57.5%) | 946 (60.4%) | 1323 (59.5%) | |
| Missing | 11 | 17 | 28 | |
| Ethnicity, n (%) | .323 a | |||
| Hispanic or Latino | 156 (23.5%) | 328 (20.8%) | 484 (21.6%) | |
| Not Hispanic or Latino | 475 (71.4%) | 1173 (74.5%) | 1648 (73.6%) | |
| Other | 34 (5.1%) | 74 (4.7%) | 108 (4.8%) | |
| Missing | 2 | 8 | 10 | |
| Current smoker, n (%) | .053 a | |||
| Present | 47 (7.0%) | 79 (5.0%) | 126 (5.6%) | |
| Absent | 620 (93.0%) | 1504 (95.0%) | 2124 (94.4%) | |
| Diabetes, n (%) | .152 a | |||
| Present | 56 (8.4%) | 164 (10.4%) | 220 (9.8%) | |
| Absent | 611 (91.6%) | 1419 (89.6%) | 2030 (90.2%) | |
| COPD/emphysema, n (%) | .322 a | |||
| Present | 10 (1.5%) | 16 (1.0%) | 26 (1.2%) | |
| Absent | 657 (98.5%) | 1567 (99.0%) | 2224 (98.8%) | |
| Asthma, n (%) | .819 a | |||
| Present | 58 (8.7%) | 133 (8.4%) | 191 (8.5%) | |
| Absent | 609 (91.3%) | 1450 (91.6%) | 2059 (91.5%) | |
| Chronic lung disease, n (%) | .787 a | |||
| Present | 18 (2.7%) | 46 (2.9%) | 64 (2.8%) | |
| Absent | 649 (97.3%) | 1537 (97.1%) | 2186 (97.2%) | |
| Congestive heart failure, n (%) | .026 a | |||
| Present | 4 (0.6%) | 29 (1.8%) | 33 (1.5%) | |
| Absent | 663 (99.4%) | 1554 (98.2%) | 2217 (98.5%) | |
| Coronary artery disease, n (%) | <.001 a | |||
| Present | 6 (0.9%) | 59 (3.7%) | 65 (2.9%) | |
| Absent | 661 (99.1%) | 1524 (96.3%) | 2185 (97.1%) | |
| End stage liver disease, n (%) | .980 a | |||
| Present | 3 (0.4%) | 7 (0.4%) | 10 (0.4%) | |
| Absent | 664 (99.6%) | 1576 (99.6%) | 2240 (99.6%) | |
| Obesity, n (%) | .286 a | |||
| Present | 29 (4.3%) | 86 (5.4%) | 115 (5.1%) | |
| Absent | 638 (95.7%) | 1497 (94.6%) | 2135 (94.9%) | |
| Chest pain or tightness, n (%) | <.001 a | |||
| Present | 106 (15.9%) | 143 (9.0%) | 249 (11.1%) | |
| Absent | 561 (84.1%) | 1440 (91.0%) | 2001 (88.9%) | |
| Lightheadedness and/or dizziness, n (%) | .731 a | |||
| Present | 6 (0.9%) | 12 (0.8%) | 18 (0.8%) | |
| Absent | 661 (99.1%) | 1571 (99.2%) | 2232 (99.2%) | |
| Cough, n (%) | <.001 a | |||
| Present | 346 (51.9%) | 666 (42.1%) | 1012 (45.0%) | |
| Absent | 321 (48.1%) | 917 (57.9%) | 1238 (55.0%) | |
| Fever, n (%) | .378 a | |||
| Present | 183 (27.4%) | 406 (25.6%) | 589 (26.2%) | |
| Absent | 484 (72.6%) | 1177 (74.4%) | 1661 (73.8%) | |
| Chills, n (%) | <.001 a | |||
| Present | 163 (24.4%) | 282 (17.8%) | 445 (19.8%) | |
| Absent | 504 (75.6%) | 1301 (82.2%) | 1805 (80.2%) | |
| Myalgia, n (%) | <.001 a | |||
| Present | 284 (42.6%) | 522 (33.0%) | 806 (35.8%) | |
| Absent | 383 (57.4%) | 1061 (67.0%) | 1444 (64.2%) | |
| Sore throat, n (%) | <.001 a | |||
| Present | 180 (27.0%) | 309 (19.5%) | 489 (21.7%) | |
| Absent | 487 (73.0%) | 1274 (80.5%) | 1761 (78.3%) | |
| Headache, n (%) | <.001 a | |||
| Present | 337 (50.5%) | 555 (35.1%) | 892 (39.6%) | |
| Absent | 330 (49.5%) | 1028 (64.9%) | 1358 (60.4%) | |
| Anosmia/dysguesia, n (%) | <.001 a | |||
| Present | 667 (100.0%) | 0 (0.0%) | 667 (29.6%) | |
| Absent | 0 (0.0%) | 1583 (100.0%) | 1583 (70.4%) | |
Chi‐square p value.
Kruskal–Wallis p value.
The mean age of patients with loss of taste or smell was 37.2 years versus 41.9 years without smell or taste loss (p = .002) (Table 1). A 32.5% of SARS‐CoV‐2 positive females reported loss of smell or taste versus 26.4% males (p = .002) (Table 1). By race, the highest reporting group was Asian with 34.8%, Other 33.2%, White 28.5%, and Black/African American 26.9% (p = .046) (Table 1). When the Hawaiian/Pacific Island group and American Indian/Alaskan Native group are removed from Chi‐Square analysis, p = .098. The four largest groups, which makes up 98.4% of the cohort, were compared to one another and found no statistical difference between any two groups. White versus Other (p = .069), White versus Black (p = .561), Black versus Other (p = .059), White versus Asian (p = .123), Black versus Asian (p = .086), and Other versus Asian (p = .739).
Symptoms of chest pain or tightness, cough, chills, myalgia, sore throat, and headache (all p < .001) were associated with loss of taste or smell on univariate analysis (Table 1). Notably, fever was not associated with loss of taste or smell in patients who had tested positive for SARS‐CoV‐2 (p = .378, Table 1). Congestive heart failure and CAD were associated with not reporting loss of taste or smell on univariate analysis (p = .026 and <.001, respectively, Table 1).
Multivariate analysis of the data was conducted as described above (Table 2). CAD (OR 0.325, p = .01) and older age (OR 0.985, p = .0001) were associated with not reporting loss of taste or smell (Figure 1). Female sex (OR 1.237, p = .03), chest pain/tightness (OR 1.558, p = .002), cough (OR 1.384, p = .001), and headache (OR 1.636, p < .0001) were all associated with higher odds of reporting loss of taste or smell (Figure 1). After multivariate analysis, COPD/emphysema did not reach statistical significance (p = .06), and only 26 patients reported having COPD. However, a history COPD/emphysema was correlated with an odds ratio of 2.26 of reporting loss of taste or smell (Figure 1). This effect size was present after accounting for all other variables.
FIGURE 1.
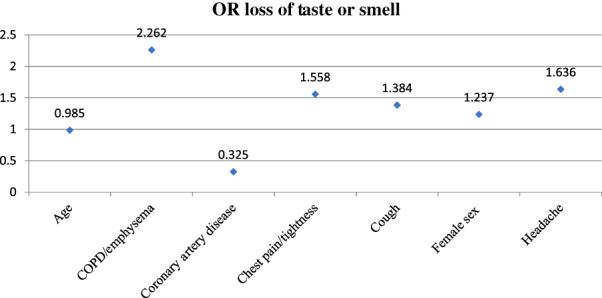
Graph depicting odds ratios of reporting loss of taste or smell by clinical factor
4. DISCUSSION
Olfactory and gustatory dysfunction have been widely reported as symptoms of COVID‐19. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 Published rates of subjective altered smell or taste with COVID‐19 vary widely, from 48% and 41%, respectively in one systematic review and meta‐analysis, to as high as 86% and 88%, respectively in a study that utilized a focused olfactory and gustatory dysfunction questionnaires. 1 , 3 Our reported rate (29.6%) of subjective smell or taste loss as assessed by a physician survey of newly SARS‐CoV‐2 positive patients is lower. Our findings of higher rates of reported olfactory or gustatory dysfunction in females and younger patients agree with several recently published studies. 1 , 5 , 7 , 8 Among the recorded races, there is a statistical difference when all groups are included in Chi‐square analysis. However, with removal of the Native Hawaii/Pacific Islander and American Indian/Alaskan Native groups (7 total patients of 2250), no statistical difference is seen between the remaining four groups, or when comparing the largest four groups to each other. A 34.8% of the Asian group reported loss of taste or smell, which is consistent with a meta‐analysis of 30,000 COVID‐19 patients that found Asians to have the highest rate of chemosensory dysfunction, though not found to be statistically significant in our data. 12
In the patient interview via phone with a physician, patients were asked “have you noticed any loss of taste or smell?” This was a single question in a questionnaire obtaining clinical and symptomatic data. It may be that the lower reported rate of loss of smell or taste at our institution is related to this being an isolated, subjective question among a lengthy interview. One study that utilized objective olfactory testing reported 98% of patients having at least some olfactory dysfunction. 10 Another possible influence on the observed rate was the Mayo Clinic patient screening process. To safely care for patients during the timeframe of the study, SARS‐CoV‐2 PCR nasopharyngeal testing was conducted on all patients, regardless of clinical presentation, within 7 days of an outpatient clinic visit and within 2 days of a planned surgical procedure. It is possible that this high volume of pre‐visit screening of asymptomatic patients contributes to our lower observed rate of reported chemosensory dysfunction. However, data recording the indication for administering the SARS‐CoV‐2 PCR test completed as part of patient screening was not collected by the CFCT. Previously published studies include independently answered patient surveys and physician‐led patient survey. 1 , 2 , 13 Length of time between testing positive and data collection in these studies was not clearly defined and not uniform from patient to patient. Early in the COVID‐19 outbreak in the United States, it was noted that symptoms may lag 2–14 days behind infection. 11 In more recent studies, it was reported that onset of anosmia or ageusia was 4–5 days after the onset of other symptoms of COVID‐19. 14 , 15 There is some variability in the literature however, with 13%–79% of patients having olfactory or gustatory symptoms prior to the onset of other symptoms of COVID‐19. 16 In our study, data was collected within 24 h of positive test result, which could contribute to our lower observed rate, with testing of symptomatic patients potentially occurring prior to the onset of alteration of smell or taste or testing of asymptomatic patients prior to onset of symptoms due to screening protocols for patients.
By clinical risk groups, the low‐risk group reported the highest rate of loss of smell or taste. These patients had zero of the listed risk factors potentially associated with poor outcomes with COVID infection as listed in the methods section. Notably, the rate of reported loss of smell or taste decreased in each of the higher risk groups. This represents decreased reporting of loss of taste or smell in patients with more reported chronic medical conditions. In 2011–2012, it was observed that patients with history of heart failure, heart attack, and liver disease reported taste disorder in the previous year, and those with asthma and emphysema reported smell disorder in the previous year. 17 It is also well documented that many medications, including antibiotics, antihistamines, antihypertensives, and statins can impair smell and taste. 18 At baseline, older patients have decreased olfactory and gustatory function, and this may be further complicated by comorbidities and increased medication use in older patients. 18 , 19 In our cohort, it may be that younger patients are more sensitive to acute change in taste or smell due to higher baseline chemosensory function.
Our findings regarding CAD in relation to smell and taste symptoms associated SARS‐CoV‐2 infection are, to the best of our knowledge, the first recognition of these relationships in the literature. On multivariate analysis, the presence of comorbid CAD was associated with lower rates of reporting loss of smell or taste (OR 0.325, p < .0001). Review of the literature does not reveal a clear connection between CAD and loss of taste or smell, though one notes the opposite association, that those with olfactory dysfunction were more likely to be free of cardiovascular disease. 20 It has been described that in patients with CAD, ACE2 expression is increased, both in circulating plasma, as well as in cardiac tissue. 21 The role of circulating ACE2 and its effect on smell and taste are unclear. No data are published regarding the relationship between circulating ACE2 and nasal ACE2 expression. It has been shown that ACE2 is expressed on the apical sustentacular cells of the olfactory neuroepithelium, rather than the olfactory neurons and is thought to be the site of binding of SARS‐CoV2. 22 This gives credence to the theory that olfactory loss is caused due to damage of the supporting cells within the olfactory epithelium. 9 , 22 It has also been reported that use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers, which are common in patients with CAD, does not affect the expression of nasal ACE2 expression. 23 Though the data in this study conflicts with other published data, the mechanism of effect of cardiovascular disease on loss of smell or taste in SARS‐CoV‐2 infection remains not fully understood and represents a possible area of further research.
COPD was associated with an OR of 2.26 for reporting of taste and smell loss, although this did not reach statistical significance (p = .06), and only 26 patients reported having COPD. This data is included because of the magnitude of correlation, which means that a patient having COPD was correlated with a 226% greater likelihood of reporting loss of taste or smell. It has been described that the ACE2 receptor is the binding site of cellular entry for SARS‐CoV‐2, and data has demonstrated that the ACE2 is most highly expressed in the nasal cavity. 24 There are also several recently published studies that found increased lung expression of ACE2 in those with COPD. 24 , 25 , 26 Although the correlation between lung and nasal ACE2 expression has not been reported in patients with COPD, if nasal ACE2 expression is increased with COPD, this may explain the association of COPD with reporting loss of taste and smell in our study. 27 In our study, younger patients were more likely to report loss of smell or taste. It is possible though that older patients, with reduced olfactory function, may not have noticed a change in sense of smell compared to younger patients. 19 However, this is confounded by the fact that children express lower levels of ACE2 in their nasal epithelium compared to adults. 28 The conflicting nature of published data highlights the need to further investigate the pathogenesis of alteration of smell and taste caused by SARS‐CoV‐2 infection. Overall, any conclusions regarding COPD and loss of smell or taste must be made with caution, given the non‐significant nature of the data and the few number of patients (n = 26) reporting COPD. Characterization of nasal expression of ACE2 in COPD and CAD may allow for better understanding of the pathophysiology of smell and taste dysfunction, or lack thereof, in these patients.
This data is unique to the literature due to the volume of patients directly interviewed and the data collection model. The data reported here represents the largest single institution study investigating COVID‐19 related olfactory and gustatory dysfunction, with 192,683 tests performed, and 2250 SARS‐CoV‐2 positive patients included. This is the only study that we are aware of that includes patient data directly collected from the patient by a physician within 24 h after testing positive. We believe that this may improve reliability of the data and reduce recall bias.
The collection of this data was not completed with the primary goal of investigating change in taste or smell, and this data is now 2 years old which limits applicability to more recent Coronavirus variants Objective testing of smell and taste function was not assessed. This limits our study in that reported chemosensory dysfunction was investigated only by asking whether symptoms of loss of smell or taste were present. In addition, the combination of asking about taste and smell together in one question does not allow for differentiation between olfactory and gustatory dysfunction. Some biases could include the short time frame from positive test to interview, testing of asymptomatic patients, perceived pressure with questions from a physician, and possible social stigma associated with reporting COVID‐19 symptoms.
5. CONCLUSION
Loss of smell and/or taste are widely reported as a part of the clinical syndrome of COVID‐19. This study details the clinical comorbidities and symptoms associated with reporting loss of taste or smell in a large patient cohort. Among 2250 SARS‐CoV‐2 positive patients at a single institution, 667 (29.6%) reported having symptoms of loss of taste or smell within 24 h of a positive test. These patients were younger, female and more likely to report cough, chest pain, headache, and had fewer high‐risk comorbidities. Those who were older, male, or reported a history of CAD were less likely to report chemosensory dysfunction. ACE2 has been identified as the cellular entry point for SARS‐CoV‐2, and further understanding of nasal expression of ACE2 may reveal the basis for our observations with regard to CAD. Our data are the largest single institution data investigating COVID‐19 associated loss of smell or taste, and the first to find a positive association of COPD and a positive association of CAD as factors that affect rates of reported chemosensory dysfunction.
FUNDING INFORMATION
This work was funded by the Kerry Olsen Professorship Joseph I and Barbara Ashkins Professor of Surgery Award, as part of the Mayo Clinic Department of Otorhinolaryngology.
CONFLICTS OF INTEREST
No conflicts of interest for any of the authors.
ACKNOWLEDGMENTS
We would like to acknowledge the COVID Care Frontline Team at Mayo Clinic, as well as all healthcare providers, and all their work caring for those with COVID‐19.
Johnson BJ, Salonen B, O'Byrne TJ, et al. Patient factors associated with COVID‐19 loss of taste or smell patient factors in smell/taste loss COVID‐19. Laryngoscope Investigative Otolaryngology. 2022;7(6):1688‐1694. doi: 10.1002/lio2.911
REFERENCES
- 1. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibekwe TS, Fasunla AJ, Orimadegun AE. Systematic review and meta‐analysis of smell and taste disorders in COVID‐19. OTO Open. 2020;4(3):2473974X20957975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori‐Asenso R. Smell and taste dysfunction in patients with COVID‐19: a systematic review and meta‐analysis. Mayo Clin Proc. 2020;95(8):1621‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID‐19): a meta‐analysis of 27,492 patients. Laryngoscope. 2021;131(4):865‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 7. Martinez AG, Galvez MA, Sanz SR, Ruiz PH, Morillas AG, Sanchez TE. Incidence of smell and taste disorders and associated factors in patients with mild to moderate COVID‐19. Otolaryngol Pol. 2020;75(2):1‐5. [DOI] [PubMed] [Google Scholar]
- 8. Husain Q, Kokinakos K, Kuo YH, Zaidi F, Houston S, Shargorodsky J. Characteristics of COVID‐19 smell and taste dysfunction in hospitalized patients. Am J Otolaryngol. 2021;42(6):103068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID‐19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021;748:135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10(8):944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the new York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis reveals significant ethnic differences. ACS Chem Nerosci. 2020;11(19):2944‐2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fantozzi PJ, Pampena E, Di Vanna D, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID‐19. Am J Otolaryngol. 2020;41(6):102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19. Med Mal Infect. 2020;50(5):436‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID‐19 patients: single‐center experience on 72 cases. Head Neck. 2020;42(6):1252‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santos REA, da Silva MG, do Monte Silva MCB, et al. Onset and duration of symptoms of loss of smell/taste in patients with COVID‐19: a systematic review. Am J Otolaryngol. 2021;42(2):102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiue I. Adult taste and smell disorders after heart, neurological, respiratory and liver problems: US NHANES, 2011‐2012. Int J Cardiol. 2015;179:46‐48. [DOI] [PubMed] [Google Scholar]
- 18. Schiffman SS. Influence of medications on taste and smell. World J Otorhinolaryngol Head Neck Surg. 2018;4(1):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doty RL. Age‐related deficits in taste and smell. Otolaryngol Clin North Am. 2018;51(4):815‐825. [DOI] [PubMed] [Google Scholar]
- 20. Mercier J, Osman M, Bouiller K, et al. Olfactory dysfunction in COVID‐19, new insights from a cohort of 353 patients: the ANOSVID study. J Med Virol. 2022;94:4762‐4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burrell LM, Harrap SB, Velkoska E, Patel SK. The ACE2 gene: its potential as a functional candidate for cardiovascular disease. Clin Sci (Lond). 2013;124(2):65‐76. [DOI] [PubMed] [Google Scholar]
- 22. Chen M, Shen W, Rowan NR, et al. Elevated ACE‐2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS‐CoV‐2 entry and replication. Eur Respir J. 2020;56(3):2001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee IT, Nakayama T, Wu CT, et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 2020;11(1):5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sungnak W, Huang N, Becavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J. 2020;55(5):2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: implication for COVID‐19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy. 2020;50:1313‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hou YJ, Okuda K, Edwards CE, et al. SARS‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429‐446 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saheb Sharif‐Askari N, Saheb Sharif‐Askari F, Alabed M, et al. Airways expression of SARS‐CoV‐2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


