Abstract
Background
Diagnosis of influenza in patients admitted to hospital is delayed due to long turnaround times with laboratory testing, leading to inappropriate and late antiviral treatment and isolation facility use. Molecular point-of-care tests (mPOCTs) are highly accurate, easy to use, and generate results in less than 1 h, but high-quality evidence for their effect on management and clinical outcomes is needed. The aim of this study was to assess the clinical impact of an mPOCT on influenza detection, antiviral use, infection control measures, and clinical outcomes in adults admitted to hospital with acute respiratory illness.
Methods
In this multicentre, pragmatic, open-label, randomised controlled trial (FluPOC), we recruited adults admitted to hospital with acute respiratory illness during influenza seasons from two hospitals in Hampshire, UK. Eligible patients were aged 18 years and older, with acute respiratory illness of 10 days or fewer duration before admission to hospital, who were recruited within 16 h of admission to hospital. Participants were randomly assigned (1:1), using random permuted blocks of varying sizes (4, 6 and 8), to receive mPOCT for influenza or routine clinical care (control group). The primary outcome was the proportion of patients infected with influenza who were treated appropriately with antivirals (neuraminidase inhibitors) within 5 days of admission. Safety was assessed in all patients. Secondary outcomes included time to antivirals, isolation facility use, and clinical outcomes. This study is registered with the ISRCTN registry, ISRCTN17197293, and is now complete.
Findings
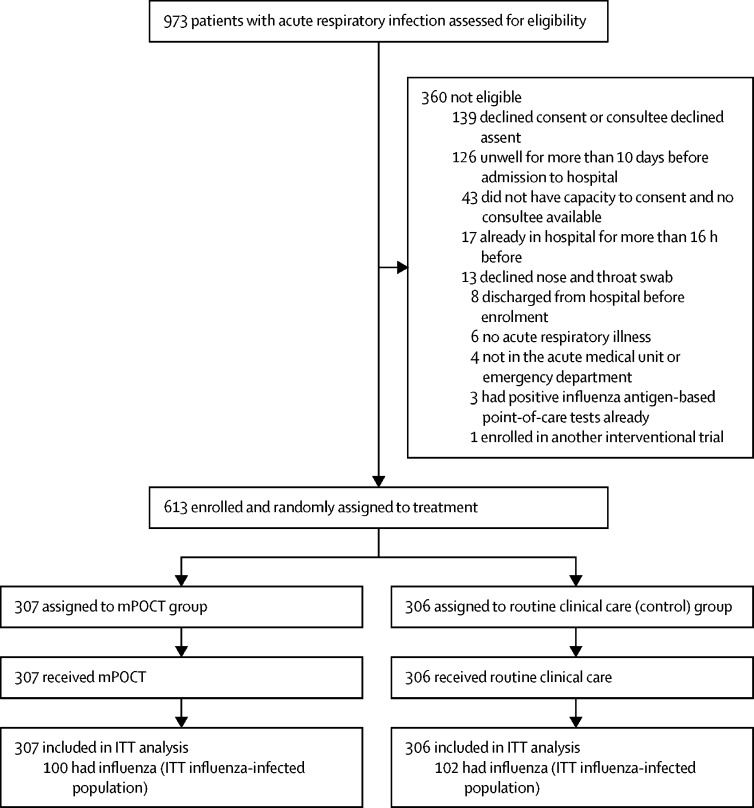
Between Dec 12, 2017, and May 3, 2019, over two influenza seasons, 613 patients were enrolled, of whom 307 were assigned to the mPOCT group and 306 to the control group, and all were analysed. Median age was 62 years (IQR 45–75) and 332 (54%) of 612 participants with data were female. 100 (33%) of 307 patients in the mPOCT group and 102 (33%) of 306 in the control group had influenza. 100 (100%) of 100 patients with influenza were diagnosed in the mPOCT group and 60 (59%) of 102 were diagnosed though routine clinical care in the control group (relative risk 1·7, 95% CI 1·7–1·7; p<0·0001). 99 (99%) of 100 patients with influenza in the mPOCT group were given antiviral treatment within 5 days of admission versus 63 (62%) 102 in the control group (relative risk 1·6, 95% CI 1·4–1·9; p<0·0001). Median time to antivirals was 1·0 h (IQR 0·0 to 2·0) in the mPOCT group versus 6·0 h (0·0 to 12·0) in the control group (difference of 5·0 h [95% CI 0·0–6·0; p=0·0039]). 70 (70%) of 100 patients with influenza in the mPOCT group were isolated to single-room accommodation versus 39 (38%) of 102 in the control group (relative risk 1·8 [95% CI 1·4–2·4; p<0·0001]). 19 adverse events occurred among patients with influenza in the mPOCT group compared with 34 events in the control group. No patients with influenza died in the mPOCT group and two (2%) died in the control group (p=0·16).
Interpretation
Routine mPOCT for influenza was associated with improved influenza detection and improvements in appropriate and timely antiviral and isolation facility use. Routine mPOCT should replace laboratory-based diagnostics for acute admissions to hospital during the influenza season.
Funding
National Institute for Health Research.
Introduction
The influenza virus causes seasonal epidemics of acute respiratory illness every winter in temperate climates, leading to large numbers of admissions to hospital, principally among adults (ie, aged ≥18 years).1, 2, 3, 4 Adults who are admitted to hospital with influenza are frequently admitted to critical care units and 3–15% will die in hospital.5 Influenza often remains undiagnosed in patients admitted to hospital due to the absence of universal testing for influenza in patients with acute respiratory illness and the inaccuracy of testing based on clinical suspicion of infection.6 Therefore, many patients infected with the influenza virus are not identified and treated appropriately with antivirals or isolated correctly in single-room accommodation to prevent the spread of influenza to others. Even when patients are tested for influenza, diagnosis is often delayed due to the long turnaround times of centralised laboratory PCR testing.7 This delay also contributes to the inappropriate and late use of antivirals and isolation facilities and impairs patient flow through acute areas, reducing hospital operational capacity.
Research in context.
Evidence before this study
We searched PubMed, the Cochrane Controlled Clinical Trials Register, and ClinicalTrials.gov, for relevant published articles and ongoing trials assessing the clinical impact of molecular point-of-care tests (mPOCTs) for influenza in adults (aged ≥18 years) admitted to hospital with acute respiratory illness. We used the search terms (”point-of-care testing” OR “rapid PCR testing” OR ‘'rapid molecular testing'’) AND “influenza” AND “hospital” AND “adult” AND (“clinical trial” OR “randomised controlled trial” OR ‘'trial'’ OR “study”). We restricted our search to studies published between Jan 1, 1980, and Jan 1, 2020, in English. We excluded studies using antigen-based point-of-care tests for influenza (including digital immunoassays), studies in children, and studies reporting only diagnostic accuracy. We found no Cochrane systematic reviews for mPOCT in adults. We found two randomised controlled trials of mPOCT for influenza and other respiratory viruses assessing clinical outcomes and one quasi-randomised trial of low quality. One of these studies did not specifically report the effect of influenza detection on outcomes. The other two studies suggested improvement in use of neuraminidase inhibitors and isolation facilities with mPOCT but were constrained by the low number of patients infected with influenza or other methodological issues. We found several retrospective observational studies in adults admitted to hospital assessing the effect of mPOCTs on clinical outcomes including neuraminidase inhibitor use, antibiotics, length of hospital stay, and number of investigations. These studies all broadly suggested improvements in neuraminidase inhibitor use and investigations but did not reliably assess use of isolation facilities and were conflicting regarding the effect on antibiotic use. One study suggested a reduction in nosocomial spread of influenza. No studies reported on the effect of mPOCT for influenza on clinical outcomes other than length of hospital stay. We found a systematic review of studies assessing the clinical impact and diagnostic accuracy of rapid molecular tests for respiratory viruses, only some of which were done at the point of care and all were already identified in our initial literature search.
Added value of this study
To our knowledge, this is the first multicentre, pragmatic, randomised control trial of mPOCT for influenza that was designed and powered to assess influenza-specific outcomes in adults admitted to hospital. Retrospective testing for influenza in all patients in the control group allowed a direct assessment of missed diagnoses and comparison of outcomes in all patients with influenza tested with mPOCT or routine clinical care. The use of clinical outcome measures such as time to clinical improvement, time on supplementary oxygen, and the hospital recovery scale score, allowed assessment of the effect of mPOCT on clinical outcomes in addition to the effect on clinical management (eg, use of antivirals, antibiotics, isolation facilities). Although this study does not definitively exclude the possibility that routine laboratory PCR testing could lead to some of the improvements seen with mPOCTs, such improvements would require substantial reductions in current turnaround times that are unlikely to be achievable in most laboratories due to batch testing and other constraints.
Implications of all the available evidence
Routine mPOCTs for influenza in adults admitted to hospital during influenza season improves the detection rate of influenza, and the appropriate use of influenza antivirals and isolation facilities. Routine use of mPOCTs for influenza should become standard of care in hospitals during the influenza season.
Antiviral treatment with neuraminidase inhibitors is recommended for all patients admitted to hospital with suspected and proven influenza by UK and US national guidelines8, 9 and evidence suggests that earlier use is associated with better outcomes.10, 11 New rapid molecular point-of-care tests (mPOCTs) using PCR for influenza are highly accurate, easy to use, and can generate a result in less than 1 h,12, 13, 14, 15 compared with the current turnaround time of approximately 24 h for laboratory-based PCR testing.7 Routinely testing patients for influenza at the point of care might improve influenza detection and use of antivirals and isolation facilities by allowing confident real-time decision making.7, 16, 17, 18, 19, 20
Results of our previous trial7 suggested that the use of routine mPOCT for respiratory viruses improves the use of antivirals and isolation facilities for influenza, but was hampered by small numbers of patients who were positive for influenza and an absence of universal viral testing in the control group.7 Several retrospective observational studies have also suggested that mPOCT might improve the detection of influenza, the use of influenza antivirals and isolation facilities, and might reduce nosocomial transmission;15, 16, 17, 18, 19 however, such studies are highly prone to bias. Because mPOCTs for influenza are now starting to be used in some UK National Health Service (NHS) hospitals and in other countries around the world, high-quality evidence for their clinical impact is urgently needed. We aimed to prospectively assess the effect of a routine molecular point-of-care test-and-treat strategy for influenza in adults admitted to hospital with acute respiratory illness on clinical management and patient outcomes.
Methods
Study design and participants
In this multicentre, pragmatic, parallel group, open-label, randomised, controlled trial (FluPOC), patients were recruited from two acute hospitals in Southampton and Winchester, Hampshire, UK. The trial took place over two successive winter seasons in 2017–18 and 2018–19 when influenza was circulating according to national (ie, Public Health England) surveillance systems. All patients were recruited from the acute medical unit and emergency department of Southampton General Hospital, Southampton, UK (a large, acute-care, teaching hospital in the south of the UK serving a population of 650 000 for secondary care) run by University Hospital Southampton Foundation NHS Trust, and from Royal Hampshire County Hospital (Winchester, UK) a large district general hospital in Hampshire run by Hampshire Hospitals Foundation NHS Trust.
Patients were eligible if they: were aged 18 years or older; had the capacity to give informed, written consent, or if the patient did not have the capacity to consent for themselves, consultee assent was obtained; were admitted to the hospital and physically in either the acute medical unit or emergency department; could be recruited to the study within 16 h of admission; had an acute respiratory illness; and had been ill for 10 days or fewer before admission to hospital. An episode of acute respiratory illness was defined as a provisional diagnosis of acute pulmonary illness including pneumonia, bronchitis (non-pneumonic lower respiratory tract infection) and influenza-like illness, or an acute exacerbation of a chronic respiratory illness (including exacerbation of chronic obstructive pulmonary disease, asthma, or bronchiectasis). Patients were excluded if a palliative approach was being taken by the treating clinicians; the patient declined nasal or pharyngeal swabbing; and if they had previously been included in this study and were re-presenting within 30 days of hospital discharge.
The study was approved by the South Central − Hampshire A Research Ethics Committee (reference 17/SC/0368, approved on the Sept 7, 2017). The protocol has been published previously.20 The protocol was amended once on Nov 23, 2017, to allow the inclusion of a second study site (Hampshire Hospitals Foundation NHS Trust) and this change was communicated to the trial registry.
Randomisation and masking
Participants were consecutively assigned a unique participant identification number by study team members (NJB, AKM, SP, CC, SM, KRB, EN, and LP) who then, using an internet-based randomisation service (Sealed envelope, which uses random permuted blocks of varying sizes; 4, 6 and 8) to generate the allocation sequence. Using the allocation code, participants were assigned (1:1) to either the mPOCT group (intervention group) or the routine clinical care group (control group). Due to the nature of the intervention, participants, research staff, and clinical care providers were unmasked to assignment. Data analysts and statisticians (including SE) were masked to group allocation.
Procedures
Participants randomly assigned to the intervention (mPOCT) group had a combined nose and throat swab sample (and sputum samples where available) taken by research staff according to standard protocols, which was then analysed immediately using the FilmArray Respiratory Panel 2 (BioFire Diagnostics, a bioMérieux company, Salt Lake City, UT, USA)—ie, the mPOCT. The FilmArray Respiratory Panel 2 uses nested PCR to detect a panel of respiratory viruses and atypical bacteria, including influenza A and B. The BioFire 2.0 testing units were located in the acute medical units and the test took around 45 min to generate a result. The clinical and infection control teams were informed directly of all results (positive and negative). Participants randomly assigned to the control group were managed according to routine clinical care, with testing for respiratory viruses being at the discretion of the responsible clinical team, and any testing was done using laboratory PCR by conventional methods in the on-site laboratory facilities. Patients in the control group also had a nose and throat swab obtained at enrolment and stored for subsequent analysis at least 30 days after enrolment (using the FilmArray Respiratory Panel 2) to allow retrospective assessment of missed diagnoses. Clinical management decisions were made independently by the responsible clinical team and if influenza was detected by mPOCT, research teams directed clinical teams to national guidelines for the treatment of influenza.9
Demographic and clinical data were collected at recruitment and outcome data were collected retrospectively from paper case notes, electronic medical records, and electronic prescribing systems. All data were collected on a standardised case report form. Serious adverse events were actively monitored and reported. A serious adverse event was defined as any adverse event that: results in death, was life threatening, required admission to hospital or extension of stay in hospital, resulted in persistent or clinically significant disability or incapacity, or consisted of a congenital anomaly or birth defect. Participants already admitted to an acute medical unit and emergency department but with a decision already made to admit were considered already admitted to hospital. However, an adverse event leading to an extended ongoing stay in hospital was counted as a serious adverse event.
Outcomes
The primary outcome measure was the proportion of patients with confirmed influenza (ie, positive PCR test) who were treated with antivirals (ie, neuraminidase inhibitors) during their hospital stay, within 5 days of admission. This outcome measure was selected because Public Health England guidelines recommend that all adults requiring admission to hospital for influenza should receive neuraminidase inhibitors. This guidance is consistent with that in several other countries.8, 9 Although, to our knowledge, no placebo-controlled trials of neuraminidase inhibitors have been done in adults who have been admitted to hospital, there is observational evidence showing that use of neuraminidase inhibitors are associated with improvements in clinical outcomes in this group.10,11,21–23
Secondary outcomes were as follows: the proportion of patients with influenza (ie, PCR positive) identified during their time in hospital, turnaround time for respiratory virus testing, proportion of all antiviral (ie, neuraminidase inhibitors) use occurring in patients with influenza and without influenza, time to antiviral commencement, duration of antiviral use in patients with influenza and without influenza, total doses of antiviral used (not reported here), proportion of patients with and without influenza treated with antibiotics, proportion of patients with and without influenza treated with single doses or brief courses (<24 h) of antibiotics, duration of antibiotic use, proportion of patients using isolation facilities, duration of isolation facility use, proportion of patients with influenza who were isolated, time to isolation for patients with influenza, time from admission to isolation of patients without influenza (not reported here), duration of stay in hospital for all patients, time on supplementary oxygen for all patients, time to clinical improvement (originally defined as time to clinical stability in the published protocol20 and subsequently changed to time to clinical improvement, which is a newly developed outcome measure for trials of influenza antivirals; full details and relevant references are provided in the appendix [p 2]), proportion of patients requiring admission to an intensive care unit (ICU) or high dependency unit (HDU), median duration of stay in ICU or HDU (not reported here), proportion of patients re-presenting to hospital within 30 days, proportion of patients readmitted to hospital within 30 days, in-hospital and 30-day all-cause mortality, and 60-day all-cause mortality (not reported here).
Exploratory outcomes reported here were median hospital recovery score at days 4 and 7 (the hospital recovery scale is a 6-point ordinal scale developed as an outcome measure for trials of influenza therapeutics, with higher scores denoting a worse outcome; appendix p 2). Additional exploratory outcomes not reported here are difference in influenza viral load between upper and lower respiratory tract samples; proportion of patients with influenza RNA detected in blood; and changes in influenza viral load over time (kinetics) in respiratory tract samples and blood. These will be reported elsewhere.
All outcomes were measured for the duration of hospital stay or up to 30 days (whichever was shortest) unless otherwise specified and the duration of antimicrobials includes medication (antibiotics and antivirals) that patients were discharged home with.
Statistical analysis
The sample size for our study was based on the primary outcome measure of the proportion of patients testing positive for the influenza virus and treated with neuraminidase inhibitors (within 5 days of hospital admission). We initially aimed to recruit up to 840 participants (420 patients per group) over up to three influenza seasons, on the conservative assumption of a 25% positivity rate for influenza during influenza seasons (based on our previous local studies over several seasons7)—ie, approximately 100 patients being positive for influenza in each group. These group sizes would give a 90% power at a 0·05 significance level to detect a difference of 20% in neuraminidase inhibitor use for patients with influenza between the groups (ie, from 65% to 85%).8 Because 100 patients or more with confirmed influenza were recruited to each group by the end of the second season, the trial was stopped at this point.
Analysis was by intention to treat (ITT) and the framework was superiority. Analyses were done by a dedicated statistician from the University of Southampton, Southampton, UK (SE) who was masked to group allocation. We had complete data for the primary outcome and, overall, we had minimal missing data (<1%). We measured the primary outcome only in patients with influenza (ie, the ITT influenza-infected population). We used difference in proportions and unadjusted risk ratios (ie, relative risk) to compare the groups. We calculated the number needed to test (1/absolute risk ratio) for the primary outcome to allow assessment of the number of patients required to receive mPOCT to have a clinical impact on one person, above routine care. The prespecified plan was to analyse antiviral use in a logistic regression model; however, the model could not be fitted due to separation, at least partly as a result of only one person testing positive for influenza in the mPOCT not receiving antiviral treatment. No further multivariable analyses were attempted given the strong signal. Secondary outcome measures were measured both in all patients (ITT population) and in the ITT influenza-infected population as appropriate. Difference in proportions was assessed using the χ2 test or Fisher's exact test as appropriate, depending on group size. For secondary outcomes, we compared the intervention and control groups for equality of proportions for binary data and used Student's t tests or Mann-Whitney U tests for continuous data (eg, turnaround time), as appropriate; the choice between these two analyses was based on the distribution of the observed data and the sample size. Time-to-event analysis data were compared using the log-rank test. Where 95% CIs are presented, we used Stata defaults.
We did a post-hoc analysis to assess median time to receipt of antivirals, excluding patients treated empirically, and the proportion of patients isolated, excluding those discharged rapidly or nursed in areas without isolation facilities.
All analyses were done using Prism version 7.0 (GraphPad Software, La Jolla, CA, USA) and Stata version 16.0. This study was prospectively registered with the ISRCTN registry, ISRCTN17197293.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all of the data and the final responsibility to submit for publication.
Results
Between Dec 12, 2017, and May 3, 2018, and between Dec 3, 2018, and May 3, 2019, over two influenza seasons, 973 patients were assessed for eligibility and 613 were randomly assigned to the mPOCT (n=307) or routine clinical care group (n=306; figure 1 ). Because 100 or more patients with confirmed influenza were recruited to each group by the end of the second season, the trial was stopped at this point. Overall the median age of study population was 62 years (IQR 45–75) and 332 (54%) of 612 participants were female (data missing for one patient). Baseline characteristics for all patients (ITT population) and influenza-infected patients (ITT influenza-infected population) are shown in table 1 .
Figure 1.
Study profile
ITT=intention-to-treat. mPOCT=molecular point-of-care test.
Table 1.
Baseline characteristics of all patients (ITT population) and ITT influenza-infected population
|
All patients (ITT) |
ITT influenza-infected population |
|||||
|---|---|---|---|---|---|---|
| mPOCT group (n=307) | Control group (n=306) | mPOCT group (n=100) | Control group (n=102) | |||
| Age, years | 62 (41–74) | 63 (47–76) | 63 (42–73) | 65 (51–78) | ||
| Sex | ||||||
| Female | 175 (57%) | 157 (51%) | 53 (53%) | 47 (46%) | ||
| Male | 132 (43%) | 148 (48%) | 47 (47%) | 55 (54%) | ||
| Missing | 0 | 1 (<1%) | 0 | 0 | ||
| Ethnic origin | ||||||
| White British | 285 (93%) | 284 (93%) | 95 (95%) | 94 (93%) | ||
| Other | 22 (7%) | 21 (7%) | 5 (5%) | 7 (7%) | ||
| Missing | 0 | 1 (<1%) | 0 | 1 (2%) | ||
| Current smoker | ||||||
| Yes | 71 (23%) | 70 (23%) | 23 (23%) | 24 (24%) | ||
| No | 235 (77%) | 235 (77%) | 77 (77%) | 78 (76%) | ||
| Missing | 1 (<1%) | 0 | 0 | 0 | ||
| Influenza vaccine* | ||||||
| Yes | 199 (65%) | 185 (60%) | 60 (60%) | 56 (55%) | ||
| No | 107 (35%) | 117 (38%) | 40 (40%) | 46 (45%) | ||
| Missing | 1 (<1%) | 4 (1%) | 0 | 0 | ||
| Pregnant | ||||||
| Yes | 4 (1%) | 9 (3%) | 1 (1%) | 2 (2%) | ||
| No | 302 (98%) | 297 (97%) | 99 (99%) | 100 (98%) | ||
| Missing | 1 (<1%) | 0 | 0 | 0 | ||
| Duration of symptoms, days† | 4 (3–6) | 4 (3–7) | 4 (3–7) | 4 (3–6) | ||
| Antibiotics within 14 days before presentation | ||||||
| Yes | 95 (31%) | 99 (33%) | 30 (30%) | 34 (33%) | ||
| No | 209 (68%) | 205 (67%) | 70 (70%) | 68 (67%) | ||
| Missing | 3 (1%) | 2 (1%) | 0 | 0 | ||
| Antivirals within 14 days before presentation | ||||||
| Yes | 0 | 2 (1%) | 0 | 1 (1%) | ||
| No | 305 (99%) | 302 (99%) | 100 (100%) | 101 (99%) | ||
| Missing | 2 (1%) | 2 (1%) | 0 | 0 | ||
| Comorbidities | ||||||
| Hypertension | 82 (27%) | 89 (29%) | 26 (26%) | 29 (28%) | ||
| Cardiovascular disease | 61 (20%) | 64 (21%) | 22 (22%) | 17 (17%) | ||
| Respiratory disease | 216 (70%) | 189 (62%) | 60 (60%) | 49 (48%) | ||
| Renal disease | 16 (5%) | 18 (6%) | 4 (4%) | 6 (6%) | ||
| Liver disease | 2 (1%) | 6 (2%) | 1 (1%) | 0 | ||
| Diabetes | 36 (12%) | 57 (19%) | 12 (12%) | 15 (15%) | ||
| Cancer | 18 (6%) | 21 (7%) | 7 (7%) | 9 (9%) | ||
| Immunosuppression | 14 (5%) | 12 (4%) | 3 (3%) | 4 (4%) | ||
| Charlson Comorbidity Index score24 | 4 (4–7) | 4 (3–8) | 4 (0–4) | 4 (0–7) | ||
| Observations at admission | ||||||
| Temperature, °C | 37·1 (36·5–38·1) | 37·1 (36·4–37·9) | 37·3 (36·7–38·1) | 37·5 (36·7–38·3) | ||
| ≥38°C | 82 (27%) | 70 (23%) | 30 (30%) | 35 (34%) | ||
| Pulse rate, beats per min | 100 (88–117) | 100 (85–115) | 103 (90–116) | 100 (85–115) | ||
| Respiratory rate, breaths per min | 23 (20–26) | 22 (19–27) | 22 (19–27) | 22 (19–28) | ||
| Oxygen saturation, % | 95 (92–97) | 95 (92–97) | 95 (93–97) | 95 (91–97) | ||
| Supplementary oxygen | 54 (18%) | 71 (23%) | 21 (21%) | 22 (22%) | ||
| Blood pressure, mm Hg | ||||||
| Systolic | 134 (120–150) | 135 (120–148) | 135 (122–148) | 136 (117–146) | ||
| Diastolic | 74 (63–84) | 75 (65–83) | 71 (64–81) | 75 (65–84) | ||
| NEWS2 score | 4 (3–6) | 4 (3–6) | 4 (3–7) | 5 (3–7) | ||
| Laboratory and radiology | ||||||
| C-reactive protein, mg/L | 48 (13–127) | 38 (14–99) | 51 (17–138) | 49 (18–98) | ||
| White blood cell count, 109 per L | 10·5 (7·9–14·6) | 9·9 (7·3–13·3) | 8·7 (6·7–11·5) | 8·1 (5·8–11·0) | ||
| Neutrophils, 109 per L | 8·1 (5·8–12·1) | 7·8 (5·2–10·9) | 6·6 (5·1–9·6) | 6·0 (4·0–8·1) | ||
| Lymphocytes, 109 per L | 1·1 (0·7–1·6) | 1·1 (0·7–1·7) | 0·9 (0·6–1·4) | 0·8 (0·6–1·4) | ||
| Chest x-ray | ||||||
| Yes | 302 (98%) | 299 (98%) | 98 (98%) | 102 (100%) | ||
| No | 5 (2%) | 25 (8%) | 2 (2%) | 0 | ||
| Chest CT | ||||||
| Yes | 19 (6%) | 25 (8%) | 2 (2%) | 4 (4%) | ||
| No | 288 (94%) | 281 (92%) | 98 (98%) | 98 (96%) | ||
| Final diagnosis based on hospital discharge summary | ||||||
| Asthma | 59 (19%) | 55 (18%) | 22 (22%) | 14 (14%) | ||
| COPD exacerbation | 77 (25%) | 79 (26%) | 19 (19%) | 23 (23%) | ||
| Exacerbation of another chronic lung disease | 8 (3%) | 9 (3%) | 2 (2%) | 1 (1%) | ||
| Pneumonia | 88 (29%) | 71 (23%) | 23 (23%) | 20 (20%) | ||
| Influenza-like illness | 27 (9%) | 38 (12%) | 21 (21%) | 32 (31%) | ||
| NPLRTI | 31 (10%) | 38 (12%) | 12 (12%) | 11 (11%) | ||
| Other | 17 (6%) | 16 (5%) | 1 (1%) | 1 (1%) | ||
Data are n (%) or median (IQR). COPD=chronic obstructive airways disease. ITT=intention-to-treat. mPOCT=molecular point-of-care test. NEWS2=national early warning score 2. NPLRTI=non-pneumonic lower respiratory tract infection.
For the current influenza season.
Before admission to hospital.
In the analysis of all patients (ie, the ITT population), the median turnaround time for influenza test results was 1·2 h (IQR 1·1–1·4) in the mPOCT group and 22·6 h (16·0–28·7) in the control group (difference of 21·4 h [95% CI 20·6–22·4; p<0·0001]; table 2 ). All antiviral use was with the neuraminidase inhibitor oseltamivir and no difference was seen in the proportion of patients given antivirals between the groups (relative risk 1·1 [95% CI 0·9–1·3; p=0·49]; table 2); however, more patients given antivirals in the mPOCT group than in the control group tested positive for influenza (99 [71%] of 139 vs 63 [48%] of 130; relative risk 1·5 [1·2–1·8; p<0·0001]; table 2). Patients who tested negative for influenza who were given antivirals had a median course length of 0·1 days (IQR 0·1–0·5; ie, a single dose) in the mPOCT group versus 1·5 days (0·5–4·5) in the control group (difference of 1·4 days [95% CI 0·7–2·1; p<0·0001]; table 2). 106 (35%) of 307 patients in the mPOCT group were isolated in single-room accommodation versus 67 (22%) of 306 in the control group (relative risk 1·6 [95% CI 1·2–2·1; p=0·0006]; table 2). Information on antibiotic use in the mPOCT and control groups can be found in the appendix (p 4).
Table 2.
Influenza testing, antiviral use, and isolation facility use in all patients (ITT population)
| mPOCT group (n=307) | Control group (n=306) | Difference or relative risk (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Tested for influenza during admission | 307 (100%) | 212 (69%) | 1·4 (1·4 to 1·5) | <0·0001 | ||
| Turnaround time for results, h* | 1·2 (1·1 to 1·4) | 22·6 (16·0 to 28·7) | 21·4 (20·6 to 22·4) | <0·0001 | ||
| Influenza infected | 100 (33%) | 102 (33%) | 1·0 (0·9 to 1·2) | 0·86 | ||
| Influenza A | 63/100 (63%) | 76/102 (75%) | .. | .. | ||
| Influenza B | 37/100 (37%) | 24/102 (24%) | .. | .. | ||
| Influenza A and B | 0/100 (0%) | 2/102 (2%) | .. | .. | ||
| Antiviral use | ||||||
| Total antiviral use | 139 (45%) | 130 (42%) | 1·1 (0·9 to 1·3) | 0·49 | ||
| Oseltamivir | 139 (45%) | 130 (42%) | .. | .. | ||
| Other | 0 | 0 | .. | .. | ||
| Patients testing positive for influenza | 99/139 (71%) | 63/130 (48%) | 1·5 (1·2 to 1·8) | <0·0001 | ||
| Duration of antivirals, days | ||||||
| All patients treated with antivirals | 4·7 (0·7 to 5·1) | 4·5 (1·0 to 5·0) | −0·2 (−0·5 to 0·0) | 0·11 | ||
| Influenza positive | 4·8 (4·6 to 5·5) | 4·5 (4·2 to 5·0) | −0·3 (−0·6 to −0·1) | 0·0024 | ||
| Influenza negative | 0·1 (0·1 to 0·5) | 1·5 (0·5 to 4·5) | 1·4 (0·7 to 2·1) | <0·0001 | ||
| Isolation facility use† | ||||||
| Isolated at presentation | 30 (10%) | 26 (9%) | 1·2 (0·7 to 1·9) | 0·58 | ||
| Isolated at any time | 106 (35%) | 67 (22%) | 1·6 (1·2 to 2·1) | 0·0006 | ||
| Influenza positive | 70/106 (66%) | 39/67 (58%) | 1·1 (0·9 to 1·5) | 0·33 | ||
| Duration of isolation, days | ||||||
| All isolated patients | 2·5 (1·3 to 4·5) | 1·8 (1·0 to 4·4) | −0·7 (−0·9 to 0·3) | 0·29 | ||
| Influenza positive | 2·8 (1·9 to 4·5) | 2·4 (1·0 to 4·7) | −0·4 (−1·2 to 0·3) | 0·21 | ||
| Influenza negative | 1·4 (0·5 to 3·5) | 1·4 (1·0 to 3·9) | 0·0 (−0·5 to 0·8) | 0·42 | ||
Data are n (%), n/N (%), or median (IQR) unless otherwise stated. p values were calculated using χ2 test or Fischer's exact test. ITT=intention-to-treat. mPOCT=molecular point-of-care test.
Based on those that were tested; for mPOCT group, this is the turnaround time for mPOCT results, for the control group, it is for laboratory PCR results.
Isolation facility use relates to the use of single-room accommodation or dedicated influenza cohort bays for patients.
The ITT influenza-infected population comprised 100 (33%) of 307 patients in the mPOCT group and 102 (33%) of 306 patients in the control group who were infected with influenza. 100 (100%) of 100 patients with influenza in the mPOCT group were diagnosed with influenza while in hospital (all by PCR testing) but only 60 (59%) of 102 in the control group were diagnosed while in the hospital, of whom 55 (54%) were PCR positive and five (5%) had a clinical diagnosis of influenza but had no confirmatory test performed (relative risk 1·7 [95% CI 1·7 to 1·7; p<0·0001]; table 3 ). 42 patients in the control group not diagnosed during admission were retrospectively diagnosed with influenza after testing of their stored samples using the FilmArray Respiratory Panel 2.
Table 3.
Outcomes in patients in the ITT influenza-infected population
| mPOCT group (n=100) | Control group (n=102) | Difference or relative risk (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Diagnosis of influenza during admission | 100 (100%) | 60 (59%) | 1·7 (1·7 to 1·7) | <0·0001 | ||
| PCR positive | 100 (100%) | 55 (54%) | .. | .. | ||
| Clinical diagnosis* | 0 | 5 (5%) | .. | .. | ||
| Turnaround time of results, h | 1·2 (1·1 to 1·4) | 23·1 (13·8 to 31·5) | 21·9 (20·2 to 23·8) | <0·0001 | ||
| Antiviral use | 99 (99%) | 63 (62%) | 1·6 (1·4 to 1·9) | <0·0001 | ||
| Empirical antiviral use† | 30/99 (30%) | 41/63 (65%) | 0·6 (0·4 to 0·7) | <0·0001 | ||
| Directed use‡ | 69/99 (70%) | 22/63 (35%) | 2·0 (1·4 to 2·9) | .. | ||
| Time to antiviral use, h | 1·0 (0·0 to 2·0) | 6·0 (0·0 to 12·0) | 5·0 (0·0 to 6·0) | 0·0039 | ||
| Time to antiviral use, excluding those treated empirically, h | 2·0 (1·0 to 3·0) | 30·5 (12·0 to 59·0) | 28·5 (18·0 to 31·0) | <0·0001 | ||
| Isolation facility use§ | ||||||
| Isolated empirically at presentation | 10 (10%) | 9 (9%) | 1·1 (0·5 to 2·7) | 0·77 | ||
| Isolated during hospital stay | 70 (70%) | 39 (38%) | 1·8 (1·4 to 2·4) | <0·0001 | ||
| Isolated during hospital stay, excluding exceptions¶ | 70/76 (92%) | 39/90 (43%) | 2·1 (1·7 to 2·8) | <0·0001 | ||
| Time to isolation, h | 4·5 (1·9 to 9·8) | 25·0 (3·6 to 45·1) | 20·5 (6·0 to 26·5) | 0·0003 | ||
| Time to isolation, excluding those isolated empirically, h | 5·0 (2·0 to 12) | 32·0 (16·0 to 55·0) | 27·0 (17·0 to 33·0) | <0·0001 | ||
| Antibiotic use | ||||||
| Antibiotic use (any) | 90 (90%) | 98 (96%) | 0·9 (0·9 to 1·0) | 0·089 | ||
| Duration of antibiotics, days | 6·1 (4·1 to 7·2) | 5·8 (4·2 to 7·4) | −0·3 (−0·8 to 0·8) | 0·96 | ||
| Single dose of antibiotics only | 9/90 (10%) | 6/98 (6%) | 0·9 (0·9 to 1·1) | 0·32 | ||
| Antibiotics given for <24 h | 13/90 (14%) | 7/98 (7%) | 0·9 (0·8 to 1·1) | 0·10 | ||
| Intravenous antibiotics | 68 (68%) | 69 (68%) | 1·0 (0·8 to 1·2) | 0·96 | ||
| Duration of intravenous antibiotics, days | 0·5 (0·1 to 1·8) | 1·1 (0·1 to 2·0) | 0·6 (0 to 0·5) | 0·31 | ||
| Single dose of intravenous antibiotics only | 29/68 (43%) | 19/68 (28%) | 0·8 (0·6 to 1·0) | 0·064 | ||
| Intravenous antibiotics given for <24 h | 40/68 (59%) | 34/68 (50%) | 0·8 (0·6 to 1·2) | 0·26 | ||
| Clinical outcome measures | ||||||
| Length of hospital stay, days | 2·7 (1·1 to 4·2) | 2·7 (1·1 to 5·4) | 0·0 (−0·4 to 0·9) | 0·37 | ||
| Discharged within 24 h | 23 (23%) | 18 (18%) | 0·9 (0·8 to 1·1) | 0·34 | ||
| Duration of supplementary oxygen, h | 32·0 (15·0 to 59·0) | 33·0 (14·0 to 81·0) | 1·0 (−8·0 to 18·0) | 0·48 | ||
| Time to clinical improvement, h‖ | 8·8 (3·2 to 23·9) | 13·9 (5·1 to 42·5) | 5·1 (−0·3 to 9·9) | 0·077 | ||
| Hospital recovery score at day 7 after admission‖ | ||||||
| 1 Home | 90 (90%) | 82 (80%) | NA | 0·045 | ||
| 2 Hospital ward not requiring oxygen | 8 (8%) | 12 (12%) | NA | .. | ||
| 3 Hospital ward requiring oxygen | 2 (2%) | 5 (5%) | NA | .. | ||
| 4 ICU, not ventilated | 0 | 1 (1%) | NA | .. | ||
| 5 ICU, ventilated | 0 | 1 (1%) | NA | .. | ||
| 6 Death | 0 | 1 (1%) | NA | .. | ||
Data are n (%), n/N (%), or median (IQR) unless otherwise stated. p values for the difference between proportions were calculated using the χ2 or Fisher's exact test as appropriate, and for continuous variables (ie, difference in medians) using the Mann-Whitney U test. ICU=intensive care unit. ITT=intention-to-treat. NA=not applicable.
A clinical diagnosis of influenza is defined as either influenza listed as a diagnosis on the discharge summary or a full 5-day course of oseltamivir given, in the absence of diagnostic testing being done.
Empirical antiviral use denotes antivirals started in patients with a presumptive clinical diagnosis of influenza before diagnostic results were available.
Directed antiviral use denotes antiviral use started once diagnosis has been confirmed by a positive diagnostic test; all antiviral use was with oseltamivir.
Isolation facility use relates to the use of single room accommodation or dedicated influenza cohort bays for patients.
Exceptions are patients discharged rapidly or nursed in areas without isolation facilities.
Time to clinical improvement and the hospital recovery scale (HRS) are defined in the supplementary appendices.
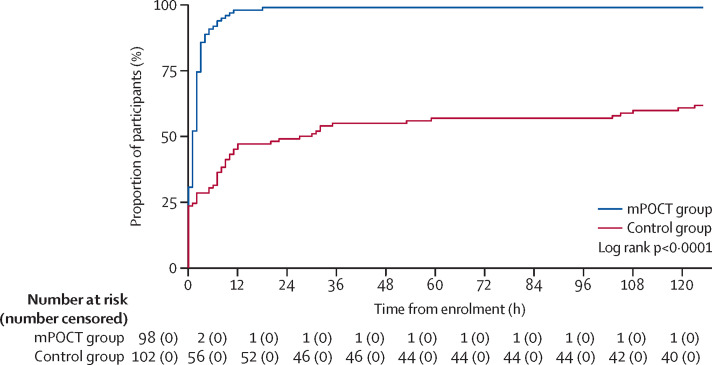
For the primary outcome, 99 (99%) of 100 patients with influenza in the mPOCT group were given antiviral treatment within 5 days versus 63 (62%) of 102 in the control group (relative risk 1·6 [95% CI 1·4 to 1·9; p<0·0001]; number needed to test was 2·7; table 3). Median time to receipt of antivirals was 1·0 h (0·0 to 2·0) in the mPOCT group versus 6·0 h (0·0 to 12·0) in the control group (difference of 5·0 h [95% CI 0·0 to 6·0; p=0·0039; table 3, figure 2 ). In a post-hoc analysis, excluding patients treated empirically (ie, before results were available) the median time to receipt of antivirals was 2·0 h (IQR 1·0 to 3·0) in the mPOCT group versus 30·5 h (12·0 to 59·0) in the control group (difference of 28·5 h [95% CI 18·0 to 31·0; p<0·0001]; table 3; appendix p 6). Duration of antiviral treatment in patients who tested positive for influenza was 4·8 days (IQR 4·6 to 5·5) in the mPOCT group and 4·5 days (4·2 to 5·0) in the control group (difference of −0·3 days [95% CI −0·6 to −0·1; p=0·0024]; table 2).
Figure 2.
Antiviral use over time in patients with influenza (ITT influenza-infected population)
ITT=intention-to-treat. mPOCT=molecular point-of-care test.
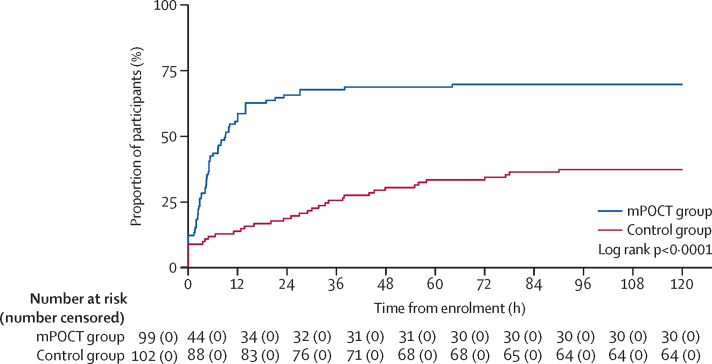
At presentation, few patients who were infected with influenza were correctly isolated in single-room accommodation or dedicated influenza cohort bays empirically: ten (10%) of 100 in the mPOCT group and nine (9%) of 102 in the control group (relative risk 1·1 [95% CI 0·5–2·7; p=0·77]; table 3). During their hospital stay, 70 (70%) of 100 patients with influenza in the mPOCT group were correctly isolated in single-room accommodation or dedicated influenza cohort bays versus 39 (38%) of 102 in the control group (relative risk 1·8 [1·4–2·4; p<0·0001]; table 3). In the mPOCT group, 76 patients who were infected with influenza and 90 patients in the control group were admitted for more than 24 h and nursed in an area with side rooms (appendix p 6). Excluding those rapidly discharged on the same day as they were admitted or cared for in an area without single-room accommodation (eg, high dependency areas), 70 (92%) of 76 patients with influenza in the mPOCT group were correctly isolated to single-room accommodation versus 39 (43%) of 90 in the control group (relative risk 2·1 [1·7–2·8; p<0·0001]). Patients infected with influenza were isolated after a median of 4·5 h (IQR 1·9–9·8) in the mPOCT group versus 25·0 h (3·6–45·1) in the control group, (difference of 20·0 h [95% CI 6·0–26·5; p=0·0003]; table 3, figure 3 ). For patients who were infected with influenza who were not initially isolated but who were subsequently isolated, isolation occurred after a median of 5·0 h (IQR 2·0–12·0) in the mPOCT group versus 32·0 h (16·0–55·0) in the control group (difference of 27·0 h [95% CI 17·0–33·0; p<0·0001]; table 3; appendix p 6).
Figure 3.
Isolation facility use over time in patients with influenza (ITT influenza-infected population)
ITT=intention-to-treat. mPOCT=molecular point-of-care test.
90 (90%) of 100 patients with influenza in the mPOCT group were given antibiotics versus 98 (96%) of 102 in the control group (relative risk 0·9 [95% CI 0·9 to 1·0; p=0·089]; table 3). Patients in the mPOCT group received antibiotics for a median of 6·1 days (IQR 4·1 to 7·2) versus 5·8 days (4·2 to 7·4) in the control group (difference of −0·3 [95% CI −0·8 to 0·8; p=0·96]; table 3). 13 (14%) of 90 patients who were given antibiotics in the mPOCT group received antibiotics for less than 24 h versus seven (7%) of 98 in the control group (relative risk 0·9 [95% CI 0·8 to 1·1; p=0·10]; table 3).
Patients testing positive for influenza had a median hospital stay of 2·7 days (IQR 1·1 to 4·2) in the mPOCT group versus 2·7 days (1·1 to 5·4) in the control group (difference of 0·0 days [95% CI −0·4 to 0·9; p=0·37]; table 3). The exploratory outcome of median hospital recovery scale score at 4 days after admission to hospital was no different between groups (p=0·099), but at 7 days after admission it was lower in the mPOCT group than in the control group (p=0·045; table 3; appendix p 7). Median time to clinical improvement was 8·8 h (IQR 3·2 to 23·9) in the mPOCT group versus 13·9 h (5·1 to 42·5) in the control group (difference of 5·1 h [95% CI −0·3 to 9·9; p=0·077; table 3). Time-to-event analysis showed a non-significantly reduced time to clinical improvement in the mPOCT group compared with in the control group (log-rank test p=0·056; appendix p 8). Patients with influenza were treated with supplemental oxygen for a median of 32·0 h (IQR 15·0 to 59·0) versus 33·0 h (14·0 to 81·0) in the control group (difference of 1·0 h [95% CI −8·0 to 18·0; p=0·48]; table 3; appendix p 9). Time-to-event analysis did not show any difference in the time to hospital discharge between the groups (in patients with influenza, log-rank test p=0·22; appendix p 10).
19 adverse events occurred among patients with influenza in the mPOCT group compared with 34 events in the control group (table 4 ). One (1%) of 100 patients with influenza in the mPOCT group was admitted to a critical care unit compared with six (6%) of 102 in the control group (relative risk 0·2 [95% CI 0·0–1·1; p=0·058; table 4). No patients with influenza died in the mPOCT group and two (2%) died in the control group (p=0·16). Ten (10%) patients with influenza in the mPOCT group had stayed in hospital for more than 7 days versus 20 (20%) in the control group (relative risk 0·5 [0·3–1·0; p=0·055]; table 4). Details of adverse events in the entire ITT population are in the appendix (p 5).
Table 4.
Adverse events in the ITT influenza-infected population
| mPOCT group (n=100) | Control group (n=102) | Relative risk (95% CI) | p value | ||
|---|---|---|---|---|---|
| Total number of adverse events | 19 | 34 | .. | .. | |
| Patients with adverse events | 16 (16%) | 23 (23%) | 0·7 (0·4–1·3) | 0·24 | |
| ICU or HDU admission | 1 (10%)* | 6 (6%)† | 0·2 (0·0–1·1) | 0·058 | |
| Death | |||||
| In hospital | 0 | 2 (2%)‡ | NA | 0·16 | |
| Within 30 days | 0 | 2 (2%)‡ | NA | 0·16 | |
| Readmission within 30 days | 6 (6%) | 5 (5%) | 1·2 (0·4–3·8) | 0·76 | |
| Re-presentation to hospital but without admission, within 30 days | 2 (2%) | 1 (1%) | 2·0 (0·2–21·7) | 0·56 | |
| Hospital stay of >7 days | 10 (10%) | 20 (20%) | 0·5 (0·3–1·0) | 0·055 | |
Data are n or n (%) unless otherwise stated. p values for the difference between proportions were calculated using the χ2 or Fisher's exact test as appropriate, and for continuous variables (ie, difference in medians) using the Mann-Whitney U test. HDU=high dependency unit. ICU=intensive care unit. ITT=intention-to-treat. mPOCT=molecular point-of-care test. NA=not applicable.
Influenza A H3.
Influenza A H3 n=2, influenza A H1 n=1, influenza A untypable n=1, and influenza B n=2.
Influenza B n=1 and influenza A untypable n=1.
Discussion
The routine use of a molecular point-of-care test-and-treat strategy for influenza during influenza season led to improved detection of patients infected with influenza who had been admitted to hospital and delivery of results in near real time to clinical and infection control teams. Use of neuraminidase inhibitors and isolation facilities were improved with mPOCT, such that patients testing positive for influenza were rapidly treated with antivirals and appropriately isolated. Additionally, routine mPOCT was also associated with improvements in the exploratory clinical outcome measure of hospital recovery scale in patients with influenza.
The high proportion of patients with influenza who remained undiagnosed in the control group is likely to relate to low rates of test requests by clinicians in patients not displaying the classic symptoms or signs of influenza. Influenza testing in most institutions is not done routinely but is based on clinical suspicion of influenza, which is known to be highly inaccurate, even during periods of intense transmission.6 The risk of nosocomial transmission of influenza from patients who are undiagnosed to staff and other patients is high, and undiagnosed patients do not have the opportunity to benefit from antiviral therapy. These factors make a compelling argument for health-care centres to institute routine mPOCT for influenza in all patients admitted to hospital with acute respiratory illness during influenza season.
Our study showed rapid time to results with mPOCT of just over 1 h compared with almost 1 day with laboratory PCR in the control group, a typical laboratory turnaround time reported in other studies.7, 25 The importance of rapid turnaround times in improving clinical management has been shown in several studies26, 27 and is supported by similar findings in studies of other diagnostics in emergency departments.28 Some of the benefits associated with mPOCT in this study might also be achieved by routine laboratory PCR testing if turnaround times could be substantially reduced. However, because turnaround times of 1 h or less are unlikely to be achievable in most laboratory settings, testing for influenza in hospitals should move to the point of care. The mPOCT platform used in this study generated a result in 45 min but other mPOCT platforms can generate results within 20 min or less (eg, GeneXpert [Cepheid, Sunnyvale, CA, USA] and the Cobas Liat (Roche Diagnostics, Basel, Switzerland]29) and might further reduce time to therapeutic and infection control interventions.
The use of influenza antivirals was significantly improved with routine mPOCT compared with routine clinical care, such that nearly all patients with influenza were given appropriate antiviral treatment within a few hours of admission. Patients not initially suspected to have influenza who subsequently had a positive mPOCT were rapidly started on neuraminidase inhibitors compared with a delay of over 24 h in the control group. Conversely, patients who were started on neuraminidase inhibitors empirically because of clinical suspicion of them having influenza, but who subsequently tested negative by mPOCT, had their neuraminidase inhibitors stopped after a single dose compared with an extended duration of unnecessary drug use in the control group. These findings are consistent with previous randomised controlled trials and observational studies of mPOCT for influenza that consistently show improved use of directed neuraminidase inhibitors in patients tested by mPOCT.7,16–19 With the use of even faster mPOCTs than that used in this study, done at the time of presentation, the need for empirical therapy could be removed altogether without compromising the speed of administration of antivirals and ushering in an era of real-time pathogen-directed therapy for influenza and other infections.30
Single-room accommodation in hospitals, used for isolating patients who are potentially infectious, is a scarce resource in most UK hospitals. Our study shows that in routine clinical care most patients with influenza are not isolated appropriately in single-room accommodation or in influenza cohort bays, and are hence a high risk for nosocomial transmission to other patients. In comparison, most patients with influenza in the mPOCT group were correctly isolated. Additionally, patients with influenza in the control group who were not initially isolated but subsequently had a positive laboratory PCR result for influenza were only isolated after a delay of nearly 1·5 days (reflecting the long turnaround time of laboratory PCR tests) compared with just a few hours in the mPOCT group. The rational and appropriate use of isolation facilities is hugely important for UK hospitals to prevent nosocomial transmission of influenza and to maintain patient flow though acute areas during periods of intense influenza activity.
Routine mPOCT use was not associated with major reductions in antibiotic use in patients with influenza in this trial and most patients with influenza in both groups were given antibiotics, consistent with other similar trials.7, 21 Other studies of mPOCT for respiratory viruses have shown similar results with either no effect on antibiotic use or only slight reductions.7,15–19 The identification of a virus by PCR does not by itself rule out the presence of a concomitant bacterial infection and so clinicians tend to continue to treat patients admitted to hospital with an acute respiratory illness with antibiotics. Alternative strategies to reduce unnecessary antibiotics are still urgently needed in this group and include combining pathogen detection with biomarker measurement (such as C-reactive protein or procalcitonin) or with other markers of host response, all integrated in a robust antibiotic stewardship programme.
In addition to observing the above improvements in clinical management, to our knowledge, this is the first randomised trial to show improvements in clinical outcomes in patients with influenza through the use of mPOCTs. Although this study cannot directly attribute the cause of these changes, the possible trend towards improvement in time to clinical improvement and the improvement in hospital recovery score are likely to relate to the rapid and near universal use of oseltamivir in patients infected with influenza in the mPOCT group compared with frequently absent or delayed use with routine care. Although no placebo controlled trials of neuraminidase inhibitors for influenza have ever been done in adults admitted to hospital, our findings are consistent with a large body of observational evidence showing that use of neuraminidase inhibitors is associated with improvements in clinical outcomes, including length of stay and mortality, in this group10, 11, 22, 23, 31 and that early use of neuraminidase inhibitors is associated with the best outcomes.10, 11
The strengths of this study include the multicentre and pragmatic design of the study, that it was run over two influenza seasons, the broad inclusion criteria representing typical patients admitted to UK secondary care, the simple intervention, and comparison to routine clinical care. These factors suggest that the findings of this study are likely to be generalisable to other similar UK and international centres. The trial was designed and powered to assess influenza-specific outcomes in adults admitted to hospital and universal sampling with retrospective testing for influenza in the control group using the same platform allowed a direct assessment of missed diagnoses and comparison of outcomes in all patients with influenza. The use of clinical outcome measures such as time on supplementary oxygen, time to clinical improvement, and hospital recovery score allowed the assessment of the effect of mPOCT on clinical outcomes in addition to changes in clinical management.
Our study has several limitations. Because the study took place only during winter months when influenza was circulating, the findings cannot be extrapolated to times outside of this period, when mPOCT for influenza is unlikely to have an effect. We made no attempt to mask clinical teams or participants to the allocated groups. Because the study required clinical teams to be informed of the mPOCT results in real time, they could not be masked to which group a participant had been randomly assigned. In our trial, the mPOCT was done by research staff rather than clinicians, and so uncertainties remain about how testing should be best delivered in routine care. Various implementation models exist including physician-delivered or nurse-delivered testing and technician-delivered testing in a POCT hub, embedded in an acute admission area. These models should be studied in implementation trials alongside obtaining real-world data to confirm the clinical impact of mPOCTs. Another uncertainty regarding the implementation of mPOCTs is the choice between syndromic testing for a comprehensive panel of respiratory viruses or testing for influenza viruses alone. Our previous work32 suggests that the effect on antibiotic use and length of stay in hospital will be greatest when the syndromic approach is used, although this approach must be balanced against the current higher cost and longer turnaround times of most current systemic panels than tests for influenza alone.32
In summary, a routine molecular point-of-care test-and-treat strategy for influenza in adults who have been admitted to hospital with an acute respiratory illness improved detection of influenza, appropriate use of influenza antivirals, and the rational use of isolation facilities, and was associated with improvements in clinical outcomes in patients infected with influenza. Routine mPOCT for influenza should become the standard of care in hospitals during the influenza season.
Data sharing
All de-identified participant data analysed and presented in this study are available from the corresponding author following publication, on reasonable request.
Acknowledgments
Acknowledgments
We thank all the patients recruited at the Southampton General Hospital and Hampshire County Hospital and all the clinical staff involved, including doctors, nurses, and laboratory technicians. We thank the directors, research nurses, data managers, clinical trials assistants, and laboratory staff at the National Institute for Health Research (NIHR) Clinical Research Facility and the NIHR Southampton Biomedical Research Centre. We thank the staff at the research and development department, University Hospital Southampton NHS Foundation Trust, and the NIHR Clinical Research Network, Wessex, for their support throughout the trial. In particular, we thank the data analysts Florina Borca and Hang Phan at the Clinical Informatics Research Unit - Access Extract Integrate Safe data, NIHR Southampton Biomedical Research Centre team for their help with data analysis. Finally, we thank Kordo Saeed, consultant microbiologist, and Ana Maria Arias, research nurse, at the Royal Hampshire County Hospital for all their assistance during the trial. This study was funded by the NIHR Post Doctoral Fellowship Programme and was supported by the NIHR Southampton Respiratory Biomedical Research Centre and the NIHR Clinical Research Network, Wessex. The molecular analysers were leased and the test kits purchased independently from the UK distributor bioMérieux UK. The manufacturer of the molecular test platform (BioFire Diagnostics, a bioMérieux company, Salt Lake City, Utah, USA) had no role in the study conception, design, data analysis, or manuscript preparation. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Contributors
TWC reviewed the medical literature, conceived of and designed the study, oversaw the conduct of the study, participated in the collection of data and interpretation of results, and drafted and wrote the manuscript. KRB reviewed the medical literature, participated in the trial design, recruited patients, generated and collected data, and assisted with drafting the manuscript. NJB, AKM, SM, CC, SP, EN, and LP screened and recruited patients and collected data. NC acted as principal investigator for the second trial site and oversaw the running of the study there. SE designed the statistical analysis plan and analysed the data. All authors reviewed and contributed to the manuscript during its development. TWC and SE verified the underlying data for this study.
Declaration of interests
TWC has received speaker fees, honoraria, travel reimbursement, and equipment and consumables free of charge for the purposes of research outside of this submitted study, from BioFire Diagnostics and bioMérieux; received consultancy fees from Synairgen Research, Randox Laboratories, and Cidara therapeutics; is a member of an advisory board for Roche and of two independent data monitoring committees for trials sponsored by Roche; and has acted as the UK chief investigator for an investigational medicinal product study sponsored by Janssen. SM has received speaker fees and travel reimbursement from bioMérieux. All other authors declare no competing interests.
Supplementary Material
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Dao CN, Kamimoto L, Nowell M, et al. Adult hospitalizations for laboratory-positive influenza during the 2005–2006 through 2007–2008 seasons in the United States. J Infect Dis. 2010;202:881–888. doi: 10.1086/655904. [DOI] [PubMed] [Google Scholar]
- 3.Troeger CE, Blacker BF, Khalil IA, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauskopf J, Klesse M, Lee S, Herrera-Taracena G. The burden of influenza complications in different high-risk groups: a targeted literature review. J Med Econ. 2013;16:264–277. doi: 10.3111/13696998.2012.752376. [DOI] [PubMed] [Google Scholar]
- 6.Dugas AF, Valsamakis A, Atreya MR, et al. Clinical diagnosis of influenza in the ED. Am J Emerg Med. 2015;33:770–775. doi: 10.1016/j.ajem.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): a pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5:401–411. doi: 10.1016/S2213-2600(17)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Public Health England . Public Health England; London: September, 2019. Guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza Version 10.0, September 2019.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833572/PHE_guidance_antivirals_influenza_201920.pdf [Google Scholar]
- 9.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68:895–902. doi: 10.1093/cid/ciy874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. doi: 10.1016/S2213-2600(14)70041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzen J, Kohn R, Houk JL, Ison MG. Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis. 2019;69:52–58. doi: 10.1093/cid/ciy860. [DOI] [PubMed] [Google Scholar]
- 12.Brendish NJ, Schiff HF, Clark TW. Point-of-care testing for respiratory viruses in adults: the current landscape and future potential. J Infect. 2015;71:501–510. doi: 10.1016/j.jinf.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang HS, Tsai CL, Chang J, Hsu TC, Lin S, Lee CC. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin Microbiol Infect. 2018;24:1055–1063. doi: 10.1016/j.cmi.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merckx J, Wali R, Schiller I, et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167:394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 15.Vos LM, Bruning AHL, Reitsma JB, et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis. 2019;69:1243–1253. doi: 10.1093/cid/ciz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trabattoni E, Le V, Pilmis B, et al. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an ED. Am J Emerg Med. 2018;36:916–921. doi: 10.1016/j.ajem.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Vecino-Ortiz AI, Goldenberg SD, Douthwaite ST, et al. Impact of a multiplex PCR point-of-care test for influenza A/B and respiratory syncytial virus on an acute pediatric hospital ward. Diagn Microbiol Infect Dis. 2018;91:331–335. doi: 10.1016/j.diagmicrobio.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egilmezer E, Walker GJ, Bakthavathsalam P, et al. Systematic review of the impact of point-of-care testing for influenza on the outcomes of patients with acute respiratory tract infection. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youngs J, Marshall B, Farragher M, et al. Implementation of influenza point-of-care testing and patient cohorting during a high-incidence season: a retrospective analysis of impact on infection prevention and control and clinical outcomes. J Hosp Infect. 2019;101:276–284. doi: 10.1016/j.jhin.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Beard K, Brendish N, Malachira A, et al. Pragmatic multicentre randomised controlled trial evaluating the impact of a routine molecular point-of-care ‘test-and-treat’ strategy for influenza in adults hospitalised with acute respiratory illness (FluPOC): trial protocol. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branche AR, Walsh EE, Vargas R, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. J Infect Dis. 2015;212:1692–1700. doi: 10.1093/infdis/jiv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesan S, Myles PR, Bolton KJ, et al. Neuraminidase inhibitors and hospital length of stay: a meta-analysis of individual participant data to determine treatment effectiveness among patients hospitalized with nonfatal 2009 pandemic influenza A(H1N1) virus infection. J Infect Dis. 2020;221:356–366. doi: 10.1093/infdis/jiz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Chu HY, Englund JA, Huang D, et al. Impact of rapid influenza PCR testing on hospitalization and antiviral use: a retrospective cohort study. J Med Virol. 2015;87:2021–2026. doi: 10.1002/jmv.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brendish NJ, Malachira AK, Beard KR, Ewings S, Clark TW. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a post hoc analysis from a randomised controlled trial. Eur Respir J. 2018;52 doi: 10.1183/13993003.00555-2018. [DOI] [PubMed] [Google Scholar]
- 27.Wabe N, Li L, Dahm MR, et al. Timing of respiratory virus molecular testing in emergency departments and its association with patient care outcomes: a retrospective observational study across six Australian hospitals. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Georgiou A, Vecellio E, et al. The effect of laboratory testing on emergency department length of stay: a multihospital longitudinal study applying a cross-classified random-effect modeling approach. Acad Emerg Med. 2015;22:38–46. doi: 10.1111/acem.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee D, Kanwar N, Hassan F, Essmyer C, Selvarangan R. Comparison of six sample-to-answer influenza A/B and respiratory syncytial virus nucleic acid amplification assays using respiratory specimens from children. J Clin Microbiol. 2018;56:e00930–e01018. doi: 10.1128/JCM.00930-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brendish NJ, Clark TW. Molecular point-of-care testing for influenza to improve early neuraminidase inhibitor treatment and outcomes in hospitalized adults. Clin Infect Dis. 2019;68:2154–2155. doi: 10.1093/cid/ciy1042. [DOI] [PubMed] [Google Scholar]
- 31.Lee N, Chan PK, Choi KW, et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12:501–508. [PubMed] [Google Scholar]
- 32.Brendish NJ, Mills S, Ewings S, Clark TW. Impact of point-of-care testing for respiratory viruses on antibiotic use in adults with exacerbation of airways disease. J Infect. 2019;79:357–362. doi: 10.1016/j.jinf.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All de-identified participant data analysed and presented in this study are available from the corresponding author following publication, on reasonable request.